Abstract
Global warming and climate changes have a detrimental impact on poultry production, causing substantial economic losses. This study investigated the effects of incorporating dietary betaine (BT) and organic minerals (OMs) on broilers’ performance as well as their potential to mitigate the negative impacts of heat stress (HS). Six hundred 1-day-old Ross 308 chicks were randomly allocated to 12 experimental treatments with 5 replicates of 10 birds each (5 male + 5 female). The birds were provided with diets containing BT (0 and 2,000 ppm) and OMs (0, 250, and 500 ppm), either individually or in combination, under both thermoneutral and HS-inducing temperatures. The HS conditions involved exposing the birds to cyclic periods of elevated temperature (35°C ± 2°C) for 6 h daily, from 10:00 am to 4:00 pm, starting from d 10 and continuing until d 35. The exposure to HS deteriorated birds’ growth performance; however, dietary BT and OMs inclusion improved the growth performance parameters bringing them close to normal levels. Carcass traits were not affected by dietary supplementation of BT, OMs, HS, or their interaction. Interestingly, while HS led to increased (P < 0.05) levels of total cholesterol, LDL-cholesterol, and hepatic malondialdehyde (MDA), these adverse effects were mitigated (P < 0.05) by the addition of BT and OMs. Moreover, dietary BT supplementation led to elevated serum total protein and globulin concentrations. Cyclic HS did not alter Mn, Zn, and Cu contents in the pectoral muscle. However, the incorporation of OMs at both levels increased concentrations of these minerals. Notably, the combination of 500 ppm OMs and 2,000 ppm BT improved Mn, Zn, Cu, and Fe digestibility, which has been compromised under HS conditions. Cyclic HS upregulated gene expression of interleukin-1β, heat shock protein 70, and Toll-like receptor-4 while downregulated the expression of claudin-1, uncoupling protein, growth hormone receptor, superoxide dismutase 1, glutathione peroxidase 1 and insulin-like growth factor 1. The aforementioned gene expressions were reversed by the combination of higher dietary levels of BT and OMs. In conclusion, the dietary supplementation of 500 ppm OMs along with 2,000 ppm BT yielded significant improvements in growth performance and mineral digestibility among broiler chickens, regardless of thermal conditions. Moreover, this combination effectively restored the expression of growth-related genes even under heat-stress conditions.
Key words: betaine, organic mineral, growth performance, heat stress, broiler
INTRODUCTION
In intensive broiler production, birds are exposed to various stress factors, mainly heat stress (Saleh et al., 2021). Heat stress is one of the severe stress factors that negatively influence broiler productivity in tropical and subtropical regions, especially in humid environments (Abdel-Moneim et al., 2021). At the same time, heat stress causes physiological abnormalities in the endocrine and immune systems and electrolyte imbalances, which are detrimental to the health and production of broilers (Nawab et al., 2018; Abdel-Moneim et al., 2022). Heat stress also damages gut structure and function (including low intestinal epithelium regeneration and integrity), decreasing broilers’ growth performance and metabolic rate (Quinteiro-Filho et al., 2010, 2012; Hirakawa et al., 2020; Guo et al., 2021). Maintaining proper temperature and humidity in modern poultry houses can help minimize heat stress. However, this approach is expensive, increases production costs, and is unavailable for most regions of third-world countries (Nawab et al., 2018). Alternatively, several studies reported that nutritional intervention such as the addition of probiotics, prebiotics, antioxidants, vitamins, organic minerals (OMs), and other natural active substances is a beneficial function strategy for mitigating the adverse effects of heat stress in broilers (Nawab et al., 2018; Abdel-Moneim et al., 2021; Saleh et al., 2021). Other practices relied on balancing the dietary energy level and supplementing antioxidants, OMs, and vitamins (Saleh et al., 2020a,b; Ashour et al., 2021).
The interest in employing natural plant extracts in poultry nutrition has recently increased. Betaine (BT) is a trimethyl glycine derivative present in various natural extracts such as sugar beet (Palmonari et al., 2021). The BT is a methyl donor that has osmoprotective properties, which help maintain cell water homeostasis without interfering with the cell's metabolism (Sakomura et al., 2013; Willingham et al., 2020; Al-Sagan et al., 2021). The BT acts as an extracellular osmolyte and chaperone, preventing or delaying stress-induced protein denaturation while assisting proteins that have already been denatured (Saeed et al., 2017; Al-Tamimi et al., 2019). Furthermore, the methyl group must be included in the poultry diet as it is required for metabolism (Summers, 2013; Chen et al., 2020), and it may contribute to increasing broilers’ body weight and meat quality (Sun et al., 2008; Attia et al., 2009). The BT also improved the regeneration of the intestinal mucosa, enhancing nutrient absorption and utilization (Mahmoudnia and Madani, 2012) and relieving the adverse effects of high environmental temperature (Hassan et al., 2011; Shakeri et al., 2018).
OMs are widely accepted and becoming more popular than inorganic counterparts to be supplemented in broiler diets. Several investigations revealed that OMs had better bioavailability, readily transported, and retained for more extended periods than their inorganic minerals, which consequently improved the bioavailability and digestibility of broilers (Maiorka et al., 2002; Saleh et al., 2020b). Adding OMs to broiler diets increased growth performance in broilers under heat-stress conditions (Laganá et al., 2007). Organic mineral sources can also help increase trace elements’ absorption by reducing interference from agents that create insoluble complexes with ionic trace elements (Van der Klis and Kemme, 2002).
Additionally, OMs have numerous biological activities, including elevated serum concentrations of immunoglobulins (IgG, IgM, and IgA) (Feng et al., 2010), reducing lipid peroxidation (Bun et al., 2011), and improving antioxidant enzyme activities (superoxide dismutase “SOD” and glutathione peroxidase “GPx”) and gut morphology (Ma et al., 2011). Saleh et al. (2020b) demonstrated that dietary OMs supplementation reduced lipid peroxidation and enhanced antioxidative properties and immune response under high ambient temperatures. Based on the beneficial effects of BT and OMs, it could be hypothesized that there may be a synergism between them to ameliorate the negative impacts of heat stress and enhance growth performance, digestibility, and immunity in heat-stressed broiler chickens.
Therefore, this study aimed to investigate the effect of dietary BT and/or OMs on growth performance, carcass traits, muscle minerals profile, immunological response, and digestibility of broilers reared on thermoneutral and chronic heat-stress conditions.
MATERIALS AND METHODS
Ethical Statement
This work was executed under the approval of the Ethics Committee of the Local Experimental Animals Care Committee and carried out in compliance with Kafrelsheikh University, Egypt (Number 4/2016EC) guidelines.
Birds, Experimental Design, and Husbandry
A total of 600 one-day-old mixed-sex Ross-308 (50% male + 50% female) chicks were housed in pens (stocking density was 10 birds/m²) and randomly allotted into 12 experimental treatments with 5 replicates (10 birds each, 5 male + 5 female) to equalize the average body weight in each group. The birds were provided with diets containing BT (0 and 2,000 ppm) and OMs (0, 250, and 500 ppm), either individually or in combination, under both thermoneutral and HS-inducing temperatures. The HS conditions involved exposing the birds to cyclic periods of elevated temperature (35°C ± 2°C) for 6 h daily, from 10:00 am to 4:00 pm, starting from d 10 and continuing until d 35. Relative humidity inside the poultry house was kept between 50 and 70%).
All diets were formulated (Table 1) in mash form of 3 phases (starter “1–10 d of age,” grower “11–25 d of age,” and finisher “26–35 d of age”) to meet the requirements of broilers (Aviagen, 2014). The experimental dietary supplementations were provided from 1 to 35 d of age. Birds were raised in a climate-controlled area with unrestricted access to food and water and subjected to the same environmental management (temperature, moisture, ventilation, and light). Chicks were reared according to the Ross Broiler Commercial Management Guide's recommendations from 1 to 10 d of age. From 10 to 35 d of age, the thermoneutral groups were continued according to Ross Broiler Commercial Management Guide's recommendations. In contrast, the other experimental groups were subjected to cyclic heat-stress conditions. The BT and OMs were provided by VESMARK Company, Tanta City, Egypt. The OMs contents were 240 g zinc glycinate, 26 g copper glycinate, 300 g manganese, and 100 g iron glycinate, while BT was a nature betaine HCL 98%.
Table 1.
Composition of the experimental starter, grower, and finisher diets.
| Ingredient, g/kg | Starter | Grower | Finisher |
|---|---|---|---|
| (1–10 d) | (11–25 d) | (26–35 d) | |
| Yellow corn | 526 | 568 | 627 |
| Soybean meal, 46% | 370 | 322 | 244 |
| Corn gluten meal, 60% | 35 | 42 | 51 |
| Soya oil | 27 | 25 | 37 |
| Calcium carbonate | 11 | 11 | 10.5 |
| Dicalcium phosphate | 18 | 18 | 15 |
| Salt | 3 | 3 | 3 |
| Sodium bicarbonate | 1.5 | 1.5 | 2 |
| DL-Methionine, 99% | 2.8 | 3.2 | 3 |
| L-Lysine HCl, 98% | 1.8 | 2.1 | 3.2 |
| L-Threonine | 0.5 | 0.8 | 0.9 |
| Choline chloride, 60% | 0.4 | 0.4 | 0.4 |
| Premix1 | 3 | 3 | 3 |
| Chemical analysis on DM basis | |||
| AME kcal | 3000 | 3050 | 3150 |
| Crude protein, % | 23.02 | 21.05 | 19.04 |
| Fat, % | 5.3 | 5.5 | 5.9 |
| Digestible LYS, % | 1.38 | 1.34 | 1.25 |
| Digestible M and C, % | 0.95 | 0.92 | 0.87 |
| Digestible THR, % | 0.86 | 0.83 | 0.77 |
| Digestible ARG, % | 1.37 | 1.33 | 1.25 |
| Digestible ILE, % | 0.90 | 0.87 | 0.85 |
| Digestible LEU, % | 1.87 | 1.83 | 1.84 |
| Digestible VAL, % | 0.96 | 0.93 | 0.91 |
| Calcium, % | 0.96 | 0.96 | 0.87 |
| Available P, % | 0.48 | 0.48 | 0.44 |
| Sodium, % | 0.18 | 0.18 | 0.18 |
| Chloride, % | 0.24 | 0.24 | 0.24 |
Hero mix (Hero pharm, Cairo, Egypt). Composition (per 3 kg): Vitamin A 12,000,000 IU, vitamin D3 2,500,000 IU, vitamin E 10,000 mg, vitamin K3 2,000 mg, vitamin B1 1,000 mg, vitamin B2 5,000 mg, vitamin B6 1,500 mg, vitamin B12 10 mg, niacin 30,000 mg, biotin 50 mg, folic acid 1,000 mg, pantothenic acid 10,000 mg, manganese 60,000 mg, zinc 50,000 mg, iron 30,000 mg, copper 4,000 mg, iodine 300 mg, selenium 100 mg, and cobalt 100 mg.
