Abstract
Background
Precision prevention involves using the unique characteristics of a particular group to determine their responses to preventive interventions. This study aimed to systematically evaluate the participant characteristics associated with responses to interventions in gestational diabetes mellitus (GDM) prevention.
Methods
We searched MEDLINE, EMBASE, and Pubmed to identify lifestyle (diet, physical activity, or both), metformin, myoinositol/inositol and probiotics interventions of GDM prevention published up to May 24, 2022.
Results
From 10347 studies, 116 studies (n = 40940 women) are included. Physical activity results in greater GDM reduction in participants with a normal body mass index (BMI) at baseline compared to obese BMI (risk ratio, 95% confidence interval: 0.06 [0.03, 0.14] vs 0.68 [0.26, 1.60]). Combined diet and physical activity interventions result in greater GDM reduction in participants without polycystic ovary syndrome (PCOS) than those with PCOS (0.62 [0.47, 0.82] vs 1.12 [0.78–1.61]) and in those without a history of GDM than those with unspecified GDM history (0.62 [0.47, 0.81] vs 0.85 [0.76, 0.95]). Metformin interventions are more effective in participants with PCOS than those with unspecified status (0.38 [0.19, 0.74] vs 0.59 [0.25, 1.43]), or when commenced preconception than during pregnancy (0.21 [0.11, 0.40] vs 1.15 [0.86–1.55]). Parity, history of having a large-for-gestational-age infant or family history of diabetes have no effect on intervention responses.
Conclusions
GDM prevention through metformin or lifestyle differs according to some individual characteristics. Future research should include trials commencing preconception and provide results disaggregated by a priori defined participant characteristics including social and environmental factors, clinical traits, and other novel risk factors to predict GDM prevention through interventions.
Subject terms: Gestational diabetes, Preventive medicine
Plain language summary
An individual’s characteristics, such as medical, biochemical, social, and behavioural may affect their response to interventions aimed at preventing gestational diabetes, which occurs during pregnancy. Here, we evaluated the published literature on interventions such as diet, lifestyle, drug treatment and nutritional supplement and looked at which individual participant characteristics were associated with response to these interventions. Certain participant characteristics were associated with greater prevention of gestational diabetes through particular treatments. Some interventions were more effective when started prior to conception. Future studies should consider individual characteristics when assessing the effects of preventative measures.
Lim et al. perform a systematic review and meta-analysis to identify participant characteristics associated with response to gestational diabetes prevention. Characteristics such as BMI, polycystic ovary syndrome and being in the preconception phase could determine responses to certain preventive interventions.
Introduction
Gestational diabetes mellitus (GDM) is characterized by glucose intolerance first identified during pregnancy and is associated with perinatal and long-term adverse health outcomes in both the pregnant individual and the offspring. The physiologic reduction in insulin sensitivity during pregnancy is the hallmark metabolic feature that leads to the onset of glucose intolerance and GDM in predisposed individuals1. Established risk factors for GDM include previous GDM, advanced maternal age, parity, overweight/obesity, and family history of diabetes2,3. GDM increases perinatal complications including preeclampsia, operative deliveries, stillbirth, neonatal risks of large-for-gestational age, hypoglycemia, and respiratory distress syndrome4. GDM confers an increased lifetime risk of type 2 diabetes mellitus for both mother and offspring5,6. GDM rates vary considerably, with geographic differences and varying diagnostic criteria accounting for the 1–30% incidence7. Nonetheless, rates of GDM are increasing across all populations8,9, commensurate to worldwide increasing rates of overweight and obesity.
Prevention of GDM involves reducing hyperglycemia and insulin resistance, factors that are also highly correlated with obesity10,11. Weight reduction prior to pregnancy and prevention of excessive gestational weight gain (GWG) are important features of diabetes prevention12,13. Insulin resistance is affected by a number of factors: weight, lifestyle, physical activity, dietary intake and supplement use. Several meta-analyses of randomized controlled trials (RCTs) investigating lifestyle interventions have reported on diet and physical activity interventions, metformin, and supplements as either primary GDM prevention strategies or secondary prevention strategies for trials targeting weight management and/or reduction as a primary outcome. Results of these meta-analyses have not been unanimous in the reporting of findings suggesting heterogeneity in the intervention response, perhaps due to the characteristics of the study population, and/or the timing and type of intervention14–17.
Individual characteristics such as clinical, psychosocial and biochemical factors may influence the effectiveness of interventions in preventing GDM. The prevention of GDM results from an interaction between behavioural factors, such as the ability to adhere to the intervention, and physiological factors, such as the biological responsiveness towards reducing insulin resistance. Hence, clinical traits, such as overweight/obesity, age, history of GDM or polycystic ovary syndrome (PCOS), along with social determinants of health, for example, socioeconomic status, cultural background, race or ethnicity, are potential sources of heterogeneity of the intervention effect18. These clinical, biochemical, social and environmental traits could affect GDM prevention through behavioural or physiological pathways, or both. Given that interventions to prevent GDM are unlikely to be effective for individuals as a ‘one-size-fits-all’ approach, there is a need to elucidate the most effective mode of prevention for each population. To date, there has not been a comprehensive meta-analysis of GDM prevention, accounting for participant characteristics to inform precision medicine.
