Abstract
This study evaluated the tolerance and potential pharmacokinetic interactions between clarithromycin (500 mg every 12 h) and rifabutin (300 mg daily) in clinically stable human immunodeficiency virus-infected volunteers with CD4 counts of <200 cells/mm3. Thirty-four subjects were randomized equally to either regimen A or regimen B. On days 1 to 14, subjects assigned to regimen A received clarithromycin and subjects assigned to regimen B received rifabutin, and then both groups received both drugs on days 15 to 42. Of the 14 regimen A and the 15 regimen B subjects who started combination therapy, 1 subject in each group prematurely discontinued therapy due to toxicity, but 19 of 29 subjects reported nausea, vomiting, and/or diarrhea. Pharmacokinetic analysis included data for 11 regimen A and 14 regimen B subjects. Steady-state pharmacokinetic parameters for single-agent therapy (day 14) and combination therapy (day 42) were compared. Regimen A resulted in a mean decrease of 44% (P = 0.003) in the clarithromycin area under the plasma concentration-time curve (AUC), while there was a mean increase of 57% (P = 0.004) in the AUC of the clarithromycin metabolite 14-OH-clarithromycin. Regimen B resulted in a mean increase of 99% (P = 0.001) in the rifabutin AUC and a mean increase of 375% (P < 0.001) in the AUC of the rifabutin metabolite 25-O-desacetyl-rifabutin. The usefulness of this combination for prophylaxis of Mycobacterium avium infections is limited by frequent gastrointestinal adverse events. Coadministration of clarithromycin and rifabutin results in significant bidirectional pharmacokinetic interactions. The resulting increase in rifabutin levels may explain the increased frequency of uveitis observed with concomitant use of these drugs.
Mycobacterium avium complex (MAC) disease is a frequent cause of morbidity and mortality in patients with late-stage human immunodeficiency virus (HIV) infection (3, 4). In common with other mycobacterial infections, treatment of MAC infections with combinations of drugs appears to be necessary to improve efficacy and to prevent the emergence of resistance. Clarithromycin and rifabutin are agents commonly used for both the treatment and the prophylaxis of MAC infection. Clarithromycin is a macrolide antibiotic with a high level of activity against MAC, with MICs at which 90% of isolates are inhibited (MIC90s) reportedly ranging between 1 and 4 μg/ml for clinical isolates (2, 17, 19). MAC strains isolated from patients without previous macrolide therapy are uniformly susceptible to clinically achievable clarithromycin levels. A dose-ranging, single-agent-treatment trial of clarithromycin demonstrated impressive clinical activity in the reduction or elimination of MAC bacteremia (8). The MIC90s of rifabutin range between 0.25 and 2 μg/ml, although typically ≤60% of the strains are susceptible to the drug at levels obtained with present dosing practices (18, 37). The 25-O-desacetyl metabolite of rifabutin has in vitro activity similar to that of rifabutin (41). Rifabutin was effective for MAC prophylaxis in a placebo-controlled, double-blind trial (31). The combination of rifabutin and clarithromycin was found to have additive activity in vitro (23, 26) in macrophages (27) and the beige mouse model (24).
Clarithromycin is a known inhibitor (22) and rifabutin is a known inducer (5) of hepatic microsomal cytochrome P-450 enzymes. The effect of enzyme inhibition by clarithromycin appears with the first dose, but it is not maximized until after several doses (15). Enzyme induction by rifabutin was demonstrated at 7 days by the increased metabolism of antipyrine (32), and autoinduction of rifabutin metabolism was detected at 10 days in studies with healthy volunteers (28). Pharmacokinetic interactions between clarithromycin and rifabutin could cause significant changes in the systemic exposure of both parent compounds and their primary metabolites and could have important implications for the safety and effectiveness of therapy against MAC. In a group of patients treated with clarithromycin for lung disease due to MAC, mean levels of clarithromycin were decreased in those also receiving rifabutin at 600 mg/day, indicating that this dosage of rifabutin appeared to induce clarithromycin metabolism (43). This study was designed to evaluate the tolerance of combination therapy and the potential pharmacokinetic interactions between clarithromycin and rifabutin in a population with late-stage HIV infection.
MATERIALS AND METHODS
Subjects.
Thirty-four clinically stable HIV-infected adult volunteers provided written, informed consent according to the institutional guidelines of the participating centers prior to enrollment. Patients were excluded for known MAC bacteremia or a compatible syndrome, CD4 counts of >200 cells/mm3, acute opportunistic infection or malignancy, a serum creatinine level of >2.0 mg/dl, a bilirubin level of >2.0 mg/dL, aspartate aminotransferase and alanine aminotransferase levels more than five times the upper limit of normal, a history of sensitivity or intolerance to the study drugs, or recent use of clarithromycin (within 14 days) or rifabutin (within 30 days). Patients requiring drugs likely to interact pharmacokinetically with the study agents were excluded, with the exception that azoles for acute treatment or maintenance therapy for fungal infections were permitted.
Study design.
Volunteers were randomized equally to one of two regimens. Regimen A consisted of clarithromycin, at 500 mg every 12 h for 2 weeks (days 1 to 14), followed by a combination of the same clarithromycin dose plus rifabutin, 300 mg once daily for an additional 4 weeks (days 15 to 42). Regimen B used the same doses of study drugs used for regimen A, but the subjects received rifabutin during days 1 to 14 and the combination of rifabutin and clarithromycin during days 15 to 42. Subjects were instructed to take the daily rifabutin dose with the morning dose of clarithromycin. Clinical evaluations and hematologic and biochemical profiles were repeated every 2 weeks over an 8-week period, with the last study visit occurring 2 weeks after the discontinuation of study drugs. To monitor compliance, missed doses were self-reported by the study participants. Subjects experiencing a possible drug-related toxicity of grade 3 or higher, as defined by the Division of AIDS Table for Grading Severity of Adult Adverse Experiences, were permanently discontinued from study therapy and were followed until the resolution of the toxicity.
Pharmacokinetic sampling occurred on day 14, the time at which steady state for either clarithromycin alone (regimen A) or rifabutin alone (regimen B) is presumed to occur, day 15 (day 1 of combination therapy), and day 42 (presumed combination steady state). Subjects fasted for 12 h before and 2 h after study drug administration, but they were allowed water and concomitant medications. For subjects receiving regimen A, blood was obtained predosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 12 h after dosing on days 14 and 15. For days 42 and 43, blood was also obtained at hours 16, 24, 36, and 48. For subjects receiving regimen B, blood was obtained predosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 h after dosing on days 14 and 15. For days 42 to 45, blood was also obtained at hours 36, 48, 72, and 96. At weeks 3, 4, and 5, blood was obtained within 1 hour prior to the administration of the next dose of combination study medication for determination of trough levels. Blood samples for drug level assays were centrifuged within 1 h of collection, and the plasma was aliquotted and frozen at −70°C until it was assayed.