Growth Performance and Carcass Traits
Initial and final weight (IBW and FBW), feed intake (FI), and body weight gain (BWG) were recorded. The FCR was calculated following Abdel-Moneim et al. (2020). The health status and mortality rates were monitored daily during the whole trial. At 35 d of age, 10 birds from each treatment (2 birds per duplicate, 1 male and 1 female) were randomly selected and slaughtered. Giblets (liver, heart, spleen, and gizzard), abdominal fat, empty carcass, breast, and thigh were weighed.
Serum Biochemical Parameters and Hepatic Lipid Peroxidation
Blood samples (5 mL) were collected from the wing vein immediately before slaughtering, gathered into heparinized test tubes, and then rapidly centrifuged (3,000 rpm for 20 min at 5°C) to separate the plasma. Plasma was stored at −20°C pending analysis.
Total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride (TG), glucose, aspartate aminotransferase (AST), total protein, albumin, and globulin were all measured colorimetrically using commercial kits (Diamond Diagnostics Inc. 333 Fiske Street Holliston, MA 01746-2048, USA) according to the manufacturer's instructions. Malondialdehyde (MDA) was used to detect lipid peroxidation in the liver following Richard et al. (1992), and the SOD and GPX activities were determined according to the method of Marklund and Marklund (1974). The hemagglutination inhibition test was used to assess serum antibody titers against Newcastle disease (ND) and avian influenza (H9N1) following OIE (2008).
Muscle Minerals Profile
Muscle mineral contents, including Mn, Zn, Cu, and Fe, were assessed utilizing gas-liquid chromatography (GLC) following Saleh et al. (2013) and AOAC (2003). Ten grams of pectoral muscle were homogenized in 40 mL of 0.1 N HCl for 45 s at 4°C, then centrifuged 15,000 × g for 50 min at 4°C. The supernatants were filtered and examined using a gas chromatograph (GC-4 CM-PFE, Shimadzu gas chromatograph, Tokyo, Japan) with a flame ionization detector (GC-4 CM-PFE, Shimadzu gas chromatograph, Tokyo, Japan).
Nutrients Digestibility Coefficients
During the final 3 d of the investigation, excreta from each cage replicate were collected and weighed for digestibility testing. All samples were dried for 24 h at 60°C in a drying oven following the digestibility test. The dried samples were then homogenized in their entirety according to (AOAC, 2003) for scrutiny and finely ground. The Kjeldahl method was used to determine the nitrogen digestibility of the crude protein component in the feed and excreta (CP, Method 968.06). An adiabatic bomb calorimeter was used to calculate gross energy (GallenkampAutobomb, London, UK) standardized with benzoic acid (AOAC, 2003). The digestibility of Mn, Zn, Cu, and Fe minerals was measured following the method described by Bao et al. (2009).
Messenger RNA Expression of Growth-Related Genes
Liver and duodenal samples were collected after slaughter, kept in liquid nitrogen, and stored at −80°C pending analysis. Hepatic and duodenal tissues’ RNA was extracted, and its purity was checked using a 260/280 nm spectrophotometer. To produce single-stranded complementary DNA (cDNA), the QuantiTect reverse transcription kit was used with 2 μg of total RNA, and a random primer hexamer was used in the 2-step RT-PCR reaction. The routine PCR and gel electrophoresis were performed to evaluate all the primers before real-time PCR (TaKaRa PCR Thermal Cycler Dices, Takara, Shiga, Japan). Table 2 shows the primers of genes involved in this study. In this case, to quantify gene expression, a real-time PCR technique using the 2−ΔΔCT method was employed. GAPDH was used to normalize against the examined genes as a standard internal gene since it was confirmed that the expression level was not altered under the current experimental conditions. CT values were used to compare the relative intensities of the genes and mRNA expression for each sample.
Table 2.
Primers for gene expression by RT-PCR.
| Gene | Direction | Primer sequence | Accession number | bp | Tm (°C) | Efficiency (%) |
|---|---|---|---|---|---|---|
| TLR4 | Sense | AGGCACCTGAGCTTTTCCTC | NM_001030693.1 | 188 | 59 | 92.219 |
| Antisense | TACCAACGTGAGGTTGAGCC | |||||
| IL-1b | Sense | CCGAGGAGCAGGGACTTT | XM_015297469.1 | 270 | 59 | 96.124 |
| Antisense | GGACTGTGAGCGGGTGTAG | |||||
| CLDN1 | Sense | CATACTCCTGGGTCTGGTTGGT | NM_001013611.2 | 140 | 59 | 95.325 |
| Antisense | GACAGCCATCCGCATCTTCT | |||||
| SOD1 | Sense | AGGAGTGGCAGAAGTAGAAA | NM_205064.1 | 110 | 60 | 93.154 |
| Antisense | TAAACGAGGTCCAGCAT | |||||
| GPX1 | Sense | GCCCGCACCTCTGTCATAC | NM_001277853.1 | 165 | 60 | 90.124 |
| Antisense | TGCTTCTCCAGGCTGTTCC | |||||
| UCP | Sense | GAGAAACAGAGCGGGATTTGAT | AB088685.1 | 135 | 58 | 94.581 |
| Antisense | GCTCCTGGCTCACGGATAGA | |||||
| GAPDH | Sense | AGAACATCATCCCAGCGTCC | NM_204305 | 105 | 58 | 96.256 |
| Antisense | CGGCAGGTCAGGTCAACAAC | |||||
| HSP70 | Sense | ATTCTTGCGTGGGTGTCTTC | NM_001006685.1 | 152 | 58 | 97.481 |
| Antisense | GATGGTGTTGGTGGGGTTC | |||||
| GHr | Sense | TCAGAAAGGATGGATTACTCTGGAGTATG | NM_001001293·1 | 145 | 60 | 95.213 |
| Antisense | CGGAGGTACGTTGTCTTGATCGGAC | |||||
| IGF-1 | Sense | GGTGCTGAGCTGGTTGATGC | JN942578 | 140 | 60 | 92.154 |
| Antisense | CGTACAGAGCGTGCAGATTTAGGT |
TLR4, Toll-like receptor 4; 1b, interleukin 1 beta; CLDN1, claudin-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; SOD1, superoxide dismutase 1; GPX1, glutathione peroxidase; UCP, uncoupling protein; HSP70; heat shock protein 70; GHr, growth hormone receptor; IGF-1: insulin growth factor-1.
Statistical Analysis
The data on the effect of BT and OMs on broiler performance under thermoneutral or high ambient temperature were analyzed using the GLM procedure of SAS (2002) for a completely randomized design. The SAS software's general linear model (GLM) approach evaluated the collected data under 1-way ANOVA (Ver 9.4; SAS Institute, 2016). Tukey's test was used to determine the significance of mean differences, and all differences were judged significant at P < 0.05.
RESULTS
Growth Performance and Carcass Traits
The effects of dietary supplementation of BT, OMs, and exposure to HS, as well as their interaction effects, on the growth performance of broiler chickens, are presented in Table 3. The findings indicate that exposure to HS deteriorated all FBW, WG, FI, and FCR. However, the inclusion of BT and OMs in the diets resulted in improvements in these paramters. Dietary incorporation of BT and OMs nearly restored these indices to almost normal levels, mainly when used at higher concentrations. No significant interactions were observed among the 3 factors that affected the outcomes.
Table 3.
Effect of dietary supplementation of betaine (BT) and organic minerals (OMs) on growth performance in broilers under heat stress (HS).
| AT | BT, g/ton | OMs, g/ton | Initial body weight, g | Final body weight 35 d, g | Weight gain 35 d, g | Feed intake 35 d, g | FCR, 35 d, g:g |
|---|---|---|---|---|---|---|---|
| TN | 0 | 0 | 42.42 | 2169 | 2126 | 3337 | 1.538 |
| 250 | 42.46 | 2262 | 2219 | 3379 | 1.494 | ||
| 500 | 42.40 | 2283 | 2241 | 3368 | 1.476 | ||
| 2000 | 0 | 42.68 | 2246 | 2204 | 3320 | 1.478 | |
| 250 | 42.58 | 2315 | 2272 | 3378 | 1.460 | ||
| 500 | 42.72 | 2335 | 2292 | 3356 | 1.437 | ||
| HS | 0 | 0 | 42.48 | 1884 | 1842 | 3066 | 1.628 |
| 250 | 42.64 | 1926 | 1883 | 3068 | 1.598 | ||
| 500 | 42.56 | 2077 | 2035 | 3252 | 1.566 | ||
| 2000 | 0 | 42.70 | 2035 | 1992 | 3199 | 1.576 | |
| 250 | 42.42 | 2107 | 2064 | 3424 | 1.627 | ||
| 500 | 42.66 | 2140 | 2097 | 3251 | 1.524 | ||
| SEM | 0.400 | 40.15 | 40.18 | 54.88 | 0.030 | ||
| P value | |||||||
| HS | 0.886 | >0.001 | >0.001 | >0.001 | >0.001 | ||
| BT | 0.567 | >0.001 | >0.001 | >0.001 | 0.073 | ||
| OMs | 0.976 | >0.001 | >0.001 | 0.073 | 0.044 | ||
| HS × BT | 0.668 | 0.135 | 0.134 | 0.009 | 0.534 | ||
| HS × OMs | 0.997 | 0.446 | 0.447 | 0.526 | 0.514 | ||
| BT × OMs | 0.854 | 0.496 | 0.495 | 0.065 | 0.468 | ||
| HS × BT × OMs | 0.965 | 0.595 | 0.595 | 0.092 | 0.727 |
Values provided represent means that have been deemed statistically significant at (P < 0.05). SEM = standard error of means; AT = ambient temperature; TN = thermoneutral temperature. n = 50, 50 birds per treatment (10 birds per duplicate, 5 male and 5 female).
