ABSTRACT.
Globally, half of patients with pulmonary tuberculosis (PTB) are diagnosed clinically without bacteriologic confirmation. In clinically diagnosed PTB patients, we assessed both the proportion in whom PTB could be bacteriologically confirmed by reference standard diagnostic tests and the prevalence of diseases that mimic PTB. We recruited adult patients beginning treatment of bacteriologically unconfirmed PTB in Moshi, Tanzania, in 2019. We performed mycobacterial smear, Xpert MTB/RIF Ultra, and mycobacterial culture, fungal culture, and bacterial culture on two induced sputum samples: fungal serology and computed tomography chest scans. We followed participants for 2 months after enrollment. We enrolled 36 (63%) of 57 patients with bacteriologically unconfirmed PTB. The median (interquartile range) age was 55 (44–67) years. Six (17%) were HIV infected. We bacteriologically confirmed PTB in 2 (6%). We identified pneumonia in 11 of 23 (48%), bronchiectasis in 8 of 23 (35%), interstitial lung disease in 5 of 23 (22%), pleural collections in 5 of 23 (22%), lung malignancy in 1 of 23 (4%), and chronic pulmonary aspergillosis in 1 of 35 (3%). After 2 months, 4 (11%) were dead, 21 (58%) had persistent symptoms, 6 (17%) had recovered, and 5 (14%) were uncontactable. PTB could be bacteriologically confirmed in few patients with clinically diagnosed PTB and clinical outcomes were poor, suggesting that many did not have the disease. We identified a high prevalence of diseases other than tuberculosis that might be responsible for symptoms.
INTRODUCTION
Tuberculosis (TB) is one of the leading causes of global morbidity and mortality, with > 1.4 million deaths during 2019.1 The World Health Organization (WHO) case definitions of TB include bacteriologically confirmed cases, where a biologic specimen is positive by smear microscopy, culture, or approved rapid diagnostic tests. Alternatively, TB cases can be clinically diagnosed where the patient has not been bacteriologically confirmed but has been diagnosed with active TB by a clinician who has decided to give TB treatment.2 In 2019, approximately 6 million, or 84% of global TB cases, were pulmonary TB (PTB), with the remainder extrapulmonary TB. Of those with PTB, 57% were bacteriologically confirmed, with the remainder clinically diagnosed.1 The proportion of PTB that is bacteriologically unconfirmed varies substantially by country, but in many well-resourced settings less than 10% of PTB is unconfirmed.3 Documented outcomes of patients with TB are frequently worse among those with clinically diagnosed TB compared with those with bacteriologically confirmed TB, with recent data indicating up to 10% mortality at 6 months among those diagnosed clinically compared with < 5% among bacteriologically confirmed cases.4 Data suggest this increased mortality cannot be fully explained by comorbidities,4,5 raising the possibility that unrecognized diseases mimicking TB are contributing to individual poor outcomes and compromising programmatic goals.
Nearly half of all PTB cases are clinically diagnosed because of limitations among diagnostic tests as well as the lack of availability.6 Notably, these tests rely on the collection of sputum, which is not feasible in all patients. Furthermore, each test has its limitations. Mycobacterial culture of multiple sputum samples, the reference standard diagnostic test for PTB,7 is resource intense and technically challenging, and consequently widely unavailable in many low-resource, high-TB incidence countries. Sputum smear microscopy, historically the most commonly used diagnostic test for PTB, has low sensitivity among patients with paucibacillary disease, such as children and those with HIV infection.8 Molecular detection by Xpert MTB/RIF or Xpert MTB/RIF Ultra have higher sensitivity compared with sputum microscopy, but the sensitivity remains lower than that of culture.9 Taken together, clinicians in high-TB incidence settings are often faced with making PTB treatment decisions in the context of a limited or negative diagnostic workup. To avoid undertreatment of PTB, clinical diagnosis and treatment of chronic respiratory diseases as PTB is common.1 This approach is understandable given the infection risk to others and potential morbidity of untreated PTB along with the recognition that the best available diagnostic tools may not diagnose all patients with PTB when treatment decisions are needed.10
Therefore, the symptoms of some patients with clinically diagnosed PTB may in fact be due to diseases other than TB,11 but knowledge of the epidemiology of diseases that mimic PTB in low-resource, high-TB incidence settings is limited. Failure to diagnose and treat these conditions may explain the poorer treatment outcomes for these patients. Available reports suggest that other diseases with similar manifestations, such as aspergillosis,12 bronchiectasis,13 histoplasmosis,14 and Pneumocystis jiroveci pneumonia are underrecognized.15 Standard diagnostic tests for these conditions are typically unavailable in many high-TB incidence countries; and current diagnostic and treatment guidelines, such as those provided by the WHO, do not promote consideration or testing for these conditions among patients suspected of having PTB. For these reasons, an understanding of the epidemiology of diseases that mimic PTB is an important step toward redesigning present diagnostic and treatment pathways, and such efforts may improve clinical outcomes for patients across many low-resource settings.
