Abstract
Background
A substantial observational literature relating specific fatty acid classes to chronic disease risk may be limited by its reliance on self-reported dietary data.
Objectives
We aimed to develop biomarkers for saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acid densities, and to study their associations with cardiovascular disease (CVD), cancer, and type 2 diabetes (T2D) in Women’s Health Initiative (WHI) cohorts.
Methods
Biomarker equations were based primarily on serum and urine metabolomics profiles from an embedded WHI human feeding study (n = 153). Calibration equations were based on biomarker values in a WHI nutritional biomarker study (n = 436). Calibrated intakes were assessed in relation to disease incidence in larger WHI cohorts (n = 81,894). Participants were postmenopausal women, aged 50–79 when enrolled at 40 United States Clinical Centers (1993–1998), with a follow-up period of ∼20 y.
Results
Biomarker equations meeting criteria were developed for SFA, MUFA, and PUFA densities. That for SFA density depended somewhat weakly on metabolite profiles. On the basis of our metabolomics platforms, biomarkers were insensitive to trans fatty acid intake. Calibration equations meeting criteria were developed for SFA and PUFA density, but not for MUFA density. With or without biomarker calibration, SFA density was associated positively with risk of CVD, cancer, and T2D, but with small hazard ratios, and CVD associations were not statistically significant after controlling for other dietary variables, including trans fatty acid and fiber intake. Following this same control, PUFA density was not significantly associated with CVD risk, but there were positive associations for some cancers and T2D, with or without biomarker calibration.
Conclusions
Higher SFA and PUFA diets were associated with null or somewhat higher risk for clinical outcomes considered in this population of postmenopausal United States women. Further research is needed to develop even stronger biomarkers for these fatty acid densities and their major components.
This study is registered with clinicaltrials.gov identifier: NCT00000611.
Keywords: biomarker, cancer, cardiovascular disease, type 2 diabetes, metabolomics, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids
Introduction
In recent concurrent work, we report chronic disease associations for diets relatively high in biomarker-calibrated total fat, as estimated using biomarkers derived primarily from serum and urine metabolomics profiles, in Women’s Health Initiative (WHI) cohorts. In analyses that control for calibrated total energy, a higher ratio of calories from fat to total calories, hereafter total fat density, was associated with elevated risks for breast, colon, and total invasive cancer; for CHD, stroke, total CVD, and heart failure; and for type 2 diabetes (T2D). With control for additional dietary variables including fiber, the CVD associations were no longer evident, whereas those for cancer and T2D mostly remained. However, metabolomics-based biomarkers were not available for specific fatty acids densities.
The relationship between types of fat and CVDs, cancer, and other major outcomes remains unsettled despite decades of observational and experimental studies, and a related large literature. Briefly, meta-analyses of cohort studies report a lack of significant association between saturated fat intake and risk of CHD or CVD more generally [1], and between saturated fat and risk of all-cause mortality, CHD, CVD, ischemic stroke, and T2D [2]. Although an early large prospective study [3] reported positive associations of CHD risk with SFA and trans unsaturated fatty acid intake, and inverse associations with MUFA and PUFA, only the positive association with trans unsaturated fat and the inverse association with PUFA were significant with longer (20-y) follow-up [4]. Meta-analyses of randomized dietary intervention trials generally support replacement of SFA by PUFA for CHD risk reduction [5], and support reduction in SFA for CVD risk reduction more generally [6]. However, uncertainty persists concerning associations of these major fatty acids categories and chronic disease risks. For example, a major review [7] of the sources summarized above concluded that higher omega-6 fatty acids is associated with lower CHD risk, whereas a recent large case-cohort analysis in 9 European countries [8] found no association of (self-reported) total fat density or specific fatty acid densities with CHD risk. A limitation in nutritional epidemiology methods is the lack of objective measures of actual fatty acids intake.
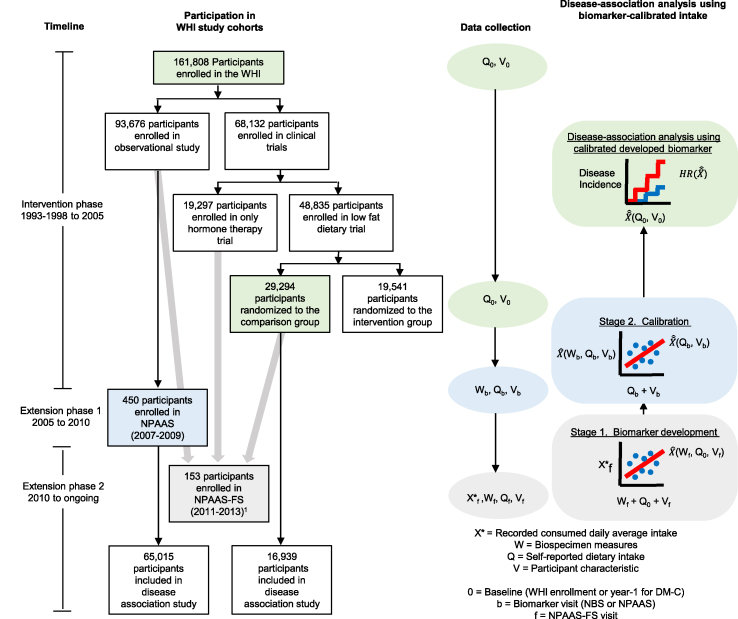
In this report, we explore the use of serum and urine metabolomics for potential development of biomarkers for fatty acid densities, use resulting biomarkers to explore the development of biomarker-calibrated intakes that allow for measurement error in fatty acid densities from self-reported dietary data, and assess associations between fatty acids densities and chronic disease risks in WHI cohorts. As depicted in Figure 1, and elaborated below, these developments involve 3 stages: a biomarker development stage in a WHI feeding study [9], a calibration equation development stage in a WHI nutritional biomarker study [10], and an application stage in larger WHI cohorts.
FIGURE 1.
Study design for biomarker development, dietary intake calibration, and disease-association analysis. Postmenopausal women were aged 50–79 y at enrollment during 1993–1998 at 40 United States clinical centers. Green, blue, and gray boxes indicate cohort (disease association, calibration, and biomarker development), timing of data collection (WHI enrollment for OS or year 1 for DM-C, NPAAS, NPAAS-FS) and corresponding analysis stage, respectively. Gray arrows indicate parent-cohorts of participants who were recruited for NPAAS-FS. Participants in the disease-association analyses were without prior personal history of the disease category under analysis, and had all data needed for intake calibration and confounding control. Notation for data collected (X∗, W, Q, V) and analysis were based on Huang [23] with pertinent regression variables shown along the X-axis (predictor variables), Y-axis (response variables), and line plots (developed biomarker; calibrated developed biomarker; estimated HR) of each regression-icon. 1NPAAS-FS includes n = 14 women who had previously participated in NPAAS. DM-C, DM comparison group; FS, feeding study; NPAAS, Nutrition and Physical Activity Assessment Study; OS, Observational Study; WHI, Women’s Health Initiative.
Methods
The context, resources, and methods for the dietary fatty acid analyses reported here are similar to those for our earlier reports on the intakes of carbohydrate and protein [11] and their components [12].
Study cohorts
Briefly, during 1993–1998, 48,835 participants were randomly assigned in the WHI Dietary Modification (DM) trial, with 29,294 assigned to the usual diet comparison group (DM-C), and 93,676 participants were enrolled in the companion prospective WHI Observational Study (OS) [13]. All participants were postmenopausal and in the age range 50–79 y when enrolled at 40 United States clinical centers. The WHI FFQ [14] targeted dietary intake over the preceding 3-mo period and was administered at baseline and year 1 in the DM trial, and approximately every 3 y thereafter during the trial intervention period (ended March 31, 2005), and was administered at baseline and at year 3 in the OS. Here, we used FFQs collected at 1 y after randomization in the DM-C, rather than at enrollment, to reduce assessment biases because of the trial eligibility criterion of FFQ % of energy from fat of at least 32%. The 1-y FFQ assessment is referred to here as “baseline FFQ.” FFQs at enrollment provide “baseline” self-reported diet in the OS. All nutrient content estimates from self-report assessments were derived from the University of Minnesota’s Nutrition Data System for Research (NDSR® version 2005). Participants completed core questionnaires at WHI enrollment including medical history, reproductive history, family history, personal habits, medications and dietary supplements, and provided a fasting blood sample [13].
Nutrition and Physical Activity Assessment Study
After an initial Nutrition Biomarker Study in the DM trial cohort [15], we conducted a Nutrition and Physical Activity Assessment Study (NPAAS) [10] among 450 OS participants during 2007–2009. Its purposes were to examine the measurement properties of dietary self-report data for nutritional variables having an established biomarker, and to use biomarker data to correct corresponding dietary self-report data for measurement error in disease-association analyses. We recruited WHI participants at 9 clinical centers to NPAAS, with an overrepresentation of Black and Hispanic women and of women having BMI >30.0 kg/m2. Our study protocol required 2 clinic visits separated by 2 wk and included various at-home activities. A 20% reliability subsample repeated the protocol ∼6 mo after their initial study participation. The first NPAAS visit included measured height and weight, doubly labeled water (DLW) dosing for total energy consumption assessment [16], completion of FFQ, dietary supplement, and other questionnaires, and collection of a blood specimen. Participants received instructions and a kit for 24-h urine collection for home completion. At the second clinic visit, participants brought 24-h urine specimens collected over the preceding day, provided a fasting blood specimen, and provided additional spot urine specimens to complete the DLW protocol. Baseline characteristics in the NPAAS cohort have been presented [10]. Participants were similar in age to other WHI participants, 60% were overweight or obese (that is, BMI ≥25.0 kg/m2), 95% were nonsmokers (never or past smokers); 51% had a college degree or higher education; 19%, 14%, and 64% self-classified, respectively, as being of Black, Hispanic, or non-Hispanic White race/ethnicity.
NPAAS-feeding study (NPAAS-FS)
We recruited 153 WHI women living in the Seattle area to the NPAAS-feeding study (NPAAS-FS) during 2011–2013 [9]. Of the 153, 14 had previously participated in NPAAS. Participants were provided food and beverages over a 2-wk feeding period, with individualized diets that were intended to approximate their usual diets, so that blood and urine concentrations would stabilize quickly and so that intake variations in the study cohort would be substantially retained during the feeding period. Biomarkers developed for the macronutrient intakes studied here rely on metabolomics profiles from the second clinic visit serum and 24-h urine specimens, along with the inclusion of readily available participant characteristic measures. Baseline demographic and lifestyle characteristics for participants in the NPAAS-FS have been reported [9]. Participants were well educated (83% college degree or higher), and mostly nonsmokers (98%). Most self-identified as White (95%), were overweight or obese (60%), and were of similar ages to other WHI enrollees.