The various carcass traits, including percentages of carcass, breast, thigh, liver, heart, spleen, gizzard, and abdominal fat, showed no notable alterations due to dietary supplementation of BT, OMs, HS, or their interactions (Table 4). The exceptions were spleen percentage, which decreased due to HS, and abdominal fat percentage, which was reduced due to BT supplementation.
Table 4.
Effect of dietary supplementation of betaine (BT) and organic minerals (OMs) on carcass traits (%) in broilers under heat stress (HS).
| AT | BT, g/ton | OMs, g/ton | Carcass | Breast muscle | Thigh muscle | Liver | Heart | Spleen | Gizzard | Abdominal fat |
|---|---|---|---|---|---|---|---|---|---|---|
| TN | 0 | 0 | 63.40 | 22.14 | 16.62 | 2.162 | 0.510 | 0.132 | 1.466 | 1.388 |
| 250 | 64.01 | 22.51 | 16.48 | 2.248 | 0.548 | 0.140 | 1.464 | 1.374 | ||
| 500 | 63.80 | 22.41 | 16.59 | 2.128 | 0.522 | 0.152 | 1.438 | 1.294 | ||
| 2000 | 0 | 63.89 | 22.86 | 16.43 | 2.180 | 0.532 | 0.124 | 1.470 | 1.214 | |
| 250 | 64.26 | 22.35 | 16.42 | 2.214 | 0.488 | 0.126 | 1.474 | 1.164 | ||
| 500 | 63.29 | 22.55 | 16.01 | 2.114 | 0.512 | 0.118 | 1.436 | 1.112 | ||
| HS | 0 | 0 | 62.73 | 21.97 | 16.17 | 2.130 | 0.492 | 0.082 | 1.420 | 1.530 |
| 250 | 62.98 | 21.97 | 16.08 | 2.272 | 0.518 | 0.086 | 1.436 | 1.434 | ||
| 500 | 62.41 | 21.95 | 16.16 | 2.210 | 0.534 | 0.086 | 1.468 | 1.410 | ||
| 2000 | 0 | 63.28 | 21.83 | 16.10 | 2.096 | 0.516 | 0.126 | 1.456 | 1.346 | |
| 250 | 62.33 | 22.02 | 16.24 | 2.206 | 0.516 | 0.118 | 1.454 | 1.274 | ||
| 500 | 62.48 | 22.01 | 16.78 | 2.238 | 0.504 | 0.118 | 1.482 | 1.254 | ||
| SEM | 1.550 | 0.650 | 0.552 | 0.134 | 0.032 | 0.020 | 0.055 | 0.148 | ||
| P value | ||||||||||
| HS | 0.235 | 0.177 | 0.600 | 0.818 | 0.738 | 0.020 | 0.883 | 0.165 | ||
| BT | 0.968 | 0.768 | 0.953 | 0.825 | 0.578 | 0.501 | 0.713 | 0.037 | ||
| OMs | 0.928 | 0.997 | 0.980 | 0.602 | 0.975 | 0.990 | 0.996 | 0.605 | ||
| HS×BT | 0.960 | 0.746 | 0.430 | 0.927 | 0.683 | 0.033 | 0.797 | 0.895 | ||
| HS×OMs | 0.928 | 0.985 | 0.747 | 0.690 | 0.874 | 0.944 | 0.697 | 0.963 | ||
| BT×OMs | 0.928 | 0.932 | 0.970 | 0.951 | 0.461 | 0.802 | 0.987 | 0.997 | ||
| HS×BT×OMs | 0.942 | 0.829 | 0.749 | 0.966 | 0.664 | 0.955 | 0.990 | 0.989 |
Values provided represent means that have been deemed statistically significant at (P < 0.05). SEM = standard error of means; AT = ambient temperature; TN = thermoneutral temperature. n = 10, 10 birds per treatment (2 birds per duplicate, 1 male and 1 female).
Serum Biochemical Parameters and Hepatic Lipid Peroxidation
The impacts of dietary supplementation of BT, OMs, and HS on the serum biochemical parameters of broilers are shown in Table 5. Concentrations of glucose, AST, and HDL-cholesterol remained unaffected by the main factors as well as their interactions. Total cholesterol and LDL-cholesterol levels were influenced (P < 0.05) by HS, BT, and OMs, while TG levels were reduced (P < 0.05) only by the inclusion of BT. The interaction between BT, OMs, and HS did not exert a significant effect on these 3 parameters. Notably, heat stress led to an increase in total cholesterol and LDL-cholesterol values (P < 0.01), while the dietary inclusion of BT and OMs resulted in a reduction (P < 0.01) in the levels of these indices.
Table 5.
Effect of dietary supplementation of betaine (BT) and organic minerals (OMs) on serum parameters in broilers under heat stress (HS).
| AT | BT, g/ton | OMs, g/ton | TC, mg/dL | TG, mg/dL | LDL, mg/dL | HDL, mg/dL | Glucose, mg/dL | AST, mg/dL |
|---|---|---|---|---|---|---|---|---|
| TN | 0 | 0 | 121.0 | 62.20 | 64.80 | 52.20 | 145.8 | 240.4 |
| 250 | 112.2 | 50.80 | 57.00 | 51.60 | 147.8 | 246.0 | ||
| 500 | 117.6 | 58.80 | 58.60 | 54.00 | 153.0 | 247.0 | ||
| 2000 | 0 | 115.8 | 46.00 | 58.20 | 54.20 | 150.8 | 246.6 | |
| 250 | 107.2 | 50.40 | 51.80 | 52.00 | 142.4 | 231.2 | ||
| 500 | 119.4 | 53.80 | 63.00 | 52.60 | 148.4 | 226.6 | ||
| HS | 0 | 0 | 135.8 | 69.20 | 76.00 | 52.60 | 165.4 | 251.2 |
| 250 | 125.4 | 59.20 | 66.40 | 54.60 | 155.2 | 267.2 | ||
| 500 | 121.8 | 54.60 | 65.60 | 53.20 | 152.2 | 230.6 | ||
| 2000 | 0 | 121.4 | 52.00 | 66.00 | 52.40 | 156.4 | 244.8 | |
| 250 | 118.8 | 50.40 | 63.20 | 51.80 | 142.8 | 240.8 | ||
| 500 | 118.2 | 51.00 | 59.60 | 52.80 | 145.2 | 232.0 | ||
| SEM | 3.810 | 5.671 | 3.418 | 0.934 | 11.45 | 9.372 | ||
| P value | ||||||||
| HS | 0.001 | 0.467 | 0.001 | 0.804 | 0.462 | 0.411 | ||
| BT | 0.016 | 0.012 | 0.029 | 0.458 | 0.398 | 0.089 | ||
| OMs | 0.025 | 0.511 | 0.025 | 0.614 | 0.643 | 0.160 | ||
| HS×BT | 0.225 | 0.686 | 0.323 | 0.177 | 0.551 | 0.945 | ||
| HS×OMs | 0.112 | 0.433 | 0.157 | 0.245 | 0.672 | 0.346 | ||
| BT×OMs | 0.264 | 0.222 | 0.306 | 0.233 | 0.913 | 0.359 | ||
| HS×BT×OMs | 0.781 | 0.817 | 0.441 | 0.256 | 0.941 | 0.393 |
Values provided represent means that have been deemed statistically significant at (P < 0.05). SEM = standard error of means; AT = ambient temperature; TN = thermoneutral temperature; TC = total cholesterol; LDL = low-density lipoprotein cholesterol; HDL = high-density lipoprotein cholesterol; TG = triglyceride; AST = aspartate aminotransferase. n = 10, 10 birds per treatment (2 birds per duplicate, 1 male and 1 female).
In conditions of heat stress, concentrations of hepatic MDA were elevated (P < 0.05), but these levels were lowered (P < 0.05) by the incorporation of BT and OMs, as demonstrated in Table 6. The interaction between HS, BT, and OMs did not significantly impact MDA levels. Hepatic GPX levels were reduced (P < 0.01) due to exposure to HS without being affected by BT and OMs supplementation or their interactions, regardless of the presence or absence of HS. A similar pattern was observed in hepatic SOD levels, which were notably reduced (P < 0.01) in birds subjected to heat stress. However, the dietary inclusion of BT and OMs, along with their interaction, led to a reduction (P < 0.05) in hepatic SOD levels.
Table 6.
