Abstract
We have recently described GS 4071, a carbocyclic transition-state analog inhibitor of the influenza virus neuraminidase, which has potent inhibitory activity comparable to that of 4-guanidino-Neu5Ac2en (GG167; zanamivir) when tested against influenza A virus replication and neuraminidase activity in vitro. We now report that GS 4071 is active against several strains of influenza A and B viruses in vitro and that oral GS 4104, an ethyl ester prodrug which is converted to GS 4071 in vivo, is active in the mouse and ferret models of influenza virus infection. Oral administration of 10 mg of GS 4104 per kg of body weight per day caused a 100-fold reduction in lung homogenate viral titers and enhanced survival in mice infected with influenza A or B viruses. In ferrets, a 25-mg/kg dose of GS 4104 given twice daily reduced peak viral titers in nasal washings and eliminated constitutional responses to influenza virus infection including fever, increased nasal signs (sneezing, nasal discharge, mouth breathing), and decreased activity. Consistent with our demonstration that the parent compound is highly specific for influenza virus neuraminidases, no significant drug-related toxicity was observed after the administration of oral dosages of GS 4104 of up to 800 mg/kg/day for 14 days in nonclinical toxicology studies with rats. These results indicate that GS 4104 is a novel, orally active antiviral agent with the potential to be used for the prophylaxis and treatment of influenza A and B virus infections.
Influenza continues to be a serious health concern causing substantial morbidity and mortality, particularly among very young people, elderly people, and persons with chronic cardiovascular and respiratory diseases (22). Current options for the prevention and treatment of influenza virus infections have limitations. Vaccine development is only partially effective in the control of influenza epidemics due, at least in part, to the rapid change in the antigenic sites of the surface proteins of the influenza virus (7). In addition, there is concern that it will not be possible to generate and manufacture new vaccines rapidly enough to protect against future pandemic influenza virus strains, which arise due to major changes in the antigenic determinants. Thus, effective antiviral agents would provide an attractive therapeutic option, particularly in the event of the occurrence of a pandemic strain. However, amantadine and rimantadine, the only antiviral agents approved for the prophylaxis and treatment of influenza A virus infections, are not active against influenza B viruses, and their clinical utility is limited by significant adverse side effects and the rapid emergence of resistant strains in the clinical setting (11, 12). As a result, there has been a great deal of interest in identifying novel antiviral agents directed against influenza viruses.
Recent studies have demonstrated that the influenza virus neuraminidase (sialidase), which is one of the two glycoproteins expressed on the virion surface, is a valid target for antiviral intervention (23, 30, 31). This enzyme, which cleaves terminal sialic acid residues from glycoproteins, glycolipids, and oligosaccharides, is essential for influenza virus replication and infectivity. It is thought that the influenza virus neuraminidase is required for elution of newly synthesized virions from infected cells (21, 23, 24) and that it aids the movement of the virus through the mucus of the respiratory tract (2, 19). The influenza virus neuraminidase is an attractive antiviral target because the enzyme active site is highly conserved among all influenza A and B virus strains investigated (5, 6) and the enzymatic mechanism of activity has been studied at the structural level (3, 4, 36, 42), facilitating the possibility of rationally based drug design.
On the basis of X-ray crystallographic studies of the influenza virus neuraminidase cocrystallized with sialic acid and the unsaturated sialic acid analog Neu5Ac2en (1, 39, 40), several sialic acid analogs have been synthesized and tested as potential inhibitors of this enzyme. Zanamivir (GG167; 4-guanidino-Neu5Ac2en) (Fig. 1), the most potent of these sialic acid-based inhibitors, is a selective inhibitor of influenza A and B virus neuraminidases (15, 37, 40, 44). The efficacy of zanamivir has been demonstrated with animal models of influenza virus infection (30, 31) and in studies with humans (14, 16), and it is in clinical development for the treatment of influenza A and B virus infections. However, due to poor oral bioavailability and rapid renal elimination, zanamivir is applied topically to the respiratory tract via an intranasal spray or by inhalation (16, 30, 31).
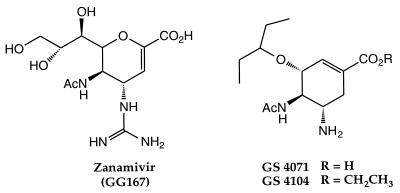
FIG. 1.
Structures of zanamivir, GS 4071, and GS 4104. Ac, acetyl.