Tanzania is a lower middle-income country in East Africa, and in 2019 had an estimated incidence of TB of 237 (uncertainty interval 112–408) cases per 100,000 population.1 TB culture is not widely available, so sputum smear microscopy and more recently Xpert MTB/RIF are used to bacteriologically confirm TB. Approximately half of all PTB diagnoses are not bacteriologically confirmed, and this proportion has not fallen since the widespread introduction of Xpert MTB/RIF testing of sputum.1,16 In this context, we sought to investigate patients beginning treatment of PTB without bacteriologic confirmation, to assess whether PTB could be confirmed via induced sputum specimens and to systematically assess the presence of diseases that might mimic PTB.
MATERIALS AND METHODS
We conducted a prospective cohort study at two referral hospitals, Mawenzi Regional Referral Hospital and Kilimanjaro Christian Medical Center (KCMC) in Moshi, Tanzania from July 9 through October 21, 2019. Moshi (population > 180,000) is the administrative center of the Kilimanjaro region (population > 1.6 million). Mawenzi Regional Referral Hospital is a 300-bed regional hospital that serves the Kilimanjaro region and KCMC is a 630-bed zonal referral hospital that serves the northern zone of Tanzania (population > 15 million). Xpert MTB/RIF of sputum samples was the standard diagnostic test for PTB at our study sites during the study period.
Enrollment criteria.
We prospectively screened all patients beginning TB treatment at each site. Patients were eligible for enrollment if they were beginning treatment of PTB as a clinically diagnosed case without bacteriologic confirmation2; that is, they did not have a biological specimen that was positive by smear microscopy, culture, or a WHO-approved rapid diagnostic test (such as Xpert MTB/RIF). We included any patient diagnosed according to the treating physician’s judgment, and included patients whose sputum samples had tested negative and those who were unable to provide sputum samples. Patients with impaired consciousness, requiring > 3 L/minute supplemental oxygen, with evidence of shock, or with uncontrolled asthma were excluded because of concerns they would be unable to safely complete sputum induction. Participants were enrolled within 24 hours of beginning treatment.
Study procedures.
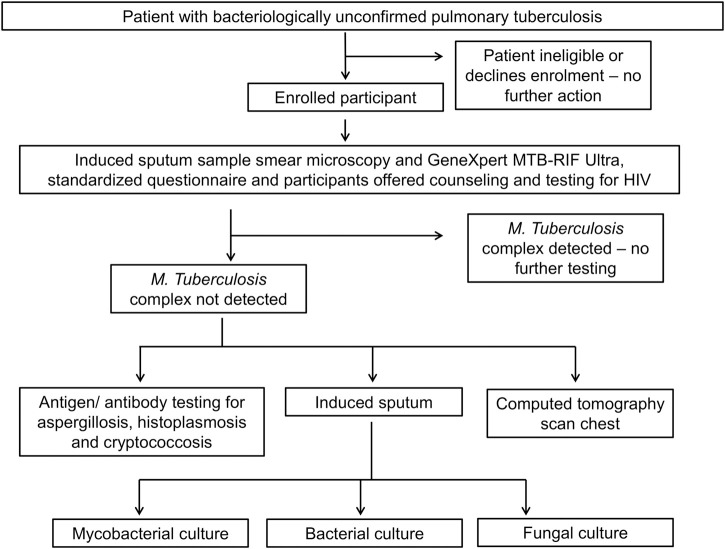
After providing informed written consent, participants were administered a standardized questionnaire of symptoms, treatments taken, past medical history, and social risk factors for respiratory disease. Standardized physical examination was performed by a trained clinical officer. Participants underwent a cascade of investigations as shown in Figure 1. First, sputum induction was performed using 7% hypertonic saline via the DeVilbiss UltraNeb 2000 (DeVilbiss, Port Washington, NY) ultrasonic nebulizer. We considered an adequate sputum volume to be ≥ 5 mL.17 Two samples were collected at least 2 hours apart, pooled, and tested as one.18 Participants with negative initial mycobacterial tests (sputum microscopy and molecular tests as described below) were requested to provide blood for fungal serology and undergo a computed tomography (CT) chest scan, and induced sputum was processed for bacterial and fungal pathogens. All participants were offered counseling and testing for HIV by the treating clinicians immediately prior to enrollment, with our study recording participant-reported HIV infection status.
Figure 1.
Investigation protocol for participants with bacteriologically unconfirmed pulmonary tuberculosis, northern Tanzania, 2019.