Study drugs.
Clarithromycin was provided as 500-mg tablets by Abbott Laboratories (Abbott Park, Ill.), and rifabutin was provided as 150-mg capsules by Adria Laboratories (currently Pharmacia & Upjohn, Kalamazoo, Mich.).
Drug assays.
The concentrations of the drugs in plasma were determined by validated high-pressure liquid chromatographic techniques (10, 25). The concentrations of clarithromycin and 14 (R)-hydroxyclarithromycin (14-OH-clarithromycin) in plasma were determined by Harris Laboratories (Lincoln, Nebr.); rifabutin and 25-O-desacetyl-rifabutin concentrations in plasma were determined by BAS Analytics (West Lafayette, Ind.).
The clarithromycin assay had a sensitivity of 0.0156 μg/ml and was linear from 0.0156 to 8.00 μg/ml. The between-day percent coefficients of variation were 10.1% at 0.0606 μg/ml, 10.7% at 0.25 μg/ml, and 6.4% at 2.00 μg/ml. The sensitivity and linearity range were the same for 14-OH-clarithromycin. For this metabolite, the between-day percent coefficients of variation of assay were 5.1% at 0.065 μg/ml, 10.9% at 0.25 μg/ml, and 9.5% at 2.00 μg/ml.
The rifabutin and 25-o-desacetyl-rifabutin assays each required two standard curves. Linearity was acceptable within each standard curve range. The rifabutin low standard curve ranged from 0.005 to 0.060 μg/ml and the high standard curve ranged from 0.060 to 0.800 μg/ml. The between-day percent coefficients of variation for rifabutin were 17.4% at 0.00726 μg/ml, 10.8% at 0.029 μg/ml, 9.82% at 0.144 μg/ml, and 8.2% at 0.726 μg/ml. For the 25-o-desacetyl-rifabutin metabolite, the low standard curve ranged from 2.5 to 30 ng/ml and the high standard curve ranged from 30 to 400 ng/ml. The between-day percent coefficients of variation for 25-o-desacetyl-rifabutin were 19.7% at 0.00319 μg/ml, 12.0% at 0.0127 μg/ml, 10.9% at 0.0634 μg/ml, and 12.1% at 0.319 μg/ml.
Statistical considerations.
It was projected that a sample of 12 evaluable subjects would provide at least an 80% power (with α = 0.05) for one-sided tests of the hypothesis that combination therapy would be tolerated by 67% or more of the subjects receiving each regimen against the alternative that only 33% could tolerate the therapy. The baseline characteristics of the subject groups were compared by the Student t test and the chi-square test. The study was also designed to determine if apparent drug clearance for each agent, as measured by the mean change in the area under the plasma concentration-time curve (AUC), would be significantly modified in the presence of the other drug.
The LAGRAN software of Rocci and Jusko (34) was used to compute AUC. The maximal concentration (Cmax) and the time to Cmax (Tmax) were determined by inspection of interpolated curves. λz, the terminal elimination rate constant, was determined by fitting a log-linear regression to the data for the last four time points of the terminal phase; in cases of a delayed Tmax, fewer points were used. AUC0–τ is the presumed steady-state value for each drug and its metabolite during the period between doses. For day 15, the AUC0–∞ (the AUC from time zero to infinity) of the drug initiated on that day was calculated as AUC0–Cn + Cn/λz, where Cn is the concentration at the last measurable time point. Treatment arms were compared by two-sample t tests.
RESULTS
Subject characteristics.
A total of 34 volunteers, 17 receiving each regimen, were enrolled into the study at six participating centers between 16 June 1992 and 27 October 1992. Thirty-two subjects began the single-drug therapy to which they were randomized, and 29 of these subjects initiated combination therapy on day 15. Data for 11 volunteers randomized to regimen A and 14 volunteers randomized to regimen B were included in the pharmacokinetic analysis (Table 1).
TABLE 1.
Study enrollment, withdrawal, and completion ratesa
| Subject characteristic | No. of subjects
|
||
|---|---|---|---|
| Regimen A | Regimen B | Total | |
| Subjects who were randomized | 17 | 17 | 34 |
| Subject withdrawal for acute illness before treatment with a single drug was initiated | 1 | 1 | 2 |
| Subjects evaluable for single-drug tolerance | 16 | 16 | 32 |
| Subject withdrawal of consent before two drug combination was initiated | 1 | 1 | 2 |
| Subject noncompliance before two-drug combination was initiated | 1 | 0 | 1 |
| Subjects evaluable for combination drug tolerance | 14 | 15 | 29 |
| Subject withdrawal for adverse event after two-drug combination was initiated | 1 | 1 | 2 |
| Subject noncompliance during two-drug combination | 1 | 0 | 1 |
| Subjects completing the study | 12 | 14 | 26 |
| Subjects for whom no pharmacokinetic data were available because of technical problems | 1 | 0 | 1 |
| Subjects for whom data were evaluable for pharmacokinetic analysis | 11 | 14 | 25 |
Regimen A, clarithromycin on days 1 to 14 and both study drugs on days 15 to 42; regimen B, rifabutin on days 1 to 14 and both study drugs on days 15 to 42.
The baseline characteristics of the 32 study volunteers who started a study drug are presented in Table 2. At enrollment, the mean CD4 cell counts were 67 and 82 cells/mm3 (P = 0.25) for regimen A subjects and regimen B subjects, respectively. The only characteristic for which there was a significant difference at the baseline was weight (P = 0.02), but there was no significant difference in body surface area (P = 0.13). There were no statistically significant differences in the baseline characteristics of the 26 subjects who completed the study compared to those of the 6 subjects who did not complete the study (data not shown).
TABLE 2.
