ABSTRACT.
Diarrhea and respiratory illness are leading causes of mortality and morbidity among young children. We assessed the impact of a homestead food production intervention on diarrhea and acute respiratory infection (ARI) in children in Bangladesh, secondary outcomes of the Food and Agricultural Approaches to Reducing Malnutrition (FAARM) cluster-randomized trial. The trial enrolled 2,705 married women and their children 3 years or younger in 96 rural settlements (geographic clusters) in Sylhet Division, Bangladesh. The intervention promoted home gardening and poultry rearing alongside child nutrition and health counseling over 3 years (2015–2018). An 8-month food hygiene behavior change component using emotional drivers was delivered beginning in mid-2017. Caregiver-reported diarrhea and symptoms of ARI in the week preceding the survey were recorded every 2 months. We analyzed 32,460 observations of 3,276 children over 4 years and found that 3.9% of children had diarrhea and 3.4% had an ARI in the prior 7 days. There was no overall effect of the intervention on 7-day diarrhea period prevalence (odds ratio [OR], 0.92; 95% CI, 0.71–1.19), diarrhea point prevalence (OR, 1.03; 95% CI, 0.78–1.36), or 7-day ARI period prevalence (OR, 1.18; 95% CI, 0.88–1.60). There was no impact on diarrhea severity or differences in health-seeking behaviors. Our findings suggest that this homestead food production program was insufficient to reduce morbidity symptoms among children in a rural setting. More comprehensive water, sanitation, and hygiene measures, and behavioral recommendations may be needed to achieve impacts on child health.
INTRODUCTION
Diarrhea and respiratory illness are leading, preventable causes of death among children younger than 5 years, contributing to an estimated 1.2 million deaths in 2015.1 The majority of deaths from diarrhea and acute respiratory infection (ARI) occur in the first 2 years of life2 and are greatest in sub-Saharan Africa and South Asia.3,4 In addition to contributing to mortality, child diarrhea is associated with poor child growth5 and cognitive development.6,7 Acute respiratory infections in childhood can impair lung development.2,8 Undernutrition, unsafe water and sanitation, poor hygiene practices, suboptimal breastfeeding practices, and zinc deficiency increase children’s risk of diarrhea and ARI.2,3,9 Household air pollution and crowding also increase children’s susceptibility to ARI.4,9 Furthermore, prior studies have found that diarrhea increases the risk of subsequent ARI,10–12 possibly as a result of increased malnutrition and reduced immune function.
Global child mortality has declined notably during the past several decades; nevertheless, further progress in reducing deaths from diarrhea and respiratory infection is needed to meet Sustainable Development Goal 3.2 of ending preventable child deaths and reducing under-five mortality to less than 25 per 1,000 live births.13–15 In Bangladesh, the under-five mortality rate was 29 per 1,000 in 2020.13 Respiratory infections are the leading cause of death among children younger than 5 years in Bangladesh, contributing to 18% of deaths, whereas diarrhea contributes to approximately 3% of deaths.16 Having been one of the primary causes of mortality in Bangladesh in the past, diarrhea mortality has declined significantly as a result of improvements in sanitation and nutrition, as well as the treatment of sick children with oral rehydration solution (ORS) and therapeutic zinc supplementation.17 Nevertheless, according to the most recent 2017/2018 Bangladesh Demographic and Health Survey,16 31% of children younger than 5 years remain stunted, only 38% of children 6 to 23 months old consume adequately diverse diets, and the average child younger than 6 months of age is only exclusively breastfed up until 4.1 months of age, in contrast to the WHO18 recommendations to breastfeed all children exclusively until 6 months. Furthermore, only half of the population has access to a private, improved sanitation facility, although access to a pit latrine is nearly ubiquitous, and only 39% have a handwashing facility with soap and water.16 Addressing these risk factors is essential to reducing child deaths and related life-long health consequences from diarrhea and ARI.
Interventions to reduce diarrhea and ARI morbidity have focused primarily on improving water, sanitation, and hygiene (WASH), although several recent large-scale cluster-randomized trials have found little to no impacts on diarrhea or ARI.19–22 A meta-analysis of WASH interventions found evidence for benefits from handwashing promotion on diarrhea reduction, but not from using an improved water source or sanitation.23 A systematic review of hygiene interventions on ARI found evidence for reduced childhood ARI symptoms from handwashing promotion and soap provision in urban, but not rural, domestic settings.24 Reducing contamination of complementary food may also be an important factor in reducing child morbidity, given a high burden of child diarrhea from foodborne pathogens; however, there are limited investigations of food hygiene interventions on diarrhea outcomes.25 A recent integrated handwashing plus food hygiene intervention in Malawi delivered over 31 weeks found reductions in self-reported diarrhea among children younger than 5 years old, and reductions in ARI among children who additionally received a water and sanitation intervention component.26 In The Gambia, a community-level food hygiene intervention found a reduced risk of mother-reported diarrhea and ARI in children 6 to 24 months old.27
An additional area of focus for reducing diarrhea and ARI is addressing child undernutrition. Wasting and stunting have been associated with increased severity of diarrheal disease and pneumonia, as well as increased risk of diarrhea- and pneumonia-associated mortality.28–30 Emerging evidence shows that undernutrition impairs innate and adaptive immune function in children.31 Deficiencies in micronutrients, including vitamin A and zinc, also increase the risk of infectious illness.32 In Bangladesh, improvements in child nutrition, as marked by reductions in stunting, are estimated to be one of the most important factors for reducing diarrhea-related mortality.17 Nutrition-sensitive agricultural interventions, which aim to improve child feeding behaviors and reduce undernutrition, may thus contribute to reducing child morbidity and mortality.3
During the past 30 years, the international nonprofit organization Helen Keller International developed a homestead food production (HFP) program to reduce undernutrition and promote household food security through year-round production and consumption of micronutrient-rich foods, and to promote optimal nutrition and hygiene practices.33 To date, there is some evidence that the HFP program may reduce diarrhea prevalence in children, from evaluations after 1 to 2 years in Burkina Faso34 and Nepal.35 However, other nutrition-sensitive agricultural interventions, ranging from 18 months to 4 years, have shown no impacts on diarrhea36–38 or ARI.37 This mixed evidence suggests that nutrition-sensitive agricultural interventions may reduce child morbidity in some settings, although the short duration and infrequent surveillance of the majority of these trials limits the understanding of potential longer term impacts of agricultural interventions on child health. Furthermore, no study (to our knowledge) has examined the impacts of a combined agriculture and food hygiene intervention on child diarrhea and ARI. Helen Keller International’s HFP program has focused primarily on promoting optimal handwashing behaviors, with limited elements targeting feeding and food hygiene behaviors. Using social and behavior-change techniques, we added a food hygiene module to the HFP program to improve caregivers’ food hygiene practices around food preparation and child feeding, and to reduce microbial contamination of food at the household level.
Thus, the objective of our study was to evaluate the impact of a multiyear HFP and food hygiene intervention on the prevalence of diarrhea and ARI among children younger than 3 years.