In an attempt to identify potentially orally bioavailable influenza virus neuraminidase inhibitors, we have designed and synthesized a series of carbocyclic transition-state analogs in which lipophilic side chains replace the polar glycerol moiety of the sialic acid-based inhibitors such as zanamivir. Among these, GS 4071 (18) (Fig. 1) is as potent an inhibitor of influenza virus replication and neuraminidase activity as zanamivir. GS 4071 lacks both the polar glycerol and guanidino groups which are responsible for the high affinity of zanamivir for the influenza virus neuraminidases but, rather, relies on strong hydrophobic interactions between its 3-pentyloxy side chain and an induced hydrophobic pocket (18) located in the region of the enzyme active site occupied by the glycerol side chain of sialic acid and the sialic acid-based inhibitors such as zanamivir. Because two highly polar groups, the guanidino and glycerol functionalities, have been eliminated, we have postulated that GS 4071 may be an orally available influenza virus neuraminidase inhibitor with antiviral activity comparable to that of zanamivir. We now report our characterization of the potent and selective in vitro activity of GS 4071 against several laboratory-derived and recent clinical strains of influenza virus and present evidence that oral administration of GS 4104 (Fig. 1), an ethyl ester prodrug which is converted to GS 4071 in vivo (9, 20, 43), protects mice and ferrets against the effects of influenza virus infection.
MATERIALS AND METHODS
Materials.
GS 4071, GS 4104, and zanamivir were synthesized at Gilead Sciences by previously published procedures (18, 41). L-(Tosylamido 2-phenyl)ethyl chloromethyl ketone-treated trypsin was from Worthington Biochemicals (Freehold, N.J.). Tissue culture medium and fetal bovine serum were from Irvine Scientific (Santa Ana, Calif.), GIBCO Laboratories (Grand Island, N.Y.), or Hyclone Laboratories (Logan, Utah). Purified neuraminidases from Vibrio cholerae and Clostridium perfringens were purchased from Boehringer Mannheim (Indianapolis, Ind.). Frozen human liver was purchased from Keystone Skinbank (Exton, Pa.). Unless otherwise stated, all other reagents were from Sigma Chemical Co. (St. Louis, Mo.).
Cells and viruses.
MDCK canine kidney cells, from the American Type Culture Collection (ATCC; Rockville, Md.), and MA-104 African green monkey kidney cells, from BioWhittaker (Walkersville, Md.), were grown in Eagle’s minimum essential medium containing Earle’s salts and 10% fetal bovine serum. Laboratory strains of influenza A and influenza B viruses were from ATCC and were propagated in the allantoic cavities of fertilized pathogen-free hen eggs (SPAFAS, Norwich, Conn.). The clinical isolates influenza A/Texas/36/91 (H1N1), influenza A/Johannesburg/33/94 (H3N2), and influenza B/Harbin/07/94 were provided by the Centers for Disease Control and Prevention (Atlanta, Ga.). Influenza A/Johannesburg/33/94 was propagated in fertilized hen eggs. Influenza A/Texas/36/91 and influenza B/Harbin/07/94 were propagated in MDCK cells by using serum-free tissue culture medium containing trypsin at a final concentration of 10 U/ml and EDTA at a final concentration of 1 μg/ml. The influenza A/NWS/33 (H1N1) virus used in the mouse studies was provided by K. W. Cochran (University of Michigan, Ann Arbor) and was propagated in MDCK cells. Parainfluenza virus type 3 (strain C243) was from ATCC and was propagated in MA-104 cells in serum-free tissue culture medium. Newcastle disease virus (strain NJ-Roakin) was from ATCC and was propagated in the allantoic cavities of fertilized hen eggs.
Tissue culture assays.
Plaque reduction assays were performed as described previously (11, 18) with confluent monolayers of MDCK cells in six-well tissue culture plates. The concentration of compound required to reduce the number of plaques by 50% relative to the number of plaques in untreated wells (IC50) was determined. Inhibition of viral cytopathic effect (CPE) assays were performed as described previously (17) with confluent MDCK cells in 96-well microtiter plates. IC50s were the concentrations of compound required to inhibit the extent of CPE by 50% relative to the CPE observed in infected but untreated wells. Toxicity was assessed in duplicate wells containing cells exposed to compound but not infected with virus. All visual determinations of the extent of CPE were confirmed on the basis of measurements of neutral red dye uptake (17).
Influenza virus neuraminidase enzymatic assay.
Influenza virus neuraminidase enzyme activity was assayed by previously described modifications (18) to the method of Potier et al. (26) in 100-μl reaction volumes containing 33 mM MES [2-(N-morpholino)ethanesulfonic acid; pH 6.5] and 4 mM CaCl2 and with the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid. Briefly, equal volumes of inhibitor and virus were incubated at room temperature for 30 min prior to the addition of substrate to a final concentration of 50 μM. The reactions (100 μl) were stopped after 30 min at 37°C with the addition of 150 μl of 14 mM NaOH in 83% ethanol. Fluorescence was quantitated in a Perkin-Elmer fluorimeter (model LS50B) with an excitation wavelength of 360 nm, an emission wavelength of 448 nm, and slit widths of 2.5 nm. IC50s were taken to be the concentration of inhibitor necessary to reduce the activity by 50% relative to the activity in a reaction mixture containing virus but no inhibitor.
