Abstract
Susceptibility of 230 penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647), a new ketolide, was tested by agar dilution, and results were compared with those of erythromycin, azithromycin, clarithromycin, roxithromycin, rokitamycin, clindamycin, pristinamycin, ciprofloxacin, sparfloxacin, trimethoprim-sulfamethoxazole, doxycycline, chloramphenicol, cefuroxime, ceftriaxone, imipenem, and vancomycin. HMR 3647 was very active against all strains tested, with MICs at which 90% of the strains were inhibited (MIC90s) of 0.03 μg/ml for erythromycin-susceptible strains (MICs, ≤0.25 μg/ml) and 0.25 μg/ml for erythromycin-resistant strains (MICs, ≥1.0 μg/ml). All other macrolides yielded MIC90s of 0.03 to 0.25 and >64.0 μg/ml for erythromycin-susceptible and -resistant strains, respectively. The MICs of clindamycin for 51 of 100 (51%) erythromycin-resistant strains were ≤0.125 μg/ml. The MICs of pristinamycin for all strains were ≤1.0 μg/ml. The MIC90s of ciprofloxacin and sparfloxacin were 4.0 and 0.5 μg/ml, respectively, and were unaffected by penicillin or erythromycin susceptibility. Vancomycin and imipenem inhibited all strains at ≤1.0 μg/ml. The MICs of cefuroxime and cefotaxime rose with those of penicillin G. The MICs of trimethoprim-sulfamethoxazole, doxycycline, and chloramphenicol were variable but were generally higher in penicillin- and erythromycin-resistant strains. HMR 3647 had the best kill kinetics of all macrolides tested against 11 erythromycin-susceptible and -resistant strains, with uniform bactericidal activity (99.9% killing) after 24 h at two times the MIC and 99% killing of all strains at two times the MIC after 12 h for all strains. Pristinamycin showed more rapid killing at 2 to 6 h, with 99.9% killing of 10 of 11 strains after 24 h at two times the MIC. Other macrolides showed significant activity, relative to the MIC, against erythromycin-susceptible strains only.
The worldwide incidence of infections caused by pneumococci resistant to penicillin G and other antimicrobials has increased at an alarming rate during the past two decades and, in particular, during the past 5 years (4, 6, 11, 13). The main foci of penicillin-resistant pneumococci are currently South Africa, Spain, and Eastern Europe. However, wherever susceptibility testing is performed by appropriate methods, resistant strains are almost universally found (4). The spread of penicillin-resistant clones from country to country and from continent to continent demonstrates the capability of these strains to spread rapidly throughout the world (19). In the United States, recent surveys (7) have shown an increase in resistance to penicillin from <5% of isolates before 1989 (including <0.02% of isolates for which the MICs were ≥2.0 μg/ml) to 6.6% of isolates in 1991–1992 (with 1.3% of isolates for which the MICs were ≥2.0 μg/ml). In another recent survey, 23.6% of 1,527 clinically significant pneumococcal isolates (i.e., 360 isolates) were not susceptible to penicillin (8).
Pneumococcal strains with intermediate and especially full resistance to penicillin G are often resistant to erythromycin. In the United States, Breiman and coworkers in 1991 to 1992 demonstrated erythromycin resistance rates of 3.7 and 2.2% in patients of 1 to 2 and ≥4 years of age, respectively (7). A recent study by Doern and coworkers (8) documented erythromycin resistance rates of 19 to 20 and 49% in penicillin-intermediately-resistant and -resistant strains, respectively. In Europe, erythromycin resistance rates are generally higher. For example, 27.5% of all pneumococci studied in France during 1992 (63% of penicillin-resistant strains) were erythromycin resistant (12). Although clarithromycin generally demonstrates MICs for pneumococci which are 1 or 2 dilutions lower than those of other macrolides (5, 8, 16, 18, 21), erythromycin-resistant pneumococci are resistant to all other existing macrolides (8, 10, 13). Until now, the only member of the macrolide-lincosamide-streptogramin B group that is consistently active against all pneumococci, irrespective of their penicillin or erythromycin susceptibility status, has been RP 59500 (quinupristin or dalfopristin), a parenteral streptogramin (22, 23, 25). HMR 3647 (RU 66647) is a new ketolide (15). The ketolides are semisynthetic broad-spectrum macrolides characterized by a 3-keto function which replaces the cladinose moiety of other members of the macrolide group (1–3). The present study examined the susceptibility of 230 penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647) (15) in comparison to susceptibilities to erythromycin, azithromycin, clarithromycin, roxithromycin, clindamycin, rokitamycin, pristinamycin, trimethoprim-sulfamethoxazole, ciprofloxacin, sparfloxacin, doxycycline, chloramphenicol, cefuroxime, ceftriaxone, imipenem, and vancomycin. Additionally, the activity of HMR 3647, erythromycin, azithromycin, clarithromycin, roxithromycin, clindamycin, and pristinamycin against a selected number of erythromycin-susceptible and -resistant pneumococci was tested by time-kill methodology.
MATERIALS AND METHODS
Bacterial strains.