MATERIALS AND METHODS
Study design and participants.
The Food and Agricultural Approaches to Reducing Malnutrition (FAARM) trial was a cluster-randomized controlled trial conducted from 2015 to 2020 in Habiganj District, Sylhet Division, Bangladesh, to evaluate the impact of an HFP program on children’s undernutrition. We used a cluster-randomized design to facilitate the delivery of the intervention, using a woman farmer group approach, and to minimize the risk of contamination. The target group of the intervention was children 3 years of age or younger born to enrolled women during the intervention period. To be eligible, women had to report being 30 years old or younger, be married, have access to at least 40 m2 of land, and be interested in participating in the HFP program. The FAARM study protocol provides further information on the trial design, intervention, conceptual framework, and data collection procedures.39
Randomization and masking.
Households in Baniachong and Nabiganj Subdistricts, Habiganj District, were enumerated to identify women eligible for inclusion in the trial. After enumeration, 96 settlements (geographic clusters of households with 10–65 eligible women) were formed that were separated by at least 400 m. The baseline survey was then conducted in which women and their youngest child less than 3 years provided written consent for participation and were enrolled in the trial. After the baseline survey, clusters were randomized 1:1 using covariate-constrained randomization as described in the study protocol,39 with 48 clusters allocated to the intervention group and 48 to the control group. Children born during the intervention period were enrolled shortly after birth and were monitored until shortly after their third birthday. Participants were not informed explicitly of their allocation to the intervention group. Outcome assessors were not informed of settlements’ allocation to the intervention or control groups; however, they could have observed materials provided to participants of the intervention during data collection. Data analysts were not blinded to enable the project to track outcomes and adapt based on data from the field.
Procedures.
The FAARM HFP program was implemented by Helen Keller International over a 3-year period—from mid-2015 to late-2018. The HFP program promoted the production and consumption of nutrient-dense foods through home gardening, poultry rearing, and nutrition and hygiene counseling using a “women farmer groups” approach. Women participating in the intervention received training on year-round vegetable gardening as well as improved poultry production and management practices. Every 2 months, project staff held trainings during which participants and their families were provided with information on nutrition, health, and hygiene topics. These group training sessions were supplemented with individual household visits every 2 months to review and discuss the topics covered in the trainings. From June 2017 to February 2018, an additional food hygiene component was added to the HFP intervention to promote four specific food hygiene behaviors: washing feeding utensils with soap and water, washing hands with soap and water, safe storage of food and water, and cooking fresh or reheating food before feeding. Using a behavior-centered approach, the food hygiene intervention was implemented with four 1-hour participatory group sessions and four home visits during which project staff provided practical support and helped families rearrange their cooking area to promote the key behaviors. Further information on the food hygiene intervention is provided in the FAARM study protocol39 and description of the intervention by Sobhan et al.40
Data collection.
Trained data collection officers collected survey data using face-to-face interviews and the tablet-based application Open Data Kit.41 The FAARM baseline survey was conducted from March to May 2015 and targeted eligible women and their youngest biological child. It included questions on household, woman, and child characteristics; WASH; and children’s morbidity symptoms in the 15-days prior to the survey date based on caregiver recall. A household wealth index was calculated using principal components analysis of household assets, in line with standard Demographic and Health Survey techniques.42 Women’s education was categorized into those who had no formal education, partial primary education, complete primary education, or any secondary education based on the reported number of school years completed. Water facilities (e.g., tube well, public tap) and sanitation facilities (e.g., pit latrine, flush toilet) were defined in accordance with the WHO/UNICEF Joint Monitoring Program for Water Supply, Sanitation and Hygiene definitions.43 Data collectors observed and recorded the availability of a handwashing facility with water and soap.
From September 2015 to September 2019, routine assessments were conducted every 2 months among all trial participants as part of the FAARM surveillance system. During routine assessments, participants were surveyed on key diet and morbidity indicators as well as other indicators along the hypothesized program impact pathway. Data on morbidity were collected on all children until roughly 3 years of age. Caregivers were asked to recall whether their child had symptoms of illness in the 7 days prior to the survey, including whether the child was sick with loose or mushy stools, a runny or blocked nose, cough, difficulty breathing, or short and rapid breaths. If loose or mushy stools were reported, women were also asked to report the number of stools their child passed on the worst day. Women were also asked to recall the number of loose or mushy stools on the day before or 2 days before the day of the survey. Women were asked about the severity of morbidity symptoms and about health-seeking behaviors in response to the child’s sickness.
The FAARM end line survey was conducted from October to December 2019 with continued follow-up until February 2020, approximately 1 year after the conclusion of the intervention activities, and included all children who were born after September 1, 2016 (in the 3 years before the start of the survey). Mothers were asked about their child’s morbidity symptoms in the same way as in the routine assessment surveys.
Outcomes.
The primary outcomes of this analysis were period prevalence of diarrhea (7-day recall), point prevalence of diarrhea (2-day recall), and period prevalence of ARI (7-day recall) in children, which are prespecified secondary outcomes of the FAARM trial. Period prevalence of diarrhea was defined as maternal report of the child having three or more loose or mushy stools on at least 1 day in the 7 days prior to the survey. Point prevalence of diarrhea was calculated based on the child having at least three loose or mushy stools on the day before or 2 days before the day of the survey. Acute respiratory infection was defined as maternal report of the child having a cough with short, rapid breathing or difficulty breathing, excluding children with only a blocked nose.44 We also analyzed data on severity of symptoms in the prior 7 days, including maternal report of blood in the child’s stools, whether the child had fever or vomiting, and whether the child refused to eat or drink, cried a lot, or was weak or drowsy, as well as maternal report of health-seeking behaviors in response to the child’s illness, including whether the child received health care, was given ORS, or received zinc supplements.
Statistical analysis.
The sample size of the FAARM trial was based on the primary trial outcome, children’s length/height-for-age z-score, as described in the study protocol.39 All statistical analysis was conducted using Stata MP version 16.1 (StataCorp, College Station, TX). The FAARM trial is registered with ClinicalTrials.gov (ID: NCT025-05711).
We conducted descriptive analyses of household and mother characteristics at baseline, and children’s age and sex in the control and intervention groups using proportions for categorical variables, and means and standard deviations for continuous variables. The effects of the FAARM intervention on individual-level diarrhea and ARI outcomes were evaluated by intention-to-treat analysis. We used mixed-effects logistic regression models with repeat measures to analyze the primary outcomes, with a random effect at the settlement level to account for clustering. An interaction term was used to calculate effects by trial time periods: intervention year 1 (September 2015–August 2016), year 2 (September 2016–August 2017), year 3 (September 2017–August 2018), scale-down (September 2018–September 2019), and postintervention (October 2019–February 2020). During the scale-down of the intervention, field activities were phased out and stopped by December 2018, with only one training in April 2019. We used the lincom command in Stata software to calculate point estimates for each period, and exponentiated the coefficients to obtain odds ratios (ORs) and 95% CIs. Models for the primary outcomes included all children age 36 months and younger at each of the routine surveillance assessments and at end line. In sensitivity analyses, we adjusted the models for child age and sex, month of the survey, and baseline cluster-average diarrhea and ARI prevalence to increase precision, and wealth quintile to account for the slight imbalance in baseline wealth between the groups. We used stratified analysis to examine the effect of the intervention on the primary outcomes by age group. Diarrhea severity, illness symptoms accompanying a diarrhea episode, and health-seeking behaviors were compared between intervention and control groups using descriptive statistics.