Experiments to determine Ki values for GS 4071 against influenza virus neuraminidase subtypes were performed in a similar fashion by using influenza virions purified on sucrose gradients and solubilized with the addition of 0.2% Nonidet P-40 (NP-40). Enzyme, in the form of solubilized virions, was preincubated with GS 4071 or no inhibitor for 1 h at room temperature prior to the addition of substrate and transfer of the reaction mixture to 37°C. Data obtained at three time points within the linear rate of the reaction were used to determine reaction velocities. A total of four inhibitor concentrations, including no inhibitor, and three substrate concentrations were used to calculate kinetic constants based on Dixon plots (8) generated with the KinetAsyst program (Think Technologies). GS 4071 was a competitive inhibitor of all influenza virus neuraminidoses tested.
Enzymatic assay for non-influenza virus neuraminidases.
Solubilized Newcastle disease virus, in allantoic fluid containing 0.1% NP-40, was used without further modification as the source of enzyme. Tissue culture medium containing parainfluenza virus, solubilized with the addition of NP-40 to a final concentration of 0.1%, was concentrated 10-fold with an Amicon stirred cell concentrator fitted with an XM-30 membrane and was used as the source of parainfluenza virus neuraminidase. Human liver neuraminidase was prepared as described previously (10). Briefly, 5 g of human liver was homogenized in 4 volumes of ice-cold isotonic buffer (10 mM Tris [pH 7.0], 250 mM sucrose), and the homogenate was centrifuged at 400 × g for 15 min to remove nuclei and cellular debris. The supernatant fraction was then centrifuged at 7,500 × g for 1 h, and the supernatant fraction of the second centrifugation was further centrifuged at 100,000 × g for 1 h. The pellet from this third centrifugation was resuspended in 1 ml of isotonic buffer and was used without further modification as the “microsomal” fraction containing neuraminidase activity. The bacterial neuraminidases were prepared according to the manufacturer’s specifications.
Newcastle disease virus neuraminidase activity was assayed under conditions identical to those used for the assay of influenza virus neuraminidase activity. Assays to determine the inhibitory activity of GS 4071 against neuraminidases from human liver, parainfluenza virus, and bacterial sources were performed as described above for the influenza virus enzyme, except that the reactions were performed at the pH optima for these enzymes in a reaction buffer containing 50 mM sodium acetate (pH 4.6), 4 mM CaCl2, and, in the case of the bacterial enzymes, bovine serum albumin at a final concentration of 100 μg/ml. The fluorogenic substrate was included in all assays at a concentration comparable to the Km value determined for each of the enzymes (500 μM for the parainfluenza virus enzyme and 100 μM for the other enzymes). Reactions were carried out at 37°C over a time period during which the reaction velocities remained constant. Reactions were stopped with the addition of 1.5 volumes of stop buffer containing 140 mM NaOH in 83% ethanol.
In vivo efficacy studies.
Studies with mice were performed as described previously (32, 33). Briefly, female BALB/c mice (weight, 13 to 15 g) were infected intranasally with a 90% lethal dose of mouse-adapted influenza A/NWS/33 (H1N1), influenza A/Victoria/3/75 (H3N2), or influenza B/Hong Kong/5/72 virus. GS 4104, prepared as an aqueous solution in saline, or saline alone was administered by oral gavage twice daily for 5 days beginning 4 h prior to infection. Mice were observed daily for 21 days after infection for the occurrence of death. In a separate study, groups of 6 treated mice and 10 untreated mice infected with influenza A/NWS/33 (H1N1) virus were killed on days 1, 2, 4, and 6 postinfection, and their lungs were removed and assayed for virus titer as described previously (33).
Adult ferrets (average weight, 1.4 kg) were inoculated intranasally with 106 50% egg infective doses of the influenza A England/939/69 (H3N2) [R] clone 7a virus, a reassortant of influenza A/Puerto Rico/8/34 and influenza A/England/939/69, as described previously (38). GS 4104 was administered twice daily for 3 days beginning 2 h after inoculation with the virus. Clinical signs of infection (including fever, activity, nasal signs [sneezing, nasal discharge, mouth breathing], and inflammatory cell counts in nasal washings), as well as virus titers in nasal washings, were determined as described previously (29, 38).