Effect of dietary supplementation of betaine (BT) and organic minerals (OMs) on broilers’ hepatic contents of MDA, SOD, and GPX under heat stress (HS).
| AT | BT, g/ton | OMs, g/ton | MDA, nmol/g | SOD, U/mg | GPX, U/mg |
|---|---|---|---|---|---|
| TN | 0 | 0 | 8.450 | 3.988 | 1.988 |
| 250 | 6.380 | 4.740 | 2.740 | ||
| 500 | 6.740 | 5.040 | 3.040 | ||
| 2000 | 0 | 7.436 | 4.760 | 2.760 | |
| 250 | 6.870 | 4.720 | 2.720 | ||
| 500 | 6.370 | 5.140 | 3.140 | ||
| HS | 0 | 0 | 10.61 | 2.200 | 1.060 |
| 250 | 7.870 | 3.420 | 1.420 | ||
| 500 | 7.556 | 3.740 | 1.740 | ||
| 2000 | 0 | 7.800 | 3.900 | 1.900 | |
| 250 | 7.126 | 3.520 | 1.520 | ||
| 500 | 7.280 | 3.520 | 1.520 | ||
| SEM | 0.233 | 0.129 | 0.115 | ||
| P value | |||||
| HS | 0.018 | >0.001 | >0.001 | ||
| BT | 0.050 | 0.015 | 0.109 | ||
| OMs | 0.003 | 0.007 | 0.096 | ||
| HS×BT | 0.235 | 0.454 | 0.891 | ||
| HS×OMs | 0.900 | 0.875 | 0.352 | ||
| BT×OMs | 0.156 | 0.003 | 0.064 | ||
| HS×BT×OMs | 0.625 | 0.285 | 0.830 |
Values provided represent means that have been deemed statistically significant at (P < 0.05). SEM = standard error of means; AT = ambient temperature; TN = thermoneutral temperature; MDA = malondialdehyde; SOD = superoxide dismutase; GPX = glutathione peroxidase. n = 10, 10 birds per treatment (2 birds per duplicate, 1 male and 1 female).
Breast Meat Minerals Content
The influence of incorporating dietary BT and OMs, as well as exposure to HS, on the mineral composition of the pectoral muscle is elucidated in Table 7. The exposure to HS did not induce any notable changes in Mn, Zn, and Cu levels within the pectoral muscle, although there was a marginal reduction in Fe content (P < 0.05). The inclusion of BT in the diet led to an increase (P < 0.01) in Mn content within the breast meat, while the levels of Zn, Cu, and Fe remained unaffected. Moreover, the dietary inclusion of OMs resulted in enhanced (P < 0.01) levels of all minerals in the pectoral muscle. The interactions between HS, BT, and OMs did not significantly impact the mineral content of the breast muscle, with the exception of Cu levels, which were increased (P < 0.05) due to the interaction between BT and OMs.
Table 7.
Effect of dietary supplementation of betaine (BT) and organic minerals (OMs) on broilers’ muscle mineral profile (mg/kg) under heat stress (HS).
| AT | BT, g/ton | OMs, g/ton | Mn | Zn | Cu | Fe |
|---|---|---|---|---|---|---|
| TN | 0 | 0 | 0.126 | 3.762 | 0.296 | 2.260 |
| 250 | 0.198 | 6.066 | 0.482 | 2.474 | ||
| 500 | 0.204 | 6.354 | 0.500 | 2.640 | ||
| 2000 | 0 | 0.128 | 4.856 | 0.336 | 2.252 | |
| 250 | 0.250 | 6.702 | 0.402 | 2.766 | ||
| 500 | 0.252 | 6.466 | 0.414 | 2.510 | ||
| HS | 0 | 0 | 0.122 | 3.562 | 0.256 | 2.060 |
| 250 | 0.168 | 5.764 | 0.436 | 2.106 | ||
| 500 | 0.180 | 6.520 | 0.420 | 2.374 | ||
| 2000 | 0 | 0.194 | 4.092 | 0.414 | 1.926 | |
| 250 | 0.246 | 6.266 | 0.426 | 2.260 | ||
| 500 | 0.274 | 6.340 | 0.428 | 2.352 | ||
| SEM | 0.024 | 0.465 | 0.442 | 0.238 | ||
| P value | ||||||
| HS | 0.740 | 0.300 | 0.744 | 0.028 | ||
| BT | >0.001 | 0.096 | 0.845 | 0.851 | ||
| OMs | >0.001 | >0.001 | 0.001 | 0.049 | ||
| HS×BT | 0.074 | 0.536 | 0.070 | 0.847 | ||
| HS×OMs | 0.315 | 0.720 | 0.705 | 0.773 | ||
| BT×OMs | 0.525 | 0.412 | 0.040 | 0.583 | ||
| HS×BT×OMs | 0.787 | 0.945 | 0.928 | 0.914 |
Values provided represent means that have been deemed statistically significant at (P < 0.05). SEM = standard error of means; AT = ambient temperature; TN = thermoneutral temperature. n = 10, 10 birds per treatment (2 birds per duplicate, 1 male and 1 female).
Immune Response
Table 8 illustrates the impact of dietary supplementation with BT and OMs, as well as exposure to HS, on the immune response of broiler chickens. The antibody titer against H9N1 and ND remained unaffected by BT, OMs, HS, or their interactions, except for the ND titer, which displayed an increase (P < 0.05) due to BT supplementation. Serum concentrations of total protein and globulins were not altered in response to BT, OMs, HS, or their interactions, except they were elevated (P < 0.05) due to BT and the interaction between BT and OMs. Conversely, serum albumin levels were reduced (P < 0.01) upon OMs inclusion while remaining unaffected by the other factors or their interactions.
Table 8.
Effect of dietary supplementation of betaine (BT) and organic minerals (OMs) on immunity in broilers under heat stress (HS).
| AT | BT, g/ton | OMs, g/ton | Total protein, mg/dL | Albumin, mg/dL | Globulin, mg/dL | H9N1, titer | ND, titer |
|---|---|---|---|---|---|---|---|
| TN | 0 | 0 | 3.700 | 1.180 | 2.520 | 7.400 | 6.000 |
| 250 | 3.660 | 1.140 | 2.520 | 8.000 | 6.800 | ||
| 500 | 3.920 | 1.120 | 2.800 | 7.600 | 6.200 | ||
| 2000 | 0 | 3.720 | 1.200 | 2.520 | 7.200 | 6.600 | |
| 250 | 4.380 | 1.280 | 3.100 | 7.600 | 6.400 | ||
| 500 | 3.820 | 1.160 | 2.660 | 8.400 | 6.800 | ||
| HS | 0 | 0 | 3.820 | 1.220 | 2.640 | 6.600 | 6.200 |
| 250 | 3.860 | 1.320 | 2.440 | 7.200 | 6.600 | ||
| 500 | 3.720 | 1.180 | 2.540 | 8.200 | 6.000 | ||
| 2000 | 0 | 4.060 | 1.260 | 2.800 | 8.400 | 6.800 | |
| 250 | 4.180 | 1.280 | 2.900 | 8.000 | 7.000 | ||
| 500 | 4.040 | 1.060 | 2.980 | 8.000 | 7.000 | ||
| SEM | 0.145 | 0.058 | 0.138 | 0.551 | 0.379 | ||
| P value | |||||||
| HS | 0.400 | 0.152 | 0.699 | 0.916 | 0.540 | ||
| BT | 0.002 | 0.630 | 0.002 | 0.176 | 0.036 | ||
| OMs | 0.266 | 0.002 | 0.334 | 0.252 | 0.518 | ||
| HS×BT | 0.547 | 0.058 | 0.186 | 0.251 | 0.359 | ||
| HS×OMs | 0.299 | 0.264 | 0.208 | 0.865 | 0.909 | ||
| BT×OMs | 0.039 | 0.381 | 0.050 | 0.709 | 0.299 | ||
| HS×BT×OMs | 0.203 | 0.272 | 0.186 | 0.143 | 0.753 |
Values provided represent means that have been deemed statistically significant at (P < 0.05). SEM = standard error of means, AT = ambient temperature; TN = thermoneutral temperature. n = 10, 10 birds per treatment (2 birds per duplicate, 1 male and 1 female).
Digestibility
Table 9 provides insight into the impact of HS, as well as dietary supplementation with BT and OMs, on the digestibility of CP, ME, Mn, Zn, Cu, and Fe. Across treatments, no significant changes were observed in CP and ME digestibility nor their interaction with the main factors. However, it is worth noting that CP digestibility experienced an increase (P < 0.01) due to OMs supplementation. Notably, including OMs in the diet led to increased (P < 0.01) digestibility for Mn, Zn, Cu, and Fe. Conversely, exposure to HS resulted in reduced (P < 0.05) digestibility for Mn and Fe, while BT supplementation led to an increased (P < 0.05) digestibility of Mn. The interaction among HS, BT, and OMs did not exert a discernible effect on the digestibility of the aforementioned parameters.
Table 9.
Effect of dietary supplementation of betaine (BT) and organic minerals (OMs) on nutrients and minerals digestibility coefficients (%) in broilers under heat stress (HS).
| AT | BT, g/ton | OMs, g/ton | CP | ME | Mn | Zn | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| TN | 0 | 0 | 66.00 | 55.33 | 28.33 | 27.67 | 24.33 | 29.00 |
| 250 | 65.66 | 54.67 | 34.00 | 32.00 | 30.00 | 34.33 | ||
| 500 | 67.00 | 55.67 | 38.00 | 35.67 | 34.00 | 37.33 | ||
| 2000 | 0 | 65.00 | 55.33 | 27.33 | 27.67 | 26.00 | 27.67 | |
| 250 | 66.33 | 55.33 | 33.33 | 32.67 | 31.00 | 33.67 | ||
| 500 | 66.33 | 54.67 | 37.00 | 35.00 | 33.00 | 36.33 | ||
| HS | 0 | 0 | 64.66 | 55.00 | 27.00 | 26.33 | 24.67 | 28.00 |
| 250 | 66.66 | 54.00 | 32.33 | 31.67 | 29.33 | 32.67 | ||
| 500 | 67.00 | 55.33 | 36.00 | 35.00 | 34.00 | 35.33 | ||
| 2000 | 0 | 65.33 | 53.67 | 25.67 | 26.00 | 25.00 | 26.67 | |
| 250 | 66.66 | 56.33 | 27.67 | 27.67 | 26.33 | 28.00 | ||
| 500 | 67.00 | 55.67 | 35.33 | 35.33 | 32.67 | 36.67 | ||
| SEM | 0.634 | 0.861 | 1.265 | 1.357 | 1.384 | 1.470 | ||
| P value | ||||||||
| HS | 0.651 | 0.739 | 0.004 | 0.066 | 0.198 | 0.041 | ||
| BT | 0.880 | 0.739 | 0.043 | 0.362 | 0.630 | 0.146 | ||
| OMs | 0.005 | 0.714 | >0.001 | >0.001 | >0.001 | >0.001 | ||
| HS×BT | 0.453 | 0.579 | 0.369 | 0.362 | 0.248 | 0.747 | ||
| HS × OMs | 0.417 | 0.496 | 0.438 | 0.483 | 0.374 | 0.328 | ||
| BT × OMs | 0.742 | 0.177 | 0.557 | 0.693 | 0.478 | 0.409 | ||
| HS × BT × OMs | 0.436 | 0.411 | 0.438 | 0.347 | 0.630 | 0.323 |
Values provided represent means that have been deemed statistically significant at (P < 0.05). SEM = standard error of means; AT = ambient temperature; TN = thermoneutral temperature; CP = crude protein; ME = metabolizable energy. n = 10, 10 birds per treatment (2 birds per duplicate, 1 male and 1 female).