Metabolite profiling
Serum and 24-h urine metabolomics profiles, obtained using specimens collected at the end of the NPAAS-FS feeding period, were derived as described by Zheng et al. [17].
Serum metabolite measurements
Briefly, serum samples from NPAAS-FS participants were analyzed by targeted LC-MS/MS using liquid chromatography coupled to a Sciex Triple Quad 6500+ mass spectrometer. A total of 303 metabolites were targeted, of which 155 were detected with <20% missing values. Separately, lipid metabolites were measured using the Sciex QTRAP 5500 Lipidyzer platform including the SelexION differential mobility spectrometry method that targeted 1070 lipids in 13 major lipid classes, resulting in 664 serum lipids that had <20% missing values.
Urine metabolite measurements
Metabolite profiles from 24-h urine samples were analyzed by NMR spectroscopy using a Bruker Avance III 800 MHz NMR spectrometer. Relative concentrations for 57 targeted metabolites were obtained. None of the metabolites had missing values. Urine metabolites were also analyzed by untargeted gas chromatography-mass spectrometry method using an Agilent 7890A/5875C instrument resulting in the identification of 275 metabolites with <20% missing values.
Total fat density biomarker
In our concurrently submitted manuscript, a biomarker equation for total fat density was developed by subtracting carbohydrate density and protein density biomarkers and an estimate of alcohol density from unity.
Outcome ascertainment, follow-up, and disease categories
Clinical outcomes were reported biannually in the DM trial and annually in the OS, by self-administered questionnaire [18] throughout the time from enrollment in 1993–1998 to the end of the intervention period (March 31, 2005), and annually thereafter in both cohorts. An initial report of CVD during cohort follow-up was confirmed by review of medical records by physician-adjudicators. In addition, CHD (defined as nonfatal MI plus CHD death), stroke (ischemic plus hemorrhagic), heart failure, and all deaths were centrally reviewed by expert physician investigator committees. All invasive cancers, except nonmelanoma skin cancer, were centrally coded using the NCI’s Surveillance, Epidemiology, and End Results (SEER) procedures. Prevalent, treated T2D (by oral agents or insulin) at baseline was self-reported during eligibility screening. Incident treated T2D during follow-up was documented by self-report at annual contacts throughout the follow-up period. These sources have been shown to be consistent with medication inventories of oral agents or insulin [19], and with known inflammatory and endothelial dysfunction biomarkers [20,21].
After the intervention period, WHI participants had the opportunity to enroll for additional follow-up through September 30, 2010, and subsequently for additional open-ended follow-up, with more than 80% of women doing so on each occasion. Cancer, diabetes, and all-cause mortality (including National Death Index matching) outcomes through December 31, 2020, are included here. Follow-up for CVD incidence is included only through September 30, 2010, because self-reports for most WHI participants were not adjudicated after that date. Also, heart failure adjudication in WHI cohorts stopped after March 31, 2005. The median follow-up duration is 11.3 y for CVD incidence, 7.8 y for heart failure, and ∼20 y for cancer incidence, diabetes incidence, and mortality outcomes. Disease outcome categories are those reported in our previous report on biomarker-calibrated protein and carbohydrate intake [11].
Statistical methods
Biomarker development for fatty acid classes in NPAAS-FS
Biomarker linear regression equations for SFA, MUFA, and PUFA densities, all log-transformed, used the same approach as for earlier [11] carbohydrate and protein biomarker development. The potential SFA, MUFA, and PUFA density biomarker equations also considered the total fat density, carbohydrate density, and protein density biomarkers for possible inclusion. The established DLW total energy biomarker, and the urinary nitrogen total protein biomarker [22] were also considered for possible inclusion in these biomarker equations, again with all dietary variables log-transformed. Participant characteristics as well as baseline FFQ measures were considered for inclusion, as in our previous work [11]. The potential inclusion of baseline FFQ measures prepares the biomarker for use in disease-association analyses. Specifically doing so is intended to avoid a bias that may otherwise occur if the biomarker is “noisy,” because then baseline FFQ estimates may have an association with outcomes in the presence of the related calibrated intake, and disease association estimates conceptually condition on the pertinent baseline FFQ values [23]. Also as in our previous work [11], LASSO procedures [24] were used for variable selection and cross-validation was used to reduce overfitting in biomarker equation model building, with all metabolite concentrations log-transformed.
The participant characteristics considered for inclusion were dietary supplement use, self-reported race/ethnicity, season of FFQ completion, education, age, measured BMI, and self-reported leisure activity (Metabolic Equivalent Unit (MET) h/wk). As in our previous work [11], cross-validated fraction of provided dietary intake variation explained (CV-R2) values were calculated as averages of R2 values over 100 random splits of the NPAAS-FS dataset into 2 approximately equal sized subsets. A 36% or larger CV-R2 was a criterion for a suitable biomarker.
Our goal is to define a biomarker so that actual log-fatty acids density can be written as corresponding log-fatty acids density biomarker plus error that is unrelated to the biomarker. Such independence entails traditional biomarker sensitivity and specificity considerations that can be difficult to ensure with dietary intakes that may involve many fatty acids within SFA, PUFA, and MUFA classes and with potential biomarkers that may involve multiple metabolites and other variables. To some extent the justification for the biomarkers we propose here relies on serum and urine metabolomics profiles being sufficiently comprehensive to support a sensitive and specific assessment of the dietary intakes under consideration. Supplemental Table 1 shows the set of serum and urine metabolites used for biomarker development.
Calibration equation development for fatty acids in NPAAS
Calibration equations for log-transformed fatty acids densities likewise used the same methods as for carbohydrate and protein [11] calibration equation development, while also considering calibrated total energy and calibrated total fat density for possible inclusion in linear regression equations. Biomarker equations meeting CV-R2 criteria were used to calculate biomarker-based intake estimates for log-transformed fatty acids density estimates for the 436 participants in NPAAS who were not enrolled in NPAAS-FS. These biomarker values were regressed linearly on corresponding concurrent log-transformed FFQ assessments, and on a disease category-specific set of personal characteristics listed in Supplemental Table 2 for development of potential calibration equations for estimating fatty acids densities in larger WHI cohorts. Briefly, among other variables CVD analyses considered (P < 0.10 for inclusion and retention) age, BMI, season of FFQ completion, race/ethnicity, family income, education, cigarette smoking history, alcohol intake (drinks/wk), leisure physical activity (metabolic equivalent units/wk), any dietary supplement use, prior menopausal hormone use, antihypertension medication use, antidiabetic medication use, history of treated diabetes, history of treated hypertension, personal history of CVD, and family history of MI, stroke, or diabetes. Invasive cancer analyses included these same variables, exclusive of personal history of CVD and of family history of CVD, stroke, or diabetes, and inclusive of Gail model 5-y breast cancer risk score, family history of colorectal cancer, and personal history of colon polyp removal. T2D analyses included the same variables as the CVD analyses except for family history of MI or stroke. The percent of variation explained in the response variable in these equations is reduced by temporal variation in the biomarker values. On the basis of the extent of variation between biomarker values for the 14 participants in both NPAAS and NPAAS-FS, we conservatively divide R2 values by 0.53, the largest of the sample correlations for the (log-transformed) fatty acids densities for these 14 replicate measurements, which are based on specimens collected by ∼4 y apart. A resulting adjusted R2 value of 36% or greater was a criterion for a suitable calibration equation.
Disease-association analyses in the DM-C and OS using Biomarker-Calibrated FFQ Data
Table 1 presents baseline demographic and lifestyle characteristics for the 81,954 participants, 16,939 from the DM-C, and 65,015 from the OS, considered for these analyses as in our previous work [11]. Participants averaged ∼62 y of age at baseline. Approximately 60% were overweight or obese, 85% were White, over 40% had a college degree or higher, and 94% were current nonsmokers. Participants having CVD, invasive cancer, or treated T2D before enrollment were excluded from respective CVD, cancer, or diabetes analyses.
TABLE 1.