Toxicological studies.
Sprague-Dawley rats (age, 5 to 7 weeks; Simonsen Laboratories, Gilroy, Calif.) received once-daily doses of GS 4104 (40, 160, or 800 mg/kg of body weight per day) or sterile water via oral gavage (10 ml/kg of body weight) for 14 consecutive days. Animals were observed daily for clinical signs of toxicity during the dosing phase. Twenty-four hours after administration of the final dose, blood was withdrawn for analysis of clinical chemistry and hematology parameters. Animals were then killed and were further evaluated for signs of toxicity. Evaluations consisted of measurements of body and organ weight changes, gross necropsy findings, and histopathological assessment of at least 14 preselected tissues.
RESULTS
GS 4071 is a specific inhibitor of influenza virus replication and neuraminidase activity in vitro.
GS 4071 was tested for its ability to inhibit the replication of several strains of human influenza virus in tissue culture by two assays: a plaque reduction assay and a CPE assay. Zanamivir was included in all experiments as a control. Neither compound showed any signs of cellular toxicity even at 1 mM, the highest concentration tested.
As indicated in Table 1, GS 4071 is a potent inhibitor of the growth of influenza A (H1N1), influenza A (H3N2), and influenza B viruses, with IC50s comparable to those of zanamivir for both the laboratory strains and the recent clinical isolates. Interestingly, GS 4071 appears to be a particularly potent inhibitor of the growth of influenza A (H3N2) viruses, with IC50s consistently below those of zanamivir. To directly test the inhibitory activity of GS 4071, the susceptibilities of the neuraminidases from the different virus strains were tested in an in vitro enzymatic assay. As indicated in Table 1, the neuraminidases of all the viruses tested were sensitive to GS 4071, with IC50s comparable to those of zanamivir. Consistent with its observed potency against influenza A (H3N2) viruses in tissue culture, GS 4071 was a more potent inhibitor of the N2 neuraminidases than zanamivir.
TABLE 1.
GS 4071 inhibits the replication and neuraminidase activity of influenza A and B viruses in vitro
| Virus | IC50a (nM) of GS 4071
|
IC50 (nM) of zanamivir
|
||
|---|---|---|---|---|
| Culture assayb | Enzymatic assayc | Culture assay | Enzymatic assay | |
| Laboratory strains | ||||
| A/WS/33 (H1N1) | 22 | 1.0 | 19 | 0.7 |
| A/FM/1/47 (H1N1) | 24 | NDd | 20 | ND |
| A/Victoria/3/75 (H3N2) | 0.6 | 0.5 | 3.5 | 1.7 |
| A/Port Chalmers/1/73 (H3N2) | 0.8 | 0.3 | 3.1 | 1.1 |
| B/Mass/3/66 | 117 | 0.8 | 90 | 1.7 |
| B/Hong Kong/5/72 | 155 | 1.7 | 150 | 1.0 |
| Clinical isolates | ||||
| A/Texas/36/91 (H1N1) | 94 | 0.5 | 70 | 0.3 |
| A/Johannesburg/33/94 (H3N2) | 40 | 0.8 | 241 | 4.6 |
| B/Harbin/07/94 | 91 | 2.0 | 30 | 2.1 |
IC50s are taken to be the concentration of compound necessary to reduce the plaque number or the extent of cell killing by 50% in tissue culture assays or the enzymatic activity by 50%, relative to duplicate samples assayed in the absence of inhibitor.
Replication in tissue culture was determined by a plaque reduction assay for the laboratory strains and by a CPE assay for the clinical isolates. Values represent the means obtained from at least three independent experiments except in the case of A/Johannesburg/33/94 (H3N2) which was tested only twice.
Enzymatic assays were performed as described in the text with tissue culture medium harvested from cultures in which full cell death occurred. Values represent the means of two independent experiments.
ND, not determined.
GS 4071 inhibited both virus replication and neuraminidase activity for all influenza virus subtypes tested. However, compared to enzymatic inhibition, there was considerably more variability in the susceptibility to both GS 4071 and zanamivir in tissue culture assays (Table 1). A similar observation, particularly among recent clinical isolates, has previously been reported for zanamivir (15, 44). Importantly, the in vivo susceptibilities of different influenza virus isolates to zanamivir have been shown to correlate with their susceptibilities in the enzymatic assay, not the culture assay (25, 44). These results indicate that the tissue culture assay cannot necessarily be used to predict the relative susceptibilities of different isolates to a given neuraminidase inhibitor, although it can be used to compare the susceptibility of a single isolate to different neuraminidase inhibitors.
