ABSTRACT.
In 2017, Sri Lanka experienced its largest dengue epidemic and reported severe and unusual presentations of dengue with high morbidity. This outbreak was associated with the reemergence of dengue virus-2 (DENV-2), with the responsible strain identified as a variant of the previously circulating DENV-2 cosmopolitan genotype. In this study, we characterized the DENV-2 cosmopolitan genotype from patients during this epidemic. Also, we identified host factors that contributed to the severity of dengue infection in patients infected with this particular virus. Ninety-one acute serum samples from patients at the National Hospital in Kandy were randomly selected. Of these, 40.2% and 48.9% were positive for dengue IgM and IgG, respectively. NS1 antigen levels were significantly higher in primary infections. The severe dengue (SD) and dengue with warning signs (DWWS) groups exhibited significantly higher viral genome and infectivity titers than the dengue without warning signs (DWoWS) group. The highest viremia level was observed in SD patients. As for host cytokine response, interferon α (IFN-α) levels were significantly higher in the DWoWS group than in the DWWS and SD groups, whereas interleukin (IL)-12p40 and tumor necrosis factor α (TNF-α) levels in SD patients were significantly higher than in the other two groups. The TNF-α, IL-4, and monocyte chemoattractant protein-1 concentrations were positively correlated with NS1 antigen levels. From whole-genome analysis, NS4 had the highest frequency of amino acid variants, followed by the E gene. Our study suggests that viremia levels and immune responses contributed to SD outcomes, and these findings may help in identifying an effective therapeutic strategy against SD infection.
INTRODUCTION
Dengue is currently regarded as one of the most common arthropod-borne viral diseases in tropical and subtropical areas.1 Its causative agent, the dengue virus (DENV), is a single-stranded, positive-sense RNA virus approximately 11 kb in genome length. Dengue virus is categorized into four serotypes (DENV-1–4) based on genetic and antigenic characteristics, and their inter-serotype nucleotide variability is about 30%.2–5 Each serotype of DENV is subdivided into distinct genotypes based on 6–8% nucleotide and 3% amino acid sequence differences.5 The DENV genome encodes three structural proteins (capsid [C], membrane [M], and envelope [E]) and seven nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5).6 The primary role of the structural proteins is to contribute to the pathogenic functions of host attachment, virulence, and replication.7 Nonstructural proteins are involved in the regulation of protein synthesis, viral replication, pathogenesis, and control of host viral responses.8 Nonstructural proteins are essential for the evolution of DENV in endemic areas. Increases in severity and transmission are associated with amino acid variations in NS proteins.9
The first DENV case in Sri Lanka was identified serologically in 1962. During the epidemics that occurred between 1965 and 1968, DENV-1 and -2 were the circulating serotypes, and DENV-4 was detected for the first time in 1978 in Sri Lanka.10 In the 1990s and early 2000s, all four serotypes of DENV were circulating. Dengue virus–2 and DENV-3 were not detected from 2009 to mid-2016. During 2009, emergence of DENV-1 occurred. In 2017, Sri Lanka encountered its largest dengue outbreak, with an extremely high morbidity level compared with previous years.11 The present study site, Kandy (Figure 1), is the second largest city and the capital of the central province in Sri Lanka. The population of this city was over 1,500,000. In the 2017 dengue outbreak, there were a total of 186,101 confirmed dengue cases and 440 dengue-related deaths. The dengue incidence rate was 6.1-fold higher than consecutive data from the previous 5 years.11 This unusual increase was associated with the reemergence of DENV-2, and the responsible strain was a variant of the previously circulating DENV-2 cosmopolitan genotype.12
Figure 1.
Map of Kandy, Sri Lanka, including National Hospital Kandy (red circle) where serum samples were collected from dengue-infected patients.
Several mechanisms are suggested to explain severe forms of DENV clinical manifestations: virulence of the DENV strain, antibody-dependent enhancement of the infection, cell-mediated immune response, and quantity and type of cytokines present during infection.13 In particular, DENV-2 contributes substantially to the dengue burden and dengue-related mortality in the tropics and subtropics.14 Dengue virus–2 comprises six genotypes, among which the cosmopolitan genotype is the most widespread worldwide.15–17 Different genotypes demonstrate variable adaptability that accompanies genetic modifications in DENV, enhancing virus dispersion and epidemic potential in different geographical regions. Among the four serotypes, DENV-1 and -2 have been most commonly associated with known outbreaks.1 Furthermore, according to empirical data, the highest pooled mortality rate was reported during DENV-2 outbreaks. Some studies have demonstrated that DENV-2 causes more severe secondary infections than the other serotypes as observed in fluid accumulation and bleeding manifestation.5,18–20 A study based on the Thai population indicated that the pleural index was higher in DENV-2–infected children with secondary infection.20 These reports highlight the importance of DENV-2 in dengue epidemiology and global health burdens.1 Interestingly, some researchers have hypothesized that the DENV-2 cosmopolitan genotype has evolved parallel to its global dispersal to achieve a higher heterogeneity than other DENV-2 genotypes.1
Even though DENV-2 had not been detected in Sri Lanka for over a decade, the findings from the 2017 outbreak indicate that DENV-2 was the causative serotype.11 The present study aimed to characterize the clinical, virological, and immunological features of patients infected with cosmopolitan genotype DENV-2 in this outbreak.
MATERIALS AND METHODS
Samples and patients.