Messenger RNA Expression of Growth-Related Genes
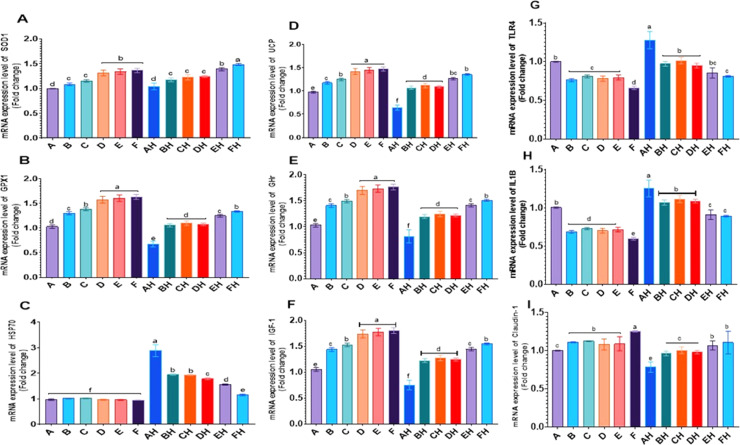
Significant upregulation of hepatic gene expression of SOD1, GPX1, UCP, GHr, and IGF-1 proteins were observed in response to HS and BT and OMs dietary treatments (Figure 1A, B, D, E, and F). A key finding of this study is the substantial normalization and recovery of hepatic mRNA expression levels for SOD1, GPX1, UCP, GHr, and IGF-1 in groups subjected to similar treatments under heat-stress conditions. Conversely, there was a notable increase in hepatic HSP70 mRNA expression within the heat-stress groups compared to other treated groups (Figure 1C). This elevation was mitigated by different BT and OMs treatments under heat-stress conditions.
Figure 1.
The mRNA expression of (A) hepatic SOD1, (B) hepatic GPX1, (C) hepatic HSP70, (D) hepatic UCP, (E) hepatic GHr, (F) hepatic IGF-1, (G) intestinal TLR, (H) intestinal IL-1β, (I) intestinal claudin-1. Different letters in the different columns are significantly different (P < 0.05). Values are expressed as means ± SEM. 1 A, control fed a basal diet; B and C, the diet mixed with 250 or 500 ppm OMs; D, the diet mixed with 2,000 ppm BT; E and F, the diet mixed with 250 or 500 ppm OMS plus 2,000 ppm BT. The same treatments were used for the groups AH to FH. 2 A–F groups were reared under thermoneutral conditions (26 + 1 C), while AH–FH groups were under heat stress (35°C ± 2°C for 6 h daily) from d 10 to d 35. n = 10, 10 samples from each treatment (2 birds per duplicate, 1 male and 1 female).
Results depicted in Figures (1G and H) underscore a pronounced elevation in mRNA expression levels of intestinal TLR4 and IL-1β in the heat-stressed group compared to other treated groups. Intriguingly, various dietary treatments involving BT and OMs under heat-stressed conditions demonstrated a significant reduction in intestinal TLR4 and IL-1β gene expression. Furthermore, there was a noteworthy increase in claudin-1 mRNA expression due to dietary BT and OMs treatments, with a substantial surge attributed to the interaction between BT and high levels of OMs (Figure 1I). Claudin-1 mRNA expression was significantly decreased (P < 0.01) in the heat-stressed groups but exhibited a marked increase (P < 0.01) due to the dietary incorporation of BT and OMs, as well as their interaction.
DISCUSSION
Growth Performance
Several studies demonstrated that heat stress reduces broilers’ growth performance, including FI, BWG, and FCR (Lu et al., 2017; Liu et al., 2019). The current study also indicates that exposure to HS decadent all FBW, WG, FI, and FCR. However, the inclusion of BT and OMs in the diets resulted in an enhancement in performance parameters (Table 3). Notably, the dietary combination of BT and OMs enhanced FBW and BWG in broilers under heat-stress conditions. However, no significant interactions were observed among the 3 factors (HS, BT, OMs) that affected the results. Also, current results agree with Chand et al. (2017), who found that dietary 0.20% BT supplementation significantly improved FI, BWG, and FCR in broilers under heat-stress conditions. On the same path, other studies showed that dietary 0.10% BT supplementation decreased the detrimental impact of high ambient temperature on performance and increased production efficiency (He et al., 2015; Al-Sagan et al., 2021). The reduction in broilers’ growth under heat-stress conditions might be due to the reduced FI, a defensive strategy to limit body heat increment (Song et al., 2013).
Furthermore, broilers underuse high energy to adjust the increased ambient temperature, reducing the energy required for development and resulting in inferior growth performance (Nawab et al., 2018). Temperature loss via radiation can be occurred by blood moving from central to peripheral (body's surface) circulation (Attia and Hassan, 2017; Rizk et al., 2019). Also, heat stress reduced the jejunal transepithelial resistance affecting the digestion and absorption processes and, perchance jeopardizing feed efficiency. Moreover, dietary supplementation of OMs may reduce the environmental impact of excessive excretion by reducing the inclusion of inorganic forms and improving absorption (Aksu et al., 2010). At the same time, dietary BT supplementation increased jejunum permeability and relieved the adverse effects of heat stress on FI, BWG, and breast muscle weight in broiler chickens (Shakeri et al., 2018). Furthermore, disrupting the intestinal barrier might provide a pathogen entry point, raising the risk of sickness (Dunshea et al., 2013; Ebeid et al., 2021). A similar set of results was discovered by Laganá et al. (2007), who found that supplementing OMs increased broiler performance by causing a drop in FI, which resulted in a higher FCR, regardless of the environmental conditions. Due to heat stress exacerbates a minor mineral shortage or raises the nutrient demand, and these nutrients have interrelationships, which could improve nutritional digestibility (Sahin and Kucuk, 2001).
Similarly, Petrovič et al. (2010) and Zhao et al. (2010) found that OMs are more bioavailable, resulting in lower food inclusion, excretion, and environmental pressure. The results might differ for various reasons, including different environmental circumstances and dietary treatments such as BT and OMs in broiler diets. The disparities in experimental results suggested that more research was needed to close the gap in contradicting results caused by the varying doses, experiment environment, and poultry species.
Carcass Traits
Results of carcass traits in the present study agreed with those of Sakomura et al. (2013), who found no differences in the carcass, breast yield, or abdominal fat in heat-stressed broilers given BT as a dietary supplement. Furthermore, Al-Sagan et al. (2021) reported that BT supplements did not affect the percentage of carcass pieces. Additionally, the results of Zhao et al. (2010) and Mishra et al. (2013) established that the OMs supplemented into broilers’ diets did not affect carcass traits. Several studies reported that conditional heat stress deteriorates carcass traits and abdominal fat content (Rao et al., 2011; Lu et al., 2017; Al-Sagan et al., 2021). On the other hand, other researchers found that supplementing broilers’ diets with BT increased their eviscerated carcass % and breast muscle yield when exposed to high ambient temperature (Liu et al., 2019). The variation between experimental results might most likely be linked to the observed severity of the heat stress, BT and OMs concentrations, and broilers’ genetic background.
Breast Meat Minerals Content
The dietary mixture of BT plus OMs may enhance OMs bioavailability and increase the deposition of Mn and Zn in breast meat in the present study (Table 6). Also, significant disparities between breast muscle concentrations of Mn, Zn, and Cu in Ross-308 broiler chickens received the various experimental diets. These findings agree with those of Wedekind et al. (1992) and Rabiee et al. (2010), which stated that the bioavailability of OMs is higher than that of inorganic minerals. El-Husseiny et al. (2012) noted that higher performance and carcass traits and lower excretion of these organic minerals were noticed in birds-fed diets with 100% of the requirements of inorganic form. Furthermore, Manangi et al. (2012) noted that Zn, Cu, and Mn litter levels were low in birds fed the low concentration of OMs compared to industry-standard mineral supplementation derived from inorganic sources.
Serum Biochemical Parameters
Results presented in Table 5 revealed that dietary BT and OMs combination did not affect serum concentrations of lipid profile, glucose, and AST. However, total cholesterol and LDL-cholesterol values were numerically reduced in groups B, D, and E and B, C, D, and E, respectively, compared to the control group (A). Similar outcomes were reported by Ratriyanto and Mosenthin (2018), who observed that dietary BT improved lipase activity and reduced the quantities of triacylglycerols and cholesterol in the serum of laying hens. Ghasemi and Nari (2020) reported that supplementing BT in diets did not affect blood biochemical parameters. Moreover, Echeverry et al. (2016) revealed no marked changes caused by the supplementation of OMs into broilers’ diets in the overall analysis of biochemical blood indices, including total protein, globulin, glucose, and cholesterol. The use of suitable amounts of BT and OMs, which may be a valuable nutritional intervention, especially under heat-stress conditions, might be contributed to the improvement in serum parameters of broilers in the present study.