The specificity of GS 4071 as an influenza virus neuraminidase inhibitor was investigated by determining its inhibitory activity against neuraminidases from a variety of sources in an in vitro enzymatic assay. As indicated in Table 2, the Ki values for GS 4071 against the influenza virus neuraminidases ranged from 2 × 10−10 to 1.2 × 10−9 M. Consistent with observations obtained in tissue culture assays, GS 4071 was a particularly potent inhibitor of the N2 neuraminidase subtype. However, GS 4071 was at least a 106-fold less potent inhibitor when it was tested against neuraminidases from other sources, including those of parainfluenza virus type 3 and Newcastle disease virus, which share homology with the influenza virus neuraminidases in their enzyme active sites (6), with little or no activity at concentrations up to 1 mM. Importantly, 1 mM GS 4071 did not inhibit neuraminidase activity in the “microsomal” fraction of human liver.
TABLE 2.
GS 4071 is a selective inhibitor of influenza virus neuraminidases
| Neuraminidase source | Ki (μM) |
|---|---|
| Influenza virus | |
| A/PR/8/34 (H1N1) | 0.0005 |
| A/Victoria/3/75 (H3N2) | 0.0002 |
| B/Lee/40 | 0.0012 |
| Human liver | >500 |
| Viral | |
| Parainfluenza virus | >500 |
| Newcastle disease virus | >500 |
| Bacterial | |
| C. perfringens | 370 |
| V. cholerae | >500 |
Oral administration of GS 4104, a prodrug of GS 4071, protects mice and ferrets against the effects of influenza virus infection.
Although GS 4071 was initially designed with the goal of maintaining potency while increasing the likelihood of being orally bioavailable, initial experiments demonstrated that the oral bioavailability of GS 4071 in rats was low and was similar to that of zanamivir (20). However, oral administration of GS 4104 (Fig. 1), an ethyl ester prodrug of GS 4071, produced high and sustained concentrations of GS 4071 in the plasma of the four animal species tested (20), including mice and ferrets, which are commonly used to test the efficacies of anti-influenza virus compounds.
On the basis of the favorable pharmacokinetic profile observed for the prodrug, the efficacy of orally administered GS 4104 was tested in the mouse and ferret models of influenza virus infection. In the mouse influenza virus infection model, the virus inoculum initially infects the lower respiratory tract (28, 45). Viral replication then proceeds in the respiratory epithelium, causing an immune response which eventually leads to the development of pneumonia and, ultimately, death. The efficacy of orally administered GS 4104 in this model was evaluated on the basis of the survival rate, measured at 21 days postinfection, for treated, infected animals relative to that for untreated, infected (control) animals. Oral administration of GS 4104 provided protection against the lethal effects of influenza A/NWS/33 (H1N1), influenza A/Victoria/3/75 (H3N2), and influenza B/Hong Kong/5/72 virus infection in a dose-dependent fashion (Table 3). For each of the viruses, a 10-mg/kg/day oral dosage of GS 4104, administered beginning 4 h prior to infection and continuing for 5 days, provided nearly complete protection against the lethal effects of each of the viruses, whereas the majority of the control animals died, with mean times to death of between 9 and 11 days postinfection. Lower doses of GS 4104 also increased the survival rates for infected animals, although the effect was only significant for the mice infected with the influenza A (H1N1) virus or the influenza B virus. No signs of drug-related toxicity were observed in any of the animals treated with GS 4104.
TABLE 3.
Oral administration of GS 4104 protects mice against a lethal challenge with influenza virus
| Virus | Dosage (mg/kg/day)a | No. of survivors/total no. | % Survivors |
|---|---|---|---|
| A/NWS/33 (H1N1) | 10 | 8/8b | 100 |
| 1 | 7/8c | 88 | |
| 0.1 | 2/8 | 25 | |
| 0d | 2/16 | 13 | |
| A/Victoria/3/75 (H3N2) | 10 | 8/8c | 100 |
| 1 | 5/8 | 63 | |
| 0.1 | 3/8 | 38 | |
| 0d | 5/16 | 31 | |
| B/Hong Kong/5/72 | 10 | 8/10c | 80 |
| 3.2 | 9/10c | 90 | |
| 1 | 1/10 | 10 | |
| 0d | 2/18 | 11 |
GS 4104 was administered orally twice daily for 5 days beginning 4 h prior to infection with the indicated influenza virus.
P < 0.001 compared to control animals in the same experiment.
P < 0.01 compared to control animals in the same experiment.
Control.