Acute serum samples were collected from patients who were admitted to the National Hospital Kandy in Kandy, Sri Lanka, during March 2017−January 2018. This hospital had the largest number of dengue cases in the Central Province. A total of 91 acute serum samples were randomly selected from patients infected with DENV-2 cosmopolitan genotype as confirmed in a previous study.10 Following the 2009 WHO guidelines, patients were classified as dengue without warning signs (DWoWS), dengue with warning signs (DWWS), or severe dengue (SD) on the basis of clinical findings and laboratory tests. All patients visited the hospital 2 to 6 days after disease onset, and their serum samples were collected on their first visit or admission day. For cytokine analysis, 10 serum samples collected from healthy individuals who were negative for dengue NS1 antigen (Ag), anti-DENV IgM, and anti-DENV IgG were used as the control group.
Serological tests.
Detection of DENV NS1 Ag in the serum samples was done by using the Platelia Denv NS1 Ag (Bio-Rad, Hercules, CA) kit following the manufacturer’s protocol. The optical densities (ODs) of the processed samples were read by a plate reader at 450/620 nm, and the results were expressed as ratios after application of the following formula: sample ratio = OD/cutoff value. Sample ratio ≥ 1.00 was considered NS1 Ag positive. Positive samples were subjected to in-house DENV IgM capture ELISA. Optical density was read at 492 nm; a positive/negative (P/N) ratio ≥ 2 was considered positive.21 An in-house DENV IgG indirect ELISA was used to determine primary or secondary DENV infection. A sample titer ≥ 3,000 was used as the threshold value for positive DENV IgG. An IgG titer of ≥ 29,000 or < 29,000 was considered a secondary or primary infection, respectively, following the cutoff value our laboratory previously established to highly correlate with the WHO gold standard, dengue hemagglutination inhibition test.22,23
Quantification of DENV genome levels.
The RNA was extracted from 140 μL of serum by using a viral RNA mini-kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. For the quantitative real-time polymerase chain reaction (RT-PCR), 5 μL of RNA was used. Envelope gene amplification was performed by using 20 μL of the reaction mixture (5 μL of TaqMan Master Mix, 9 μL of nuclease water, 0.5 μL of 10 µM forward and reverse primers, and 0.25 μL of 10 µM of probe with DENV serotype-specific primer) with TaqMan Fast Virus 1-Step Master Mix (Life Technologies, Carlsbad, CA). Ten-fold serial dilutions of standard RNA (102–108 genome copies) were applied for the quantification of viral genome levels.24,25 The detection limit for the viral genome was 100 copies, and the viral genome levels were expressed as log10 genome copies/mL.25
Quantification of DENV infectivity titer.
A focus formation assay was performed to determine DENV viremia levels in patient sera. Serially diluted serum samples were inoculated onto Vero cells, which were then incubated at 37°C for 1 hour, followed by the overlaying of 1.25% methylcellulose 4000 in 2% fetal calf serum minimum essential medium (MEM). After incubation at 37°C for 72 hours, cells were fixed with 4% paraformaldehyde phosphate buffer and permeabilized with 1% Nonidet P-40 in phosphate-buffered saline (PBS) without magnesium and calcium (PBS−). Then, at 1-hour intervals, sequential adding of Block Ace, pooled human sera containing high-titered anti-dengue IgG, and horseradish peroxidase-conjugated goat anti-human IgG (American Qualex, San Clemente, CA) was done. Positive results were visualized by adding a substrate (diaminobenzidine-tetrahydrochloride; Wako, Tokyo, Japan). The stained foci of cells were counted under the microscope to calculate focus-forming units per milliliter (FFU/mL) for the virus titer.25
Virus isolation, RNA extraction, and DENV serotyping by RT-PCR.
Cultured Aedes albopictus mosquito clone cells (C6/36) were resuspended in Eagle’s MEM supplemented with 2% fetal bovine serum and 0.2 mM non-essential amino acid. The cells were seeded in Nunclon Delta (flat) tubes (Thermo Fisher Scientific, Walton, MA), mixed with 10 µL of patient serum, and incubated at 28°C for 7 days.25 The cell culture supernatant was collected, and viral RNA was extracted and subjected to RT-PCR. Briefly, RNA was extracted by QIAamp RNA mini kits (Qiagen GmbH, Hilden, Germany) and amplified by using Takara One-Step RT-PCR kits (Takara Bio Inc., Shiga, Japan) added with consensus and DENV serotype-specific primers.26
Analysis of nucleotide and amino acid variants by whole-genome sequencing.
Whole transcriptome libraries (Ion Total RNA-Seq Kit v2, Life Technologies) were prepared by using the RNA extracted from infected culture fluid. Sequencing was conducted with next generation sequencing (NGS), Ion Proton (Life Technologies). Low-quality reads containing < 75% of bases with a quality score of < 20 were removed by the FASTX-Toolkit from the input data file, and the sequence quality was checked by FastQC before and after quality trimming. De novo assembly was performed by using Trinity,27 and BLASTN was used to assemble the de novo contig.28 Burrows-Wheeler Aligner was used for mapping the trimmed FastQ data set,29 and the reference sequence was chosen by BLASTN; variants were detected by LoFreq30 and VarScan.31 Samtools constructed the consensus DENV sequence by using the output of VarScan. Dengue virus sequences in the International Nucleotide Sequence Database Consortium were collected with Entrez Direct and annotated by SeqKit. Bootstrap values were obtained after 1,000 replications. The substitution model was selected by jModelTest.32
Identification and quantification of cytokines.