A total of 230 isolates of Streptococcus pneumoniae (see Tables 1 and 2) isolated from blood, cerebrospinal fluid, ear, nasopharynx, or sputum during 1995 to 1997 were examined by agar dilution MIC. Forty-eight strains susceptible to penicillin (MICs, <0.1 μg/ml) were isolated from various hospitals in the United States. One hundred twenty-six isolates resistant to penicillin (MICs, ≥2.0 μg/ml) and most of the 56 strains intermediately resistant to penicillin (MICs, 0.1 to 1.0 μg/ml) were isolated in the United States, South Africa, France, Spain, Eastern Europe (Hungary, Slovakia, and Bulgaria), Japan, and Korea. One hundred thirty strains were erythromycin susceptible (MICs, ≤0.25 μg/ml), and 100 randomly selected strains were erythromycin resistant (MICs, ≥1.0 μg/ml) (20). For time-kill studies, activities of HMR 3647, erythromycin, azithromycin, clarithromycin, roxithromycin, clindamycin, and pristinamycin against five erythromycin-susceptible (MICs, ≤0.25 μg/ml) and six erythromycin-resistant (MICs, ≥2.0 μg/ml) pneumococcal strains were examined.
TABLE 1.
Susceptibility of pneumococci to antimicrobial agents by penicillin susceptibility
| Antimicrobial and penicillin susceptibilitya | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Penicillin | |||
| S | ≤0.004–0.06 | 0.03 | 0.06 |
| I | 0.125–1.0 | 0.25 | 1.0 |
| R | 2.0–8.0 | 4.0 | 4.0 |
| HMR 3647 | |||
| S | ≤0.004–0.125 | 0.016 | 0.03 |
| I | ≤0.004–0.5 | 0.016 | 0.06 |
| R | ≤0.004–1.0 | 0.03 | 0.25 |
| Erythromycin | |||
| S | ≤0.004–>64.0 | 0.03 | 1.0 |
| I | 0.016–>64.0 | 0.03 | >64.0 |
| R | 0.008–>64.0 | 1.0 | >64.0 |
| Azithromycin | |||
| S | 0.03–>64.0 | 0.125 | 1.0 |
| I | 0.03–>64.0 | 0.125 | >64.0 |
| R | 0.03–>64.0 | 1.0 | >64.0 |
| Clarithromycin | |||
| S | 0.008–>64.0 | 0.016 | 0.25 |
| I | 0.016–>64.0 | 0.03 | >64.0 |
| R | ≤0.004–>64.0 | 0.5 | >64.0 |
| Roxithromycin | |||
| S | 0.03–>64.0 | 0.125 | 4.0 |
| I | 0.03–>64.0 | 0.125 | >64.0 |
| R | 0.016–>64.0 | 2.0 | >64.0 |
| Rokitamycin | |||
| S | 0.03–>64.0 | 0.125 | 0.25 |
| I | 0.016–>64.0 | 0.125 | 0.5 |
| R | 0.016–>64.0 | 0.25 | 8.0 |
| Clindamycin | |||
| S | 0.008–>64.0 | 0.03 | 0.125 |
| I | ≤0.004–>64.0 | 0.06 | 8.0 |
| R | 0.016–>64.0 | 0.06 | >64.0 |
| Pristinamycin | |||
| S | 0.06–0.5 | 0.25 | 0.25 |
| I | 0.06–1.0 | 0.25 | 0.5 |
| R | 0.06–1.0 | 0.25 | 0.5 |
| Trimethoprim-sulfamethoxazole | |||
| S | 0.125–>16.0 | 0.5 | 4.0 |
| I | ≤0.004–>16.0 | 1.0 | 16.0 |
| R | 0.25–>16.0 | 16.0 | >16.0 |
| Ciprofloxacin | |||
| S | 0.5–16.0 | 2.0 | 4.0 |
| I | 0.5–4.0 | 1.0 | 4.0 |
| R | 0.125–8.0 | 2.0 | 4.0 |
| Sparfloxacin | |||
| S | 0.125–1.0 | 0.25 | 0.5 |
| I | 0.125–0.5 | 0.25 | 0.5 |
| R | 0.06–0.5 | 0.25 | 0.5 |
| Doxycycline | |||
| S | 0.03–32.0 | 0.25 | 8.0 |
| I | 0.06–16.0 | 0.25 | 16.0 |
| R | 0.06–32.0 | 4.0 | 16.0 |
| Chloramphenicol | |||
| S | 0.06–32.0 | 4.0 | 16.0 |
| I | 1.0–32.0 | 4.0 | 16.0 |
| R | 1.0–32.0 | 8.0 | 16.0 |
| Cefuroxime | |||
| S | ≤0.004–0.25 | 0.03 | 0.06 |
| I | 0.125–4.0 | 0.5 | 2.0 |
| R | 2.0–32.0 | 4.0 | 16.0 |
| Ceftriaxone | |||
| S | 0.008–0.25 | 0.03 | 0.06 |
| I | 0.03–1.0 | 0.25 | 0.5 |
| R | 0.25–4.0 | 1.0 | 2.0 |
| Imipenem | |||
| S | ≤0.004–0.03 | 0.008 | 0.016 |
| I | 0.008–1.0 | 0.03 | 0.25 |
| R | 0.03–1.0 | 0.25 | 0.5 |
| Vancomycin | |||
| S | 0.03–1.0 | 0.25 | 0.5 |
| I | 0.125–1.0 | 0.25 | 0.5 |
| R | 0.125–1.0 | 0.25 | 0.5 |
The susceptibilities to various antimicrobials of pneumococci that were penicillin susceptible (S), intermediately resistant (I), or resistant (R) were determined.
TABLE 2.
