Abstract
Autophagy is the process by which cells degrade and recycle proteins and organelles to maintain intracellular homeostasis. Generally, autophagy plays a protective role in cells, but disruption of autophagy mechanisms or excessive autophagic flux usually leads to cell death. Despite recent progress in the study of the regulation and underlying molecular mechanisms of autophagy, numerous questions remain to be answered. How does autophagy regulate cell death? What are the fine-tuned regulatory mechanisms underlying autophagy-dependent cell death (ADCD) and autophagy-mediated cell death (AMCD)? In this article, we highlight the different roles of autophagy in cell death and discuss six of the main autophagy-related cell death modalities, with a focus on the metabolic changes caused by excessive endoplasmic reticulum-phagy (ER-phagy)-induced cell death and the role of mitophagy in autophagy-mediated ferroptosis. Finally, we discuss autophagy enhancement in the treatment of diseases and offer a new perspective based on the use of autophagy for different functional conversions (including the conversion of autophagy and that of different autophagy-mediated cell death modalities) for the clinical treatment of tumors.
Subject terms: Macroautophagy, Cancer metabolism
Facts
Autophagy is involved in the regulation of almost all modes of cell death in disease contexts.
Autophagy plays a role in protecting cells against most diseases, and lethal autophagy is usually induced by pharmacological or genetic treatment.
Excessive autophagy-induced cell death is caused by excessive degradation of cellular contents and involves various metabolic mechanisms and organelles.
Open questions
Protective autophagy and lethal autophagy may be simultaneously triggered in cells. How do cells balance the regulatory mechanisms of these processes?
What is the biological mechanism explaining why cells undergo excessive autophagy?
What is the role of autophagy in the phagocytosis of organelles in cells undergoing ferroptosis?
Can controlling the conversion of autophagy function contribute to the treatment of cancer, neurodegenerative diseases, inflammation, cell senescence and cardiovascular diseases.
Introduction
Death is the end of all living things. In recent decades, scientists have gradually unraveled the mystery of cell death [1]. Moreover, the roles of autophagy in cell death have received increasing attention, but the precise mechanisms underlying the roles are still unclear. Macroautophagy (hereafter referred to as autophagy) is a process by which cellular contents are encircled by the membrane-bound structures known as autophagic vesicles, which eventually fuse with lysosomes; in these vesicles, the cellular contents are broken down into small molecules [2]. Autophagy plays a dual role in various diseases. For example, in the early stages of tumorigenesis, autophagy plays a tumor-suppressing role by helping maintain genomic integrity and inhibiting tissue damage and inflammation through processes involving quality control systems and oxidative stress responses [3, 4]. However, in the advanced stage of tumor development, autophagy provides nutrients to cancer cells and promotes their immune escape (by promoting the degradation of MHC-I on the surface of cancer cells [5]), as well as other functions [2, 6]. The effects of autophagy on organ tissues appear to be related to the presence of disease or health of an organism [7]. Moreover, autophagy seems to determine whether a cell lives or dies [8–10]. The roles of autophagy in cell death are mainly classified into autophagy-dependent cell death (ADCD) (or autophagic cell death, ACD), which depends on the autophagy mechanism, and autophagy-mediated cell death (AMCD), which depends on other modes of cell death. In ADCD, the unrestricted degradation of cellular contents leads to a disrupted cellular environment; however, is the mechanism truly a simple case of excessive self-eating? In AMCD, autophagy drives different modes of death as the basis for the initiation of other death modes; however, does autophagy also regulate the switching to different modalities of cell death after clinical treatment? In this paper, we highlight the various forms of autophagy and the mechanisms that mediate cell death, explaining the shift in cells undergoing AMCD.
Autophagosome formation
Since the discovery of the autophagy-related genes (ATGs) in brewer’s yeast by Yoshinori Ohsumi in the 1990s [11], scholars have identified more than 40 ATGs in yeast by genetic screening, and approximately one-half of these genes show clear homology to ATGs in mammals. AMPK and mTOR signaling initiate autophagy, and their function is mediated through cellular responses to stress, such as starvation or oxidation (Fig. 1). ULK1 Ser757 is dephosphorylated by protein phosphatase 2A (PP2A) [12] and protein phosphatase 1D magnesium-dependent delta isoform (PPM1D) [13] after the dissociation of mTOR from ULK1, and AMPK phosphorylates ULK1 Ser 317 and Ser 777 to promote the formation of the ULK1 complex [14]. OGT co-mediates the phosphorylation of ATG14 Ser29 with GABARAPs after the acetylation of ULK1 Ser757 by O-GlcNAc [15, 16]. After activation of the ULK1 complex, PIKfyve Ser1548 is phosphorylated to promote the conversion of PI to PI(5)P, with the latter subsequently binding WIPI2 to promote separation of the isolation membrane from the ER membrane [2, 17, 18]. To continue the extension of the isolation membrane, the PI3KC3 complex binds to the ATG5-ATG12 complex and the ATG8/LC3 system via WIPI2, promoting ATG5-ATG12 complex and ATG8/LC3 system ubiquitination via a positive feedback loop until the isolation membrane closes, forming an autophagosome [19, 20]. Then, autophagosomes recruit lysosomal fusion proteins, while ATGs on the outer autophagosome membrane are successively removed. STX17 is deacetylated, and C-terminal hairpin-like structures are inserted into intact autophagosomes, where they interact with SNAP29 and HOPS to facilitate autophagosome–lysosome binding [21, 22]. Ultimately, the cellular cargo is degraded into small molecules by the lysosomes and recycled.
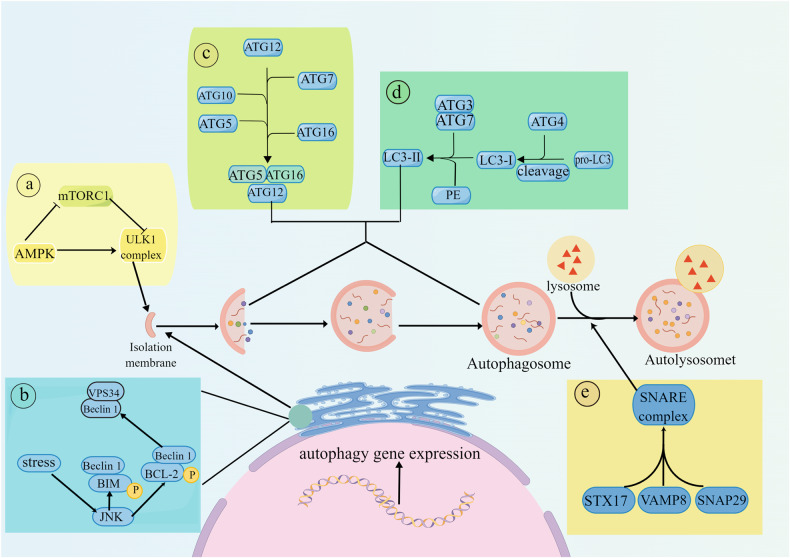
Fig. 1. Mechanism of autophagy.
Autophagy is a complex self-degradation process involving the following key steps: ①Signaling pathways regulate the initiation of autophagy. AMPK inhibits the formation of the mTORC1 complex, which weakens the inhibitory effect of mTORC1 on the formation of the ULK1 complex, thereby promoting the production of autophagic vesicles. ② The Beclin-1/VPS34 complex promotes the extension of autophagic vesicles. The activated kinase JNK destroys the Beclin1/BCL-2 and Beclin1/BIM complexes by phosphorylating BCL-2 and BIM to free Beclin1. Free Beclin1 activates VPS34 and binds to it to form a complex, and the PI3P that is produced promotes the extension of autophagic vesicles. ③ The ATG12-ATG5 complex binds to ATG16 and completes polymerization. The polymer complex ATG5-ATG12-ATG16L is formed by a series of ATG5, ATG12 and ATG16L actions, and then, the polymer complex is fused with autophagic vesicles. ④ LC3 is inserted into autophagosomes through a series of reactions. The cysteine protease ATG4 cleaves LC3 into LC3-I, which is then processed by ATG3, ATG7 and phosphatidylethanolamine to form LC3-II. Subsequently, LC3-II is inserted into the autophagosomes. ⑤ Autophagosomes and lysosomes fuse to form autolysosomes. STX17 binds to SNAP29 and VAMP8 to form a SNARE complex that is transferred to the autophagosomal membrane, allowing lysosomes to fuse with autophagosomes and form autolysosomes. The graph was drawn with Figdraw.
Autophagy and cell death
In addition to maintaining intracellular energy and metabolic homeostasis, autophagy can mediate cell death under certain conditions. The following two main types of autophagy-related cell death modes have been described: 1) ADCD, which is highly dependent on autophagy components and is independent of other forms of programmed death. For cell death to be attributed to ADCD, ① the autophagic flux must be elevated during cell death; ② the cell death process must be reversible via genetic or pharmacological inhibition of autophagy; ③ the death process depends on at least two autophagy molecules, which prevents individual molecules from mediating cell death independent of autophagy; and ④ the death process must not be accompanied by other modes of death [23, 24]. 2) In AMCD, autophagic molecules interact directly with cell death molecules or autophagy triggers apoptosis, necrosis, and ferroptosis via dynamic autophagy functions [8, 25]. The two forms of autophagy-related cell death are not independent of each other and can be triggered simultaneously, and cell death processes may switch between these two modalities under certain conditions [26]. Herein, we describe the six modes of cell death mediated by ADCD (endoplasmic reticulum-phagy [ER-phagy], mitophagy, and autosis) and AMCD (apoptosis, necroptosis, and ferroptosis).
Autophagy-dependent cell death
ADCD is involved in the degradation of the larval midgut during Drosophila development, and knockdown of the associated ATG-encoding genes significantly delays midgut degradation [27]. In embryonic model mice with Bax/Bak knocked out, autophagy facilitated elimination of interdigital web cells. Autophagosomes appear to predominantly breakdown cell matrix components with no apparent commensurate degradation of organelles [28]. ADCD is important to epidermal keratin-forming cells in adult mammalian skin, and autophagy regulates terminal cell death by degrading organelles, such as the ER or mitochondria, or intracellular membranes [29]. In recent years, excessive autophagy in tumor or cardiovascular disease cells has been widely reported and classified into ER-phagy, excessive mitophagy, and autosis. These three types of autophagy are described in this section.
Excessive ER-phagy
Introduction to excessive ER-phagy
The ER is the largest organelle in eukaryotic cells. It can form a complex cell quality control network by contacting other organelle membranes and participates in Ca2+ homeostasis and protein synthesis [30]. The ER activates protective mechanisms under stress conditions, such as a lack of nutrients or oxygen, including the unfolded protein response (UPR), ER-associated degradation (ERAD) and ER-phagy. Although ER-phagy was observed as early as 1965 [31], how the isolation membrane specifically recognizes the ER was not known until more recently. In 2015, FAM134B was the first ER-phagy receptor to be identified. In mammalian cells, FAM134B carries an LC3 interaction region (LIR), which specifically recognizes the LC3/GABARAP family, and an ER homology domain (RHD), which is involved in fragmenting the ER into small segments, enabling engulfment by the autophagosome [32]. In yeast, the FAM134B homologs ATG39 and ATG40 also regulate ER homeostasis via ER-phagy [33].
The primary function of ER-phagy under stress condition is degradation of the expanded ER membrane to maintain cellular quality and balance [34]. The UPR produces upstream activation signaling that triggers autophagy [35], which activates ATF4, inositol-requiring enzyme 1 (IRE1), and other molecules to mediate ADCD after pharmacological treatment [36, 37]. Eleven ER-phagy receptors have been identified [34], and two of these proteins have been reported to be involved in ADCD. Loperamide treatment of glioblastoma (GBM) promotes the UPR, and downstream ATF4 mediates the activation of the ER-phagy receptors FAM134B and TEX264 to promote ER degradation [38]. ER function is impaired by the continuous degradation of ER fragments, eventually leading to cell death. However, some studies have described different mechanisms to explain ER-phagy. In stressed environments, FAM134B activity is upregulated to promote ER-phagy, continuously degrading the ER and impairing its function until the UPR is activated (Fig. 2) [39]. Although the study explaining this process did clearly explain the relationship between ER-phagy receptors, autophagy and the UPR, it is commonly thought that the UPR sends upstream activation signals to trigger autophagy and induce autophagy receptor expression [35, 38, 40], as is discussed below.
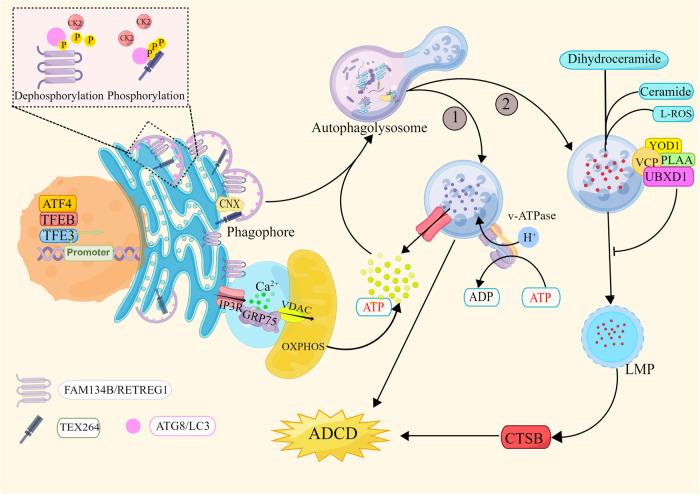
Fig. 2. Excessive ER-phagy-mediated cell death.