Laboratory testing.
Sputum testing and cryptococcal antigen testing were performed at the Kilimanjaro Clinical Research Institute, Moshi, Tanzania. Aspergillus and Histoplasma serological testing was performed by New South Wales Pathology, Sydney, Australia.
Sputum mycobacterial testing.
Approximately two-thirds of the sputum sample was apportioned for mycobacterial testing. Sputum was tested for Mycobacterium tuberculosis complex using microscopy with TB Fluorescent Stain (Becton Dickinson, Franklin Lakes, NJ) using the standard technique; the Xpert MTB/RIF Ultra assay (Cepheid, Sunnyvale, CA) according to manufacturer protocols; and mycobacterial culture using the BACTEC Mycobacteria Growth Indicator Tube (MGIT) system on the MGIT BACTEC 960 (Becton Dickinson) with a maximum incubation of 42 days. Positive mycobacterial cultures were confirmed as TB using the GenoType MTBDRplus (Hain Life Sciences, Nehren, Germany).
Sputum bacterial culture.
Approximately one-third of the sputum sample was apportioned for bacterial and fungal testing. Sputum was processed using standard streak plate methods. Gram stain was performed directly on the most purulent portion of sputum samples. A purulent portion of sputum was inoculated onto sheep blood agar, MacConkey agar, chocolate agar, and colistin nalidixic acid agar and then cultured at 35–37°C in 5% CO2. Isolate identification was performed using standard biochemical methods.
Sputum fungal culture.
Sputum was inoculated onto Sabouraud dextrose agar and cultured at 30 and 36°C.
Fungal serology.
Cryptococcal antigen level was measured using the Latex Cryptococcal Antigen Detection System assay (Immuno-Mycologics, Norman, OK). Serum samples were stored at −80°C and tested for Aspergillus IgG using the CAP fluoroenzyme immunoassay system and for total Histoplasma antibody by immunodiffusion.
CT chest scanning.
Participants underwent CT scanning of the chest using the Siemens Somatom Definition AS+ scanner (Siemens AG, Munich, Germany). CT scans were read by two radiologists (A. S. and R. T.). A single radiologist (R. T.) categorized CT results according to a priori case definitions (Table 1). Participants and the treating clinicians received a report of the results of the scan.
Table 1.
Case definitions for diseases among patients who began treatment of PTB without bacterial confirmation
| Disease | Relevant investigations | Definition |
|---|---|---|
| Mycobacterial infection | ||
| TB | Mycobacterial culture | Positive Xpert MTB/RIF or culture of Mycobacterium tuberculosis complex |
| Nontuberculous mycobacterial infection | Mycobacterial culture | Culture of nontuberculous mycobacteria |
| Bacterial infection | ||
| Bacterial pneumonia | CT chest, sputum culture, blood culture | Consolidation on CT chest, with negative tests for M. tuberculosis complex and fungal lung disease |
| Bronchiectasis | CT chest | On CT chest: the internal diameter of the bronchus is larger than that of its accompanying vessel, or the bronchus fails to taper in the periphery of the chest |
| Lung abscess | CT chest, sputum culture | Evidence of abscess formation on CT chest, with or without culture of bacterial pathogen on sputum culture. Negative tests for M. tuberculosis complex and fungal lung disease |
| Nocardia pneumonia | Sputum gram stain and culture, blood culture | Presence of Nocardia on gram stain or culture |
| Fungal infection | ||
| CPA | Aspergillus IgG ELISA | Radiological findings in keeping with CPA definitions, plus either culture of Aspergillus species or a positive Aspergillus IgG |
| Cryptococcosis | Cryptococcal antigen test | Culture of Cryptococcus from sputum or a positive cryptococcal antigen test |
| Histoplasmosis | Histoplasma IgG serology | Culture of Histoplasma from sputum or a positive Histoplasma serology |
| Diffuse noninfectious | ||
| HP | CT chest, exposure history | CT chest findings in keeping with HP (with or without relevant exposure history), and negative M. tuberculosis complex and fungal tests |
| Interstitial lung disease | CT chest | CT chest findings in keeping with interstitial lung disease, and negative M. tuberculosis culture and fungal tests |
| Pneumoconiosis | CT chest, exposure history | CT chest findings in keeping with pneumoconiosis, occupational mining exposure history, and negative M. tuberculosis complex and fungal tests |
| Malignancy | CT chest | CT chest findings in keeping with malignancy |
CPA = chronic pulmonary aspergillosis; CT = computed tomography; HP = hypersensitivity pneumonitis; PTB = pulmonary tuberculosis.
Case definitions.
We assigned diagnoses using standardized disease definitions as described in Table 1.19–26 Participants could accrue > 1 diagnosis.