Susceptibility of pneumococci to antimicrobial agents by erythromycin susceptibility
| Antimicrobial and erythro- mycin susceptibilitya | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| Penicillin | |||
| S | 0.008–8.0 | 0.5 | 4.0 |
| R | ≤0.004–8.0 | 2.0 | 4.0 |
| HMR 3647 | |||
| S | ≤0.004–0.06 | 0.016 | 0.03 |
| R | 0.008–1.0 | 0.06 | 0.25 |
| Erythromycin | |||
| S | ≤0.004–0.25 | 0.03 | 0.03 |
| R | 1.0–>64.0 | >64.0 | >64.0 |
| Azithromycin | |||
| S | 0.03–1.0 | 0.125 | 0.125 |
| R | 1.0–>64.0 | >64.0 | >64.0 |
| Clarithromycin | |||
| S | ≤0.004–0.25 | 0.03 | 0.06 |
| R | 0.5–>64.0 | 32.0 | >64.0 |
| Roxithromycin | |||
| S | 0.016–8.0 | 0.125 | 0.25 |
| R | 1.0–>64.0 | >64.0 | >64.0 |
| Rokitamycin | |||
| S | 0.016–0.25 | 0.125 | 0.25 |
| R | 0.016–>8.0 | 1.0 | >64.0 |
| Clindamycin | |||
| S | ≤0.004–0.125 | 0.03 | 0.125 |
| R | 0.008–>64.0 | 0.125 | >64.0 |
| Pristinamycin | |||
| S | 0.06–0.5 | 0.25 | 0.5 |
| R | 0.06–1.0 | 0.25 | 0.5 |
| Trimethoprim-sulfamethoxazole | |||
| S | ≤0.004–>16.0 | 2.0 | >16.0 |
| R | ≤0.004–>16.0 | 8.0 | >16.0 |
| Ciprofloxacin | |||
| S | 0.125–16.0 | 2.0 | 4.0 |
| R | 0.25–8.0 | 1.0 | 4.0 |
| Sparfloxacin | |||
| S | 0.06–1.0 | 0.25 | 0.5 |
| R | 0.125–1.0 | 0.25 | 0.5 |
| Doxycycline | |||
| S | 0.03–32.0 | 0.25 | 8.0 |
| R | 0.06–32.0 | 8.0 | 16.0 |
| Chloramphenicol | |||
| S | 1.0–32.0 | 4.0 | 16.0 |
| R | 1.0–32.0 | 8.0 | 16.0 |
| Cefuroxime | |||
| S | 0.016–16.0 | 1.0 | 8.0 |
| R | ≤0.004–32.0 | 4.0 | 16.0 |
| Ceftriaxone | |||
| S | 0.008–4.0 | 0.25 | 2.0 |
| R | 0.008–4.0 | 1.0 | 2.0 |
| Imipenem | |||
| S | ≤0.004–1.0 | 0.06 | 0.5 |
| R | ≤0.004–1.0 | 0.25 | 0.5 |
| Vancomycin | |||
| S | 0.06–1.0 | 0.25 | 0.5 |
| R | 0.03–1.0 | 0.25 | 0.5 |
The susceptibilities to various antimicrobials of pneumococci that were erythromycin susceptible (S) or resistant (R) were determined.
Antimicrobial agents.
Antimicrobial agents were supplied as laboratory powders of known potency by the manufacturers indicated as follows: HMR 3647, roxithromycin, rokitamycin, and pristinamycin by Roussel Uclaf, Paris, France; erythromycin and clarithromycin by Abbott Laboratories, Chicago, Ill.; azithromycin by Pfizer Inc., New York, N.Y.; clindamycin by The Upjohn Co., Kalamazoo, Mich.; trimethoprim, sulfamethoxazole, penicillin G, doxycycline, and chloramphenicol by Sigma Chemical Co., St. Louis, Mo.; ciprofloxacin by Bayer Corp., West Haven, Conn.; sparfloxacin by Rhône-Poulenc Rorer, Collegeville, Pa.; cefuroxime and vancomycin by Eli Lilly & Co., Indianapolis, Ind.; ceftriaxone by Roche Laboratories, Nutley, N.J.; and imipenem by Merck & Co., Rahway, N.J.
Agar dilution MIC testing.
Agar dilution MICs were determined for 230 strains by using Mueller-Hinton agar (BBL Microbiology Systems, Cockeysville, Md.) supplemented with 5% sheep blood (13). Suspensions with a turbidity equivalent to that of a 0.5 McFarland standard were prepared by suspending growth from blood agar plates in 2 ml of Mueller-Hinton broth (BBL). Suspensions were further diluted 1:10 to obtain a final inoculum of 104 CFU/spot. The plates were inoculated with a Steers replicator and incubated overnight in ambient air at 37°C. Because these strains were subcultured repeatedly, no growth failures were observed and good growth was observed in every case. Standard quality control strains, including S. pneumoniae ATCC 49619 (20), were included in each run.
Broth microdilution MICs.
Eleven pneumococcal strains (see Table 3) were studied by broth microdilution MIC and time-kill methods. Microbroth dilution MIC assays were performed in accordance with standard methods (20) in Mueller-Hinton broth supplemented with 5% lysed horse blood. Inocula were prepared by suspending growth from overnight cultures in sterile saline to a turbidity of approximately 0.5 McFarland standard. Final inocula contained 5 × 105 CFU/ml. The lowest concentration of antibiotic resulting in no growth was read as the MIC. Quality control organisms (see above) were included in each run. Antibiotic-containing plates were frozen at −70°C prior to use.
TABLE 3.