RESULTS
At baseline, 1,321 women were enrolled in the control group and 1,302 women were enrolled in the intervention group (Figure 1). An additional 47 women in the control group and 35 women in the intervention group, who were newly married into already enrolled households, were enrolled in year 2. At baseline, 1,547 children were identified for inclusion (control, n = 773; intervention, n = 774). During the 24 surveillance rounds, conducted every 2 months from September 2015 to September 2019, another 1,923 children born to participating women were included (control, n = 963; intervention, n = 960). An additional 262 children were identified for inclusion at end line (control, n = 136; intervention, n = 126). Data on 3,284 children were collected over the surveillance period and at end line. In total, we analyzed 32,460 observations of 3,276 children with 7-day and 2-day recall data collected over the surveillance period and at end line (control, 87.9% of ever-eligible children; intervention, 87.6% of ever-eligible children). There were no differences in baseline household or maternal characteristics between children who were targeted for inclusion compared with those who were analyzed (Supplemental Table 1). Children who were targeted but not included in the analysis were older (born in 2012), because these children aged out of the surveillance system before it began in September 2015, and we further restricted our analyses to those children younger than 36 months. More targeted than analyzed children were also born in 2019 or 2020, because these children were born after morbidity indicators were collected for the last time. The total number of children who were included in data analysis at each round are included in Supplemental Table 2.
Figure 1.
Trial profile. aWomen and their children were enrolled before randomization. For children born after the baseline survey, parental consent was acquired soon after birth. bIn year 2, women who were newly married into enrolled households were recruited for participation. In the control arm, 47 women in 26 settlements were enrolled out of 61 women in 30 settlements identified for recruitment. In the intervention arm, 35 women in 21 settlements were enrolled out of 53 women in 30 settlements identified for recruitment.
Characteristics of participating children, their households, and their mothers are presented in Table 1 by intervention group. Baseline maternal age and education as well as household religion were similar between the intervention and control groups. The basic WASH infrastructure was similar between the two groups. Forty percent of intervention households and 38% of control households had access to an improved sanitation facility, and 51% and 47% of intervention and control households, respectively, had a handwashing facility with soap and water. Access to an improved drinking water source, mainly from tube wells, was nearly universal (98%). At baseline, prevalence of diarrhea in children was collected for the prior 15 days and was found to be 11.4% in the control group and 13.5% in the intervention group. Children in the intervention group had a lower prevalence of ARI (4.3%) at baseline compared with those in the control group (9.8%). Averaged across all data collection rounds, the average age of children was the same between the two groups (19 months).
Table 1.
Household, maternal, and child characteristics of study sample
| Characteristic | Control* | n | Intervention* | n |
|---|---|---|---|---|
| Baseline household characteristics | ||||
| No. of household members | 7.0 ± 3.3 | 1,598 | 7.4 ± 3.6 | 1,579 |
| Religion | – | 1,646 | – | 1,630 |
| Muslim | 70.6 | – | 75.5 | – |
| Hindu | 29.4 | – | 24.5 | – |
| Wealth quintile | – | 1,643 | – | 1,620 |
| Lowest | 27.6 | – | 21.5 | – |
| Low | 22.4 | – | 21.7 | – |
| Middle | 19.7 | – | 20.3 | – |
| High | 16.6 | – | 19.9 | – |
| Highest | 14.1 | – | 16.5 | – |
| Improved basic sanitation facility† | 37.9 | 1,598 | 39.6 | 1,577 |
| Handwashing facility with water and soap | 47.1 | 1,597 | 50.5 | 1,577 |
| Improved drinking water source‡ | 98.2 | 1,598 | 97.8 | 1,579 |
| Baseline maternal characteristics | ||||
| Age, years | 23.4 ± 3.9 | 1,646 | 23.4 ± 3.8 | 1,630 |
| Education | – | 1,646 | – | 1,630 |
| None | 14.9 | – | 14.5 | – |
| Partial primary | 23.8 | – | 23.3 | – |
| Complete primary | 23.8 | – | 21.8 | – |
| Any secondary | 37.6 | – | 40.4 | – |
| Child-level characteristics | ||||
| Sex | – | 1,646 | – | 1,630 |
| Male | 51.3 | – | 50.4 | – |
| Female | 48.7 | – | 49.6 | – |
| Age, months§ | 18.7 ± 10.5 | 16,239 | 18.7 ± 10.5 | 16,221 |
| Diarrhea at baseline‖ | 11.4 | 737 | 13.5 | 741 |
| Acute respiratory infection at baseline‖ | 9.8 | 737 | 4.3 | 741 |
Values are mean ± SD for continuous variables or percentages for categorical variables.
Basic improved sanitation facilities include flush/pour flush toilets connected to piped sewer systems, septic tanks, or pit latrines; pit latrines with slabs (including ventilated pit latrines); and composting toilets that are not shared with other households, as defined by the WHO/UNICEF Joint Monitoring Program for Water Supply, Sanitation and Hygiene (JMP).
Improved drinking water source includes piped water, boreholes or tube wells, protected dug wells, protected springs, rainwater, tanker water, and bottled water as defined by the WHO/UNICEF JMP.
Age is averaged across all survey rounds, excluding baseline. In the control group, this includes 16,239 observations of 1,646 children; in the intervention group, this includes 16,221 observations of 1,630 children.
Diarrhea and acute respiratory infection were assessed with a 15-day recall period at baseline.
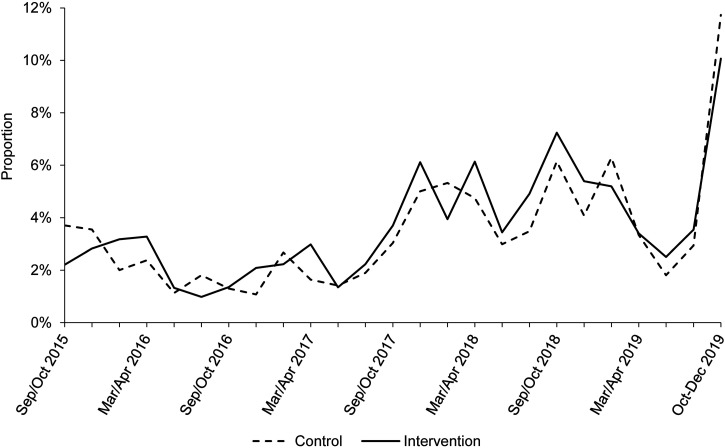
Figure 2 shows the period prevalence of diarrhea over the FAARM trial’s duration by survey round (every 2 months) and intervention group. Overall, diarrhea period prevalence was 4.0% in the control group and 3.8% in the intervention group (Table 2). Diarrhea prevalence was greater in both groups at the beginning of surveillance data collection in trial year 1 (control, 9.0%; intervention, 9.6%) and at end line (control, 6.9%; intervention, 9.0%). The overall diarrhea point prevalence was 1.7% in both groups (Table 2). Figure 3 shows the period prevalence of ARI by survey round and intervention group. The overall period prevalence of ARI was 3.3% in the control group and 3.5% in the intervention group (Table 2). There was a notable increase in ARI prevalence at end line (control, 11.7%; intervention, 10.1%).