① The transcription factors ATF4, TFEB, and TFE3 induce the expression of the ER-phagy receptors FAM134B/RETREG1 and TEX264 after pharmacological treatment, thereby initiating the ER-phagy program. Dephosphorylated CK2 phosphorylates the LIR in FAM134B and TEX264 to enhance their ability to bind LC3. The ER is cleaved into small fragments, which are wrapped by the isolation membrane and eventually degraded by lysosomes. Excessive ER consumption eventually leads to cell death. ②Excessive ER-phagy leads to the disruption of lipid and cholesterol metabolism, and lipid accumulation in lysosomes results in the accumulation of Lipid-ROS and the release of LMP, with CTSB leading to ADCD. The lysosomal receptor VCP and its cofactors YOD1, PLAA, and UBXD1 induce the degradation of damaged lysosomes to mitigate ADCD. OXPHOS provides energy for ER-phagy, and FAM134B protects mitochondria by inhibiting ROS, degrading CNX, and inhibiting Ca2+ conductance mediated by the IP3R-GRP75-VDAC complex. The graph was drawn with Figdraw.
The energy context of excessive ER-phagy
Neither ER stress nor autophagy is mediated by a complex regulatory signaling network, and the cell metabolism context of excessive ER-phagic environments cannot be fully explained by UPR signaling, ER-phagy or ER damage. During excessive ER-phagy, approximately fivefold more autophagic vacuoles are produced than that which are produced in cells under physiological conditions, and twice as many as those in the stressed state [39]; twice as many autophagosomes implies doubled energy expenditures. After 30%-50% of ATP is consumed, the autophagosome volume decreases by 70% [41]. How do cells maintain constant autophagic flux when energy is constantly depleted? The functions of two receptors mediating ADCD, FAM134B and TEX264, reveal the answer [38, 39]. To attenuate the disruption to energy homeostasis in cells undergoing excessive autophagic flux, activation of the transcription factors TFEB and TFE3 promotes the expression of FAM134 family proteins and TEX264, and the regulation of casein kinase 2 (CK2) mediated via phosphorylation enhances the affinity of autophagy receptors for LC3 (Fig. 2). FAM134B and TEX264 at the ER lamellae and tubule junctions, respectively, are activated to mediate autophagosome envelopment of ER fragments and their further breakdown into small-molecule metabolites [42–45]. Thus, FAM134B and TEX264 mediate the degradation of more than 50% of ER fragments within 15 min [42, 46], effectively balancing the energy consumed via excessive autophagic flux. However, pharmacological treatments cause high stress levels in cells, and the energy recycled via autophagy alone is clearly not sufficient to sustain the energy expenditure associated with cell quality control and the repair of damaged proteins or DNA. Meyer et al. observed that mitochondria remained unexpectedly intact during excessive ER-phagy [47], suggesting that mitochondria may be regulated via protective mechanisms that enable them to function. A genome-wide CRISPR interference (CRISPRi) reporter gene screening with the HCT116 colorectal cancer cell line by Liang et al. revealed that the oxidative phosphorylation system (OXPHOS) stimulated ER-phagy through a nonclassical energy-regulating pathway [48]. The metabolism in tumor cells easily changes from aerobic glycolysis to OXPHOS through metabolic reprogramming [49], and this efficient metabolic shift addresses the energy balance discussed above. However, how do cells ensure that mitochondria safely undergo OXPHOS in a high-stress context? To ensure mitochondrial function, FAM134B reduces mitochondrial damage by inhibiting Ca2+ and reactive oxygen species (ROS) production. FAM134B inhibits Ca2+ hypo-transduction in the mitochondria-associated ER membrane (MAM) by the inositol-1,4,5-trisphosphate receptor (IP3R)-glucose regulatory protein 75 (Grp75)-voltage-dependent anion channel (VDAC) complex, which is involved in MAM formation. VDAC and IP3R are channels through which Ca2+ enters mitochondria. Excessive conduction of Ca2+ may lead to mitochondrial dysfunction, and FAM134B reduces Ca2+ transfer between MAMs by inhibiting IP3R activity, but the exact mechanism is not clear. In addition, FAM134B induces ER-phagy to directly degrade calmodulin and CNX and attenuates mitochondrial Ca2+ damage [50, 51]. Additionally, FAM134B overexpression mitigates ROS accumulation [52]. In contrast, although FAM134B has been suggested to interact with the mitochondrial inner membrane protein OPA1 and the FAM134B LIR motif plays a role in mitochondria degradation by assembling phagocytic factors around mitochondria [53], cells in models of excessive ER-phagy preferentially protect energy-producing structures [47].
Other mechanisms underlying excessive ER-phagy
Loperamide and pimozide induce excessive ER-phagy in patients with GBM and upregulation of cholesterol and lipid anabolism, leading to the accumulation of cholesterol and lipids (predominantly ceramide) in lysosomes [47]. The accumulation of lipids leads to lysosomal membrane permeabilization (LMP), which contributes to lipid-ROS production and cathepsin B (CTSB) release [47]. Moreover, if a rapid lysosomal repair mechanism mediated by phosphoinositide and the ER does not function properly due to high Lipid-ROS production rates and the continuous depletion of ER function [54], this can ultimately lead to autophagy-mediated LMP-related death. Interestingly, p97/VAP is translocated to the surface of lysosomes after cells are damaged and recruits cofactors YOD1, UBXD1, and PLAA to induce lysophagy, through which the damaged lysosomes are degraded, promoting GBA cell survival (Fig. 2) [47, 55]. This outcome indicates that protective and lethal autophagy may both be triggered and balanced and that triggering the activation of certain molecules may lead to cell death mediated by an imbalance in autophagy.
The membrane source of excessive ER-phagy is unknown and an interesting area of inquiry. Isolation membranes usually form at the ER, Golgi apparatus or MAM [18], and different membrane sources may accelerate excessive ER-phagy-mediated ADCD. When the predominant membrane source is the ER, ER-phagy promotes ER membrane consumption, and isolation membrane formation accelerates this process. Interestingly, FAM134B knockdown reduced the volume of the Golgi apparatus by 40% without altering the cell volume [56], suggesting that FAM134B may influence the source of membranes of autophagosomes by regulating the volume of organelles. In conclusion, the dynamic structure of the ER dictates that its size needs to be coordinated with that of other organelles to maintain physiological cell functions [30], and further investigation into the fine-tuned regulatory mechanisms among organelles in the context of excessive ER-phagy may facilitate the development of new ADCD-related therapeutic strategies.
Mitophagy
Mitochondria are involved in almost all critical steps in tumor development, including the production of ATP and ROS, functioning as hubs for anabolism and regulated cell death (RCD) signaling [57]. Mitochondria have complex quality-regulatory mechanisms, which primarily balance the removal of damaged or depolarized mitochondria and mitochondria biogenesis, and excessive mitophagy leads to defects in mitochondrial function and cell death.
Mitophagy-triggered ADCD was first identified in 2006, when Reef et al. found that overexpression of an alternative translation product of ARF (p19), namely, short mitochondrial ARF (smARF), led to an abnormal mitochondrial membrane potential and promoted mitophagy-mediated ADCD [58]. Budina-Kolomets et al. further found that full-length ARF induced autophagy, but smARF bound Parkin to induce mitophagy [59, 60].
AT-101, a natural compound from cotton seeds, induced excessive mitophagy in glioma cells by upregulating the expression of heme oxygenase 1 (HMOX1) and the mitophagy receptors BNIP3 and BNIP3L [61]. 1-(3,4,5-Trihydroxyphenyl) nonan-1-one (THPN) drives excessive mitophagy in melanoma. The nuclear receptor TR3 (Nur77 or NGFI-B) is translocated to the outer mitochondrial membrane (OMM), interacts with NIX, and enters the mitochondrial space through the channel proteins Tom40 and Tom70, promoting alteration of the mitochondrial membrane potential by permeability transition pore complex ANT1-VDAC1 and induction of excessive mitophagy (Fig. 3) [62]. Akt2 phosphorylation of TR3 inhibits its translocation and reduces the therapeutic sensitivity of cancer cells to THPN [63].
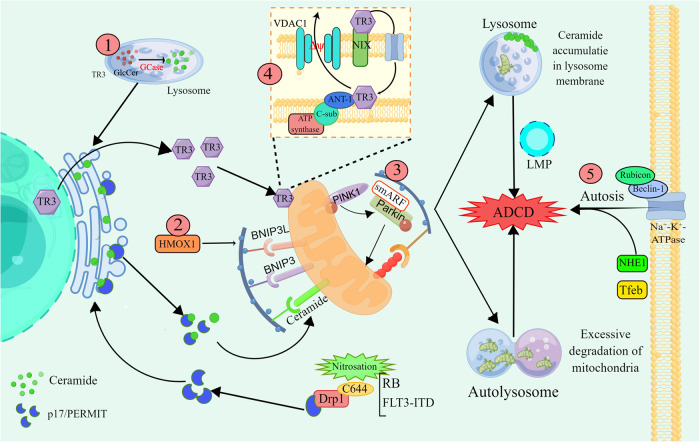
Fig. 3. Mechanisms underlying lethal mitophagy and autosis.
① GCase overexpression promotes lysosomal glucose ceramide catabolism into glucose and lysosomal ceramide, which are recycled in the ER. Under conditions of RB or FLT3-ITD inhibition, Drp1 C644 nitrosylation leads to the release of the transport protein p17/PERMIT. Ceramide is transported from the ER to mitochondria by p17/PERMIT, which functions as a mitophagy receptor, and its binding to lipidated LC3 promotes lethal mitophagy. In addition, ceramide or dihydroceramide accumulates in autophagosomal membranes to trigger LMP. ②AT-101 induces the transcription and translation of HMOX1, BNIP3L and BNIP3 and promotes excessive mitophagy. ③smARF promotes excessive mitophagy by binding to Parkin. ④ TR3 is translocated from the nucleus to mitochondria, interacts with NIX and enters the mitochondrial membrane space via Tom40 and Tom70. TR3 interacts directly with ANT-1 and promotes VDAC1 action to alter the MMP and thus induce mitophagy. ⑤ Rubicon and Beclin-1 bind directly to Na+-K+-ATPase to promote autosis. This graph was drawn with Figdraw.
In recent years, sphingolipids have been identified as key molecules in the induction of lethal mitophagy [64, 65]. Dasari et al. treated A549 non-small cell lung cancer (NSCLC) cells with resveratrol and identified GBA1 as a “switch” that induces ADCD, as discovered by short hairpin RNA (shRNA) survival screening [65]. GCase catalyses the conversion of glucose ceramide to glucose and lysosomal ceramide, the latter of which can be reconverted to ceramide in the ER after lysosomal breakdown. Under stress conditions, the mitochondrial fission molecule Dynamin-related protein 1 (Drp1) is nitrosylated at C644, releasing p17/PERMIT. p17/PERMIT moves near the ER and binds to CerS1 in the ER, facilitating CerS1-induced ceramide-dependent mitophagy via the OMM [64, 66]. In resveratrol-treated A549 cells, knockdown of GCase significantly inhibited ADCD, and its overexpression upregulated C16:0, C18:0, C20:0 and C24:1 ceramide levels [65]. Furthermore, knockdown of GBA1 delays midgut degradation in Drosophila larvae, suggesting that GBA1-mediated ADCD is a conserved mechanism [67]. In addition, sphingolipids affect the stability of autophagolysosomes. Δ9-Tetrahydrocannabinol (THC) inhibits the translocation of dihydroceramide from the ER to the Golgi apparatus, and accumulated dihydroceramide is enriched in autophagosomes to promote LMP [68]. Notably, in contrast to loperamide, which causes impaired lysosomal degradation leading to sphingolipid accumulation [47], the small-molecule anticancer drug ABTL0812 reduces DEGS1 activity to increase the level of dihydroceramide, which mediates cytotoxic autophagy via the activation of ER stress/ the UPR [69]. Studies have shown that Drp1-ceramide activation induces excessive mitophagy. Thomas et al. found that HPV-E7 promoted ceramide-induced mitophagy and alleviated head and neck squamous cell carcinoma development by inhibiting RB activity to activate Drp1 [70]. Inhibition of FLT3-ITD has been shown to promote cell death through Drp1 activation of CerS1/C18 ceramide synthesis in acute myeloid leukemia samples [71].
Alterations in lipid metabolism may contribute to the imbalance between protective lysophagy and lethal mitophagy or ER-phagy, and this alteration is evident in almost all types of ADCD (ER-phagy, mitophagy and normal autophagy). Notably, excessive autophagy may activate other processes to promote cell death. In conclusion, excessive degradation of organelles by either ER-phagy or mitophagy is not the only cause of cell death.
Autosis
Autosis is a novel form of ADCD. Notably, whether autosis is truly a form of ADCD is debated, and although autosis-mediated death cannot be completely rescued after pharmacological inhibition of autophagy, we include a description of autosis as a form ADCD in this paper. Autosis has recently been identified and is characterized by an increase in the number of autophagic vacuoles and focal swelling of the perinuclear space, with cell death mainly associated with Na+, K+-ATPase activity (Fig. 3) [72]. Liu et al. named this phenomenon “autosis” after observing it while treating HeLa cells with the autophagy-specific activator Tat-Beclin1 [73]. Autosis mediates a unique mode of death that does not depend on other modes of death or on excessive “self-eating”. Research on autosis is focused on the cardiovascular system. Nah et al. found that ischemia‒reperfusion (I/R) induced myocardial cell autosis in mice, with changes in autophagic flux occurring in two phases, an early increase and a late decrease, with both phases promoting autosis [74]. Mechanistically, the transcription factor EB (Tfeb) is activated in both early and late stage autosis-related autophagic flux, and the inhibition of late autophagy is mainly mediated by Rubicon [74, 75]. Interestingly, Rubicon inhibits autophagy mainly by binding to Beclin1 [76, 77], but Beclin1 binds directly to Na+, K+-ATPase during ischemia to promote autosis [78]. Therefore, Rubicon may play other roles in autosis as a cofactor of Beclin1, and exploring the relationship among Rubicon, Beclin1 and Na+, K+-ATPase in the process of autosis will help to further elucidate the mechanism underlying autosis.