The field of precision medicine recognizes that examining the heterogeneity of individual responses to intervention is important for optimizing health-enhancing interventions and minimizing exposure to specific risk factors, to delay or prevent the onset of a given disease18,19. The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for better diabetes prevention and care through precision medicine18. This review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine20. To inform a precision medicine approach to diabetes prevention, the primary focus of this review was to assess the contribution of various participant characteristics to the effectiveness of interventions for GDM prevention. To this end, this systematic review and meta-analysis examined the effectiveness of interventions employing lifestyle modification, metformin, or dietary supplements within the preconception, pregnant and postpartum/interconception periods for reducing the risk of developing GDM. We find that certain participant characteristics such as BMI, having polycystic ovary syndrome or history of GDM or being in the preconception phase may determine responses to particular interventions.
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement21. The protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews (CRD42022320513).
Search Strategy
A comprehensive search strategy was developed by a professional medical librarian (AF) in consultation with the authors (SL, LR, KV, JJ). The search strategy included keywords and Medical Subject Headings (MeSH), as shown in Supplementary Table 1. We searched the following databases: Embase (Elsevier), Ovid Medline, and PubMed from the inception of the database to May 24, 2022. Results were limited to studies in human and in English-language. No limit was placed on publication date. Endnote (Clarivate) was used to compile records and remove duplicates. Covidence (Veritas Health Innovation, Melbourne, Australia) was then used for title/abstract screening and full text review. Hand-searches including the reference list of related reviews were also examined for additional eligible trials.
Selection criteria
Randomised and non-randomized controlled trials (RCTs and non-RCTs) investigating the effects of lifestyle (diet, exercise, or both), metformin, or dietary supplements (fish oil, myoinositol/inositol, probiotics) on prevention of GDM in women of childbearing age (including preconception cohorts) were included (Supplementary Table 2). Control conditions included usual care or minimal intervention (no more than a single intervention session in the case of diet and exercise interventions). Studies without a control group (usual care or placebo), those that did not report GDM, observational studies, editorials, commentaries, conference abstracts, reviews, meta-analyses and study protocols were excluded. Titles and abstracts were evaluated independently and in duplicate to identify articles for full-text review. Full-text review was conducted independently and in duplicate with reasons for exclusion recorded. Discrepancies were resolved by consensus by two or more authors.
Data extraction
Data were extracted using an extraction template developed for this study with GDM as the primary outcome. Study characteristics (authors, year of publication, country, setting, sample size, design, diagnostic criteria, diagnosis time point, intervention commencement, and outcome of interest), participant characteristics (age, race/ethnicity, BMI, education status, employment status, parity, prior GDM, smoking status and other medical history), intervention type (diet, physical activity, diet and physical activity, metformin, types of dietary supplement), and outcome of intervention (GDM incidence) were extracted. Study characteristics were determined based on known GDM risk factors and other relevant factors identified by the precision medicine report19. Authors were contacted for missing information. One author conducted the data extraction, and a second author conducted a 10% sub-sample data extraction to establish reliability. An agreement of 89% was achieved between the two authors with discussion to resolve discrepancies.
Quality assessment
Quality of the included studies was critically appraised using a relevant tool for each study design. The Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2.0)22 was used for RCTs to assess bias arising from the randomization process, deviations from the protocol, missing data, measurement of the outcome and selective reporting. The ROBINS-I tool was used for non-RCTs to assess bias from confounding, participant selection, classification of interventions, missing data, deviations from intended interventions, measurement of outcomes, and selection of reported results23. Two reviewers independently conducted the methodological quality and bias assessment for individual studies. Differences were resolved by consensus.
The GRADE process (Grading of Recommendations Assessment, Development and Evaluation), which rates the quality of evidence from a study in a systematic approach was conducted for the primary outcome of GDM24. Risk of bias, along with consistency, directness, precision and publication bias were considered for GRADE appraisal to determine the quality of evidence.
Statistical analysis
The outcome was the incidence of GDM. Data were pooled and GDM incidence was expressed as risk ratios (RR) with 95% confidence intervals (CI). Heterogeneity between studies was assessed by the I2 test where I2 > 50% indicated substantial heterogeneity. Potential sources of heterogeneity by participant characteristics (e.g. obesity, age, PCOS) were explored through subgroup analyses and meta-regression, as conducted in other systematic reviews and meta-analyses25,26. Significant (p < 0.05) Egger’s test and funnel plot (asymmetry) was used to declare publication bias. Estimates (RR) were pooled using random-effects model with the DerSimonian and Laird estimator27. Subgroup analyses were conducted if there was at least one trial present in at least two comparative subgroups. Sensitivity analyses were conducted by excluding non-randomized controlled trials. P < 0.05 was taken as the level of statistical significance. All analyses were conducted in Stata Version 17 (STATA Corporation, College Station, Texas, USA).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
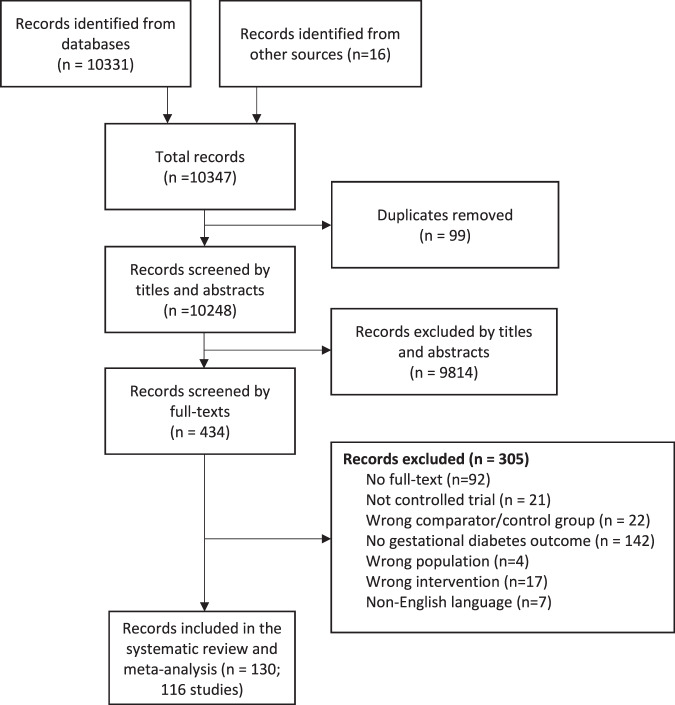
We screened 10,347 records for eligibility and 434 records were reviewed as full texts (Fig. 1). Overall, 130 articles were deemed eligible representing 116 unique studies (117 comparisons due to multiple intervention arms in Luoto et al) and were included in the meta-analysis. Reasons for exclusion included lack of an appropriate control group where the only difference between the treatment and control group was with or without the interventions of interest, no GDM outcome, or active intervention in the control group (Fig. 1).