Interleukin (IL)-1β, interferon (IFN)-γ, tumor necrosis factor α (TNF-α), IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p40, and monocyte chemoattractant protein-1 (MCP-1) levels were detected by the MILLIPLEX MAP human cytokine/chemokine magnetic panel (EMD Millipore Corporation, Burlington, MA) according to the manufacturer’s protocol and measured with the Bio-Rad 200 system. The data were analyzed with Bio-Plex Manager 6.2 Standard Software, and IFN-α and IFN-β were measured by using MyBioSource (San Diego, CA) according to the manufacturer’s instructions.
Statistical analysis.
The data were analyzed by using SPSS for Windows, version 16.0 (IBM Corp., Armonk, NY) and GraphPad Prism 9.0.0 software (GraphPad Software, La Jolla, CA). Continuous variables were presented as median (interquartile range) and mean ± SD values. A Kruskal–Wallis or one-way analysis of variance was performed among groups. Continuous variables were compared by using the Mann–Whitney U test between two groups. The correlation between laboratory parameters and cytokines was established by using Spearman’s rank correlation, and the results were given as correlation coefficients (i.e., ρ [−1 to +1]). For all analyses, P values < 0.05 were considered statistically significant.
RESULTS
General characteristics of the study population.
Virological features along with serological and cytokine profiles of 91 patients diagnosed with cosmopolitan genotype DENV-2 infection during the 2017 dengue outbreak in Sri Lanka were assessed. According to the 2009 WHO classification, 13 patients were DWoWS, 37 patients were DWWS, and 41 were SD. The median ages for DWoWS, DWWS, and SD patients were 19, 20, and 24 years, respectively. NS1 Ag was detected in all patients. Positive results for the detection of DENV IgM were found in 40.6% of the sample population. Fifty patients (54.9%) exhibited primary infections, and 41 patients (45.1%) presented with secondary infections (Table 1).
Table 1.
Characteristics of study population and laboratory parameters of patients infected with DENV-2, 2017
| Parameters | DWoWS | DWWS | SD | Total |
|---|---|---|---|---|
| Median (IQR) age, years | 19 (14–31) | 20 (16–30) | 24 (16.5–26) | – |
| Male | 6 | 19 | 15 | 40 |
| Female | 7 | 18 | 26 | 51 |
| DENV NS1 Ag(+) | 13 | 37 | 41 | 91 |
| IgM(+) | 6 | 14 | 17 | 37 |
| Primary infection | ||||
| < 5 years | 2 | 3 | 3 | 8 |
| 6–12 years | 0 | 0 | 0 | 0 |
| 13–20 years | 3 | 8 | 6 | 17 |
| > 20 years | 2 | 9 | 14 | 25 |
| Secondary infection | ||||
| < 5 years | 0 | 0 | 1 | 1 |
| 6–12 years | 0 | 0 | 1 | 1 |
| 13–20 years | 2 | 8 | 6 | 16 |
| > 20 years | 4 | 9 | 10 | 23 |
Ag = antigen; DENV = dengue virus; DWoWS = dengue without warning signs; DWWS = dengue with warning signs; IQR = interquartile range; NS = nonstructural; SD = severe dengue.
Serological examination.
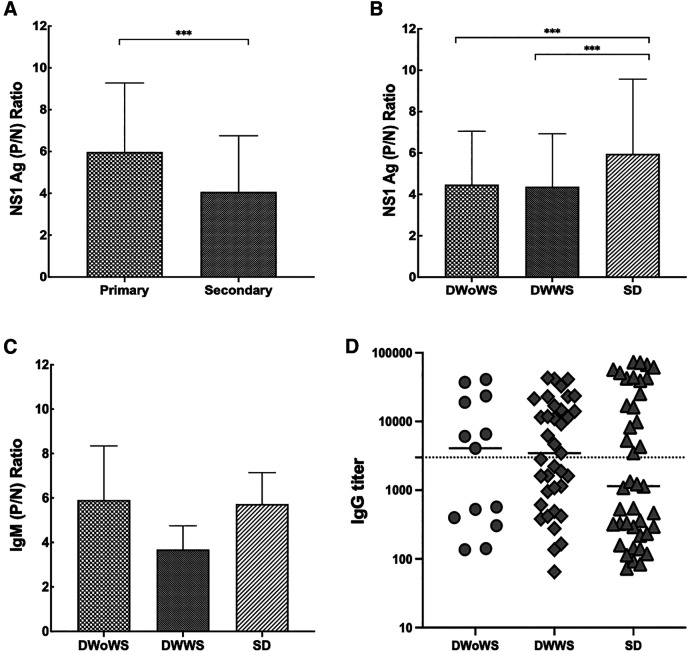
In our study, DENV IgM positivity was observed in 6 DWoWS patients, 14 DWWS patients, and 17 SD patients (Table 1). Patterns of NS1 Ag levels (P/N ratio), IgM ratio (P/N ratio), and IgG titers were also documented. Patients with primary infections had significantly higher levels of NS1 Ag than those with secondary infections (Figure 2A), and the highest level was observed in the SD group (Figure 2B). The IgM ratios and IgG titers were not significantly different among the DWoWS, DWWS, and SD groups (Figure 2C and D).
Figure 2.