The sodium-glucose cotransporter 2 (SGLT2) inhibitor EMPA significantly alleviates myocardial ischemic injury by inhibiting excessive autophagy and autosis in cardiomyocytes through the downregulation of Na/hex exchanger 1 (NHE1) activity [79]. Activation of autophagy in cardiomyocytes under stress conditions induces both apoptosis and autosis, and crosstalk between these death pathways is evident before p62 activation [80]. Studies on autosis are still in their infancy, and the mechanism leading to cell death may be related to excessive membrane depletion, disruption of cholesterol metabolism in the ER/external nuclear membrane or altered ion transport and osmotic pressure in the plasma membrane.
The mechanisms by which drugs or compounds mediate ADCD have been summarized in Table 1.
Table 1.
Drugs and other compounds that regulate ADCD.
| Intervention Factors | Mechanism | Mode | Citation |
|---|---|---|---|
| RCD | – | Normal autophagy | [25, 27] |
| Bax/Bak KO | – | [26] | |
| SH003 | Activates ATF4 and inhibit G9a | [34] | |
| Kaempferol | Activates IRE1-JNK-CHOP and inhibits G9a | [35] | |
| Tunicamycin | Promotes ceramide synthesis through GBA1 action | [40] | |
| Resveratrol | Activates GBA1 to promote ceramide synthesis | [65] | |
| THC | Promotes dihydroceramide accumulation in lysosomes, which leads to LMP | [68] | |
| ABTL0812 | Promotes dihydroceramide accumulation, which activates the ATF4-DDIT3-TRIB3 pathway | [71] | |
| Loperamide | Activates the ATF4-FAM134B axis and TEX264, leads to lipid accumulation in lysosomes and lipid ROS production | ER-phagy | [36, 47] |
| Z36 | Activates FAM134B and the UPR | [37] | |
| Sorafenib | Activates FAM134B | [38] | |
| Activate smARF | smARF bind to parkin to promote mitophagy | Mitophagy | [58–60] |
| AT-101 | Activate BNIP3, BNIP3L and HMOX1 | [61] | |
| THPN | TR3 translocation to mitochondrial gap change membrane potential | [62] | |
| C18-Pyr-Cer | Activate CerS1/C18-Drp1 | [64] | |
| HPV | HPV E7-E2F5-Drp1-ceramide | [69] | |
| Inhibition of FLT3-ITD | Activate Drp1-ceramide | [70] | |
| Tat-Beclin | Activates Na+-K+ATPase | Autosis | [72] |
| Ischemia/Reperfusion | Activates Tfeb and inhibits Rubicon | [74, 75] | |
| Tat-Beclin | Beclin1 binds to Na + -K + ATPase | [78] | |
| Homocysteine and copper | Activates p22phox and NOX-mediated p62 upregulation | [80] |
Autophagy-mediated cell death
Apoptosis
Apoptosis is classified as intrinsic or extrinsic apoptosis based on the mechanism that triggers it [24].
Intrinsic apoptosis is activated by various stress conditions and is mediated mainly by the anti-apoptotic protein Bcl-2 and the proapoptotic proteins Bax and Bak. Extrinsic apoptosis is mediated by membrane death receptors such as Fas cell surface death receptor (FAS/CD95/APO-1) and TNF receptor superfamily member 1 A (TNFRSF1A), which are activated by binding to their cognate ligands. Both pathways lead to the release of cytochrome c, triggering a cascade of caspase signaling [24].
Autophagy and apoptosis are critical processes that regulate cell survival and death, and the interactions involved in apoptosis and autophagy differ in different biological contexts. The following three theories describe autophagy-mediated apoptosis: 1) autophagy inhibition of apoptosis; 2) autophagy promotion of apoptosis; and 3) autophagy is merely coactivated with apoptosis, and there is no interaction between the two pathways.
The following two main mechanisms explain autophagy-mediated apoptosis: ① Autophagy leads to cell death through the phagocytosis of apoptotic molecules or organelles. The tyrosine phosphatase Fap-1 is a negative regulator of Fas (Fig. 4). In Fas-induced apoptosis, p62 recognizes and binds to Fap-1, leading to its specific degradation, increasing Fas phosphorylation levels and enhancing cell sensitivity to apoptosis [81]. Autophagosomes in BAX-/BAK-mediated apoptosis inhibit IFN-γ secretion by phagocytosing mitochondria, ensuring immune-silencing during apoptosis [82]. In SKI-1-treated cells, FADD is translocated to ATG5-ATG12-positive autophagosomal membranes to form a complex that continuously recruits and activates Caspase8 to promote apoptosis [25]. ② Autophagy molecules promote apoptosis by directly binding to apoptotic molecules. During autophagy, ATG12-ATG5 coupling plays a key role in the lipidation of LC3. Interestingly, both ATG12 and ATG5 promote apoptosis and exert anticancer effects independent of each other. Under physiological conditions, free ATG12 is degraded in a proteasome-dependent manner [83]. Following cell treatment with proteasome inhibitors, stably expressed ATG12 binds to and inactivates the antiapoptotic proteins Bcl-2 and Mcl-1 via a BH3-like motif, resulting in BAX activation, mitochondrial release of cytochrome c, and enhanced chemosensitivity [84]. ATG5-induced apoptosis is associated with calpain, which cleaves ATG5 to produce a short amino-terminal truncated fragment. Truncated ATG5 is translocated to mitochondria to block Bcl-2 and Mcl-1, altering the degree of mitochondrial outer membrane permeabilization (MOMP) and causing a cascade of activation of caspases [85].
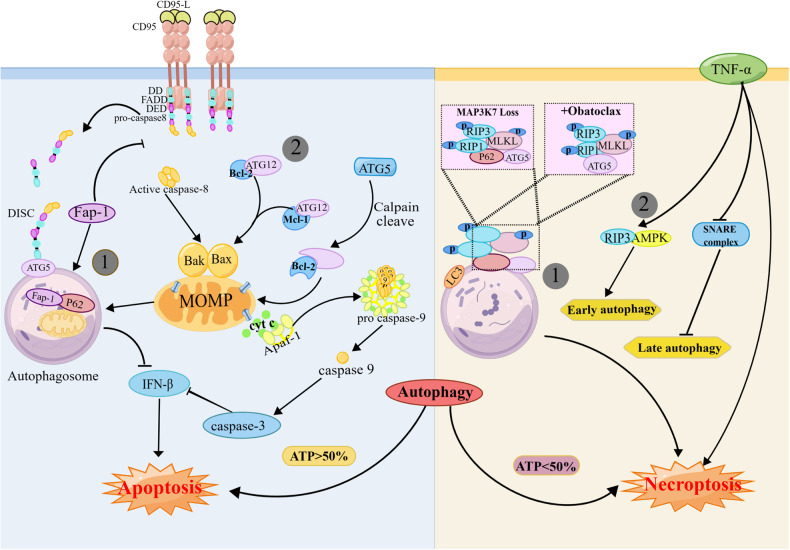
Fig. 4. The relationship between autophagy and apoptosis or necroptosis.
Autophagy mediates apoptosis and necroptosis in two main ways. ① Both forms of RCD rely on the dynamic functioning of autophagy. Autophagosomes engulf mitochondria and Fap-1 or function as platforms for the assembly of necrosis complexes, which promote cell death. ② Autophagy molecules bind to apoptotic or necrotic molecules to promote cell death. During apoptosis, ATG5 and ATG12 bind to Bcl-2 and Mcl-1 to promote apoptosis. In necroptosis, RIPK3 activates early autophagy via AMPK. This graph was drawn with Figdraw.
Necroptosis
Necroptosis is an inflammatory form of RCD that is induced by death receptors such as TNFRSF1A/TNFR1. When Caspase8 is inhibited, phosphorylation of receptor-interacting protein kinase 1 (RIPK1) and receptor-interacting protein kinase 3 (RIPK3) activates the downstream protein mixed lineage kinase domain-like protein (MLKL), driving its translocation to the plasma membrane and causing cell content leakage [24, 86]. Additionally, because of the partial overlap in the mechanisms underlying necroptosis and apoptosis, the machinery can be interchanged in some cases.
This mode of cell death is not static, and autophagy can switch from mediating apoptosis to necroptosis in specific cellular environments. ATP is the mediator of this death mode switching and initiates necrotic apoptosis when the amount of ATP available in a cell is less than 50% [87]. The switch between necroptosis and apoptosis is achieved by increasing cellular ATP utilization through “self-eating” (when ATP reserves are greater than 50%) [88]. The autophagosome membrane, an apoptotic “signal tower”, is at the core of another apoptotic mode conversion mechanism. MAP3K7 is a tumor suppressor gene. In the absence of MAP3K7, p62 recruits and mediates the translocation of RIPK1 to the autophagosomal membrane, inducing necrosis by assembling the necrosis complex, and the death modality is converted to apoptosis when p62 is knocked out [89].
In contrast to apoptosis, autophagy-mediated necroptosis usually depends on the structure or function of the autophagosome; for example, autophagy-mediated necroptosis is mediated via the aforementioned “signal tower”. Whether autophagy and necroptosis molecules engage in crosstalk is debated, as autophagy and necroptosis are regulated by separate mechanisms after different pharmacological treatments. Treatment of rhabdomyosarcoma cells with obatoclax (GX15-070), a small-molecule inhibitor of Bcl-2, stimulates ATG5 recruitment signals at the autophagosomal membrane, which serves as a platform where RIPK1, RIPK3 and FADD assembly mediates necrosis, but knockdown of RIPK1 does not inhibit autophagy (Fig. 4) [90]. In contrast, in coxsackievirus B3-infected intestinal epithelial cells, RIPK3 positively regulates autophagy, and its deletion inhibits autophagic flux and leads to the accumulation of autophagosomes [91]. Some evidence suggests that necroptosis promotes early autophagy and inhibits late autophagy. RIPK3 binds to AMPK to trigger autophagy during TNF-induced necroptosis, but TNF disrupts the SNARE complex and inhibits autophagosome–lysosome fusion [92]. There are several questions about these modes of cell death that remain unanswered, including the following: 1. What is the relationship between autophagy and necroptosis? 2. Is the activation of autophagy mediated by necroptosis, or is it an independent process? 3. The autophagosome membrane functions as a signaling platform for the necrosis complex; does it also recruit other regulatory molecules that participate in signal transduction. Autophagy is essential for necroptosis [93], and further exploration of the crosstalk between autophagy and necroptosis may help us discover potential therapeutic approaches.
Ferroptosis
Ferroptosis, which is a form of programmed necrosis that has been identified in recent years, is activated mainly by the accumulation of iron and the production of lipid peroxides (lipid-ROS). No indicators of autophagy were detected in early studies of ferroptosis [94]. Fortunately, autophagy has been found to play a key role in iron uptake and export, redox homeostasis, and lipid metabolism; therefore, ferroptosis has been identified as a form of ADCD [95–97].
Selective autophagy is the main mode of autophagy-dependent ferroptosis, including (but not limited to) 1) degradation of intracellular ferritin (FTH1, FTL, and FTMT) and iron export protein (SLC40A1) via mitophagy and NCOA4 (Nuclear receptor coactivator 4)-mediated ferritinophagy [97–100]; 2) RAB7A-dependent lipophagy of lipid droplets [101]; 3) degradation of ARNTL by SQSTM1-dependent clocophagy [102]; 4) degradation of Glutathione peroxidase 4 (GPX4) by chaperone-mediated autophagy [103]; and 5) degradation of Cadherin 2 (CDH2) by Hippocalcin like 1 (HPCAL1)-mediated selective autophagy [104]. As the aforementioned factors have been previously described in excellent articles [95, 96], we will not discuss them here (Fig. 5).
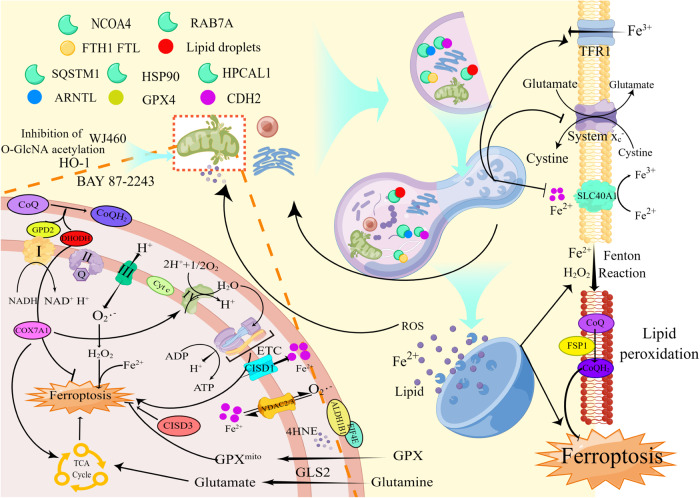
Fig. 5. The relationship between ferroptosis and autophagy.