Follow-up procedures.
Participants attended a follow-up visit 2 months after enrollment. A study clinical officer administered a standardized questionnaire covering current symptoms, health status, and additional treatment and performed a standardized physical examination. For patients unable to attend a follow-up in person, a telephone questionnaire was administered. If study staff were unable to contact a participant, their next of kin was contacted to ascertain vital status.
Data handling and statistical analysis.
Questionnaire and physical examination data were entered onto the Open Data Kit platform.27 Laboratory testing data were entered using the Cardiff OpenText TeleForm System (Waterloo, Canada). Radiology data were entered into a Microsoft Excel spreadsheet (Microsoft Corp, Seattle, WA). All data were analyzed in Stata version 16 (StataCorp, College Station, TX). Continuous variables were expressed using medians and interquartile ranges (IQR). Categorical variables were expressed as frequencies and percentages.
RESULTS
Baseline sociodemographic and clinical characteristics.
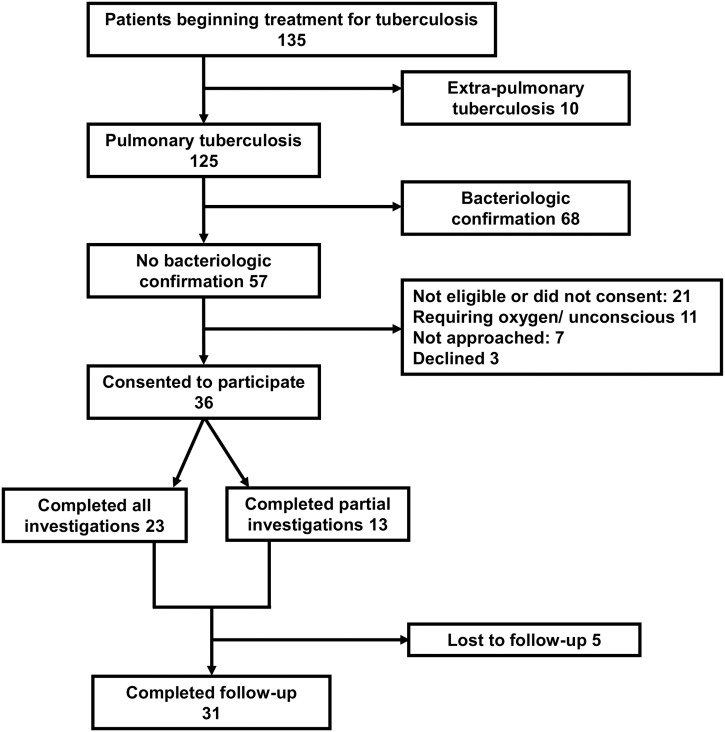
Among 135 screened patients beginning treatment of TB, 125 (93%) had been diagnosed with PTB and 57 (42%) were bacteriologically unconfirmed and therefore eligible for our study (Figure 2). Of those eligible, 36 (63%) consented to participate. The median (IQR) age was 55 (44–67) years, with 21 (58%) male (Table 2). All participants reported a cough for a median (IQR) duration of 30 (21–90) days, with 19 (53%) reporting spontaneous sputum production. Twenty (56%) reported fevers, and 17 (47%) reported weight loss. Thirty-three (92%) reported taking antibacterial medication for their current illness, with 18 (50%) taking two courses and two (6%) taking at least three courses. The most commonly reported antimicrobial drugs consumed were amoxicillin (10; 28%) and ampicillin-cloxacillin (9; 25%), with two (6%) taking ciprofloxacin, an antibacterial with some expected activity against M. tuberculosis complex. Three (8%) participants reported taking antifungal medication, and three (8%) reported taking a previous course of TB medication during the current illness.
Figure 2.
Study flow diagram indicating recruitment and follow-up of patients beginning treatment of tuberculosis, northern Tanzania, 2019.
Table 2.
Characteristics of patients who began treatment of PTB without bacterial confirmation (N = 36)
| Characteristic | n (%) |
|---|---|
| Median (IQR) age (years) | 55 (44–67) |
| Male sex | 21 (58) |
| Occupation | |
| Subsistence farmer | 21 (58) |
| Business person | 7 (19) |
| Healthcare worker | 2 (6) |
| Other | 6 (17) |
| Current or ex-miner | 8 (22) |
| Cough | 36 (100) |
| Sputum production | 19 (53) |
| Hemoptysis | 9 (25) |
| Median (IQR) duration of cough (days) | 30 (21–90) |
| Dyspnea | 26 (72) |
| Fever | 20 (56) |
| Median (IQR) fever duration (days) | 7 (5–21) |
| Weight loss | 17 (47) |
| Sweats | 8 (22) |
| Median (IQR) body mass index | 21.6 (18.2–23.8) |
| Median (IQR) SpO2 (%) | 94 (81–98) |
| Digital clubbing | 1 (2.8) |
| Palpable lymphadenopathy | 3 (8.3) |
| Median (range) mid-upper arm circumference | 25.0 (21.5–28.5) |
IQR = interquartile range; N = number of study participants with relevant data available; n = number of participants displaying the relevant characteristic, symptom, or sign; PTB = pulmonary tuberculosis; SpO2 = peripheral oxygen saturation; TB = tuberculosis.