Serological characteristics of patients according to types of infection and severity. (A) Mean NS1 Ag (P/N) ratios in different types of infection, (B) mean NS1 Ag (P/N) ratios in different severity groups, (C) mean IgM anti-dengue antibody (P/N) ratios in different severity groups, and (D) mean IgG anti-dengue antibody titer in different severity groups. Dotted line describes the IgG-positive value of 3,000 titers. Kruskal–Wallis one-way analysis of variance was performed among groups. Continuous variables were compared by using the Mann–Whitney U test between two groups (***P < 0.001). Error bars describe standard deviation of the mean. Ag = antigen; DWoWS = dengue without warning signs; DWWS = dengue with warning signs; P/N = positive/negative; SD = severe dengue.
Detection of infectivity titer and viral genome levels.
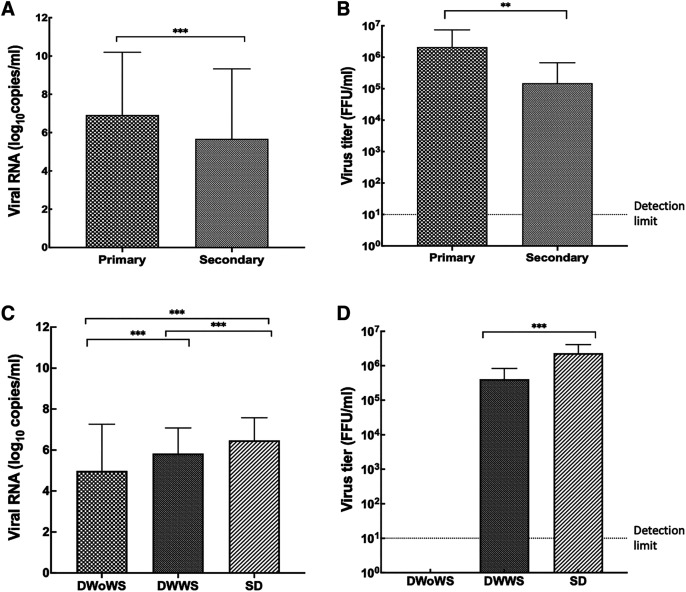
Viral RNA levels were significantly higher in primary infections than in secondary infections (Figure 3A). The highest viral genome level was measured in the SD group (Figure 3C). When the different types of infections were compared, the infectivity titer was significantly higher in primary infections (Figure 3B). Furthermore, although the infectivity titer of the DWoWS group was below the level of detection (1:9) dilution of serum, it was significantly higher in the SD group than in the DWWS group (Figure 3D).
Figure 3.
Viremia levels in different types of infection and in different severity groups. (A) Mean viral genome (copies/mL) levels in different types of infection. (B) Mean infectivity titer (FFU/mL) in different types of infection. (C) Mean viral genome (copies/mL) levels in different severity groups. (D) Mean infectivity titer (FFU/mL) in different severity groups. Kruskal–Wallis one-way analysis of variance was performed among groups. Continuous variables were compared using the Mann–Whitney U test between two groups (**P > 0.01; ***P < 0.001). Error bars describe standard deviation of the mean. DWoWS = dengue without warning signs; DWWS = dengue with warning signs; FFU = focus-forming units; SD = severe dengue.
Whole-genome analysis, genetic variation, and disease severity.
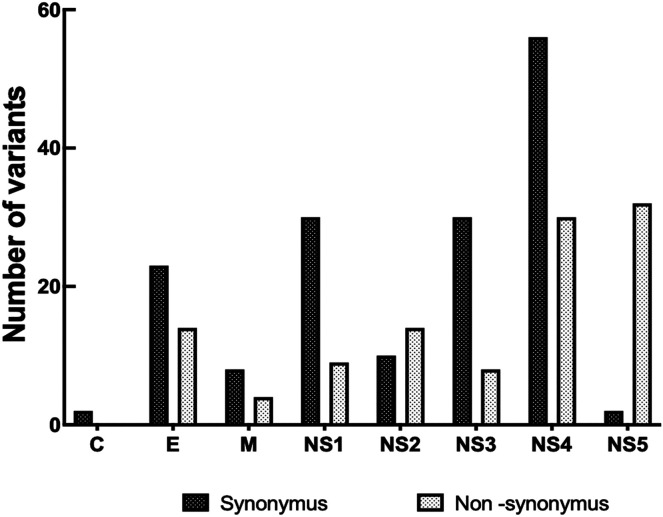
The whole-genome nucleotide variants in 50 isolates of the DENV-2 cosmopolitan genotype (34, 11, and 5 isolates from SD, DWWS, and DWoWS patients, respectively) were analyzed by NGS strategy. Nonsynonymous (NSY) and synonymous (SY) variants were identified through comparisons to the reference strain MT180479 as used in a previous study.33 Nucleotide changes were identified in 27 sites throughout the complete coding region of the 50 DENV-2 isolates selected for this study. Across the entire genome, the number of positions with variant frequencies > 1% revealed 2 SY sites in C, 23 SY and 14 NSY sites in E, 8 SY and 4 NSY sites in M, 30 SY and 9 NSY sites in NS1, 10 SY and 14 NSY sites in NS2, 30 SY and 8 NSY sites in NS3, 56 SY and 30 NSY sites in NS4, and 2 SY and 32 NSY sites in NS5 among the DENV isolates (Figure 4, Table 2, and Supplemental Table 1). Variant frequencies > 50% indicated that these variants became predominant in a particular patient. The total SY and NSY alterations in each gene were analyzed independently from the frequency. Greater variability was observed among the NS genes than with the structural genes. Higher proportions of SY and NSY changes were observed in the NS4 gene compared with the other genes assessed. NS2:I1137T, NS4:W3194R, S3254N, NS5:S3397L, T3414A, K3432E, and R3504G were the most frequently identified mutations in the DWoWS, DWWS, and SD groups. Among the NSY mutations with multiple frequencies observed only in the SD group were NS1:K891R, NS3:A1704V, V2334A, and N2387T in the NS4 gene (Table 2).