Baseline characteristics of the 32 subjects who started study drugsa
| Characteristic | Regimen A (n = 16) | Regimen B (n = 16) |
|---|---|---|
| Gender (no. of subjects) | ||
| Male | 14 | 15 |
| Female | 2 | 1 |
| Race (no. of subjects) | ||
| White | 9 | 15 |
| Black | 4 | 1 |
| Other | 3 | 0 |
| HIV risk factors (no. of subjects) | ||
| Male homosexual or bisexual contact(s) | 12 | 12 |
| Male homosexual or bisexual contact(s) and intravenous drug use | 2 | 1 |
| Heterosexual contact or sex with intravenous drug user | 1 | 2 |
| Unknown | 1 | 1 |
| Mean (SD) age (yr) | 36.3 (8.0) | 36.8 (7.8) |
| Mean (SD) wt (kg) | 68.7 (10.3) | 74.3 (8.2) |
| Mean (SD) body surface area (m2) | 1.8 (0.2) | 1.9 (0.1) |
| Mean (SD) CD4 cell count (cells/mm3) | 67 (50) | 82 (52) |
Regimen A, clarithromycin on days 1 to 14 and both study drugs on days 15 to 42; regimen B, rifabutin on days 1 to 14 and both study drugs on days 15 to 42.
Drug tolerance and adverse events.
After 1 week of combination therapy, one regimen A subject experienced grade 3 myalgia and one regimen B subject experienced grade 3 leukopenia; each subject discontinued the study drugs. The 95% confidence intervals for the proportion tolerating combination therapy are as follows: for regimen A subjects (13 of 14 subjects), 80.7 to 99.6%; for regimen B subjects (14 of 15 subjects), 81.9 to 99.7%. In neither regimen was the hypothesis of at least 67% tolerance rejected (P ≥ 0.97).
Gastrointestinal (GI) symptoms were reported frequently, with 19 of 29 (65.5%) subjects reporting nausea, vomiting, or diarrhea events during combination therapy. All except three of these GI events were grade 1; one subject had a single report of grade 2 nausea (defined as four to five episodes per day or vomiting for 1 week or longer) at week 1, and two others had reports of grade 2 diarrhea (defined as five to seven loose stools per day or nocturnal loose stools) at week 3. Of these 19 subjects, 9 had only one type of GI symptom (3 subjects had nausea and 6 subjects had diarrhea) and 11 had multiple symptoms (5 subjects had nausea and diarrhea, 2 subjects had nausea and vomiting, and 4 subjects had all three symptoms). The GI complaints were persistent in 16 volunteers who reported the same symptom on 2 or more consecutive weekly visits during combination therapy. HIV-related conditions occurring during the study included herpes simplex in 1 subject and oral candidiasis in 12 subjects.
Effect of rifabutin on clarithromycin pharmacokinetics.
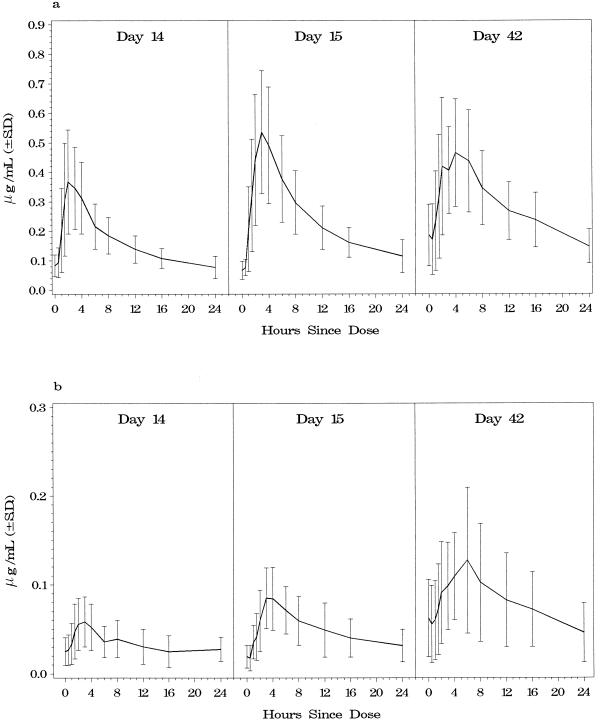
Data for three regimen A subjects who received combination therapy were not used in the pharmacokinetics analysis; one subject had poor study drug compliance, one subject prematurely withdrew from the study due to an adverse event, and technical problems prevented the use of one subject’s samples. Table 3 presents the estimated values of the pharmacokinetic parameters, including the AUC0–12, Cmax, and Tmax of clarithromycin and 14-OH-clarithromycin, for regimen A subjects on days 14, 15, and 42. Figure 1 displays the AUCs of clarithromycin (Fig. 1a) and 14-OH-clarithromycin (Fig. 1b) for each of the three sampling periods.
TABLE 3.
Pharmacokinetic parameters of clarithromycin and 14-OH-clarithromycin for 11 subjects receiving regimen Aa
| Drug | AUC0–τ (μg · h/ml)
|
Mean % change in AUC0–τ between days 14 and 42 (P value) |
Cmax (μg/ml)
|
Mean % change in Cmax between days 14 and 42 (P value) |
Tmax (h)
|
Mean % change in Tmax between days 14 and 42 (P value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 14 | Day 15 | Day 42 | Day 14 | Day 15 | Day 42 | Day 14 | Day 15 | Day 42 | ||||
| Clarithromycin | 36.5 ± 15.0 | 35.5 ± 15.8 | 16.6 ± 5.4 | −44 ± 37 (0.003) | 5.7 ± 2.4 | 4.6 ± 1.7 | 2.8 ± 1.0 | −41 ± 35 (0.003) | 3.0 ± 2.3 | 3.2 ± 2.2 | 3.5 ± 3.2 | 34 ± 100 (0.287) |
| 14-OH-Clarithromycin | 9.1 ± 3.4 | 9.2 ± 3.7 | 13.4 ± 5.0 | 57 ± 51 (0.004) | 1.1 ± 0.4 | 1.0 ± 0.4 | 1.7 ± 0.6 | 69 ± 49 (<0.001) | 3.6 ± 2.8 | 3.1 ± 2.4 | 4.6 ± 3.6 | 52 ± 106 (0.136) |
| Rifabutin | 8.6 ± 8.2b | 6.1 ± 1.9 | 0.59 ± 0.33 | 0.54 ± 0.19 | 3.7 ± 1.1 | 3.8 ± 3.4 | ||||||
| 25-O-Desacetyl-rifabutin | 2.3 ± 1.2b | 1.5 ± 0.7 | 0.10 ± 0.06 | 0.12 ± 0.06 | 5.3 ± 1.2 | 4.4 ± 3.0 | ||||||
Study day 14 is steady state for clarithromycin alone, day 15 is the first day that rifabutin was added to the regimen, and day 42 is steady state for both drugs. All values are means ± standard deviations.
The AUC reported for rifabutin and its metabolite on day 15 represents AUC0–∞.
FIG. 1.
