Abstract
Introduction
Early afterdepolarizations (EADs) are secondary voltage depolarizations associated with reduced repolarization reserve (RRR) that can trigger lethal arrhythmias. Relating EADs to triggered activity is difficult to study, so the ability to suppress or provoke EADs would be experimentally useful. Here, we use computational simulations to assess the feasibility of subthreshold optogenetic stimulation modulating the propensity for EADs (cell-scale) and EAD-associated ectopic beats (organ-scale).
Methods
We modified a ventricular ionic model by reducing rapid delayed rectifier potassium (0.25–0.1 × baseline) and increasing L-type calcium (1.0–3.5 × baseline) currents to create RRR conditions with varying severity. We ran simulations in models of single cardiomyocytes and left ventricles from post-myocardial infarction patient MRI scans. Optogenetic stimulation was simulated using either ChR2 (depolarizing) or GtACR1 (repolarizing) opsins.
Results
In cell-scale simulations without illumination, EADs were seen for 164 of 416 RRR conditions. Subthreshold stimulation of GtACR1 reduced EAD incidence by up to 84.8% (25/416 RRR conditions; 0.1 μW/mm2); in contrast, subthreshold ChR2 excitation increased EAD incidence by up to 136.6% (388/416 RRR conditions; 50 μW/mm2). At the organ scale, we assumed simultaneous, uniform illumination of the epicardial and endocardial surfaces. GtACR1-mediated suppression (10–50 μW/mm2) and ChR2-mediated unmasking (50–100 μW/mm2) of EAD-associated ectopic beats were feasible in three distinct ventricular models.
Conclusions
Our findings suggest that optogenetics could be used to silence or provoke both EADs and EAD-associated ectopic beats. Validation in animal models could lead to exciting new experimental regimes and potentially to novel anti-arrhythmia treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12195-023-00781-z.
Keywords: Cardiac optogenetics, Channelrhodopsin, GtACR1, ChR2, Cardiac electrophysiology, Reduced repolarization reserve, Early afterdepolarization, Myocardial infarction, Cardiomyocyte, Computational modeling
Introduction
Early afterdepolarizations (EADs) are spontaneous depolarizations during cardiac action potential (AP) repolarization that are strongly associated with dangerous arrhythmogenic events like torsade de pointes (TdP) and sudden cardiac death [60, 77]. It is understood that EADs occur in the presence of conditions that increase inward ionic currents and decrease outward ionic currents, known as reduced repolarization reserve (RRR) [60, 73, 77]. RRR can result from either congenital factors, such as ion channelopathy (e.g., long QT syndrome [LQTS]) [25, 49] or acquired arrhythmogenic drivers such as action potential duration (APD) -prolonging drugs [50, 73], cardiac ischemia [24], or heart failure [36]. However, the link between EADs and TdP is not fully understood. EADs occur stochastically in single cells and RRR alone does not explain EAD genesis [60]. Mechanistically linking these cell-scale events to organ-scale phenomena (i.e., propagating ectopic wavefronts leading to extra heartbeats) is difficult. Consequently, the ability to suppress or provoke EAD-mediated triggered activity in tissue and organ scale environments would be extremely useful to experimentalists. For instance, whole organ preparations like Langendorff-perfused mouse hearts could be a viable and consistent experimental platform for studying EAD-associated ectopic beat propensity. Currently available methods, such as direct current injection or pharmaceutical intervention, lack spatiotemporal specificity and disrupt underlying EAD behavior.
Optogenetic stimulation of cardiac tissue [10, 11] could provide a means of exploring EAD mechanisms. This method incorporates microbial light-sensitive ion channels and pumps called opsins into cardiomyocytes to allow light-mediated, spatiotemporally precise stimulation [27]. Numerous studies have explored novel applications using this technique, including control of spiral wave chirality [15], neuromodulation of the heart [20, 44, 83], arrhythmia termination [13, 14, 54, 55], and modulation of AP and repolarization properties [32, 38, 45]. Notably, nearly all of these studies used optogenetic stimulation to elicit sustained depolarizing currents. This is not conducive for studying EADs, since the constant light-mediated stimulation would disrupt the underlying electrical phenomena. More recently, scientists have begun examining the use of subthreshold (i.e., non-AP evoking) optogenetic stimulation to alter tissue electrophysiological properties without eliciting a propagating response. Examples include: control of spiral wave drift and termination [35], creating electric turbulence in previously stable environments [43], and modulating electrical alternans dynamics to destabilize reentrant arrhythmias [7]. We hypothesized that subthreshold optogenetic stimuli could be used to modulate EAD propensity at the cell scale and EAD-associated ectopic beat emergence at the organ scale.
In the present study, we use computational simulations to evaluate this hypothesis in RRR-afflicted models of ventricular cardiomyocytes and infarcted human ventricles. We incorporate biophysically realistic models of two opsins: an outward current anion channelrhodopsin (GtACR1) [29, 31] and an inward current cation channelrhodopsin-2 (ChR2) [9, 79]. Stimulation of GtACR1 or ChR2 is used to attenuate or exacerbate factors underlying EADs, respectively. First, we conduct simulations in a realistic ventricular cell model to down- or up-regulate EAD occurrence across a parameter space of RRR conditions. Then, we simulate RRR-afflicted patient-derived left ventricular (LV) models to examine down- and up-regulation of EAD-associated ectopic beat propensity. We provide proof-of-concept evidence that subthreshold optogenetic stimulation can be used to suppress or evoke EAD phenomena (i.e., EADs in simulated cardiomyocytes and ectopic beats in LV models) during RRR conditions across multiple biophysical scales.
Methods
EADs in Simulated Human Ventricular Cardiomyocytes
We used the ventricular action potential model developed by O’Hara et al. (ORd) [56] in all cell and organ-scale simulations. The ORd fast sodium current (INa) was replaced with the ten Tusscher et al. [70] formulation to facilitate compatibility with organ-scale modeling, as advised by the ORd developers [56, 64]. To generate EAD-favoring RRR conditions, we varied scaling factors of conductance for two ionic currents in a manner similar to previous publications [72, 85]. The rapid delayed rectifier K+ current (IKr) conductance (gKr) was varied from 0.25–0.10 × baseline gKr by steps of 0.01, and the L-type Ca2+ current (ICaL) conductance (gCaL) was varied from 1.0–3.5 × baseline gCaL by steps of 0.1.
Single cell simulations were conducted using 416 permutations of these two scaling factors. Each virtual cardiomyocyte was paced (pulse duration: 1 ms; stimulus amplitude: 60 pA/pF) at different frequencies: basic cycle lengths (BCL) of 800 ms (0.83 Hz), 1000 ms (1 Hz), or 1200 ms (1.25 Hz). Each simulation was conducted twice, with either 290 or 291 stimuli, then evaluated for a final screening window of 8 to 9 cycle lengths for EAD occurrence using a custom Matlab script (total simulation duration: 240, 300, or 360 s). EADs were classified by any positive deflection in the membrane voltage (Vm) time derivative (dVm/dt) during repolarization after each stimulus (evaluated from 100 ms post-stimulus to the end of the BCL interval). An RRR condition was classified as “EAD” if afterdepolarization(s) were detected in either simulation. It was necessary to conduct and evaluate two different screening windows (i.e., 290 or 291 stimuli) because some RRR conditions had frequent EAD fluctuations persisting for > 1000 ms that were terminated by the subsequent stimulus, which would lead to an erroneous “no EADs” classification.
For simulations involving electric pacing at BCL = 800 ms with ChR2 stimulation, the response to pacing had to be manually inspected in some cases to confirm the presence of EADs. This was because rapid pacing sometimes pushed the cell into an operating mode where EAD occurred for most of the simulation but were transiently suppressed at some point when the cell was entrained at a faster-than-normal beating rate. This led to “no EAD” misclassification in a few cases, which were manually corrected.
Electrophysiological Simulation of Human Post-myocardial Infarction Ventricles
Simulations were conducted in three finite element LV models (Fig. 1) reconstructed from late gadolinium enhanced magnetic imaging resonance (LGE-MRI) scans as part of a prior study [47, 48]. These models were freely available from a published data set of 24 ischemic cardiomyopathy patients with prior myocardial infarction (MI) [48]. In all three cases, the mesh resolution of the publicly available version was increased via a single iteration of tetrahedral subdivision (edge length in publicly available versions ~ 1 mm; for models used in this study: ~ 500 µm), which moved the models into the agreed-upon tolerable range for numerical convergence [52]. The temporal discretization was 25 μs, as in past studies [2, 57].
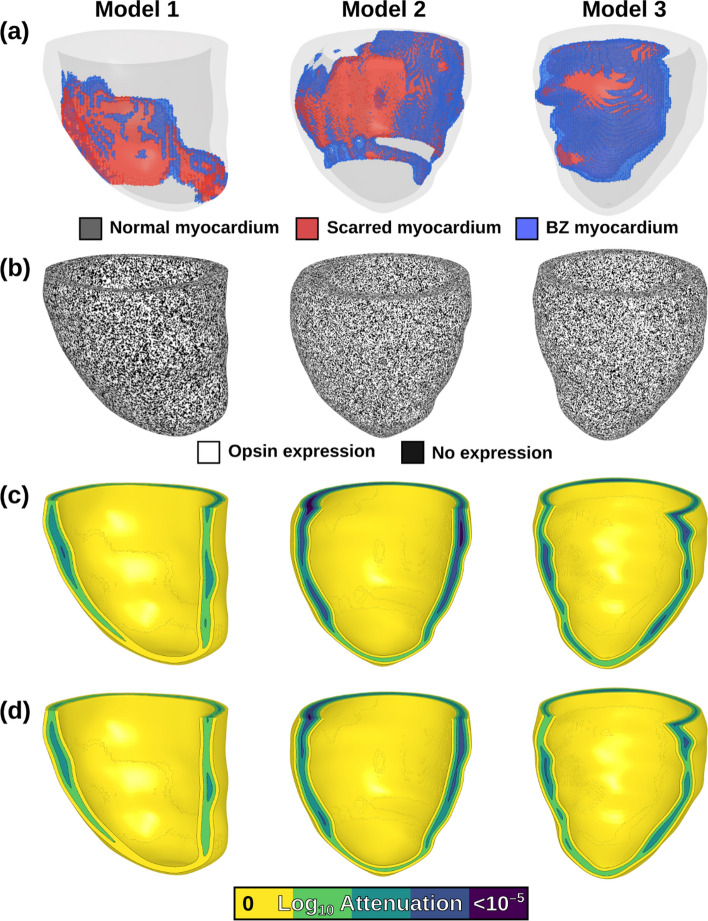
Fig. 1.