Figure 4.
Nonsynonymous and synonymous variants in the sequenced regions. Reference strain (Gen Bank accession no: MT180479) was used. C = capsid; E = envelope; M = membrane.
Table 2.
Nonsynonymous variant with multiple mutation (> 1%) alleles shared among the cosmopolitan genotype DENV-2 isolates based on dengue severity
| Sample ID | WHO classification | Protein region | Nucleotide position | AA position | Reference allele | Alter allele | Frequency | Change |
|---|---|---|---|---|---|---|---|---|
| S-10, 13 | SD | NS1 | 2768 | 891 | A | G | 9% | K-R |
| S-5, 9 | SD | NS3 | 5207 | 1704 | C | T | 97% | A-V |
| S-8, 10 | SD | NS4B | 7097 | 2334 | T | C | 49% | V-A |
| S-12, 17, 30 | SD | NS4B | 7256 | 2387 | A | C | 8% | N-T |
| S-11, 16, 20 | SD | NS4B | 9676 | 3194 | T | C | 7% | W-R |
| S-20 | SD | NS4B | 9857 | 3254 | G | A | 86% | S-N |
| S-6, 11, 32 | SD | NS5 | 10286 | 3397 | C | T | 99% | S-L |
| S-23 | SD | NS5 | 10336 | 3414 | A | G | 98% | T-A |
| S-22, 27 | SD | NS5 | 10390 | 3432 | A | G | 98% | K-E |
| S-2, 8, 13, 18, 23, 29, 31, 33 | SD | NS5 | 10606 | 3504 | C | G | 7% | R-G |
| S-7, 28 | DWWS | NS2B | 4208 | 1371 | T | C | 94% | I-T |
| S-1 | DWWS | NS4B | 9676 | 3194 | T | C | 8% | W-R |
| S-1, 3 | DWWS | NS5 | 10286 | 3397 | C | T | 100% | S-L |
| S-3 | DWWS | NS5 | 10336 | 3414 | A | G | 99% | T-A |
| S-26 | DWWS | NS5 | 10390 | 3432 | A | G | 99% | K-E |
| S-3, 15, 25 | DWWS | NS5 | 10606 | 3504 | C | G | 21% | R-G |
| S-4, 19, 25 | DWoWS | NS5 | 10606 | 3504 | C | G | 19% | R-G |
| S-21 | DWoWS | NS4B | 9857 | 3254 | G | A | 95% | S-N |
| S-40 | DWoWS | NS5 | 10286 | 3397 | C | T | 99% | S-L |
| S-40 | DWoWS | NS5 | 10336 | 3414 | A | G | 99% | T-A |
AA position = amino acid position; DWoWS = dengue without warning signs; DWWS = dengue with warning signs; NS = nonstructural; SD = severe dengue. Reference strain (GenBank accession number: MT180479) was used.
Cytokine expression regarding disease severity, infection type, and viral factors.
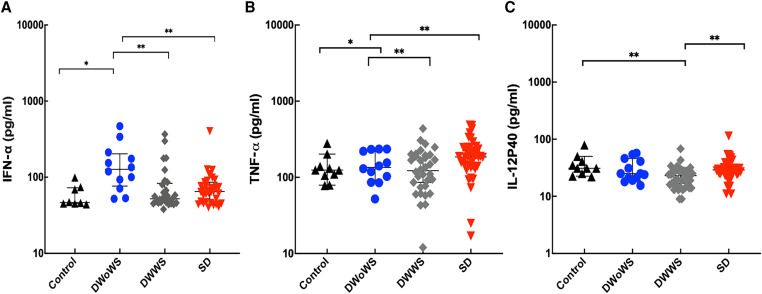
Cytokine expression patterns were analyzed in the 91 acute serum samples of confirmed dengue patients. The IFN-α was significantly higher in DWoWS than in DWWS and SD groups (Figure 5A), and TNF-α and IL-12p40 were significantly higher in SD than in DWoWS and DWWS groups (Figure 5B and C). The IFN-γ, TNF-α, IFN-β, IL-2, IL-4, IL-6, IL-8, IL-10, and MCP-1 concentrations were not significantly different among the tested groups. No significant differences were detected in cytokine concentrations between primary and secondary infections. Tumor necrosis factor α, IL-4, and MCP-1 were positively correlated with NS1 Ag levels. In addition, IL-4 and MCP-1 were positively correlated with viral genome levels (Table 3). Among the 12 cytokines tested, only IFN-γ levels were dependent on sampling time after onset of fever between day 2 and day 6 (Supplemental Figure 1).
Figure 5.
Cytokine expression patterns in healthy controls and different severity groups of dengue-infected patients. (A) IFN-α, (B) TNF-α, and (C) IL-12p40. Kruskal–Wallis one-way analysis of variance were performed among groups. Continuous variables were compared by using the Mann–Whitney U test between two groups (*P < 0.05, **P < 0.01). Error bars describe median with interquartile range. DWoWS = dengue without warning signs; DWWS = dengue with warning signs; IFN-α = interferon-α IL = interleukin; SD = severe dengue; TNF-α = tumor necrosis factor α.