Microbroth MICs (μg/ml) of 11 pneumococcal strains studied by time-kill assay
| Drug | MIC (μg/ml) at microdilution (fold) MIC of:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Penicillin G | 0.5 | 0.03 | 1.0 | 0.5 | 0.5 | 2.0 | 2.0 | 8.0 | 2.0 | 0.25 | 0.125 |
| HMR 3647 | 0.016 | 0.008 | 0.016 | 0.008 | 0.016 | 0.016 | 0.125 | 0.125 | 0.125 | 0.016 | 0.125 |
| Erythromycin | 0.03 | 0.03 | 0.06 | 0.03 | 0.03 | 2 | >64 | >64 | >64 | 2 | 2 |
| Azithromycin | 0.125 | 0.125 | 0.25 | 0.06 | 0.125 | 16 | >64 | >64 | >64 | 0.125 | 1 |
| Clarithromycin | 0.016 | 0.016 | 0.125 | 0.016 | 0.03 | 0.5 | >64 | >64 | >64 | 0.5 | 1 |
| Roxithromycin | 0.125 | 0.25 | 0.25 | 0.125 | 0.06 | 8 | >64 | >64 | >64 | 8 | 4 |
| Clindamycin | 0.06 | 0.125 | 0.125 | 0.03 | 0.03 | 8 | >64 | >64 | >64 | 8 | 0.008 |
| Pristinamycin | 0.25 | 0.5 | 0.5 | 0.25 | 0.125 | 0.25 | 0.25 | 0.25 | 0.5 | 0.25 | 0.125 |
Time-kill studies.
For time-kill experiments, glass tubes containing 5 ml of cation-adjusted Mueller-Hinton broth (Difco) plus 5% lysed horse blood with doubling antibiotic concentrations were inoculated with approximately 5 × 105 to 5 × 106 CFU of organism per ml and incubated at 35°C in a shaking water bath. Antibiotic concentrations were chosen to comprise 3 doubling dilutions above and 3 dilutions below the microdilution MIC (22, 23).
Lysed horse blood was prepared by freezing and thawing horse blood (Cleveland Scientific, Bath, Ohio) as described previously (20, 21). Appropriate amounts of 50% lysed blood were then added to the cation-adjusted Mueller-Hinton broth to yield a final concentration of 5% lysed horse blood. The bacterial inoculum was prepared by diluting a 16-h broth (medium as above) culture in the same medium. Dilutions required to obtain the correct inoculum (approximately 5 × 105 CFU/ml) were determined by prior viability studies with each strain (22, 23).
To inoculate each tube of serially diluted antibiotic, 50 μl of diluted inoculum was delivered by pipette beneath the surface of the broth and then vortexed and plated for viability counts (0 h). Only tubes containing an initial inoculum within the range of 5 × 105 to 5 × 106 CFU/ml were acceptable. Viability counts of antibiotic-containing suspensions were performed at 0, 2, 4, 6, 12, and 24 h by plating 0.1-ml aliquots from 10-fold dilutions onto Trypticase soy agar–5% sheep blood agar plates (BBL). Recovery plates were incubated for up to 48 h. Colony counts were performed on plates yielding 30 to 300 colonies.
Time-kill assays were analyzed by determining the number of strains which yielded a decrease in viable count of −1, −2, and −3 log10 CFU/ml compared to counts at time 0 h for all compounds at all time periods. With the sensitivity threshold and inocula used in these studies, 99.9% killing (>3 log10 CFU decrease in viability count per ml) could readily be determined when present. The problem of bacterial carryover was minimized by dilution of inocula, as described previously (22, 23). Time-kill assays for erythromycin, azithromycin, clarithromycin, roxithromycin, and clindamycin were performed only with strains for which the erythromycin MICs were ≤2.0 μg/ml.
RESULTS
The results of agar dilution MIC testing are presented in Tables 1 and 2. In Table 1, MICs are shown by penicillin susceptibility. As shown by these data, resistance to macrolide and clindamycin was found mainly in penicillin-intermediately-resistant and penicillin-resistant strains. In all cases, however, the MICs of HMR 3647 were ≤1.0 μg/ml, with MICs at which 90% of the strains were inhibited (MIC90s) of 0.03, 0.06, and 0.25 μg/ml for penicillin-susceptible, -intermediately-resistant, and -resistant strains, respectively. By contrast, the MIC90s of erythromycin, azithromycin, clarithromycin, roxithromycin, and rokitamycin were 0.25 to 4.0, 0.5 to >64.0, and 8 to >64.0 μg/ml, respectively, for these three strain groups. All strains were susceptible to pristinamycin at MICs of ≤1.0 μg/ml. Quinolone activity was independent of penicillin susceptibility, with sparfloxacin more active than ciprofloxacin (MIC90s of 0.5 and 4.0 μg/ml, respectively). The MICs of cefuroxime, ceftriaxone, and imipenem increased with those of penicillin G. However, all strains were inhibited by ceftriaxone at MICs of ≤4.0 μg/ml and by imipenem at MICs of ≤1.0 μg/ml. Susceptibilities of strains to trimethoprim-sulfamethoxazole, doxycycline, and chloramphenicol were variable. As in the case of macrolides, however, resistance to the latter three drugs was seen more often in penicillin-intermediately-resistant and penicillin-resistant strains. All strains were susceptible to vancomycin at MICs of ≤1.0 μg/ml.