Twenty-seven (75%) of participants reported no comorbidities. Reported comorbidities included six (17%) with HIV infection, all of whom were taking antiretroviral medication, and asthma, three (8%). Of those with HIV, two (33%) had a productive cough, and for those without HIV, 17 (57%) had a productive cough. We identified six (17%) participants who had completed at least one course of TB medication for a previous illness, and two (6%) who had completed two courses. Sixteen (44%) were current or ex-smokers, with a median (IQR) exposure of 10 (4–16) pack-years. Twenty-one (58%) participants were subsistence farmers, and eight (22%) were current or ex-miners.
Ten (28%) participants had a body mass index (BMI) < 18.5 kg/m2, and nine (25%) had a mid-upper arm circumference < 22 cm. One (3%) participant had a temperature ≥ 38.0°C. Eleven (31%) participants had a peripheral oxygen saturation (SpO2) < 92% when measured on ambient air.
Prevalence of PTB and other diseases.
We successfully induced an adequate sputum sample from 35 (97%) participants. The median (range) sputum volume was 9 (5–20) mL. We bacteriologically confirmed TB in two (6%). Of 34 participants eligible to proceed to further investigations, chest CT scans were completed and able to be interpreted in 23 (68%), with seven (20%) not completing chest CT scans; data storage or transfer problems precluded classification of scans in four (12%).
The most commonly identified diseases among the 23 participants who completed the full investigations were pneumonia (11; 48%) and bronchiectasis (8; 35%), all of whom also had evidence of pneumonic consolidation (Table 3). In addition, 10 (44%) had radiologic evidence of previous exposure to TB, including six (75%) of those with bronchiectasis. In the 11 participants diagnosed with pneumonia, we cultured Pseudomonas aeruginosa in three (27%), Klebsiella pneumoniae in three (27%), and other gram-negative organisms in three (27%). Pseudomonas aeruginosa was also cultured in two (25%) of the participants with bronchiectasis and two (40%) with pleural disease. In addition, P. aeruginosa was cultured from six people who did not meet criteria for any diagnosis, of whom five (83%) had not had a CT chest scan.
Table 3.
Diagnoses of patients who began treatment of PTB without bacterial confirmation
| Diagnosis | N | n | % |
|---|---|---|---|
| TB | 35 | 2 | 6 |
| Nontuberculous mycobacterial infection | 35 | 0 | 0 |
| Bacterial pneumonia (without bronchiectasis) | 23 | 11 | 48 |
| Bronchiectasis | 23 | 8 | 35 |
| Lung abscess | 23 | 1 | 4 |
| Nocardia pneumonia | 34 | 0 | 0 |
| Chronic pulmonary aspergillosis | 34 | 1 | 3 |
| Cryptococcosis | 34 | 0 | 0 |
| Histoplasmosis | 34 | 0 | 0 |
| Hypersensitivity pneumonitis | 23 | 2 | 9 |
| Interstitial lung disease | 23 | 2 | 9 |
| Pneumoconiosis | 23 | 1 | 4 |
| Thoracic malignancy | 23 | 1 | 4 |
| Pleural disease | 23 | 5 | 22 |
N = number of study participants with relevant data available; n = number of participants displaying the relevant characteristic, symptom, or sign; PTB = pulmonary tuberculosis; TB = tuberculosis.
Participant follow-up.
We completed follow-up at 2 months for 31 (86%) participants, with 20 (56%) completing in-person follow-up, seven (19%) completing telephone follow-up, and four (11%) confirmed as deceased, none of whom had bacteriologically confirmed TB. Among decedents, we diagnosed lung abscess in one (25%) and lung malignancy and concomitant pneumonia in one (25%). The other two (50%) did not undergo CT chest scans, and no diagnoses were reached. Of living participants who completed follow-up, 21 (78%) had persistent symptoms, and six (22%) reported resolution of symptoms. Both participants in whom we bacteriologically confirmed PTB reported resolution of symptoms. Participants with persistent symptoms included nine (81%) of 11 diagnosed with bacterial pneumonia, four (50%) of eight diagnosed with bronchiectasis, one (100%) with chronic pulmonary aspergillosis, one (100%) diagnosed with pneumoconiosis, one (50%) of two with hypersensitivity pneumonitis, and one (50%) of two diagnosed with interstitial lung disease. Persistent symptoms included cough (13; 48%), dyspnea (7; 26%), night sweats (5; 19%), and hemoptysis (1; 4%). In those with in-person follow-up who had a physical examination, 13 (65%) had a lower BMI than at enrollment, and 11 (55%) had a reduced mid-upper arm circumference.