Fig. 1. PRISMA flow diagram.
Flowchart demonstrating the process of the identifying the papers included in this review. This maps out the number of records identified, included and excluded at each stage of assessment. Reasons for full text exclusions are provided.
Study characteristics
A summary of the characteristics of included studies are shown in Supplementary Data 1. Studies were published from 1997 to 2022. Sample sizes ranged from 31 to 4631. Of the included studies, 92 (79%) involved lifestyle (diet, physical activity, or both), 13 (11%) involved metformin, and 12 (10%) involved dietary supplement interventions. One study included a comparison of lifestyle, probiotics with diet and control28 and was included as both a lifestyle and dietary supplement study in the meta-analysis. Types of lifestyle intervention included diet only (n = 17), physical activity only (n = 19) or a combination of diet and physical activity (n = 59). The types of dietary supplement interventions included myoinositol/inositol (n = 7), probiotics-only (n = 4), probiotics coupled with diet (n = 1), probiotics with fish oil (n = 1) and fish-oil only (n = 1). Interventions commenced from preconception to 26 weeks gestation.
The definition of the participant characteristics is shown in Supplementary Table 3. Detailed description of the participant characteristics of the included studies is shown in Supplementary Table 4. One hundred and five studies commenced the intervention during pregnancy, seven studies reported commencing the intervention prior to pregnancy while four studies did not provide information on pregnancy status at recruitment. Two studies were conducted in women who were nulliparous. A number of studies included only participants with certain medical conditions or medical history: overweight (BMI 25–29.9 kg/m2) or obesity (BMI > 29.9 kg/m2) (n = 57), PCOS (n = 9), prediabetes (n = 1) or family history of diabetes (n = 2); whereas some studies excluded participants with certain medical conditions or medical history: hypertension (n = 27), prediabetes (n = 52), PCOS (n = 3), history of stillbirth (n = 2), family history of diabetes (n = 2), previous macrosomia infant (n = 3), past history of GDM (n = 20), hypertensive disorders of pregnancy (n = 4), history of cardiovascular disease (n = 14), smoking (n = 11). The mean age of the participants ranged from 25 to 34 years, and mean BMI at baseline ranged from 21 to 39 kg/m2. Of the included studies, 25 (22%) had mostly participants with tertiary education (as defined in Supplementary Table 3) and 25 (22%) had mostly participants in employment (Supplementary Table 3). Of the 54 studies which reported race, 18 studies were predominantly conducted among White participants, 10 were predominantly conducted among non-White participants, and 26 among mixed populations.
Each included study reported GDM as a primary or secondary outcome (Supplementary Data 1). The criteria used for GDM diagnosis varied across the studies and included one-step (most commonly a single 75-gram, 2-h oral glucose tolerance test) and two-step methods (commonly the 50 gram one-hour oral glucose challenge test, followed by a 2 or 3-h oral glucose tolerance test if the oral glucose challenge test was abnormal). The most frequently reported diagnostic criteria (n = 37) were those of the International Association of the Diabetes and Pregnancy Study Groups (IADPSG), the World Health Organization (WHO) in 1999 (prior to WHO adopting those of the IADPSG) (n = 9), Carpenter & Coustan (C&C, n = 7), and National Diabetes Data Group (NDDG, n = 6). The method of GDM diagnosis was not reported by 34 studies and 13 studies used a method that could not be categorized by one set of diagnostic criteria. Of the 37 studies using IADPSG criteria, 22 tested diet and physical activity interventions, 9 supplements, 3 metformin, 2 diet, and 1 physical activity. Within those studies that used IADPSG, 26 identified GDM as a primary outcome. Of the 7 studies initiated in the preconception period (2 diet, 1 diet+physical activity, 4 metformin), GDM was the primary outcome for 4, 2 of which were non-randomized clinical studies of metformin use initiated prior to pregnancy targeting women with PCOS and the only 2 that used the same criteria for diagnosing GDM (NDDG).
Meta-analysis
Meta-analysis of all lifestyle interventions (diet only, physical activity only, and combined diet+physical activity) showed a significant reduction in the risk of GDM with moderate heterogeneity (RR 0.78, 95%CI 0.72, 0.85, I2 = 45). (Table 1).
Table 1.