Table 3.
Cytokines associated with NS1 Ag and viral genome level
| Cytokine | Correlation coefficient | P value |
|---|---|---|
| Correlation of cytokines and NS1 Ag ratio | ||
| TNF-α | 0.363 | 0.0001*** |
| IL-4 | 0.221 | 0.035* |
| MCP-1 | 0.409 | 0.0001*** |
| Correlation of cytokines and viral genome level | ||
| IL-4 | 0.299 | 0.004** |
| MCP-1 | 0.531 | < 0.001*** |
Ag = antigen; IL-4 = interleukin-4; MCP-1 = monocyte chemoattractant protein-1, NS = nonstructural; TNF-α = tumor necrosis factor α.
*P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
The 2017 dengue outbreak in Sri Lanka was the largest in its history.33 In this study, we investigated virological features and immunological responses in detail, including the cytokine expression patterns of patients who were infected with DENV-2 cosmopolitan genotype in 2017 and who were from Kandy, Sri Lanka.
Primary infections represented approximately 56% of the cases in the present study. The mean NS1 ratio among cases of primary infection was significantly higher than that found in cases of secondary infection. The lower NS1 Ag level during secondary infection can be attributed to immune complex formation with circulating anti-DENV antibodies, which prevents the detection of free NS1.34,35
The highest viremia level was measured in the SD group. This observation indicates that the virus directly contributes to severe outcomes.36 In this study, higher viremia levels were detected in primary infections, suggesting that the duration of dengue viremia was prolonged in patients experiencing primary DENV infections compared with those experiencing secondary infections.20,35,37 Different DENV strains have been linked to outbreaks of mild or severe disease, and the viral hypothesis contends that SD disease is the result of infection with a more virulent strain.36 One report described that DENV-2 was associated with severe illness and more serious clinical symptoms.38 Higher magnitudes of viral infectivity were seen in patients with more severe disease in the present study. This finding aligns with the positive correlation between peak viral load and disease severity reported in studies focusing on the early stages of infection.36
The role of genetic variation in severe disease occurrence was clarified by performing complete nucleotide sequencing to compare different isolates obtained from DWoWS, DWWS, and SD cases from the outbreak area. The analysis of nucleotide changes in SY and NSY alterations revealed that the NS4 gene had the highest number of SY variants. NS4 proteins are involved in replication complex formation. A transmembrane peptide, 2K, connects NS4A and Ns4B together; this peptide is then cleaved during polyprotein maturation. In DENV infection, stabilization of the replication complex is mediated by the interaction of NS4A with another molecule called the vimentin scaffold. NS4B modulates the viral replication by its interaction with the helicase domain of NS3 to assist its dissociation from the viral RNA.38 NS4B interacts with other viral proteins, including NS1, NS2B, NS3, and NS4A, and these interactions are critical for viral replication.39
Both SY and NSY variants were more frequently observed among the NS genes of DENV-2. The presence of these variants is one factor proposed to be involved in driving genotype/serotype turnover and enhanced transmission, leading to larger outbreaks. Various groups have defined molecular determinants of DENV virulence. One group demonstrated that NSY amino acid replacement in structural and NS proteins related to disease severity.40 Our results also demonstrated more frequent NSY alterations according to disease severity. Thus, complete genome sequencing can provide a better understanding of DENV genomic diversity and can reveal the consequences of genetic variation.41 The characteristics and contribution of mutations that we observed only in SD patients require further study.
The highest concentration of IFN-α was detected in the DWoWS group. IFN-α, a type I interferon, is involved in orchestrating innate and adaptive immune responses against viral infections. It is also responsible for the control of DENV replication and contributes to rapid development of adaptive immune responses that eliminate the virus and reduce severe effects induced by the virus.8 In this study, no significant differences were observed in measured cytokines from the serum of patients with primary and secondary infections, suggesting that the preexisting immunity to DENV in this study population did not influence cytokine expression. The pro-inflammatory cytokines, the expressions of which resulted in the failure of endothelial function and high levels of enhancing antibodies, further augmented the pathogenesis of SD.23
According to our findings, TNF-α was highest in the SD group from among the different severity groups. This observation was in line with numerous studies revealing TNF-α to be elevated in patients with SD.34,42–44 In particular, elevated levels of TNF-α in dengue hemorrhagic fever patients were documented in a previous report, suggesting that it participates in the process leading to plasma leakage.34,40 Thus, TNF-α may play an important role in two main pathological processes in SD plasma leakage and coagulopathy.45 Increased levels of IL-4 were found in SD patients. This cytokine has been indicated to play a role in vascular permeability. The MCP-1 chemokine has been associated with permeability changes in endothelial cells.46–48 Elevated levels of MCP-1 were found in patients with warning signs, suggesting that these dengue patients would develop more severe clinical outcomes.49 Furthermore, the current investigation documented increased levels of TNF-α, IL-4, and MCP-1 that were also correlated with increased levels of NS1 Ag. Hence, these three cytokines and NS1 Ag are associated with severe outcomes.
A significantly higher level of IL-12p40 was observed in the SD group compared with the DWoWS, DWWS, and control groups. This finding is in agreement with previous studies that reported the presence of high levels of IL-12 subunits (IL-12p40 and IL-12p70) in dengue patients compared with healthy subjects in correlation with severe cases of the disease.46 In our study, a significantly higher level of IFN-γ was seen in patients 2 days after infection, and this is consistent with other reports. Hence, this cytokine can be used to predict the development of SD.48,50 Increased serum levels of IL-4, IL-6, and IL-10 were observed mainly in more severe forms of dengue infection.