DISCUSSION
Despite routine use of sensitive molecular techniques to diagnose PTB in our setting, nearly half of patients were presumptively initiated on treatment of PTB without bacteriologic confirmation. In all but two participants in this group, we were unable to culture M. tuberculosis complex from induced sputum samples. In those in whom we could not bacteriologically confirm PTB, poor clinical outcomes including death, deteriorating mid-upper arm circumference, and persistent symptoms were the norm. Recent estimates are that mycobacterial culture of induced sputum has a sensitivity for PTB of 0.72 (95% CI, 0.66–0.77).28 Therefore, it is likely that some participants in our study had PTB despite negative testing. However, we interpret our findings as strongly suggesting that diseases other than TB were causing many participants’ symptoms. Using standard criteria, we diagnosed most patients with diseases that are neglected in national programmatic and WHO recommendations for PTB diagnosis and management.29–31
We uncovered a broad range of diseases masquerading as PTB, but pneumonia and bronchiectasis were the most common. Bronchiectasis is a disease that reduces quality of life and causes hospitalization and death. Diagnosis has important implications for patients because it has specific treatments that improve quality of life and prognosis. The underappreciation of bronchiectasis in sub-Saharan Africa has long been recognized,13 but the relative lack of CT scanners, the primary diagnostic tool in well-resourced healthcare systems, means it continues to be neglected for diagnosis and treatment. Although bronchiectasis can be caused by a range of underlying conditions, CT findings and the high prevalence of a past history of TB treatment suggested that prior TB may be the predominant cause in our cohort. There is increasing recognition of the persistent symptoms after PTB,32 and our study suggests differentiating post-PTB bronchiectasis from active PTB is challenging with available diagnostic tools.
We also identified a substantial proportion of participants with pulmonary consolidation and persistent symptoms indicative of bacterial pneumonia despite prior antibacterial drugs. Although radiographic consolidation may take several weeks to resolve after appropriate antimicrobial treatment, the persistence of symptoms may suggest unresolved infection. In this group, we identified a high prevalence of gram-negative bacteria in the sputum, particularly Pseudomonas aeruginosa, an organism usually resistant to commonly taken antibacterial medications, and K. pneumoniae.
An important finding is that although most participants in our study did not have bacteriologically confirmed PTB, presumptive treatment of their illness as PTB was supported by present national programmatic and WHO guidelines.29–31 Notably, almost all participants experienced symptoms compatible with PTB, had abnormal chest imaging, and continued to experience symptoms despite a course of antibacterial medication. Our study, in conjunction with other recent evidence, suggests limited specificity of clinical algorithms for the diagnosis of PTB.33 The concerning clinical outcomes in our study of those in whom PTB was not confirmed may reflect the lack of treatment directed at the causative disease and highlight the impact of the limited specificity of current algorithms. Our findings suggest that for patients who test negative on Xpert MTB/RIF on adequate sputum samples the probability of PTB is low, and improved diagnostic pathways for patients who test negative on Xpert MTB/RIF are needed. Such pathways must be applicable at lower-level health centers and district hospitals and not exclusively PTB focused.
A strength of our study was a systematic approach that minimized bias. However, our diagnostic testing algorithm had limitations. Although mycobacterial culture of induced sputum is among the most sensitive diagnostic tests,34 it is imperfectly sensitive and may have underestimated the prevalence of PTB. In addition, because our protocol did not include pleural sampling, TB may been responsible for some of the pleural disease identified. Study limitations may have also altered the estimated prevalence of non-TB diagnoses. Some conditions, particularly cardiac or renal conditions that cause dyspnea and cough, cannot be adequately diagnosed with microbiological and radiographic testing. For some diseases, such as bacterial pneumonia, our definition may include patients with noninfective causes of consolidation. In addition, we did not test for every potential pathogen. Pneumocystis jiroveci is one notable example, an important TB mimic among HIV-infected individuals.15 Similarly, the use of standard media for sputum culture may underestimate the contribution of Burkholderia species and Legionella species, and the high prevalence of antibacterial use may have influenced the success of bacterial isolation. For other diseases, for example malignancies, the definitive test, histological examination of lung tissue, was not performed.