Patient-specific, post-MI LV models reconstructed from LGE-MRI scans. A Spatial distribution of tissue is shown; normal myocardium (gray), non-conductive scar (red), and electrically remodeled border zone (BZ; blue). b Opsin transfection patterns are shown in each model, where white denotes opsin expression and black denotes lack of expression. C, D Light attenuation for green (C) and blue (D) light stimuli applied uniformly and simultaneously to the epicardial and endocardial surfaces
When selecting three LV models from the available twenty-four, we analyzed the extent of myocardial scar and the relative location of infarcted tissue within the myocardial wall (e.g.: epicardial dominant, endocardial dominant, or transmural) for all possible candidates (Fig. S1). The first model (Model 1) was selected for its moderate scar burden (15.4% scar, 2.09% BZ) and balanced transmural scar distribution. Other two models selected had larger scar burden with predominantly sub-endocardial infarcts: Model 2 (19.1% scar, 2.84% BZ) and Model 3 (17.5% scar, 6.61% BZ). This was contrasted against the average for all 24 possible models (11.4% scar, 2.95% BZ).
These models had representations of normal myocardium, peri-infarct border zone (BZ), and infarct scar segmented from patient-specific LGE-MRI scans, as described by the authors of the original study [47, 48]. Realistic fiber orientations were represented using a rules-based approach, as originally described by Bayer et al. [5]. We modeled scar as non-conductive, as in prior work [2, 14, 47, 48, 57]. BZ tissue was modeled with identical ionic properties to normal myocardium [2] to eliminate possible confounding effects from RRR conditions. As in prior studies [37, 57], tissue scale electrical conductivity in the longitudinal direction (i.e., parallel to fibers; σL) in normal myocardium and BZ was identical (0.255 S/m). In the transverse direction (i.e., perpendicular to fibers; σT) this value was reduced in normal myocardium to reflect anisotropy of conduction (0.0775 S/m), and reduced even further in BZ tissue (7.75 mS/m) to represent effects of electrophysiological remodeling in the infarct periphery [2].
Electrical conduction at tissue scale was simulated using a finite element approximation of the monodomain equation [59, 75]; this approach for modeling cardiac electrophysiology has been extensively validated in prior work [2, 3, 14, 22, 42]. Ordinary differential equations associated with action potential simulations were solved using the Rush-Larsen scheme [63] for ion channel gating variables and forward Euler integration elsewhere. The governing partial differential equation for the monodomain formulation was solved with the full (non-lumped) mass matrix using a Crank-Nicholson scheme to improve model stability [75].
All simulations were conducted using freely available software (openCARP) [58]. All information necessary for reproducing these models and the simulations described in this study, including openCARP source files and driver scripts, can be found at the following permanent link: 10.6084/m9.figshare.23994354.
EAD-Associated Ectopic Beats in Organ Scale Models
Six RRR conditions were selected from the gKr-gCaL parameter space (BCL: 1000 ms) to examine their effects in organ-scale models. These were selected at regular intervals in a line perpendicular to the approximate threshold between non-EAD and EAD domains to span an appropriate range of RRR conditions. We ran simulations in LV models using methods described above, with the RRR condition applied to normal myocardium and BZ tissue. In each case, an initial electrical stimulus (t = 0 ms) was applied to the entire LV endocardial surface to approximate sinus activation via excitation of the His-Purkinje system. Subsequent spatiotemporal evolution of Vm was monitored for the occurrence of a sustained and propagating EAD-associated ectopic excitation (simulation duration: 1500 ms). We classified presence of an ectopic beat for a given RRR condition as any organ-wide depolarization during the repolarization period following the initial endocardial stimulus.
Optogenetic Modulation of Electrophysiology via Light Stimulation of GtACR1 or ChR2
The response to light stimulation of two opsins (GtACR1 and ChR2) was simulated using previously published photocurrent models. IGtACR1 was represented using a 2-state Markov chain model [57] created from measurements taken from neonatal rat ventricular myocytes [29]. Simulated voltage clamp experiments found agreement between the source experimental data and the IGtACR1 model output. IChR2 was represented using a 4-state model generated from ChR2(H134R)-expressing HEK293 cells [79]. In this study, simulated cell responses of myocytes to ChR2 stimulation agreed well with comparison data from in vitro guinea pig ventricular myocytes under action potential clamp conditions. As in our past study, peak GtACR1 channel conductance was modeled as 1.4 mS/cm2 [57], assuming a membrane capacitance of 100 pF (within physiological range) [33]. ChR2 channel conductance was modeled as 0.17 mS/cm2, to match the recorded peak ChR2(H134R) photocurrent from an earlier study [76]. Based on different studies examining opsin transfection for 1-12 months following viral injection [40, 76], opsin expression was represented in 58.2% of non-infarct (normal and BZ) tissue in a diffuse pattern (Fig. 1B) [14]. The spatial patterns of expression for the two opsins (i.e., GtACR1 and ChR2) were identical within each patient-derived model. In organ-scale simulations where optogenetic stimulation was applied, global illumination began at the simulation start (t = 0 ms) and was maintained for the entire simulation duration.
Modeling of Light Attenuation
Realistic light attenuation via scattering and absorption in myocardium was simulated using the exponential decay approximation, as in prior modeling work [12, 38, 57]. In brief, we defined parameter (value between 0 and 1; Eq. 1) using coefficients for light scattering (), light absorption (), and anisotropy factor (), which was then used to calculate the diffusion coefficient (Eq. 2) [62].
| 1 |
| 2 |
As in previous work [57], we used = 0.1, = 1.42, = 0.9 [69] for green light (515 nm) and = 0.52 and = 0.183 [8] for blue light (488 nm). These parameters resulted in exponential decay constants (δ = ) of 519.6 μm and 593.2 μm for green and blue light, respectively.
In most cases of organ-scale optogenetic stimulation, we represented concurrent illumination of the endocardial and epicardial LV surfaces. We modeled the process in this manner because we anticipated the effect of light attenuation would be too large for sub-threshold optogenetic stimuli to affect organ-scale EAD propensity if only one surface was illuminated. Light stimulation was represented by imposing a surface irradiance (), then modifying that value in the myocardial volume based on distance () from the nearest point on the illuminated surface (Eq. 3) [12, 38]:
| 3 |
Ee distributions in each LV model for the endo- and epicardial surfaces were calculated separately, then summed together (i.e., superimposed) to accurately represent the net illumination effect from dual surface illumination (Fig. 1C, D). In a subset of simulations which either endocardial- or epicardial-only illumination was applied, the respective Ee distribution was modeled.
Illumination Protocols for Optogenetic Modulation of EAD and Ectopic Beat Propensity
To assess the feasibility of modulating EAD propensity, we repeated cell-scale simulations for all RRR configurations and pacing protocols described above, but with the addition of either constant GtACR1 or ChR2 stimulation. In all cases, light onset was at simulation initialization (t = 0 ms) and persisted for the entire duration (300 s); each of these simulations was evaluated for the presence of EADs as described above. For EAD suppression, we tested constant GtACR1 illumination strengths of 1 nW/mm2 to 1 µW/mm2 (order of magnitude steps); for evoking EADs, we used different amplitudes of 1–500 µW/mm2 (half-order of magnitude steps), owing to the distinct energy requirements of ChR2.
For organ scale simulations, six RRR conditions were selected from the cell-scale simulations (see highlighted green and blue boxes in Fig. 3A). Without optogenetic stimulation, three of these conditions had EADs (green boxes), while the other three did not (blue boxes). This allowed a framework suitable for testing the efficacy of EAD suppression or provocation via subthreshold GtACR1 and ChR2 stimulation, respectively. RRR conditions were applied uniformly to all normal myocardium and BZ tissue in organ-scale simulations. Simulated illumination began at t = 0 ms and continued for the entire simulation duration (1500 ms).
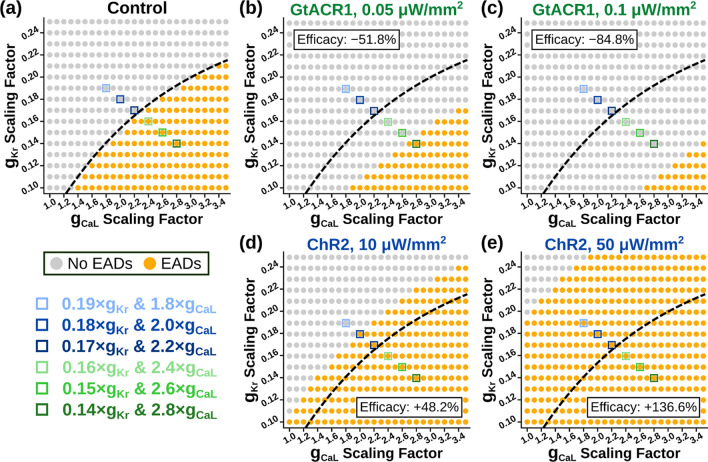
Fig. 3.
EAD occurrence at various RRR conditions in a simulated ventricular cardiomyocyte at BCL = 1000 ms. A Baseline EAD incidence in control condition (no optogenetic stimulation). B, C When GtACR1 stimulation is applied at 0.05 μW/mm2 (B) or 0.1 μW/mm2 (C), overall EAD incidence is reduced. D, E When ChR2 stimulation is applied at 10 μW/mm2 (D) or 50 μW/mm2 (E), overall EAD incidence is increased. Dashed line denotes the threshold between conditions without and with EADs in (A). Shaded blue and green boxes correspond to the six RRR conditions shown in Fig. 2 Vm(t) traces and tested in organ-scale simulations. Optogenetic modulation efficacy is calculated as the percent change in pro-EAD RRR conditions compared to the no light condition
Metrics to Predict Success or Failure of Ectopic Beat Modulation via Optogenetic Stimulation
To increase the generalizability and translational potential of our findings, we derived quantitative metrics exclusively from values that could be measured experimentally. Specifically, we only used ventricular anatomy (i.e., chamber dimensions; total wall thickness, including myocardium, BZ, and scar), and light stimulus characteristics (i.e., intensity, attenuation rate). The first metric was conceived to approximate the total irradiance absorbed by the entire ventricular myocardium in response to a particular light stimulus (Eq. 4):
| 4 |
where N is the number of nodes in the finite element grid; Ee,i is the irradiance delivered to the ith node in the grid based on Eq. 3; and Vol is the total myocardial tissue volume. The second metric was identical to the first, but with base 10 logarithmic scaling of the node-wise irradiance values (Eq. 5):
| 5 |
For each organ-scale simulation conducted in the study, we classified the outcome as success (i.e., if the stimulus suppressed or provoked an ectopic beat for GtACR1- and ChR2-expressing experiments, respectively) or failure. We then calculated TVI and TVLI for the corresponding optogenetic stimuli and applied receiver operating characteristic (ROC) analysis to assess the predictive capacity of both metrics.