Baseline demographic and lifestyle characteristics of the analytic sample (n = 81,954) comprised of 16,939 women from the Women’s Health Initiative (WHI) Dietary Modification Trial Comparison Group (DM-C) and 65,015 from the Observational Study (OS), enrolled during 1993–1998 at 40 United States clinical centers
| OS (n = 65,015) |
DM-C (n = 16,939) |
|||
|---|---|---|---|---|
| Characteristics | n | % | n | % |
| Age (y) | ||||
| 50–54 | 9126 | 14.0 | 1522 | 9.0 |
| 55–59 | 12,573 | 19.3 | 3634 | 21.5 |
| 60–64 | 14,381 | 22.1 | 4286 | 25.3 |
| 65–69 | 14,204 | 21.8 | 3902 | 23.0 |
| 70–74 | 10,259 | 15.8 | 2518 | 14.9 |
| ≥75 | 4472 | 6.9 | 1077 | 6.4 |
| BMI (kg/m2) | ||||
| <25 | 27,020 | 41.6 | 4579 | 27.0 |
| 25 to <30 | 22,140 | 34.1 | 6013 | 35.5 |
| ≥30 | 15,855 | 24.4 | 6347 | 37.5 |
| Self-identified race/ethnicity | ||||
| White | 56,032 | 86.2 | 14,250 | 84.1 |
| Black | 4122 | 6.3 | 1401 | 8.3 |
| Hispanic | 2022 | 3.1 | 536 | 3.2 |
| American Indian | 223 | 0.3 | 58 | 0.3 |
| Asian/PI | 1799 | 2.8 | 477 | 2.8 |
| Unknown | 817 | 1.3 | 217 | 1.3 |
| Education | ||||
| <High school | 2414 | 3.7 | 607 | 3.6 |
| High school/GED | 10,223 | 15.7 | 2876 | 17.0 |
| School after high school | 23,573 | 36.3 | 6648 | 39.2 |
| College degree or higher | 28,805 | 44.3 | 6808 | 40.2 |
| Family income (USD/y) | ||||
| <$20k | 9118 | 14.0 | 2258 | 13.3 |
| $20k to <$35k | 14,967 | 23.0 | 4084 | 24.1 |
| $35k to <$50k | 13,278 | 20.4 | 3664 | 21.6 |
| $50k to <$75k | 13,584 | 20.9 | 3671 | 21.7 |
| ≥$75k | 14,068 | 21.6 | 3262 | 19.3 |
| Season of FFQ completion | ||||
| Spring | 16,755 | 25.8 | 4406 | 26.0 |
| Summer | 18,135 | 27.9 | 4172 | 24.6 |
| Fall | 15,148 | 23.3 | 4180 | 24.7 |
| Winter | 14,977 | 23.0 | 4181 | 24.7 |
| Current smoker | ||||
| No | 61,120 | 94.0 | 15,917 | 94.0 |
| Yes | 3895 | 6.0 | 1022 | 6.0 |
| Alcohol1 | ||||
| Nondrinker | 18,410 | 28.3 | 5830 | 34.4 |
| <1 drink/wk | 20,583 | 31.7 | 4934 | 29.1 |
| 1 to <7 drinks/wk | 17,424 | 26.8 | 4591 | 27.1 |
| ≥7 drinks/wk | 8598 | 13.2 | 1584 | 9.4 |
| Any dietary supplement use | 36,358 | 55.9 | 8349 | 49.3 |
| Medication use | ||||
| Antihyperlipidemic medication | 5996 | 9.2 | 1562 | 9.2 |
| Antidiabetic medication | 1916 | 2.9 | 686 | 4.0 |
| Antihypertensive medication | 19,098 | 29.4 | 5611 | 33.1 |
| Postmenopausal hormone use | ||||
| Never | 25,334 | 39.0 | 6782 | 40.0 |
| Past | 9637 | 14.8 | 3357 | 19.8 |
| Estrogens-alone | 16,451 | 25.3 | 3932 | 23.2 |
| Estrogens+Progestin | 13,593 | 20.9 | 2868 | 16.9 |
| Recreational physical activity, MET2-h/wk | ||||
| None | 8318 | 12.8 | 2952 | 17.4 |
| >0 to ≤9.5 | 22,703 | 34.9 | 6910 | 40.8 |
| >9.5 to ≤20.5 | 18,017 | 27.7 | 4110 | 24.3 |
| >20.5 | 15,977 | 24.6 | 2967 | 17.5 |
| History of CVD3 | ||||
| No | 61,934 | 95.3 | 16,263 | 96.0 |
| Yes | 3081 | 4.7 | 676 | 4.0 |
| History of MI | 1410 | 2.2 | 330 | 1.9 |
| History of CABG/PCI | 1139 | 1.8 | 215 | 1.3 |
| History of CHF | 643 | 1.0 | 136 | 0.8 |
| History of stroke | 833 | 1.3 | 184 | 1.1 |
| History of cancer | ||||
| No | 56,826 | 87.4 | 16,104 | 95.1 |
| Yes | 8189 | 12.6 | 835 | 4.9 |
| Breast | 3743 | 5.8 | 74 | 0.4 |
| Colorectal | 586 | 0.9 | 15 | 0.1 |
| Ovary | 427 | 0.7 | 72 | 0.4 |
| Endometrium | 1120 | 1.7 | 158 | 0.9 |
| Thyroid | 354 | 0.5 | 64 | 0.4 |
| Cervix | 794 | 1.2 | 211 | 1.2 |
| Melanoma | 877 | 1.3 | 113 | 0.7 |
| Liver | 24 | 0.0 | 1 | 0.0 |
| Lung | 145 | 0.2 | 15 | 0.1 |
| Brain | 32 | 0.0 | 6 | 0.0 |
| Bone | 42 | 0.1 | 9 | 0.1 |
| Stomach | 34 | 0.1 | 1 | 0.0 |
| Leukemia | 58 | 0.1 | 6 | 0.0 |
| Bladder | 120 | 0.2 | 12 | 0.1 |
| Non-Hodgkin’s lymphoma | 148 | 0.2 | 6 | 0.0 |
| Hodgkin’s lymphoma | 42 | 0.1 | 6 | 0.0 |
| History of treated hypertension | 15,954 | 24.5 | 5197 | 30.7 |
| History of treated type 2 diabetes | 2360 | 3.6 | 826 | 4.9 |
| Family history of MI | 33,803 | 52.0 | 8740 | 51.6 |
| Family history of stroke | 24,694 | 38.0 | 6404 | 37.8 |
| Family history of breast cancer | 9882 | 15.9 | 2333 | 14.4 |
| Family history of colorectal cancer | 10,831 | 16.7 | 2687 | 15.9 |
| Family history of diabetes | 20,889 | 32.1 | 5859 | 34.6 |
| Gail model breast cancer risk score (tertiles) | ||||
| <1.26 | 18,972 | 29.2 | 5607 | 33.1 |
| 1.27–1.80 | 22,329 | 34.3 | 5900 | 34.8 |
| >1.80 | 23,714 | 36.5 | 5432 | 32.1 |
Abbreviations: CABG/PCI, coronary artery bypass graft or percutaneous coronary intervention; CHF, congestive heart failure; MET, metabolic equivalent units.
Drinks of alcohol defined as serving in mL (345 for beer, 177 for wine, and 43 for liquor).
Metabolic equivalent unit.
Nonfatal MI, CABG/PCI, CHF, or stroke.
We entered calibrated intake values into Cox regression models [25], along with disease-specific potential confounding factors. We assumed a linear modeling of log-HR on log-transformed fatty acid intake densities, implying a fixed HR for a fractional increase in each such density. For display purposes, we present HR estimates for a doubling in fatty acids densities, and for a 20% increment in total fat density. For comparison, the geometric means (95% confidence range) from baseline FFQs in the combined cohorts (n = 81,954) were 9.8% (4.6%, 17.4%) for SFA density, 11.2% (5.3%, 18.7%) for MUFA density, and 6.2% (3.2%, 11.3%) for PUFA density. For example, doubling of SFA density and PUFA density, respectively, imply increments of ∼9.8% and 6.2% of total energy.
Fatty acid density analyses include biomarker-calibrated log-total energy intake, and were conducted both with and without biomarker-calibrated total fat density in the HR model. Corresponding analyses were also carried out on the basis of FFQ assessments without biomarker calibration and resulting HRs are presented in main tables.
HR analyses for SFA and PUFA density were also carried out that included FFQ assessments of additional dietary variables that may help to explain observed fatty acids and disease associations. Specifically, log-total trans fatty acids (TFAs), vegetable servings, fruit servings, and log-fiber were added to the outcome HR models for these analyses. In particular, these HRs can be viewed as separating out TFA-related disease associations from those for SFA and PUFA.
For analyses having a more specific interpretation as conceptual macronutrient substitutions (calibrated) protein density and FFQ alcohol density were added to the HR models described above thereby emulating substitution of the fat intake variables by carbohydrate. Other analyses replaced SFA density by the sum of SFA density and PUFA density for conceptual PUFA for SFA substitution analyses.
As in our previous work [11], we stratified baseline hazard rates in the Cox model analyses on baseline age (that is, year 1 in DM-C, enrollment in OS) in 5-y categories, race/ethnicity, on cohort (DM-C or OS), and in the DM-C also on participation in the WHI hormone therapy trials (estrogen, estrogen placebo, estrogen plus progestin, estrogen plus progestin placebo, not randomized). Log-total energy, with or without biomarker calibration, was also included in the regression model. This implies that HRs for fatty acid densities estimate an HR factor beyond that for their contributions to estimated total energy intake. The set of disease-specific potential confounding factors considered are those shown in Supplemental Table 2 and listed above for calibration equation model building. Missing data rates were generally low for specific covariates, but 20% or more participants had missing data on 1 or more modeled covariates in some analyses. Participants were excluded from outcome-specific analyses if any modeled covariate was missing. On the basis of sensitivity analyses that dropped covariates having relatively high missingness rates, thereby including additional participants, this exclusion is not expected to materially affect disease-association HR estimates.
As in our previous work [11], we defined disease occurrence time for a “case” developing a study outcome as days from “baseline” (year 1 in the DM-C and enrollment in the OS) to diagnosis. We defined censoring time for “noncases” as days from baseline to the earliest of date of death without the outcome under study, last contact, or March 31, 2005, for heart failure, September 30, 2010, for other CVD incidence outcomes, or December 31, 2020, for cancer incidence, diabetes incidence, and mortality outcomes. Because of uncertainty in the coefficients in the calibrated intake estimating equations, a “sandwich-type” estimator was used for the variance for the log-HR parameter estimates in calibrated intake analyses [[26], [27], [28]]. We present disease rates and numbers of included participants with disease events during follow-up in Supplemental Table 3.
Linearity of the associations between log-HR and log-transformed fatty acids densities was studied by adding quadratic terms in fatty acids densities to the log-HR regression equations, and examining evidence for nonzero quadratic coefficients.
Ethics
The WHI is funded primarily by the NHLBI. Participants provided written informed consent for their overall WHI, NPAAS, and NPAAS-FS activities. The biomarker data generation in NPAAS and NPAAS-FS was funded by the NCI. Related protocols were approved by the Institutional Review Boards at the Fred Hutchinson Cancer Research Center and at each participating clinical center (clinicaltrials.gov identifier: NCT00000611).
Results
Table 2 shows results from biomarker development for SFA, MUFA, and PUFA densities. The CV-R2 for each of these log-transformed variables exceeds the prespecified 36% threshold. Note that each biomarker equation is built on multiple metabolites, mostly from serum lipidomic analyses. In addition, the biomarker equation for log-SFA density incorporates inverse contributions from baseline FFQ protein density and leisure activity; and that for log MUFA incorporates an inverse association with our carbohydrate density biomarker.
TABLE 2.