We recruited approximately two-thirds of those eligible, with critical illness the primary reason for exclusion, and the causes of illness in the critically unwell may differ. The patients enrolled in our study are broadly typical of those beginning treatment in Tanzania. Data from the Tanzanian electronic TB register show participants in our study had an approximately 10-year older median age but otherwise had very similar demographic characteristics, bacterial confirmation prevalence, and mortality to the contemporaneous national cohort.16 An analysis of this national dataset identified lack of bacterial confirmation as an independent risk factor for all-cause mortality.16 One important feature of our cohort is that the HIV prevalence was approximately half of that seen in the national TB registry. Because patients with HIV are less likely to have TB confirmed bacteriologically and are more likely to die of their disease,35 our findings should be interpreted cautiously in patients with HIV infection.
In conclusion, our study highlights the importance of diseases that mimic TB in patients beginning treatment of PTB. Our study is from a single region in Tanzania, has a small sample size, and has focused on respiratory and infectious mimics of PTB. Therefore, replication of our work in other settings, in larger cohorts and including diagnostic testing for cardiac and renal disease, is warranted.
ACKNOWLEDGMENTS
We thank those involved in the recruitment, laboratory work, data management, and study administration, including Flora F. Mboya, Lillian E. Mgowe, Frank M. Kimaro, Robert S. Chuwa, Tumsifu G. Tarimo, Rose Oisso, Cynthia A. Asiyo, and Caroline Allen. We also thank the patients and the clinical and administrative staff at Kilimanjaro Christian Medical Centre and Mawenzi Regional Referral Hospital for their support during the study.
REFERENCES
- 1. World Health Organization , 2020. Global Tuberculosis Report 2020. Geneva, Switzerland: WHO. [Google Scholar]
- 2. World Health Organization , 2020. Definitions and Reporting Framework for Tuberculosis—2013 Revision. Updated December 2014 and January 2020. Geneva, Switzerland: WHO. [Google Scholar]
- 3. World Health Organization , 2022. Global Tuberculosis Case Notifications Dataset. Geneva, Switzerland: WHO. [Google Scholar]
- 4. Abdullahi O, Moses N, Sanga D, Annie W, 2021. The effect of empirical and laboratory-confirmed tuberculosis on treatment outcomes. Sci Rep 11: 14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hargreaves N, Kadzakumanja O, Whitty C, Salaniponi F, Harries A, Squire S, 2001. ‘Smear-negative’ pulmonary tuberculosis in a DOTS programme: poor outcomes in an area of high HIV seroprevalence. Int J Tuberc Lung Dis 5: 847–854. [PubMed] [Google Scholar]
- 6. Teo AKJ, Singh SR, Prem K, Hsu LY, Yi S, 2021. Duration and determinants of delayed tuberculosis diagnosis and treatment in high-burden countries: a mixed-methods systematic review and meta-analysis. Respir Res 22: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, Keane J, Lewinsohn DA, Loeffler AM, Mazurek GH, 2017. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 64: e1–e33. [DOI] [PubMed] [Google Scholar]
- 8. Matee M, Mtei L, Lounasvaara T, Wieland-Alter W, Waddell R, Lyimo J, Bakari M, Pallangyo K, Von Reyn CF, 2008. Sputum microscopy for the diagnosis of HIV-associated pulmonary tuberculosis in Tanzania. BMC Public Health 8: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ling DI, Flores LL, Riley LW, Pai M, 2008. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression. PLoS One 3: e1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bates M, Mudenda V, Shibemba A, Kaluwaji J, Tembo J, Kabwe M, Chimoga C, Chilukutu L, Chilufya M, Kapata N, 2015. Burden of tuberculosis at post mortem in inpatients at a tertiary referral centre in sub-Saharan Africa: a prospective descriptive autopsy study. Lancet Infect Dis 15: 544–551. [DOI] [PubMed] [Google Scholar]
- 11. Jayasooriya S, Dimambro-Denson F, Beecroft C, Balen J, Awokola B, Mitchell C, Kampmann B, Campbell F, Dodd P, Mortimer K, 2023. Patients with presumed tuberculosis in sub-Saharan Africa that are not diagnosed with tuberculosis: a systematic review and meta-analysis. Thorax 78: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oladele R, Irurhe N, Foden P, Akanmu A, Gbaja-Biamila T, Nwosu A, Ekundayo H, Ogunsola F, Richardson M, Denning D, 2017. Chronic pulmonary aspergillosis as a cause of smear-negative TB and/or TB treatment failure in Nigerians. Int J Tuberc Lung Dis 21: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 13. Lawn SD, 1999. Bronchiectasis: frequent misdiagnosis in sub-Saharan Africa. Trop Doct 29: 120–121. [DOI] [PubMed] [Google Scholar]
- 14. Lofgren SM. et al. , 2012. Histoplasmosis among hospitalized febrile patients in northern Tanzania. Trans R Soc Trop Med Hyg 106: 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hargreaves N, Kadzakumanja O, Phiri S, Lee C-H, Tang X, Salaniponi F, Harries A, Squire S, 2001. Pneumocystis carinii pneumonia in patients being registered for smear-negative pulmonary tuberculosis in Malawi. Trans R Soc Trop Med Hyg 95: 402–408. [DOI] [PubMed] [Google Scholar]
- 16. Bukundi EM, Mhimbira F, Kishimba R, Kondo Z, Moshiro C, 2021. Mortality and associated factors among adult patients on tuberculosis treatment in Tanzania: a retrospective cohort study. J Clin Tuberc Other Mycobact Dis 24: 100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Association of Public Health Laboratories , 2018. Guidelines for Submission of Sputum Specimens for Tuberculosis Testing. Available at: https://stoptb.org/wg/gli/assets/documents/srt/APHL%20-%20Guidelines%20for%20Submission%20of%20Sputum%20Specimens%20for%20Tuberculosis%20Testing_April2018.pdf. Accessed March 19, 2022.
