Abstract
Background
The variability in the effectiveness of type 2 diabetes (T2D) preventive interventions highlights the potential to identify the factors that determine treatment responses and those that would benefit the most from a given intervention. We conducted a systematic review to synthesize the evidence to support whether sociodemographic, clinical, behavioral, and molecular factors modify the efficacy of dietary or lifestyle interventions to prevent T2D.
Methods
We searched MEDLINE, Embase, and Cochrane databases for studies reporting on the effect of a lifestyle, dietary pattern, or dietary supplement interventions on the incidence of T2D and reporting the results stratified by any effect modifier. We extracted relevant statistical findings and qualitatively synthesized the evidence for each modifier based on the direction of findings reported in available studies. We used the Diabetes Canada Clinical Practice Scale to assess the certainty of the evidence for a given effect modifier.
Results
The 81 publications that met our criteria for inclusion are from 33 unique trials. The evidence is low to very low to attribute variability in intervention effectiveness to individual characteristics such as age, sex, BMI, race/ethnicity, socioeconomic status, baseline behavioral factors, or genetic predisposition.
Conclusions
We report evidence, albeit low certainty, that those with poorer health status, particularly those with prediabetes at baseline, tend to benefit more from T2D prevention strategies compared to healthier counterparts. Our synthesis highlights the need for purposefully designed clinical trials to inform whether individual factors influence the success of T2D prevention strategies.
Subject terms: Disease prevention, Diabetes, Clinical trials
Plain language summary
Clinical trials to prevent development of type 2 diabetes (T2D) that test dietary and lifestyle interventions have resulted in different results for different study participants. We hypothesized that the differing responses could be because of different personal, social and inherited factors. We searched different databases containing details of published research studies investigating this to look at the effect of these factors on prevention of the development of T2D. We found a small amount of evidence suggesting that those with poorer health, particularly those with a higher amount of sugar in their blood, tend to benefit more from T2D prevention strategies compared to healthier counterparts. Our results suggest that further clinical trials that are designed to examine the effect of personal and social factors on interventions for T2D prevention are needed to better determine the impact of these factors on the success of diet and lifestyle interventions for T2D.
Bodhini et al. systematically review the evidence on sociodemographic, clinical, behavioral, and molecular factors that modify the effect of interventions for type 2 diabetes prevention. The certainty of evidence that such factors modify the effectiveness of lifestyle and behavioral interventions is low to very low.
Introduction
Diabetes affects over 530 million people worldwide1. Around 90% of all diabetes is estimated to be type 2 diabetes (T2D), a non-autoimmune condition with marked pathophysiological heterogeneity2. In many cases, diet and physical activity interventions targeted at bodyweight reduction or preventing weight gain have demonstrated to delay progression3–6, yet T2D remains a major cause of morbidity and mortality globally7. Chronic inadequate control of hyperglycemia causes downstream microvascular and macrovascular complications that drive the costly and debilitating T2D public health burden7. Coupled with its increasing incidence, public health and clinical efforts need to optimize effective upstream strategies for T2D prevention.
Landmark randomized intervention trials have demonstrated the effectiveness of intensive lifestyle interventions and glucose-lowering drug therapies for delaying the onset of T2D in patients at high risk3–6. However, T2D incidence has only escalated in the decades since, despite the success of early clinical trials. Thus, implementation strategies for diabetes prevention in the real-world setting involving more practical ways of identifying high-risk individuals and precision prevention research may contribute to understanding this gap8.
Precision prevention of T2D serves to minimize an individual’s T2D risk factor profile and maximize the effectiveness of new or established strategies for disease prevention through targeting biological interactions and/or removing barriers to access and adherence to lifestyle modification9. For example, precision prevention approaches might use clinical (e.g., age, sex, body mass index [BMI]), social (e.g., education attainment, socioeconomic status), or molecular (e.g., genetic, ‘omic’ traits) characteristics to inform strategies likely to elicit the most effective or sustainable response for an individual, resulting in tailored prevention strategies9–11.
The purpose of this systematic review is to critically appraise the accumulated experimental evidence underpinning the feasibility and effectiveness of the clinical translation of precision prevention of T2D. The scope of our investigation included studies reporting the effect modification of lifestyle and dietary interventions for T2D prevention by any of the following individual-level factors, including sociodemographics, clinical risk factors, behavior, or molecular traits. This work was undertaken as part of a series of systematic reviews conducted by the ADA/EASD Precision Medicine in Diabetes Initiative12, an international collaboration of global leaders in precision diabetes medicine13.
Through this systematic review, we found low certainty evidence that those with poorer health status, particularly those with prediabetes at baseline, tend to benefit more from T2D prevention strategies compared to healthier counterparts. Clinical trials specifically designed to inform whether individual factors influence the success of T2D prevention strategies are needed in the future.
Methods
The systematic review protocol was pre-registered on the International Prospective Register of Systematic Reviews (PROSPERO; CRD42021267686).
Data sources and search
Our search included MEDLINE, Embase, and Cochrane Central Register of Controlled Trials databases for studies reporting on the efficacy of lifestyle or behavioral interventions with T2D incidence, published from 1/1/2000 to 7/15/2021. Lifestyle interventions were defined as interventions ranging from interventions on single behavioral factors including diet, physical activity, smoking, and body weight loss, to multi-component modification programs focused on different behavioral components. An experienced librarian developed a search strategy (Supplementary Note 1), which included combinations of keywords related to lifestyle intervention for preventing T2D (diet, lifestyle, physical activity, body weight), study design, and health outcome, and was limited to the English language. We also scanned the references of included manuscripts and the reference list of systematic reviews published within the past 2 years to identify additional relevant studies.
Study selection
We included studies reporting the effect of a lifestyle, dietary pattern, or dietary supplement interventions vs. other active comparators or control on the incidence of T2D and reporting the results stratified by any eligible factor. Lifestyle interventions included either single-component (exercise, smoking, education through text messaging to the mobile phone, etc) or multi-component modification programs involving weight loss through diet or supplementation, physical activity, awareness education etc. Eligible stratification factors, or effect modifiers, included individual-level sociodemographic (i.e., race/ethnicity, socioeconomic status/education, location, age, sex), clinical factors (i.e., BMI, dysglycemia, presence of comorbidities), behavioral (i.e., baseline diet, physical activity) or molecular traits (i.e., genetics, metabolites). We did not review population-level exposures such as built environment, pollution, or climate. Off-label pharmaceutical interventions and bariatric surgery were beyond the scope of the review. We limited inclusion to studies in adults aged >18 years and enrolling at least 100. We included non-randomized and randomized clinical studies delivering an eligible intervention, comparing against another active intervention, usual care, placebo control, or non-control group. The majority of studies (N = 76 or 94%) included in this review are RCTs to examine the effect on the intervention on T2D incidence. However, as our focus is on the modification of the intervention effect by sociodemographic, clinical, behavioral and molecular factors, none of these trials can be considered randomized for the purpose of this review, as the randomization block is not conserved. Studies exclusively among individuals with a current or history of gestational diabetes were excluded because they overlapped in scope with another PMDI consortium review.
Screening, data extraction, and quality assessment
We used the Covidence online systematic review platform14 for literature screening, data extraction, and consensus. Screening consisted of two stages: (1) title and abstract and (2) full text. At each screening stage, two independent reviewers determined the eligibility of the citation, and in the case of disagreement, a third reviewer resolved the discrepancy. Among the full papers accepted for inclusion in the review, two independent reviewers extracted detailed information on the study design, participant characteristics, interventions, comparators, effect modifiers, follow-up for T2D, and analytic approach. We extracted findings related to the effect modification of treatment vs. comparator on T2D risk, including strata-specific treatment groups’ T2D cases and incidence rates, or strata-specific treatment-comparator incidence rate ratios, relative risks, risk differences, etc., including measures of variance. We also extracted data on different available measurements for the interaction of the effect modifier with the intervention effect on T2D, including interaction term estimates, interaction term p-value, stratified estimates, heterogeneity test and noted any text referring to tests performed with “data not shown”. We developed and piloted the data extraction template (Supplementary Table 1), and discrepancies were ruled on by a third reviewer. The relevant statistical results extracted for each effect modifier has been provided as Supplementary Data 1.
We evaluated the studies’ risk of bias using a modified JBI Critical Appraisal Checklist for randomized controlled trials15, performed by two independent reviewers and disagreements resolved by a third reviewer. We modified the 13-item checklist to 9 questions tailored to evaluating the quality of the study design but with consideration for our primary interest in stratified results rather than the total intervention effect for T2D risk. These 9 questions were mainly based on randomization, interventions, treatment, and assessor blindness to outcome assessment. Our evaluation corresponded to color coding in a heat map organized by intervention type and effect modifier (Supplementary Fig. 1).