Meta-analysis of the effect of diet, physical activity, metformin or dietary supplement on gestational diabetes prevention.
| Intervention type | Number of studies | Number of participants | Risk Ratio (95% CI) | I2 (%) | Certainty by GRADE | Downgrade explanations |
|---|---|---|---|---|---|---|
| Diet | 17 | 7509 | 0.78 (0.65–0.94) | 45.6 | Moderate | Most studies have Some Concerns or High risk of bias |
| Physical activity | 19 | 6701 | 0.70 (0.57–0.87) | 28.6 | Moderate | Most studies have Some Concerns or High risk of bias |
| Diet and physical activity | 59 | 22,026 | 0.82 (0.73–0.91) | 47 | Low |
Most studies have Some Concerns or a High risk of bias; High levels of heterogeneity |
| Metformin | 13 | 3120 | 0.66 (0.47–0.93) | 73 | Very low |
Most studies have Some Concerns or High risk of bias; High levels of heterogeneity; Egger test suggests significant publication bias |
| Myoinositol | 7 | 1313 | 0.39 (0.23–0.66) | 79 | Very low |
Most studies have Some Concerns or High risk of bias; Very high levels of heterogeneity |
| Probiotics | 5 | 1105 | 0.87 (0.52–1.46) | 73.2 | Very low |
Most studies have Some Concerns or High risk of bias; Very high levels of heterogeneity; Pooled CI crosses 1.0 suggesting imprecision |
Diet-only
In the random effects model, diet-only interventions showed a significant reduction in the risk of GDM (RR 0.78, 95%CI 0.65, 0.94, 17 studies, I2 = 45.6%, moderate-quality evidence) with moderate heterogeneity.
Risk differences in the subgroup and meta-regression analyses by participant characteristics such as sociodemographic (e.g. educational status) and medical history (e.g. prediabetes and hypertension) was not observed (Supplementary Data 2 and 3).
Physical activity-only
Meta-analysis of physical activity-only interventions showed a significant reduction in the risk of GDM (RR 0.70, 95%CI 0.57, 0.87, 19 studies, I2 = 28.6%, moderate-quality evidence) with moderate heterogeneity.
Subgroup analyses showed that physical activity-only interventions resulted in greater reduction in risk for GDM in studies involving women with normal BMI compared with other BMI groups, and in interventions commencing before 12 gestation weeks (Supplementary Data 4).
Diet and physical activity
Meta-analysis of interventions with both diet and physical components showed a significant reduction in the risk of GDM (RR 0.82, 95%CI 0.73, 0.91, 59 studies, I2 = 47%, low-quality evidence) with significant heterogeneity.
Subgroup analyses showed that diet and physical activity interventions were effective in studies involving women with overweight or obesity, but not in studies involving women with normal weight (Supplementary Data 5). Diet and physical activity interventions were more effective in reducing GDM in studies involving women without PCOS compared to those with PCOS, and in studies involving women without a history of GDM compared with those to unspecified history of GDM (Supplementary Data 5). Meta-regression showed that diet and physical activity interventions had greater reduction in GDM with increasing age (Supplementary Data 3).
Metformin
Meta-analysis of all metformin interventions showed a significant reduction in the risk of GDM (RR 0.66, 95%CI 0.47, 0.93, 13 studies, I2 = 73%, very low-quality evidence) with significant heterogeneity.
Subgroup analyses showed that metformin interventions were more effective when commenced preconception compared with during pregnancy (Supplementary Data 6). Metformin interventions were also more effective in reducing GDM in studies involving women with PCOS than those with unspecified status, and less effective in studies involving women without a history of GDM than those with unspecified history of GDM (Supplementary Data 6). Meta-regression showed that metformin interventions were more effective in reducing GDM with increasing age or higher fasting blood glucose at baseline (Supplementary Data 3).
Myoinositol/Inositol
Meta-analysis of myoinositol/inositol interventions showed a significant reduction in the risk of GDM (RR 0.39, 95%CI 0.23, 0.66, 7 studies, I2 = 79%, very low-quality evidence) with significant heterogeneity.
Subgroup analyses showed that myoinositol/inositol interventions were more effective in reducing GDM in studies involving mostly White women compared with women from various ethnic backgrounds (Supplementary Data 7).
Probiotics
Meta-analysis of probiotics interventions showed no reduction in the risk of GDM (RR 0.88, 95%CI 0.52, 1.47, 5 studies, I2 = 74%, very low-quality evidence) with significant heterogeneity. Of these, one study with diet plus probiotics resulted in significant reduction in GDM (RR 0.36, 95%CI 0.18,0.72)28, however four studies with probiotics-only29–32 and one study with fish oil-plus probiotics32 did not reduce GDM (RR ranging from 0.59 to 1.5).
Subgroup analyses and meta-regression were not conducted in probiotics interventions due to small numbers in each type of intervention.
Fish oil
The single fish oil intervention study found did not reduce risk for GDM (RR 1.09, 95%CI 0.64, 1.85)32.
Sensitivity analysis
After excluding two non-RCT studies, diet-only interventions remained significant in preventing GDM (RR 0.75; 95% CI; 0.64, 0.88; I2 = 23%). After excluding six non-RCT studies, combined diet and physical activity interventions remained significant in reducing GDM (RR 0.83; 95% CI; 0.74, 0.93; I2 = 64.8%). After excluding five non-RCT studies, the effect of metformin on reducing the incidence of GDM was no longer significant (RR 1.05, 95% CI 0.89, 1.23; I2 = 45.1%).
Sensitivity analysis was not conducted on physical activity-only, probiotics and myoinositol/inositol studies as they all were RCTs.