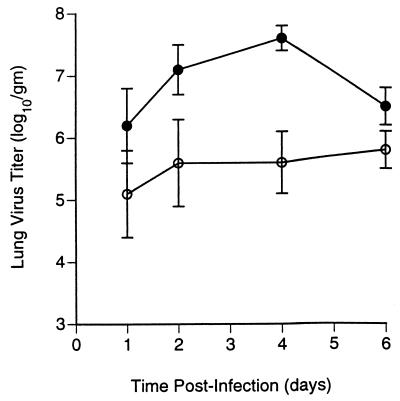
The effect of GS 4104 treatment on virus replication was also tested in mice infected with the influenza A/NWS/33 (H1N1) virus. In addition to increasing the survival rates for the infected animals, oral administration of GS 4104 caused a decrease in the virus titers detected in lung homogenates of these animals (Fig. 2). Virus titers in the lungs of untreated animals increased rapidly, reaching a maximum of 107.6 50% tissue culture infective doses (TCID50s) on day 4 after infection. Mice treated with a 10-mg/kg/day dosage of GS 4104, which was sufficient to completely protect the mice against the lethal effects of infection with this virus, had at least a 100-fold decrease in peak virus titers in their lungs and substantial reductions in virus titers in their lungs on each of the days tested. On the basis of measurements of the area under the virus titer-day curves for days 1 through 6, the 10-mg/kg/day dosage of GS 4104 reduced the virus titers in the lungs by almost 2 logs relative to those in infected, untreated animals. These results demonstrate that GS 4104, administered orally, has potent antiviral activity in vivo.
FIG. 2.
Oral administration of GS 4104 causes a reduction in the virus titers in the lungs of mice infected with influenza A/NWS/33 (H1N1) virus. Virus titers in lung homogenates of animals sacrificed at the indicated times after infection were determined as described in Materials and Methods for infected mice given oral dosages of 10 mg of GS 4104 per kg per day (○) or saline (•). Values are means ± standard deviations for at least six animals.
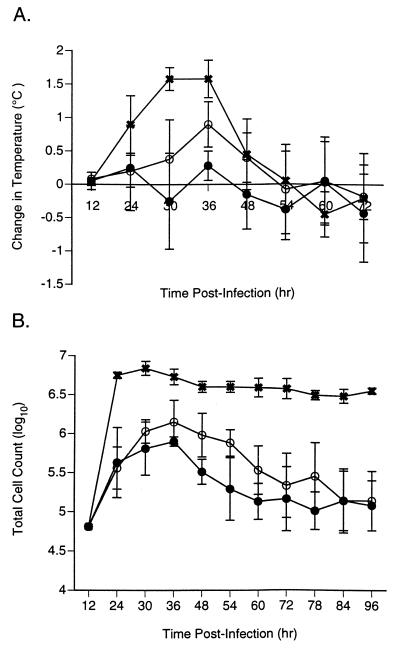
In the ferret model, infected animals develop a self-limited disease with signs similar to those observed clinically in humans: fever, increased nasal signs (sniffles, discharge, breathing by mouth), and general lethargy. The febrile response caused by the virus used in these experiments begins about 15 h after infection and lasts approximately 36 h. Twice-daily oral administration of either 5 or 25 mg of GS 4104 per kg for 3 days beginning 2 h after infection caused a substantial reduction in the febrile responses of infected animals, with the two doses causing 58 and 93% reductions, respectively, in the area-under-the-curve measurement for the increase in body temperature over the baseline values compared to those for the infected, untreated control animals (Fig. 3A). Oral administration of GS 4104 also effectively blocked the other signs of virus infection in this model; treated animals exhibited no increase in nasal signs (sneezing, nasal discharge, mouth breathing) or decrease in activity.
FIG. 3.
Oral administration of GS 4104 reduces the febrile and inflammatory responses of ferrets infected with an influenza A (H3N2) virus. (A) The change in rectal temperature relative to the baseline temperatures obtained for the same animals prior to infection was determined at the indicated times after infection. (B) The total number of inflammatory cells was determined in nasal wash samples obtained from infected animals at the indicated times after infection. Ferrets were left untreated (×) or were treated twice daily with oral doses of GS 4104 of 5 mg/kg (○) or 25 mg/kg (•) for 3 days beginning 2 h after infection with influenza virus clone 7a. Values are means ± standard deviations for four animals.