Synthesis of results
We collated the literature according to intervention type as lifestyle intervention programs (single or multi-component), dietary pattern interventions (involving modifications in diet only), or supplement intervention and effect modifier analyzed (e.g., sex, age strata) to synthesize results. We determined that a meta-analysis was not feasible among the studies included in our review due to paucity and marked differences in the nature of the study populations, interventions and comparators, study designs, and effect modifiers analyzed. We qualitatively evaluated the direction and magnitude of results and statistical tests among each prevention strategy for each effect modifier. We weighed these qualitative and quantitative results against their risk of bias. We qualitatively synthesized the evidence for each modifier based on the direction of findings reported in available studies. We used the Diabetes Canada Clinical Practice Scale to assess the certainty of the evidence for a given effect modifier16. A level of evidence was assigned following the approach and criteria described in Supplementary Table 2. For example, higher levels were assigned if the study was a systematic overview or meta-analysis of high-quality RCTs or an appropriately designed RCT with adequate power to answer the question posed by the investigators. Then, each recommendation was assigned a grade from A to D. Two reviewers independently assessed the certainty of the evidence and resolved disagreements through consensus discussion.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
The results of our systematic literature search are presented in the Fig. 1 PRISMA flow diagram. Of the 10,880 citations identified through database searches and other sources, 1047 abstracts were retrieved for full-text review. From these, 81 publications met our inclusion criteria, and data were extracted.
Fig. 1. PRISMA flow diagram.
Stepwise screening stages adapted for selecting the studies of interest using Covidence software. Screening at all stages was done by two independent reviewers, and a third reviewer resolved conflicts.
Study characteristics
The 81 publications included in our review represented 33 unique intervention studies (Table 1 and Supplementary Table 3). Twenty-eight studies were randomized clinical trials (RCTs), three were nonrandomized parallel group trials, and two were single-arm clinical interventions. Fourteen intervention studies took place in Asia, 11 in Europe, seven in North America, and one was a multicenter study that took place in Asia and Europe. Intervention enrollment sample sizes ranged from 302 to 48,835 participants (Table 1). Twenty-two studies included individuals at high risk for T2D, two studies at increased cardiovascular risk, and other studies included the general population or other specific groups. The active intervention times ranged from one lifestyle counseling visit to active interventions lasting up to 10 years (Supplementary Fig. 2).
Table 1.
Description of study population and study design of the included trials grouped according to the type of intervention.
| Study population | Study design | Main trial info PMIDs | Included studies (PMDIs) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial/Study name | Country | Total enrolled; inclusion criteria | Baseline enrollment years | Intervention design | Active intervention duration | Intervention(s) | Comparator/control intervention | ||
| Lifestyle interventions | |||||||||
| Chae et al.27 | South Korea | N = 7233; General population | 2007/11 | Non-randomized, parallel arm | 6 months | Physical activity program | Usual care | 2268854927 | 2268854927 |
| Da Qing IGT and Diabetes Study | China | N = 577; Prediabetes | 1986 | Cluster-randomized trial | 6 years |
(i) Diet: Low-calorie, low-fat (25–30% kcal) healthy pattern; (ii) Increase exercise; (iii) Diet + Exercise |
Provided with diabetes education materials at baseline | 90969775 | 2473167428, 3421246517, 1241377929 |
| Diabetes Community Lifestyle Improvement Program (D-CLIP) | India | N = 578; Prediabetes | 2009/12 | Randomized, parallel arm | 3 years | Lifestyle diabetes prevention program + Metformin | Lifestyle diabetes prevention program at baseline | 2750401418 | 2750401418 |
| Diabetes in Europe—Prevention using Lifestyle, Physical Activity and Nutritional - Catalonia (DE-PLAN-CAT)a | Spain | N = 544; Prediabetes | 2006 | Non-randomized, parallel arm | 4 years | Lifestyle weight loss and diabetes prevention program | Provided with diabetes education materials at baseline | 2232292130 | 2232292130 |
| Diabetes Prevention Program (DPP)a | US | N = 3234; Prediabetes | 1996/99 | Randomized, parallel arm | Mean 2.8 years | (i) Lifestyle weight loss and diabetes prevention program; (ii) Metformin | Usual care + placebo | 118325273 | 2602485131; 3344415832; 3331762933; 2845378034; 1964096035; 2386072236; 1685526437; 1901775138; 1806066039; 118325273; 2351295140; 1707720241; 1987898642; 3339454543; 2902120744; 2137817545; 2068268746; 1736374047; 2569749448; 2527738949 |
| EDIPS-Newcastle | UK | N = 102; Prediabetes | Randomized, parallel arm | 5 years | Lifestyle diabetes prevention program | Usual care | 1975842850 | 2345116651 | |
| Finnish Diabetes Prevention Study (DPS)a | Finland | N = 522; Prediabetes | 1993/98 | Randomized, parallel arm | Mean 3.2 years | Lifestyle and weight loss diabetes prevention program | Provided with diabetes education materials at baseline | 113339904 | 1675931352; 1727758553; 1687369954; 1824921955; 1965191956; 1763611457; 113339904; 2098041258; 1214517459; 1512720360; 1825290061; 1561602462; 1530929263; 1598323064; 1809102365, 1512651466 1743708067 |
| Indian Diabetes Prevention Program 2013 (IDPP-2013) | India | N = 537; Men with prediabetes | 2009 | Randomized, parallel arm | 24 months | SMS-delivered lifestyle diabetes prevention education | Provided with diabetes education materials at baseline | 2462236768 | 2677387169; 163919036 |
| Indian Diabetes Prevention Programme (IDPP-1) | India | N = 531; Prediabetes | 2001/02 | Randomized, parallel arm | 3 years |
(i) Lifestyle diabetes prevention program; (ii) Metformin; (iii) Lifestyle + Metformin |
Usual care | 163919036 | 163919036, 2051966370, 2677387169 |
| Indian Diabetes Prevention Programme (IDPP-2) | India | N = 407; Prediabetes | 2003/05 | Randomized, parallel arm | 3 years | Lifestyle diabetes prevention program + Pioglitazone | Lifestyle diabetes prevention program + Placebo | 1927760271 | 2051966370 |
| Japan Diabetes Prevention Program (Japan DPP)a | Japan | N = 304; Prediabetes | 1999/02 | Randomized, parallel arm | 3 years | Lifestyle weight loss and diabetes prevention program | Provided with diabetes education materials at baseline | 2545285472 | 2545285472; 2123582520 |
| Kerala Diabetes Prevention Program (K-DPP) | India | N = 1007; Prediabetes, rural | 2013 | Cluster-randomized trial | 12 months | Peer-led lifestyle diabetes prevention program | Provided with diabetes education materials at baseline | 2418031673 | 2987423674 |
| Kosaka et al.a75 | Japan | N = 458; Men with prediabetes | 1990/92 | Randomized, parallel arm | 4 years | Lifestyle weight loss and diabetes prevention program | Lifestyle weight loss and diabetes prevention information only | 1564957575 | 1564957575 |
| Let’s Prevent Diabetes | UK | N = 880; Prediabetes | 2009/11 | Cluster-randomized trial | 36 months | Lifestyle diabetes prevention program | Provided with diabetes education materials at baseline | 2260716076 | 2674034677 |
| Multiple Risk Factor Intervention Trial (MRFIT) | US | N = 12,866; Men with high cardiovascular risk | 1973/76 | Randomized, parallel arm | 6 years | Lifestyle modifications for heart disease prevention | Usual care | 1573845078 | 1573845078 |