Assessment of bias and quality of evidence
Some concerns or high risk of bias were found in 12 (80%) of diet-only, 16 (94%) physical activity-only, 46 (78%) of diet and physical activity, 10 (77%) of metformin, 5 (71%) in myoinositol/inositol, 3 (60%) in probiotics (Supplementary Table 5). Most studies assigned a high risk of bias had insufficient or non-blinding of participants as the main reason, which for lifestyle intervention is essentially not possible.
The quality of evidence rated by the GRADE approach found that the overall quality for diet-only or physical activity-only interventions were moderate, downgraded mainly due to most studies contributing to the outcome having high or some concerns in risk of bias. (Supplementary Table 5). The quality of evidence for diet and physical activity interventions were low, due to risk of bias and inconsistency. The quality of evidence for metformin interventions was very low, due to risk of bias, inconsistency and publication bias. The quality of evidence for myoinositol/inositol interventions was very low, due to risk of bias and inconsistency. The quality of evidence for probiotics interventions was very low due to risk of bias, inconsistency and imprecision.
Publication bias
Funnel plot and Egger’s test suggested the presence of small studies publication bias for metformin studies (P = 0.001) (Supplementary Fig. 1).
No significant publication bias was detected for studies on physical activity-only, diet-only, diet and physical activity, or probiotics (Supplementary Figs. 2–6).
Discussion
This study aimed to determine the effect of participant characteristics in interventions for GDM prevention. Our analyses showed that lifestyle interventions, metformin and myoinositol/inositol reduced the risk of GDM. For physical activity-only interventions, greater risk reduction for GDM was seen in studies involving women with normal BMI. Combined diet and physical activity interventions were more effective in GDM reduction in those with overweight or obesity, without PCOS, without history of GDM and with increasing age. Metformin interventions were more effective in GDM reduction in women with a history of PCOS and with increasing age and fasting blood glucose. Metformin or physical activity-only interventions were more effective when commenced preconception or in early gestation (before 12 gestation weeks).
Diet and physical activity interventions were more effective in lowering the risk for GDM among women without a history of GDM. This may be because women with a prior history of GDM have impaired beta-cell compensatory response during pregnancy and persistent or ongoing decline in insulin sensitivity post-GDM pregnancy11. Lifestyle modification alone may have limited ability to overcome these impairments in glycemic control in these individuals. In addition, lower adherence to a healthy diet, as lower dietary quality, has been observed among women with a history of GDM compared with women without a history of GDM, which may have also contributed to this finding33. Similarly, we also found that lifestyle interventions were more effective in lowering GDM risks in women without a history of PCOS. Like GDM, PCOS is also associated with increased insulin resistance34. In addition to the physiological challenges of insulin resistance, women with PCOS may also face further challenges with adhering to a healthy lifestyle which ranges from physiological barriers such as alteration in gut hormone regulation to psychological barriers such as a high prevalence of disordered eating in this population35. Further, we found that physical activity interventions were only effective in individuals with normal BMI but not in those with obese BMI. Similar observations have been reported in other meta-analyses of GDM prevention in individuals with excess body weight36,37. As obesity is associated with increased insulin resistance, it is possible that physical activity interventions alone could not reduce insulin resistance sufficiently for the prevention of GDM in these individuals. Further research is needed to determine if more intensive lifestyle intervention or additional co-intervention such as metformin or supplementation is needed to prevent GDM in women with conditions of high insulin resistance including prior GDM, PCOS and/or excess body weight. This is important so as not to provide unfounded expectations on the benefit of lifestyle on GDM prevention for certain groups. It is also of particular pertinence to these specific populations outlined, given the stigma associated with obesity and diabetes, resulting from the perception that these health outcomes are caused by personal failures38,39.
To date, systematic reviews are inconsistent in the observed effect of metformin on GDM prevention16,40. Heterogeneity in participant characteristics across studies, which undermines the power to detect a significant effect within a small number of studies, may contribute to this inconsistency16,40. Past studies conducted in select populations with homogeneous characteristics, such as studies in women with PCOS, have found a consistent benefit in GDM prevention with metformin41,42. By increasing the number of included metformin trials from 3 in the previous meta-analysis16 to 13, the current review revealed a significant reduction in GDM with metformin. To further explore the sources of heterogeneity by participant characteristics, our meta-regression additionally found that metformin is more effective in studies involving women with an older mean age at baseline, or in women with higher baseline fasting blood glucose. Increasing age is associated with greater insulin resistance, while higher baseline fasting glucose indicates early signs of failure of beta-cell compensatory response in insulin production43. The greater benefit of metformin in women with increased insulin resistance is in line with the known mechanisms of metformin, which is to reduce glucose production in the liver, improve peripheral glucose uptake and increase insulin sensitivity44. Our findings suggest that metformin may be the intervention of choice for preventing GDM in populations at high risk of insulin resistance, including in women with advanced age, higher fasting blood glucose, history of GDM or PCOS, along with healthy antenatal lifestyle advice. However, we found that the quality of evidence for metformin in preventing GDM was very low, thus further high-quality RCTs are needed to confirm these findings.