Results
Early After depolarizations in Simulated Ventricular Myocytes Under Reduced Repolarization Reserve
To establish RRR conditions for EAD testing in simulated single cardiomyocytes, we adapted a previously published approach [71, 85]. Maximal channel conductance values for IKr and ICaL ionic currents were down- and up-regulated, respectively. Specifically, scaling factors tested were 0.25–0.10 × baseline gKr (steps of 0.01), and 1.0–3.5 × baseline gCaL (steps of 0.1). We also determined the minimum single cell irradiance values necessary to elicit action potentials; the purpose here was to define the range of optogenetic stimuli defined as subthreshold (i.e., below that minimum irradiance). These were found to be ≤ 0.1 µW/mm2 for GtACR1 and ≤ 50 µW/mm2 for ChR2.
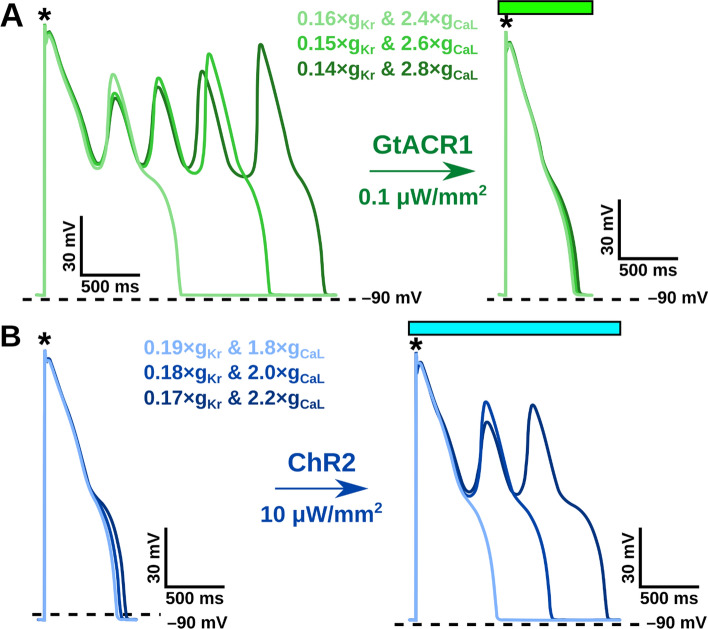
Representative voltage traces are highlighted in Fig. 2 to demonstrate EAD suppression using GtACR1 (Fig. 2A) and EAD provocation using ChR2 (Fig. 2B) across different RRR conditions. Across the RRR range, there were noteworthy differences in the morphologies of EAD-afflicted action potentials (e.g, number of “humps” following initial repolarization), despite the deterministic nature of the representation of ectopy. For the parameter space at BCL = 1000 ms, we found that EADs developed in 164 of 416 RRR conditions (Fig. 3A). When constant optogenetic stimulation was applied to the simulated cardiomyocytes, the proportion of EAD-occurring RRR conditions reduced under subthreshold GtACR1 stimulation (Fig. 3B, C) and increased under subthreshold ChR2 stimulation (Fig. 3D, E). Specifically, GtACR1 stimulation at very low irradiance (0.05 μW/mm2) reduced EAD incidence by 51.8% (79/416 conditions; Fig. 3B), while a stronger stimulus (0.1 μW/mm2) led to more dramatic ectopy suppression (84.8% reduction; 25/416 conditions; Fig. 3C). Conversely, low irradiance ChR2 stimulation (10 μW/mm2) increased EAD incidence by 48.2% (243/416 conditions; Fig. 3D), and this trend was amplified by the use of a stronger stimulus (136.6% increase at 50 μW/mm2; 388/416 conditions; Fig. 3E).
Fig. 2.
EAD occurrence can be down- or up-regulated in a simulated cardiomyocyte using subthreshold optogenetic stimulation. A Representative traces of membrane voltage over time [Vm(t)] for conditions with EADs that are suppressed by constant GtACR1 stimulation (0.1 μW/mm2; BCL: 1000 ms). B Representative Vm(t) for traces without EADs are evoked by constant ChR2 stimulation (10 μW/mm2; BCL: 1000 ms). Black asterisks indicate paced electrical stimuli and green/blue boxes indicate light stimulation at respective wavelengths. Colored bars correspond to illumination duration. The different shades of blue and green trace colors correspond to the six RRR conditions highlighted in Fig. 3A and tested in organ-scale simulations
We also evaluated the impact of optogenetic EAD modulation in cells electrically paced at faster (BCL: 800 ms) or slower (BCL: 1200 ms) frequencies compared to the baseline pacing rate discussed above. In the absence of optogenetic stimulation, accelerated pacing decreased EAD prevalence (Fig. S2A; 127/416 vs. 164/416 at baseline), while slowed pacing increased the total number of RRR conditions that produced EADs (Fig. S3A; 208/416). These general findings remained true in the context of optogenetic stimulation via GtACR1 or ChR2 was applied (see Figs. S2B-E and S3B-E). Interestingly, the efficacy of optogenetic modulation (i.e., percent change in EAD prevalence compared to non-illuminated cells) improved as a function of pacing frequency. For instance, at baseline pacing rate (1 Hz) with weak GtACR1 stimulation (0.05 µW/mm2), the efficacy of EAD suppression was − 51.8% (79 EAD cases vs. 164 in the absence of illumination; Fig. 3A, B). For the identical configuration in the contexts of hastened or slowed pacing, the respective efficacy values were − 59.1% (see Fig. S2A-B) and − 42.8% (see Fig. S3A-B). The same trend occurred for ChR2: under 50 µW/mm2 stimulation, BCL = 800 ms had EAD prevalence increases from 127 to 202 (efficacy: + 59.1%; Fig. S2A, D), BCL = 1000 ms increased from 164 to 243 (efficacy: + 48.2%; Fig. 3A, D), and BCL = 1200 ms increased from 208 to 289 (efficacy: + 38.9%; Fig. S3A,D).
Ectopic Beats in Organ-Scale LV Models Under Reduced Repolarization Reserve
We conducted organ-scale simulations in three patient-derived LV models (Fig. 1). Due to differences in electrical source-sink dynamics compared to the cell-scale milieu, first we established the organ-scale subthreshold optogenetic stimulation irradiances for both opsins. The GtACR1- and ChR2-expressing LV models were illuminated simultaneously from the epicardial and endocardial surfaces, then examined at steady state (chosen as t = 2000 ms). Using this method, we found that the subthreshold optogenetic stimulation irradiances were 1–10 μW/mm2 for GtACR1 and 10–100 μW/mm2 for ChR2 (half order-of-magnitude steps for both; Fig. S4).
From the cell-scale parameter analysis, a subset of six RRR conditions with and without EADs at baseline were selected (see colored boxes superimposed on grid in Fig. 3 for specific combinations of gKr and gCaL multipliers). Due to differences in source-sink mismatch at the cellular versus organ scales, EAD presence at a specific RRR condition in simulated cardiomyocytes does not guarantee that an ectopic beat will occur in LV model simulations with the same RRR settings. Nevertheless, all six RRR conditions we selected did result in consistent organ-scale behavior in all three LV models (i.e., EAD-prone RRR conditions did lead to ectopic beats at baseline while EAD-lacking conditions did not show ectopy at baseline).
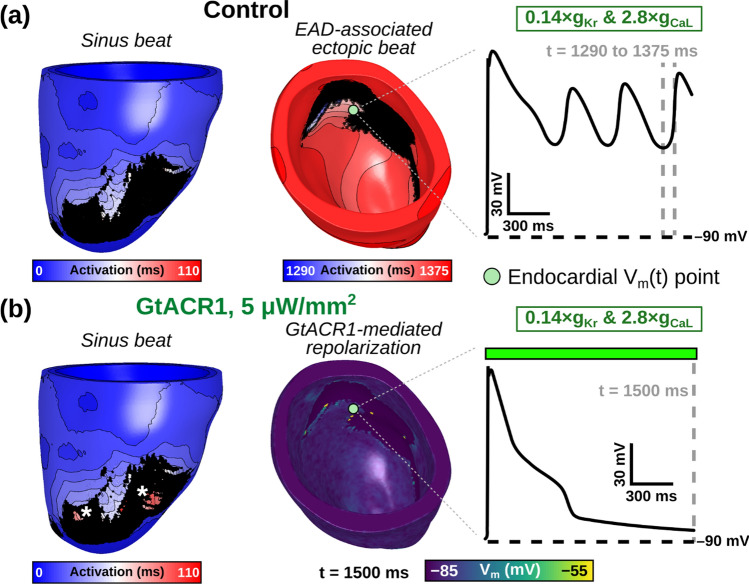
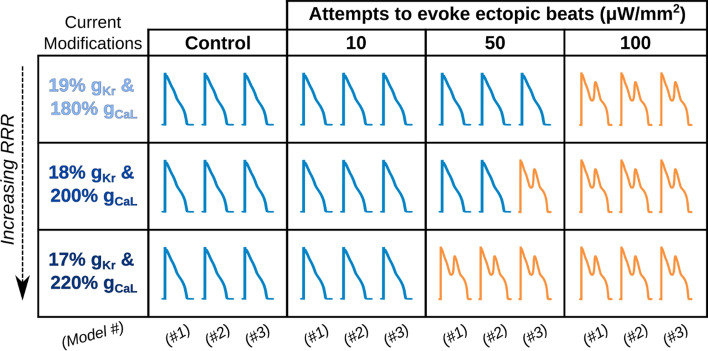
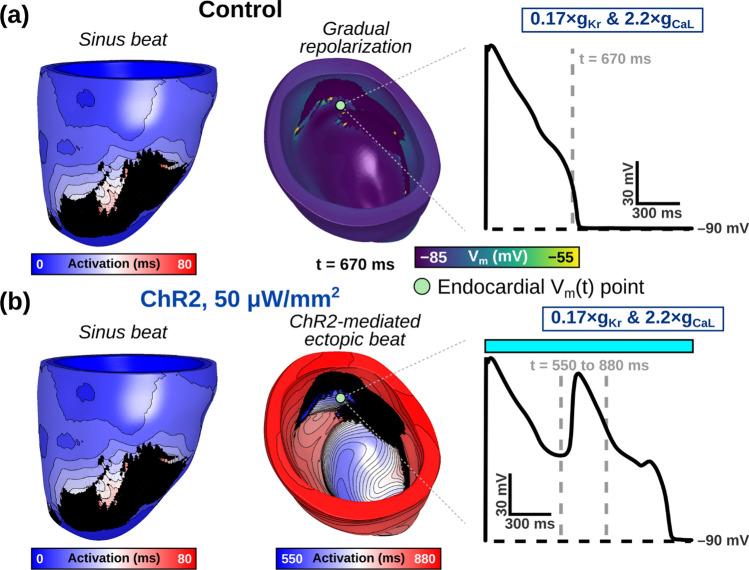
When subthreshold epi- and endocardial green light illumination of GtACR1-expressing LV models was simulated, we found that 5 and 10 μW/mm2 irradiances suppressed ectopic beats in 17 of 18 cases (2 irradiances × 3 RRR conditions × 3 models; Fig. 4). A representative example of an optogenetically-suppressed ectopic beat is shown in Fig. 5 and Supplemental Video 1. When subthreshold epi- and endocardial blue light illumination of ChR2-expressing LV models was simulated, we found that 50 and 100 μW/mm2 irradiances provoked ectopic beats in 13 of 18 cases (2 irradiances × 3 RRR conditions × 3 models; Fig. 6). A representative example of an optogenetically-provoked ectopic beat is shown in Fig. 7 and Supplemental Video 2. In simulations presenting ectopic beats, we consistently observed EADs and related re-excitations occurring in tissue proximal to scar tissue (see Vm(t) insets in Figs. 5A and 7B).