| Nanditha et al. 79 | India, UK | N = 2062; Prediabetes | 2012/17 | Randomized, parallel arm | 24 months | SMS-delivered lifestyle diabetes prevention education | Provided with diabetes education materials at baseline | 3191953979 | 3191953979 |
| National Program for the Prevention of Type 2 Diabetes (FIN-D2D)a | Finland | N = 2798; Prediabetes | 2004/07 | Population-wide intervention | Mean 14 months | Lifestyle weight loss and diabetes prevention program | – | 2066402080 | 2298378581; 3377151582; 2178115383; 2066402080; 2162267784 |
| Niyantrita Madhumeha Bharata Abhiyaan (NMB-Trial) | India | N = 4450; Prediabetes | 2017 | Cluster-randomized trial | 3 months | Yoga-based lifestyle diabetes prevention program | Presentation on lifestyle for diabetes prevention at baseline | 3417780585 | 3417780585 |
| Norfolk Diabetes Prevention Study (NDPS) | UK | N = 1028; Prediabetes | 2011/18 | Randomized, parallel arm | 12–46 months | (i) Lifestyle diabetes prevention program; (ii) Lifestyle diabetes prevention program with peer support | Provided with diabetes education materials at baseline | 3313611986 | 3313611986 |
| Prevention of Diabetes in Euskadi (PreDE)a | Spain | N = 1088; Prediabetes | 2011/13 | Cluster-randomized trial | 24 months | Lifestyle weight loss and diabetes prevention program | Usual care | 2947688887 | 2947688887 |
| Tehran Lipid and Glucose Study (TLGS) | Iran | N = 10,368; General population | 1999/01 | Non-randomized, cluster | Mean 3.6 years | Lifestyle program for chronic disease prevention | Usual care | 2049423988 | 2502936889; 2049423988 |
| Thai Diabetes Prevention Program (Thai DPP) | Thailand | N = 1903; Prediabetes | 2013 | Cluster-randomized trial | 24 months | Lifestyle diabetes prevention program | Provided with diabetes education materials at baseline | 3107951719 | 3107951719 |
| Västerbotten Intervention Programme (VIP) | Sweden | N = 113, 203; General population | 1987-present | Population-wide intervention | Ongoing | Lifestyle CVD and diabetes prevention program | – | 2033947990 | 2553267891 |
| Zensharen Study for Prevention of Lifestyle Diseasesa | Japan | N = 641; Prediabetes | 2004/06 | Randomized, parallel arm | 36 months | Lifestyle weight loss program + Frequent engagement | Lifestyle weight loss program + Minimal engagement | 2182494892 | 2182494892 |
| Dietary pattern interventions | |||||||||
| CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (CORDIOPREV) | Spain | N = 1002; Prevalent heart disease | 2009/12 | Randomized, parallel arm | Median 7 years | Mediterranean dietary pattern | AHA low-fat pattern (<30% kcal) | 2729784893 | 3272350894 |
| Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts (PREDIMED) | Spain | N = 7447; High cardiovascular risk | 2003/09 | Randomized, parallel arm | 4.8 years |
(i) Mediterranean pattern + extra-virgin olive oil; (ii) Mediterranean pattern + mixed nuts |
Low-fat pattern | 2989786695 | 2966301196, 2673999697; 2303496298; 3137717999; 24573661100; 20929998101 |
| Shahbazi et al. 102 | Iran | N = 336; Prediabetes | 2012 | Randomized, parallel arm | 2 years |
(i) High-fat diet from olive oil (45% kcal); (ii) Normal fat diet (30% kcal) |
Standard low-fat diet (<30% kcal) | DOI 10.1007/s13410-017-0548-3 | DOI 10.1007/s13410-017-0548-3102 |
| Women’s Health Initiative Dietary Modification Trial (WHI-DM) | US | N = 48,835; Healthy postmenopausal women | 1993/98 | Randomized, parallel arm | Mean 8.1 years | Low-fat (20% kcal) healthy pattern | Provided with healthy diet materials at baseline | 18663162103 | 29282203104 |
| Dietary supplement interventions | |||||||||
| Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study (ATBC) | Finland | N = 29,133; Men, smokers | 1985/88 | Randomized, parallel arm | Median 6.1 years | 2 × 2 factorial: (i) alpha-tocopherol (50 mg/day), (ii) beta-carotene (20 mg/day) | Placebo | 8205268105 | 17994292106 |
| Vitamin D and Type 2 Diabetes Trial (D2d) | US | N = 2423; Prediabetes | 2013/17 | Randomized, parallel arm | Median 2.5 years | Vitamin D supplementation (4000 IU/day) | Placebo | 31173679107 | 31173679107 |
| Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS) | US | N = 5442; Women with cardiovascular disease | 1998 | Randomized, parallel arm | Median 7.3 years | Folic acid (2.5 mg/day), vitamin B6 (50 mg/day), and vitamin B12 (1 mg/day) combined supplementation | Placebo | 19491213108 | 19491213108 |
| Women’s Antioxidant Cardiovascular Study (WACS) | US | N = 8171; Women with cardiovascular disease | 1995/96 | Randomized, parallel arm | Median 9.2 years | 2 × 2 × 2 factorial: (i) vitamin C (500 mg/day), (ii) vitamin E (600 IU/day), (iii) beta-carotene (50 mg/eod) supplementation | Placebo | 19491386109 | 19491386109 |
| Women’s Health Study (WHS) | US | N = 39,876; Healthy women | 1992/95 | Randomized, parallel arm | 10.1 years | 2 × 2 Factorial, every other day: Aspirin (100 mg); (ii) Vitamin E supplementation (600 IU) | Placebo | 15998891110 | 17003353111 |
aTrials which aimed at weight loss and prevention of T2D.
Twenty-four of the included studies assessed the effect of a multi-component lifestyle intervention program focused on changes in diet, physical activity, smoking, or body weight loss. Four studies implemented a dietary intervention, and five administered supplements. Across multi-component lifestyle intervention studies, the comparator consisted of a less intensive lifestyle program consisting of usual care or general lifestyle advice administered at baseline. Active comparator groups for dietary intervention studies focused on high-fat diets consisted of a low-fat intervention. The active comparator for supplement studies consisted of a placebo intervention. T2D was diagnosed in person with an oral glucose tolerance test (OGTT) in 27 studies, whereas in 6 studies, T2D was ascertained via self-report or through linkage with a healthcare registry database. The primary endpoint was T2D incidence in 21 studies or a composite cardiovascular event in six studies (Table 1 and Supplementary Table 3).
All except seven studies of a multi-component lifestyle intervention program showed evidence that a lifestyle intervention reduces the risk of T2D, with estimated relative risk reduction ranging from 60 to 23% (Supplementary Table 3). Available evidence also suggests that a high-fat diet (Mediterranean pattern diet with extra-virgin olive oil/ mixed nuts or high-fat diet from olive oil), reduces the relative risk of T2D when compared to a diet with a lower amount of fat. Evidence from studies using supplements showed a null effect on T2D risk reduction.
Our certainty of evidence assessment determined that the primary study design and approach was generally low, particularly for the RCTs, owing to randomization methods and uniform outcome assessment (Supplementary Fig. 1). However, common concerns for bias were due to non-blinding of participants, deliverers, and outcomes assessors to treatment assignment. Nonrandomized interventions and RCTs having additional concerns for study design did have ratings of high risk of bias.
Sociodemographic and clinical factors
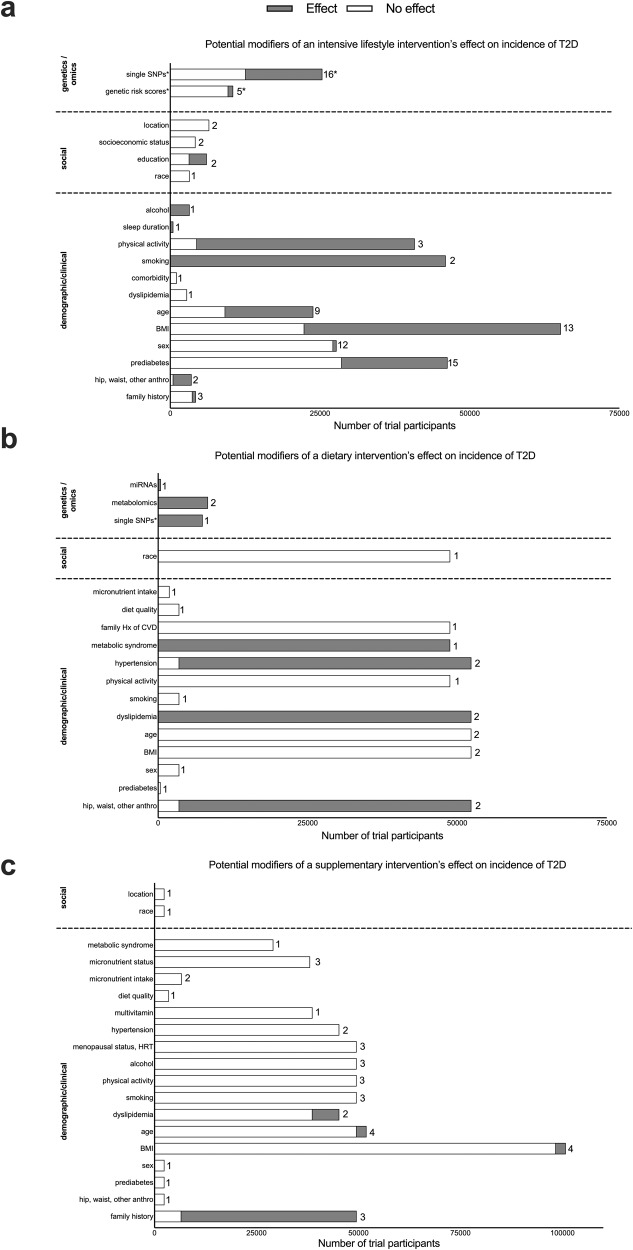
Some clinical trials, such as the Diabetes Prevention Program (DPP), the Finnish Diabetes Prevention Study (DPS), or the PREDIMED study, were highly represented, with 20, 16, and 6 different publications from each study, respectively. Certainty of evidence to indicate different effects for sociodemographic and clinical characteristics such as age, sex, race/ethnicity, socioeconomic status or geographic location in response to lifestyle intervention was low. Study-specific numeric estimates for the effect modification are provided in the extended data file. Evidence from studies investigating sociodemographic interaction effects in dietary modification or supplementation trials showed no significant heterogeneity in response to intervention according to these characteristics (Table 2 and Fig. 2).