Potential biomarker equations for fatty acids densities based on linear regression of log-fatty acid density values from the NPAAS-FS (2011–2014; n = 153) on log-transformed metabolomics concentrations, log-transformed established dietary biomarkers, participant characteristics and baseline FFQ measures1
| Coef | R2 (%) | CV-R2 (%) | |
|---|---|---|---|
| SFA density | |||
| Intercept | −4.426 | ||
| TG (TG 51:2, FA 15:0) (serum) | 0.288 | 21.8 | 12.7 |
| Baseline protein density (FFQ) | −0.391 | 21.0 | 12.2 |
| Carbohydrate density biomarker | −0.451 | 1.6 | 0.9 |
| Leisure physical activity (metabolic equivalent units/wk) | −0.004 | 6.0 | 3.5 |
| Sphingomyelin (SM 14:0) (serum) | 0.268 | 3.1 | 1.8 |
| Ceramides (CER 22:0) (serum) | 0.493 | 5.0 | 2.9 |
| Cholesterol ester (CE 12:0) (serum) | 0.045 | 2.7 | 1.6 |
| TG (TG 52:2, FA 20:0) (serum) | 0.115 | 1.8 | 1.0 |
| D-Talose (urine) | −0.038 | 0.7 | 0.4 |
| Energy intake (kcal) from DLW | 0.072 | 0.1 | 0.0 |
| Urinary nitrogen | −0.041 | 0.2 | 0.1 |
| TG (TG 51:2, FA 18:1) (serum) | −0.039 | 0.0 | 0.0 |
| Diacylglycerol (DAG16:0, 18:1) (serum) | 0.038 | 0.1 | 0.0 |
| Cholesterol ester (CE 15:0) (serum) | −0.016 | 0.0 | 0.0 |
| Total | 63.9 | 37.2 | |
| MUFA density | |||
| Intercept | −11.356 | ||
| Carbohydrate density biomarker | −0.463 | 25.7 | 14.9 |
| TG (TG 52:3, FA 18:0) (serum) | −0.410 | 7.7 | 4.5 |
| N-Acetylalanine (serum) (serum) | 0.314 | 8.3 | 4.8 |
| 1/3-Methylhistidine (serum) | −0.036 | 6.7 | 3.9 |
| Phosphatidylethanolamine (PE 18:1, 18.1) (serum) | 0.114 | 4.9 | 2.8 |
| Niacinamide (serum) | 0.085 | 3.6 | 2.1 |
| Phosphatidylcholine (PC 16:0, 20:2) (serum) | −0.141 | 2.8 | 1.6 |
| 3-Hydroxypropionic acid (serum) | 0.110 | 1.9 | 1.1 |
| Cholesterol ester (CE 20:1) (serum) | −0.155 | 2.0 | 1.1 |
| Lysophosphatidylethanolamine (LPE 18:1) (serum) | 0.103 | 1.7 | 1.0 |
| Urinary nitrogen | 0.069 | 0.5 | 0.3 |
| TG (TG 54:2, FA 18:1) (serum) | 0.082 | 1.1 | 0.6 |
| Diacylglycerol (DAG 18:1, 18.1) (serum) | 0.096 | 0.3 | 0.2 |
| TG (TG 52:3, FA 20:1) (serum) | −0.029 | 0.1 | 0.0 |
| Total energy (kcal) from DLW | 0.005 | 0.0 | 0.0 |
| Total | 67.2 | 38.8 | |
| PUFA density | |||
| Intercept | −3.353 | ||
| TG (TG 49:2, FA 16:1) (serum) | 0.239 | 30.3 | 21.2 |
| TG (TG 50:3, FA 16:1) (serum) | −0.345 | 0.0 | 0.0 |
| Cholesterol ester (CE 15:0) (serum) | −0.358 | 3.1 | 2.2 |
| TG (TG 50:2, FA 14:1) (serum) | −0.156 | 1.8 | 1.2 |
| Cholesterol ester (CE 18:1) (serum) | −0.586 | 14.9 | 10.4 |
| Total energy (kcal) from DLW | 0.375 | 1.4 | 1.0 |
| Hexosylceramides (HCER 22:0) (serum) | 0.397 | 4.6 | 3.2 |
| Urinary nitrogen | −0.158 | 1.8 | 1.2 |
| Cholesterol ester (CE 18:0) (serum) | −0.362 | 1.9 | 1.3 |
| TG (TG 52:1, FA 20:1) (serum) | −0.079 | 1.8 | 1.3 |
| Cholesterol ester (CE 22:4) (serum) | −0.153 | 1.2 | 0.8 |
| TG (TG 54:6, FA 22:5) (serum) | −0.068 | 0.4 | 0.3 |
| TG (TG 54:7, FA 18:3) (serum) | 0.043 | 0.2 | 0.2 |
| TG (TG 52:2, FA 20:1) (serum) | 0.012 | 0.0 | 0.0 |
| Cholesterol ester (CE 16:0) (serum) | 0.020 | 0.0 | 0.0 |
| Total | 63.2 | 44.2 | |
Abbreviations: NPAAS-FS, Nutrition and Physical Activity Assessment Study feeding study; R2, percent of variation explained.
In (TG X1:Y1, FA X2:Y2), X1 indicates total number of carbons and Y1 indicates total number of double bonds in the 3 fatty acid chains. X2 indicates number of carbons and Y2 indicates number of double bonds in the fatty acid chain. In (DAG X1:Y1, X2:Y2); (PE X1:Y1, X2:Y2), X1 and X2 indicate number of carbons and Y1 and Y2 indicate number of double bonds in the fatty acid chains. In (SM X:Y); (HCER X:Y); (CE X:Y); (CER X:Y); (LPE X:Y); (FFA X:Y); (LPC X:Y); (PC X:Y), X indicates number of carbons and Y indicates number of double bonds in the fatty acid chain.
The 3 metabolomics-based biomarkers were used for calibration equation development in NPAAS (n = 436). Table 3 shows resulting potential calibration equations using the CVD set of confounding factors. A 36% adjusted R2 criterion is met for SFA and PUFA densities, but not for MUFA density. The partial R2 values for the log-FFQ contributions to these linear regression equations are substantial for both SFA and PUFA densities. Note also the inverse contribution from leisure physical activity for the SFA calibration equation, as well as contributions from racial/ethnic and socioeconomic variables for each equation. Corresponding potential calibration equations using the cancer and T2D set of covariates are similar to those shown in Table 3. These also satisfy a 36% cross-validated R2 criterion for SFA and PUFA but not for MUFA, and are given in Supplemental Table 4.
TABLE 3.
Calibration equations for fatty acid densities based on linear regression analysis of biomarker-based log-intake estimates on corresponding self-report (FFQ) log-intake estimates and personal characteristics among 436 participants in the NPAAS Biomarker Study, using the CVD covariate set
| FFQ (n = 303) |
|||||
|---|---|---|---|---|---|
| Covariates1 | β | SE (β) | P value | R2 (%) | adj2R2 (%) |
| SFA density with CVD covariates3 | |||||
| Log-SFA density biomarker | |||||
| Intercept | −2.3842 | 0.0183 | |||
| log-SFA density self-report | 2.2529 | 0.413 | <0.0001 | 18.51 | 34.92 |
| log PUFA density self-report | −2.71 | 0.6704 | 0.0013 | 0.07 | 0.13 |
| log-total fat density calibrated | 0.66 | 0.2337 | 0.0429 | 1.84 | 3.47 |
| Black | −0.0748 | 0.0276 | 0.0535 | 2.01 | 3.79 |
| Other race | −0.1072 | 0.0542 | 0.069 | 0.49 | 0.92 |
| Income: $50k–$75k | −0.0472 | 0.0234 | 0.0624 | 0.20 | 0.38 |
| Alcohol intake: 7+ drinks/wk | 0.0726 | 0.0398 | 0.069 | 0.02 | 0.04 |
| Leisure physical activity | −0.0042 | 0.0007 | <0.0001 | 8.31 | 15.68 |
| Total R2 | 31.5 | 59.34 | |||
| MUFA density with CVD covariates3 | |||||
| Log MUFA density biomarker | |||||
| Intercept | −3.0058 | 0.0126 | |||
| log MUFA density self-report | 0.888 | 0.3049 | 0.0025 | 2.95 | 5.57 |
| Income: $20k–$35k | −0.065 | 0.0233 | 0.0126 | 2.10 | 3.96 |
| Income: $50k–$75k | −0.0452 | 0.0218 | 0.03 | 0.35 | 0.66 |
| Alcohol intake: 7+ drinks/wk | 0.0424 | 0.0246 | 0.086 | 1.15 | 2.17 |
| Postmenopausal hormone use | 0.0771 | 0.0412 | 0.0679 | 1.89 | 3.57 |
| Total R2 | 8.4 | 15.91 | |||
| PUFA density with CVD covariates3 | |||||
| Log PUFA density biomarker | |||||
| Intercept | −2.5115 | 0.0153 | |||
| log PUFA density self-report | 4.955 | 0.6627 | <0.0001 | 12.02 | 22.68 |
| log-SFA density self-report | −1.6919 | 0.3954 | <0.0001 | 1.68 | 3.17 |
| Age | −0.0045 | 0.002 | 0.0017 | 2.09 | 3.94 |
| Black | 0.0738 | 0.0319 | 0.0338 | 5.46 | 10.30 |
| Income: $50k–$75k | 0.0492 | 0.0272 | 0.0718 | 0.59 | 1.11 |
| Alcohol intake: 7+ drinks/wk | −0.0686 | 0.032 | 0.0156 | 2.01 | 3.79 |
| Total R2 | 23.80 | 44.98 | |||
Abbreviations: NPAAS, Nutrition and Physical Activity Assessment Study; R2, linear regression percent of variation explained.
Potential covariates for inclusion: log PUFA density (centered), log MUFA density (centered), calibrated log fat density (centered), calibrated log FFQ total energy (centered), race/ethnicity (White = ref, Black, Hispanic, Other), age, BMI, education (≤HS/GED = ref, post-HS, college+), income (<$20k, $20k–$35k, $35k–$50k = ref, $50k–$75k, ≥$75k), season of FFQ completion (Summer, Spring, Fall, Winter = ref), current smoking, alcohol intake (Non/Past Drinker = ref, <1 drinks/wk, 1 to <7 drinks/wk, ≥7 drinks/wk), total recreational physical activity (MET h/wk), any dietary supplement use, postmenopausal hormone use, antihypertensive medication use, anti-lipid medication use, antidiabetic medication use, history of CVD (MI, stroke, congestive heart failure, CABG/PCI), history of treated diabetes, history of treated hypertension and family history of diabetes; age, BMI, self-reported SFA density, self-reported MUFA density, self-reported PUFA density, calibrated total fat density, calibrated total energy were centered at the NPAAS sample mean.
Adjusted for correlation between the log-fatty acid biomarker values in NPAAS-FS and NPAAS overlap sample by dividing by 0.53, the largest of the correlations for the log-fatty acid variables.
Covariate selection using stepwise approach, with P value for entry and removal = 0.10.
Table 4 (left side) gives CVD HRs (95% CIs) for a doubling of SFA and PUFA density. Significant risk elevations (P ≤ 0.05) at higher calibrated SFA density were observed for nonfatal MI, coronary death, total CHD, ischemic stroke, total stroke, combined CHD and stroke, total CVD, and heart failure, in analyses that also included calibrated total energy. However, these estimated risk elevations are quite small; for example, the HR (95% CI) for CHD is 1.10 (1.04, 1.17), for total CVD is 1.06 (1.02, 1.10), and for heart failure is 1.12 (1.02, 1.22). Note that HRs for SFA density without biomarker calibration of the dietary variables were similar, and even a little larger than those with calibration.
TABLE 4.