Autophagy promotes ferroptosis by altering cellular iron content, lipid storage, redox homeostasis and energy status. During autophagy, the selective autophagy receptors NCOA4, RAB7A, and p62 bind and degrade substrates such as FTH1, lipid droplets, ARNTL, and SLC40A1. Moreover, autophagy can affect the expression of TFR1 and System XC-. The TCA cycle, ETC and the input of Fe2+ promote ferroptosis in mitochondria, and the input of GPX inhibits mitochondrial lipid peroxidation. DHODH1 and FSP1 are involved in inhibiting lipid peroxidation at the mitochondrial membrane and plasma membrane, respectively. This graph was drawn with Figdraw.
The following tree main characteristics distinguish mitophagy and ferroptosis: (1) the role of mitochondria; (2) the relationship between mitophagy and ROS levels; and (3) the significance of organelle phagocytosis via autophagy.
The role of mitochondria in ferroptosis
Mitochondria appear to play different roles in different models of ferroptosis [105]. Energy stress effectively inhibits ferroptosis induced by cysteine deprivation or GPX4 inhibition [106]. Glutamine metabolism, the TCA cycle, and the electron transport chain (ETC) play important roles in cysteine deprivation-induced ferroptosis, suggesting that mitochondria promote ferroptosis. Notably, deletion of fumarate hydratase (FH), an enzyme involved in the TCA cycle, renders renal cancer cells resistant to ferroptosis [107]. Under these conditions, mitophagy is a negative regulator of ferroptosis in most cases. The action of CISD3, a cytochrome c oxidase subunit, is inhibited during reprogrammed leukemia cell metabolism, which is promoted via glutamine catabolism and OXPHOS. Activation of mitophagy inhibits CISD3 knockdown-induced ferroptosis [108]. COX7A1 increases the sensitivity of NSCLC cells to cysteine deprivation-induced ferroptosis by enhancing the activity of the TCA cycle and ETC complex IV [109].
In contrast, pharmacological treatment showed that mitophagy promotes ferroptosis. The mitochondrial complex I inhibitor BAY 87-2243 increased mitophagy-dependent ROS levels, leading to the ferroptosis of melanoma cells [93]. Overexpression of heme oxygenase-1 (HO-1) in BAY 11-7085-treated cells caused mitochondrial dysfunction and mitophagy, which promoted ferroptosis [110]. Treatment of breast cancer cells with mitochondrion- targeted deferoxamine (mitoDFO) impaired iron–sulfur [Fe-S] cluster/heme biogenesis and inhibited mitochondrial respiration, inducing mitophagy and promoting ferroptosis [111]. Ferroptosis was also induced by targeting myoferlin and mitochondrial iron metabolism, which promoted mitophagy [112]. However, the use of the mitophagy inhibitor Mdivi1 inhibited ROS production and restored cell viability (described in the section “The relationship between mitophagy and ROS in ferroptosis”) [112]. There are two possible reasons for the different roles of the mitochondria in ferroptosis as follows: (1) Mitochondria are metabolism hubs, and the mechanisms of ferroptosis may vary greatly under different experimental conditions; and (2) the energy metabolism and quality control in cells undergoing autophagy are coordinated, but the opposite situation is observed in ferroptosis [113]. Since autophagy recycles small molecules such as amino acids, it can theoretically inhibit ferroptosis by inducing energy stress. In contrast to this theory, it has been posited that autophagy is the main mechanism mediating ferroptosis. Activation of the autophagy initiation signal AMPK and inhibition of mTOR both mitigate the onset of ferroptosis [114, 115]; furthermore, it has been reported that the type of selective autophagy induced differs under ferroptotic and starvation conditions [116]. These results suggest that selective autophagy may be an independent mechanism that promotes ferroptosis.
The relationship between mitophagy and ROS in ferroptosis
Mitochondria promote cell survival by regulating processes such as energy metabolism and redox homeostasis during ferroptosis [114, 117]. Although mitophagy can trigger the onset of ferroptosis [118, 119], the relationship between ROS levels and mitophagy initiation during ferroptosis remains unclear. H2O2 (a type of ROS) and Fe2+ are important substrates for the Fenton reaction, but some studies have shown that ROS function as upstream signaling molecules that activate mitophagy to promote ferroptosis [93, 112, 119], which contradicts the function of mitophagy in scavenging ROS [97, 119, 120]. This contradiction may explain ROS production from the perspective of Fe2+. Fe2+ reacts with H2O2, which produces lethal free radicals that peroxidize unsaturated fatty acids [94]; therefore, cells maintain free iron at very low levels through sophisticated regulatory processes. During ferroptosis, autophagy facilitates the production of free iron by affecting the levels of iron input and output, storage, and transport [99, 100, 120]. Moreover, mitochondria function as buffers for cytoplasmic free Fe2+ to inhibit ferroptosis, while mitochondrial cleavage and autophagy are promoted following inhibition of O-GlcNAcylation [98]. Thus, mitochondria function as iron pools involved in redox, are capable of storing a certain amount of iron in the physiological state [121], and function as buffers to attenuate iron toxicity in cells [98]. Mitophagy may also contribute to the clearance of certain ferroptosis inhibitors, such as ALDH1B1, which inhibits ferroptosis by scavenging 4-hydroxynonenal (4HNE) [122]. After mitophagy induction, autophagolysosomes breakdown antioxidant substances and ferritin [123], allowing for the release of free Fe2+ and the Fenton reaction to proceed with unreduced ROS [124]. Fe2+ and Lipid-ROS are released from lysosomes and stimulate mitophagy, leading to a vicious cycle of iron leakage. In summary, mitophagy may be triggered by cells that help maintain balance. Compared with ROS degradation via mitophagy, increased ROS production may be promoted via the decomposition of ferroptosis inhibitors such as antioxidant substances or a decrease in the ferritin level in mitochondria. Of course, this hypothesis is applicable only to the inhibition of ferroptosis via mitochondria and cannot explain lipid peroxidation in mitochondria.
Autophagy and mitochondrial lipid peroxidation
Lipid peroxidation occurs not only at the plasma membrane but also at the mitochondrial membrane [125]. The relationship between autophagy and the organelles involved in lipid peroxidation is worth considering. Knocking down CDGSH iron sulfur domain 1 (CISD1) promotes mitochondrial iron accumulation and lipid peroxide production [125]. The lipid transporter protein Sterol carrier protein 2 (SCP2) transports hydroperoxide species and phospholipid hydroperoxide families to the mitochondria during ferroptosis, promoting lipid peroxidation at the mitochondrial membrane [126, 127]. In contrast, mitochondria can trigger a set of antioxidant mechanisms to counteract lipid peroxidation. Dihydroorotate dehydrogenase (DHODH) and G3P dehydrogenase 2 (GPD2) promote the conversion of mitochondrial membrane CoQ to CoQH2 and thus protect mitochondrial membrane integrity [128, 129]. The specific protective mechanism of the mitochondrial membrane suggests that redox homeostasis may be necessary in the organelles of other subcellular compartments, such as the ER [130], peroxisomes [131], lysosomes [132], and the Golgi apparatus [133]. Interestingly, although the prevailing theory suggests that autophagy positively regulates ferroptosis [97, 98, 100], there is no plausible explanation for the facilitation of ferroptosis via the autophagosome phagocytosis of organelles. Thus, does autophagy function as only one of the mechanisms that regulates the redox of these organelles to promote ferroptosis? Cell and organelle membranes include unsaturated fatty acids that can undergo lipid peroxidation; therefore, autophagosomal membranes derived from organelle membranes (e.g., ER and Golgi) can be subjected to lipid peroxidation. Can autophagosomes shuttling through cells function as “mobile bombs,” causing serious damage to cells? Exploring the precise regulatory mechanisms of autophagy and the roles of organelles will help us further understand the significance of autophagy in ferroptosis.
The mechanisms by which drugs or compounds mediate AMCD have been summarized in Table 2.
Table 2.
Drugs and other compounds that regulate cellular AMCD.
| Intervention Factors | Mechanism | Mode | Citation |
|---|---|---|---|
| SKI and Bortezomib | Autophagosome provides a platform for Caspase-8 recruitment and activation | Apoptosis | [23] |
| Homocysteine and copper | Cleavage of ATG5 to block Bcl-2 | [80] | |
| Fas Ligand | Autophagosome phagocytosis of Fap-1 Inhibits Fas | [81] | |
| ABT-737 | Autophagosomes engulf mitochondria and inhibit IFN-β secretion | [82] | |
| MG132 | ATG12 binds to and inactivates Bcl-2 | [83, 84] | |
| Calpain | Calpain cleaves ATG5 to block Bcl-2 | [85] | |
| TRAIL and MAP3K7 KO,GX15-070 | Autophagosome membrane platform promotes assembly of the necrosis complex | Necroptosis | [89] |
| Coxsackievirus B3 | Activates RIPK3 | [91] | |
| TNF-α | RIPK3 activates AMPK to promote early autophagy and inhibit late autophagy | [92] | |
| BAY 87-2243 | Promotes ROS-dependent mitophagy | [93] | |
| Erastin | Autophagosomes degrade FTH1, FTL, FTMT | Ferroptosis | [96–98] |
| Autophagosomes degradation SLC40A1 | [99] | ||
| Autophagosomes degrade lipid droplets through RAB7A action | [100] | ||
| Autophagosomes degrade ARNTL through the action of SQSTM1 | [101] | ||
| Partner-mediated autophagosome degradation of GPX4 | [102] | ||
| Autophagosomes degrades CDH2 through the action of HPCAL1 | [103] | ||
| ROS activates autophagy, upregulates TFR1 level | [116] | ||
| BAY 87-2243 | Promotes ROS-dependent mitophagy | [93] | |
| Inhibition of O-GlcNA acetylation | Induces mitophagy and iron-specific autophagy and promotes F2+ release | [97] | |
| WJ460 | Induces mitophagy | [111] | |
| Celastrol and erastin | Induces autophagy and mitophagy | [115] |
Autophagy in the clinic: challenges and hope
Currently, the clinical application of autophagy mainly involves inhibiting autophagy and combining it with chemotherapeutic drugs to promote cell death in tumors, and there is no strategy to cure the disease by inducing ACD. However, this paper shows that autophagy is involved in almost all cell death modes, which indicates that targeting autophagy is a powerful strategy for curing diseases. However, it will take some time for ACD to be applied in the clinic, and we propose three questions based on the possible future development of ACD: 1) How can the functions of autophagy be identified? 2) How can an ideal autophagy-targeting drug be developed? 3) Is there a more effective clinical treatment based on the different functions of autophagy?
How can the functions of autophagy be identified?
Autophagy exhibits dual effects in most diseases, and identifying the effects of autophagy in pathological tissues is a prerequisite for targeted autophagy-based therapy. However, the identification of autophagy effects in clinical trials is based on findings from cellular or animal experiments. Considering recent studies, we classified autophagy into the following two categories according to its mechanism of action: monophasic autophagy and biphasic autophagy. (1) Monophasic autophagy. Autophagy can promote either cell survival or death in tissues, which is the common model in most studies. We can determine the effects of autophagy by detecting protection-related (Sirt1 [134]) or lethality-related (β-Thujaplicin [135] and GBA1 [65]) markers of autophagy. However, marker sensitivity and specificity need to be evaluated, and their development for clinical use in molecular diagnostics is still in its early stages. (2) Biphasic autophagy. Although biphasic autophagy has not been systematically investigated, this model of autophagy has been reported. Loperamide induced excessive ER-phagy to cause cell death [38], while it also eliminated lysosomes during LMP through lysophagy [47], suggesting that autophagy may be involved in different processes in the same cells. In sorafenib-treated hepatocellular carcinoma (HCC) cells, autophagy was activated to inhibit apoptosis and mediate cellular chemoresistance [136], but sorafenib also induced autophagy-dependent ferroptosis in HCC cells [130]. Furthermore, the involvement of different molecules in the formation of autophagosomes induced by starvation or erastin (a ferroptosis inducer) suggested that the different roles autophagy may be mediated via different regulatory mechanisms [116]. Therefore, identifying the “tipping point” between autophagy-mediated cell protection and killing or identifying and quantifying the expression of marker molecules for different autophagy-related functions to identify which autophagy functions dominate cell survival may be a strategy to determine the predominant role of autophagy under different conditions.
How can ideal autophagy-targeting drugs be developed?
Current therapeutic strategies are mainly based on autophagy inhibition and are limited to tumors; one of the main reasons for this phenomenon is the lack of clinically applicable autophagy activators that have been developed. Theoretically, the following two clinical outcomes are based on lethal autophagy: (1) The promotion of autophagy to kill tumors, bacteria or viruses, and (2) the inhibition of autophagy to protect tissues and cells. However, lethal autophagy in cells under physiological or pathological conditions is rarely triggered, and it is not practical to treat diseases by inhibiting lethal autophagy in clinical applications. Furthermore, the induction of lethal autophagy to destroy tumors and pathogens is only one of the therapeutic purposes of autophagy activation; therefore, we add to the scope of this discussion by suggesting the strategy of promoting autophagy to treat disease. Previous studies have used rapamycin, metformin, carbamazepine, cardiac glycosides, and statins to promote autophagy for the treatment of disease [137]. However, these drugs act mainly on the upstream signaling of autophagy pathways and enhance autophagy while also playing a role in other pathways, such as apoptosis, the immune response and cell growth, which can lead to side effects. Therefore, the development of selective activators of autophagy is more promising than a treatment based on the pan-active activation of autophagy.