Table 2.
Efficacy of T2D preventive interventions according to sociodemographic effect modifiers.
| T2D preventive strategies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lifestyle intervention | Dietary pattern intervention | Dietary supplements intervention | |||||||
| Modifier | Number of studies | Effect modificationa | Certainty of evidenceb | Number of studies | Effect modificationa | Certainty of evidenceb | Number of studies | Effect modificationa | Certainty of evidenceb |
| Age | 12 |
Yes: 7 studies No: 5 studies |
Grade D | 3 | No: 3 studies | Grade D | 4 |
Yes: 1 study No: 3 studies |
Grade D |
| Sex | 16 |
Yes: 1 study No: 15 studies |
Grade D | 2 | No: 2 studies | Grade D | 1 | Yes: 1 study | Grade D |
| Race/ethnicity | 3 | No: 3 studies | Grade D | 1 | No: 1 study | Grade D | 1 | No: 1 study | Grade D |
| Socioeconomic status/ Education | 4 |
Yes: 1 study No: 3 studies |
Grade D | – | – | – | – | – | – |
| Location | 2 | No: 2 studies | Grade D | – | – | – | 1 | No: 1 study | – |
Overview of the included studies investigating whether sociodemographic factors modify the response to T2D preventive intervention strategies.
aYes/No corresponds to significant/nonsignificant effect modification, as reported in the study.
bCertainty of evidence denotes consistency, Grading based on Diabetes Canada scale A to D.
Fig. 2. Potential effect modifiers of lifestyle, diet, and diet supplements intervention on the incidence of T2D.
General overview of potential effect modifiers of lifestyle (a), dietary (b), and supplement (c) interventions on the incidence of type 2 diabetes. The Y axes indicate potential effect modifiers, and the X axes illustrate the total number of trial participants included in the studies investigating each modifier. The proportion of gray or white in each bar indicates the number of trial participants included in the studies where there was (gray) or was not (white) an effect by the effect modifier. Caution is warranted because whether an effect modifier did (or did not) have an effect is based on statistical significance from the publication’s summary statistics. It is improbable that the effect modifier strictly did (or did not) have an effect on every participant included in that publication. The number of trials and trial participants are plotted because some trials (e.g., DPP) had multiple studies published using the same participants, so that the participant number would be heavily skewed. There was no instance where the same trial had multiple published studies evaluating the same effect modifier showing different results (e.g., there was no difference between sexes on the PREDIMED trial’s effect on T2D incidence in their primary vs. subgroup studies/publications). The number at the end of each bar represents the number of trials for each potential effect modifier. *indicates an exception for genetics because the effect modifiers (SNPs or GRS) were all uniquely distinct but are presented together under the categories of “SNP” or “GRS” here.
Fourteen studies investigated whether BMI modified the efficacy of multi-component lifestyle interventions. Nine of these studies showed that BMI is not associated with different responses to a lifestyle program, but five studies showed suggestive evidence that individuals with low BMI could benefit most from a lifestyle intervention. Four of these five studies presenting evidence of the differential effect of a lifestyle intervention according to BMI were conducted in Asia (Table 3). No appreciable evidence for interactions with BMI was observed in studies that implemented a dietary or supplement intervention (Table 3). Eighteen studies tested the efficacy of an intensive lifestyle intervention for preventing T2D stratified based on baseline glucose levels, impaired glucose tolerance, or prediabetes status. Evidence presented in eight of these studies indicated statistically different effects based on baseline dysglycemia, but other studies did not find evidence of effect modifications. Three studies investigated family history of T2D as a potential lifestyle intervention effect modifier, and only one provided suggestive evidence of heterogenous treatment responses. Studies stratified by baseline cardiometabolic risk factors reported that individuals with poorer health status, particularly those with dyslipidemia and metabolic syndrome, tend to benefit more from dietary or supplement interventions than healthier individuals (Table 3).
Table 3.
Efficacy of T2D preventive interventions according to clinical effect modifiers.
| T2D preventive strategies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lifestyle intervention | Dietary pattern intervention | Dietary supplements intervention | |||||||
| Modifier | Number of studies | Effect modificationa | Certainty of evidenceb | Number of studies | Effect modificationa | Certainty of evidenceb | Number of studies | Effect modificationa | Certainty of evidenceb |
| BMI | 14 |
Yes: 5 studies No: 9 studies |
Grade D | 3 | No: 3 studies | Grade D | 4 |
Yes: 1 study No: 3 studies |
Grade D |
| Prediabetes | 18 |
Yes: 8 studies No: 10 studies |
Grade D | 1 | No: 1 study | Grade D | 1 | No: 1 study | Grade D |
| Family history | 3 |
Yes: 1 study No: 2 studies |
Grade D | – | – | 3 |
Yes: 2 studies No: 1 study |
Grade D | |
| Dyslipidemia/medications | 1 | No: 1 study | Grade D | 2 | Yes: 2 studies | Grade D | 2 |
Yes: 1 study No: 1 study |
Grade D |
| Hypertension | – | – | – | 2 |
Yes: 1 study No: 1 study |
Grade D | 2 | No: 2 studies | Grade D |
| Metabolic syndrome | – | – | – | 1 | Yes: 1 study | Grade D | 1 | No: 1 study | Grade D |
| Menopausal status, HRT use | – | – | – | – | – | – | 3 | No: 3 studies | Grade D |
Overview of the included studies investigating whether clinical factors modify the response to T2D preventive intervention strategies.
aYes/No corresponds to significant/nonsignificant effect modification, as reported in the study.
bCertainty of evidence denotes consistency, Grading based on Diabetes Canada scale A to D.
Behavioral factors
Several secondary studies have assessed whether baseline lifestyle factors (i.e., overall dietary quality, alcohol intake, physical activity, and/or smoking) influence the efficacy of T2D prevention interventions. Evidence presented in studies investigating the effect of a lifestyle intervention according to baseline smoking status and physical activity indicates statistically different effects, suggesting that smokers and those with lower physical activity levels benefited less from a lifestyle program (Table 4). Available studies reported no interactions of baseline smoking status and physical activity levels with dietary or supplement interventions on the risk of T2D. Among the four studies that focused on alcohol intake, only one found that the lifestyle intervention was more effective in individuals who drink alcohol frequently than in those who rarely drink. Six studies tested whether baseline diet modified the association between supplements and the risk of T2D and found no evidence of significant interactions (Table 4).
Table 4.
Efficacy of T2D preventive interventions according to behavioral effect modifiers.
| T2D preventive strategies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lifestyle intervention | Dietary pattern intervention | Dietary supplements intervention | |||||||
| Modifier | Number of studies | Effect modificationa | Certainty of evidenceb | Number of studies | Effect modificationa | Certainty of evidenceb | Number of studies | Effect modificationa | Certainty of evidenceb |
| Smoking | 2 | Yes: 2 studies | Grade D | 1 | No: 1 study | Grade D | 3 | No: 3 studies | Grade D |
| Physical activity | 3 |
Yes: 2 studies No: 1 study |
Grade D | 1 | No: 1 study | Grade D | 3 | No: 3 studies | Grade D |
| Alcohol intake | 1 | Yes: 1 study | Grade D | – | – | – | 3 | No: 3 studies | Grade D |
| Diet and supplements | – | – | – | 2 | No: 2 studies | Grade D | 6 | No: 6 studies | Grade D |
Overview of the included studies investigating whether behavioral factors at baseline modify the response to T2D preventive intervention strategies.
aYes/No corresponds to significant/nonsignificant effect modification, as reported in the study.
bCertainty of evidence denotes consistency, Grading based on Diabetes Canada scale A to D.
Molecular factors
The extent to which genetic predisposition modifies the efficacy of interventions to prevent T2D was reported in 22 publications. Most of them were based on data from the DPP and the DPS. Genetic predisposition was defined based on single genetic variants in 17 studies or genetic risk scores in five. While many of the T2D-associated loci identified in the earlier GWAS studies have been examined for their potential roles as effect modifiers, some reported evidence that individuals with specific genotypes could benefit the most from a lifestyle intervention, but these studies rarely corrected for the number of performed tests. Of the five studies that reported on the role of polygenic scores for T2D, only one study showed that lifestyle intervention was more effective among individuals with a high genetic risk.
Besides genetics, other molecular markers such as plasma branched-chain amino acids and miRNAs have been studied. The evidence that these molecular features modify the efficacy of dietary interventions in the prevention of T2D has only low to very-low certainty (Table 5 and Fig. 2).
Table 5.