In contrast to the infection which occurs in mice, influenza virus infection in ferrets is primarily limited to the upper respiratory tract (27). Virus replicates in the nasal epithelium, with the virus titers recovered in nasal washes reaching peak values of 104 to 105 TCID50s/ml at 30 h postinfection and then dropping rapidly. Although GS 4104 treatment did not reduce the areas under the curves for the virus titers in nasal washes over the course of the infection, the 5- and 25-mg/kg oral doses of GS 4104 did reduce peak virus titers two- and eightfold, respectively, with the eightfold drop being significant (P < 0.01) (Table 4). GS 4104 treatment also caused a decrease in the inflammatory response at the site of the infection. In untreated, infected animals the number of inflammatory cells in nasal washings increased to levels approximately 100-fold above those in uninfected animals by 24 h postinfection, and these levels were sustained for at least an additional 72 h. Animals treated with GS 4104 exhibited only 1/10 of the increase in the peak number of inflammatory cells in the nasal washings and a more rapid return to preinfection levels (Fig. 3B). The effect on the inflammatory response was specific for influenza virus infection since GS 4104 had no effect on the inflammatory response in ferrets given endotoxin as a nonspecific stimulator of inflammation (17a).
TABLE 4.
GS 4104 reduces peak nasal virus titers in ferrets infected with influenza A virus
| Time (h) postinfection | Nasal wash virus titer (log10 TCID50s/ml)a
|
||
|---|---|---|---|
| Untreated | GS 4104 (25 mg/kg) | GS 4104 (5 mg/kg) | |
| 12 | 0.29 ± 0.81 | 0.21 ± 0.60 | 0.29 ± 0.83 |
| 24 | 0.31 ± 0.87 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 30b | 4.70 ± 0.30 | 3.83 ± 0.29 | 4.46 ± 0.35 |
| 36 | 4.14 ± 0.34 | 2.91 ± 0.53 | 3.70 ± 0.45 |
| 48 | 3.59 ± 0.42 | 3.62 ± 0.38 | 3.18 ± 1.31 |
| 54 | 2.35 ± 1.49 | 3.20 ± 0.48 | 1.89 ± 1.68 |
| 60 | 3.03 ± 0.84 | 2.24 ± 0.53 | 2.78 ± 0.68 |
| 72 | 3.14 ± 0.86 | 2.33 ± 1.07 | 2.53 ± 0.97 |
| 78 | 2.49 ± 1.31 | 2.49 ± 1.32 | 2.50 ± 1.18 |
Data represent the means ± standard deviations for duplicate measurements with the nasal wash samples obtained from four animals.
Peak virus titers were detected at 30 h postinfection.
GS 4104 is well tolerated in animal models.
No sign of drug-related toxicity was detected in the animals used in the efficacy studies. However, a more complete evaluation of the nonclinical toxicity of GS 4104 was performed with rats. GS 4104, even at dosages as high as 800 mg/kg/day for 14 consecutive days, which are at least 50-fold higher than the dosages needed to protect mice against the lethal effect of influenza virus infection, was not associated with any drug-related toxicity.
DISCUSSION
We have previously reported on the design and synthesis of a series of carbocyclic transition-state analog inhibitors of the influenza virus neuraminidase (18). These compounds, which, among other changes, replace the polar glycerol moeity of the sialic acid-based inhibitors with lipophilic side chains and replace the guanidino group of zanamivir with an amino group, were designed with the intent of identifying potent influenza virus neuraminidase inhibitors which have the potential of being orally bioavailable. One compound from this series, GS 4071, was found to be as potent an inhibitor of influenza A (H1N1) virus neuraminidase activity and virus replication in tissue culture as zanamivir (18). In this report we have demonstrated that the ability of GS 4071 to specifically inhibit the in vitro replication and neuraminidase activities of several laboratory strains and recent clinical isolates of human influenza A (H1N1) and influenza B viruses is similar to that of zanamivir and that GS 4071 is more potent than zanamivir when tested against influenza A (H3N2) virus. Recently, analogs of zanamivir containing alkylcarboxyamide side chains have been reported to be potent inhibitors of influenza A virus replication in vitro even without the guanidino substitution (35). However, in marked contrast to the results obtained for GS 4071, these zanamivir analogs are selective inhibitors of influenza A virus neuraminidase, with poor activity against influenza B virus neuraminidase (34, 35).
Although GS 4071 itself has low oral bioavailability, oral administration of GS 4104, an ethyl ester prodrug of GS 4071, did result in high, sustained levels of GS 4071 in the plasma of mice and ferrets, with calculated oral bioavailability values of 30% for mice and 11% for ferrets (20). For both species, the levels of GS 4071 in plasma remained above 100 nM, a concentration which substantially inhibits virus replication in tissue culture, for approximately 12 h. Even at 24 h postdosing, the levels of GS 4071 in plasma remained approximately 40 times higher than those required to inhibit influenza virus neuraminidases in vitro (Ki, ∼1 nM). A similar pharmacokinetic profile for GS 4071 following oral administration of the prodrug was also seen in rats (bioavailability = 35%) and dogs (bioavailability = 73%) (20) and in humans (43).