Fig. 4.
Subthreshold GtACR1 stimulation can suppress ectopic beats under RRR conditions. Orange Vm(t) trace represents an ectopic beat occurrence and blue Vm(t) trace represents no ectopic beat occurrence for a given RRR condition and irradiance. Within a specific column and row, the three traces correspond to patient Models 1, 2, and 3 in order
Fig. 5.
Representative examples of LV model simulations without (control) and with subthreshold GtACR1 stimulation. A Activation sequence for simulated sinus activation beginning at t = 0 (left) is followed by multiple ectopic re-excitations originating from an endocardial focus (right), which are the consequence of local EADs (inset panel). B When 5 μW/mm2 illumination is applied to the GtACR1-expressing model from (A), all triggered activity is suppressed. During sinus activation (left) some previously unexcited epicardial regions surrounded by scar (*) are activated via direct optogenetic stimulation. Illumination (colored box) is applied uniformly and simultaneously to the epicardial and endocardial surfaces of Model 1 at t = 0 ms. Vm(t) trace location is consistent between (A) and (B)
Fig. 6.
Subthreshold ChR2 stimulation can evoke ectopic beats under RRR conditions. Orange Vm(t) trace represents an ectopic beat occurrence and blue Vm(t) trace represents no ectopic beat occurrence for a given RRR condition and irradiance. Within a specific column and row, the three traces correspond to patient Models 1, 2, and 3 in order
Fig. 7.
Representative examples of organ-scale simulations without (control) and with ChR2-mediated subthreshold stimulation. A At baseline, sinus activation beginning at t = 0 (left) leads to gradual repolarization (right) without EADs developing (inset panel). B When 50 μW/mm2 illumination is added to the ChR2-expressing model from (A), an ectopic beat emerges (right) after a brief delay (inset panel). Illumination (colored box) is applied uniformly and simultaneously to the epicardial and endocardial surfaces of Model 1 at t = 0 ms. Vm(t) trace location is consistent between (A) and (B)
To contrast dual surface illumination against single surface endocardial or epicardial illumination, simulations were conducted using the maximal subthreshold irradiance for both opsins (10 μW/mm2 for GtACR1 and 100 μW/mm2 for ChR2). We found that both single surface illumination strategies were completely ineffective in preventing ectopic beats (GtACR1 expression; 0 of 9 cases), regardless of the irradiance or RRR condition tested (Fig. S5 and representative examples shown in Supplemental Video 3). However, for ChR2-expressing left ventricles, epicardial-only illumination had identical success to dual surface illumination (i.e., 9 of 9 cases promoted ectopic beats); endocardial-only illumination had mixed results (6 of 9 cases promoted ectopic beats; Fig. S6 and examples in Supplemental Video 4).
It was also initially unclear whether and to what extent the presence of scar and/or BZ substrate prompted the initiation of ectopic beats in RRR-afflicted LV models. Thus, we conducted a subset of simulations in models that were scar-only (i.e., BZ replaced by normal myocardium) or homogenized (i.e., both scar and BZ replaced by myocardium). Two extremes of the RRR range were selected to reflect the weakest and strongest RRR conditions used in organ-scale simulations: 19% gKr and 180% gCaL (with 100 μW/mm2 ChR2 stimulation) for low RRR; 14% gKr and 280% gCaL (with 1 μW/mm2 GtACR1 stimulation) for high RRR. EAD-induced ectopic beats provoked by ChR2 stimulation under weak RRR conditions were sensitive to the inclusion of MI-related remodeling (see top row of Fig. S7). Specifically, removal of BZ effects eliminated the ChR2-provoked ectopic in one of three ventricular models; when scar was also converted to excitable and conductive myocardium, ectopic beats in this configuration were eliminated altogether. In contrast, under high RRR conditions (bottom row of Fig. S7), the inability of GtACR1 to prevent EAD-induced ectopic beats was preserved across all tested configurations (left: both scar and BZ; middle: scar only; right: neither scar nor BZ). This suggests that susceptibility to EAD-induced ectopic beats in organ-scale models may be moderately affected by the presence, extent, and spatial pattern of MI-related remodeling; however, in the context of severe RRR, these effects appear to be inconsequential.
Outcomes of Optogenetic Modulation of Ectopic Beat can be Predicted
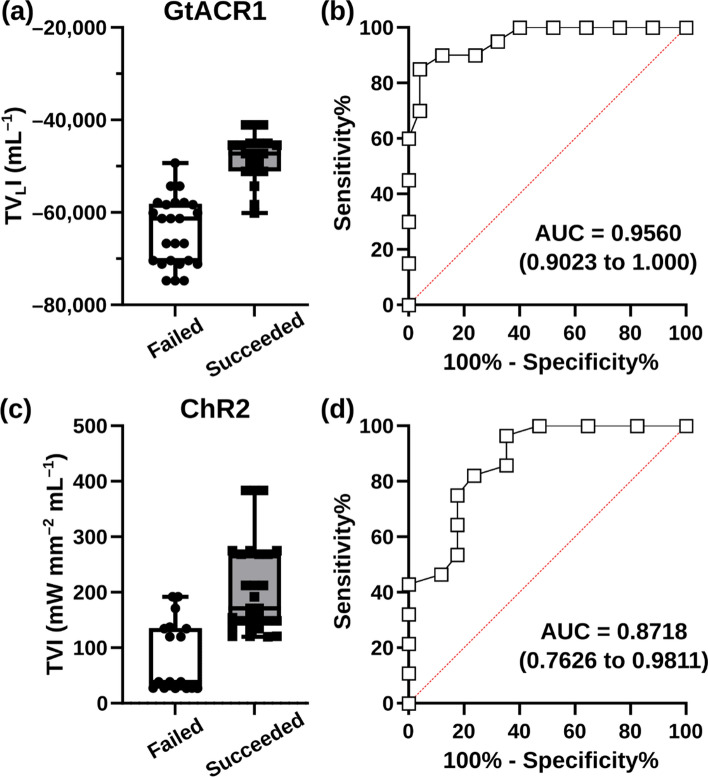
As described in “Methods” section, for each simulated configuration in our study we calculated two metrics (TVI and TVLI) based solely on ventricular anatomy and light stimulus characteristics. We found that TVLI was an excellent predictor of ectopic beat suppression via GtACR1-based stimulation (ROC area under the curve [AUC] = 0.956; Fig. 8A, B), but the TVI metric had inferior performance (AUC = 0.722; Figs. S8A-B). In contrast, for attempts to provoke ectopic beats via blue light stimulation of ChR2, the TVI metric was superior (AUC = 0.872 vs. 0.538; Figs. 8C, D and S8C-D).
Fig. 8.
Total volumetric irradiance metrics can predict optogenetic modulation success rates. A Raw TVLI values for all simulations in which GtACR1-based suppression of ectopic beating failed or succeeded. B ROC analysis of the latter. C, D Same as (A, B) but for TVI metric applied in the context of ChR2-based ectopic beat provocation
Discussion
In this study, we used subthreshold optogenetic stimulation to modulate EADs and EAD-associated ectopic beats in computational models of ventricular myocytes and patient-derived LV models. The main findings are as follows: (1) both EADs and ectopic beats could be suppressed (with GtACR1) or evoked (with ChR2) using very low irradiances (0.01–100 μW/mm2) under RRR conditions; (2) dual surface epi- and endocardial illumination was required for ectopic beat suppression (GtACR1) but both dual surface and epicardial-only illumination strategies were sufficient to provoke an ectopic beat (ChR2); and (3) total volumetric irradiance calculations demonstrated combining ventricular anatomy and illumination strategy can broadly distinguish between effective and ineffective outcomes. Our study has demonstrated that optogenetic stimulation at very low irradiances can be used to modulate EAD occurrence and ectopic beat propensity under specific RRR conditions.
Early studies in cardiac optogenetics [26, 78] considered the possibility of applying low intensity optogenetic stimuli, too weak to evoke or overtly suppress action potentials, but strong enough to elicit subtle electrophysiological changes in cardiac environments. Our study defines this effect as “subthreshold” optogenetic stimulation and adds to this growing recent trend within cardiac optogenetics. An early group to capitalize upon this phenomenon was Hussaini et al., who demonstrated control of spiral wave dynamics in cardiac tissue [35]. The authors found that a low-amplitude ChR2 current caused the spiral core to drift towards illuminated regions in silico. This technique was exploited to manipulate the direction and speed of the spiral wave core trajectory using selectively patterned and timed illumination. A different spiral wave study by Majumder et al. used uniform ChR2 stimulation to initiate wave break in simulated cardiac tissue using pulsed light [43]. By reducing the excitable gap of repolarizing tissue between waves, this subthreshold perturbation affected membrane voltage enough to cause electrical turbulence in a previously stable state. Finally, the group of Leonardo Sacconi has recently systematically varied and characterized subthreshold ChR2 stimulation in Langendorff-perfused mouse hearts [7, 45]. The authors found that subthreshold illumination increased the incidence of cardiac alternans, generally thought of as pro-arrhythmic but here the net effect was deemed cardioprotective, since it prompted an apparent increase in the rate of VT self-termination [7]. Our work differs from prior studies in that it uses subthreshold optogenetic modulation to affect EAD and ectopic beat propensities under various RRR conditions. Use of GtACR1 or other inhibitory opsins to suppress or reduce electrical activity may prove consequential in other forthcoming studies.