Composite curves of mean clarithromycin concentrations (a) and 14-OH-clarithromycin concentrations (b) for regimen A subjects during the three pharmacokinetic sampling periods. Regimen A was clarithromycin, 500 mg every 12 h during days 1 to 14 and then the same clarithromycin dose with rifabutin, 300 mg daily during days 15 to 42.
The mean decrease in the clarithromycin AUC0–12 between days 14 and 42 was 44% (P < 0.003) (Table 3). A significant decrease was observed when comparing the mean clarithromycin AUC0–12 between days 15 and 42 (P < 0.004) but not between days 14 and 15 (P = 0.678). The individual clarithromycin AUC0–12 estimates decreased 23.4 to 83.5% between days 14 and 42 for all except one subject; the AUC0–12 for one subject increased by 49%. The mean increase in the 14-OH-clarithromycin AUC0–12 between days 14 and 42 was 57% (P = 0.004). The increase was also significant between days 15 and 42 (P = 0.006) but not between days 14 and 15 (P = 0.795).
The mean decrease in the clarithromycin Cmax between days 14 and 42 was 41% (P = 0.003), and a significant decrease was seen in the mean Cmax estimates between days 15 and 42 (P < 0.008). The mean increase in the Cmax of the 14-OH-clarithromycin metabolite between days 14 and 42 was 69% (P = 0.001). No statistically significant changes were seen in the Tmax of clarithromycin or the 14-OH-clarithromycin metabolite among any of the three time points.
Effect of clarithromycin on rifabutin pharmacokinetics.
One regimen B subject who received combination therapy prematurely withdrew from the study due to an adverse event, and data for that subject were not used in the pharmacokinetic analysis. The AUC0–24, Cmax, and Tmax values for rifabutin and 25-O-desacetyl rifabutin on days 14, 15, and 42 for regimen B subjects are presented in Table 4. Figure 2 displays the AUCs of rifabutin (Fig. 2a) and 25-O-desacetyl rifabutin (Fig. 2b) for each of the three sampling periods.
TABLE 4.
Pharmacokinetic parameters of rifabutin and 25-O-desacetyl-rifabutin for 14 subjects receiving regimen Ba
| Drug | AUC0–τ (μg · h/ml)
|
Mean % change in AUC0–τ between days 14 and 42 (P value) |
Cmax (μg/ml)
|
Mean % change in Cmax between days 14 and 42 (P value) |
Tmax (h)
|
Mean % change in Tmax between days 14 and 42 (P value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 14 | Day 15 | Day 42 | Day 14 | Day 15 | Day 42 | Day 14 | Day 15 | Day 42 | ||||
| Rifabutin | 4.0 ± 1.5 | 5.9 ± 1.9 | 7.1 ± 2.6 | 99 ± 89 (0.001) | 0.43 ± 0.20 | 0.94 ± 1.1 | 0.60 ± 0.20 | 69 ± 87 (0.011) | 2.4 ± 0.7 | 3.2 ± 1.1 | 3.9 ± 1.7 | 80 ± 104 (0.013) |
| 25-O-Desacetyl-rifabutin | 0.59 ± 0.44 | 1.1 ± 0.56 | 2.0 ± 1.1 | 375 ± 326 (<0.001) | 0.06 ± 0.03 | 0.09 ± 0.04 | 0.11 ± 0.07 | 237 ± 374 (0.034) | 2.9 ± 1.7 | 4.9 ± 3.1 | 4.7 ± 1.9 | 108 ± 147 (0.017) |
| Clarithromycin | 13.3 ± 5.5b | 21.6 ± 9.0 | 3.2 ± 1.8 | 3.2 ± 1.0 | 2.3 ± 1.4 | 2.2 ± 0.8 | ||||||
| 14-OH-Clarithromycin | 12.4 ± 4.1b | 14.5 ± 2.5 | 1.7 ± 1.1 | 1.7 ± 0.4 | 3.0 ± 3.0 | 2.2 ± 1.3 | ||||||
Study day 14 is steady state for rifabutin alone, day 15 is the first day that clarithromycin was added to the regimen, and day 42 is steady state for both drugs. All values are means ± standard deviations.
The AUC reported for clarithromycin and its metabolite on day 15 represents AUC0–∞.
FIG. 2.
Composite curves of mean rifabutin concentrations (a) and 25-O-desacetyl rifabutin concentrations (b) for regimen B subjects during the three pharmacokinetic sampling periods. Regimen B was rifabutin, 300 mg daily during days 1 to 14 and then the same rifabutin dose with clarithromycin, 500 mg every 12 h during days 15 to 42.
The mean increase in the rifabutin AUC0–24 between days 14 and 42 was 99% (P = 0.001) (Table 4). Most of this change occurred between days 14 and 15 (P < 0.001), while the change between days 15 and 42 was not significant (P = 0.152). The individual rifabutin AUC0–24 estimates increased 19 to 266% between days 14 and 42 for all except one subject; the AUC0–24 for one subject decreased by 39%. The mean increase in the 25-O-desacetyl rifabutin AUC0–24 between day 14 and day 42 was 375% (P < 0.001). This increase was also significant between days 14 and 15 (P = 0.003) and between days 15 and 42 (P = 0.002).
The mean increase in the rifabutin Cmax between days 14 to 42 was 69% (P = 0.011). The changes in Cmax between days 14 and 15 and between days 15 and 42 were not significant (P ≥ 0.20) because of the large variability in the Cmax estimate at day 15 (Table 4). The mean increase in the Cmax for the rifabutin metabolite between days 14 to 42 was statistically significant (P = 0.034). As also shown in Table 4, the mean percent change in the rifabutin Tmax showed a significant increase between days 14 and 42 (P = 0.013). The mean percent change in the 25-O-desacetyl rifabutin Tmax also increased significantly between days 14 and 42 (P = 0.017).
Concomitant medications.
Ten subjects (five subjects from each regimen) received an azole antifungal agent (fluconazole, n = 8; ketoconazole, n = 2) for some period of time during their participation in the study. For regimen A, the mean clarithromycin AUCs at day 14 were 37.1 and 36.1 μg · h/ml for the subjects receiving azoles (n = 4) and those not receiving azoles (n = 7), respectively. At day 42, the mean clarithromycin AUCs were 17.8 and 15.6 μg · h/ml for the azole recipients (n = 5) and those not receiving azoles (n = 6), respectively. For regimen B, the mean rifabutin AUCs at day 14 were 3.8 and 4.4 μg · h/ml for the subjects receiving azoles (n = 4) and those not receiving azoles (n = 10), respectively. At day 42, the mean rifabutin AUCs for the azole recipients (n = 5) and those not receiving azoles (n = 9) were 6.9 and 7.2 μg · h/ml, respectively. For both regimens, these mean study drug AUCs for patients receiving azoles compared to those for patients not receiving azoles at both day 14 and day 42 were not significantly different. The regimen A subject with an atypical AUC response was receiving fluconazole throughout the study, and the regimen B subject with an atypical AUC response did not receive any azole therapy. Other concomitant medications included zidovidine (23 subjects), ddI (14 subjects), dapsone (11 subjects), ddC (4 subjects), and trimethoprim-sulfamethoxazole (7 subjects).