Susceptibility results analyzed by erythromycin susceptibility are presented in Table 2. As can be seen, the MICs of azithromycin, clarithromycin, roxithromycin, rokitamycin, and clindamycin for erythromycin-susceptible strains (MICs, ≤0.25 μg/ml) were low, with the MICs of clarithromycin being 1 or 2 dilutions lower than those of erythromycin, azithromycin, roxithromycin, and rokitamycin. Of all macrolides-ketolides tested, HMR 3647 had the lowest MICs for erythromycin-susceptible strains (MIC90, 0.03 μg/ml). Strains which were resistant to erythromycin (MICs, ≥1.0 μg/ml) were also resistant to azithromycin, clarithromycin, and roxithromycin, and rokitamycin MICs were increased. By comparison, HMR 3647 had MICs which, although higher than those for erythromycin-susceptible strains, were all ≤1.0 μg/ml (MIC90, 0.25 μg/ml). Although many macrolide-resistant strains were also resistant to clindamycin, 51 of 100 strains (51%) were susceptible to clindamycin (MICs, ≤0.125 μg/ml). Pristinamycin and quinolone MICs were identical for erythromycin-susceptible and -resistant strains. The MICs of β-lactams, trimethoprim-sulfamethoxazole, doxycycline, and chloramphenicol were generally higher for erythromycin-resistant than for erythromycin-susceptible strains.
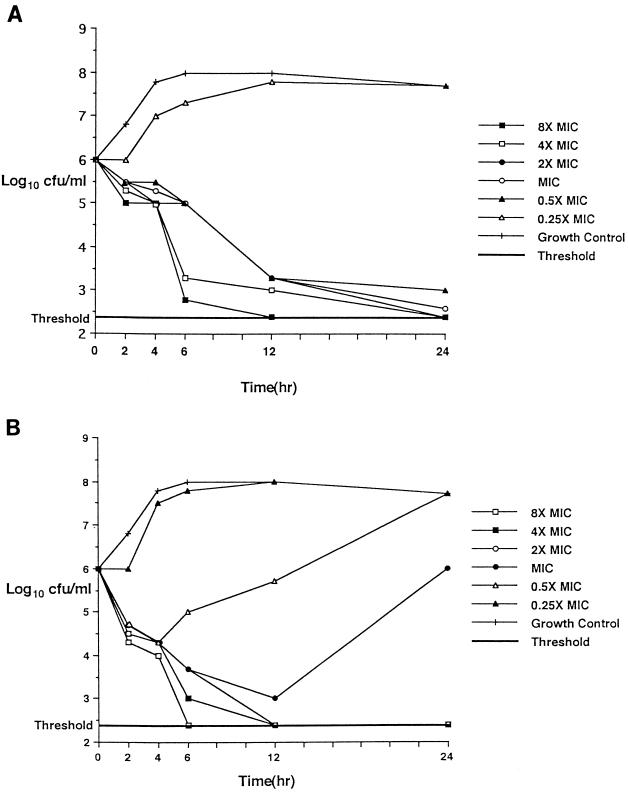
Microbroth MICs of the 11 pneumococcal strains used for time-kill experiments are presented in Table 3, and time-kill results are shown in Tables 4 and 5. Microbroth MICs for individual strains were within 1 dilution of those obtained by agar dilution. HMR 3647 was uniformly bactericidal (irrespective of the strain’s erythromycin susceptibility) after 24 h at twice the MIC (≤0.25 μg/ml) and showed 99% killing of all strains at twice the MIC after 12 h. Pristinamycin killed all 11 strains more rapidly at earlier time periods, with 90% killing of all strains at twice the MIC after 4 h and 99% killing of 10 of 11 strains at twice the MIC after 6 h; pristinamycin was bactericidal against all 5 erythromycin-susceptible strains at the MIC and against all 6 erythromycin-resistant strains at four times the MIC (≤2.0 μg/ml) after 24 h. Erythromycin, azithromycin, clarithromycin, roxithromycin, and clindamycin showed slower kill kinetics than the ketolides and pristinamycin did at 2 to 6 h against erythromycin-susceptible strains. After 24 h, the five compounds were bactericidal against all five erythromycin-susceptible strains at four times the MIC and against all three erythromycin-resistant strains with MICs of 2.0 μg/ml at microbroth dilutions of four to eight times the MIC. Time-kill results with the MICs of both HMR 3647 and pristinamycin for strain 9 (penicillin and macrolide resistant) are depicted graphically in Fig. 1.
TABLE 4.