Figure 2.
Diarrhea period prevalence (7-day recall) by survey round during the Food and Agricultural Approaches to Reducing Malnutrition (FAARM) trial comparing intervention and control groups. The FAARM baseline survey, conducted in March to May 2015, assessed diarrhea in the past 15 days and is not shown. Routine assessments on morbidity symptoms among all children age 3 years and younger were conducted every 2 months from September 2015 to September 2019. End line morbidity data on all children age 3 years and younger were collected between October and December 2019, with continued follow-up until February 2020.
Table 2.
Effect of the FAARM intervention on morbidity outcomes among children 0 to 36 months old
| Time period* | Con. | Int. | OR (95% CI) | P value |
|---|---|---|---|---|
| Diarrhea period prevalence, 7-day recall | ||||
| Overall | 4.0% | 3.8% | 0.92 (0.71–1.19) | 0.53 |
| Year 1 | 5.9% | 5.4% | 0.91 (0.68–1.23) | 0.55 |
| Year 2 | 3.2% | 3.2% | 1.01 (0.72–1.41) | 0.96 |
| Year 3 | 2.8% | 2.6% | 0.91 (0.63–1.32) | 0.62 |
| Scale-down | 3.7% | 2.8% | 0.72 (0.50–1.04) | 0.08 |
| Postintervention | 6.9% | 9.0% | 1.28 (0.77–2.10) | 0.34 |
| Total no. of observations | 32,460 | – | – | – |
| Total no. of children | 3,276 | – | – | – |
| Diarrhea point prevalence, 2-day recall | ||||
| Overall | 1.7% | 1.7% | 1.03 (0.78–1.36) | 0.82 |
| Year 1 | 2.2% | 2.4% | 1.08 (0.79–1.49) | 0.63 |
| Year 2 | 1.4% | 1.6% | 1.12 (0.78–1.60) | 0.53 |
| Year 3 | 1.2% | 1.2% | 1.01 (0.68–1.49) | 0.96 |
| Scale-down | 1.5% | 1.3% | 0.81 (0.55–1.20) | 0.30 |
| Postintervention | 3.3% | 3.7% | 1.09 (0.65–1.82) | 0.76 |
| Total no. of observations | 64,920 | – | – | – |
| Total no. of children | 3,276 | – | – | – |
| ARI period prevalence, 7-day recall | ||||
| Overall | 3.3% | 3.5% | 1.18 (0.88–1.60) | 0.27 |
| Year 1 | 2.5% | 2.3% | 1.09 (0.74–1.62) | 0.66 |
| Year 2 | 1.7% | 2.0% | 1.36 (0.89–2.08) | 0.15 |
| Year 3 | 4.1% | 4.7% | 1.26 (0.88–1.80) | 0.21 |
| Scale-down | 4.2% | 4.6% | 1.20 (0.83–1.74) | 0.33 |
| Postintervention | 11.7% | 10.1% | 0.93 (0.58–1.50) | 0.78 |
| Total no. of observations | 32,460 | – | – | – |
| Total no. of children | 3,276 | – | – | – |
ARI = acute respiratory infection; CI = confidence interval; Con. = control; FAARM = Food and Agricultural Approaches to Reducing Malnutrition; Int. = intervention; OR = odds ratio.
The first year of the intervention was from September 2015 to August 2016; the second year, from September 2016 to August 2017; and the third year, from September 2017 to August 2018. The scale-down of the intervention was from September 2018 to September 2019, during which field activities were phased out and stopped by December 2018, and only a nutrition counseling refresher training was provided in May 2019. The postintervention end line survey was conducted from October 2019 to February 2020.
Values are raw percentages, and ORs and 95% CIs are calculated using mixed-effects logistic regression models with a random effect at the settlement level to account for clustering. An interaction term was used to calculate effects by year. Analyses are intention-to-treat.
Figure 3.
Acute respiratory infection period prevalence (7-day recall) by survey round during the Food and Agricultural Approaches to Reducing Malnutrition (FAARM) trial comparing intervention and control groups. The FAARM baseline survey, conducted in March to May 2015, assessed acute respiratory infection in the past 15 days and is not shown. Routine assessments on morbidity symptoms among all children age 3 years and younger were conducted every 2 months from September 2015 to September 2019. End line morbidity data on all children age 3 years and younger were collected between October and December 2019, with continued follow-up until February 2020.
The effects of the HFP intervention on the primary outcomes are shown in Table 2. We found no overall effect of the HFP intervention on diarrhea period prevalence (OR, 0.92; 95% CI, 0.71–1.19; P = 0.53) nor on diarrhea point prevalence (OR, 1.03; 95% CI, 0.78–1.36; P = 0.82). Diarrhea period prevalence was marginally lower in the intervention group during the scale-down period of the trial, when intervention activities were phased out (OR, 0.72; 95% CI, 0.50–1.04; P = 0.08), but no other effects by year were observed. The intracluster correlation coefficient at the settlement level, which reflects the correlation between observations within clusters, was 0.087 for the diarrhea period prevalence and 0.097 for the diarrhea point prevalence (including repeat child measures). There was also no evidence of an overall effect of the HFP intervention on ARI period prevalence (OR, 1.18; 95% CI, 0.88–1.60; P = 0.27), nor were there any effects by year of the trial (Table 2). The intracluster correlation coefficient for ARI period prevalence was 0.119 at the settlement level.
As a sensitivity analysis, we assessed the overall intervention effects on each of the primary outcomes adjusting for child age and sex, month of the survey, cluster-average baseline diarrhea and ARI prevalence, and baseline wealth quintile given the slightly higher proportion of intervention households in the two highest wealth quintiles compared with control at baseline, and found no meaningful differences in the effect estimates compared with unadjusted models (Supplemental Table 3).
Across the trial period, diarrhea prevalence was highest among children age 6 to 11 months and lowest in children who were 2 to 3 years old (Table 3). Acute respiratory infection prevalence was highest in the youngest children (age, 0–5 months) and decreased as children aged. In stratified analyses by child age, we did not observe any differential overall effects of the intervention on the primary outcomes (Table 3).
Table 3.