Premature ventricular complexes (PVCs), which are often synonymous with ectopic (extra) beats, have remained an area of active study [1, 34, 84]. It has been previously established that increased PVC burden is associated with increased risk of heart failure, reduced left ventricular ejection, and mortality [46]. Despite advances in care, it has remained difficult to proactively differentiate between PVC-presenting individuals who experience adverse consequences from those without negative outcomes. Furthermore, not all PVCs are necessarily triggered by EAD events. We argue that, at a minimum, the methodology that we simulate in this study could be used to characterize mechanisms of the EAD-associated PVCs. The ability to either forcibly lower membrane voltage or hasten spontaneous depolarizations could tip the balance away from or towards PVC occurrence, regardless of the underlying initiation mechanism. This would be a potentially helpful experimental tool to enable probing and dissection of mechanisms underlying PVCs, given the fact that ectopic excitations are stochastic in nature and their occurrence can be inconsistent.
It is generally accepted that EADs and PVCs are interrelated in structurally abnormal tissue (e.g., patients with LQTS, previous MI, or APD-prolonging pharmaceuticals). It is believed that dispersion of repolarization in these conditions can be arrhythmogenic, due to differences in repolarization time leading to conduction block and reentry. Transmural dispersion of repolarization exists in healthy adult hearts [28] and this effect can be exacerbated APD-prolonging genetic mutations as in LQTS [34, 67, 73]. In our study, we empirically observed longer repolarization times occurring at tissue in close proximity to scar regions. Since we used homogeneous light distribution to the entire endocardium and/or epicardium, spatially targeted illumination strategies were not attempted to correct these repolarization differences. Experimentalists and modelers alike have previously used spatiotemporally controlled light stimulation to achieve aims such as correcting APD in short QT syndrome [38], defibrillate VT using a triple barrier pattern [21], and terminate atrial fibrillation using various optrode densities [10, 11]. If future research emerges suggesting that EAD-associated ectopic beats could be mitigated by reducing dispersion of repolarization, then use of spatially delineated optical stimuli could be further explored.
Previous studies have attempted to link EAD mechanisms at cellular scales to triggered activity at tissue and organ scales [16, 17, 24, 71, 72, 80, 85]. Our findings in simulated ventricular cardiomyocytes suggest that cell-scale optogenetic modulation of these arrhythmic phenomena should be quite feasible via subthreshold stimulation of GtACR1 or ChR2. An important caveat is that both opsins have repolarizing and depolarizing effects that depend on action potential phase (during which Vm traverses the range between ≈− 85 mV and ≈ 30 mV). Phase dependency of optogenetic current arises from the intrinsic property of opsin reversal potential (GtACR1: − 40 mV; ChR2: 0 mV). This point was emphasized in two recent studies [32, 39], which showed that in some situations GtACR1 can be used to pace zebrafish hearts and ChR2 stimulation can silence electrical activity. Despite these interesting findings, the cellular configurations evaluated in our study still found that the rate of EAD occurrence was robustly decreased by GtACR1 stimulation or increased by ChR2 stimulation, as compared to non-illuminated virtual myocytes. Since EADs can be challenging to study experimentally, an interesting potential application of our findings would be for use in patch clamp experiments [65, 66]. Given the findings from our study, we anticipate that the propensity of EADs in such preparations could be modulated using subthreshold optogenetic stimuli.
Our methodology for modeling EADs is deterministic (i.e., EADs occur consistently on every beat), which is distinct from the stochastic process that underlie the phenomena in nature [60, 65, 66, 80]. This reductionist approach was a necessary simplification for our study, since it allowed us to establish whether optogenetic stimulation could dynamically modulate the occurrence of cell- or organ-scale arrhythmia triggers under tightly controlled conditions. The precise boundary separating EAD occurrence and absence is very likely affected by this methodology. Indeed, we observed cycle-to-cycle variability in EAD occurrence, which required our methodology to examine two different screening windows. When the identical set of model parameters was incorporated in cell- and LV-scale models, we previously hypothesized that a corresponding outcome was not predetermined (i.e., RRR conditions that produced EADs did not necessarily guarantee PVCs at the organ level), due to the large differences in source-sink relationship between these two contexts. Of note, we found that all six of the RRR conditions highlighted in Fig. 3A did have corresponding effect in cell- and organ-scale models (i.e., the presence of lack of EADs or ectopy, respectively). Future work may benefit from incorporating a more realistic methodology reflecting beat-to-beat variability in EAD morphology as well as incorporating more accurate stochasticity [66]. This would be important to validate prior to considering this approach for clinical use.
In the organ-scale simulations conducted, all three LV models had combinations of RRR severity and irradiance that resulted in provoked or suppressed ectopic beats compared to the baseline state (Figs. 4, 6). We found that conditions with more RRR generally required higher irradiances for consistent ectopy suppression and lower irradiances for evoking ectopic beats. This is consistent with the idea that optogenetic ectopy modulation has higher energy demands than evoking ectopic beats under the evaluated RRR conditions. An interesting example was failed ectopic beat suppression simulation in Model 3 (14% IKr & 280% ICaL, 5 μW/mm2 GtACR1 stimulation; Fig. 4). In this simulation, micro re-entry occurred via epicardial conduction through a narrow isthmus of BZ and healthy tissue, nestled in a dense mass of scar tissue, leading to re-excitation and ectopic beat propagation on the endocardial surface in BZ tissue held at elevated Vm (≈− 50 mV) by continuous GtACR1 stimulation. This example highlights that optogenetic stimulation at less-than-maximal subthreshold irradiances may occasionally fail due to patient-specific anatomical substrate but does not contradict the evidence that near maximal subthreshold stimuli (10 μW/mm2 for GtACR1, 100 μW/mm2 for ChR2; Figs. 4, 6) consistently suppressed and evoked ectopic beats in this study.
A noteworthy aspect of our modeling is that dual endo- and epicardial illumination was initially simulated in all six RRR conditions. When we compared these results to single surface illumination strategies, we found that ectopy suppression via GtACR1 stimulation was no longer possible (0 of 18 successes for both single surface strategies using 10 μW/mm2; Fig. S5). This was consistent with our prior expectations, since single surface illumination would not prevent the formation of a larger repolarization gradient between the lit and unlit surfaces, leading to re-excitation and ultimately an ectopic beat. However, dual surface illumination would be logistically difficult and somewhat far-fetched, even if recent advances in light delivery offer promising possibilities [4]. Interestingly, for evoking ectopic beats via ChR2 stimulation we found that epicardial only illumination was as effective as dual surface illumination (9 of 9 in both cases; Fig. S6). This finding is exciting because it suggests a new experimental technique wherein PVC “hotspots” (i.e., areas where ectopic beats could occur but do not necessarily occur frequently) could be revealed via constant ChR2 stimulation in Langendorff-perfused mouse hearts, potentially in the context of genetic or pharmacological intervention(s) to prolong APD (i.e., RRR). Light attenuation concerns have been previously raised about optogenetic stimulation in human hearts [14, 37], due to their thicker walls compared to those of small animals [13, 14, 54]. Our success here with epicardial-only illumination in simulated human hearts opens the door for an exciting new approach that could be applied in large heart, providing a more spatiotemporally controllable complement to existing methods for eliciting arrhythmia triggers to study arrhythmia mechanisms (e.g., subepicardial injection of norepinephrine) [51].
An ongoing difficulty with research examining EADs and ectopic beats is establishing firm mechanistic links between cell, tissue, and organ-scale phenomena. In our study, we observed that ectopic beat triggers tended to localize near regions of scar tissue. We believe that this was due to spatial heterogeneity in source-sink mismatch [68], as proximity to non-conductive scar reduces the electrotonic sink and consequently the necessary threshold for the initiation of a propagating response. Previous research has highlighted that the minimum number of synchronized cells presenting EADs decreases as source-sink mismatch increases [80]. EADs have also been observed in isolated BZ cardiomyocytes, albeit during isoproterenol infusion [23]. However, it is also worth noting that our BZ tissue modeling differs from the methodology used in previous studies [2, 57]. Whereas these studies incorporated reductions to the IKr, ICaL, slow delayed outward potassium rectifier current IKs, and inward sodium current INa, our study did not implement any ionic current changes in BZ regions. Here, our intent was to avoid interference with the already model-wide changes to IKr and ICaL as a part of RRR conditions. Consequently, the only difference between healthy myocardium and BZ tissue was 90% reduction of conductivity in BZ as compared to myocardium, consistent with experimental conduction velocity measurements [82]. These tradeoffs were necessary to keep consistency between the RRR conditions applied to the patient LV models, as otherwise differences observed in the BZ tissue would be difficult to attribute to prolonged APDs alone. Our findings from simulations with variations in ventricular substrate (i.e., scar only; neither scar nor BZ) suggest RRR severity may have greater impact on vulnerability to ectopy than the extent, burden, and pattern of MI-related remodeling. Nevertheless, as evidenced by our findings in the context of low RRR (top row of Fig. S7), these factors clearly still play some role in creating a milieu that allows cell-scale EADs to overcome source-sink mismatch and initiate organ-scale ectopic beats.
A key finding of our study is that the outcome of organ-scale optogenetic trigger modulation can be predicted using metrics based exclusively on cardiac anatomy, optical stimulus parameters, and myocardial light attenuation properties. These metrics did not include information about opsin distribution or expression level, nor did they consider the extent or spatial distribution of infarct or border zone. Interestingly, while there was a relatively straightforward association between TVI and optogenetic PVC provocation success, the predictive metric for GtACR1-based PVC suppression was the version involving summation of log10 scaled Ee values (TVLI). This recalls prior work assessing the feasibility of defibrillation via low-energy pulsed electrical stimuli, which showed a power law relationship between field strength and elicited activations [41].
Future work in this area will benefit from a rich environment of new optogenetic constructs and light delivery techniques. Recent advances in optogenetics established a new class of naturally occurring, K+-conducting channelrhodopsins, including kalium rhodopsins (HcKCR1 and HcKCR2) [30, 74] and Wobblia inhibitory channelrhodopsin (WiChR) [74]. These discoveries may prompt a departure from the use of chloride (Cl–)-conducting anion channelrhodopsins (e.g., GtACR1), in part because of ongoing concerns about sufficient intracellular Cl– recovery in cardiomyocytes following continuous illumination. K+-conducting channelrhodopsins have high ratios of K+ to Na+ conduction, but further characterization in mammalian cardiomyocytes will be needed to progress the field. Although WiChR was expressed in atrial-like human induced pluripotent stem cell-derived cardiomyocytes [74], it is still unclear if non-negligible Na+ conduction could trigger action potentials upon illumination and how continuous stimulation might disrupt K+ homeostasis.