Antioxidative Properties and Lipid Peroxidation
Interestingly, adding BT with OMs was more effective in reducing hepatic MDA levels (as the index of lipid peroxidation) under heat-stress conditions (Figure1A). These improvements might be attributed to enhancing the antioxidative enzyme activities. This assumption is confirmed in the present study, whereas gene expression of SOD1 and GPX1 was upregulated by BT and OMs dietary treatments (Figure 1B and C). It was reported that heat stress increased the level of MDA in broiler breast muscle (Azad et al., 2010) by increasing substrate oxidation and mitochondrial membrane potential, as well as decreasing uncoupling protein production (Mahmoud and Edens, 2003). Dietary BT was shown to facilitate antioxidative status in the breast muscle of broilers fed a low-methionine diet depending on its role within tissue as a methyl donor, which might be used for methionine synthesis (Alirezaei et al., 2012). Based on the findings of Alirezaei et al. (2011), BT may protect cells from oxidative injury by replenishing S-adenosyl methionine, which helps increase substrate availability for the manufacture of GSH, which shields cells against oxidative damage. Wang et al. (2019) proved that methionine is involved in regulating the antioxidant-related gene expression in the liver and intestine, including thioredoxin (Trx), glutaredoxin (Grx), glutathione reductase (GSR), and glutathione synthetase (GSS) via transsulfuration pathway in glutathione system in broiler chickens.
Even though BT serves as a methyl group donor in protein structure, enzyme function, and cellular function, its osmoregulatory properties could affect the growth of broiler chickens in hot environments (Craig, 2004). Furthermore, BT reduces the fear response in broilers exposed to heat stress by constraining oxidative stress and growing broiler feed intake (Ratriyanto and Mosenthin, 2018). During heat stress, administration of BT reduced broiler chickens’ tonic immobility, linked to higher serum levels of SOD and GPX activities. These observations supported our data concerning the gene expression of SOD1, GPX1, GHr, and IGF-1.
Heat stress stimulated free radicals production and decreased the UCP mRNA expression with a significant reduction in GPX1 (Del Vesco et al., 2015; Habashy et al., 2018). In this study, hepatic gene expressions of GPX1 and UCP in heat-stressed broilers were increased by BT supplement, which may reduce oxidative damage in the liver. Moreover, the methionine-saving properties of BT may be vital, allowing more methionine to be used for antioxidant gene expression, including phosphatidylinositol 3-kinase, regulatory 1 in the liver, and atrogin 1 and cathepsin L2 in breast muscle in broiler chickens (Del Vesco et al., 2015). Furthermore, BT positively affected mitochondrial respiration, which may also be a factor in increased UCP mRNA expression (Lee, 2015). Additionally, BT may help maintain intestinal integrity and barrier function, resulting in better digestion and nutritional absorption. Recently, Wu et al. (2020) discovered that adding BT to lipopolysaccharide boosted CLDN1 mRNA expression in porcine epithelial cells, in line with our findings.
Immune Response
Surprisingly, there were numerical differences in serum antibody titers against H9N1 and ND when broilers were fed a combination of BT and OMs under heat-stress conditions. At the same time, total protein, albumin, and globulin were numerically enhanced by high levels of dietary supplementations of BT and OMs compared to the other treatments under either thermoneutral or heat-stress conditions. These findings are consistent with prior results, which indicated that the increase in blood albumin due to BT integration was connected with its capacity to contribute methyl groups, which are required for protein metabolism, and its involvement in boosting immunity (Attia et al., 2005; Hassan et al., 2011; Chand et al., 2017). Moreover, OMs supplementation may benefit intestinal development and immune system function (Echeverry et al., 2016). Conversely, Dardenne et al. (1985) reported that Zn, as one of the trace minerals, plays a vital role in regulating the immune system function and its relationship with enzymes required to preserve the integrity of cells engaged in the immunological response. It is worth noting that an adequate supply of trace minerals in the diet is essential for a healthy immune system that can deal with environmental challenges and infections (Maggini et al., 2007; Tomlinson et al., 2008).
Digestibility
According to previous evidence, heat exposure has been demonstrated to stress osmotic cells in broilers, causing a water imbalance and modifying cell permeability through dehydration (Ratriyanto and Mosenthin, 2018). Furthermore, changes in the digestive system's structure and function may happen due to fluid movement from the digestive system during heat stress (Song et al., 2013). There were no noteworthy differences in CP or metabolism energy digestibility in the present study between dietary BT and OMs treatments under thermoneutral or heat-stress conditions. However, the supplementation of OMs in combination with BT significantly increased Mn, Zn, Cu, and Fe trace minerals’ digestibility (Table 8). These results align with the findings of Liu et al. (2019). Therefore, it could be supposed that a dietary combination of BT plus OMs may enhance OMs bioavailability and stabilize the normal mineral balance, improving the growth performance and antioxidative properties in broilers subjected to stress conditions. Moreover, enhancing OMs bioavailability may be resulted in increasing the deposition of Mn and Zn in breast meat in the present study.
Messenger RNA Expression of Growth-Related Genes
The cells of all animals include heat shock genes, which produce HSPs that aid in cell protection from heat-induced injury. Many HSPs were induced because of their well-documented roles in cells as a defense against heat stress (Dangi et al., 2016). In the present study, HSP70 mRNA was increased during heat anxiety with significant normalization with the dietary treatment of BT and OMs. In agreement with the results of the present study, Dangi et al. (2015) reported that the expression of HSPs is significantly reduced by BT. The gene expression of HSP70 can also be inhibited by BT, which may help to stabilize cellular proteins and protect them from denaturation due to heat stress (Sheikh-Hamad et al., 1994). These results might be attributed to the fact that under heat-stress conditions, dietary BT supplementation is involved in reducing rectal temperature and respiration rates in broiler chickens (Singh et al., 2015) and growing rabbits (Hassan et al., 2011) which may lead to alleviating some of the adverse effects of heat stress including expression of HSP70.
The intestinal mucosal immune system relies on TLRs to transmit signals and mediates the proinflammatory effect (Chow et al., 1999; Vallabhapurapu and Karin, 2009). The TLR4, NF-kβ, and IL-1β mRNA expression in broilers’ intestines were reduced by BT treatment (Song et al., 2021). In addition, the NF-kβ pathway was inhibited or downregulated by BT to alleviate stress in rats (Yang et al., 2018). Furthermore, Wu et al. (2020) found that treating intestinal porcine epithelial cells with BT abridged the mRNA expression of the proinflammatory cytokine interleukin 6, and S-adenosylmethionine synthesis was increased by BT, which reduced inflammation. An adenosylmethionine averted the initiation of inducible nitric oxide synthase and lessened NF-kβ synthesis (Purohit et al., 2007; Dou et al., 2018). The increased intestinal barrier and integrity function may be connected with lower mRNA expressions of intestinal genes such as TLR4, NF-kβ, and IL-1β.
CONCLUSIONS
Heat stress negatively affected the growth performance and physiological homeostasis of broiler chickens. The dietary supplementation of 500 ppm OMs along with 2,000 ppm BT yielded significant improvements in growth performance and mineral digestibility among broiler chickens, regardless of thermal conditions. Moreover, this combination effectively restored the expression of growth and immune-related genes under heat-stress conditions.
ACKNOWLEDGMENTS
The authors wish to acknowledge the helpful suggestions of members of the Department of Poultry Production, Faculty of Agriculture, Kafrelsheikh University, Egypt. The authors acknowledge the financial support through the Researchers Supporting Project number (RSPD2023R581), King Saud University, Riyadh, Saudi Arabia.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (NRF-RS-2023-00275307)
DISCLOSURES
The authors declare that they have no conflict of interest.
Contributor Information
Hossam M. El-Tahan, Email: Hossam.eltahan@dankook.ac.kr.
In Ho Kim, Email: inhokim@dankook.ac.kr.
Sungbo Cho, Email: sungbocho@dankook.ac.kr.