CVD incidence HRs and 95% CIs for a doubling of fatty acids densities, with and without biomarker calibrations of FFQ assessments in Women’s Health Initiative cohorts (n = 81,894) of postmenopausal United States women enrolled during 1993–1998 at 40 United States clinical centers and followed through December 20201
| No additional baseline FFQ measures |
Additional baseline FFQ measures2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| With biomarker calibration |
Without biomarker calibration |
With biomarker calibration |
Without biomarker calibration |
|||||
| Outcome (participants with events) | HR (95% CI)3 | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| Nonfatal MI (2102) | ||||||||
| SFA density | 1.11 (1.03, 1.19) | 0.005 | 1.16 (1.04, 1.30) | 0.01 | 1.01 (0.92, 1.11) | 0.82 | 1.04 (0.91, 1.18) | 0.59 |
| PUFA density | 1.05 (1.01, 1.10) | 0.02 | 0.98 (0.88, 1.09) | 0.76 | 0.99 (0.94, 1.05) | 0.83 | 0.94 (0.84, 1.06) | 0.32 |
| Coronary death (897) | ||||||||
| SFA density | 1.13 (1.02, 1.26) | 0.02 | 1.25 (1.06, 1.48) | 0.009 | 1.11 (0.96, 1.28) | 0.15 | 1.18 (0.96, 1.44) | 0.11 |
| PUFA density | 1.04 (0.98, 1.11) | 0.19 | 0.89 (0.75, 1.04) | 0.15 | 1.05 (0.96, 1.15) | 0.29 | 0.96 (0.81, 1.15) | 0.66 |
| Total CHD (2869) | ||||||||
| SFA density | 1.10 (1.04, 1.17) | 0.001 | 1.18 (1.07, 1.29) | <0.001 | 1.04 (0.96, 1.12) | 0.35 | 1.07 (0.96, 1.20) | 0.22 |
| PUFA density | 1.04 (1.01, 1.08) | 0.02 | 0.95 (0.87, 1.04) | 0.25 | 1.01 (0.96, 1.06) | 0.71 | 0.95 (0.86, 1.05) | 0.32 |
| Ischemic stroke (1776) | ||||||||
| SFA density | 1.09 (1.01, 1.18) | 0.02 | 1.13 (1.01, 1.28) | 0.04 | 1.04 (0.94, 1.15) | 0.42 | 1.06 (0.92, 1.23) | 0.40 |
| PUFA density | 1.05 (1.00, 1.10) | 0.03 | 1.01 (0.90, 1.13) | 0.91 | 1.02 (0.96, 1.09) | 0.54 | 0.99 (0.87, 1.12) | 0.83 |
| Hemorrhagic stroke (395) | ||||||||
| SFA density | 1.02 (0.89, 1.17) | 0.79 | 1.05 (0.82, 1.34) | 0.71 | 0.98 (0.81, 1.18) | 0.84 | 1.01 (0.75, 1.36) | 0.96 |
| PUFA density | 1.00 (0.92, 1.08) | 0.96 | 0.94 (0.74, 1.20) | 0.63 | 0.97 (0.86, 1.09) | 0.58 | 0.89 (0.68, 1.17) | 0.41 |
| Total stroke (2425) | ||||||||
| SFA density | 1.08 (1.01, 1.15) | 0.02 | 1.12 (1.01, 1.24) | 0.03 | 1.02 (0.94, 1.11) | 0.62 | 1.05 (0.92, 1.18) | 0.48 |
| PUFA density | 1.04 (1.00, 1.08) | 0.06 | 0.99 (0.89, 1.09) | 0.78 | 1.00 (0.95, 1.06) | 0.94 | 0.95 (0.86, 1.07) | 0.41 |
| CHD + stroke (5023) | ||||||||
| SFA density | 1.09 (1.04, 1.14) | <0.001 | 1.14 (1.06, 1.22) | <0.001 | 1.03 (0.97, 1.09) | 0.39 | 1.05 (0.97, 1.14) | 0.25 |
| PUFA density | 1.04 (1.01, 1.07) | 0.004 | 0.97 (0.91, 1.04) | 0.45 | 1.01 (0.97, 1.04) | 0.78 | 0.96 (0.89, 1.03) | 0.28 |
| CABG + PCI (3119) | ||||||||
| SFA density | 1.02 (0.96, 1.08) | 0.55 | 0.99 (0.91, 1.09) | 0.91 | 0.97 (0.89, 1.04) | 0.36 | 0.94 (0.84, 1.05) | 0.25 |
| PUFA density | 1.03 (0.99, 1.06) | 0.11 | 1.09 (1.00, 1.19) | 0.05 | 0.99 (0.94, 1.04) | 0.66 | 1.04 (0.95, 1.15) | 0.40 |
| Total CVD4 (6964) | ||||||||
| SFA density | 1.06 (1.02, 1.10) | 0.002 | 1.09 (1.02, 1.16) | 0.006 | 1.00 (0.95, 1.05) | 0.89 | 1.01 (0.94, 1.09) | 0.78 |
| PUFA density | 1.03 (1.01, 1.06) | 0.004 | 1.01 (0.95, 1.07) | 0.82 | 1.00 (0.97, 1.03) | 0.92 | 0.98 (0.92, 1.05) | 0.59 |
| Heart failure (1381) | ||||||||
| SFA density | 1.12 (1.02, 1.22) | 0.02 | 1.19 (1.04, 1.36) | 0.01 | 1.07 (0.95, 1.2) | 0.25 | 1.12 (0.95, 1.32) | 0.16 |
| PUFA density | 1.05 (0.99, 1.11) | 0.08 | 0.95 (0.83, 1.08) | 0.44 | 1.03 (0.95, 1.10) | 0.48 | 0.95 (0.82, 1.10) | 0.48 |
Abbreviations: CABG/PCI, coronary artery bypass graft or percutaneous coronary intervention.
Covariates considered for inclusion are age (linear), BMI, season of FFQ completion, race/ethnicity, family income, education, cigarette smoking history, alcohol intake (drinks/wk), leisure physical activity (metabolic equivalent units/wk), any dietary supplement use, prior menopausal hormone use, antihypertension medication use, antidiabetic medication use, history of treated diabetes, history of treated hypertension, personal history of CVD, family history of MI, stroke, or diabetes.
Additional covariates in this model include log (total trans fatty acids (g)+1) vegetable servings/d, fruit servings/d, and log(total fiber(g)+1).
HR estimates and 95% CIs are based on Cox models with baseline hazard rates stratified on study component (DM-C or OS), hormone therapy trial status (estrogen plus progestin, estrogen plus progestin placebo, estrogen-alone, estrogen-alone placebo, not randomized), age at enrollment (50–54, 55–59, 60–64, 65–69, 70–74, ≥75), and race/ethnicity, and with adjustment for a disease-specific set of potential confounding factors listed above.
Total CVD comprised of CHD + CABG + PCI + stroke.
However, as shown on the right side of Table 4, these HRs are greatly attenuated toward the null, and nonsignificant, when baseline FFQ value for TFA, vegetable servings, fruit servings, and fiber were added to the HR model. Further analyses (not shown) indicate that this attenuation is primarily because of the inclusion of fiber in the HR model.
Estimated HRs for a doubling of calibrated PUFA density were also small, with significant elevations for nonfatal MI, total CHD, ischemic stroke, combined CHD and stroke, and total CVD. Fewer HRs are significantly elevated without biomarker calibration. All significant PUFA density associations ceased to be significant after including FFQ assessments for the 4 additional dietary variables in the HR model.
Table 5 (left side) gives corresponding calibrated fatty acid density HRs (95% CIs) for the incidence of various cancers. Doubling of fatty acids densities was associated with small HR elevations for invasive breast cancer with HRs (95% CIs) of 1.03 (1.00, 1.06) for SFA, and 1.03 (1.01, 1.05) for PUFA. Similarly, there was a small colon cancer risk elevation with HR (95% CI) of 1.07 (1.01, 1.14) for SFA, and small risk elevations for total invasive cancer with HRs (95% CIs) of 1.03 (1.01, 1.05) for SFA and 1.02 (1.01, 1.03) for PUFA, along with other risk elevations including that for leukemia, and a possible risk reduction with SFA density for rectum cancer. HRs were fairly similar without biomarker calibration, although that for SFA density and breast cancer was nonsignificant.
TABLE 5.