Effect of the FAARM intervention on morbidity outcomes, stratified by child age
| Age in months | Obs. | n | Con., % | Int., % | OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Diarrhea period prevalence, 7-day recall | ||||||
| 0–5 | 4,527 | 1,789 | 3.0 | 2.1 | 0.73 (0.44–1.20) | 0.22 |
| 6–11 | 5,040 | 2,025 | 6.6 | 6.6 | 1.00 (0.72–1.39) | 1.00 |
| 12–23 | 10,806 | 2,390 | 4.8 | 4.6 | 0.93 (0.70–1.22) | 0.60 |
| 24–36 | 11,213 | 2,452 | 2.8 | 2.6 | 0.89 (0.65–1.21) | 0.45 |
| Diarrhea point prevalence, 2-day recall | ||||||
| 0–5 | 9,054 | 1,789 | 1.5 | 1.3 | 1.00 (0.49–2.02) | 1.00 |
| 6–11 | 10,082 | 2,025 | 3.1 | 3.2 | 0.96 (0.65–1.41) | 0.83 |
| 12–23 | 21,610 | 2,390 | 1.9 | 2.0 | 1.01 (0.70–1.45) | 0.96 |
| 24–36 | 22,426 | 2,452 | 0.9 | 1.0 | 1.12 (0.79–1.60) | 0.52 |
| ARI period prevalence, 7-day recall | ||||||
| 0–5 | 4,527 | 1,789 | 4.4 | 4.2 | 0.99 (0.68–1.44) | 0.96 |
| 6–11 | 5,040 | 2,025 | 3.7 | 4.5 | 1.24 (0.79–1.93) | 0.35 |
| 12–23 | 10,806 | 2,390 | 3.4 | 3.7 | 1.14 (0.81–1.59) | 0.45 |
| 24–36 | 11,213 | 2,452 | 2.5 | 2.7 | 1.10 (0.81–1.51) | 0.54 |
ARI = acute respiratory infection; CI = confidence interval; Con. = control; FAARM = Food and Agricultural Approaches to Reducing Malnutrition; Int. = intervention; Obs. = observations; OR = odds ratio.
Values are raw percentages, and ORs and 95% CIs are calculated using mixed-effects logistic regression models with a random effect at the settlement level to account for clustering. Analyses are intention-to-treat.
There were no overall differences in diarrhea severity and accompanying illness symptoms between the control and intervention groups (Table 4), nor were there differences by year of the trial (Supplemental Table 4). In half of the diarrhea episodes, children had an accompanying fever; in slightly more than one quarter, they were reported to have vomited; and in approximately 7%, they were reported to have bloody stools. Health care was received in about 70% of child diarrhea episodes and in 86% of ARI episodes, according to the caregivers’ report. Slightly more diarrhea episodes in the intervention group (56%) than in the control group (47%) were treated with ORS, although this difference was marginal and may have been a result of chance. About half of diarrhea episodes in children were treated with zinc in both trial groups.
Table 4.
Diarrhea severity and health-seeking behaviors among intervention and control groups in the FAARM trial
| Indicator | Control | Intervention | ||
|---|---|---|---|---|
| freq/n | % | freq/n | % | |
| Diarrhea severity | ||||
| Proportion of diarrhea episodes in which bloody stool was reported | 44/657 | 6.7 | 45/615 | 7.3 |
| Proportion of diarrhea episodes in which child refused to eat or drink | 430/657 | 65.4 | 442/615 | 71.9 |
| Proportion of diarrhea episodes in which child cried a lot | 371/657 | 56.5 | 362/615 | 58.9 |
| Proportion of diarrhea episodes in which child was weak and drowsy | 362/657 | 55.1 | 379/615 | 61.6 |
| Proportion of diarrhea episodes in which child had a fever | 326/657 | 49.6 | 287/615 | 46.7 |
| Proportion of diarrhea episodes in which child was vomiting | 192/657 | 29.2 | 178/615 | 28.9 |
| Health-seeking behaviors | ||||
| Proportion of diarrhea episodes for which child received health care | 458/657 | 69.7 | 437/615 | 71.1 |
| Proportion of diarrhea episodes in which child was given ORS* | 310/657 | 47.2 | 346/615 | 56.3† |
| Proportion of diarrhea episodes in which child was given zinc‡ | 301/619 | 48.6 | 286/564 | 50.7 |
| Proportion of ARI episodes for which child received health care | 449/531 | 84.6 | 500/573 | 87.3 |
ARI = acute respiratory infection; FAARM = Food and Agricultural Approaches to Reducing Malnutrition; Freq = frequency; ORS = oral rehydration solution.
ORS includes packaged ORS and homemade ORS.
P < 0.1 using mixed-effects logistic regression models accounting for clustering at the settlement level.
Data on zinc supplementation were not collected at end line.
DISCUSSION
Our findings suggest that an HFP and food hygiene intervention implemented in Sylhet Division, Bangladesh, had no impact on morbidity symptoms in children younger than 3 years. Bimonthly data collected across the trial showed an overall low prevalence of diarrhea (3.9%) and ARI (3.4%) over 1-week periods. We found no evidence of an effect of the intervention on diarrhea or ARI outcomes overall or by year of the trial. There were also no differences between the intervention and control groups with regard to the severity of diarrhea symptoms, such as accompanying fever or vomiting, nor were there any differences in caregivers’ health-seeking behaviors, such as treatment of diarrhea with ORS or zinc.
Prior evaluations of nutrition-sensitive agricultural interventions show mixed evidence for impacts on diarrhea and ARI in children. Similar to our study, several agricultural interventions that included WASH components alongside home gardening and livestock production found no impacts on these morbidity outcomes. An 18-month chicken production intervention in Kenya36 that provided households with improved, vaccinated chicks with or without seeds for home gardening, and behavior-change communication on nutrition, women’s empowerment, and WASH found no difference in the 2-week diarrhea prevalence among children (0–36 months old at baseline) in either intervention group compared with the control group. Similarly, a 2-year trial conducted in Cambodia37 that promoted cash-crop production of rice, chickens, or vegetables among farmer groups in addition to nutrition, food safety, and hygiene education and provision of soap found no impacts on the 2-week diarrhea prevalence or ARI prevalence among children younger than 2 years. In Zambia, a 4-year intervention38 that promoted home gardening, chicken and goat rearing, and women’s empowerment activities, with or without nutrition and hygiene behavior-change communication, also found no difference in the 2-week diarrhea prevalence among children younger than 5 years compared with the control group, although diarrhea reduced in all groups compared with baseline. In contrast to these studies and our study, prior randomized, controlled trials of Helen Keller International’s HFP program show potential beneficial impacts on diarrhea. A 2-year HFP evaluation conducted in Burkina Faso34 found a 10- and 16-percentage-point reduction from baseline in 7-day diarrhea prevalence among children (3–12.9 months at baseline) in the two intervention arms compared with the change from diarrhea baseline prevalence in the control group, although diarrhea was substantially less at baseline in the control arm (17% versus 27% and 31%), and end line diarrhea prevalence was similar across all arms (12–14%). In Nepal, an 11-month substudy of an HFP program35 conducted biweekly surveillance of morbidity symptoms among 306 children age 6 to 9 months and found statistical evidence for a lower 2-week longitudinal prevalence of diarrhea (defined as at least one loose stool) among children receiving only the HFP program (2.4%) compared with the control group (3.1%), but not among those who received the HFP program plus micronutrient powder supplementation (2.6%). The positive impacts on diarrhea seen in these Helen Keller International HFP interventions—if they indeed imply real reductions—contrast with ours and the other studies mentioned. This may be a result of differences in the delivery of the behavior-change communication component. The FAARM implementation team conducted one-on-one home visits in addition to group sessions on similar content and overall similar dosage to other Helen Keller International interventions; however, women were visited less frequently (every 2 months compared with twice monthly) over a longer time scale, and FAARM visits were used to support both the HFP implementation, and nutrition and health education. The FAARM trial sought to strengthen health behavior change through an additional food hygiene component delivered by separate staff, making the food hygiene messaging stronger overall compared with other HFP interventions. However, although several food hygiene practices were improved (including using clean feeding utensils, cooking fresh food or reheating stored food, and washing hands with soap and water before feeding children), handwashing practices were rare overall and practiced inconsistently (Sobhan et al., submitted). Furthermore, we observed no reduction in complementary food contamination with Escherichia coli (Huda et al., to be published). Thus, if no reduction in pathogen ingestion was achieved through our intervention, we would only expect to see reductions in diarrhea susceptibility through improved nutrition.