REFERENCES
- Abdel-Moneim A.-M.E., Sabic E., Abu-Taleb A., Ibrahim N. Growth performance, hemato-biochemical indices, thyroid activity, antioxidant status, and immune response of growing Japanese quail fed diet with full-fat canola seeds. Trop. Anim. Health Prod. 2020;52:1853–1862. doi: 10.1007/s11250-020-02200-1. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., Shehata A.M., Khidr R.E., Paswan V.K., Ibrahim N.S., El-Ghoul A.A., Aldhumri S.A., Gabr S.A., Mesalam N.M., Elbaz A.M., Elsayed M.M.Wakwak, Ebeid T.A. Nutritional manipulation to combat heat stress in poultry–A comprehensive review. J. Therm. Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102915. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., Shehata A.M., Selim D.A., El-Saadony M.T., Mesalam N.M., Saleh A.A. Spirulina platensis and biosynthesized selenium nanoparticles improve performance, antioxidant status, humoral immunity and dietary and ileal microbial populations of heat-stressed broilers. J. Therm. Biol. 2022;104 doi: 10.1016/j.jtherbio.2022.103195. [DOI] [PubMed] [Google Scholar]
- Aksu D.S., Aksu T., Baytok E. The effects of replacing inorganic with a lower level of organically complexed minerals (Cu, Zn and Mn) in broiler diets on lipid peroxidation and antioxidant defense systems. Asian-Aust. J. Anim. Sci. 2010;23:1066–1072. [Google Scholar]
- Alirezaei M., Gheisari H.R., Ranjbar V.R., Hajibemani A. Betaine: a promising antioxidant agent for enhancement of broiler meat quality. Br. Poult. Sci. 2012;53:699–707. doi: 10.1080/00071668.2012.728283. [DOI] [PubMed] [Google Scholar]
- Alirezaei M., Jelodar G., Niknam P., Ghayemi Z., Nazifi S. Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. J. Physiol. Biochem. 2011;67:605–612. doi: 10.1007/s13105-011-0107-1. [DOI] [PubMed] [Google Scholar]
- Al-Sagan A.A., Al-Yemni A.H., Abudabos A.M., Al-Abdullatif A.A., Hussein E.O. Effect of different dietary betaine fortifications on performance, carcass traits, meat quality, blood biochemistry, and hematology of broilers exposed to various temperature patterns. Animals. 2021;11:1555. doi: 10.3390/ani11061555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tamimi H., Mahmoud K., Al-Dawood A., Nusairat B., Bani Khalaf H. Thermotolerance of broiler chicks ingesting dietary betaine and/or creatine. Animals. 2019;9:742. doi: 10.3390/ani9100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 17th ed. AOAC; Gaithersburg, MD: 2003. Official Methods of Analysis of AOAC. [Google Scholar]
- Ashour E.A., Farsi R.M., Alaidaroos B.A., Abdel-Moneim A.-M.E., El-Saadony M.T., Osman A.O., Abou Sayed-Ahmed E.T., Albaqami N.M., Shafi M.E., Taha A.E. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021;20:1357–1372. [Google Scholar]
- Attia Y.A., Hassan S.S. Broiler tolerance to heat stress at various dietary protein/energy levels. Eur. Poult. Sci. 2017;81:1612–9199. [Google Scholar]
- Attia Y., Hassan R., Qota E. Recovery from adverse effects of heat stress on slow-growing chicks in the tropics 1: effect of ascorbic acid and different levels of betaine. Trop. Anim. Health Prod. 2009;41:807–818. doi: 10.1007/s11250-008-9256-9. [DOI] [PubMed] [Google Scholar]
- Attia Y., Hassan R., Shehatta M., Abd-El-Hady S.B. Growth, carcass quality and serum constituents of slow growing chicks as affected by betaine addition to diets containing 2. Different levels of methionine. Int. J. Poult. Sci. 2005;4:856–865. [Google Scholar]
- Aviagen R. Aviagen Limited; Newbridge Midlothian, Scotland, UK: 2014. Ross Broiler Management Manual. [Google Scholar]
- Azad M., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Bao Y., Choct M., Iji P., Bruerton K. The digestibility of organic trace minerals along the small intestine in broiler chickens. Asian-Aust. J. Anim. Sci. 2009;23:90–97. [Google Scholar]
- Bun S., Guo Y., Guo F., Ji F., Cao H. Influence of organic zinc supplementation on the antioxidant status and immune responses of broilers challenged with Eimeria tenella. Poult. Sci. 2011;90:1220–1226. doi: 10.3382/ps.2010-01308. [DOI] [PubMed] [Google Scholar]
- Chand N., Naz S., Maris H., Khan R.U., Khan S., Qureshi M.S. Effect of betaine supplementation on the performance and immune response of heat stressed broilers. Pak. J. Zool. 2017;49 [Google Scholar]
- Chen R., Wen C., Gu Y., Wang C., Chen Y., Zhuang S., Zhou Y. Dietary betaine supplementation improves meat quality of transported broilers through altering muscle anaerobic glycolysis and antioxidant capacity. J. Sci. Food. Agric. 2020;100:2656–2663. doi: 10.1002/jsfa.10296. [DOI] [PubMed] [Google Scholar]
- Chow J.C., Young D.W., Golenbock D.T., Christ W.J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Craig S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- Dangi S.S., Dangi S.K., Chouhan V., Verma M., Kumar P., Singh G., Sarkar M. Modulatory effect of betaine on expression dynamics of HSPs during heat stress acclimation in goat (Capra hircus) Gene. 2016;575:543–550. doi: 10.1016/j.gene.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Dangi S.S., Gupta M., Dangi S.K., Chouhan V.S., Maurya V., Kumar P., Singh G., Sarkar M. Expression of HSPs: an adaptive mechanism during long-term heat stress in goats (Capra hircus) Int. J. Biometeorol. 2015;59:1095–1106. doi: 10.1007/s00484-014-0922-5. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Savino W., Berrih S., Bach J.-F. A zinc-dependent epitope on the molecule of thymulin, a thymic hormone. Proc. Natl. Acad. Sci. 1985;82:7035–7038. doi: 10.1073/pnas.82.20.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vesco A.P., Gasparino E., de Oliveira Grieser D., Zancanela V., Soares M.A.M., de Oliveira Neto A.R. Effects of methionine supplementation on the expression of oxidative stress-related genes in acute heat stress-exposed broilers. Br. J. Nutr. 2015;113:549–559. doi: 10.1017/S0007114514003535. [DOI] [PubMed] [Google Scholar]
- Dou X., Li S., Hu L., Ding L., Ma Y., Ma W., Chai H., Song Z. Glutathione disulfide sensitizes hepatocytes to TNFα-mediated cytotoxicity via IKK-β S-glutathionylation: a potential mechanism underlying non-alcoholic fatty liver disease. Exp. Mol. Med. 2018;50:1–16. doi: 10.1038/s12276-017-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunshea F.R., Leury B.J., Fahri F., DiGiacomo K., Hung A., Chauhan S., Clarke I.J., Collier R., Little S., Baumgard L. Amelioration of thermal stress impacts in dairy cows. Anim. Prod. Sci. 2013;53:965–975. [Google Scholar]
- Ebeid T.A., Al-Homidan I.H., Fathi M.M. Physiological and immunological benefits of probiotics and their impacts in poultry productivity. World's Poult. Sci. J. 2021;77:883–899. [Google Scholar]
- Echeverry H., Yitbarek A., Munyaka P., Alizadeh M., Cleaver A., Camelo-Jaimes G., Wang P., Rodriguez-Lecompte J. Organic trace mineral supplementation enhances local and systemic innate immune responses and modulates oxidative stress in broiler chickens. Poult. Sci. 2016;95:518–527. doi: 10.3382/ps/pev374. [DOI] [PubMed] [Google Scholar]
- El-Husseiny O.M., Hashish S.M., Ali R.A., Arafa S.A., Abd El-Samee L.D., Olemy A.A. Effects of feeding organic zinc, manganese and copper on broiler growth, carcass characteristics, bone quality and mineral content in bone, liver and excreta. Int. J. Poult. Sci. 2012;11:368–377. [Google Scholar]
- Feng J., Ma W., Niu H., Wu X., Wang Y. Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol. Trace. Elem. Res. 2010;133:203–211. doi: 10.1007/s12011-009-8431-9. [DOI] [PubMed] [Google Scholar]
- Ghasemi H., Nari N. Effect of supplementary betaine on growth performance, blood biochemical profile, and immune response in heat-stressed broilers fed different dietary protein levels. J. Appl. Poult. Res. 2020;29:301–313. [Google Scholar]
- Guo Y., Balasubramanian B., Zhao Z.-H., Liu W.-C. Heat stress alters serum lipid metabolism of Chinese indigenous broiler chickens - a lipidomics study. Environ. Sci. Pollut. Res. 2021;28:10707–10717. doi: 10.1007/s11356-020-11348-0. [DOI] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Rekaya R., Aggrey S.E. Expression of genes that encode cellular oxidant/antioxidant systems are affected by heat stress. Mol. Biol. Rep. 2018;45:389–394. doi: 10.1007/s11033-018-4173-0. [DOI] [PubMed] [Google Scholar]
- Hassan R.A., Ebeid T.A., Abd El-Lateif A.I., Ismail N.B. Effect of dietary betaine supplementation on growth, carcass and immunity of New Zealand White rabbits under high ambient temperature. Livest. Sci. 2011;135:103–109. [Google Scholar]
- He S., Zhao S., Dai S., Liu D., Bokhari S.G. Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim. Sci. J. 2015;86:897–903. doi: 10.1111/asj.12372. [DOI] [PubMed] [Google Scholar]
- Hirakawa R., Nurjanah S., Furukawa K., Murai A., Kikusato M., Nochi T., Toyomizu M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020;7:46. doi: 10.3389/fvets.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganá C., Ribeiro A.M.L., Kessler A.d.M., Kratz L.R., Pinheiro C.C. Effect of the supplementation of vitamins and organic minerals on the performance of broilers under heat stress. Braz. J. Poult. Sci. 2007;9:39–43. [Google Scholar]
- Lee I. Betaine is a positive regulator of mitochondrial respiration. Biochem. Biophys. Res. Commun. 2015;456:621–625. doi: 10.1016/j.bbrc.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Liu W., Yuan Y., Sun C., Balasubramanian B., Zhao Z., An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals. 2019;9:506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Ma W., Niu H., Feng J., Wang Y., Feng J. Effects of zinc glycine chelate on oxidative stress, contents of trace elements, and intestinal morphology in broilers. Biol. Trace Elem. Res. 2011;142:546–556. doi: 10.1007/s12011-010-8824-9. [DOI] [PubMed] [Google Scholar]
- Maggini S., Wintergerst E.S., Beveridge S., Hornig D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007;98:S29–S35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- Mahmoud K.Z., Edens F. Influence of selenium sources on age-related and mild heat stress-related changes of blood and liver glutathione redox cycle in broiler chickens (Gallus domesticus) Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2003;136:921–934. doi: 10.1016/s1096-4959(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Mahmoudnia N., Madani Y. Effect of betaine on performance and carcass composition of broiler chicken in warm weather - a review. Int. J. AgriSci. 2012;2:675–683. [Google Scholar]
- Maiorka A., Laurentiz A., Santin E., Araujo L., Macari M. Dietary vitamin or mineral mix removal during the finisher period on broiler chicken performance. J. Appl. Poult. Res. 2002;11:121–126. [Google Scholar]
- Manangi M.K., Vazquez-Anon M., Richards J.D., Carter S., Buresh R.E., Christensen K.D. Impact of feeding lower levels ofchelated trace minerals versus industry levels of inorganic traceminerals on broiler performance, yield, footpad health, and littermineral concentration. J. Appl. Poult. Res. 2012;21:881–890. [Google Scholar]
- Marklund S., Marklund G. Involment of the superoxideanion radical in the autooxidation of pyrogallol and aconvenient assay for super oxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mishra S., Swain R., Behura N., Das A., Mishra A., Sahoo G., Dash A. Effect of supplementation of organic minerals on the performance of broilers. Indian J. Anim. Sci. 2013;83:1335–1339. [Google Scholar]
- Nawab A., Ibtisham F., Li G., Kieser B., Wu J., Liu W., Zhao Y., Nawab Y., Li K., Xiao M. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018;78:131–139. doi: 10.1016/j.jtherbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- OIE Terrestrial Manual, 2008.