DISCUSSION
In 1992, plans for large randomized clinical trials were developed to optimize MAC prophylaxis by comparing the most promising new therapies. One of these trials, trial ACTG 196/CPCRA 009, was designed to compare clarithromycin, rifabutin, and both drugs in combination. The combination arm was included to determine if effectiveness could be increased by preventing clarithromycin resistance, because relapses of MAC bacteremia with macrolide-resistant strains occurred frequently during a trial of clarithromycin alone for the treatment of disseminated MAC disease (8). The tolerance of this combination in the target population and the possibility of bidirectional pharmacokinetic drug interactions were important issues to be considered in the design and conduct of the ACTG/CPCRA phase III prophylaxis trial. Trial DATRI 001A was designed to address these questions directly with a population of subjects with late-stage HIV infection.
The outcome of this study validated the hypothesis that >67% of the volunteers could tolerate the combination of clarithromycin and rifabutin over a total of 28 days. Only 2 (7%) of the 29 subjects receiving combination therapy discontinued the study drugs due to toxicities, but adverse events were frequently reported. Nineteen subjects (66%) reported GI complaints of nausea, vomiting, and/or diarrhea during the combination-therapy period. Given the relatively short period of evaluation, the combination therapy may be much less well tolerated over the longer term. Also, while these adverse events were not serious and may be tolerated during treatment of disseminated MAC infection, they may be less often tolerated during prophylaxis.
The single-drug pharmacokinetic profiles are consistent with those from previous studies of HIV-negative and HIV-infected subjects receiving single-agent therapy (9, 38, 42). Both pharmacokinetic interactions demonstrated in this study were extensive, with the mean day 42 clarithromycin AUC0–12 decreasing by 44% and the mean rifabutin AUC0–24 increasing by 99% (P values for both comparisons, ≤0.003). These two-way pharmacokinetic interactions between clarithromycin and rifabutin are consistent with the ability of rifabutin to induce clarithromycin metabolism and, conversely, the inhibitory effect of clarithromycin on rifabutin metabolism. The observed time course for the clarithromycin AUC0–12 reduction, which was not appreciable at day 15 but which was highly significant at day 42, is consistent with cytochrome P-450 enzyme induction by rifabutin. The increase in the 14-OH-clarithromycin AUC0–12 was also only apparent at day 42, further supporting an enzyme induction mechanism. Rifabutin levels increased significantly following the administration of a single clarithromycin dose, and most of the change between days 14 and 42 (60%) occurred between days 14 and 15. This finding is consistent with clarithromycin inhibition of enzyme metabolism. The identity of the responsible enzymes is in question. While evidence from one in vitro study indicates that CYP3A4 has a major role (20), other evidence indicates that rifabutin is not metabolized by CYP3A4 (39).
The mean percent increases in the 25-O-desacetyl-rifabutin metabolite AUC0–24 observed between days 14 and 42 were greater than the mean increases in the rifabutin AUC0–24 over the same time periods, suggesting that the enzyme responsible for conversion of rifabutin to the 25-O-desacetyl metabolite is not inhibited. In addition, this finding also suggests that clarithromycin inhibits conversion of the parent compound to alternative primary metabolites and the further metabolism of 25-O-desacetyl-rifabutin (for example, by inhibiting conversion of both the parent and 25-O-desacetyl forms to their 31-hydroxy metabolites) (11).
The data indicating decreased clarithromycin levels at day 42 were consistent among all except one of the regimen A subjects; for one subject the clarithromycin AUC0–12 had increased by 57% at day 42. The AUC change for this subject cannot be explained by concomitant medications (i.e., fluconazole use predated study entry), but additional doses of clarithromycin may have been taken. An increase in rifabutin levels at day 42 was a consistent finding for all regimen B subjects except one subject for whom the AUC decreased by 39%. The day 14, day 21, and day 42 trough levels for this subject were 0.144 μg/ml 0.341 μg/ml, and undetectable (<0.050 μg/ml), respectively, suggesting decreased compliance with the study drug regimen prior to the final pharmacokinetic sampling session. Both subjects with atypical AUC changes reported excellent compliance. These outlying results lessen the magnitude of the pharmacokinetic interaction estimates and suggest that the observed mean changes in AUC0–τ are conservative estimates. The day 42 AUC values for both the drugs and the measured metabolites were not significantly different when the AUCs for subjects receiving regimen A (clarithromycin initiated first) and those receiving regimen B (rifabutin initiated first) were compared. This indicates that steady-state values had been reached by day 42 and that the order of addition of these drugs did not affect their pharmacokinetic disposition at steady state (all P values were ≥0.120); i.e., no period effect was apparent.
There have been several reports of uveitis occurring in patients receiving rifabutin, usually at dosages of ≥450 mg/day and often in combination with clarithromycin (13, 14, 30, 35, 36). Preliminary data from trial ACTG 196/CPCRA 009, a randomized comparison of clarithromycin, rifabutin, and the combination for prophylaxis of MAC infection, support a specific association of uveitis with this drug combination. In the preliminary analysis of 1,178 eligible patients, uveitis occurred in 23 patients initially assigned to the combination of clarithromycin (500 mg twice daily) and rifabutin (450 mg daily, which was later reduced to 300 mg daily) and in 5 patients assigned to rifabutin and 2 patients assigned to clarithromycin at the same doses (13). No episodes of uveitis were reported for volunteers in the present study, but uveitis has been most commonly reported after receiving at least 4 weeks of combination therapy and appears to be very infrequent in patients receiving rifabutin at dosages of ≤300 mg/day in combination with clarithromycin.