Cancer incidence HRs and 95% CIs for a doubling of fatty acid densities, with and without biomarker calibration of FFQ assessments in Women’s Health Initiative cohorts (n = 81,894) of postmenopausal United States women enrolled during 1993–1998 at 40 United States clinical centers and followed through December 20201
| No additional baseline FFQ measures |
Additional baseline FFQ measures2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| With biomarker calibration |
Without biomarker calibration |
With biomarker calibration |
Without biomarker calibration |
|||||
| Cancer site (participants with events) | HR (95% CI)3 | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| Breast (5311) | ||||||||
| SFA density | 1.03 (1.00, 1.06) | 0.03 | 1.04 (0.97, 1.11) | 0.30 | 1.01 (0.97, 1.05) | 0.66 | 0.99 (0.91, 1.08) | 0.84 |
| PUFA density | 1.03 (1.01, 1.05) | <0.001 | 1.09 (1.02, 1.17) | 0.01 | 1.02 (1.00, 1.04) | 0.10 | 1.08 (1.00, 1.16) | 0.06 |
| Colon (1101) | ||||||||
| SFA density | 1.07 (1.01, 1.14) | 0.02 | 1.15 (0.99, 1.34) | 0.06 | 1.06 (0.98, 1.15) | 0.14 | 1.14 (0.95, 1.37) | 0.16 |
| PUFA density | 1.03 (1.00, 1.07) | 0.07 | 1.04 (0.90, 1.21) | 0.57 | 1.02 (0.97, 1.07) | 0.39 | 1.00 (0.85, 1.18) | 0.97 |
| Rectum (162) | ||||||||
| SFA density | 0.84 (0.71, 0.99) | 0.04 | 0.58 (0.39, 0.86) | 0.007 | 0.93 (0.75, 1.15) | 0.50 | 0.70 (0.43, 1.14) | 0.15 |
| PUFA density | 1.03 (0.94, 1.13) | 0.51 | 1.53 (1.03, 2.26) | 0.03 | 1.09 (0.97, 1.22) | 0.14 | 1.69 (1.12, 2.56) | 0.01 |
| Endometrium (916) | ||||||||
| SFA density | 0.99 (0.92, 1.06) | 0.74 | 0.91 (0.77, 1.08) | 0.27 | 0.99 (0.90, 1.09) | 0.85 | 0.91 (0.75, 1.12) | 0.39 |
| PUFA density | 1.03 (0.99, 1.08) | 0.12 | 1.20 (1.01, 1.42) | 0.04 | 1.04 (0.98, 1.09) | 0.20 | 1.20 (1.00, 1.45) | 0.05 |
| Ovary (479) | ||||||||
| SFA density | 0.97 (0.88, 1.07) | 0.54 | 0.96 (0.76, 1.20) | 0.69 | 1.15 (1.02, 1.31) | 0.02 | 1.34 (1.02, 1.77) | 0.03 |
| PUFA density | 0.98 (0.93, 1.03) | 0.43 | 0.95 (0.75, 1.19) | 0.63 | 1.06 (0.99, 1.14) | 0.09 | 1.06 (0.83, 1.36) | 0.63 |
| Leukemia (456) | ||||||||
| SFA density | 1.19 (1.08, 1.31) | <0.001 | 1.34 (1.05, 1.70) | 0.02 | 1.16 (1.02, 1.32) | 0.02 | 1.28 (0.96, 1.71) | 0.09 |
| PUFA density | 1.11 (1.05, 1.17) | <0.001 | 1.26 (1.00, 1.59) | 0.05 | 1.10 (1.03, 1.18) | 0.01 | 1.26 (0.97, 1.62) | 0.08 |
| Lung (1500) | ||||||||
| SFA density | 1.00 (0.95, 1.06) | 0.94 | 1.00 (0.88, 1.13) | 0.98 | 0.97 (0.91, 1.04) | 0.36 | 0.92 (0.79, 1.07) | 0.29 |
| PUFA density | 1.00 (0.97, 1.04) | 0.77 | 1.02 (0.90, 1.16) | 0.78 | 1.00 (0.96, 1.04) | 0.85 | 1.03 (0.90, 1.18) | 0.64 |
| Lymphoma (852) | ||||||||
| SFA density | 1.09 (1.02, 1.17) | 0.009 | 1.21 (1.02, 1.44) | 0.03 | 1.12 (1.03, 1.23) | 0.01 | 1.25 (1.02, 1.55) | 0.03 |
| PUFA density | 1.04 (0.99, 1.08) | 0.10 | 1.02 (0.86, 1.21) | 0.80 | 1.06 (1.00, 1.11) | 0.04 | 1.08 (0.90, 1.30) | 0.42 |
| Bladder (179) | ||||||||
| SFA density | 1.05 (0.89, 1.24) | 0.58 | 1.19 (0.82, 1.72) | 0.37 | 1.22 (0.98, 1.51) | 0.07 | 1.57 (1.00, 2.45) | 0.05 |
| PUFA density | 0.98 (0.89, 1.07) | 0.65 | 0.84 (0.58, 1.21) | 0.35 | 1.06 (0.94, 1.19) | 0.32 | 0.97 (0.65, 1.45) | 0.87 |
| Kidney (326) | ||||||||
| SFA density | 1.05 (0.93, 1.19) | 0.42 | 1.12 (0.84, 1.49) | 0.44 | 1.01 (0.86, 1.18) | 0.90 | 1.01 (0.72, 1.42) | 0.95 |
| PUFA density | 1.02 (0.95, 1.09) | 0.58 | 1.01 (0.76, 1.33) | 0.97 | 1.01 (0.93, 1.10) | 0.81 | 1.03 (0.76, 1.39) | 0.84 |
| Pancreas (433) | ||||||||
| SFA density | 1.02 (0.93, 1.12) | 0.63 | 1.12 (0.88, 1.42) | 0.35 | 0.98 (0.87, 1.11) | 0.76 | 1.03 (0.77, 1.37) | 0.83 |
| PUFA density | 0.97 (0.92, 1.03) | 0.34 | 0.84 (0.66, 1.07) | 0.16 | 0.95 (0.89, 1.02) | 0.19 | 0.82 (0.63, 1.06) | 0.13 |
| Obesity related4 (7563) | ||||||||
| SFA density | 1.03 (1.00, 1.05) | 0.02 | 1.03 (0.97, 1.09) | 0.30 | 1.01 (0.98, 1.04) | 0.47 | 1.00 (0.93, 1.07) | 0.93 |
| PUFA density | 1.03 (1.01, 1.04) | <0.001 | 1.09 (1.03, 1.16) | 0.003 | 1.02 (1.00, 1.04) | 0.03 | 1.08 (1.01, 1.15) | 0.02 |
| Total invasive (13,290) | ||||||||
| SFA density | 1.03 (1.01, 1.05) | <0.001 | 1.06 (1.01, 1.11) | 0.009 | 1.02 (1.00, 1.05) | 0.08 | 1.03 (0.98, 1.09) | 0.22 |
| PUFA density | 1.02 (1.01, 1.03) | <0.001 | 1.04 (1.00, 1.09) | 0.07 | 1.01 (1.00, 1.03) | 0.03 | 1.04 (0.99, 1.09) | 0.13 |
Covariates considered for inclusion are age (linear), BMI, season of FFQ completion, race/ethnicity, family income, education, cigarette smoking history, alcohol intake (drinks/wk), leisure physical activity (metabolic equivalent units/wk), any dietary supplement use, prior menopausal hormone use, antihypertension medication use, antidiabetic medication use, history of treated diabetes, history of treated hypertension, Gail model 5-y breast cancer risk score, family history of colorectal cancer, personal history of colon polyp removal.
Additional covariates in this model include log (total trans fatty acids (g)+1), vegetable servings/d, fruit servings/d, and log(total fiber(g)+1).
HR estimates and 95% CIs are based on Cox models with baseline hazard rates stratified on study component (DM-C or OS), hormone therapy trial status (estrogen plus progestin, estrogen plus progestin placebo, estrogen-alone, estrogen-alone placebo, not randomized), age at enrollment (50–54, 55–59, 60–64, 65–69, 70–74, ≥75), and race/ethnicity, and with adjustment for a disease-specific set of potential confounding factors.
Obesity-related cancer defined here as breast, colon, rectum, endometrium or kidney cancer.
As shown on the right side of Table 5, the SFA density and PUFA density HRs were mostly not changed materially after the inclusion of TFA, vegetables, fruits, and fiber in the outcome model, although the SFA density associations were then no longer significant for breast cancer or for total invasive cancer, with or without biomarker calibration of the fatty acids variables.
As shown in Table 6 (left side), a doubling of the fatty acids densities was associated with small T2D risk elevations, with HRs (95% CIs) of 1.06 (1.04, 1.09) for calibrated SFA density and 1.04 (1.03, 1.06) for calibrated PUFA density. These elevations were a bit larger without biomarker calibration, and they remained (Table 6, right side) after control for the additional 4 dietary variables.
TABLE 6.
Type 2 diabetes (T2D) incidence HRs and 95% CIs for a doubling of fatty acids densities, with and without biomarker calibration of FFQ in Women’s Health Initiative cohorts (n = 81,894) of postmenopausal United States women enrolled during 1993–1998 at 40 United States clinical centers and followed through December 20201
| No additional baseline FFQ measures |
Additional baseline FFQ measures2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| With biomarker calibration |
Without biomarker calibration |
With biomarker calibration |
Without biomarker calibration |
|||||
| Outcome (participants with outcome) | HR (95% CI)3 | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| T2D (12,605) | ||||||||
| SFA density | 1.06 (1.04, 1.09) | <0.001 | 1.09 (1.04, 1.14) | <0.001 | 1.05 (1.02, 1.08) | 0.0015 | 1.06 (1.01, 1.12) | 0.03 |
| PUFA density | 1.04 (1.03, 1.06) | <0.001 | 1.08 (1.03, 1.13) | 0.001 | 1.04 (1.02, 1.06) | <0.001 | 1.09 (1.01, 1.14) | <0.001 |
Covariates considered for inclusion are age (linear), BMI, season of FFQ completion, race/ethnicity, family income, education, cigarette smoking history, alcohol intake (drinks/wk), leisure physical activity (metabolic equivalent units/wk), any dietary supplement use, prior menopausal hormone use, antihypertension medication use, antidiabetic medication use, history of treated diabetes, history of treated hypertension, personal history of CVD, family history of diabetes.
Additional covariates in this model include log (total trans fatty acids (g) +1), vegetable servings/d, fruit servings/d, and log (total fiber(g)+1).
HR estimates and 95% CIs are based on Cox models with baseline hazard rates stratified on study component (DM-C or OS), hormone therapy trial status (estrogen plus progestin, estrogen plus progestin placebo, estrogen-alone, estrogen-alone placebo, not randomized), age at enrollment (50–54, 55–59, 60–64, 65–69, 70–74, ≥75), and race/ethnicity, and with adjustment for a disease-specific set of potential confounding factors.
The log-HR functions were approximately linear as a function of the modeled SFA density and PUFA density variables in TABLE 4, TABLE 5, TABLE 6 analyses. Specifically, none of 44 tests for null values for quadratic components in the variables listed in Table 4 was significant at the 0.05 level. Only 2 of the 52 tests for quadratic coefficients in Table 5 were significant, both for PUFA density and leukemia without calibration (both with P = 0.05), and Table 6 quadratic coefficient P values were far from significant.
TABLE 4, TABLE 5, TABLE 6 examine disease associations for SFA density and PUFA density relative to other sources of energy, including other fats, alcohol, and principally carbohydrate. Supplemental Tables 5–7 present corresponding HRs with log-total fat density included in the HR model. In these analyses, the total fat density HRs involve comparisons of fats with carbohydrate, protein, and alcohol as sources of energy, whereas the HRs for SFA density and PUFA density examine whether there are additional associations with these fatty acid categories, beyond their contribution to total fat. With total fat density included, the HRs for doubling of SFA density and PUFA density are nonsignificant for CVD outcomes (Supplemental Table 5), whereas the estimated HRs for a 20% increment in total fat density are also nonsignificant with the exception of that for ischemic stroke for which the HR (95% CI) was 2.18 (1.04, 4.56) with biomarker calibration and 1.20 (1.02, 1.41) without biomarker calibration, neither of which was much altered when the additional 4 dietary variables were included in the HR model. On adding total fat density to the HR model, the cancer HRs for SFA density and PUFA density (Supplemental Table 6) are similarly mostly nonsignificant with or without biomarker calibration and with or without the inclusion of the additional 4 dietary variables. As an exception, a doubling of the fatty acids densities gives a leukemia HR (95% CI) of 1.60 (1.04, 2.45) with calibration and 2.27 (1.04, 4.94) without calibration for SFA density; and 1.32 (1.03, 1.71) with calibration and 1.81 (1.04, 3.17) without calibration for PUFA density, again with little change when the 4 additional dietary variables are also considered. These elevations carry over to give corresponding small risk elevations for total invasive cancer. Finally, T2D HRs were not significant for SFA density or PUFA density, beyond their contributions to total fat density (Supplemental Table 8).