Time-kill results of five erythromycin-susceptible strains
| Drug and microdilution MIC | No. of strains with viable count decrease at:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h
|
4 h
|
6 h
|
12 h
|
24 h
|
|||||||||||
| −1a | −2a | −3a | −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | |
| HMR 3647 | |||||||||||||||
| Four times MIC | 2 | 0 | 0 | 4 | 2 | 1 | 5 | 5 | 2 | 5 | 5 | 4 | 5 | 5 | 5 |
| Two times MIC | 2 | 0 | 0 | 3 | 2 | 1 | 5 | 4 | 2 | 5 | 5 | 3 | 5 | 5 | 5 |
| MIC | 2 | 0 | 0 | 3 | 1 | 0 | 4 | 2 | 1 | 5 | 3 | 2 | 4 | 4 | 4 |
| One-half MIC | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 3 | 1 | 1 | 4 | 3 | 2 |
| Erythromycin | |||||||||||||||
| Four times MIC | 1 | 0 | 0 | 2 | 1 | 1 | 4 | 1 | 1 | 5 | 3 | 2 | 5 | 5 | 5 |
| Two times MIC | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 1 | 4 | 3 | 2 | 5 | 5 | 4 |
| MIC | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 4 | 1 | 1 | 4 | 2 | 2 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 1 |
| Azithromycin | |||||||||||||||
| Four times MIC | 1 | 0 | 0 | 2 | 1 | 1 | 4 | 1 | 1 | 4 | 3 | 2 | 5 | 5 | 5 |
| Two times MIC | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 4 | 3 | 2 | 5 | 5 | 4 |
| MIC | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 4 | 2 | 1 | 5 | 4 | 3 |
| One-half MIC | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 3 | 1 | 1 | 3 | 3 | 2 |
| Clarithromycin | |||||||||||||||
| Four times MIC | 0 | 0 | 0 | 2 | 0 | 0 | 5 | 1 | 1 | 5 | 4 | 2 | 5 | 5 | 5 |
| Two times MIC | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 3 | 1 | 1 | 3 | 3 | 2 |
| MIC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| Roxithromycin | |||||||||||||||
| Four times MIC | 1 | 0 | 0 | 1 | 1 | 1 | 4 | 2 | 1 | 5 | 3 | 2 | 5 | 5 | 5 |
| Two times MIC | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 2 | 1 | 3 | 2 | 2 | 4 | 4 | 3 |
| MIC | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 3 | 1 | 1 | 4 | 3 | 0 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
| Clindamycin | |||||||||||||||
| Four times MIC | 0 | 0 | 0 | 2 | 1 | 1 | 4 | 1 | 1 | 5 | 3 | 3 | 5 | 5 | 5 |
| Two times MIC | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 4 | 3 | 2 | 5 | 5 | 3 |
| MIC | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 4 | 1 | 1 | 5 | 2 | 1 |
| One-half MIC | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 1 |
| Pristinamycin | |||||||||||||||
| Four times MIC | 4 | 1 | 0 | 5 | 4 | 1 | 5 | 5 | 3 | 5 | 5 | 3 | 5 | 5 | 5 |
| Two times MIC | 3 | 0 | 0 | 5 | 3 | 1 | 5 | 5 | 1 | 5 | 5 | 3 | 5 | 5 | 5 |
| MIC | 1 | 0 | 0 | 5 | 2 | 0 | 5 | 4 | 1 | 5 | 5 | 3 | 5 | 5 | 5 |
| One-half MIC | 1 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 1 |
ΔLog10 CFU/ml lower than value at time 0 h.
TABLE 5.
Time-kill results of six erythromycin-resistant strainsa
| Drug and microdilution MIC | No. of strains with viable count decrease at:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h
|
4 h
|
6 h
|
12 h
|
24 h
|
|||||||||||
| −1b | −2b | −3b | −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | |
| HMR 3647 | |||||||||||||||
| Four times MIC | 3 | 0 | 0 | 6 | 1 | 0 | 6 | 6 | 4 | 6 | 6 | 6 | 6 | 6 | 6 |
| Two times MIC | 2 | 0 | 0 | 6 | 1 | 0 | 6 | 3 | 0 | 6 | 6 | 3 | 6 | 6 | 6 |
| MIC | 1 | 0 | 0 | 2 | 0 | 0 | 4 | 2 | 0 | 6 | 5 | 3 | 5 | 5 | 5 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 5 | 3 | 1 | 4 | 4 | 4 |
| Erythromycin | |||||||||||||||
| Four times MIC | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 3 | 0 | 3 | 3 | 3 | 3 | 3 | 3 |
| Two times MIC | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 1 | 0 | 3 | 3 | 2 | 3 | 3 | 2 |
| MIC | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| Azithromycin | |||||||||||||||
| Four times MIC | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 2 | 0 | 3 | 3 | 1 | 3 | 2 | 2 |
| Two times MIC | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 3 | 2 | 1 | 3 | 2 | 1 |
| MIC | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Clarithromycin | |||||||||||||||
| Four times MIC | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 2 | 0 | 3 | 3 | 2 | 3 | 3 | 3 |
| Two times MIC | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 3 | 3 | 0 | 3 | 3 | 2 |
| MIC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Roxithromycin | |||||||||||||||
| Four times MIC | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 3 | 0 | 3 | 3 | 3 | 3 | 3 | 3 |
| Two times MIC | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 2 | 0 | 3 | 3 | 2 | 3 | 3 | 3 |
| MIC | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 3 | 1 | 1 | 1 | 1 | 2 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
| Clindamycin | |||||||||||||||
| Four times MIC | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 2 | 0 | 3 | 3 | 3 | 3 | 3 | 2 |
| Two times MIC | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 3 | 0 | 2 | 2 | 1 |
| MIC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| One-half MIC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Pristinamycin | |||||||||||||||
| Four times MIC | 6 | 3 | 1 | 6 | 4 | 3 | 6 | 6 | 5 | 6 | 6 | 6 | 6 | 6 | 6 |
| Two times MIC | 5 | 2 | 0 | 6 | 4 | 3 | 6 | 5 | 3 | 6 | 6 | 5 | 6 | 6 | 5 |
| MIC | 4 | 1 | 0 | 5 | 3 | 0 | 5 | 3 | 2 | 5 | 5 | 3 | 3 | 3 | 1 |
| One-half MIC | 3 | 1 | 0 | 4 | 1 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
Time-kill assays were not performed for erythromycin, azithromycin, clarithromycin, roxithromycin, and clindamycin with MICs for strains of ≥64 μg/ml.
ΔLog10 CFU/ml lower than value at time 0 h.
FIG. 1.