Efficacy of T2D preventive interventions according to molecular effect modifiers.
| T2D preventive strategies | ||||||
|---|---|---|---|---|---|---|
| Lifestyle intervention | Dietary pattern intervention | |||||
| Modifier | Number of studies | Effect modificationa | Certainty of evidenceb | Number of Studies | Effect modificationa | Certainty of evidenceb |
| T2D single SNPs | 17 |
Yes: 9 studies No: 7 studies Not reported: 1 study |
Grade D | 1 | Yes: 1 study | Grade D |
| Diabetes polygenic score | 5 |
Yes: 1 study No: 4 studies |
Grade D | – | – | – |
| Metabolites/miRNA | – | – | – | 3 | Yes: 3 studies | Grade D |
Overview of the included studies investigating whether genetic and molecular factors at baseline modify the response to T2D preventive intervention strategies.
aYes/No corresponds to significant/nonsignificant effect modification, as reported in the study.
bCertainty of evidence denotes consistency, Grading based on Diabetes Canada scale A to D.
Grading of evidence certainty
Although our systematic review included intervention studies, most RCTs with low risk of bias, we evaluated certainty through our hypothesis of identifying valid effect modifiers to inform precision prevention. None of the studies included a priori consideration of intervention interactions with individual-level characteristics or risk factors in their study design, which were largely conducted as post hoc analyses. As a result, statistical power was often limited. Further, most did not adjust for individual-level risk factors, undermining the validity of interpreting effect modifiers’ role independent of other traits. These considerations were factored into the major downgrading of the evidence (Tables 2–5).
Discussion
We performed a comprehensive systematic review to identify individual-level sociodemographic, clinical, behavioral, or molecular factors that could modify the efficacy of T2D prevention strategies. Overall, we find low to very low certainty of evidence that traits such as age, sex, BMI, race/ethnicity, socioeconomic status, baseline lifestyle factors, or genetics consistently and validly modify the effectiveness of lifestyle and behavioral interventions. Individuals with prediabetes at baseline benefit slightly more from prevention interventions than those without prediabetes, but the certainty of the evidence was low. This can be explained by relative and absolute risk differences among people with/without prediabetes. However, whether the modest benefit reported in these studies was due to poor health status or other correlated risk factors cannot be ascertained based on the available evidence.
Large randomized clinical trials have consistently demonstrated that a healthy lifestyle or dietary interventions can prevent or delay T2D3,4,6,17. However, there is large inter-individual variability in response to these preventive interventions, in which some people seem to greatly benefit from T2D preventive interventions. Precision prevention aims to identify participant characteristics that determine this variability in response to ultimately tailor preventive strategies to subgroups of individuals that are likely to benefit the most. So far, no studies exist that were prospectively designed to determine interactions by a baseline trait or factor with an intervention to prevent T2D. We evaluated the evidence base and identified several stratified post hoc analyses of existing prevention intervention trials. In post hoc analyses, the participant population is stratified by a potential effect modifier, and the efficacy of the intervention is tested within each stratum and compared across the strata, which reduces statistical power and increases type 2 error.
Furthermore, precision prevention strategies may be optimized by incorporating several individual-level factors into decision-making, whereas the current literature predominantly evaluates one stratified trait at a time. For example, correlated behaviors, such as physical activity, diet, and smoking, might provide more information when considered collectively than individually. Clinical trials specifically designed to investigate the influence of sociodemographic, clinical, behavioral, or molecular factors on the response to T2D preventive strategies are needed to generate valid and robust evidence before the implementation of T2D precision prevention strategies.
One area of promise warranting further research is the presence of prediabetes at baseline and whether this may be targeted in future precision prevention research. Low certainty evidence suggests that individuals at risk of T2D or with prediabetes at baseline benefit slightly more from prevention interventions than those not at risk of T2D3–6. However, the evidence is inconsistent, even though the studies report that a lifestyle intervention, compared to standard care, results in higher T2D reduction rates among studies conducted in Asia17–20. Beyond the methodological limitations of the available evidence, an additional reason for inconsistent evidence supporting the greater effectiveness of lifestyle interventions for the prevention of T2D among individuals with prediabetes is due to the heterogeneity that characterizes this condition. Prediabetes refers to a pathophysiological state of early alterations in glucose metabolism that precedes the development of diabetes. Still, the mechanisms by which glucose is elevated are very different and could range from those with primary alterations in insulin secretion pathways to those with primary insulin resistance21. Clinical trials specifically designed to capture the nuances and complexity of early glycemic alterations and whether individuals with distinct pathophysiological features benefit from more targeted preventive interventions are needed to fill the gap in current T2D precision prevention evidence.
Even though there are far more lifestyle intervention trials for the prevention of T2D than diet alone and diet supplementation trials, collectively, however, results for effect modification by any one factor are sparsely reported or arising from an evidence base of very different trials and patient populations. Further, many secondary analyses in this systematic review are derived from two single clinical interventions viz, the DPP and the DPS. Findings from available evidence contrast with recent clinical studies documenting variable responses to identical foods, diets, or lifestyle interventions based on inter-individual differences in demographic, clinical, genetic, gut microbiota, and lifestyle characteristics22–24. While these studies offer insights into variable postprandial metabolic response, their short follow-up periods, the lack of time-series data and changes in parameters that could influence response to interventions, and the inclusion of relatively young and healthy individuals preclude the generalizability to T2D prevention efforts. Whether the promise of T2D precision prevention is matched by evidence of the long-term beneficial impact remains uncertain. Still, interest and activity in this field are proliferating to identify factors underlying variable nutritional responses and develop algorithms to predict individual responses to nutrients, foods, and dietary patterns.
While recent studies support the benefits of losing body weight loss on the risk of developing T2D regardless of the mechanisms underlying T2D, there is still enormous variability in individual response to weight-loss interventions. For example, the DIETFITS study25, showed that weight change varied widely within each study group, ranging from a loss of ~30 kg to a gain of ~10 kg. While weight loss is critical in T2D prevention, these findings reinforce the continued effort to identify molecular, environmental and social characteristics underlying the variable response to diabetes prevention interventions.
Our systematic review had some limitations. The scope of our literature review as part of the PDMI was broad and inclusive of diverse study designs, T2D prevention strategies, study populations, and effect modification analyses. Although this resulted in a heterogeneous evidence base and did not provide an opportunity for meta-analysis, we qualitatively synthesized the evidence for precision prevention. Our hypothesis originally spanned to include observational studies, which were ultimately excluded due to the uncertainty of their being readily related to clinical interventions. Protocol amendments were registered to reflect these decisions prior to study screening and extraction. Moreover, as our scope only included moderators of the intervention efficacy on T2D, which are typically measured prior to or at baseline26, important mediators of the intervention effects on T2D as e.g., weight loss was not addressed and discussed. This will be important to address in future studies to gain a deeper understanding of heterogenous lifestyle interventions responses.
In conclusion, our systematic review and synthesis of the T2D prevention literature provide low to very low certainty evidence that sociodemographic, clinical, lifestyle, or molecular factors are more useful, valid, and consistent in informing T2D precision prevention strategies than current interventions. We also uncover several areas of potential for growth in the precision medicine field, including prospectively designed interventions and clinical trials incorporating the investigation of treatment response heterogeneity.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Hugo Fitipaldi, Esther González-Padilla, Alisha Sha, and Jiaxi Yang for attending some of the working group meetings and/or for reviewing some of the abstracts. The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for better diabetes prevention and care through precision medicine (Nolan et al.13). This Systematic Review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine (Tobias et al.12). The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden), for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), the University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL). D.B. was supported through an Early Career Research grant (ECR/2017/000640) from Science and Engineering Research Board (SERB), India. J.M. was partially supported by funding from the American Diabetes Association (7-21-JDFM-005) and the National Institutes of Health (P30 DK40561 and UG1 HD107691). R.J.F.L. received support through NNF18CC0034900; NNF20OC0059313 (Laureate Award), and DNRF161 (Chair).
Author contributions
D.B., R.W.M., S.L.F., J.S.P., P.W.F., ADA/EASD PMDI, D.K.T., J.M., V.M., and R.J.F.L. contributed to the conception and design of the research questions. D.B., R.W.M., V.S., M.N., H.P.M., C.C., S.L.F., M.G.F., J.S.P., M.R.L., D.K.T., J.M., V.M., and R.J.F.L. contributed to the study screening and data extraction. D.K.T. and J.M. did the quality assessment; D.B., R.W.M., V.S., M.N., S.L.F., M.G.F., J.S.P., M.R.L., D.K.T., J.M., V.M., and R.J.F.L. summarized and interpreted the data. D.B., J.M., and R.J.F.L. drafted the paper; D.K.T. and V.M. revised it substantively. All authors edited the manuscript and approved the final version.
Peer review
Peer review information
Communications Medicine thanks Lisa Moran and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
This systematic review compiles data available in clinical studies. The PMIDs of included studies are available in Table 1. The study-specific numeric estimates for the effect modification has been given in Supplementary Data 1. The source data for Fig. 2 is provided in Supplementary Data 2. All other extracted data have been summarized in the figures and tables presented in the manuscript and are available from the corresponding author on reasonable request.