Only one study, to our knowledge, has investigated the impacts of an intervention including comprehensive nutrition-sensitive agriculture plus WASH inputs. In Kenya, Wegmüller et al.45 compared the impacts of a basic agricultural intervention to an agricultural intervention plus monthly distribution of micronutrient powders, ORS, and soap; a twice-yearly provision of chlorine solution; a provision of laying hens; and monthly nutrition and WASH group trainings. The 2-week prevalence of diarrhea among children age 6 to 35 months at baseline, assessed quarterly, was significantly less compared with the control arm after 1 year, but not after 2 years, when diarrhea was substantially lower in both groups, which the authors explained as an age effect or increased hygiene measures to prevent coronavirus disease in early 2020. Acute respiratory infection was significantly less after 1 and 2 years of the intervention. Although these results provide some evidence that adding WASH inputs to agricultural interventions may be more effective than just WASH messaging in reducing child morbidity, prior WASH interventions find limited evidence of impacts on morbidity. Of three large-scale combined WASH randomized controlled trials conducted in Bangladesh, in four districts in the Dhaka Division,19 Kenya,20 and Zimbabwe,21 only the trial conducted in Bangladesh19 showed reductions in children’s diarrhea prevalence. The trials showed marginal to no impacts on ARI.21,22 Based on this evidence, Cumming et al.46 suggested that basic household-level WASH interventions are likely insufficient to stop the multiple routes of pathogen transmission in settings where there is high environmental contamination. Nevertheless, a recent meta-analysis suggests that WASH interventions could reduce diarrhea substantially in low-income settings.47 However, responder and observer bias may have contributed to the large effects observed in the meta-analysis48 and may explain why these results have not been confirmed in more rigorous trials in Kenya, Bangladesh, and Zimbabwe.19–21
Treatment of diarrhea with ORS and zinc as well as timely health-care seeking can reduce diarrhea mortality and the duration of diarrhea episodes.9,14 Bangladesh, a pioneer in ORS treatment, has one of the highest levels of ORS use in the world (76% of children with diarrhea received ORS in 2017)49; nevertheless, many more lives could be saved if universal coverage is to be achieved.17 In our study, we observed a marginal increase in the use of ORS to treat diarrhea. In the intervention group, slightly more than half (56%) of diarrhea episodes were treated with ORS (control group, 47%), which is similar to the reported percentage of children with diarrhea receiving ORS in Sylhet Division in 2019 (62%), but less than the national average.50 Intervention studies on the effectiveness of ORS promotion are limited.51 A 6-month multiple behavior-change intervention, which included clinic events, community events, and radio messaging, found no increase in the use of ORS to treat diarrhea among children in Zambia.52 Messaging alone thus may be insufficient to increase ORS use. In the FAARM trial, a yearly session on care of sick and recovering children was held as part of the HFP intervention, so participants may have heard about ORS three to four times. In the study in Kenya conducted by Wegmüller et al.45 described earlier, the authors show large increases in ORS and zinc treatment compared with the control group, which may be because the intervention supplied caregivers with ORS and zinc throughout the trial. Further research is needed to understand how to increase ORS use for diarrhea in Bangladesh, where ORS packets are relatively accessible and inexpensive.
Many nutrition-sensitive agricultural interventions include small-scale livestock rearing to improve dietary quality.53 However, recent studies highlight concerns about the risks of livestock rearing, particularly of poultry, for children’s health. A study in Ethiopia found a negative association between poultry rearing and children’s height-for-age z-score when poultry were kept in the household dwelling at night, likely because of exposure to fecal pathogens.54 Other research corroborates this hypothesis, finding that children who are exposed to chickens, particularly indoors, have a greater likelihood of infection with certain zoonotic enteropathogens55–57 and have elevated markers of environmental enteric dysfunction (EED).58 Our study promoted poultry production but found no evidence of a negative impact on children’s morbidity outcomes. Other poultry production trials similarly show no evidence of increased diarrhea among children receiving the poultry intervention.34–36,45 These results suggest that agricultural interventions may be able to promote poultry production for dietary benefits without increasing infectious risks. An important aspect to poultry interventions may be training to improve hygienic poultry practices. Our study promoted safe poultry management through improved poultry sheds meant to separate poultry from children, particularly at night, and to promote poultry health. More than two thirds of households in the intervention group of our study had an “improved” poultry shed at end line, and three quarters of poultry owners used the shed for keeping poultry.59 However, it is important to interpret these findings with caution. Children may have experienced subclinical infections from poultry exposure that were not observed in this analysis of morbidity outcomes. Enteropathogen infection without diarrhea is common among young children living in low- and middle-income countries, with a multisite cohort study finding at least one enteropathogen in 65% of nondiarrheal stool samples.60 In FAARM, analysis of EED biomarkers among a subsample of children younger than 24 months showed greater levels of myeloperoxidase in the intervention as compared with the control group at end line (Müller-Hauser et al., submitted). In the intervention group, myeloperoxidase levels were associated with poultry keeping only if poultry were not kept in a shed. These findings further indicate the importance of training and facilitation of safe poultry production practices in interventions in low-resource contexts. Further research is needed on the potential subclinical infectious risks of poultry interventions.