Accordingly, we conclude that severe manifestations during the 2017 dengue outbreak can be attributed to genetic variation in the DENV-2 cosmopolitan genotype and the higher viremia levels and differential cytokine expression of the host population. Thus, these parameters will be highly supportive of diagnostic, therapeutic, and preventive approaches concerning SD infections.
Supplemental Materials
ACKNOWLEDGMENTS
We thank all the patients who gave their consent to participate in this study and to all the members of the Department of Virology, Institute of Tropical Medicine, Nagasaki University, Japan, as well as to the medical professionals of National Hospital Kandy, Kandy, Sri Lanka for their assistance in our study.
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1. Yenamandra SP, Koo C, Chiang S, Lim HSJ, Yeo ZY, Ng LC, Hapuarachchi HC, 2021. Evolution, heterogeneity and global dispersal of cosmopolitan genotype of Dengue virus type 2. Sci Rep 11: 13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barde P, Shukla M, Joshi P, Sahare L, Ukey M, 2019. Molecular studies on dengue viruses detected in patients from central India. Indian J Med Microbiol 37: 12–18. [DOI] [PubMed] [Google Scholar]
- 3. Putri DH, Sudiro TM, Yunita R, Jaya UJ, Dewi BE, Sjatha F, Konishi E, Hotta H, Sudarmono P, 2015. Immunogenicity of a candidate DNA vaccine based on the prM/E genes of a dengue type 2 virus cosmopolitan genotype strain. Jpn J Infect Dis 68: 357–363. [DOI] [PubMed] [Google Scholar]
- 4. Chen R, Vasilakis N, 2011. Dengue–quo tu et quo vadis? Viruses 3: 1562–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang EH, Kim G, Oh H, An YJ, Kim J, Kim JH, Hwang ES, Park JH, Hong J, Koo BS, 2020. Molecular and evolutionary analysis of dengue virus serotype 2 isolates from Korean travelers in 2015. Arch Virol 165: 1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chambers TJ, Hahn CS, Galler R, Rice CM, 1990. Flavivirus genome organization, expression, and replication. Annu Rev Microbio 44: 649–688. [DOI] [PubMed] [Google Scholar]
- 7. Owens RJ, Limn C, Roy P, 2004. Role of an arbovirus nonstructural protein in cellular pathogenesis and virus release. J Virol 78: 6649–6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castillo Ramirez JA, Urcuqui-Inchima S, 2015. Dengue virus control of type I IFN responses: a history of manipulation and control. J Interferon Cytokine Res 35: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez-Roche R, Gould EA, 2013. Understanding the dengue viruses and progress towards their control. Biomed Res Int 2013: 690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ngwe Tun MM. et al. , 2020. Unusual, neurological and severe dengue manifestations during the outbreak in Sri Lanka, 2017. J Clin Virol 125: 104304. [DOI] [PubMed] [Google Scholar]
- 11. Ali S, Khan AW, Taylor-Robinson AW, Adnan M, Malik S, Gul S, 2018. The unprecedented magnitude of the 2017 dengue outbreak in Sri Lanka provides lessons for future mosquito-borne infection control and prevention. Infect Dis Health 23: 114–120. [DOI] [PubMed] [Google Scholar]
- 12. Tissera HA. et al. , 2020. Severe dengue epidemic, Sri Lanka, 2017. Emerg Infect Dis 26: 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patro ARK, Mohanty S, Prusty BK, Singh DK, Gaikwad S, Saswat T, Chattopadhyay S, Das BK, Tripathy R, Ravindran B, 2019. Cytokine signature associated with disease severity in dengue. Viruses 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo C. et al. , 2017. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front Cell Infect Microbiol 7: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Twiddy SS, Farrar JJ, Vinh Chau N, Chau NV, Wills B, Ernest AE, Gritsun GT, Lloyd G, Holmes EC, 2002. Phylogenetic relationships and differential selection pressures among genotypes of dengue-2 virus. Virology 298: 63–72. [DOI] [PubMed] [Google Scholar]
- 16. Khan MA. et al. , 2013. Emergence and diversification of dengue 2 cosmopolitan genotype in Pakistan, 2011. PLoS One 8: e56391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Añez G, Morales-Betoulle ME, Rios M, 2011. Circulation of different lineages of dengue virus type 2 in Central America, their evolutionary time-scale and selection pressure analysis. PLoS One 6: e27459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aguas R, Dorigatti I, Coudeville L, Luxemburger C, Ferguson NM, 2019. Cross-serotype interactions and disease outcome prediction of dengue infections in Vietnam. Sci Rep 9: 9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon I-K, Jarman RG, Green S, Rothman AL, Cummings DAT, 2010. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis 4: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vaughn DW. et al. , 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181: 2–9. [DOI] [PubMed] [Google Scholar]
- 21. Bundo K, Igarashi A, 1985. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J Virol Methods 11: 15–22. [DOI] [PubMed] [Google Scholar]
- 22. Inoue S. et al. , 2010. Evaluation of a dengue IgG indirect enzyme-linked immunosorbent assay and a Japanese encephalitis IgG indirect enzyme-linked immunosorbent assay for diagnosis of secondary dengue virus infection. Vector Borne Zoonotic Dis 10: 143–150. [DOI] [PubMed] [Google Scholar]