Beclin-1
Activation of Beclin-1 promotes autophagy initiation and autophagosome formation, and it has shown remarkable therapeutic potential in tumors, Parkinson’s disease (PD), and the induction of anti-pathogenic microbial responses. Initially, Levine et al. developed a peptide derived from Beclin-1, named Tat-beclin, in a study on pathogenic microorganisms, and it enhanced autophagy to effectively prevent chikungunya virus and West Nile virus infection in neonatal mice [73]. Since its discovery, Tat-beclin has been shown to mediate autosis [72]. Based on the study of Levine’s group, Zhang et al. encapsulated Tat-beclin in biodegradable lipid-coated hybrid lactic-co-glycolic acid (PLGA) nanoparticles to selectively induce autosis in HIV-infected CD4+ T cells and macrophages; notably, the uninfected cells were largely unaffected, suggesting that Tat-beclin shows great potential as an antiviral therapy [138, 139]. Wang et al. developed the following novel antitumor strategy based on the idea of “expanding revenue sources and reducing expenses”: the activation of early autophagy using Tat-beclin and the inhibition of late autophagy using hydroxychloroquine (HCQ) to induce excess autophagosome production in tumor cells [140]. Xu et al. found that knockdown of DUSP4, an upstream signaling protein involved in the Beclin-1-Bcl-2 interaction, attenuated the relationship between Beclin-1 and Bcl-2 and promoted apoptosis [141]. Furthermore, induction of Beclin-1-dependent autophagy in the context of sepsis conferred significant protection against LPS damage and reduced inflammation in mouse hearts [142].
Autophagy receptors
Targeting autophagy receptors is an emerging strategy for the clinical regulation of autophagy-selective degradation. p62 is a classical autophagy receptor that recognizes cargo and delivers it to autophagosomes. Dehydroepiandrosterone mediates p62 expression to promote ACD in HCC cells. Additionally, p62-dependent ACD is particularly effective in cells that are resistant to apoptosis [143]. Ginkgolide targets p62 to induce ACD and promote oxidative damage in NSCLC [144].
NCOA4 is an autophagy receptor for ferritinophagy. Caryophyllene oxide is a targeted regulator of NCOA4, which facilitates the interaction of NCOA4 with FTH1 and the delivery of FTH1 to lysosomes to promote ferroptosis [145]. Combined treatment with d-borneol and cisplatin promotes NCOA4-mediated ferritinophagy while upregulating PRNP expression and downregulating PCBP2 expression to promote ferroptosis in HCC cells [146]. However, increased NCOA4-mediated ferritinophagy has been shown to help maintain the growth of pancreatic cancer tumors by promoting mitochondrial iron–sulfur cluster protein synthesis and mitochondrial respiration; these different outcomes may be due to different degrees of NCOA4 activation [147]. In summary, the selection of the most appropriate dose of an NCOA4 activator to kill tumors while minimizing damage to surrounding tissues is a major challenge.
HPCAL1 is a neuronal calcium sensor that has recently been identified as an autophagy receptor that induces ferroptosis. HPCAL1 mediates the degradation of CDH2 and reduces plasma membrane tension to promote lipid peroxidation. Inhibition of HPCAL1 inhibited pancreatic cancer progression and pancreatitis development in a mouse model [104], suggesting that targeted enhancement of HPCAL1 expression may be a potential antitumor strategy.
Gcase
As previously mentioned, the gene encoding GCase, GBA1, is a positive mediator of ADCD [65], and the ability to mediate ADCD is highly conserved among organisms [67]. Clearly, GCase is the preferred target for the development of drugs targeting ADCD pathways in tumor therapy. In addition, enhanced GCase expression stimulates the lysosomal degradation of α-synuclein. In the postmortem brains of PD patients, approximately one-half of the GCase identified was located on the lysosomal surface, and this mis-localization was attributed to a pentapeptide motif mutation in GCase. This mutation inhibited chaperone-mediated autophagy, which ultimately led to the accumulation of α-synuclein [148]. As GCase does not cross the blood‒brain barrier, the development of small-molecule chaperones to correct GCase folding and thus enhance GCase activity and lysosomal function is a feasible strategy [149]. Small-molecule chaperones specifically target misfolded GCases trapped in the ER, stabilize its active form and increase the amount of GCase transported to lysosomes [150]. Several small-molecule chaperones, such as amiloride [151], AT2101 (isofagomine) [152] and histone deacetylase inhibitors [153], have been shown to increase GCase activity and reduce oxidative stress in PD fibroblasts carrying GBA1 mutations or enhance GCase activity in GBA macrophage models. In addition, through high-throughput screening, a pyrazolopyrimidine derivative that did not inhibit GCase but contributed to its translocation to lysosomes was identified, and it showed promising effects in preclinical experiments [154].
Other compounds
MTMR is a myotubular protein-related phosphatase that antagonizes the formation of autophagosome membrane structures. Autophagy enhancer-67 (AUTEN-67) and AUTEN-99, small-molecule inhibitors of MTMR, significantly increased autophagic flux in both in vitro and in vivo models. AUTEN-67 promoted longevity and protected neurons from stress-induced cell death and caused no severe side effects [155]. AUTEN-99 blocked the progression of neurodegenerative symptoms in Drosophila models of PD and Huntington’s disease [156]. Notably, both of these compounds slowed the ageing of Drosophila rhabdomyocytes [157].
TFEB coordinates lysosomal biogenesis and is a key link between the upstream signaling pathways regulating macroautophagy and lysosomes. 2-Hydroxypropyl-β-cyclodextrin chemically activated TFEB to promote autophagy-based clearance of α-syn [158, 159]. Arsenic trioxide and dihydromyricetin activated TFEB to induce ADCD in cancer cells [160, 161].
Although small molecules or compounds targeting autophagy have been rapidly developed, the development of these potential treatments from the preclinical stage to the clinical stage has been met with some resistance. (1) Autophagy-independent functions of autophagy machinery. The vast majority of autophagy-regulated molecules show autophagy-independent functions, although for some of the proteins known to be involved in autophagy, there have not been annotated non-autophagy roles (e.g., ATG2), most likely because of theoretical or technical limitations [162]. The unclear effects on non-autophagy functions are the reason that small-molecule drugs targeting autophagy molecules are not widely used in the clinic—for example, we cannot identify the other effects of small molecules involved in autophagosome formation in different patients. Two reasons for the failure to identify the autophagy-independent function of small molecules are clear. First, small molecules involved in multiple cellular functions are present in low numbers, and when one of the functions it mediates is inhibited, another function may be overactivated. For example, UVRAG interacts with an ER-tether to control COPI transport from the Golgi to the ER. However, when autophagy is activated, UVRAG dissociates from the tether and participates in autophagosome formation [163]. Second, autophagy is a redundant process that can occur independently of classical regulatory molecules; for example, degenerative autophagy, secretory autophagy and recycling autophagy can be mediated simultaneously [164]. In summary, the search for molecules that stably regulate autophagy mechanisms is a prerequisite for the development of autophagy-targeted drugs. (2) Off-target effects. Metformin, an AMPK-specific agonist, induces autophagy but also induces other signaling pathways, such as the Hedgehog [165] and ROS/JNK [166] signaling pathways, leading to off-target effects. Although wortmannin is an efficient pan-PI3K inhibitor with relatively satisfactory potency, its low selectivity for other kinases limits the expansion of its applications [167]. (3) Low efficacy Although the autophagy inducers rapamycin and alginate and the autophagy inhibitor 3-methyladenine (3-MA) have been widely used in animal or cellular disease models (to treat liver diseases, neurodegenerative diseases, etc.) and have shown clear efficacy, they are not used in the clinic because of the high concentrations needed to induce their function [168]. This limitation is similar to that of the two autophagy inhibitors currently applied in the clinic, chloroquine (CQ) and HCQ—the clinical trials based on these drugs targeting autophagy have been largely limited to cancer therapy, again due to the high concentrations required for their effectiveness [169, 170]. In summary, the following two types of problems have slowed the development of small molecules that target the autophagy pathway: methods to identify the targets of the compounds and challenges in increasing the efficacy of the compounds. The molecules involved in autophagy regulation alone are not well characterized, and therefore, the development of compounds that target multiple autophagy-regulating molecules with high potency and specificity is a potential approach to overcome these challenges.
Is there a more effective clinical treatment based on the different functions of autophagy?
The functions of autophagy are highly dependent on the biological context, which can play different roles at different autophagy stages even in the same disease. Therefore, modulating the functions of autophagy in disease states, such as switching protective autophagy to lethal autophagy in tumors or switching lethal autophagy to protective autophagy in osteoarthritis (OA), senescence or the cardiovascular system, may considerably improve therapeutic efficacy. We think that these “switches” are widespread in diseases, but the current research on these “switches” is extremely limited, and in the following, we will focus on the transformation of autophagy in cancer and senescence, while the rest are presented in Table 3.
Table 3.
Switches that mediate the functional transitions of autophagy.
| Disease/Mold | Switches | Conversion of autophagy function | Citation |
|---|---|---|---|
| Insulin withdrawal | VCP, calpain 1 and calpain 2 | ACD was induced by insulin withdrawal. The overexpression of VCP, calpain 1 and calpain 2 converted the cell death pattern to apoptosis | [26, 176] |
| I/R | – | Treatment with 3-MA for 20 min before hypoxic ischemia induction inhibited autophagy and increased the neuronal death rate. However, when 3-MA was used 4 hours after ischemia, it significantly reduced lesion formation. | [183] |
| I/R | AMPK | Ischemia induced AMPK activation and protective autophagy in the myocardium. Lethal autophagy was activated by Beclin1-dependent and AMPK-independent autophagy during the reperfusion phase | [184] |
| Amyotrophic lateral sclerosis | – | Autophagy defects in motor neurons caused denervation of the tibialis anterior muscle and episodes of hindlimb tremor in the early stages of the disease. However, the autophagy defect alleviated glial inflammation and blocked the activation of interneuron c-Jun at the late stage, extending the lifespan of the mice. | [185] |
| Virus infection | – | Autophagy in tick-borne encephalitis virus-infected macrophages was altered with an increasing duration of infection. In the early stages of infection, autophagy inhibited viral replication. In contrast, autophagy inhibited antiviral responses by limiting IFN-β production in the later stages of infection. | [186] |
| Rheumatoid arthritis | – | Osteoarthritic synovial fibroblasts were treated with an ER stress inducer and a proteasome inhibitor to activate autophagy. The former induced autophagy-mediated nonapoptotic cell death, whereas the latter induced protective autophagy to inhibit apoptosis. | [187] |
| OA | SIRT1 | Autophagy selectively cleared SIRT1 to increase LOX-1 expression, leading to chondrocyte death. In contrast, under physiological conditions, SIRT1 is normally expressed induces autophagy. | [188] |
Cancer
In tumor cells, a new set of survival mechanisms are initiated to counteract different treatment strategies. A phase II clinical trial conducted in 2013–2017 to evaluate the pharmacological treatment of advanced pancreatic ductal carcinoma showed that the autophagy inhibitor HCQ in combination with paclitaxel or gemcitabine resulted in significantly higher overall remission rates in patients [170], but more recent studies have suggested that cancer cells have evolved macropinocytosis (MP) to target and inhibit protective autophagy therapies [171]. Therefore, the need to adapt research and therapeutic strategies for autophagy treatment is urgent. Autophagy plays different roles at different stages of tumor formation, suggesting that targeting one or more key molecules may drive a switch in autophagy function. For example, in HCC cells, autophagy undergoes two transitions at different stages of hepatocarcinogenesis. First, autophagy inhibition leads to attenuated liver injury and hepatomegaly but does not affect liver fibrosis. Subsequently, liver fibrosis gradually develops into early-stage tumors, and the role of autophagy shifts from promoting liver injury to inhibiting early-stage tumor formation [172]. Finally, in the late stages of tumor development, autophagy shifts from inhibiting early tumor formation to promoting the proliferation of advanced tumors [173]. In addition, similar autophagy role switching has been identified in the development of lung cancer [174]. Some of the “switches” that mediate the transformation of autophagy function have been identified. In pancreatic cancer driven by the KrasG12D mutation, overexpression of P53 and inhibition of autophagy attenuated tumor development. However, the knockout of P53 and inhibition of autophagy promoted glucose uptake and enhanced the glycolytic and pentose phosphate pathways to induce pancreatic carcinogenesis [7]. Valproic acid (VPA) treatment promoted H4K16ac and led to the death of HeLa cells mediated through rapamycin-activated autophagy. In untreated cells in this study, rapamycin-induced autophagy failed to induce cell death [175].
Additionally, it is difficult to eradicate drug-resistant and residual cancer cells, mainly because a patient’s long-term single mode of treatment causes the cancer cells to evolve survival mechanisms. Using autophagy-based therapy, it may be possible to flexibly target key molecules at different times during tumor treatment and switch cell death patterns to increase sensitivity to treatment. Recent studies have successfully implemented examples of these therapies, such as MAP3K7 and p62, which mediated the switch between necroptosis and apoptosis (described in the section “Necroptosis”) [89]. VCP [26] and calpain [176], which mediated the switch between ADCD and apoptosis.