Advances in light delivery technology have continued to help reduce barriers to the feasibility of attempting cardiac optogenetics solutions in larger mammals. For instance, a recent proof-of-concept study successfully implanted a, battery-free optoelectrode in a conscious rat and could successfully optogenetically stimulate the entire heart (Ausra, Madrid et al. 2022). Previously, in vivo optogenetic stimulation in small animals had occurred while the animal was unconscious with battery leads exiting the chest cavity [53–55]. Another exciting advance is the emergence of wireless implantable cardiac devices that are bioresorbable without leads or batteries [18, 19]. These studies showed that energy harvesting may eliminate the need for a battery, paving the way for devices that can safely dissolve within several weeks post-implantation. It may be possible to modify this class of devices to provide illumination using micro-LEDs instead [81]. Another possible alternative to implantable devices for illumination are up-converting nanoparticles, which release photons in response to absorption of ultrasound or X-rays [6]. This has been successfully achieved by pacing rat hearts using near-infrared illumination and up-converting nanoparticle films [61]. Further characterization is needed to validate this method across a larger variety of experimental circumstances.
Conclusions
We used computational modeling to present a proof-of-concept approach for using subthreshold optogenetic stimulation to suppress (GtACR1) or provoke (ChR2) EADs across a range of RRR conditions. Ectopic beats could be consistently suppressed or provoked using subthreshold light stimuli in all three post-MI, patient-derived LV models tested. Our findings using epicardial-only illumination in ChR2-sensitized left ventricles suggest that Langendorff-perfused hearts could be used to study ectopic beat development under RRR conditions. Our characterized range of RRR conditions and irradiances in simulated single myocytes provides a novel technique for future potential experimental work.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AP
Action potential
- APD
Action potential duration
- AUC
Area under the curve
- BCL
Basic cycle length
- BZ
Peri-infarct border zone
- Ca2+
Calcium ion
- Cl–
Chloride ion
- ChR2
Channelrhodopsin-2 (H134R variant)
- EAD
Early afterdepolarization
- gCaL
Scaling conductance factor of ICaL
- gKr
Scaling conductance factor of IKr
- GtACR1
Guillardia theta Anion channelrhodopsin 1
- HcKCR
Hyphochytrium catenoides Kalium channelrhodopsin
- ICaL
L-type calcium ionic current
- IChR2
Current elicited by light activation of channelrhodopsin-2
- IGtACR1
Current elicited by light activation of Guillardia theta anion channelrhodopsin 1
- IKr
Rapid delayed rectifier potassium ionic current
- K+
Potassium ion
- LGE-MRI
Late gadolinium enhanced magnetic resonance imaging
- LQTS
Long QT syndrome
- LV
Left ventricle
- MI
Myocardial infarction
- PVC
Premature ventricular complex
- ROC
Receiver operating characteristic
- RRR
Reduced repolarization reserve
- TdP
Torsade de pointes
- TVI
Total volumetric irradiance
- TVLI
Total volumetric log10 transformed irradiance
- Vm
Membrane voltage
- Vm(t)
Membrane voltage over time
- WiChR
Wobblia inhibitory channelrhodopsin
Patrick M. Boyle
leads the Cardiac Systems Simulation (CardSS) Lab in the Department of Bioengineering at the University of Washington (UW) in Seattle. The CardSS Lab uses computational models of the human heart to cultivate new knowledge about arrhythmia mechanisms and devise novel treatments for these conditions. They develop approaches to identify patients with future risk of major complications (cardiac arrest, stroke, arrhythmia) via simulations in models reconstructed from medical imaging data (e.g., MRI or CT scans). The same modeling techniques can be used to lower translational barriers in regenerative medicine (e.g., deciphering mechanisms of arrhythmic side effects of cell therapy) or test sophisticated new biomedical devices like light-based defibrillators. The CardSS Lab also uses sophisticated explainable machine learning approaches to better predict cardiovascular risk for a wide range of individuals, including pediatric cancer survivors. The lab’s research is currently supported by the US National Institutes of Health (R01-HL158667, R21-CA277746) and the Catherine Holmes Wilkins Charitable Foundation. He was born and bred in the expansive Canadian prairies, east of the Rockies and west of the rest. He proudly attended the University of Calgary in his hometown, earning his BSc (Computer Engineering, '05) and PhD (Biomedical Engineering, '11). Before starting the CardSS Lab at UW, he spent seven years at the Johns Hopkins University in the beautiful city of Baltimore, starting as a postdoctoral fellow in Dr. Natalia Trayanova’s Computational Cardiology lab and eventually attaining the rank of Assistant Research Professor in the Department of Biomedical Engineering and the Institute for Computational Medicine. He has published over 75 papers in a wide range of peer reviewed journals, including Nature Biomedical Engineering, the Journal of Physiology, eLife, and Circulation. He is also co-inventor of three patents. Outside the lab, Dr. Boyle (Pat) lives in the Greenwood neighborhood of Seattle with his wife and partner, Soraya Bailey, and their two boys (Clare & Rhett). He is a passionate collector of vinyl LPs, he has completed the New York Times Crossword for 1000 consecutive days (and counting), he is a fierce competitor when it comes to board games and card games, and he is a long-suffering fan of the woebegone Calgary Flames.
Funding
A.R.O. was supported by a graduate student fellowship from the Institute of Stem Cell and Regenerative Medicine in Seattle, WA, USA. P.M.B. was supported by a grant from the University of Washington Royalty Research Fund and by NIH R01-HL158667.
Declarations
Conflict of interest
A.R.O. and P.M.B. declare they have no conflicts of interest.
Ethical Approval
No human studies or animal studies were carried out by the articles for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amoni M, Claus P, Dries E, Nagaraju C, De Buck S, Vandenberk B, Ingelaere S, Vermoortele D, Roderick HL, Sipido KR, Willems R. Discrete sites of frequent premature ventricular complexes cluster within the infarct border zone and coincide with high frequency of delayed afterdepolarizations under adrenergic stimulation. Heart Rhythm. 2021;18(11):1976–1987. doi: 10.1016/j.hrthm.2021.07.067. [DOI] [PubMed] [Google Scholar]
- 2.Arevalo HJ, Vadakkumpadan F, Guallar E, Jebb A, Malamas P, Wu KC, Trayanova NA. Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat. Commun. 2016;7:11437. doi: 10.1038/ncomms11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashikaga H, Arevalo H, Vadakkumpadan F, Blake RC, III, Bayer JD, Nazarian S, Muz Zviman M, Tandri H, Berger RD, Calkins H, Herzka DA, Trayanova NA, Halperin HR. Feasibility of image-based simulation to estimate ablation target in human ventricular arrhythmia. Heart Rhythm. 2013;10(8):1109–1116. doi: 10.1016/j.hrthm.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausra J, Madrid M, Yin RT, Hanna J, Arnott S, Brennan JA, Peralta R, Clausen D, Bakall JA, Efimov IR, Gutruf P. Wireless, fully implantable cardiac stimulation and recording with on-device computation for closed-loop pacing and defibrillation. Sci. Adv. 2022;8(43):eabq7469. doi: 10.1126/sciadv.abq7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayer JD, Blake RC, Plank G, Trayanova NA. A novel rule-based algorithm for assigning myocardial fiber orientation to computational heart models. Ann. Biomed. Eng. 2012;40(10):2243–2254. doi: 10.1007/s10439-012-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry R, Getzin M, Gjesteby L, Wang G. X-Optogenetics and U-optogenetics: feasibility and possibilities. Photonics. 2015;2(1):23–39. doi: 10.3390/photonics2010023. [DOI] [Google Scholar]
- 7.Biasci V, Santini L, Marchal GA, Hussaini S, Ferrantini C, Coppini R, Loew LM, Luther S, Campione M, Poggesi C, Pavone FS, Cerbai E, Bub G, Sacconi L. Optogenetic manipulation of cardiac electrical dynamics using sub-threshold illumination: dissecting the role of cardiac alternans in terminating rapid rhythms. Basic Res. Cardiol. 2022;117(1):25. doi: 10.1007/s00395-022-00933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop MJ, Rodriguez B, Eason J, Whiteley JP, Trayanova N, Gavaghan DJ. Synthesis of voltage-sensitive optical signals: application to panoramic optical mapping. Biophys. J. 2006;90(8):2938–2945. doi: 10.1529/biophysj.105.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 10.Boyle PM, Karathanos TV, Trayanova NA. Cardiac optogenetics: 2018. JACC Clin. Electrophysiol. 2018;4(2):155–167. doi: 10.1016/j.jacep.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle PM, Murphy MJ, Karathanos TV, Zahid S, Blake RC, 3rd, Trayanova NA. Termination of re-entrant atrial tachycardia via optogenetic stimulation with optimized spatial targeting: insights from computational models. J. Physiol. 2018;596(2):181–196. doi: 10.1113/JP275264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle PM, Williams JC, Ambrosi CM, Entcheva E, Trayanova NA. A comprehensive multiscale framework for simulating optogenetics in the heart. Nat Commun. 2013;4:2370. doi: 10.1038/ncomms3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruegmann T, Beiert T, Vogt CC, Schrickel JW, Sasse P. Optogenetic termination of atrial fibrillation in mice. Cardiovasc. Res. 2018;114(5):713–723. doi: 10.1093/cvr/cvx250. [DOI] [PubMed] [Google Scholar]