Competing interests
The authors declare the following competing interests: R.W.M. and P.W.F. are employees of the Novo Nordisk Foundation, a private philanthropic enterprise foundation. The opinions expressed in this article do not necessarily reflect the perspectives of the Novo Nordisk Foundation. V.M. has acted as consultant and speaker and received research or educational grants from Novo Nordisk, MSD, Eli Lilly, Novartis, Boehringer Ingelheim, Lifescan J&J, Sanofi-Aventis, Roche Diagnostics, Abbott, and several Indian pharmaceutical companies, including USV, Dr. Reddy’s Laboratories, and Sun Pharma. None of the other authors have any conflicts of interest to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Deirdre K. Tobias, Jordi Merino.
These authors jointly supervised this work: Viswanathan Mohan, Ruth J. F. Loos.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Ruth J. F. Loos, Email: ruth.loos@sund.ku.dk
ADA/EASD PMDI:
Deirdre K. Tobias, Abrar Ahmad, Catherine Aiken, Jamie L. Benham, Dhanasekaran Bodhini, Amy L. Clark, Kevin Colclough, Rosa Corcoy, Sara J. Cromer, Daisy Duan, Jamie L. Felton, Ellen C. Francis, Pieter Gillard, Véronique Gingras, Romy Gaillard, Eram Haider, Alice Hughes, Jennifer M. Ikle, Laura M. Jacobsen, Anna R. Kahkoska, Jarno L. T. Kettunen, Raymond J. Kreienkamp, Lee-Ling Lim, Jonna M. E. Männistö, Robert Massey, Niamh-Maire Mclennan, Rachel G. Miller, Mario Luca Morieri, Jasper Most, Rochelle N. Naylor, Bige Ozkan, Kashyap Amratlal Patel, Scott J. Pilla, Katsiaryna Prystupa, Sridharan Raghavan, Mary R. Rooney, Martin Schön, Zhila Semnani-Azad, Magdalena Sevilla-Gonzalez, Pernille Svalastoga, Wubet Worku Takele, Claudia Ha-ting Tam, Anne Cathrine B. Thuesen, Mustafa Tosur, Amelia S. Wallace, Caroline C. Wang, Jessie J. Wong, Jennifer M. Yamamoto, Katherine Young, Chloé Amouyal, Mette K. Andersen, Maxine P. Bonham, Mingling Chen, Feifei Cheng, Tinashe Chikowore, Sian C. Chivers, Dana Dabelea, Adem Y. Dawed, Aaron J. Deutsch, Laura T. Dickens, Linda A. DiMeglio, Monika Dudenhöffer-Pfeifer, Carmella Evans-Molina, María Mercè Fernández-Balsells, Hugo Fitipaldi, Stephanie L. Fitzpatrick, Stephen E. Gitelman, Mark O. Goodarzi, Jessica A. Grieger, Marta Guasch-Ferré, Nahal Habibi, Torben Hansen, Chuiguo Huang, Arianna Harris-Kawano, Heba M. Ismail, Benjamin Hoag, Randi K. Johnson, Angus G. Jones, Robert W. Koivula, Aaron Leong, Gloria K. W. Leung, Ingrid M. Libman, Kai Liu, S. Alice Long, William L. Lowe, Jr., Ayesha A. Motala, Suna Onengut-Gumuscu, Maleesa Pathirana, Sofia Pazmino, Dianna Perez, John R. Petrie, Camille E. Powe, Alejandra Quinteros, Rashmi Jain, Debashree Ray, Zeb Saeed, Vanessa Santhakumar, Sarah Kanbour, Sudipa Sarkar, Gabriela S. F. Monaco, Denise M. Scholtens, Elizabeth Selvin, Wayne Huey-Herng Sheu, Cate Speake, Maggie A. Stanislawski, Nele Steenackers, Andrea K. Steck, Norbert Stefan, Julie Støy, Rachael Taylor, Sok Cin Tye, Gebresilasea Gendisha Ukke, Marzhan Urazbayeva, Bart Van der Schueren, Camille Vatier, John M. Wentworth, Wesley Hannah, Sara L. White, Gechang Yu, Yingchai Zhang, Shao J. Zhou, Jacques Beltrand, Michel Polak, Ingvild Aukrust, Elisa de Franco, Sarah E. Flanagan, Kristin A. Maloney, Andrew McGovern, Janne Molnes, Pål Rasmus Njølstad, Hugo Pomares-Millan, Michele Provenzano, Cécile Saint-Martin, Cuilin Zhang, Yeyi Zhu, Sungyoung Auh, Russell de Souza, Andrea J. Fawcett, Chandra Gruber, Eskedar Getie Mekonnen, Emily Mixter, Diana Sherifali, Robert H. Eckel, John J. Nolan, Louis H. Philipson, Rebecca J. Brown, Liana K. Billings, Kristen Boyle, Tina Costacou, John M. Dennis, Jose C. Florez, Anna L. Gloyn, Maria F. Gomez, Peter A. Gottlieb, Siri Atma W. Greeley, Kurt Griffin, Andrew T. Hattersley, Irl B. Hirsch, Marie-France Hivert, Korey K. Hood, Jami L. Josefson, Soo Heon Kwak, Lori M. Laffel, Siew S. Lim, Ronald C. W. Ma, Chantal Mathieu, Nestoras Mathioudakis, James B. Meigs, Shivani Misra, Viswanathan Mohan, Rinki Murphy, Richard Oram, Katharine R. Owen, Susan E. Ozanne, Ewan R. Pearson, Wei Perng, Toni I. Pollin, Rodica Pop-Busui, Richard E. Pratley, Leanne M. Redman, Maria J. Redondo, Rebecca M. Reynolds, Robert K. Semple, Jennifer L. Sherr, Emily K. Sims, Arianne Sweeting, Tiinamaija Tuomi, Miriam S. Udler, Kimberly K. Vesco, Tina Vilsbøll, Robert Wagner, Stephen S. Rich, and Paul W. Franks
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-023-00363-0.
References
- 1.Sun H, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomi T, et al. The many faces of diabetes: a disease with increasing heterogeneity. Lancet. 2014;383:1084–1094. doi: 10.1016/S0140-6736(13)62219-9. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuomilehto J, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 5.Pan XR, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 7.Ling W, et al. Global trend of diabetes mortality attributed to vascular complications, 2000–2016. Cardiovasc. Diabetol. 2020;19:182. doi: 10.1186/s12933-020-01159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wareham NJ. Personalised prevention of type 2 diabetes. Diabetologia. 2022;65:1796–1803. doi: 10.1007/s00125-022-05774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung WK, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:1617–1635. doi: 10.2337/dci20-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie F, Chan JCN, Ma RCW. Precision medicine in diabetes prevention, classification and management. J. Diabetes Investig. 2018;9:998–1015. doi: 10.1111/jdi.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutie, P. M., Giordano, G. N. & Franks, P. W. Lifestyle precision medicine: the next generation in type 2 diabetes prevention? BMC Med.15, 171 (2017). [DOI] [PMC free article] [PubMed]
- 12.Tobias, D. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. 10.1038/s41591-023-02502-5 (2023). [DOI] [PMC free article] [PubMed]
- 13.Nolan JJ, et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diabetes Care. 2022;45:261–266. doi: 10.2337/dc21-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covidence systematic review software. Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
- 15.Barker TH, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023;21:494–506. doi: 10.11124/JBIES-22-00430. [DOI] [PubMed] [Google Scholar]
- 16.Sherifali D, et al. Methods. Can. J. Diabetes. 2018;42:S6–S9. doi: 10.1016/j.jcjd.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Gong Q, et al. Efficacy of lifestyle intervention in adults with impaired glucose tolerance with and without impaired fasting plasma glucose: a post hoc analysis of Da Qing Diabetes Prevention Outcome Study. Diabetes Obes. Metab. 2021;23:2385–2394. doi: 10.1111/dom.14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber MB, et al. The stepwise approach to diabetes prevention: results from the D-CLIP randomized controlled trial. Diabetes Care. 2016;39:1760–1767. doi: 10.2337/dc16-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aekplakorn W, et al. Evaluation of a community-based diabetes prevention program in Thailand: a cluster randomized controlled trial. J. Prim. Care Community Health. 2019;10:2150132719847374. doi: 10.1177/2150132719847374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakane N, et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health. 2011;11:40. doi: 10.1186/1471-2458-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner R, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 2021;27:49–57. doi: 10.1038/s41591-020-1116-9. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Yacov O, et al. Personalized postprandial glucose response-targeting diet versus Mediterranean diet for glycemic control in prediabetes. Diabetes Care. 2021;44:1980–1991. doi: 10.2337/dc21-0162. [DOI] [PubMed] [Google Scholar]
- 23.Berry SE, et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020;26:964–973. doi: 10.1038/s41591-020-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeevi D, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Li X, et al. Distinct factors associated with short-term and long-term weight loss induced by low-fat or low-carbohydrate diet intervention. Cell Rep. Med. 2022;3:100870. doi: 10.1016/j.xcrm.2022.100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen R, Bours MJL, Nielsen SM. Effect modifiers and statistical tests for interaction in randomized trials. J. Clin. Epidemiol. 2021;134:174–177. doi: 10.1016/j.jclinepi.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Chae JS, et al. Supervised exercise program, BMI, and risk of type 2 diabetes in subjects with normal or impaired fasting glucose. Diabetes Care. 2012;35:1680–1685. doi: 10.2337/dc11-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474–480. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 29.Li G, et al. Effects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes mellitus: the DA Qing IGT and Diabetes Study. Diabetes Res. Clin. Pract. 2002;58:193–200. doi: 10.1016/s0168-8227(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 30.Costa B, et al. Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention. Diabetologia. 2012;55:1319–1328. doi: 10.1007/s00125-012-2492-6. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien MJ, Whitaker RC, Yu D, Ackermann RT. The comparative efficacy of lifestyle intervention and metformin by educational attainment in the Diabetes Prevention Program. Prev. Med. 2015;77:125–130. doi: 10.1016/j.ypmed.