Senescence
Basal autophagy rates delay cellular senescence, mainly by removing damaged mitochondria to reduce ROS production and maintain the stemness of satellite cells, muscle stem cells, etc. [177]. Moderate overexpression of Drosophila ULK1 prolonged lifespan, but ULK1 overexpression inhibited mitochondrial metabolism and led to progressive lipid damage and shortened lifespan [178]. Narita et al. identified a specific autophagy process during H-RasV12-induced senescence, called the TOR-autophagy spatial coupling compartment (TASCC), which produced many cells that acquired the senescence-associated secretory phenotype (SASP), which promotes senescence [179]. The SASP is one of the most important causes of cellular senescence, as the secreted factors are capable of affecting not only the cell itself in an autocrine manner but also nearby cells and the tissue microenvironment. In the TASCC, autophagy degrades proteins into small molecules, such as amino acids, and these amino acids activate mTOR to induce the synthesis of various cytokines, chemokines, etc. This catabolic-anabolic coupling mechanism greatly enhances SASP factor secretion and promotes cellular senescence [180]. In addition, RAS mediates the binding of LC3 to the nuclear protein LaminB1, and some chromatin is transported with LaminB1 and degraded in the lysosome, thereby promoting cellular senescence. However, in starvation-induced autophagy, the interaction of LC3 with LaminB1 does not mediate the degradation of chromatin or LaminB1 [181]. These studies suggest that RAS may be critical in determining the different types of autophagy that are initiated. Jiang et al. found that under physiological conditions, GATA4 binds to the autophagy receptor p62 and is degraded by selective autophagy. However, selective autophagy of GATA4 was disabled in the presence of senescence-inducing stimuli, and GATA4 became stable, activating the SASP program and inducing cellular senescence [182].
Conclusions
Autophagy with dual effects is similar to a maze with two exits, and the bifurcation points of the two pathways are becoming clearer. Protective and lethal autophagy may be triggered simultaneously, but one type of autophagy predominates in different stages of disease development or under intervening conditions [47]. AMCD and ADCD are both forms of RCD, and a finely tuned system of regulation mediates these processes. Alterations in the expression or posttranslational modifications of specific molecules can determine the autophagy-mediated transition between the protection and killing of cells, but the exact mechanisms of action and the upstream molecules activating this transition remain unclear. The study and application of autophagy remain limited. For experiments, effective experimental methods to analyze the dynamic switching process are lacking, which is especially important for in vivo experiments. No specific inhibitors or activators of autophagy are available in the clinic, and the efficacy and pharmacological safety of clinical drug candidates that trigger autophagy need to be investigated further.
Investigation should not be limited to a single function of autophagy (e.g., protective, lethal, or mediating a single mode of death) but should focus on the flexibility of autophagy in regulating cell death and explore the mechanisms underlying autophagy switching in tumors. Exploring autophagy-related death is a priority for the induction of autophagy transitions. Therefore, the discovery of marker molecules of autophagy-associated death and related metabolic mechanisms, the exploration of clinically applicable target drugs, sensitive indicators of autophagic flux and autophagy outcomes, and the application of these markers in combination with autophagy transitioning in the clinic may transform disease treatment.
Acknowledgements
The authors apologize to all our colleagues whose studies we could not include in this review. We thank the members of the Y. Wang laboratory for discussions of this manuscript.
Author contributions
Concept and design: SL. Writing, review, and revision of the manuscript: SL, SY, and YW. Figure and table design: HY and SL. All authors approved the final version of the article.
Funding
This work was supported by the XinJiang TianShan Talent Project (2021233 to Y. Wang) and the Doctoral Start-up Foundation of Xinjiang Medical University (B202101 to Y. Wang).
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: ShiZuo Liu, ShuaiJie Yao.
References
- 1.Green DR. The coming decade of cell death research: five riddles. Cell. 2019;177:1094–107. doi: 10.1016/j.cell.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–64. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 3.Barnard RA, Regan DP, Hansen RJ, Maycotte P, Thorburn A, Gustafson DL. Autophagy inhibition delays early but not late-stage metastatic disease. J Pharm Exp Ther. 2016;358:282–93. doi: 10.1124/jpet.116.233908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–5. doi: 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzwalter BE, Towers CG, Sullivan KD, Andrysik Z, Hoh M, Ludwig M, et al. Autophagy inhibition mediates apoptosis sensitization in cancer therapy by relieving FOXO3a turnover. Dev Cell. 2018;44:555–65.e3. doi: 10.1016/j.devcel.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Jeong H, Yu SW. Autophagy as a decisive process for cell death. Exp Mol Med. 2020;52:921–30. doi: 10.1038/s12276-020-0455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20:1110–7. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper KF. Till death do us part: the marriage of autophagy and apoptosis. Oxid Med Cell Longev. 2018;2018:4701275. doi: 10.1155/2018/4701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–74. doi: 10.1016/0014-5793(93)80398-E. [DOI] [PubMed] [Google Scholar]
- 12.Wong PM, Feng Y, Wang J, Shi R, Jiang X. Regulation of autophagy by coordinated action of mTORC1 and protein phosphatase 2A. Nat Commun. 2015;6:8048. doi: 10.1038/ncomms9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torii S, Yoshida T, Arakawa S, Honda S, Nakanishi A, Shimizu S. Identification of PPM1D as an essential Ulk1 phosphatase for genotoxic stress-induced autophagy. EMBO Rep. 2016;17:1552–64. doi: 10.15252/embr.201642565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pyo KE, Kim CR, Lee M, Kim JS, Kim KI, Baek SH. ULK1 O-GlcNAcylation is crucial for activating VPS34 via ATG14L during autophagy initiation. Cell Rep. 2018;25:2878–90.e4. doi: 10.1016/j.celrep.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Grunwald DS, Otto NM, Park JM, Song D, Kim DH. GABARAPs and LC3s have opposite roles in regulating ULK1 for autophagy induction. Autophagy. 2020;16:600–14. doi: 10.1080/15548627.2019.1632620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karabiyik C, Vicinanza M, Son SM, Rubinsztein DC. Glucose starvation induces autophagy via ULK1-mediated activation of PIKfyve in an AMPK-dependent manner. Dev Cell. 2021;56:1961–75.e5. doi: 10.1016/j.devcel.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Manghwar H, Hu W, Liu F. Degradation mechanism of autophagy-related proteins and research progress. Int J Mol Sci. 2022;23:7301. [DOI] [PMC free article] [PubMed]
- 19.Brier LW, Ge L, Stjepanovic G, Thelen AM, Hurley JH, Schekman R. Regulation of LC3 lipidation by the autophagy-specific class III phosphatidylinositol-3 kinase complex. Mol Biol Cell. 2019;30:1098–107. doi: 10.1091/mbc.E18-11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fracchiolla D, Chang C, Hurley JH, Martens S A PI3K-WIPI2 positive feedback loop allosterically activates LC3 lipidation in autophagy. J Cell Biol. 2020;219:e201912098. [DOI] [PMC free article] [PubMed]
- 21.Xu Y, Wan W. Acetylation in the regulation of autophagy. Autophagy. 2022;19:379–87. [DOI] [PMC free article] [PubMed]
- 22.Shen Q, Shi Y, Liu J, Su H, Huang J, Zhang Y, et al. Acetylation of STX17 (syntaxin 17) controls autophagosome maturation. Autophagy. 2021;17:1157–69. doi: 10.1080/15548627.2020.1752471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605–16. doi: 10.1038/s41418-018-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young MM, Takahashi Y, Khan O, Park S, Hori T, Yun J, et al. Autophagosomal membrane serves as platform for intracellular death-inducing signaling complex (iDISC)-mediated caspase-8 activation and apoptosis. J Biol Chem. 2012;287:12455–68. doi: 10.1074/jbc.M111.309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo BK, Hong CJ, Chung KM, Woo H, Kim K, Jung S, et al. Valosin-containing protein is a key mediator between autophagic cell death and apoptosis in adult hippocampal neural stem cells following insulin withdrawal. Mol Brain. 2016;9:31. doi: 10.1186/s13041-016-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denton D, Shravage B, Simin R, Mills K, Berry DL, Baehrecke EH, et al. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr Biol. 2009;19:1741–6. doi: 10.1016/j.cub.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arakawa S, Tsujioka M, Yoshida T, Tajima-Sakurai H, Nishida Y, Matsuoka Y, et al. Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ. 2017;24:1598–608. doi: 10.1038/cdd.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koenig U, Robenek H, Barresi C, Brandstetter M, Resch GP, Gröger M, et al. Cell death induced autophagy contributes to terminal differentiation of skin and skin appendages. Autophagy. 2020;16:932–45. doi: 10.1080/15548627.2019.1646552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.English AR, Voeltz GK. Endoplasmic reticulum structure and interconnections with other organelles. Cold Spring Harb Perspect Biol. 2013;5:a013227. doi: 10.1101/cshperspect.a013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locke M, Collins JV. The structure and formation of protein granules in the fat body of an insect. J Cell Biol. 1965;26:857–84. doi: 10.1083/jcb.26.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354–8. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 33.Mochida K, Oikawa Y, Kimura Y, Kirisako H, Hirano H, Ohsumi Y, et al. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature. 2015;522:359–62. doi: 10.1038/nature14506. [DOI] [PubMed] [Google Scholar]
- 34.Mochida K, Nakatogawa H. ER-phagy: selective autophagy of the endoplasmic reticulum. EMBO Rep. 2022;23:e55192. doi: 10.15252/embr.202255192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim TW, Cheon C, Ko SG. SH003 activates autophagic cell death by activating ATF4 and inhibiting G9a under hypoxia in gastric cancer cells. Cell Death Dis. 2020;11:717. doi: 10.1038/s41419-020-02924-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TW, Lee SY, Kim M, Cheon C, Ko SG. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9:875. doi: 10.1038/s41419-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zielke S, Kardo S, Zein L, Mari M, Covarrubias-Pinto A, Kinzler MN, et al. ATF4 links ER stress with reticulophagy in glioblastoma cells. Autophagy. 2021;17:2432–48. doi: 10.1080/15548627.2020.1827780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao Y, Duan B, Zhang Y, Zhang X, Xia B. Excessive ER-phagy mediated by the autophagy receptor FAM134B results in ER stress, the unfolded protein response, and cell death in HeLa cells. J Biol Chem. 2019;294:20009–23. doi: 10.1074/jbc.RA119.008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luhr M, Torgersen ML, Szalai P, Hashim A, Brech A, Staerk J, et al. The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress. J Biol Chem. 2019;294:8197–217. doi: 10.1074/jbc.RA118.002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schellens JP, Vreeling-Sindelárová H, Plomp PJ, Meijer AJ. Hepatic autophagy and intracellular ATP. A morphometric study. Exp Cell Res. 1988;177:103–8. doi: 10.1016/0014-4827(88)90028-6. [DOI] [PubMed] [Google Scholar]
- 42.An H, Ordureau A, Paulo JA, Shoemaker CJ, Denic V, Harper JW. TEX264 is an endoplasmic reticulum-resident ATG8-Interacting protein critical for ER remodeling during nutrient stress. Mol Cell. 2019;74:891–908.e10. doi: 10.1016/j.molcel.2019.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Lorenzo G, Iavarone F, Maddaluno M, Plata-Gómez AB, Aureli S, Quezada Meza CP, et al. Phosphorylation of FAM134C by CK2 controls starvation-induced ER-phagy. Sci Adv. 2022;8:eabo1215. doi: 10.1126/sciadv.abo1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chino H, Yamasaki A, Ode KL, Ueda HR, Noda NN, Mizushima N. Phosphorylation by casein kinase 2 enhances the interaction between ER-phagy receptor TEX264 and ATG8 proteins. EMBO Rep. 2022;23:e54801. doi: 10.15252/embr.202254801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cinque L, De Leonibus C, Iavazzo M, Krahmer N, Intartaglia D, Salierno FG, et al. MiT/TFE factors control ER-phagy via transcriptional regulation of FAM134B. EMBO J. 2020;39:e105696. doi: 10.15252/embj.2020105696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shiozaki Y, Miyazaki-Anzai S, Keenan AL, Miyazaki M. MEF2D-NR4A1-FAM134B2-mediated reticulophagy contributes to amino acid homeostasis. Autophagy. 2022;18:1049–61. doi: 10.1080/15548627.2021.1968228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer N, Henkel L, Linder B, Zielke S, Tascher G, Trautmann S, et al. Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death. Autophagy. 2021;17:3424–43. doi: 10.1080/15548627.2021.1874208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang JR, Lingeman E, Luong T, Ahmed S, Muhar M, Nguyen T, et al. A genome-wide ER-phagy screen highlights key roles of mitochondrial metabolism and ER-resident UFMylation. Cell. 2020;180:1160–77.e20. doi: 10.1016/j.cell.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24:2482–90. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 50.Guo YX, Han B, Yang T, Chen YS, Yang Y, Li JY, et al. Family with sequence similarity 134 member B-mediated reticulophagy ameliorates hepatocyte apoptosis induced by dithiothreitol. World J Gastroenterol. 2022;28:2569–81. doi: 10.3748/wjg.v28.i23.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Li Y, Li Y, Du L, Zhang J, Li N, et al. FAM134B-mediated ER-Phagy in Mg(2+)-free solution-induced mitochondrial calcium homeostasis and cell death in epileptic hippocampal neurons. Neurochem Res. 2021;46:2485–94. doi: 10.1007/s11064-021-03389-9. [DOI] [PubMed] [Google Scholar]