In this review, we identified seven preconception interventions that met our inclusion criteria. Despite this small number, metformin or physical activity-only interventions were more effective in lowering the risk for GDM when commenced preconception or in the first trimester of pregnancy. Our conclusions are similar to those of a previous meta-analysis15. Earlier initiation of interventions results in a greater duration of intervention exposure prior to GDM diagnosis, with the benefit of preconception commencement providing an opportunity to optimize insulin sensitivity prior to the onset of pregnancy-induced insulin resistance. A preconception intervention is in line with the concept of GDM arising as a result of a chronic metabolic condition that antedates pregnancy. Although GDM may be the first recognition of impaired fasting glucose or glucose intolerance, data suggest that women who develop GDM are already on a trajectory of increased cardiometabolic risk prior to pregnancy45. Future research should focus on providing preconception GDM prevention in those at risk of developing GDM. The use of preconception risk prediction models for GDM may help identify the populations who will optimally benefit from early initiation interventions46.
The strength of this review includes a comprehensive assessment of the impact of participant characteristics on the effectiveness of a broad range of interventions for the prevention of GDM, with a goal to identify populations that could optimally benefit from each intervention type. Although most studies were conducted in mixed populations and did not report outcomes according to subgroup characteristics, we coded the participant subgroups according to the inclusion and exclusion criteria (e.g. if the study included only women with obesity) to allow for group comparisons. This review also has several limitations. Participant characteristics that are known risk factors for GDM, such as parity, seldom feature in the inclusion or exclusion criteria of relevant studies, yielding a low number of subgroups available for comparison. This could be partly mitigated in individual level meta-analysis, if this information were collected at an individual level by the original studies. Significant heterogeneity remained in some subgroups, suggesting that other confounding factors may have contributed to the effect sizes. One of the confounding effects that are difficult to quantify is the changing GDM diagnostic criteria over the years which may have also contributed to the heterogeneity observed in the analyses. The findings on lifestyle or metformin on GDM incidence may also be limited by possible publication bias. The certainty of the evidence was very low for metformin, myoinositol/inositol and probiotics and low for diet and physical activity combined, and should be interpreted with caution.
Conclusion
Lifestyle, metformin and myoinositol/inositol interventions reduce the risk of GDM. Lower GDM risks were seen when the intervention commenced preconception or in the first trimester of pregnancy. Diet and physical activity interventions may be associated with a greater reduction in GDM risks in women with older age or without a history of GDM or PCOS, while metformin may be more effective in preventing GDM in women with older age, having higher fasting blood glucose or with PCOS. However, these results should be interpreted with caution due to limited reporting of intervention outcomes by participant characteristics in the individual studies. Given the potentially greater effectiveness of lifestyle and metformin interventions in individuals, future research on tailored recommendations in precision GDM prevention, replacing the current ‘one-size-fits-all’ approach, is needed. To advance knowledge in precision prevention, future research should include trials commencing in the preconception period and provide results disaggregated by a priori defined participant characteristics, including social and environmental factors, clinical traits, and other novel risk factors.
Supplementary information
Acknowledgements
The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL).SL is funded by the Australian National Health and Medical Research Council (NHMRC) Fellowship. JW is funded by NHMRC Ideas Grant. WT and MC are funded by the Australian Government Research Training Program Scholarship. GGU is funded by the Monash Graduate Scholarship and Monash International Tuition Scholarship. SC is funded by the Sir George Alberti fellowship, Diabetes UK (21/0006277). LR is funded by the National Institute of Health (5R01DK124806). JJ is funded by the National Institute of Health (5R01DK118403). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person meetings (PI: L. Phillipson, University of Chicago, IL, USA).
Author contributions
S.L., J.J., L.R., and K.V. conceptualised the research question. A.F. contributed to the search of the articles. S.L., M.C., J.G., N.H., L.R., J.J., K.V., W.T., K.L., G.U., and S.C. screened the articles. J.G., N.H., W.T., G.U., G.L., S.Z., R.T., M.P., K.L., M.B., A.Q., W.H., and E.M .extracted the data and appraised the studies. W.T. and S.L. coded the participant characteristics. W.T., G.U. and M.C. contributed to data analysis, all authors contributed to the manuscript.
Peer review
Peer review information
Communications Medicine thanks Naomi Levitt, Alec Welsh and Mireille Van Poppel for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files. The list of included studies is available in Supplementary Data 1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wubet Worku Takele, Kimberly K. Vesco, Leanne M. Redman, Wesley Hannah.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Siew Lim, Email: siew.lim1@monash.edu.
Jami Josefson, Email: JJosefson@luriechildrens.org.