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriska AM, et al. The impact of physical activity on the prevention of type 2 diabetes: evidence and lessons learned from the diabetes prevention program, a long-standing clinical trial incorporating subjective and objective activity measures. Diabetes Care. 2021;44:43–49. doi: 10.2337/dc20-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allaire BT, et al. Diet quality, weight loss, and diabetes incidence in the Diabetes Prevention Program (DPP) BMC Nutr. 2020;6:74. doi: 10.1186/s40795-020-00400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billings LK, et al. Variation in maturity-onset diabetes of the young genes influence response to interventions for diabetes prevention. J. Clin. Endocrinol. Metab. 2017;102:2678–2689. doi: 10.1210/jc.2016-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crandall JP, et al. Alcohol consumption and diabetes risk in the Diabetes Prevention Program. Am. J. Clin. Nutr. 2009;90:595–601. doi: 10.3945/ajcn.2008.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruthur NM, et al. Early response to preventive strategies in the Diabetes Prevention Program. J. Gen. Intern. Med. 2013;28:1629–1636. doi: 10.1007/s11606-013-2548-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florez JC, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 2006;355:241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore AF, et al. The association of ENPP1 K121Q with diabetes incidence is abolished by lifestyle modification in the diabetes prevention program. J. Clin. Endocrinol. Metab. 2009;94:449–455. doi: 10.1210/jc.2008-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Florez JC, et al. Testing of diabetes-associated WFS1 polymorphisms in the Diabetes Prevention Program. Diabetologia. 2008;51:451–457. doi: 10.1007/s00125-007-0891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Q, et al. Variation at the melanocortin 4 receptor gene and response to weight-loss interventions in the diabetes prevention program. Obesity. 2013;21:E520–E526. doi: 10.1002/oby.20459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diabetes Prevention Program Research Group et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1075–1081. doi: 10.1093/gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diabetes Prevention Program Research Group et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghavan S, et al. Interaction of diabetes genetic risk and successful lifestyle modification in the Diabetes Prevention Programme. Diabetes Obes. Metab. 2021;23:1030–1040. doi: 10.1111/dom.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman WH, et al. Impact of lifestyle and metformin interventions on the risk of progression to diabetes and regression to normal glucose regulation in overweight or obese people with impaired glucose regulation. Diabetes Care. 2017;40:1668–1677. doi: 10.2337/dc17-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hivert M-F, et al. Updated genetic score based on 34 confirmed type 2 diabetes Loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes. 2011;60:1340–1348. doi: 10.2337/db10-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jablonski KA, et al. Common variants in 40 genes assessed for diabetes incidence and response to metformin and lifestyle intervention in the diabetes prevention program. Diabetes. 2010;59:2672–2681. doi: 10.2337/db10-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujimoto WY, et al. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes. 2007;56:1680–1685. doi: 10.2337/db07-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sussman JB, Kent DM, Nelson JP, Hayward RA. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454. doi: 10.1136/bmj.h454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diabetes Prevention Program (DPP) Research Group et al. Factors affecting the decline in incidence of diabetes in the Diabetes Prevention Program Outcomes Study (DPPOS) Diabetes. 2015;64:989–998. doi: 10.2337/db14-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penn L, et al. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. 2009;9:342. doi: 10.1186/1471-2458-9-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penn L, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PLoS ONE. 2013;8:e57143. doi: 10.1371/journal.pone.0057143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mager U, et al. Association of the Leu72Met polymorphism of the ghrelin gene with the risk of Type 2 diabetes in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention Study. Diabet Med. 2006;23:685–689. doi: 10.1111/j.1464-5491.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- 53.Laaksonen DE, et al. Physical activity, diet, and incident diabetes in relation to an ADRA2B polymorphism. Med. Sci. Sports Exerc. 2007;39:227–232. doi: 10.1249/01.mss.0000246998.02095.bf. [DOI] [PubMed] [Google Scholar]
- 54.Herder C, et al. Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the Finnish Diabetes Prevention Study. Diabetes. 2006;55:2340–2346. doi: 10.2337/db05-1320. [DOI] [PubMed] [Google Scholar]
- 55.Kilpeläinen TO, et al. Interaction of single nucleotide polymorphisms in ADRB2, ADRB3, TNF, IL6, IGF1R, LIPC, LEPR, and GHRL with physical activity on the risk of type 2 diabetes mellitus and changes in characteristics of the metabolic syndrome: the Finnish Diabetes Prevention Study. Metabolism. 2008;57:428–436. doi: 10.1016/j.metabol.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 56.Tuomilehto H, et al. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: the Finnish Diabetes Prevention Study. Diabetes Care. 2009;32:1965–1971. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kilpeläinen TO, et al. Physical activity modifies the effect of SNPs in the SLC2A2 (GLUT2) and ABCC8 (SUR1) genes on the risk of developing type 2 diabetes. Physiol. Genomics. 2007;31:264–272. doi: 10.1152/physiolgenomics.00036.2007. [DOI] [PubMed] [Google Scholar]
- 58.Uusitupa MI, et al. Impact of positive family history and genetic risk variants on the incidence of diabetes: the Finnish Diabetes Prevention Study. Diabetes Care. 2011;34:418–423. doi: 10.2337/dc10-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindi VI, et al. Association of the Pro12Ala polymorphism in the PPAR-gamma2 gene with 3-year incidence of type 2 diabetes and body weight change in the Finnish Diabetes Prevention Study. Diabetes. 2002;51:2581–2586. doi: 10.2337/diabetes.51.8.2581. [DOI] [PubMed] [Google Scholar]
- 60.Laukkanen O, et al. Common polymorphisms in the genes regulating the early insulin signalling pathway: effects on weight change and the conversion from impaired glucose tolerance to Type 2 diabetes. The Finnish Diabetes Prevention Study. Diabetologia. 2004;47:871–877. doi: 10.1007/s00125-004-1395-6. [DOI] [PubMed] [Google Scholar]
- 61.Lindström J, et al. Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care. 2008;31:857–862. doi: 10.2337/dc07-2162. [DOI] [PubMed] [Google Scholar]
- 62.Laaksonen DE, et al. Physical activity in the prevention of type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 63.Siitonen N, et al. Association between a deletion/insertion polymorphism in the alpha2B-adrenergic receptor gene and insulin secretion and Type 2 diabetes. The Finnish Diabetes Prevention Study. Diabetologia. 2004;47:1416–1424. doi: 10.1007/s00125-004-1462-z. [DOI] [PubMed] [Google Scholar]
- 64.Laukkanen O, et al. Polymorphisms in the SLC2A2 (GLUT2) gene are associated with the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes. 2005;54:2256–2260. doi: 10.2337/diabetes.54.7.2256. [DOI] [PubMed] [Google Scholar]
- 65.Kilpeläinen TO, et al. SNPs in PPARG associate with type 2 diabetes and interact with physical activity. Med. Sci. Sports Exerc. 2008;40:25–33. doi: 10.1249/mss.0b013e318159d1cd. [DOI] [PubMed] [Google Scholar]
- 66.Todorova B, et al. The G-250A promoter polymorphism of the hepatic lipase gene predicts the conversion from impaired glucose tolerance to type 2 diabetes mellitus: the Finnish Diabetes Prevention Study. J. Clin. Endocrinol. Metab. 2004;89:2019–2023. doi: 10.1210/jc.2003-031325. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, et al. Variants of transcription factor 7-like 2 (TCF7L2) gene predict conversion to type 2 diabetes in the Finnish Diabetes Prevention Study and are associated with impaired glucose regulation and impaired insulin secretion. Diabetologia. 2007;50:1192–1200. doi: 10.1007/s00125-007-0656-6. [DOI] [PubMed] [Google Scholar]
- 68.Ramachandran A, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1:191–198. doi: 10.1016/S2213-8587(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 69.Nanditha A, et al. Impact of lifestyle intervention in primary prevention of Type 2 diabetes did not differ by baseline age and BMI among Asian-Indian people with impaired glucose tolerance. Diabet. Med. 2016;33:1700–1704. doi: 10.1111/dme.13071. [DOI] [PubMed] [Google Scholar]
- 70.Ramachandran A, Arun N, Shetty AS, Snehalatha C. Efficacy of primary prevention interventions when fasting and postglucose dysglycemia coexist: analysis of the Indian Diabetes Prevention Programmes (IDPP-1 and IDPP-2) Diabetes Care. 2010;33:2164–2168. doi: 10.2337/dc09-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramachandran A, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2) Diabetologia. 2009;52:1019–1026. doi: 10.1007/s00125-009-1315-x. [DOI] [PubMed] [Google Scholar]