The Supplemental Tables 5–7 log-HR functions again showed little evidence of departure from linearity in log-SFA density and log-PUFA density, as was also the case for log-total fat density with the possible exception of that for ischemic stroke where there was some evidence for a positive quadratic coefficient with or without calibration and with or without the inclusion of the 4 additional dietary variables.
The further addition of protein density (with or without calibration) and FFQ alcohol density to the HR model, thereby yielding a more specific conceptual substitution of these fat-related variables for carbohydrate, did not materially change these results. The HR for ischemic stroke is elevated at higher total fat density, with or without calibration and with or without the further addition of the 4 dietary variables, whereas the corresponding additional PUFA density HR factor may be slightly reduced. Other CVD-related HRs are nonsignificant in these analyses. Among cancer analyses, leukemia risk is elevated at higher SFA density and higher PUFA density with or without calibration, as is also the case for total invasive cancer, whereas other HRs are nonsignificant. T2D HRs were nonsignificant for each of the modeled fat-related variables. In analyses that replace the SFA density variable in Supplemental Tables 6–8 by the sum of SFA density and PUFA density, thereby estimating HRs for PUFA density beyond its contribution to combined SFA and PUFA density, there were no significant PUFA density associations for CVD outcomes. Among cancer outcomes, there was an elevated leukemia risk for a doubling of PUFA density, with HR (95% CI) of 1.30 (1.01, 1.66) with biomarker calibration but a nonsignificant HR without biomarker calibration, as was also the case for total invasive cancer. The PUFA density HRs for T2D were far from significant in these analyses.
Discussion
This contribution has the dual purpose of exploring the ability to identify useful intake biomarkers for SFA, MUFA, and PUFA densities from resources that include serum and 24-h urine metabolomics profiles, and to use the resulting biomarkers to adjust self-reported dietary data for measurement error in chronic disease-association studies in WHI cohorts.
We have had some success in achieving these goals: from the square roots of total R2 values in Table 2 one sees that we have identified potential biomarkers having cross-validated correlations of 0.61, 0.62, and 0.66, respectively, with corresponding feeding study values for (log-transformed) SFA, MUFA, and PUFA densities. These are substantial correlations, especially when one considers that feeding study intakes also have some measurement error due, for example, to limitations in nutrient databases used to calculate nutrient intakes from foods.
To the contrary, it is a limitation of the SFA biomarker equation that protein density and leisure physical activity at WHI baseline are included in the biomarker equation, suggesting that metabolomics profiles alone from specimens collected at the end of the feeding period provide, at best, a noisy SFA density biomarker. This particular issue does not attend the MUFA or PUFA biomarker equations (Table 2). Note that these biomarkers are expected to be insensitive to TFA intake, because the fats targeted by our serum lipidomics platform do not include trans isomers. According to FFQs ∼2.8% of energy derived from TFAs at WHI baseline in the DM trial comparison group, reducing only slightly by year 6 after enrollment [29]. For interpretation however, analyses on the right sides of TABLE 4, TABLE 5, TABLE 6 (and Supplemental Tables 5–7) can be viewed as targeting fatty acids and disease associations after controlling for TFA intake.
Concerning our approach to biomarker and calibration equation development more generally, one can note that some metabolites in our biomarker equations are difficult to relate directly to the specific fatty acids classes because of the large number of metabolites from which to choose, with their possibly complex correlation structures. In the resulting biomarker equations lipids are the major contributing class of metabolites (Table 2). Fatty acids in these lipids represent different classes (MUFA, PUFA and SFA), which reflect the intake of these subclasses of lipids. For example, triglycerides that are selected for different subclasses consist of fatty acids chains that represent these subclasses as indicated by varying number of double bonds in fatty acids (see Table 2 footnote). In general the metabolite classes that appear in Table 2 seem biologically pertinent. For example, high intake of SFA increase lipotoxic species such as ceramides and saturated fatty acyl containing TGs [30,31]. In contrast, PUFAs decrease these lipids species. Biospecimens used in this study have been obtained after overnight fasting. Postprandial studies focused on metabolism and storage of dietary fat have shown that the digested dietary fat is stored in adipose tissue within ∼5 h [32,33]. While fasting, adipose tissue releases the stored fat in the form of fatty acids into the systemic plasma to meet the energy requirements of different tissue [34]. Hence, it is reasonable to assume that plasma lipids from short-term dietary exposure are reflective of longer-term exposure. Note that our biomarker equations for specific fatty acids densities may reflect lower intakes of other correlated dietary variables, such as lower carbohydrate with higher MUFA intake. As such our biomarker equations may or may not extrapolate readily to other populations, where correlation structures among nutritional variables may differ. Using the proposed biomarkers, corresponding calibration equations meeting correlational criteria could be developed for SFA and PUFA densities but not MUFA density, possibly reflecting a limited ability of FFQs to assess MUFA density.
The biomarker features and limitations described in preceding paragraphs have implications for the interpretation of corresponding disease-association analyses. First consider the association analyses based on FFQ intakes without calibration (TABLE 4, TABLE 5, TABLE 6). These analyses estimate small positive associations of SFA density with CHD, stroke, total CVD, heart failure, total invasive cancer and T2D, among a few other estimated associations. However, many of these associations are not clearly distinguishable from those for FFQ intakes for other dietary variables, such as fiber intake.
The SFA density associations after biomarker calibration tend to have similar corresponding P values with associations in the same direction, but HRs are often somewhat closer to the null than corresponding HRs without calibration, whereas deattenuation away from the null would be expected from the calibration procedure. This may reflect a limited ability of the available metabolomics profiles alone to explain feeding study SFA density variations.
Uncalibrated PUFA density was nonsignificantly associated with CVD outcomes, regardless of whether the other 4 dietary variables are included in the HR model. In comparison, uncalibrated PUFA density associations were estimated to be small but positive for breast cancer, obesity-related cancer and T2D, with or without inclusion of the other dietary variables. After biomarker calibration, positive PUFA density associations were estimated for these same cancer and T2D outcomes. This was also the case for some CVD outcomes, although these were not evident after inclusion of the other 4 dietary variables in the HR model.
With the exception of positive associations of PUFA density with total invasive cancer (as well as leukemia), none of the aforementioned associations remained significant after the inclusion of total fat density in the HR model, with or without biomarker calibration (Supplemental Tables 5–7).
It is interesting to relate these findings with other recent reports on fatty acids and health using objective measures. For example, a 2019 pooled analyses of 30 cohort studies having blood omega-6 fatty acids measures reported reduced incidence of several CVD outcomes at higher intakes of linoleic acid [35], whereas a 2017 analysis of an overlapping 20 cohorts provided similar results for T2D [36]. Also, a 2021 pooled analysis of 17 cohorts reported [37] inverse associations of blood long-chain omega-3 fatty acids concentrations with total as well as specific mortality rates. These latter serum fatty acids primarily reflect marine n-3 PUFA sources, the intake of which was small in the WHI cohorts.
Associations of total fat density and fatty acid densities with clinical outcomes in general may depend on individual characteristics, such as age [5], and also on the specific foods and food groups from which the nutrients derive. For example, the large case-cohort analysis in 9 European countries mentioned above [8] found no association of (self-reported) total fat density or fatty acid densities with CHD risk, while also reporting both positive and inverse associations with specific food sources, reinforcing the importance of diet quality and dietary patterns research in nutritional epidemiology, and suggesting caution in making broad dietary recommendations based on nutrient composition alone. Relative to United States dietary recommendations to replace SFA by PUFA [38], our analyses suggest that reduction in SFA density, in the setting of United States postmenopausal women, may have a small chronic disease risk benefit, although this risk reduction is not clearly distinguishable from that for reduction in total fat density, whereas our analyses do not suggest health benefits from an increase in PUFA density. For comparison, see Hanson et al. [39] for a recent systematic review of randomized trials, and Lawrence [40] for related biological perspectives, suggesting unfavorable PUFA associations with cancer and other chronic diseases.
Strengths of this study include the use of serum and 24-h urine metabolite profiles to assess the densities for fatty acid subtypes. Resulting biomarker equations explained considerably more of the feeding study variation than did similar analyses for total fat density [17]. In fact, the limited potential of the direct biomarker development approach used here for total fat density caused us instead to propose a total fat density biomarker formed by subtracting protein and carbohydrate density biomarkers and an alcohol density estimate from one. Also calibration equations were able to be developed for SFA and PUFA densities that enable measurement error adjustments to corresponding self-reported dietary intakes. Biomarker-calibrated SFA and PUFA densities were mostly associated with small, but positive, chronic disease risk increases in large well-characterized WHI cohorts of postmenopausal United States women having quality, long-term follow-up and outcome ascertainment. The positive associations for CVD outcomes, however, could be explained by other dietary factors, including fiber density.
Limitations include the observational nature of the cohort study results presented. For example, there is a possibility that individual diets may be modified in response to perceived health risks or clinician recommendations due to comorbidities, which may not be fully captured in our analyses. Also, the biomarkers proposed for fatty acids densities derive from a fairly small feeding study (n = 153) having a short (2-wk) feeding period, and participants were mostly of White self-reported race/ethnicity. Furthermore, the calibrated intake findings presented rely on measurement modeling assumptions for both biomarker and calibration equations. Fortunately, the error components of these models do not directly accumulate and calibrated intake associations are little affected by the magnitude of an independent error component to the biomarker equation [11]. However, an important consideration here resulting from smaller HRs for SFA and PUFA density associations with chronic disease than may be anticipated from corresponding total energy density associations is that our proposed SFA and PUFA density biomarkers may lack sensitivity for some components of these fatty acids categories. If so, such a departure from measurement model assumptions may result in HRs that are attenuated toward the null. Similarly, the disease-association analyses based on uncalibrated (FFQ) fatty acid densities can be expected to be attenuated by the noise component of these assessments, and could also be affected by systematic biases. Finally, the work is conducted in postmenopausal United States women only with dietary data collected during 1993–1998, and results may not be generalizable to more recent dietary patterns or to other populations.