Our study had several strengths, including a large sample size, bimonthly data collection over 4 years, and assessment of diarrhea using two indicators. We expect that our results are likely generalizable to other similar rural areas of Bangladesh in which undernutrition is highly prevalent. As noted earlier, this study did not capture impacts of the intervention on asymptomatic infections, as the effects on biomarkers of EED, inflammation, and infections will be reported separately. These more sensitive methods to detect infection may be useful to elucidate health impacts in settings with a low prevalence of diarrhea, as in our study. A major limitation of this study, as in most morbidity studies, is the reliance on caregiver recall for assessing the primary and secondary outcomes, which is subject to measurement error and bias. The use of a 7-day recall window and 2-day point recalls should minimize this bias compared with longer recall periods. Importantly, as participants in the intervention group could not be blinded, they may have reported less morbidity to please the implementation team. The fact that we did not observe any differences in morbidity throughout our trial thus can reassure us that such courtesy bias was not at play. A lower prevalence of ARI was found in the intervention group at baseline, and this imbalance could have biased our results toward the null; however, controlling for baseline ARI in a sensitivity analysis did not change effect estimates meaningfully, suggesting this was likely not an issue. We noted a markedly greater ARI prevalence in both the intervention and control groups at our end line survey, as well as a greater diarrhea prevalence at the beginning of surveillance and at end line. These are difficult to explain and may be a result of random variation, because there were no substantial changes in survey methodology or any clear external factors (such as infectious outbreaks) at the time of the survey that may have driven these results. It is possible that response fatigue from surveys being repeated every 2 months may explain, in part, a lower prevalence of diarrhea during surveillance compared with the end line survey, given the longer time gap between surveys from surveillance to end line and a greater overall response rate at end line.
In conclusion, our findings suggest that HFP coupled with nutrition and food hygiene training was insufficient to reduce morbidity symptoms among children in rural Bangladesh. Although there may be dietary and social benefits of introducing HFP, we found no evidence for short-term health gains related to illness. Agricultural interventions in rural, low-resource contexts may need to incorporate more comprehensive WASH measures that limit pathogen exposure from multiple routes, including animals; nutrition-specific strategies that support immune function, such as micronutrient supplementation; and frequent messaging to achieve impacts on child health.
Supplemental Materials
ACKNOWLEDGMENTS
We thank all the women and their families for participating in the Food and Agricultural Approaches to Reducing Malnutrition (FAARM) trial and data collection activities. We thank our collaborating partner, Helen Keller International, for their support in establishing and conducting the FAARM trial. We also gratefully acknowledge the project implementation and research staff, and the contributions of icddr,b and the James P Grant School of Public Health at BRAC University in Bangladesh. The authors confirm that all ongoing and related trials for this intervention are registered (#NCT025-05711). This trial is registered at ClinicalTrials.gov (#NCT025-05711, https://clinicaltrials.gov/ct2/show/NCT02505711).
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1. GBD 2015 Mortality and Causes of Death Collaborators , 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE, 2013. Global burden of childhood pneumonia and diarrhoea. Lancet 381: 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD Diarrhoeal Diseases Collaborators , 2017. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 17: 909–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2016 Lower Respiratory Infections Collaborators , 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18: 1191–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Troeger C. et al. , 2018. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health 6: e255–e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. George CM, Perin J, Parvin T, Bhuyian MSI, Thomas ED, Monira S, Zohura F, Hasan MT, Alam M, Tofail F, 2022. Diarrhea prevalence and child growth faltering are associated with subsequent adverse child developmental outcomes in Bangladesh (CHoBI7 Program). Am J Trop Med Hyg 106: 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinkerton R, Oriá RB, Lima AAM, Rogawski ET, Oriá MOB, Patrick PD, Moore SR, Wiseman BL, Niehaus MD, Guerrant RL, 2016. Early childhood diarrhea predicts cognitive delays in later childhood independently of malnutrition. Am J Trop Med Hyg 95: 1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray DM, Turkovic L, Willemse L, Visagie A, Vanker A, Stein DJ, Sly PD, Hall GL, Zar HJ, 2017. Lung function in African infants in the Drakenstein child health study: impact of lower respiratory tract illness. Am J Respir Crit Care Med 195: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhutta ZA, Das JK, Walker N, Rizvi A, Campbell H, Rudan I, Black RE, 2013. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet 381: 1417–1429. [DOI] [PubMed] [Google Scholar]
- 10. Walker CLF, Perin J, Katz J, Tielsch JM, Black RE, 2013. Diarrhea as a risk factor for acute lower respiratory tract infections among young children in low income settings. J Glob Health 3: 010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashraf S, Huque MH, Kenah E, Agboatwalla M, Luby SP, 2013. Effect of recent diarrhoeal episodes on risk of pneumonia in children under the age of 5 years in Karachi, Pakistan. Int J Epidemiol 42: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt W-P, Cairncross S, Barreto ML, Clasen T, Genser B, 2009. Recent diarrhoeal illness and risk of lower respiratory infections in children under the age of 5 years. Int J Epidemiol 38: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. UNICEF, World Health Organization, World Bank Group, United Nations , 2021. Levels & Trends in Child Mortality: Report 2021. New York, NY: UNICEF. [Google Scholar]
- 14. UNICEF , 2016. One Is Too Many: Ending Child Deaths from Pneumonia and Diarrhoea. New York, NY: UNICEF. [Google Scholar]
- 15. United Nations , 2015. Transforming Our World: The 2030 Agenda for Sustainable Development. New York, NY: United Nations. [Google Scholar]
- 16. National Institute of Population Research and Training (NIPORT), and ICF 2020. Bangladesh Demographic and Health Survey 2017–18. Dhaka, Bangladesh and Rockville, MA: NIPORT and ICF. [Google Scholar]
- 17. Billah SM. et al. , 2019. Bangladesh: a success case in combating childhood diarrhoea. J Glob Health 9: 020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization , 2013. Essential Nutrition Actions: Improving Maternal, Newborn, Infant and Young Child Health and Nutrition. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 19. Luby SP. et al. , 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health 6: e302–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Null C. et al. , 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health 6: e316–e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Humphrey JH. et al. , 2019. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health 7: e132–e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swarthout J. et al. , 2020. Effects of individual and combined water, sanitation, handwashing, and nutritional interventions on child respiratory infections in rural Kenya: a cluster-randomized controlled trial. Am J Trop Med Hyg 102: 1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Black RE, Walker CF, 2019. Do water, sanitation, and hygiene interventions prevent childhood diarrhea? J Infect Dis 22: 1241–1243. [DOI] [PubMed] [Google Scholar]
- 24. McGuinness SL, Barker SF, O’Toole J, Cheng AC, Forbes AB, Sinclair M, Leder K, 2018. Effect of hygiene interventions on acute respiratory infections in childcare, school and domestic settings in low- and middle-income countries: a systematic review. Trop Med Int Health 23: 816–833. [DOI] [PubMed] [Google Scholar]