- 52.Luo R, Li S, Li G, Lu S, Zhang W, Liu H, et al. FAM134B-mediated ER-phagy upregulation attenuates AGEs-induced apoptosis and senescence in human nucleus pulposus cells. Oxid Med Cell Longev. 2021;2021:3843145. doi: 10.1155/2021/3843145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mookherjee D, Das S, Mukherjee R, Bera M, Jana SC, Chakrabarti S, et al. RETREG1/FAM134B mediated autophagosomal degradation of AMFR/GP78 and OPA1 -a dual organellar turnover mechanism. Autophagy. 2021;17:1729–52. doi: 10.1080/15548627.2020.1783118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan JX, Finkel T. A phosphoinositide signalling pathway mediates rapid lysosomal repair. Nature. 2022;609:815–21. doi: 10.1038/s41586-022-05164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papadopoulos C, Kirchner P, Bug M, Grum D, Koerver L, Schulze N, et al. VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy. EMBO J. 2017;36:135–50. doi: 10.15252/embj.201695148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurth I, Pamminger T, Hennings JC, Soehendra D, Huebner AK, Rotthier A, et al. Mutations in FAM134B, encoding a newly identified Golgi protein, cause severe sensory and autonomic neuropathy. Nat Genet. 2009;41:1179–81. doi: 10.1038/ng.464. [DOI] [PubMed] [Google Scholar]
- 57.Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–80. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reef S, Zalckvar E, Shifman O, Bialik S, Sabanay H, Oren M, et al. A short mitochondrial form of p19ARF induces autophagy and caspase-independent cell death. Mol Cell. 2006;22:463–75. doi: 10.1016/j.molcel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 59.Budina-Kolomets A, Hontz RD, Pimkina J, Murphy ME. A conserved domain in exon 2 coding for the human and murine ARF tumor suppressor protein is required for autophagy induction. Autophagy. 2013;9:1553–65. doi: 10.4161/auto.25831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grenier K, Kontogiannea M, Fon EA. Short mitochondrial ARF triggers Parkin/PINK1-dependent mitophagy. J Biol Chem. 2014;289:29519–30. doi: 10.1074/jbc.M114.607150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer N, Zielke S, Michaelis JB, Linder B, Warnsmann V, Rakel S, et al. AT 101 induces early mitochondrial dysfunction and HMOX1 (heme oxygenase 1) to trigger mitophagic cell death in glioma cells. Autophagy. 2018;14:1693–709. doi: 10.1080/15548627.2018.1476812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang WJ, Wang Y, Chen HZ, Xing YZ, Li FW, Zhang Q, et al. Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nat Chem Biol. 2014;10:133–40. doi: 10.1038/nchembio.1406. [DOI] [PubMed] [Google Scholar]
- 63.Wang WJ, Wang Y, Hou PP, Li FW, Zhou B, Chen HZ, et al. Induction of autophagic death in cancer cells by agonizing TR3 and attenuating Akt2 activity. Chem Biol. 2015;22:1040–51. doi: 10.1016/j.chembiol.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 64.Sentelle RD, Senkal CE, Jiang W, Ponnusamy S, Gencer S, Selvam SP, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8:831–8. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dasari SK, Bialik S, Levin-Zaidman S, Levin-Salomon V, Merrill AH, Jr., Futerman AH, et al. Signalome-wide RNAi screen identifies GBA1 as a positive mediator of autophagic cell death. Cell Death Differ. 2017;24:1288–302. doi: 10.1038/cdd.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oleinik N, Kim J, Roth BM, Selvam SP, Gooz M, Johnson RH, et al. Mitochondrial protein import is regulated by p17/PERMIT to mediate lipid metabolism and cellular stress. Sci Adv. 2019;5:eaax1978. doi: 10.1126/sciadv.aax1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dasari SK, Schejter E, Bialik S, Shkedy A, Levin-Salomon V, Levin-Zaidman S, et al. Death by over-eating: The Gaucher disease associated gene GBA1, identified in a screen for mediators of autophagic cell death, is necessary for developmental cell death in Drosophila midgut. Cell Cycle. 2017;16:2003–10. doi: 10.1080/15384101.2017.1380134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernández-Tiedra S, Fabriàs G, Dávila D, Salanueva ÍJ, Casas J, Montes LR, et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy. 2016;12:2213–29. doi: 10.1080/15548627.2016.1213927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muñoz-Guardiola P, Casas J, Megías-Roda E, Solé S, Perez-Montoyo H, Yeste-Velasco M, et al. The anti-cancer drug ABTL0812 induces ER stress-mediated cytotoxic autophagy by increasing dihydroceramide levels in cancer cells. Autophagy. 2021;17:1349–66. doi: 10.1080/15548627.2020.1761651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thomas RJ, Oleinik N, Panneer Selvam S, Vaena SG, Dany M, Nganga RN, et al. HPV/E7 induces chemotherapy-mediated tumor suppression by ceramide-dependent mitophagy. EMBO Mol Med. 2017;9:1030–51. doi: 10.15252/emmm.201607088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dany M, Gencer S, Nganga R, Thomas RJ, Oleinik N, Baron KD, et al. Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood. 2016;128:1944–58. doi: 10.1182/blood-2016-04-708750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Shoji-Kawata S, Sumpter RM, Jr., Wei Y, Ginet V, Zhang L, et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci USA. 2013;110:20364–71. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–6. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nah J, Zhai P, Huang CY, Fernández ÁF, Mareedu S, Levine B, et al. Upregulation of Rubicon promotes autosis during myocardial ischemia/reperfusion injury. J Clin Invest. 2020;130:2978–91. doi: 10.1172/JCI132366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nah J, Sung EA, Zhai P, Zablocki D, Sadoshima J. Tfeb-mediated transcriptional regulation of autophagy induces autosis during ischemia/reperfusion in the heart. Cells. 2022;11:258. [DOI] [PMC free article] [PubMed]
- 76.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–80. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Liang P, Jiang B, Tang Y, Liu X, Liu M, et al. CARD9 promotes autophagy in cardiomyocytes in myocardial ischemia/reperfusion injury via interacting with Rubicon directly. Basic Res Cardiol. 2020;115:29. doi: 10.1007/s00395-020-0790-6. [DOI] [PubMed] [Google Scholar]
- 78.Fernández ÁF, Liu Y, Ginet V, Shi M, Nah J, Zou Z, et al. Interaction between the autophagy protein Beclin 1 and Na+,K+-ATPase during starvation, exercise, and ischemia. JCI Insight. 2020;5:e133282. [DOI] [PMC free article] [PubMed]
- 79.Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J, et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. 2022;13:336–59. doi: 10.1007/s13238-020-00809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin R, Wang H, Li C, Wang L, Lai S, Yang X, et al. Induction of apoptosis and autosis in cardiomyocytes by the combination of homocysteine and copper via NOX-mediated p62 expression. Cell Death Discov. 2022;8:75. doi: 10.1038/s41420-022-00870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gump JM, Staskiewicz L, Morgan MJ, Bamberg A, Riches DW, Thorburn A. Autophagy variation within a cell population determines cell fate through selective degradation of Fap-1. Nat Cell Biol. 2014;16:47–54. doi: 10.1038/ncb2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lindqvist LM, Frank D, McArthur K, Dite TA, Lazarou M, Oakhill JS, et al. Autophagy induced during apoptosis degrades mitochondria and inhibits type I interferon secretion. Cell Death Differ. 2018;25:784–96. doi: 10.1038/s41418-017-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haller M, Hock AK, Giampazolias E, Oberst A, Green DR, Debnath J, et al. Ubiquitination and proteasomal degradation of ATG12 regulates its proapoptotic activity. Autophagy. 2014;10:2269–78. doi: 10.4161/15548627.2014.981914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubinstein AD, Eisenstein M, Ber Y, Bialik S, Kimchi A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol Cell. 2011;44:698–709. doi: 10.1016/j.molcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–32. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 86.Chan FK, Luz NF, Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol. 2015;33:79–106. doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leist M, Single B, Castoldi AF, Kühnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–6. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loos B, Engelbrecht AM, Lockshin RA, Klionsky DJ, Zakeri Z. The variability of autophagy and cell death susceptibility: Unanswered questions. Autophagy. 2013;9:1270–85. doi: 10.4161/auto.25560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goodall ML, Fitzwalter BE, Zahedi S, Wu M, Rodriguez D, Mulcahy-Levy JM, et al. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev Cell. 2016;37:337–49. doi: 10.1016/j.devcel.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Basit F, Cristofanon S, Fulda S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 2013;20:1161–73. doi: 10.1038/cdd.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harris KG, Morosky SA, Drummond CG, Patel M, Kim C, Stolz DB, et al. RIP3 regulates autophagy and promotes coxsackievirus b3 infection of intestinal epithelial cells. Cell Host Microbe. 2015;18:221–32. doi: 10.1016/j.chom.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu W, Wang X, Sun Y, Berleth N, Deitersen J, Schlütermann D, et al. TNF-induced necroptosis initiates early autophagy events via RIPK3-dependent AMPK activation, but inhibits late autophagy. Autophagy. 2021;17:3992–4009. doi: 10.1080/15548627.2021.1899667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Basit F, van Oppen LM, Schöckel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol. 2020;27:420–35. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu F, Zhang Q, Liu H, Liu J, Yang S, Luo X, et al. Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov. 2022;8:40. doi: 10.1038/s41421-022-00390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509:105–9. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J, Liu J, Xu Y, Wu R, Chen X, Song X, et al. Tumor heterogeneity in autophagy-dependent ferroptosis. Autophagy. 2021;17:3361–74. doi: 10.1080/15548627.2021.1872241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, et al. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. 2019;508:997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 102.Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ, et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5:eaaw2238. doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci USA. 2019;116:2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen X, Song X, Li J, Zhang R, Yu C, Zhou Z, et al. Identification of HPCAL1 as a specific autophagy receptor involved in ferroptosis. Autophagy. 2022;19:54–74. [DOI] [PMC free article] [PubMed]
- 105.Gao M, Jiang X. To eat or not to eat-the metabolic flavor of ferroptosis. Curr Opin Cell Biol. 2018;51:58–64. doi: 10.1016/j.ceb.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma Y, Han F, Min J, Lin W. Energy metabolism as a regulator of ferroptosis. Cell Cycle. 2020;19:2960–2. doi: 10.1080/15384101.2020.1838781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of Mitochondria in Ferroptosis. Mol Cell. 2019;73:354–63.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, Wang X, Huang Z, Zhou Y, Xia J, Hu W, et al. CISD3 inhibition drives cystine-deprivation induced ferroptosis. Cell Death Dis. 2021;12:839. doi: 10.1038/s41419-021-04128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feng Y, Xu J, Shi M, Liu R, Zhao L, Chen X, et al. COX7A1 enhances the sensitivity of human NSCLC cells to cystine deprivation-induced ferroptosis via regulating mitochondrial metabolism. Cell Death Dis. 2022;13:988. doi: 10.1038/s41419-022-05430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018;416:124–37. doi: 10.1016/j.canlet.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 111.Sandoval-Acuña C, Torrealba N, Tomkova V, Jadhav SB, Blazkova K, Merta L, et al. Targeting mitochondrial iron metabolism suppresses tumor growth and metastasis by inducing mitochondrial dysfunction and mitophagy. Cancer Res. 2021;81:2289–303. doi: 10.1158/0008-5472.CAN-20-1628. [DOI] [PubMed] [Google Scholar]
- 112.Rademaker G, Boumahd Y, Peiffer R, Anania S, Wissocq T, Liégeois M, et al. Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. Redox Biol. 2022;53:102324. doi: 10.1016/j.redox.2022.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deretic V, Kroemer G. Autophagy in metabolism and quality control: opposing, complementary or interlinked functions? Autophagy. 2022;18:283–92. doi: 10.1080/15548627.2021.1933742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–34. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Conlon M, Poltorack CD, Forcina GC, Armenta DA, Mallais M, Perez MA, et al. A compendium of kinetic modulatory profiles identifies ferroptosis regulators. Nat Chem Biol. 2021;17:665–74. doi: 10.1038/s41589-021-00751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J, Liu Y, Wang Y, Li C, Xie Y, Klionsky DJ, et al. TMEM164 is a new determinant of autophagy-dependent ferroptosis. Autophagy. 2022;19:945–56. [DOI] [PMC free article] [PubMed]
- 117.Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells. 2020;9:1505. [DOI] [PMC free article] [PubMed]
- 118.Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5:e132747. [DOI] [PMC free article] [PubMed]
- 119.Liu M, Fan Y, Li D, Han B, Meng Y, Chen F, et al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol Oncol. 2021;15:2084–105. doi: 10.1002/1878-0261.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sheftel AD, Zhang AS, Brown C, Shirihai OS, Ponka P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood. 2007;110:125–32. doi: 10.1182/blood-2007-01-068148. [DOI] [PubMed] [Google Scholar]
- 122.Chen X, Huang J, Yu C, Liu J, Gao W, Li J, et al. A noncanonical function of EIF4E limits ALDH1B1 activity and increases susceptibility to ferroptosis. Nat Commun. 2022;13:6318. doi: 10.1038/s41467-022-34096-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ziegler PK, Bollrath J, Pallangyo CK, Matsutani T, Canli Ö, De Oliveira T, et al. Mitophagy in intestinal epithelial cells triggers adaptive immunity during tumorigenesis. Cell. 2018;174:88–101.e16. doi: 10.1016/j.cell.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin Y, Epstein DL, Liton PB. Intralysosomal iron induces lysosomal membrane permeabilization and cathepsin D-mediated cell death in trabecular meshwork cells exposed to oxidative stress. Invest Ophthalmol Vis Sci. 2010;51:6483–95. doi: 10.1167/iovs.10-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838–44. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 126.Bebber CM, Müller F, Prieto Clemente L, Weber J, von Karstedt S. Ferroptosis in cancer cell biology. Cancers (Basel). 2020;12:164. [DOI] [PMC free article] [PubMed]