The exact mechanism of this adverse event is unknown (30). The possible relationship between the concentrations of the parent compound and its metabolite in plasma and adverse reactions, particularly uveitis and polyarthritis, require further investigation. Previously, uveitis had been reported in subjects receiving ≥1,800 mg of rifabutin alone in a dose-escalating tolerance and pharmacokinetic study (36), suggesting that uveitis may be related to high concentrations of rifabutin or its metabolites in plasma. If this hypothesis is correct, the increased levels of rifabutin and its 25-O-desacetyl metabolite occurring in the presence of clarithromycin, as documented by this study, could explain the increased risk of uveitis associated with combination therapy. Alternatively, but less likely, uveitis may be due to increases in the levels of other rifabutin metabolites produced as a consequence of this pharmacokinetic interaction or enhancement of a toxic rifabutin effect by clarithromycin or its metabolites.
Only a small minority of the patients who receive combination therapy including rifabutin at dosages of less than 600 mg and clarithromycin develop uveitis. Considerable intersubject variability was noted in this study. The increase in the rifabutin AUC0–24 was greater than 150% for 5 of the 14 regimen B subjects, and for 2 of those the increase was greater than 200%. Increases in the 25-O-desacetyl rifabutin AUC0–24 were greater than 600% for three subjects. The patients experiencing the most substantial increases in rifabutin and 25-O-desacetyl rifabutin AUC0–24 values during combination therapy may be at the highest risk for uveitis.
Antifungal azoles are a class of drugs likely to have significant pharmacokinetic interactions with rifabutin and clarithromycin. A pharmacokinetic interaction between rifabutin and fluconazole has been reported (40). While rifabutin levels may be significantly increased in the presence of fluconazole, a specific relationship between the concomitant use of azoles and rifabutin and an increased risk of uveitis in the absence of clarithromycin has not been established. It also remains to be determined if the inhibitory effects of azoles and clarithromycin on rifabutin metabolism are additive. The data for the study volunteers who received azoles do not appear to differ from those for the remaining subjects, but a formal assessment of any additive effect was not possible. Whether concurrent therapy with fluconazole and other azoles would also alter the plasma concentration-time profile of clarithromycin or its metabolites requires further study, given the frequent use of systemic antifungal therapy in this population.
The decreased levels of clarithromycin could diminish the expected clinical efficacy of combination therapy, because in vitro studies supporting the increased activity of this combination did not take into account the observed decrease in clarithromycin concentrations. If the decrease in clarithromycin levels results in diminished clinical antimycobacterial activity, the concurrent increase in the levels of the 14-OH-clarithromycin metabolite will not provide significant compensatory activity. The mean increase in the metabolite AUC0–12 was 4.3 mg · h/ml, while the mean decrease in the clarithromycin AUC0–12 was 19.9 mg · h/ml. Also, the 14-hydroxy metabolite is approximately eightfold less active than the parent compound against MAC in vitro (19). To compensate for this interaction, the dosage of clarithromycin could be increased when it is used in combination with rifabutin. However, a higher clarithromycin dosage could exacerbate GI intolerance and may cause a further increase in the concentrations of rifabutin and its metabolites, possibly resulting in a higher incidence of uveitis. Furthermore, in treatment studies comparing the standard dosage of clarithromycin (500 mg twice daily) with higher dosages of clarithromycin (either alone or in combination regimens) for the treatment of disseminated MAC, patients assigned to the higher-dose treatment arms had poorer survival rates (8, 12). It is impossible to determine at this time whether the pharmacokinetic interaction between clarithromycin and rifabutin would have an effect on the poorer survival rate. Until this is further defined, clarithromycin either alone or in combination with rifabutin should not be administered at a dosage of greater than 500 mg twice daily for the treatment of MAC infection.
This study does not support the use of clarithromycin and rifabutin in combination for MAC prophylaxis. Combination of these drugs results in a high frequency of adverse events and bidirectional pharmacokinetic interactions. The results of the ACTG 196/CPCRA 009 study comparing clarithromycin, rifabutin, and the combination for MAC prophylaxis indicated that the use of the combination did not increase efficacy and caused more toxicity compared to the use of clarithromycin alone (13). The reduction of plasma clarithromycin levels due to pharmacokinetic interaction with rifabutin is a possible explanation for the lack of additional efficacy for this drug combination. In contrast, a large randomized study comparing rifabutin (300 mg/day), azithromycin (1,200 mg/week), and the combination of both demonstrated that the azithromycin-containing regimens were more effective than those containing rifabutin alone and that the combination regimen was significantly more effective than azithromycin alone for the prevention of disseminated MAC infection (16). One explanation for the enhanced effectiveness of azithromycin and rifabutin in combination may be that azithromycin does not undergo metabolism and therefore rifabutin does not reduce azithromycin levels (1, 29). The investigators cautioned that use of the azithromycin and rifabutin combination may be limited by its significantly poorer tolerance and increased cost and rifabutin’s pharmacokinetic interactions with medications commonly used concomitantly (16). Considering the results of these trials, monotherapy either with clarithromycin or with azithromycin once weekly is recommended as first choice for MAC prophylaxis (7).
The introduction of protease inhibitors for HIV therapy has led to additional concerns regarding the choice of drugs for MAC prophylaxis. Rifabutin increases the metabolism of protease inhibitors (21), protease inhibitors significantly inhibit rifabutin metabolism (6), and with concomitant use, adjustment of the rifabutin dosage is necessary to prevent increased adverse events (7, 21). While protease inhibitors inhibit the metabolism of clarithromycin, the clinical significance of this interaction is unknown, and dose adjustment is not advised (7, 33). Because azithromycin is not significantly metabolized and is not known to inhibit the metabolism of other drugs, pharmacokinetic interactions with the protease inhibitors are unlikely (1, 29). The occurrence of clinically significant pharmacokinetic interactions between rifabutin and protease inhibitors further support the use of clarithromycin or azithromycin alone for MAC prophylaxis.
ACKNOWLEDGMENTS
This study was supported by the Division of AIDS Treatment Research Initiative Program, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md (contract NO1-AI-15123).
We thank the volunteers for participating in this study; Carol Braun Trapnell (Food and Drug Administration, Rockville, Md.), P. K. Narang (Pharmacia & Upjohn), and Sheri Crampton (Abbott Laboratories) for helpful review and comments during the conduct and analysis of the study; Beverly B. Barber (Denver Disease Control Service and University of Colorado Health Sciences Center, Denver) and Stephanie LaCarruba (Davies Medical Center, San Francisco, Calif.) for clinical support in conducting this study; Angela Shaver (McKesson Bioservices, Rockville, Md.) for managing the investigational drug supplies; Suzanne Beckner (Westat, Rockville, Md.); and Jean King, Theresa Straut, and Mary Enama (Social & Scientific Systems, Inc., Rockville, Md.) for assisting in manuscript preparation.