- Palmonari A., Cavallini D., Sniffen C.J., Fernandes L., Holder P., Fusaro I., Giammarco M., Formigoni A., Mammi L.M.E. In vitro evaluation of sugar digestibility in molasses. Ital. J. Anim. Sci. 2021;20:571–577. [Google Scholar]
- Petrovič V., Nollet L., Kováč G. Effect of dietary supplementation of trace elements on the growth performance and their distribution in the breast and thigh muscles depending on the age of broiler chickens. Acta Vet. Brno. 2010;79:203–209. [Google Scholar]
- Purohit V., Abdelmalek M.F., Barve S., Benevenga N.J., Halsted C.H., Kaplowitz N., Kharbanda K.K., Liu Q.-Y., Lu S.C., McClain C.J. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am. J. Clin. Nutr. 2007;86:14–24. doi: 10.1093/ajcn/86.1.14. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W., Ribeiro A., Ferraz-de-Paula V., Pinheiro M., Sakai M., Sá L., Ferreira A., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Rodrigues M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M., Sá L., Ferreira A., Palermo-Neto J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012;90:1986–1994. doi: 10.2527/jas.2011-3949. [DOI] [PubMed] [Google Scholar]
- Rabiee A., Lean I., Stevenson M., Socha M. Effects of feeding organic trace minerals on milk production and reproductive performance in lactating dairy cows: a meta-analysis. J. Dairy Sci. 2010;93:4239–4251. doi: 10.3168/jds.2010-3058. [DOI] [PubMed] [Google Scholar]
- Rao S., Raju M., Panda A., Saharia P., Sunder G.S. Effect of supplementing betaine on performance, carcass traits and immune responses in broiler chicken fed diets containing different concentrations of methionine. Asian-Aust. J. Anim. Sci. 2011;24:662–669. [Google Scholar]
- Ratriyanto A., Mosenthin R. Osmoregulatory function of betaine in alleviating heat stress in poultry. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018;102:1634–1650. doi: 10.1111/jpn.12990. [DOI] [PubMed] [Google Scholar]
- Richard M.J., Portal B., Meo J., Coudray C., Hadjian A., Favier A. Malondialdehyde kit evaluated for determining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin. Chem. 1992;38:704–709. [PubMed] [Google Scholar]
- Rizk Y.S., Fahim H.N., Beshara M.M., Mahrose K.M., Awad A.L. Response of duck breeders to dietary L-Carnitine supplementation during summer season. Anais Acad. Bras. Ciênc. 2019;91(4) doi: 10.1590/0001-3765201920180907. [DOI] [PubMed] [Google Scholar]
- Saeed M., Babazadeh D., Naveed M., Arain M.A., Hassan F.U., Chao S. Reconsidering betaine as a natural anti-heat stress agent in poultry industry: a review. Trop. Anim. Health. Prod. 2017;49:1329–1338. doi: 10.1007/s11250-017-1355-z. [DOI] [PubMed] [Google Scholar]
- Sahin K., Kucuk O. Effects of vitamin E and selenium on performance, digestibility of nutrients, and carcass characteristics of Japanese quails reared under heat stress (34 C) J. Anim. Physiol. Anim. Nutr. (Berl.) 2001;85:342–348. doi: 10.1046/j.1439-0396.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- Sakomura N., Barbosa N., Longo F., Da Silva E., Bonato M., Fernandes J. Effect of dietary betaine supplementation on the performance, carcass yield, and intestinal morphometrics of broilers submitted to heat stress. Braz. J. Poult. Sci. 2013;15:105–112. [Google Scholar]
- Saleh A.A., Amber K.A., Mousa M.M., Nada A.L., Awad W., Dawood M.A., El-Moneim A., Ebeid T.A., Abdel-Daim M.M. A mixture of exogenous emulsifiers increased the acceptance of broilers to low energy diets: growth performance, blood chemistry, and fatty acids traits. Animals. 2020;10:437. doi: 10.3390/ani10030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A.A., Eltantawy M.S., Gawish E.M., Younis H.H., Amber K.A., Abd El-Moneim A.E.-M.E., Ebeid T.A. Impact of dietary organic mineral supplementation on reproductive performance, egg quality characteristics, lipid oxidation, ovarian follicular development, and immune response in laying hens under high ambient temperature. Biol. Trace. Elem. Res. 2020;195:506–514. doi: 10.1007/s12011-019-01861-w. [DOI] [PubMed] [Google Scholar]
- Saleh A.A., Hayashi K., Ohtsuka A. Synergistic effect of feeding Aspergillus awamori and Saccharomyces cerevisiae on growth performance in broiler chickens; promotion of protein metabolism and modification of fatty acid profile in the muscle. J. Poult. Sci. 2013;50:242–250. [Google Scholar]
- Saleh A.A., Shukry M., Farrag F., Soliman M.M., Abdel-Moneim A.-M.E. Effect of feeding wet feed or wet feed fermented by Bacillus licheniformis on growth performance, histopathology and growth and lipid metabolism marker genes in broiler chickens. Animals. 2021;11:83. doi: 10.3390/ani11010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri M., Cottrell J.J., Wilkinson S., Ringuet M., Furness J.B., Dunshea F.R. Betaine and antioxidants improve growth performance, breast muscle development and ameliorate thermoregulatory responses to cyclic heat exposure in broiler chickens. Animals. 2018;8:162. doi: 10.3390/ani8100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh-Hamad D., Garcia-Perez A., Ferraris J., Peters E., Burg M. Induction of gene expression by heat shock versus osmotic stress. Am. J. Physiol. Renal Physiol. 1994;267:F28–F34. doi: 10.1152/ajprenal.1994.267.1.F28. [DOI] [PubMed] [Google Scholar]
- Singh A., Ghosh T., Creswell D., Haldar S. Effects of supplementation of betaine hydrochloride on physiological performances of broilers exposed to thermal stress. Open Access Anim. Physiol. 2015;7:111–120. [Google Scholar]
- Song Y., Chen R., Yang M., Liu Q., Zhou Y., Zhuang S. Dietary betaine supplementation improves growth performance, digestive function, intestinal integrity, immunity, and antioxidant capacity of yellow-feathered broilers. Ital. J. Anim. Sci. 2021;20:1575–1586. [Google Scholar]
- Song J., Jiao L., Xiao K., Luan Z., Hu C., Shi B., Zhan X. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed Sci. Technol. 2013;185:175–181. [Google Scholar]
- Summers, J. D. 2013. Effect of choline or betaine supplementation on broilers exposed to different temperature treatments. Master's Thesis, University of Tennessee.
- Sun H., Yang W., Yang Z., Wang Y., Jiang S., Zhang G. Effects of betaine supplementation to methionine deficient diet on growth performance and carcass characteristics of broilers. Am. J. Anim. Vet. Sci. 2008;3(3):78–84. [Google Scholar]
- Statistical Analysis System (SAS) Institute (2002). SAS/STAT User's Guide. Version 8, 6th Edition, SAS Institute, Cary, 112.
- SAS Institute (2016). Statistical Analysis Software (SAS) User's Guide Version 9.4. Cary, NC: SAS Institute, Inc.
- Tomlinson D.J., Socha M.T., DeFrain J.M. Pages 39–52 in Proceedings of the Penn State Dairy Cattle Nutrition Workshop; Grantville, PA, 15–16 November. 2008. Role of trace minerals in the immune system. [Google Scholar]
- Vallabhapurapu S., Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Van der Klis, J. D., and P. A. Kemme. 2002. An appraisal of trace elements: inorganic and organic. Poultry feedstuffs: supply, composition and nutritive value, 99–108, CABI Books. CABI. doi: 10.1079/9780851994642.0099. [DOI]
- Wang Y., Yin X., Yin D., Lei Z., Mahmood T., Yuan J. Antioxidant response and bioavailability of methionine hydroxy analog relative to DL-methionine in broiler chickens. Anim. Nutr. 2019;5:241–247. doi: 10.1016/j.aninu.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedekind K., Hortin A., Baker D. Methodology for assessing zinc bioavailability: efficacy estimates for zinc-methionine, zinc sulfate, and zinc oxide. J. Anim. Sci. 1992;70:178–187. doi: 10.2527/1992.701178x. [DOI] [PubMed] [Google Scholar]
- Willingham B.D., Ragland T.J., Ormsbee M.J. Betaine supplementation may improve heat tolerance: potential mechanisms in humans. Nutrients. 2020;12:2939. doi: 10.3390/nu12102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., He C., Bu J., Luo Y., Yang S., Ye C., Yu S., He B., Yin Y., Yang X. Betaine attenuates LPS-induced downregulation of occludin and claudin-1 and restores intestinal barrier function. BMC Vet. Res. 2020;16:1–8. doi: 10.1186/s12917-020-02298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-m., Zhou R., Zhang M., Tan H.-r., Yu J.-q. Betaine attenuates monocrotaline-induced pulmonary arterial hypertension in rats via inhibiting inflammatory response. Molecules. 2018;23:1274. doi: 10.3390/molecules23061274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Shirley R., Vazquez-Anon M., Dibner J., Richards J., Fisher P., Hampton T., Christensen K., Allard J., Giesen A. Effects of chelated trace minerals on growth performance, breast meat yield, and footpad health in commercial meat broilers. J. Appl. Poult. Res. 2010;19:365–372. [Google Scholar]