Time-kills of strain 9 at the MICs of HMR 3647 (0.125 μg/ml) (A) and pristinamycin (0.5 μg/ml) (B).
DISCUSSION
The results of this study reflect the excellent activity of the ketolide HMR 3647 against pneumococci, irrespective of penicillin or erythromycin susceptibility status, and confirm previous findings with another ketolide, RU 64004 (HMR 3004) (1–3, 9, 14, 24), and preliminary findings with HMR 3647 (15). The ketolide group has a broad spectrum of activity against pneumococci, β-lactamase-positive and -negative Haemophilus influenzae, beta-hemolytic and alpha-hemolytic streptococci, enterococci, and members of the family Enterobacteriaceae (1–3, 9, 14, 24). Although ketolide MICs in our study were a few dilutions higher for erythromycin-resistant than for erythromycin-susceptible strains, values were still significantly lower than those of other macrolides. This study confirms the cross-resistance of erythromycin-resistant pneumococci to other macrolides, as well as the slightly improved activity of clarithromycin compared to those of erythromycin, azithromycin, and roxithromycin against susceptible strains (5, 16, 18, 21). However, with an NCCLS resistance breakpoint of ≥1.0 μg/ml, compared to one of ≥1.0 μg/ml for erythromycin and one of ≥2.0 μg/ml for azithromycin (20), all macrolide-resistant pneumococci would also be expected to be clinically clarithromycin resistant.
Time-kill results confirmed the excellent antipneumococcal activity of HMR 3647, irrespective of the susceptibility of strains to penicillin or other macrolides. Even for strains for which macrolide MICs were ≥64.0 μg/ml, HMR 3647 was uniformly bactericidal after 24 h at ≤0.25 μg/ml. Continued killing by HMR 3647 at one-half the MIC could be at least partially explained by a postantibiotic sub-MIC effect; this aspect is being currently investigated. Additionally, macrobroth MICs obtained by time-kill assay were all identical to, or within 1 dilution of, microbroth MICs, and thus one-half the MIC by macrobroth may have been equivalent to the microbroth MIC in these cases.
It has previously been shown that macrolide-resistant pneumococci are variably susceptible to clindamycin (9, 10, 21). Fasola and coworkers have demonstrated that incubation in CO2 or prolonged aerobic incubation of microdilution trays is necessary to obtain accurate results with clindamycin (10). Incubation of MIC plates for an additional day did not lead to significant differences in clindamycin MICs (data not shown), such that this phenomenon of dissociated clindamycin or macrolide susceptibility in pneumococci appears real. The mechanism of the latter phenomenon may be related to efflux abnormalities and is being investigated. Time-kill testing relative to the MIC showed that erythromycin, azithromycin, clarithromycin, roxithromycin, and clindamycin were active only against susceptible strains.
Pristinamycin, a streptogramin analog, yielded results which were identical to those of RP 59500 (22, 23, 25), with significant killing at earlier time periods (2 to 12 h); pristinamycin was uniformly bactericidal after 24 h at ≤2.0 μg/ml. Quinolone MICs were similar to those described previously (22, 23, 25, 27), as were those of β-lactams and vancomycin (17, 26–28). A significant percentage of penicillin-intermediately-resistant and penicillin-resistant strains were also resistant to trimethoprim-sulfamethoxazole, doxycycline, and chloramphenicol.
In summary, HMR 3647 shows great potential for treatment of infections caused by pneumococci, irrespective of their penicillin or erythromycin susceptibility status. Clinical studies will be necessary to test this hypothesis.
ACKNOWLEDGMENTS
This study was supported by grants from Hoechst-Marion Roussel, Division of Clinical Anti-Infectives, Paris, France.
REFERENCES
- 1.Agouridas C, Benedetti Y, Denis A, Fromentin C, Gouin d’Ambrieres S, le Martret O, Chantot J F. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Ketolides, a new distinct semi-synthetic class of macrolides, abstr. F-164; p. 227. [Google Scholar]
- 2.Agouridas C, Bonnefoy A, Chantot J F. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Ketolides, a new distinct semi-synthetic class of macrolides: in-vitro and in-vivo antibacterial activities, abstr. F-168; p. 227. [Google Scholar]
- 3.Agouridas C, Bonnefoy A, Chantot J F. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. In vitro antibacterial activity of RU 004, a novel ketolide highly active against respiratory pathogens, abstr. F-158; p. 140. [Google Scholar]
- 4.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 5.Barry A L, Pfaller M A, Fuchs P C, Packer R R. In vitro activities of 12 orally administered antimicrobial agents against four species of bacterial respiratory pathogens from U.S. medical centers in 1992 and 1993. Antimicrob Agents Chemother. 1994;38:2419–2425. doi: 10.1128/aac.38.10.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block S, Harrison C J, Hedrick J A, Tyler R D, Smith R A, Keegan E, Chartrand S A. Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns and antimicrobial management. Pediatr Infect Dis J. 1995;14:751–759. doi: 10.1097/00006454-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 8.Doern G V, Brueggemann A, Holley H P, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ednie L, Spangler S K, Jacobs M R, Appelbaum P C. Susceptibilities of 228 penicillin- and erythromycin-susceptible and -resistant pneumococci to RU 64004, a new ketolide, compared with susceptibilities to 16 other agents. Antimicrob Agents Chemother. 1997;41:1033–1036. doi: 10.1128/aac.41.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasola E L, Bajaksouzian S, Appelbaum P C, Jacobs M R. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae by four test methods. Antimicrob Agents Chemother. 1997;41:129–134. doi: 10.1128/aac.41.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedland I R, McCracken G H., Jr Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377–382. doi: 10.1056/NEJM199408113310607. [DOI] [PubMed] [Google Scholar]