- 72.Sakane N, et al. Effect of baseline HbA1c level on the development of diabetes by lifestyle intervention in primary healthcare settings: insights from subanalysis of the Japan Diabetes Prevention Program. BMJ Open Diabetes Res. Care. 2014;2:e000003. doi: 10.1136/bmjdrc-2013-000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sathish T, et al. Cluster randomised controlled trial of a peer-led lifestyle intervention program: study protocol for the Kerala diabetes prevention program. BMC Public Health. 2013;13:1035. doi: 10.1186/1471-2458-13-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thankappan KR, et al. A peer-support lifestyle intervention for preventing type 2 diabetes in India: a cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med. 2018;15:e1002575. doi: 10.1371/journal.pmed.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005;67:152–162. doi: 10.1016/j.diabres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Gray LJ, et al. Let’s prevent diabetes: study protocol for a cluster randomised controlled trial of an educational intervention in a multi-ethnic UK population with screen detected impaired glucose regulation. Cardiovasc. Diabetol. 2012;11:56. doi: 10.1186/1475-2840-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies MJ, et al. A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let’s Prevent Diabetes cluster randomised controlled trial. Prev. Med. 2016;84:48–56. doi: 10.1016/j.ypmed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Davey Smith G, et al. Incidence of type 2 diabetes in the randomized multiple risk factor intervention trial. Ann. Intern. Med. 2005;142:313–322. doi: 10.7326/0003-4819-142-5-200503010-00006. [DOI] [PubMed] [Google Scholar]
- 79.Nanditha A, et al. A pragmatic and scalable strategy using mobile technology to promote sustained lifestyle changes to prevent type 2 diabetes in India and the UK: a randomised controlled trial. Diabetologia. 2020;63:486–496. doi: 10.1007/s00125-019-05061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saaristo T, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D) Diabetes Care. 2010;33:2146–2151. doi: 10.2337/dc10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rautio N, et al. Do statins interfere with lifestyle intervention in the prevention of diabetes in primary healthcare? One-year follow-up of the FIN-D2D project. BMJ Open. 2012;2:e001472. doi: 10.1136/bmjopen-2012-001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rintamäki R, et al. Long-term outcomes of lifestyle intervention to prevent type 2 diabetes in people at high risk in primary health care. Prim. Care Diabetes. 2021;15:444–450. doi: 10.1016/j.pcd.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Rautio N, et al. Family history of diabetes and effectiveness of lifestyle counselling on the cardio-metabolic risk profile in individuals at high risk of Type 2 diabetes: 1-year follow-up of the FIN-D2D project. Diabet. Med. 2012;29:207–211. doi: 10.1111/j.1464-5491.2011.03388.x. [DOI] [PubMed] [Google Scholar]
- 84.Rautio N, et al. Socioeconomic position and effectiveness of lifestyle intervention in prevention of type 2 diabetes: one-year follow-up of the FIN-D2D project. Scand. J. Public Health. 2011;39:561–570. doi: 10.1177/1403494811408482. [DOI] [PubMed] [Google Scholar]
- 85.Raghuram N, et al. Effectiveness of a yoga-based lifestyle protocol (YLP) in preventing diabetes in a high-risk Indian cohort: a multicenter cluster-randomized controlled trial (NMB-Trial) Front. Endocrinol. 2021;12:664657. doi: 10.3389/fendo.2021.664657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sampson M, et al. Lifestyle intervention with or without lay volunteers to prevent type 2 diabetes in people with impaired fasting glucose and/or nondiabetic hyperglycemia: a randomized clinical trial. JAMA Intern. Med. 2021;181:168–178. doi: 10.1001/jamainternmed.2020.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanchez A, Silvestre C, Campo N, Grandes G, PredDE Group. Effective translation of a type-2 diabetes primary prevention programme into routine primary care: the PreDE cluster randomised clinical trial. Diabetes Res. Clin. Pract. 2018;139:32–42. doi: 10.1016/j.diabres.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Harati H, et al. Reduction in incidence of type 2 diabetes by lifestyle intervention in a middle eastern community. Am. J. Prev. Med. 2010;38:628–636.e1. doi: 10.1016/j.amepre.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Derakhshan A, et al. Sex specific incidence rates of type 2 diabetes and its risk factors over 9 years of follow-up: Tehran Lipid and Glucose Study. PLoS ONE. 2014;9:e102563. doi: 10.1371/journal.pone.0102563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Norberg, M., Wall, S., Boman, K. & Weinehall, L. The Västerbotten Intervention Programme: background, design and implications. Glob. Health Action3, 4643 (2010). [DOI] [PMC free article] [PubMed]
- 91.Long GH, et al. Healthy behaviours and 10-year incidence of diabetes: a population cohort study. Prev. Med. 2015;71:121–127. doi: 10.1016/j.ypmed.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 92.Saito T, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch. Intern. Med. 2011;171:1352–1360. doi: 10.1001/archinternmed.2011.275. [DOI] [PubMed] [Google Scholar]
- 93.Delgado-Lista J, et al. CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): rationale, methods, and baseline characteristics: a clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low-fat diet on cardiovascular disease in coronary patients. Am. Heart J. 2016;177:42–50. doi: 10.1016/j.ahj.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jimenez-Lucena R, et al. MiRNAs profile as biomarkers of nutritional therapy for the prevention of type 2 diabetes mellitus: from the CORDIOPREV study. Clin. Nutr. 2021;40:1028–1038. doi: 10.1016/j.clnu.2020.06.035. [DOI] [PubMed] [Google Scholar]
- 95.Estruch R, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 96.Ruiz-Canela M, et al. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: case-cohort study within the PREDIMED trial. Diabetologia. 2018;61:1560–1571. doi: 10.1007/s00125-018-4611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corella D, et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc. Diabetol. 2016;15:4. doi: 10.1186/s12933-015-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ibarrola-Jurado N, Salas-Salvadó J, Martínez-González MA, Bulló M. Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2012;96:1113–1118. doi: 10.3945/ajcn.111.033498. [DOI] [PubMed] [Google Scholar]
- 99.Liu X, et al. High plasma glutamate and low glutamine-to-glutamate ratio are associated with type 2 diabetes: case-cohort study within the PREDIMED trial. Nutr. Metab. Cardiovasc. Dis. 2019;29:1040–1049. doi: 10.1016/j.numecd.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salas-Salvadó J, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann. Intern. Med. 2014;160:1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 101.Salas-Salvadó J, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shahbazi S, Vahdat Shariatpanahi Z. Prevention of type 2 diabetes mellitus by changes in diet among subjects with abnormal glucose metabolism: a randomized clinical trial. Int. J. Diabetes Dev. Ctries. 2018;38:69–74. [Google Scholar]
- 103.Tinker LF, et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women’s Health Initiative randomized controlled dietary modification trial. Arch. Intern. Med. 2008;168:1500–1511. doi: 10.1001/archinte.168.14.1500. [DOI] [PubMed] [Google Scholar]
- 104.Howard BV, et al. A low-fat dietary pattern and diabetes: a secondary analysis from the Women’s health initiative dietary modification trial. Diabetes Care. 2018;41:680–687. doi: 10.2337/dc17-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann. Epidemiol.4, 1–10 (1994). [DOI] [PubMed]
- 106.Kataja-Tuomola M, et al. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2008;51:47–53. doi: 10.1007/s00125-007-0864-0. [DOI] [PubMed] [Google Scholar]
- 107.Pittas AG, et al. Vitamin D supplementation and prevention of type 2 diabetes. N. Engl. J. Med. 2019;381:520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effect of homocysteine-lowering treatment with folic Acid and B vitamins on risk of type 2 diabetes in women: a randomized, controlled trial. Diabetes. 2009;58:1921–1928. doi: 10.2337/db09-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am. J. Clin. Nutr. 2009;90:429–437. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee I-M, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 111.Liu S, et al. Vitamin E and risk of type 2 diabetes in the women’s health study randomized controlled trial. Diabetes. 2006;55:2856–2862. doi: 10.2337/db06-0456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
This systematic review compiles data available in clinical studies. The PMIDs of included studies are available in Table 1. The study-specific numeric estimates for the effect modification has been given in Supplementary Data 1. The source data for Fig. 2 is provided in Supplementary Data 2. All other extracted data have been summarized in the figures and tables presented in the manuscript and are available from the corresponding author on reasonable request.