Footnotes
Study DATRI 001 of the Division of AIDS Treatment Research Initiative.
REFERENCES
- 1.Amsden G W. Macrolides versus azalides: a drug interaction update. Ann Pharmacother. 1995;29:906–917. doi: 10.1177/106002809502900913. [DOI] [PubMed] [Google Scholar]
- 2.Barradell L B, Plosker G L, McTabish D. Clarithromycin, a review of its pharmacological properties and therapeutic use in Mycobacterium avium-intracellulare complex infection in patients with acquired immune deficiency syndrome. Drugs. 1993;46:289–312. doi: 10.2165/00003495-199346020-00007. [DOI] [PubMed] [Google Scholar]
- 3.Benson C. Disseminated Mycobacterium avium complex disease in patients with AIDS. AIDS Res Hum Retroviruses. 1994;10:913–916. doi: 10.1089/aid.1994.10.913. [DOI] [PubMed] [Google Scholar]
- 4.Benson C A, Ellner J J. Mycobacterium avium complex infection and AIDS: advances in theory and practice. Clin Infect Dis. 1993;17:7–20. doi: 10.1093/clinids/17.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Brogden R N, Fitton A. Rifabutin: a review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1994;47:983–1009. doi: 10.2165/00003495-199447060-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cato A, Cavanaugh J H, Shi H, Hsu A, Granneman G R, Leonard J. XI International Conference on AIDS. 1996. Assessment of multiple dose of ritonavir on the pharmacokinetics of rifabutin, abstr. Mo.B1199. [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1997 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons with human immunodeficiency virus. Morbid Mortal Weekly Rep. 1997;46:12–13. [Google Scholar]
- 8.Chaisson R E, Benson C A, Dube M P, Heifets L B, Korvick J A, Elkin S, Smith T, Craft J C, Sattler F R the AIDS Clinical Trials Group Protocol 157 Study Team. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease. Ann Intern Med. 1994;121:905–911. doi: 10.7326/0003-4819-121-12-199412150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Chu S, Wilson D S, Deaton R L, Mackenthum A V, Eason C N, Cavanaugh J H. Single- and multiple-dose pharmacokinetics of clarithromycin, a new macrolide antimicrobial. J Clin Pharmacol. 1993;33:719–726. doi: 10.1002/j.1552-4604.1993.tb05613.x. [DOI] [PubMed] [Google Scholar]
- 10.Chu S Y, Sennello L T, Sonders R C. Simultaneous determinations of clarithromycin and 14(R)-hydroxyclarithromycin in plasma and urine using high performance liquid chromatography with electrochemical detection. J Chromatogr. 1991;571:199–208. doi: 10.1016/0378-4347(91)80446-j. [DOI] [PubMed] [Google Scholar]
- 11.Cocchiara G, Strolin B M, Vicario G P, Ballabio M, Gioia B, Vioglio S, Vigevani A. Urinary metabolites of rifabutin, a new antimycobacterium agent, in human volunteers. Xenobiotica. 1989;19:769–780. doi: 10.3109/00498258909042314. [DOI] [PubMed] [Google Scholar]
- 12.Cohn D, Fischer E, Franchino B, Peng G, Hodges J, Chesnut J, Child C, Gilbert C, El-Sadr W, Hafner R, Ropka M, Heifets L, Clotfelter J, Munroe D, Caldwell R, Horsburgh R the CPCRA 027 Protocol Team. 4th Conference on Retroviruses and Opportunistic Infections. 1997. A prospective, randomized trial of four 3-drug regimens for treatment (Rx) of disseminated MAC disease in AIDS (DM): excess mortality associated with high-dose clarithromycin, abstr. 659. [Google Scholar]
- 13.Cohn D L, Benson C A, Williams P, Nevin P T, Korvick J, Hafner R, Bourland D, Kopek E, Becker S, Hojczyk P, Timmons P, Child C. XI International Conference on AIDS. 1996. A prospective, randomized, double-blind, comparative study of the safety and efficacy of clarithromycin (CLA) vs rifabutin (RBT) vs the combination for the prevention of mycobacterium avium complex (MAC) bacteremia or disseminated MAC disease (DMAC) in HIV-infected patients (pts) with CD4 counts less than or equal to 100 cells/mm3, abstr. We.B.421. [Google Scholar]
- 14.Frank M O, Graham M B, Wispelway B. Rifabutin and uveitis. N Engl J Med. 1994;330:868. doi: 10.1056/NEJM199403243301218. [DOI] [PubMed] [Google Scholar]
- 15.Gustavson L E, Blahunkg K S, Witt G F, Harris S I, Palmer R N. Evaluation of the pharmacokinetic drug interaction between terfenadine and clarithromycin. Pharm Res. 1993;10:S311. . (Abstract.) [Google Scholar]
- 16.Havlir D V, Dube M P, Sattler F R, Forthal D N, Kemper C A, Dunne M W, Parenti D M, Lavelle J P, White A C, Jr, Witt M D, Bozzette S A, McCutchan J A for the California Collaborative Treatment Group. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. N Engl J Med. 1996;335:392–398. doi: 10.1056/NEJM199608083350604. [DOI] [PubMed] [Google Scholar]
- 17.Heifets L B. Clarithromycin against Mycobacterium avium complex infections. Clarithromycin against Mycobacterium avium complex infections. Tubercle Lung Dis. 1996;77:19–26. doi: 10.1016/s0962-8479(96)90070-2. [DOI] [PubMed] [Google Scholar]
- 18.Heifets L B, Iseman M D, Lindholm-Levy P J, Kanes W. Determination of ansamycin MICs for Mycobacterium avium complex in liquid medium by radiometric and conventional methods. Antimicrob Agents Chemother. 1985;28:570–575. doi: 10.1128/aac.28.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heifets L B, Lindholm-Levy P J, Comstock R D. Clarithromycin minimal inhibitory and bactericidal concentrations against Mycobacterium avium. Am Rev Respir Dis. 1992;145:856–858. doi: 10.1164/ajrccm/145.4_Pt_1.856. [DOI] [PubMed] [Google Scholar]
- 20.Iatsimirskaia E, Tulebaev S, Storozhuk E, Utkin I, Smith D, Gerber N, Koudriakova T. Metabolism of rifabutin in human enterocyte and liver microsomes: kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents. Clin Pharmacol Ther. 1997;61:554–562. doi: 10.1016/S0009-9236(97)90135-1. [DOI] [PubMed] [Google Scholar]
- 21.Indinavir Pharmacokinetics Study Group. XI International Conference on AIDS. 1996. Indinavir (MK639) drug interaction studies, abstr Mo.B174. [Google Scholar]