- 18. Chew MY, Ng J, Lim TG, Lim TK, 2019. Diagnosing pulmonary tuberculosis by pooling induced sputum. J Clin Tuberc Other Mycobact Dis 11: 100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown JS, 2012. Community-acquired pneumonia. Clin Med (Lond) 12: 538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McShane PJ, Naureckas ET, Tino G, Strek ME, 2013. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 188: 647–656. [DOI] [PubMed] [Google Scholar]
- 21. Klompas M, 2023. Lung Abscess in Adults. Available at: https://www.uptodate.com/contents/lung-abscess?search=lung%20abscess&source=search_result&selectedTitle=1~114&usage_type=default&display_rank=1. Accessed January 11, 2023.
- 22. Menendez R, Cordero PJ, Santos M, Gobernado M, Marco V, 1997. Pulmonary infection with Nocardia species: a report of 10 cases and review. Eur Respir J 10: 1542–1546. [DOI] [PubMed] [Google Scholar]
- 23. Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C, 2016. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 47: 45–68. [DOI] [PubMed] [Google Scholar]
- 24. Jarvis JN, Harrison TS, 2008. Pulmonary cryptococcosis. Semin Respir Crit Care Med 29: 141–150. [DOI] [PubMed] [Google Scholar]
- 25. Hage CA. et al. , 2011. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 53: 448–454. [DOI] [PubMed] [Google Scholar]
- 26. Travis WD. et al. , 2013. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hartung C, Lerer A, Anokwa Y, Tseng C, Brunette W, Borriello G, 2010. Open data kit: tools to build information services for developing regions. Proceedings of the 4th ACM/IEEE International Conference on Information and Communication Technologies and Development. December 13–16, 2010, London, United Kingdom: Association for Computing Machinery, Article 18.
- 28. Luo W, Lin Y, Li Z, Wang W, Shi Y, 2020. Comparison of sputum induction and bronchoscopy in diagnosis of sputum smear-negative pulmonary tuberculosis: a systemic review and meta-analysis. BMC Pulm Med 20: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilpin C, Korobitsyn A, Migliori G, Raviglione M, Weyer K, 2018. The World Health Organization standards for tuberculosis care and management. Eur Respir J 51: 1800098. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization , 2016. Chest Radiography in Tuberculosis Detection: Summary of Current WHO Recommendations and Guidance on Programmatic Approaches. Geneva, Switzerland: WHO. [Google Scholar]
- 31. National Tuberculosis and Leprosy Programme , 2013. Manual for the Management of Tuberculosis and Leprosy, 6th edition. Dar es Salaam, Tanzania: Ministry of Health and Social Welfare. [Google Scholar]
- 32. Nightingale R. et al. , 2022. Respiratory symptoms and lung function in patients treated for pulmonary tuberculosis in Malawi: a prospective cohort study. Thorax 77: 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swai HF, Mugusi FM, Mbwambo JK, 2011. Sputum smear negative pulmonary tuberculosis: sensitivity and specificity of diagnostic algorithm. BMC Res Notes 4: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McWilliams T, Wells A, Harrison A, Lindstrom S, Cameron R, Foskin E, 2002. Induced sputum and bronchoscopy in the diagnosis of pulmonary tuberculosis. Thorax 57: 1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kang’ombe C, Harries A, Ito K, Clark T, Nyirenda T, Aldis W, Nunn P, Semba R, Salaniponi F, 2004. Long-term outcome in patients registered with tuberculosis in Zomba, Malawi: mortality at 7 years according to initial HIV status and type of TB. Int J Tuberc Lung Dis 8: 829–836. [PubMed] [Google Scholar]