ADA/EASD PMDI:
Deirdre K. Tobias, Jordi Merino, Abrar Ahmad, Catherine Aiken, Jamie L. Benham, Dhanasekaran Bodhini, Amy L. Clark, Kevin Colclough, Rosa Corcoy, Sara J. Cromer, Daisy Duan, Jamie L. Felton, Ellen C. Francis, Pieter Gillard, Véronique Gingras, Romy Gaillard, Eram Haider, Alice Hughes, Jennifer M. Ikle, Laura M. Jacobsen, Anna R. Kahkoska, Jarno L. T. Kettunen, Raymond J. Kreienkamp, Lee-Ling Lim, Jonna M. E. Männistö, Robert Massey, Niamh-Maire Mclennan, Rachel G. Miller, Mario Luca Morieri, Jasper Most, Rochelle N. Naylor, Bige Ozkan, Kashyap Amratlal Patel, Scott J. Pilla, Katsiaryna Prystupa, Sridharan Raghavan, Mary R. Rooney, Martin Schön, Zhila Semnani-Azad, Magdalena Sevilla-Gonzalez, Pernille Svalastoga, Claudia Ha-ting Tam, Anne Cathrine B. Thuesen, Mustafa Tosur, Amelia S. Wallace, Caroline C. Wang, Jessie J. Wong, Jennifer M. Yamamoto, Katherine Young, Chloé Amouyal, Mette K. Andersen, Maxine P. Bonham, Feifei Cheng, Tinashe Chikowore, Christoffer Clemmensen, Dana Dabelea, Adem Y. Dawed, Aaron J. Deutsch, Laura T. Dickens, Linda A. DiMeglio, Monika Dudenhöffer-Pfeifer, Carmella Evans-Molina, María Mercè Fernández-Balsells, Hugo Fitipaldi, Stephanie L. Fitzpatrick, Stephen E. Gitelman, Mark O. Goodarzi, Jessica A. Grieger, Marta Guasch-Ferré, Nahal Habibi, Torben Hansen, Chuiguo Huang, Arianna Harris-Kawano, Heba M. Ismail, Benjamin Hoag, Randi K. Johnson, Angus G. Jones, Robert W. Koivula, Aaron Leong, Gloria K. W. Leung, Ingrid M. Libman, Kai Liu, S. Alice Long, William L. Lowe, Jr., Robert W. Morton, Ayesha A. Motala, Suna Onengut-Gumuscu, James S. Pankow, Maleesa Pathirana, Sofia Pazmino, Dianna Perez, John R. Petrie, Camille E. Powe, Rashmi Jain, Debashree Ray, Mathias Ried-Larsen, Zeb Saeed, Vanessa Santhakumar, Sarah Kanbour, Sudipa Sarkar, Gabriela S. F. Monaco, Denise M. Scholtens, Elizabeth Selvin, Wayne Huey-Herng Sheu, Cate Speake, Maggie A. Stanislawski, Nele Steenackers, Andrea K. Steck, Norbert Stefan, Julie Støy, Rachael Taylor, Sok Cin Tye, Gebresilasea Gendisha Ukke, Marzhan Urazbayeva, Bart Van der Schueren, Camille Vatier, John M. Wentworth, Wesley Hannah, Sara L. White, Gechang Yu, Yingchai Zhang, Shao J. Zhou, Jacques Beltrand, Michel Polak, Ingvild Aukrust, Elisa de Franco, Sarah E. Flanagan, Kristin A. Maloney, Andrew McGovern, Janne Molnes, Mariam Nakabuye, Pål Rasmus Njølstad, Hugo Pomares-Millan, Michele Provenzano, Cécile Saint-Martin, Cuilin Zhang, Yeyi Zhu, Sungyoung Auh, Russell de Souza, Andrea J. Fawcett, Chandra Gruber, Eskedar Getie Mekonnen, Emily Mixter, Diana Sherifali, Robert H. Eckel, John J. Nolan, Louis H. Philipson, Rebecca J. Brown, Liana K. Billings, Kristen Boyle, Tina Costacou, John M. Dennis, Jose C. Florez, Anna L. Gloyn, Maria F. Gomez, Peter A. Gottlieb, Siri Atma W. Greeley, Kurt Griffin, Andrew T. Hattersley, Irl B. Hirsch, Marie-France Hivert, Korey K. Hood, Jami L. Josefson, Soo Heon Kwak, Lori M. Laffel, Siew S. Lim, Ruth J. F. Loos, Ronald C. W. Ma, Chantal Mathieu, Nestoras Mathioudakis, James B. Meigs, Shivani Misra, Viswanathan Mohan, Rinki Murphy, Richard Oram, Katharine R. Owen, Susan E. Ozanne, Ewan R. Pearson, Wei Perng, Toni I. Pollin, Rodica Pop-Busui, Richard E. Pratley, Maria J. Redondo, Rebecca M. Reynolds, Robert K. Semple, Jennifer L. Sherr, Emily K. Sims, Arianne Sweeting, Tiinamaija Tuomi, Miriam S. Udler, Kimberly K. Vesco, Tina Vilsbøll, Robert Wagner, Stephen S. Rich, and Paul W. Franks
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-023-00366-x.
References
- 1.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, et al. Factors associated with gestational diabetes mellitus: a meta-analysis. J. Diab. Res. 2021;2021:6692695. doi: 10.1155/2021/6692695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habibi N, et al. Maternal metabolic factors and the association with gestational diabetes: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2022;38:e3532. doi: 10.1002/dmrr.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes mellitus. Endocr. Rev. 2022;43:763–793. doi: 10.1210/endrev/bnac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe WL, Jr., et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320:1005–1016,. doi: 10.1001/jama.2018.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowe WL, Jr., et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42:372–380. doi: 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIntyre HD, et al. Gestational diabetes mellitus. Nat. Rev. Dis. Primers. 2019;5:47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 8.Shah NS, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660–669,. doi: 10.1001/jama.2021.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31:2288–2293. doi: 10.2337/dc08-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1999;180:903–916. doi: 10.1016/S0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 11.Plows, J. F., Stanley, J. L., Baker, P. N., Reynolds, C. M. & Vickers, M. H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci.19, 10.3390/ijms19113342 (2018). [DOI] [PMC free article] [PubMed]
- 12.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstetrics and gynecology. 2010;115:597–604. doi: 10.1097/AOG.0b013e3181cfce4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong C, et al. Greater early and mid-pregnancy gestational weight gain are associated with increased risk of gestational diabetes mellitus: A prospective cohort study. Clin. Nutr. ESPEN. 2017;22:48–53. doi: 10.1016/j.clnesp.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Guo XY, et al. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: a meta-analysis and meta-regression. BJOG. 2019;126:311–320. doi: 10.1111/1471-0528.15467. [DOI] [PubMed] [Google Scholar]
- 15.Song C, Li J, Leng J, Ma RC, Yang X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obes. Rev. 2016;17:960–969. doi: 10.1111/obr.12442. [DOI] [PubMed] [Google Scholar]
- 16.Griffith RJ, et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2020;6:CD012394. doi: 10.1002/14651858.CD012394.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogozinska E, Chamillard M, Hitman GA, Khan KS, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLoS One. 2015;10:e0115526. doi: 10.1371/journal.pone.0115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolan JJ, et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diab. Care. 2022;45:261–266. doi: 10.2337/dc21-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung WK, et al. Precision Medicine in Diabetes: A Consensus Report From the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diab. Care. 2020;43:1617–1635. doi: 10.2337/dci20-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobias, D. K., Merino, J., Ahmad, A. & PMDI, A. E. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. (2023). 10.1038/s41591-023-02502-5 (2023). [DOI] [PMC free article] [PubMed]
- 21.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J. P., S. J., Page M. J., Elbers RG, Sterne J. A. in Cochrane Handbook for Systematic Reviews of Interventions. 205–228 (2019).