- 14.Bruegmann T, Boyle PM, Vogt CC, Karathanos TV, Arevalo HJ, Fleischmann BK, Trayanova NA, Sasse P. Optogenetic defibrillation terminates ventricular arrhythmia in mouse hearts and human simulations. J. Clin. Invest. 2016;126(10):3894–3904. doi: 10.1172/JCI88950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton RA, Klimas A, Ambrosi CM, Tomek J, Corbett A, Entcheva E, Bub G. Optical control of excitation waves in cardiac tissue. Nat. Photonics. 2015;9(12):813–816. doi: 10.1038/nphoton.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang MG, Chang CY, de Lange E, Xu L, O'Rourke B, Karagueuzian HS, Tung L, Marban E, Garfinkel A, Weiss JN, Qu Z, Abraham MR. Dynamics of early afterdepolarization-mediated triggered activity in cardiac monolayers. Biophys. J. 2012;102(12):2706–2714. doi: 10.1016/j.bpj.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MG, Sato D, de Lange E, Lee JH, Karagueuzian HS, Garfinkel A, Weiss JN, Qu Z. Bi-stable wave propagation and early afterdepolarization-mediated cardiac arrhythmias. Heart Rhythm. 2012;9(1):115–122. doi: 10.1016/j.hrthm.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi, Y. S., H. Jeong, R. T. Yin, R. Avila, A. Pfenniger, J. Yoo, J. Y. Lee, A. Tzavelis, Y. J. Lee, S. W. Chen, H. S. Knight, S. Kim, H. Y. Ahn, G. Wickerson, A. Vazquez-Guardado, E. Higbee-Dempsey, B. A. Russo, M. A. Napolitano, T. J. Holleran, L. A. Razzak, A. N. Miniovich, G. Lee, B. Geist, B. Kim, S. Han, J. A. Brennan, K. Aras, S. S. Kwak, J. Kim, E. A. Waters, X. Yang, A. Burrell, K. San Chun, C. Liu, C. Wu, A. Y. Rwei, A. N. Spann, A. Banks, D. Johnson, Z. J. Zhang, C. R. Haney, S. H. Jin, A. V. Sahakian, Y. Huang, G. D. Trachiotis, B. P. Knight, R. K. Arora, I. R. Efimov, and J. A. Rogers. A transient, closed-loop network of wireless, body-integrated devices for autonomous electrotherapy. Science 376(6596):1006–1012, 2022. [DOI] [PMC free article] [PubMed]
- 19.Choi, Y. S., R. T. Yin, A. Pfenniger, J. Koo, R. Avila, K. Benjamin Lee, S. W. Chen, G. Lee, G. Li, Y. Qiao, A. Murillo-Berlioz, A. Kiss, S. Han, S. M. Lee, C. Li, Z. Xie, Y. Y. Chen, A. Burrell, B. Geist, H. Jeong, J. Kim, H. J. Yoon, A. Banks, S. K. Kang, Z. J. Zhang, C. R. Haney, A. V. Sahakian, D. Johnson, T. Efimova, Y. Huang, G. D. Trachiotis, B. P. Knight, R. K. Arora, I. R. Efimov, and J. A. Rogers. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat. Biotechnol. 39(10):1228–1238, 2021. [DOI] [PMC free article] [PubMed]
- 20.Cokic M, Bruegmann T, Sasse P, Malan D. Optogenetic stimulation of G(i) signaling enables instantaneous modulation of cardiomyocyte pacemaking. Front Physiol. 2021;12:768495. doi: 10.3389/fphys.2021.768495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crocini C, Ferrantini C, Coppini R, Scardigli M, Yan P, Loew LM, Smith G, Cerbai E, Poggesi C, Pavone FS, Sacconi L. Optogenetics design of mechanistically-based stimulation patterns for cardiac defibrillation. Sci. Rep. 2016;6:35628. doi: 10.1038/srep35628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng D, Arevalo H, Pashakhanloo F, Prakosa A, Ashikaga H, McVeigh E, Halperin H, Trayanova N. Accuracy of prediction of infarct-related arrhythmic circuits from image-based models reconstructed from low and high resolution MRI. Front. Physiol. 2015;6:282. doi: 10.3389/fphys.2015.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dries E, Amoni M, Vandenberk B, Johnson DM, Gilbert G, Nagaraju CK, Puertas RD, Abdesselem M, Santiago DJ, Roderick HL, Claus P, Willems R, Sipido KR. Altered adrenergic response in myocytes bordering a chronic myocardial infarction underlies in vivo triggered activity and repolarization instability. J. Physiol. 2020;598(14):2875–2895. doi: 10.1113/JP278839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dutta S, Mincholé A, Zacur E, Quinn TA, Taggart P, Rodriguez B. Early afterdepolarizations promote transmural reentry in ischemic human ventricles with reduced repolarization reserve. Prog. Biophys. Mol. Biol. 2016;120(1–3):236–248. doi: 10.1016/j.pbiomolbio.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and torsade de pointes. Pacing Clin. Electrophysiol. 2018;41(4):414–421. doi: 10.1111/pace.13296. [DOI] [PubMed] [Google Scholar]
- 26.Entcheva E. Cardiac optogenetics. Am. J. Physiol. Heart Circ. Physiol. 2013;304(9):H1179–1191. doi: 10.1152/ajpheart.00432.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Entcheva E, Kay MW. Cardiac optogenetics: a decade of enlightenment. Nat. Rev. Cardiol. 2021;18(5):349–367. doi: 10.1038/s41569-020-00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ. Res. 2010;106(5):981–991. doi: 10.1161/CIRCRESAHA.109.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Govorunova EG, Cunha SR, Sineshchekov OA, Spudich JL. Anion channelrhodopsins for inhibitory cardiac optogenetics. Sci. Rep. 2016;6:33530. doi: 10.1038/srep33530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Govorunova EG, Gou Y, Sineshchekov OA, Li H, Lu X, Wang Y, Brown LS, St-Pierre F, Xue M, Spudich JL. Kalium channelrhodopsins are natural light-gated potassium channels that mediate optogenetic inhibition. Nat. Neurosci. 2022;25(7):967–974. doi: 10.1038/s41593-022-01094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. Natural light-gated anion channels: a family of microbial rhodopsins for advanced optogenetics. Science. 2015;349(6248):647–650. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruber A, Edri O, Huber I, Arbel G, Gepstein A, Shiti A, Shaheen N, Chorna S, Landesberg M, Gepstein L. Optogenetic modulation of cardiac action potential properties may prevent arrhythmogenesis in short and long QT syndromes. JCI Insight. 2021;6(11):e147470. doi: 10.1172/jci.insight.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo W, Kamiya K, Cheng J, Toyama J. Changes in action potentials and ion currents in long-term cultured neonatal rat ventricular cells. Am. J. Physiol. 1996;271(1 Pt 1):C93–102. doi: 10.1152/ajpcell.1996.271.1.C93. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Kim TY, Koren G, Choi BR, Qu Z. Spontaneous initiation of premature ventricular complexes and arrhythmias in type 2 long QT syndrome. Am. J. Physiol. Heart Circ. Physiol. 2016;311(6):H1470–H1484. doi: 10.1152/ajpheart.00500.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussaini, S., V. Venkatesan, V. Biasci, J. M. Romero Sepúlveda, R. A. QuiñonezUribe, L. Sacconi, G. Bub, C. Richter, V. Krinski, U. Parlitz, R. Majumder, and S. Luther. Drift and termination of spiral waves in optogenetically modified cardiac tissue at sub-threshold illumination. Elife 10:e59954, 2021. [DOI] [PMC free article] [PubMed]
- 36.Johnson DM, Antoons G. Arrhythmogenic mechanisms in heart failure: linking beta-adrenergic stimulation, stretch, and calcium. Front. Physiol. 2018;9:1453. doi: 10.3389/fphys.2018.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karathanos TV, Bayer JD, Wang D, Boyle PM, Trayanova NA. Opsin spectral sensitivity determines the effectiveness of optogenetic termination of ventricular fibrillation in the human heart: a simulation study. J. Physiol. 2016;594(23):6879–6891. doi: 10.1113/JP271739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karathanos, T. V., P. M. Boyle, and N. A. Trayanova. Optogenetics-enabled dynamic modulation of action potential duration in atrial tissue: feasibility of a novel therapeutic approach. Europace 16(Suppl 4):iv69–iv76, 2014. [DOI] [PMC free article] [PubMed]
- 39.Kopton RA, Baillie JS, Rafferty SA, Moss R, Zgierski-Johnston CM, Prykhozhij SV, Stoyek MR, Smith FM, Kohl P, Quinn TA, Schneider-Warme F. Cardiac electrophysiological effects of light-activated chloride channels. Front. Physiol. 2018;9:1806. doi: 10.3389/fphys.2018.01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Wang L, Luo J, Li H, Rao P, Cheng Y, Wang X, Huang C. Optical capture and defibrillation in rats with monocrotaline-induced myocardial fibrosis 1 year after a single intravenous injection of adeno-associated virus channelrhodopsin-2. Heart Rhythm. 2021;18(1):109–117. doi: 10.1016/j.hrthm.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Luther S, Fenton FH, Kornreich BG, Squires A, Bittihn P, Hornung D, Zabel M, Flanders J, Gladuli A, Campoy L, Cherry EM, Luther G, Hasenfuss G, Krinsky VI, Pumir A, Gilmour RF, Jr, Bodenschatz E. Low-energy control of electrical turbulence in the heart. Nature. 2011;475(7355):235–239. doi: 10.1038/nature10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maguire CT, Wakimoto H, Patel VV, Hammer PE, Gauvreau K, Berul CI. Implications of ventricular arrhythmia vulnerability during murine electrophysiology studies. Physiol. Genomics. 2003;15(1):84–91. doi: 10.1152/physiolgenomics.00034.2003. [DOI] [PubMed] [Google Scholar]
- 43.Majumder R, Hussaini S, Zykov VS, Luther S, Bodenschatz E. Pulsed low-energy stimulation initiates electric turbulence in cardiac tissue. PLoS Comput. Biol. 2021;17(10):e1009476. doi: 10.1371/journal.pcbi.1009476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makowka P, Bruegmann T, Dusend V, Malan D, Beiert T, Hesse M, Fleischmann BK, Sasse P. Optogenetic stimulation of G(s)-signaling in the heart with high spatio-temporal precision. Nat. Commun. 2019;10(1):1281. doi: 10.1038/s41467-019-09322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchal GA, Biasci V, Loew LM, Biggeri A, Campione M, Sacconi L. Optogenetic manipulation of cardiac repolarization gradients using sub-threshold illumination. Front. Physiol. 2023;14:1167524. doi: 10.3389/fphys.2023.1167524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcus GM. Evaluation and management of premature ventricular complexes. Circulation. 2020;141(17):1404–1418. doi: 10.1161/CIRCULATIONAHA.119.042434. [DOI] [PubMed] [Google Scholar]
- 47.Mendonca Costa C, Neic A, Gillette K, Porter B, Gould J, Sidhu B, Chen Z, Elliott M, Mehta V, Plank G, Rinaldi CA, Bishop MJ, Niederer SA. Left ventricular endocardial pacing is less arrhythmogenic than conventional epicardial pacing when pacing in proximity to scar. Heart Rhythm. 2020;17(8):1262–1270. doi: 10.1016/j.hrthm.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendonca Costa C, Neic A, Kerfoot E, Porter B, Sieniewicz B, Gould J, Sidhu B, Chen Z, Plank G, Rinaldi CA, Bishop MJ, Niederer SA. Pacing in proximity to scar during cardiac resynchronization therapy increases local dispersion of repolarization and susceptibility to ventricular arrhythmogenesis. Heart Rhythm. 2019;16(10):1475–1483. doi: 10.1016/j.hrthm.2019.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modell SM, Lehmann MH. The long QT syndrome family of cardiac ion channelopathies: a HuGE review. Genet. Med. 2006;8(3):143–155. doi: 10.1097/01.gim.0000204468.85308.86. [DOI] [PubMed] [Google Scholar]