- 12.Geslin P, Fremaux A, Sissia G, Spicq C, Aberrane A. Epidémiologie de la résistance aux antibiotiques de Streptococcus pneumoniae en France. Réseau national de surveillance (1984–1993) Méd Mal Infect. 1994;24:948–961. [Google Scholar]
- 13.Jacobs M R. Treatment and diagnosis of infections caused by drug-resistant Streptococcus pneumoniae. Clin Infect Dis. 1992;15:119–127. doi: 10.1093/clinids/15.1.119. [DOI] [PubMed] [Google Scholar]
- 14.Jamjian C, Biedenbach D J, Jones R N. In vitro evaluation of a novel ketolide antimicrobial agent, RU-64004. Antimicrob Agents Chemother. 1997;41:454–459. doi: 10.1128/aac.41.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones R N, Biedenbach D J. Antimicrobial activity of RU-66647, a new ketolide. Diagn Microbiol Infect Dis. 1997;27:7–12. doi: 10.1016/s0732-8893(96)00181-2. [DOI] [PubMed] [Google Scholar]
- 16.Liñares J, Alonso T, Ayats J, Alcaide F, Tubau F, Hernandez J, Martín R. Program and abstracts of the 31st Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1991. In vitro activity of 7 macrolide antibiotics and 9 other antimicrobial agents against penicillin-resistant pneumococci, abstr. 191; p. 130. [Google Scholar]
- 17.Liñares J, Alonso T, Pérez J L, Ayats J, Domínguez M A, Pallarés R, Martín R. Decreased susceptibility of penicillin-resistant pneumococci to twenty-four β-lactam antibiotics. J Antimicrob Chemother. 1992;30:279–288. doi: 10.1093/jac/30.3.279. [DOI] [PubMed] [Google Scholar]
- 18.Mason E O, Jr, Kaplan S L, Lamberth L B, Tillman J. Increased rate of isolation of penicillin-resistant Streptococcus pneumoniae in a children’s hospital and in vitro susceptibilities to antibiotics of potential therapeutic use. Antimicrob Agents Chemother. 1992;36:1703–1707. doi: 10.1128/aac.36.8.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz R, Coffey T J, Daniels M, Dowson C G, Laible G, Casal J, Hakenbeck R, Jacobs M, Musser J M, Spratt B G, Tomasz A. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J Infect Dis. 1991;164:302–306. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS publication no. M7-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 21.Nelson C T, Mason E O, Jr, Kaplan S L. Activity of oral antibiotics in middle ear and sinus infections caused by penicillin-resistant Streptococcus pneumoniae: implications for treatment. Pediatr Infect Dis J. 1994;13:585–589. doi: 10.1097/00006454-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Pankuch G A, Jacobs M R, Appelbaum P C. Study of comparative antipneumococcal activities of penicillin G, RP 59500, erythromycin, sparfloxacin, ciprofloxacin, and vancomycin by using time-kill methodology. Antimicrob Agents Chemother. 1994;38:2065–2072. doi: 10.1128/aac.38.9.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankuch G A, Lichtenberger C, Jacobs M R, Appelbaum P C. Antipneumococcal activities of RP 59500 (quinupristin/dalfopristin), penicillin G, erythromycin, and sparfloxacin determined by MIC and rapid time-kill methodologies. Antimicrob Agents Chemother. 1996;40:1653–1656. doi: 10.1128/aac.40.7.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schülin T, Wennersten C B, Moellering R C, Jr, Eliopoulos G M. In vitro activity of RU 64004, a new ketolide antibiotic, against gram-positive bacteria. Antimicrob Agents Chemother. 1997;41:1196–1202. doi: 10.1128/aac.41.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spangler S K, Jacobs M R, Appelbaum P C. Susceptibilities of penicillin-susceptible and -resistant strains of Streptococcus pneumoniae to RP 59500, vancomycin, erythromycin, PD 131628, sparfloxacin, temafloxacin, Win 57273, ofloxacin, and ciprofloxacin. Antimicrob Agents Chemother. 1992;36:856–859. doi: 10.1128/aac.36.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spangler S K, Jacobs M R, Appelbaum P C. In vitro susceptibilities of 185 penicillin-susceptible and -resistant pneumococci to WY-49605 (SUN/SY 5555), a new oral penem, compared with those to penicillin G, amoxicillin, amoxicillin/clavulanate, cefixime, cefaclor, cefpodoxime, cefuroxime and cefdinir. Antimicrob Agents Chemother. 1994;38:2902–2904. doi: 10.1128/aac.38.12.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spangler S K, Jacobs M R, Pankuch G A, Appelbaum P C. Susceptibility of 170 penicillin-susceptible and -resistant pneumococci to six oral cephalosporins, four quinolones, desacetylcefotaxime, Ro 23-9424 and RP 67829. J Antimicrob Chemother. 1993;31:273–280. doi: 10.1093/jac/31.2.273. [DOI] [PubMed] [Google Scholar]
- 28.Tweardy D J, Jacobs M R, Speck W T. Susceptibility of penicillin-resistant pneumococci to eighteen antimicrobials; implications for treatment of meningitis. J Antimicrob Chemother. 1983;12:133–139. doi: 10.1093/jac/12.2.133. [DOI] [PubMed] [Google Scholar]