- 23. Ngwe Tun MM. et al. , 2021. Detection of genotype-1 of dengue virus serotype 3 for the first time and complete genome analysis of dengue viruses during the 2018 epidemic in Mandalay, Upper Myanmar. PLoS One 16: e0251314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito M, Takasaki T, Yamada KI, Nerome R, Tajima S, Kurane I, 2004. Development and evaluation of fluorogenic TaqMan reverse transcriptase PCR assays for detection of dengue virus types 1 to 4. J Clin Microbiol 42: 5935–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ngwe Tun MM. et al. , 2020. Clinical, virological, and cytokine profiles of children infected with dengue virus during the outbreak in Southern Vietnam in 2017. Am J Trop Med Hyg 102: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morita K, Tanaka M, Igarashi A, 1991. Rapid identification of dengue virus serotypes by using polymerase chain reaction. J Clin Microbiol 29: 2107–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grabherr MG. et al. , 2011. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat Biotechnol 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL, 2009. BLAST+: architecture and applications. BMC Bioinformatics 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Durbin R, 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilm A, Aw PPK, Bertrand D, Yeo GRT, Ong SH, Wong CH, Khor CC, Petric R, Hibberd ML, Nagarajan N, 2012. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res 40: 11189–11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK, 2012. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darriba D, Taboada GL, Doallo R, Posada D, 2012. JModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ngwe Tun MM, Muthugala R, Nabeshima T, Soe AM, Dumre SP, Rajamanthri L, Jayawardana D, Attanayake S, Inoue S, Morita K, 2020. Complete genome analysis and characterization of neurotropic dengue virus 2 cosmopolitan genotype isolated from the cerebrospinal fluid of encephalitis patients. PLoS One 15: e0234508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de la Cruz Hernández SI, Puerta-Guardo HN, Aguilar HF, Mateos SG, Martinez IL, Ortiz-Navarrete V, Ludert LE, del Angel RM, 2016. Primary dengue virus infections induce differential cytokine production in Mexican patients. Mem Inst Oswaldo Cruz 111: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duyen HTL, Ngoc TV, Ha DT, Hang VTT, Kieu NTT, Young PR, Farrar JJ, Simmons CP, Wolbers M, Wills BA, 2011. Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: differential effects according to serotype and immune status. J Infect Dis 203: 1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang WK, Chen H-L, Yang C-F, Hsieh S-C, Juan C-C, Chang S-M, Yu C-C, Lin L-H, Huant J-H, King C-C, 2006. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis 43: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 37. Yung CF, Lee KS, Thein TL, Tan LK, Gan VC, Wong JGX, Lye DC, Ng LC, Leo YS, 2015. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. Am J Trop Med Hyg 92: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Norazharuddin H, Lai NS. 2018. Roles and prospects of dengue virus non-structural proteins as antiviral targets: an easy digest. Malays J Med Sci 25: 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Q, Kang C. 2022. Structures and dynamics of dengue virus nonstructural membrane proteins. Membranes (Basel) 12: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vuong NL. et al. , 2021. Higher plasma viremia in the febrile phase is associated with adverse dengue outcomes irrespective of infecting serotype or host immune status: an analysis of 5642 Vietnamese cases. Clin Infect Dis 72: E1074–E1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hung S-J, Tsai H-P, Wang Y-F, Ko W-C, Wang J-R, Huang S-W, 2022. Assessment of the risk of severe dengue using intrahost viral population in dengue virus serotype 2 patients via machine learning. Front Cell Infect Microbiol 12: 831281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Talarico LB. et al. , 2017. Characterization of type I interferon responses in dengue and severe dengue in children in Paraguay. J Clin Virol 97: 10–17. [DOI] [PubMed] [Google Scholar]
- 43. Mangione JNA. et al. , 2014. The association of cytokines with severe dengue in children. Trop Med Health 42: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Masood KI, Jamil B, Rahim M, Islam M, Farhan M, Hasan Z, 2018. Role of TNF α, IL-6 and CXCL10 in dengue disease severity. Iran J Microbiol 10: 202–207. [PMC free article] [PubMed] [Google Scholar]
- 45. Srikiatkhachorn A, Mathew A, Rothman AL, 2017. Immune-mediated cytokine storm and its role in severe dengue. Semin Immunopathol 39: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuczera D, Assolini JP, Tomiotto-Pellissier F, Pavanelli WR, Ferreira Silveira GF, 2018. Highlights for dengue immunopathogenesis: antibody-dependent enhancement, cytokine storm, and beyond. J Interferon Cytokine Res 38: 69–80. [DOI] [PubMed] [Google Scholar]
- 47. Sierra B, Perez AB, Vogt K, Garcia G, Schmolke K, Aguirre E, Alvarez M, Volk HD, Guzman MG, 2010. MCP-1 and MIP-1α expression in a model resembling early immune response to dengue. Cytokine 52: 175–183. [DOI] [PubMed] [Google Scholar]
- 48. Bozza FA, Cruz OG, Zagne SMO, Azeredo EL, Nogueira RMR, Assis EF, Bozza PT, Kubelka CF, 2008. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis 8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rathakrishnan A, Wang SM, Hu Y, Khan AM, Ponnampalavanar S, Lum LC, Manikam R, Sekaran SD, 2012. Cytokine expression profile of dengue patients at different phases of illness. PLoS One 7: e52215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS, 2000. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol 28: 183–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.