- 127.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu S, Mao C, Kondiparthi L, Poyurovsky MV, Olszewski K, Gan B. A ferroptosis defense mechanism mediated by glycerol-3-phosphate dehydrogenase 2 in mitochondria. Proc Natl Acad Sci USA. 2022;119:e2121987119. doi: 10.1073/pnas.2121987119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu Z, Ma C, Wang Q, Yang H, Lu Z, Bi T, et al. Targeting FAM134B-mediated reticulophagy activates sorafenib-induced ferroptosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2022;589:247–53. doi: 10.1016/j.bbrc.2021.12.019. [DOI] [PubMed] [Google Scholar]
- 131.Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R, et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature. 2019;567:118–22. doi: 10.1038/s41586-019-0945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gao H, Bai Y, Jia Y, Zhao Y, Kang R, Tang D, et al. Ferroptosis is a lysosomal cell death process. Biochem Biophys Res Commun. 2018;503:1550–6. doi: 10.1016/j.bbrc.2018.07.078. [DOI] [PubMed] [Google Scholar]
- 133.Alborzinia H, Ignashkova TI, Dejure FR, Gendarme M, Theobald J, Wölfl S, et al. Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun Biol. 2018;1:210. doi: 10.1038/s42003-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim JY, Mondaca-Ruff D, Singh S, Wang Y. SIRT1 and autophagy: implications in endocrine disorders. Front Endocrinol (Lausanne) 2022;13:930919. doi: 10.3389/fendo.2022.930919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang G, He J, Ye X, Zhu J, Hu X, Shen M, et al. β-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma. Cell Death Dis. 2019;10:255. doi: 10.1038/s41419-019-1492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin Z, Niu Y, Wan A, Chen D, Liang H, Chen X, et al. RNA m(6) A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020;39:e103181. doi: 10.15252/embj.2019103181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, et al. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener. 2017;12:11. doi: 10.1186/s13024-017-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang G, Luk BT, Wei X, Campbell GR, Fang RH, Zhang L, et al. Selective cell death of latently HIV-infected CD4(+) T cells mediated by autosis inducing nanopeptides. Cell Death Dis. 2019;10:419. doi: 10.1038/s41419-019-1661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang G, Luk BT, Hamidy M, Zhang L, Spector SA. Induction of a Na(+)/K(+)-ATPase-dependent form of autophagy triggers preferential cell death of human immunodeficiency virus type-1-infected macrophages. Autophagy. 2018;14:1359–75. doi: 10.1080/15548627.2018.1476014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y, Tai X, Zhang L, Liu Y, Gao H, Chen J, et al. A novel antitumour strategy using bidirectional autophagic vesicles accumulation via initiative induction and the terminal restraint of autophagic flux. J Control Release. 2015;199:17–28. doi: 10.1016/j.jconrel.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Xu W, Nie C, Chen X. DUSP4 inhibits autophagic cell death and apoptosis in colorectal cancer by regulating BCL2-Beclin1/Bax signaling. Mol Biol Rep. 2023;50:3229–39. doi: 10.1007/s11033-023-08270-2. [DOI] [PubMed] [Google Scholar]
- 142.Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. 2018;138:2247–62. doi: 10.1161/CIRCULATIONAHA.117.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vegliante R, Desideri E, Di Leo L, Ciriolo MR. Dehydroepiandrosterone triggers autophagic cell death in human hepatoma cell line HepG2 via JNK-mediated p62/SQSTM1 expression. Carcinogenesis. 2016;37:233–44. doi: 10.1093/carcin/bgw003. [DOI] [PubMed] [Google Scholar]
- 144.Lou JS, Bi WC, Chan GKL, Jin Y, Wong CW, Zhou ZY, et al. Ginkgetin induces autophagic cell death through p62/SQSTM1-mediated autolysosome formation and redox setting in non-small cell lung cancer. Oncotarget. 2017;8:93131–48. doi: 10.18632/oncotarget.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiu Z, Zhu Y, Han J, Li Y, Yang X, Yang G, et al. Caryophyllene oxide induces ferritinophagy by regulating the NCOA4/FTH1/LC3 pathway in hepatocellular carcinoma. Front Pharm. 2022;13:930958. doi: 10.3389/fphar.2022.930958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li J, Yuan J, Li Y, Wang J, Xie Q, Ma R, et al. d-Borneol enhances cisplatin sensitivity via autophagy dependent EMT signaling and NCOA4-mediated ferritinophagy. Phytomedicine. 2022;106:154411. doi: 10.1016/j.phymed.2022.154411. [DOI] [PubMed] [Google Scholar]
- 147.Santana-Codina N, Del Rey MQ, Kapner KS, Zhang H, Gikandi A, Malcolm C, et al. NCOA4-Mediated Ferritinophagy Is a pancreatic cancer dependency via maintenance of iron bioavailability for iron-sulfur cluster proteins. Cancer Discov. 2022;12:2180–97. doi: 10.1158/2159-8290.CD-22-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kuo SH, Tasset I, Cheng MM, Diaz A, Pan MK, Lieberman OJ, et al. Mutant glucocerebrosidase impairs α-synuclein degradation by blockade of chaperone-mediated autophagy. Sci Adv. 2022;8:eabm6393. doi: 10.1126/sciadv.abm6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schapira AH, Gegg ME. Glucocerebrosidase in the pathogenesis and treatment of Parkinson disease. Proc Natl Acad Sci USA. 2013;110:3214–5. doi: 10.1073/pnas.1300822110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blanz J, Saftig P. Parkinson’s disease: acid-glucocerebrosidase activity and alpha-synuclein clearance. J Neurochem. 2016;139:198–215. doi: 10.1111/jnc.13517. [DOI] [PubMed] [Google Scholar]
- 151.McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, et al. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137:1481–95. doi: 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Khanna R, Benjamin ER, Pellegrino L, Schilling A, Rigat BA, Soska R, et al. The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of beta-glucosidase. FEBS J. 2010;277:1618–38. doi: 10.1111/j.1742-4658.2010.07588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang C, Rahimpour S, Lu J, Pacak K, Ikejiri B, Brady RO, et al. Histone deacetylase inhibitors increase glucocerebrosidase activity in Gaucher disease by modulation of molecular chaperones. Proc Natl Acad Sci USA. 2013;110:966–71. doi: 10.1073/pnas.1221046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patnaik S, Zheng W, Choi JH, Motabar O, Southall N, Westbroek W, et al. Discovery, structure-activity relationship, and biological evaluation of noninhibitory small molecule chaperones of glucocerebrosidase. J Med Chem. 2012;55:5734–48. doi: 10.1021/jm300063b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Papp D, Kovács T, Billes V, Varga M, Tarnóci A, Hackler L, Jr., et al. AUTEN-67, an autophagy-enhancing drug candidate with potent antiaging and neuroprotective effects. Autophagy. 2016;12:273–86. doi: 10.1080/15548627.2015.1082023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kovács T, Billes V, Komlós M, Hotzi B, Manzéger A, Tarnóci A, et al. The small molecule AUTEN-99 (autophagy enhancer-99) prevents the progression of neurodegenerative symptoms. Sci Rep. 2017;7:42014. doi: 10.1038/srep42014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Komlós M, Szinyákovics J, Falcsik G, Sigmond T, Jezsó B, Vellai T, et al. The small-molecule enhancers of autophagy AUTEN-67 and -99 delay ageing in drosophila striated muscle cells. Int J Mol Sci. 2023;24:8100. [DOI] [PMC free article] [PubMed]
- 158.Song W, Wang F, Lotfi P, Sardiello M, Segatori L. 2-Hydroxypropyl-β-cyclodextrin promotes transcription factor EB-mediated activation of autophagy: implications for therapy. J Biol Chem. 2014;289:10211–22. doi: 10.1074/jbc.M113.506246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kilpatrick K, Zeng Y, Hancock T, Segatori L. Genetic and chemical activation of TFEB mediates clearance of aggregated α-synuclein. PLoS ONE. 2015;10:e0120819. doi: 10.1371/journal.pone.0120819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tan M, Jiang B, Wang H, Ouyang W, Chen X, Wang T, et al. Dihydromyricetin induced lncRNA MALAT1-TFEB-dependent autophagic cell death in cutaneous squamous cell carcinoma. J Cancer. 2019;10:4245–55. doi: 10.7150/jca.32807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu B, Tan M, Cai W, Wang B, He P, Zhang X. Arsenic trioxide induces autophagic cell death in osteosarcoma cells via the ROS-TFEB signaling pathway. Biochem Biophys Res Commun. 2018;496:167–75. doi: 10.1016/j.bbrc.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 162.Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177:1682–99. doi: 10.1016/j.cell.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.He S, Ni D, Ma B, Lee JH, Zhang T, Ghozalli I, et al. PtdIns(3)P-bound UVRAG coordinates Golgi-ER retrograde and Atg9 transport by differential interactions with the ER tether and the beclin 1 complex. Nat Cell Biol. 2013;15:1206–19. doi: 10.1038/ncb2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumar AV, Mills J. Non-canonical autophagy in aging and age-related diseases. Front Cell Dev Biol. 2023;11:1137870. doi: 10.3389/fcell.2023.1137870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Niu C, Chen Z, Kim KT, Sun J, Xue M, Chen G, et al. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy. 2019;15:843–70. doi: 10.1080/15548627.2019.1569913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li B, Zhou P, Xu K, Chen T, Jiao J, Wei H, et al. Metformin induces cell cycle arrest, apoptosis and autophagy through ROS/JNK signaling pathway in human osteosarcoma. Int J Biol Sci. 2020;16:74–84. doi: 10.7150/ijbs.33787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–23. [PubMed] [Google Scholar]
- 168.He S, Li Q, Jiang X, Lu X, Feng F, Qu W, et al. Design of small molecule autophagy modulators: a promising druggable strategy. J Med Chem. 2018;61:4656–87. doi: 10.1021/acs.jmedchem.7b01019. [DOI] [PubMed] [Google Scholar]
- 169.Eng CH, Wang Z, Tkach D, Toral-Barza L, Ugwonali S, Liu S, et al. Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc Natl Acad Sci USA. 2016;113:182–7. doi: 10.1073/pnas.1515617113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Karasic TB, O’Hara MH, Loaiza-Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E, et al. Effect of gemcitabine and nab-paclitaxel with or without hydroxychloroquine on patients with advanced pancreatic cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:993–8. doi: 10.1001/jamaoncol.2019.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Su H, Yang F, Fu R, Li X, French R, Mose E, et al. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell. 2021;39:678–93.e11. doi: 10.1016/j.ccell.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ni HM, Chao X, Yang H, Deng F, Wang S, Bai Q, et al. Dual roles of mammalian target of rapamycin in regulating liver injury and tumorigenesis in autophagy-defective mouse liver. Hepatology. 2019;70:2142–55. doi: 10.1002/hep.30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sun K, Guo XL, Zhao QD, Jing YY, Kou XR, Xie XQ, et al. Paradoxical role of autophagy in the dysplastic and tumor-forming stages of hepatocarcinoma development in rats. Cell Death Dis. 2013;4:e501. doi: 10.1038/cddis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5:3056. doi: 10.1038/ncomms4056. [DOI] [PubMed] [Google Scholar]
- 175.Füllgrabe, Lynch-Day MA J, Heldring N, Li W, Struijk RB, Ma Q, et al. The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy. Nature. 2013;500:468–71. doi: 10.1038/nature12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chung KM, Park H, Jung S, Ha S, Yoo SJ, Woo H, et al. Calpain determines the propensity of adult hippocampal neural stem cells to autophagic cell death following insulin withdrawal. Stem Cells. 2015;33:3052–64. doi: 10.1002/stem.2082. [DOI] [PubMed] [Google Scholar]
- 177.Kwon Y, Kim JW, Jeoung JA, Kim MS, Kang C. Autophagy is pro-senescence when seen in close-up, but anti-senescence in long-shot. Mol Cells. 2017;40:607–12. doi: 10.14348/molcells.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bjedov I, Cochemé HM, Foley A, Wieser D, Woodling NS, Castillo-Quan JI, et al. Fine-tuning autophagy maximises lifespan and is associated with changes in mitochondrial gene expression in Drosophila. PLoS Genet. 2020;16:e1009083. doi: 10.1371/journal.pgen.1009083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zoncu R, Sabatini DM. Cell biology. The TASCC of secretion. Science. 2011;332:923–5. doi: 10.1126/science.1207552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–70. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dou Z, Xu C, Donahue G, Shimi T, Pan JA, Zhu J, et al. Autophagy mediates degradation of nuclear lamina. Nature. 2015;527:105–9. doi: 10.1038/nature15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66:378–89. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- 184.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 185.Rudnick ND, Griffey CJ, Guarnieri P, Gerbino V, Wang X, Piersaint JA, et al. Distinct roles for motor neuron autophagy early and late in the SOD1(G93A) mouse model of ALS. Proc Natl Acad Sci USA. 2017;114:E8294–E8303. doi: 10.1073/pnas.1704294114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Beránková Z, Kopecký J, Kobayashi S, Lieskovská J. Dual control of tick-borne encephalitis virus replication by autophagy in mouse macrophages. Virus Res. 2022;315:198778. doi: 10.1016/j.virusres.2022.198778. [DOI] [PubMed] [Google Scholar]
- 187.Kato M, Ospelt C, Gay RE, Gay S, Klein K. Dual role of autophagy in stress-induced cell death in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol. 2014;66:40–8. doi: 10.1002/art.38190. [DOI] [PubMed] [Google Scholar]
- 188.Papageorgiou AA, Goutas A, Trachana V, Tsezou A. Dual Role of SIRT1 in Autophagy and Lipid Metabolism Regulation in Osteoarthritic Chondrocytes. Medicina (Kaunas). 2021;57:1203. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.