In summary, potential metabolomics-based biomarkers have been proposed for major fatty acids classes in WHI cohorts. Corresponding biomarker-calibrated association analyses provide evidence that diets high in saturated fat and polyunsaturated fat may have small adverse associations with certain invasive cancers, and diabetes in this population of postmenopausal United States women. However, there is no clear evidence that these associations go beyond those for total fat density. Further biomarkers studies using even more comprehensive metabolomics profiles perhaps as applied to more specific fatty acids categories, and further studies of dietary self-report measurement error correction for these fatty acid subtypes are warranted to more fully understand corresponding disease associations in the WHI population as well as associations in other major cohorts.
Author contributions
The authors’ responsibilities were as follows—RLP, MLN, DR, CZ, GANG, YH, LFT, JWL: designed the research; RLP, MP, AKA, MLN, DR, GANG, CZ, LFT, YH, JLW: conducted the research, and drafted the manuscript; all authors: participated actively in critical evaluation of the manuscript, and read and approved the final manuscript; and RLP: had primary responsibility for the final content.
Data availability
Data, codebook, and analytic code used in this report may be accessed in a collaborative mode as described on the Women’s Health Initiative website (www.whi.org).
Funding
This work was supported by the NHLBI, NIH, United States Department of Health and Human Services (contracts HHSN268201600046C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C, and HHSN271201600004C); National Institute for Diabetes and Digestive and Kidney Diseases grant P30DK035816; NCI grants R01 CA119171 and P30 CA15704, and NIH instrumentation grant S10 OD021562. Decisions concerning study design, data collection and analysis, interpretation of the results, the preparation of the manuscript, and the decision to submit the manuscript for publication resided with committees comprised of WHI investigators that included NHLBI representatives. The contents of the paper are solely the responsibility of the authors.
Author disclosures
RTC reports receiving personal fees from Novartis, AstraZeneca, Pfizer Amgen, Genentech, Puma, and Up-To-Date. MLN is an Associate Editor on the Journal of Nutrition and played no role in the Journal’s evaluation of this manuscript. No potential conflicts of interest were reported by other authors.
Acknowledgments
The authors acknowledge the following investigators in the WHI Program: Program Office: (NHLBI, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross L Prentice, Andrea LaCroix, and Charles Kooperberg.
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland.
For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.05.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siri-Tarino P.W., Sun Q., Hu F.B., Krauss R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2019;91(3):535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Sousa R.J., Mente A., Maroleanu A., Cozma A., Ha V., et al. Intake of saturated and trans unsaturated fatty acids and risk of all-cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978. doi: 10.1136/bmj.h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu F.B., Stampfer M.J., Manson J.E., Rimm E., Colditz G.A., Rosner B.A., et al. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 1997;337(21):1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 4.Oh K., Hu F.B., Manson J.E., Stampfer M.J., Willett W.C. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the Nurses’ Health Study. Am. J. Epidemiol. 2005;161(7):672–679. doi: 10.1093/aje/kwi085. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D., Micha R., Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLOS Med. 2010;7(3) doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper L., Martin N., Jimoh O.F., Kirk C., Foster E., Abdelhamid A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020;5(5):CD011737. doi: 10.1002/14651858.CD011737.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris W.S., Mozaffarian D., Rimm E., Kris-Etherton P., Rudel L.L., Appel L.J., et al. Omega-6 fatty acids and risk for cardiovascular disease. A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119(6):902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 8.Steur M., Johnson L., Sharp S.J., Imamura F., Sluijs I., Key T.J., et al. Dietary fatty acids, macronutrient substitutions, food sources and incidence of coronary heart disease: findings from the EPIC-CVD case-cohort study across nine European countries. J. Am. Heart Assoc. 2021;10(23) doi: 10.1161/JAHA.120.019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampe J.W., Huang Y., Neuhouser M.L., Tinker L.F., Song X., Schoeller D.A., et al. Dietary biomarker evaluation in a controlled feeding study in women from the Women’s Health Initiative cohort. Am. J. Clin. Nutr. 2017;105(2):466–475. doi: 10.3945/ajcn.116.144840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prentice R.L., Mossavar-Rahmani Y., Huang Y., Van Horn L., Beresford S.A., Caan B., et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment using recovery biomarkers. Am. J. Epidemiol. 2011;174(5):591–603. doi: 10.1093/aje/kwr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prentice R.L., Pettinger M., Neuhouser M.L., Raftery D., Zheng C., Nagana Gowda G.A., et al. Biomarker-calibrated macronutrient intake and chronic disease risk among postmenopausal women. J. Nutr. 2021;151(8):2330–2341. doi: 10.1093/jn/nxab091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prentice R.L., Pettinger M., Zheng C., Neuhouser M.L., Raftery D., Nagana Gowda G.A., et al. Biomarkers for components of dietary protein and carbohydrate with application to chronic disease risk among postmenopausal women. J. Nutr. 2022;152(4):1107–1117. doi: 10.1093/jn/nxac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Women’s Health Initiative Study Group Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control Clin. Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 14.Patterson R.E., Kristal A.R., Tinker L.F., Carter R.A., Bolton M.P., Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann. Epidemiol. 1999;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 15.Neuhouser M.L., Tinker L., Shaw P.A., Schoeller D., Bingham S.A., Van Horn L., et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. Am. J. Epidemiol. 2008;167(10):1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 16.Schoeller D.A., Hnilicka J.M. Reliability of the doubly labeled water method for the measurement of total daily energy expenditure in free-living subjects. J. Nutr. 1996;126(1):348S–354S. [PubMed] [Google Scholar]
- 17.Zheng C., Nagana Gowda G.A., Raftery D., Neuhouser M.L., Tinker L.F., Prentice R.L., et al. Evaluation of potential metabolomics-based biomarkers of protein, carbohydrate and fat intakes using a controlled feeding study. Eur. J. Nutr. 2021;60(8):4207–4218. doi: 10.1007/s00394-021-02577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curb J.D., McTiernan A., Heckbert S.R., Kooperberg C., Stanford J., Nevitt M., et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann. Epidemiol. 2003;13(9 Suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 19.Margolis K.L., Qi L., Brzyski R., Bonds D.E., Howard B.V., Kempainen S., et al. Validity of diabetes self-reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. J. Soc. Clin. Trials. 2008;5(3):240–247. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S., Tinker L., Song Y., Rafai N., Bonds D.E., Cook N.R., et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch. Intern. Med. 2007;167(15):1676–1685. doi: 10.1001/archinte.167.15.1676. [DOI] [PubMed] [Google Scholar]
- 21.Song Y., Manson J.E., Tinker L.F., Howard B.V., Kuller L.H., Nathan L., et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women's Health Initiative Observational Study. Diabetes Care. 2007;30(7):1747–1752. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bingham S.A. Urine nitrogen as a biomarker for the validation of dietary protein intake. J. Nutr. 2003;133(Suppl 3):921S–924S. doi: 10.1093/jn/133.3.921S. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y., Zheng C., Tinker L.F., Neuhouser M.L., Prentice R.L. Biomarker-based methods and study designs to calibrate dietary intake for assessing diet–disease associations. J. Nutr. 2022;152(3):899–906. doi: 10.1093/jn/nxab420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibshirani R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. B. 1996;58:267–288. [Google Scholar]
- 25.Cox D.R. Regression analysis and life tables (with discussion) J. R. Stat. Soc. B. 1972;34:187–220. [Google Scholar]
- 26.Prentice R.L. Covariate measurement errors and parameter estimation in a failure time regression model. Biometrika. 1982;69:331–342. [Google Scholar]
- 27.Wang C.Y., Hsu L., Feng Z.D., Prentice R.L. Regression calibration in failure time regression. Biometrics. 1997;53(1):131–145. [PubMed] [Google Scholar]
- 28.Carroll R.J., Ruppert D., Stefanski L.A., Crainiceanu C.M. 2nd ed. Chapman and Hall/CRC; Boca Raton, FL: 2006. Measurement Error in Nonlinear Models: A Modern Perspective. [Google Scholar]
- 29.Howard B.V., Van Horn L., Hsia J., Manson J.E., Stefanick M.L., et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative randomized controlled dietary modification trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 30.Hammerschmidt P., Brüning J.C. Contributions of specific ceramides to obesity-associated metabolic diseases. Cell. Mol. Life Sci. 2022;79(8):395. doi: 10.1007/s00018-022-04401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaurasia B., Summers S.A. Ceramides in metabolism: key lipotoxic players. Annu. Rev. Physiol. 2021;83:303–330. doi: 10.1146/annurev-physiol-031620-093815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frayn F.N. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45(9):1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 33.Fielding B. Tracing the fate of dietary fatty acids: metabolic studies of postprandial lipaemia in human subjects. Proc. Nutr. Soc. 2011;70(3):342–350. doi: 10.1017/S002966511100084X. [DOI] [PubMed] [Google Scholar]
- 34.Detour M., Michielsen C.C.J.R., O’Donovan S., Alman L.A., Kersten S. Transcriptomic signature of fasting in human adipose tissue. Physiol. Genomics. 2020;52(10):451–467. doi: 10.1152/physiolgenomics.00083.2020. [DOI] [PubMed] [Google Scholar]
- 35.Markland M., Wu J.H.Y., Imamura F., Del Gobbo L.C., Fretts A., de Goede J., et al. Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality: an individual-level pooled analysis of 30 cohort studies. Circulation. 2019;139(21):2422–2436. doi: 10.1161/CIRCULATIONAHA.118.038908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J.H.Y., Marklund M., Imamura F., Tintle N., Ardisson Korat A.V., deGoede J., et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual–level data for 39,740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965–974. doi: 10.1016/S2213-8587(17)30307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris W.S., Tintle N.L., Imamura F., Qian F., Ardissson Korat A.V., Marklund M., et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021;12(1):2329. doi: 10.1038/s41467-021-22370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US Department of Health and Human Services and U.S. Department of Agriculture . 2015. Dietary Guidelines for Americans.https://health.gov/dietaryguidelines/2015/guidelines/ Internet. [updated December 2015; cited May 2023]. Available from: [Google Scholar]
- 39.Hanson S., Thorpe G., Winstanley L., Abdelhamid A.S., Hooper L. PUFAH group, Omega-3, omega-6, and total dietary polyunsaturated fat on cancer incidence: systemic review and meta-analysis of randomized trials. Br. J. Cancer. 2020;122(8):1260–1270. doi: 10.1038/s41416-020-0761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence G.D. Perspective: the saturated fat–unsaturated oil dilemma: relations of dietary fatty acids and serum cholesterol, atherosclerosis, inflammation, cancer, and all-cause mortality. Adv. Nutr. 2021;12(3):647–656. doi: 10.1093/advances/nmab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, codebook, and analytic code used in this report may be accessed in a collaborative mode as described on the Women’s Health Initiative website (www.whi.org).