- 50.Myles RC, Wang L, Bers DM, Ripplinger CM. Decreased inward rectifying K+ current and increased ryanodine receptor sensitivity synergistically contribute to sustained focal arrhythmia in the intact rabbit heart. J. Physiol. 2015;593(6):1479–1493. doi: 10.1113/jphysiol.2014.279638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myles RC, Wang L, Kang C, Bers DM, Ripplinger CM. Local beta-adrenergic stimulation overcomes source-sink mismatch to generate focal arrhythmia. Circ. Res. 2012;110(11):1454–1464. doi: 10.1161/CIRCRESAHA.111.262345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niederer SA, Kerfoot E, Benson AP, Bernabeu MO, Bernus O, Bradley C, Cherry EM, Clayton R, Fenton FH, Garny A, Heidenreich E, Land S, Maleckar M, Pathmanathan P, Plank G, Rodriguez JF, Roy I, Sachse FB, Seemann G, Skavhaug O, Smith NP. Verification of cardiac tissue electrophysiology simulators using an N-version benchmark. Philos. Trans. A Math. Phys. Eng. Sci. 2011;369(1954):4331–4351. doi: 10.1098/rsta.2011.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyns ECA, Jin T, Fontes MS, van den Heuvel T, Portero V, Ramsey C, Bart CI, Zeppenfeld K, Schalij MJ, van Brakel TJ, Ramkisoensing AA, Zhang G, Poelma RH, Ordog B, de Vries AAF, Pijnappels DA. Optical ventricular cardioversion by local optogenetic targeting and LED implantation in a cardiomyopathic rat model. Cardiovasc. Res. 2022;118(10):2293–2303. doi: 10.1093/cvr/cvab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nyns ECA, Kip A, Bart CI, Plomp JJ, Zeppenfeld K, Schalij MJ, de Vries AAF, Pijnappels DA. Optogenetic termination of ventricular arrhythmias in the whole heart: towards biological cardiac rhythm management. Eur. Heart J. 2017;38(27):2132–2136. doi: 10.1093/eurheartj/ehw574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nyns, E. C. A., R. H. Poelma, L. Volkers, J. J. Plomp, C. I. Bart, A. M. Kip, T. J. van Brakel, K. Zeppenfeld, M. J. Schalij, G. Q. Zhang, A. A. F. de Vries, and D. A. Pijnappels. An automated hybrid bioelectronic system for autogenous restoration of sinus rhythm in atrial fibrillation. Sci. Transl. Med. 11(481):eaau6447, 2019. [DOI] [PubMed]
- 56.O'Hara T, Virág L, Varró A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput. Biol. 2011;7(5):e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ochs AR, Karathanos TV, Trayanova NA, Boyle PM. Optogenetic stimulation using anion channelrhodopsin (GtACR1) facilitates termination of reentrant arrhythmias with low light energy requirements: a computational study. Front. Physiol. 2021;12:718622. doi: 10.3389/fphys.2021.718622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plank G, Loewe A, Neic A, Augustin C, Huang YL, Gsell MAF, Karabelas E, Nothstein M, Prassl AJ, Sanchez J, Seemann G, Vigmond EJ. The openCARP simulation environment for cardiac electrophysiology. Comput. Methods Programs Biomed. 2021;208:106223. doi: 10.1016/j.cmpb.2021.106223. [DOI] [PubMed] [Google Scholar]
- 59.Plank G, Zhou L, Greenstein JL, Cortassa S, Winslow RL, O'Rourke B, Trayanova NA. From mitochondrial ion channels to arrhythmias in the heart: computational techniques to bridge the spatio-temporal scales. Philos. Trans. A Math. Phys. Eng. Sci. 2008;366(1879):3381–3409. doi: 10.1098/rsta.2008.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu Z, Xie LH, Olcese R, Karagueuzian HS, Chen PS, Garfinkel A, Weiss JN. Early afterdepolarizations in cardiac myocytes: beyond reduced repolarization reserve. Cardiovasc. Res. 2013;99(1):6–15. doi: 10.1093/cvr/cvt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao P, Wang L, Cheng Y, Wang X, Li H, Zheng G, Li Z, Jiang C, Zhou Q, Huang C. Near-infrared light driven tissue-penetrating cardiac optogenetics via upconversion nanoparticles in vivo. Biomed. Opt. Express. 2020;11(3):1401–1416. doi: 10.1364/BOE.381480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ripoll J, Yessayan D, Zacharakis G, Ntziachristos V. Experimental determination of photon propagation in highly absorbing and scattering media. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2005;22(3):546–551. doi: 10.1364/JOSAA.22.000546. [DOI] [PubMed] [Google Scholar]
- 63.Rush S, Larsen H. A practical algorithm for solving dynamic membrane equations. IEEE Trans. Biomed. Eng. 1978;25(4):389–392. doi: 10.1109/TBME.1978.326270. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Alonso JL, Bhargava A, O'Hara T, Glukhov AV, Schobesberger S, Bhogal N, Sikkel MB, Mansfield C, Korchev YE, Lyon AR, Punjabi PP, Nikolaev VO, Trayanova NA, Gorelik J. Microdomain-specific modulation of L-type calcium channels leads to triggered ventricular arrhythmia in heart failure. Circ. Res. 2016;119(8):944–955. doi: 10.1161/CIRCRESAHA.116.308698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato D, Xie LH, Nguyen TP, Weiss JN, Qu Z. Irregularly appearing early afterdepolarizations in cardiac myocytes: random fluctuations or dynamical chaos? Biophys. J. 2010;99(3):765–773. doi: 10.1016/j.bpj.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato D, Xie LH, Sovari AA, Tran DX, Morita N, Xie F, Karagueuzian H, Garfinkel A, Weiss JN, Qu Z. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc. Natl Acad. Sci. U. S. A. 2009;106(9):2983–2988. doi: 10.1073/pnas.0809148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah SR, Park K, Alweis R, Long QT. Syndrome: a comprehensive review of the literature and current evidence. Curr. Probl. Cardiol. 2019;44(3):92–106. doi: 10.1016/j.cpcardiol.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Spector P. Principles of cardiac electric propagation and their implications for re-entrant arrhythmias. Circ. Arrhythm. Electrophysiol. 2013;6(3):655–661. doi: 10.1161/CIRCEP.113.000311. [DOI] [PubMed] [Google Scholar]
- 69.Swartling J, Palsson S, Platonov P, Olsson SB, Andersson-Engels S. Changes in tissue optical properties due to radio-frequency ablation of myocardium. Med. Biol. Eng. Comput. 2003;41(4):403–409. doi: 10.1007/BF02348082. [DOI] [PubMed] [Google Scholar]
- 70.ten Tusscher KH, Panfilov AV. Alternans and spiral breakup in a human ventricular tissue model. Am. J. PHYSIOL. Heart Circ. Physiol. 2006;291(3):H1088–1100. doi: 10.1152/ajpheart.00109.2006. [DOI] [PubMed] [Google Scholar]
- 71.Vandersickel N, Kazbanov IV, Nuitermans A, Weise LD, Pandit R, Panfilov AV. A study of early afterdepolarizations in a model for human ventricular tissue. PLoS ONE. 2014;9(1):e84595. doi: 10.1371/journal.pone.0084595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vandersickel N, Van Nieuwenhuyse E, Seemann G, Panfilov AV. Spatial Patterns of Excitation at Tissue and Whole Organ Level Due to Early Afterdepolarizations. Front Physiol. 2017;8:404. doi: 10.3389/fphys.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Varró A, Baczkó I. Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk. Br. J. Pharmacol. 2011;164(1):14–36. doi: 10.1111/j.1476-5381.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vierock, J., E. Peter, C. Grimm, A. Rozenberg, I. W. Chen, L. Tillert, A. G. Castro Scalise, M. Casini, S. Augustin, D. Tanese, B. C. Forget, R. Peyronnet, F. Schneider-Warme, V. Emiliani, O. Beja, and P. Hegemann. WiChR, a highly potassium-selective channelrhodopsin for low-light one- and two-photon inhibition of excitable cells. Sci. Adv. 8(49):eadd7729, 2022. [DOI] [PMC free article] [PubMed]
- 75.Vigmond EJ, Hughes M, Plank G, Leon LJ. Computational tools for modeling electrical activity in cardiac tissue. J. Electrocardiol. 2003;36(Suppl):69–74. doi: 10.1016/j.jelectrocard.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 76.Vogt CC, Bruegmann T, Malan D, Ottersbach A, Roell W, Fleischmann BK, Sasse P. Systemic gene transfer enables optogenetic pacing of mouse hearts. Cardiovasc. Res. 2015;106(2):338–343. doi: 10.1093/cvr/cvv004. [DOI] [PubMed] [Google Scholar]
- 77.Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm. 2010;7(12):1891–1899. doi: 10.1016/j.hrthm.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams JC, Entcheva E. Optogenetic versus electrical stimulation of human cardiomyocytes: modeling insights. Biophys. J. 2015;108(8):1934–1945. doi: 10.1016/j.bpj.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams JC, Xu J, Lu Z, Klimas A, Chen X, Ambrosi CM, Cohen IS, Entcheva E. Computational optogenetics: empirically-derived voltage- and light-sensitive channelrhodopsin-2 model. PLoS Comput. Biol. 2013;9(9):e1003220. doi: 10.1371/journal.pcbi.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys. J. 2010;99(5):1408–1415. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang, Y., M. Wu, A. Vazquez-Guardado, A. J. Wegener, J. G. Grajales-Reyes, Y. Deng, T. Wang, R. Avila, J. A. Moreno, S. Minkowicz, V. Dumrongprechachan, J. Lee, S. Zhang, A. A. Legaria, Y. Ma, S. Mehta, D. Franklin, L. Hartman, W. Bai, M. Han, H. Zhao, W. Lu, Y. Yu, X. Sheng, A. Banks, X. Yu, Z. R. Donaldson, R. W. t. Gereau, C. H. Good, Z. Xie, Y. Huang, Y. Kozorovitskiy, and J. A. Rogers. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 24(7):1035–1045, 2021. [DOI] [PMC free article] [PubMed]
- 82.Yao JA, Hussain W, Patel P, Peters NS, Boyden PA, Wit AL. Remodeling of gap junctional channel function in epicardial border zone of healing canine infarcts. Circ. Res. 2003;92(4):437–443. doi: 10.1161/01.RES.0000059301.81035.06. [DOI] [PubMed] [Google Scholar]
- 83.Yu L, Zhou L, Cao G, Po SS, Huang B, Zhou X, Wang M, Yuan S, Wang Z, Wang S, Jiang H. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J. Am. Coll. Cardiol. 2017;70(22):2778–2790. doi: 10.1016/j.jacc.2017.09.1107. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z, Liu MB, Huang X, Song Z, Qu Z. Mechanisms of premature ventricular complexes caused by QT prolongation. Biophys. J. 2021;120(2):352–369. doi: 10.1016/j.bpj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zimik S, Vandersickel N, Nayak AR, Panfilov AV, Pandit R. A comparative study of early afterdepolarization-mediated fibrillation in two mathematical models for human ventricular cells. PLoS ONE. 2015;10(6):e0130632. doi: 10.1371/journal.pone.0130632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.