- 25. Kirk MD, Angulo FJ, Havelaar AH, Black RE, 2017. Diarrhoeal disease in children due to contaminated food. Bull World Health Organ 95: 233–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morse T, Tilley E, Chidziwisano K, Malolo R, Musaya J, 2020. Health outcomes of an integrated behaviour-centred water, sanitation, hygiene and food safety intervention: a randomised before and after trial. Int J Environ Res Public Health 17: 2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manaseki-Holland S. et al. , 2021. Effects on childhood infections of promoting safe and hygienic complementary-food handling practices through a community-based programme: a cluster randomised controlled trial in a rural area of The Gambia. PLoS Med 18: e1003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caulfield LE, de Onis M, Blössner M, Black RE, 2004. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 80: 193–198. [DOI] [PubMed] [Google Scholar]
- 29. Black RE, Brown KH, Becker S, 1984. Malnutrition is a determining factor in diarrheal duration, but not incidence, among young children in a longitudinal study in rural Bangladesh. Am J Clin Nutr 39: 87–94. [DOI] [PubMed] [Google Scholar]
- 30. Walson JL, Berkley JA, 2018. The impact of malnutrition on childhood infections. Curr Opin Infect Dis 31: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bourke CD, Berkley JA, Prendergast AJ, 2016. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 37: 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Black RE. et al. , 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382: 427–451. [DOI] [PubMed] [Google Scholar]
- 33. Haselow NJ, Stormer A, Pries A, 2016. Evidence-based evolution of an integrated nutrition-focused agriculture approach to address the underlying determinants of stunting. Matern Child Nutr 12: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olney DK, Pedehombga A, Ruel MT, Dillon A, 2015. A 2-year integrated agriculture and nutrition and health behavior change communication program targeted to women in Burkina Faso reduces anemia, wasting, and diarrhea in children 3–12.9 months of age at baseline: a cluster-randomized controlled trial. J Nutr 145: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 35. Osei AK, Pandey P, Spiro D, Adhikari D, Haselow N, De Morais C, Davis D, 2015. Adding multiple micronutrient powders to a homestead food production programme yields marginally significant benefit on anaemia reduction among young children in Nepal. Matern Child Nutr 11: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Passarelli S. et al. , 2020. A chicken production intervention and additional nutrition behavior change component increased child growth in Ethiopia: a cluster-randomized trial. J Nutr 150: 2806–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reinbott A, Schelling A, Kuchenbecker J, Jeremias T, Russell I, Kevanna O, Krawinkel MB, Jordan I, 2016. Nutrition education linked to agricultural interventions improved child dietary diversity in rural Cambodia. Br J Nutr 116: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumar N, Nguyen PH, Harris J, Harvey D, Rawat R, Ruel MT, 2018. What it takes: evidence from a nutrition- and gender-sensitive agriculture intervention in rural Zambia. J Dev Effect 10: 341–372. [Google Scholar]
- 39. Wendt AS, Sparling TM, Waid JL, Mueller AA, Gabrysch S, 2019. Food and Agricultural Approaches to Reducing Malnutrition (FAARM): protocol for a cluster-randomised controlled trial to evaluate the impact of a Homestead Food Production programme on undernutrition in rural Bangladesh. BMJ Open 9: e031037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sobhan S, Müller-Hauser AA, Huda TMN, Waid JL, Gautam OP, Gon G, Wendt AS, Gabrysch S, 2022. Design, delivery, and determinants of uptake: findings from a food hygiene behavior change intervention in rural Bangladesh. BMC Public Health 22: 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hartung C, Lerer A, Anokwa Y, Tseng C, Brunette W, Borriello G, 2010. Open data kit: tools to build information services for developing regions. In: Proceedings of the 4th ACM/IEEE International Conference on Information and Communication Technologies and Development, London, UK, 1–12.
- 42. Rutstein SO, Johnson K, 2004. The DHS Wealth Index. Calverton, MD: ORC Macro. [Google Scholar]
- 43. World Health Organization, UNICEF , 2017. JMP Methodology: 2017 Update & SDG Baselines. Geneva, Switzerland: World Health Organization.
- 44. World Health Organization , 2018. 2018 Global Reference List of 100 Core Health Indicators (Plus Health-related SDGs). Geneva, Switzerland: World Health Organization. [Google Scholar]
- 45. Wegmüller R. et al. , 2022. Effectiveness of an integrated agriculture, nutrition-specific and nutrition-sensitive program on child growth in western Kenya: a cluster-randomized controlled trial. Am J Clin Nutr 116: 446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cumming O. et al. , 2019. The implications of three major new trials for the effect of water, sanitation and hygiene on childhood diarrhea and stunting: a consensus statement. BMC Med 17: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolf J. et al. , 2022. Effectiveness of interventions to improve drinking water, sanitation, and handwashing with soap on risk of diarrhoeal disease in children in low-income and middle-income settings: a systematic review and meta-analysis. Lancet 400: 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmidt W-P, Cairncross S, 2009. Household water treatment in poor populations: is there enough evidence for scaling up now? Environ Sci Technol 43: 986–992. [DOI] [PubMed] [Google Scholar]
- 49. Wiens KE. et al. , 2020. Mapping geographical inequalities in oral rehydration therapy coverage in low-income and middle-income countries, 2000–17. Lancet Glob Health 8: e1038–e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bangladesh Bureau of Statistics, UNICEF Bangladesh , 2019. Progotir Pathey, Bangladesh Multiple Indicator Cluster Survey 2019, Survey Findings Report. Dhaka, Bangladesh: Bangladesh Bureau of Statistics. [Google Scholar]
- 51. Lenters LM, Das JK, Bhutta ZA, 2013. Systematic review of strategies to increase use of oral rehydration solution at the household level. BMC Public Health 13: S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Greenland K, Chipungu J, Curtis V, Schmidt W-P, Siwale Z, Mudenda M, Chilekwa J, Lewis JJ, Chilengi R, 2016. Multiple behaviour change intervention for diarrhoea control in Lusaka, Zambia: a cluster randomised trial. Lancet Glob Health 4: e966–e977. [DOI] [PubMed] [Google Scholar]
- 53. Ruel MT, Quisumbing AR, Balagamwala M, 2018. Nutrition-sensitive agriculture: what have we learned so far? Glob Food Secur 17: 128–153. [Google Scholar]
- 54. Headey D, Hirvonen K, 2016. Is exposure to poultry harmful to child nutrition? An observational analysis for rural Ethiopia. PLoS One 11: e0160590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vasco K, Graham JP, Trueba G, 2016. Detection of zoonotic enteropathogens in children and domestic animals in a semirural community in Ecuador. Appl Environ Microbiol 82: 4218–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zambrano LD, Levy K, Menezes NP, Freeman MC, 2014. Human diarrhea infections associated with domestic animal husbandry: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 108: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Budge S, Barnett M, Hutchings P, Parker A, Tyrrel S, Hassard F, Garbitt C, Moges M, Woldemedhin F, Jemal M, 2020. Risk factors and transmission pathways associated with infant Campylobacter spp. prevalence and malnutrition: a formative study in rural Ethiopia. PLoS One 15: e0232541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. George CM. et al. , 2015. Fecal markers of environmental enteropathy are associated with animal exposure and caregiver hygiene in Bangladesh. Am J Trop Med Hyg 93: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lambrecht NJ, Waid JL, Wendt AS, Sobhan S, Kader A, Gabrysch S, 2023. Impact of a Homestead Food Production program on poultry rearing and egg consumption: a cluster-randomized controlled trial in Bangladesh. Matern Child Nutr 19: e13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Platts-Mills JA. et al. , 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3: e564–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.