- 22.Jurima-Romet M, Crawford K, Cyr T, Inaba T. Terfenadine metabolism in human liver. In vitro inhibition by macrolide antibiotics and azole antifungals. Drug Metab Dispos. 1994;22:849–857. [PubMed] [Google Scholar]
- 23.Kent R J, Bakhtiar M, Shanson D C. The in-vitro bactericidal activities of combinations of antimicrobial agents against clinical isolates of Mycobacterium avium-intracellulare. J Antimicrob Chemother. 1992;30:643–650. doi: 10.1093/jac/30.5.643. [DOI] [PubMed] [Google Scholar]
- 24.Klemens S P, DeStefano M S, Cynamon M H. Activity of clarithromycin against Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1992;36:2413–2417. doi: 10.1128/aac.36.11.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis R C, Hatfield N Z, Narang P K. A sensitive method for quantitation of rifabutin and its desacetyl metabolite in human biological fluids by high-performance liquid chromatography (HPLC) Pharm Res. 1991;8:1434–1440. doi: 10.1023/a:1015865526655. [DOI] [PubMed] [Google Scholar]
- 26.Mascellino M T, Iona E, Fattorini L, De Gregoris P, Hu C Q, Santoro C, Orefici G. In vitro activity of clarithromycin alone or in combination with other antimicrobial agents against Mycobacterium avium-intracellulare. Complex strains isolated from AIDS patients. J Chemother. 1991;3:357–362. doi: 10.1080/1120009x.1991.11739120. [DOI] [PubMed] [Google Scholar]
- 27.Mor N, Vanderkolk J, Mezo N, Heifets L. Effects of clarithromycin and rifabutin alone and in combination on intracellular and extracellular replication of Mycobacterium avium. Antimicrob Agents Chemother. 1994;38:2738–2742. doi: 10.1128/aac.38.12.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moro E, Benedetti M F, Fanfani A, Jannuzzo M G. Fifth International Congress of Toxicology. 1989. Autoinduction of rifabutin metabolism in man, abstr. [Google Scholar]
- 29.Nahata, M. 1996. Drug interactions with azithromycin and the macrolides: an overview. J. Antimicrob. Chemother. 37(Suppl. C):133–142. [DOI] [PubMed]
- 30.Nichols C W. Mycobacterium avium complex infection, rifabutin, and uveitis—is there a connection? Clin Infect Dis. 1996;22:S43–S47. doi: 10.1093/clinids/22.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 31.Nightingale S D, Cameron D W, Gordin F M, Sullam P M, Cohn D L, Chaisson R E, Eron L E, Sparti P D, Bihari B, Kaufman D L, Stern J J, Pearce D D, Weinberg W G, LaMarca A, Siegal F P. Two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993;329:828–833. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- 32.Perruca E, Grimaldi R, Frigo G M, Sardi A, Mönig Ohnhaus E E. Comparative effects of rifabutin and rifampicin on hepatic microsomal enzyme activity in normal subjects. Eur J Clin Pharmacol. 1988;34:595–599. doi: 10.1007/BF00615223. [DOI] [PubMed] [Google Scholar]
- 33.Quellet D, Hsu A, Granneman G R, et al. Assessment of the pharmacokinetic interaction between ritonavir and clarithromycin. Clin Pharmacol Ther. 1996;59:143. doi: 10.1016/S0009-9236(98)90065-0. . (Abstract PI-58.) [DOI] [PubMed] [Google Scholar]
- 34.Rocci M L, Jusko W J. LAGRAN program for area and moments in pharmacokinetic analysis. Comput Program Biomed. 1983;16:203–216. doi: 10.1016/0010-468x(83)90082-x. [DOI] [PubMed] [Google Scholar]
- 35.Shafran S D, Deschenes J, Miller M, Phillips P, Toma E. Uveitis and pseudojaundice during a regimen of clarithromycin, rifabutin, and ethambutol. N Engl J Med. 1994;330:438–439. doi: 10.1056/NEJM199402103300616. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 36.Siegal F P, Eilbott D, Burger H, Gehan K, Davidson B, Kaell A T, Weiser B. Dose-limiting toxicity of rifabutin in AIDS-related complex: syndrome of arthralgia/arthritis. AIDS. 1990;4:433–441. doi: 10.1097/00002030-199005000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Skinner M H, Blaschke T F. Clinical pharmacokinetics of rifabutin. Clin Pharmacokinet. 1995;28:115–124. doi: 10.2165/00003088-199528020-00003. [DOI] [PubMed] [Google Scholar]
- 38.Skinner M H, Hsieh M, Torseth J, Pauloin D, Bhatia G, Harkonen S, Merigan T C, Blaschke T F. Pharmacokinetics of rifabutin. Antimicrob Agents Chemother. 1989;33:1237–1241. doi: 10.1128/aac.33.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trapnell C B, Jamis-Dow C, Klecker R W, Collins J M. Metabolism of rifabutin and its 25-desacetyl metabolite, LM565, by human liver microsomes and recombinant human cytochrome P-450 3A4: relevance to clinical interaction with fluconazole. Antimicrob Agents Chemother. 1997;41:924–926. doi: 10.1128/aac.41.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trapnell C B, Narang P K, Li R, Lavelle J P. Increased plasma rifabutin levels with concomitant fluconazole in HIV-infected patients. Ann Intern Med. 1996;124:573–576. doi: 10.7326/0003-4819-124-6-199603150-00006. [DOI] [PubMed] [Google Scholar]
- 41.Unhgeri D, Franceschi G, Della Bruna C. Main human urinary metabolites of spiropiperidyl rifamycin LM 427: isolation and biological properties. In: Ishigami J, editor. Proceedings of the 14th International Congress of Chemotherapy. Tokyo: University of Tokyo Press; 1985. pp. 1917–1918. [Google Scholar]
- 42.Vance E, Watson-Bitar M, Gustavson L, Kazanjian P. Pharmacokintetics of clarithromycin and zidovudine in patients with AIDS. Antimicrob Agents Chemother. 1995;39:1355–1360. doi: 10.1128/aac.39.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace R J, Jr, Brown B A, Griffith D E, Girard W, Tanaka K. Reduced serum levels of clarithromycin in patients treated with multidrug regimens including rifampin or rifabutin for Mycobacterium avium-M. intracellulare infection. J Infect Dis. 1995;171:747–750. doi: 10.1093/infdis/171.3.747. [DOI] [PubMed] [Google Scholar]