- 23.Sterne JA, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt G, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Gillies CL, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. Bmj. 2007;334:299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim S, et al. A systematic review and meta-analysis of intervention characteristics in postpartum weight management using the TIDieR framework: A summary of evidence to inform implementation. Obes. Rev. 2019;20:1045–1056. doi: 10.1111/obr.12846. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br. J. Nutr. 2010;103:1792–1799. doi: 10.1017/S0007114509993898. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay KL, et al. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study) Am. J. Clin. Nutr. 2014;99:1432–1439. doi: 10.3945/ajcn.113.079723. [DOI] [PubMed] [Google Scholar]
- 30.Wickens KL, et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br. J. Nutr. 2017;117:804–813. doi: 10.1017/S0007114517000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callaway LK, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care. 2019;42:364–371. doi: 10.2337/dc18-2248. [DOI] [PubMed] [Google Scholar]
- 32.Pellonperä O, et al. Efficacy of Fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: a randomized, placebo-controlled, double-blind clinical trial. Diabetes Care. 2019;42:1009–1017. doi: 10.2337/dc18-2591. [DOI] [PubMed] [Google Scholar]
- 33.Xiao RS, Simas TA, Person SD, Goldberg RJ, Waring ME. Diet quality and history of gestational diabetes mellitus among childbearing women, United States, 2007-2010. Prev. Chronic Dis. 2015;12:E25. doi: 10.5888/pcd12.140360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh, S. et al. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med.12, 10.3390/jcm12041454 (2023). [DOI] [PMC free article] [PubMed]
- 35.Lim S, et al. Barriers and facilitators to weight management in overweight and obese women living in Australia with PCOS: a qualitative study. BMC Endocr. Disord. 2019;19:106. doi: 10.1186/s12902-019-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhammad HFL, Pramono A, Rahman MN. The safety and efficacy of supervised exercise on pregnant women with overweight/obesity: A systematic review and meta-analysis of randomized controlled trials. Clin. Obes. 2021;11:e12428. doi: 10.1111/cob.12428. [DOI] [PubMed] [Google Scholar]
- 37.Nasiri-Amiri F, Sepidarkish M, Shirvani MA, Habibipour P, Tabari NSM. The effect of exercise on the prevention of gestational diabetes in obese and overweight pregnant women: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019;11:72. doi: 10.1186/s13098-019-0470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speight, J. & Holmes-Truscott, E. Challenging diabetes stigma starts and ends with all of us. Lancet Diabetes Endocrinol, 10.1016/s2213-8587(23)00084-0 (2023). [DOI] [PubMed]
- 39.Hill B, Incollingo Rodriguez AC. Weight stigma across the preconception, pregnancy, and postpartum periods: a narrative review and conceptual model. Semin. Reprod. Med. 2020;38:414–422. doi: 10.1055/s-0041-1723775. [DOI] [PubMed] [Google Scholar]
- 40.Pascual-Morena, C. et al. Exercise versus Metformin to Improve Pregnancy Outcomes among Overweight Pregnant Women: A Systematic Review and Network Meta-Analysis. J. Clin. Med.10, 10.3390/jcm10163490 (2021). [DOI] [PMC free article] [PubMed]
- 41.Zhao Q, He J. Efficacy and safety of metformin in pregnant women with polycystic ovary syndrome: a systematic review with meta-analysis of randomized and non-randomized controlled trials. Gynecol. Endocrinol. 2022;38:558–568. doi: 10.1080/09513590.2022.2080194. [DOI] [PubMed] [Google Scholar]
- 42.Zhuo Z, Wang A, Yu H. Effect of metformin intervention during pregnancy on the gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and meta-analysis. J. Diabetes Res. 2014;2014:381231. doi: 10.1155/2014/381231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59:89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021;42:77–96. doi: 10.1210/endrev/bnaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Retnakaran R. Diabetes in pregnancy 100 years after the discovery of insulin: Hot topics and open questions to be addressed in the coming years. Metabolism. 2021;119:154772. doi: 10.1016/j.metabol.2021.154772. [DOI] [PubMed] [Google Scholar]
- 46.Schoenaker D, Vergouwe Y, Soedamah-Muthu SS, Callaway LK, Mishra GD. Preconception risk of gestational diabetes: Development of a prediction model in nulliparous Australian women. Diabetes Res. Clin. Pract. 2018;146:48–57. doi: 10.1016/j.diabres.2018.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Information files. The list of included studies is available in Supplementary Data 1.