Importantly, high concentrations of GS 4071 (concentrations comparable to those observed in plasma) were also detected in bronchoalveolar lavage samples from rats given an oral dose of GS 4104 (9). This observation demonstrates that oral administration of the prodrug effectively delivers GS 4071 to the secretions of the lower respiratory tracts of experimental animals. Given that natural influenza virus infection in humans commonly involves the lower respiratory tract (30), oral administration of the prodrug appears to be an effective means of delivering GS 4071 to this site of infection.
Consistent with the potent in vitro activity and favorable pharmacokinetic profile of this compound, GS 4104 was active in animal models of influenza virus infection. In the mouse model a 10-mg/kg/day dosage of GS 4104 administered for 5 days beginning 4 h prior to infection provided complete protection (or near complete protection in the case of mice infected with influenza B virus) against mortality due to the infection. In separate experiments, GS 4104 was also effective in the mouse model when treatment was initiated as late as 60 h after infection (31a). The in vivo antiviral activity of GS 4071 is evident from its ability to cause a substantial reduction in the viral titers in the lungs of infected animals. GS 4104 was also active in the ferret influenza virus infection model following oral administration of GS 4104. In infected animals, a 25-mg/kg dose of GS 4104 effectively abrogated all constitutional signs of the infection, including the characteristic febrile response. The numbers of inflammatory cells, as well as peak viral titers, detected in the nasal washings of infected animals were also lower in the treated animals. More recently, early oral treatment with GS 4104 has also been associated with significant clinical efficacy and an antiviral effect in experimental influenza A virus infection in humans (13).
In summary, we have demonstrated that GS 4071 is a specific and potent inhibitor of the replication and neuraminidase activity of influenza A (H1N1), influenza A (H3N2), and influenza B viruses, with in vitro activity comparable to that of zanamivir. We have further demonstrated that oral administration of GS 4104, a prodrug of GS 4071, produced high, sustained plasma GS 4071 levels in animals (20) and that oral administration of GS 4104 resulted in a dramatic therapeutic response in the mouse and ferret models of influenza virus infection. On the basis of these results and preliminary animal toxicity data, we conclude that GS 4104 is a novel, orally active antiviral agent with the potential to be effective for the prophylaxis and treatment of influenza A and B virus infections in humans.
ACKNOWLEDGMENTS
We thank Jay Toole for critical review of the manuscript.
This research was supported in part by contracts NO1-AI-35178 and NO1-AI-65291 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Burmeister W P, Ruigrok R W, Cusack S. The 2.2 Å resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 1992;11:49–56. doi: 10.1002/j.1460-2075.1992.tb05026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnet F M. Mucins and mucoids in relation to influenza virus action. Aust J Exp Biol Med Sci. 1948;26:381–387. doi: 10.1038/icb.1948.39. [DOI] [PubMed] [Google Scholar]
- 3.Chong A K, Pegg M S, von Itzstein M. Characterisation of an ionisable group involved in binding and catalysis by sialidase from influenza virus. Biochem Int. 1991;24:165–171. [PubMed] [Google Scholar]
- 4.Chong A K J, Pegg M S, Taylor N R, von Itzstein M. Evidence for a sialosyl cation transition-state complex in the reaction of sialidase from influenza virus. Eur J Biochem. 1992;207:335–343. doi: 10.1111/j.1432-1033.1992.tb17055.x. [DOI] [PubMed] [Google Scholar]
- 5.Colman P M. Neuraminidase: enzyme and antigen. In: Krug R M, editor. The influenza viruses. New York, N.Y: Plenum; 1989. pp. 175–218. [Google Scholar]
- 6.Colman P M, Hoyne P A, Lawrence M C. Sequence and structure alignment of paramyxovirus hemagglutinin-neuraminidase with influenza virus neuraminidase. J Virol. 1993;67:2972–2980. doi: 10.1128/jvi.67.6.2972-2980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couch R B, Keitel W, Cate T R, Quarles J A, Taber L A, Glezen W P. Prevention of influenza virus infections by current inactivated influenza virus vaccines. In: Brown L E, Hampson A W, Webster R G, editors. Options for the control of influenza III. Amsterdam, The Netherlands: Elsevier; 1996. pp. 97–106. [Google Scholar]
- 8.Dixon M. The determination of enzyme inhibitor constants. Biochem J. 1953;55:170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenberg E J, Bidgood A, Cundy K C. Penetration of GS4071, a novel influenza neuraminidase inhibitor, into rat bronchoalveolar lining fluid following oral administration of the prodrug GS4104. Antimicrob Agents Chemother. 1997;41:1949–1952. doi: 10.1128/aac.41.9.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greffard A, Pairon J C, Terzidis-Trabelsh H, Heslan J-M, Bignon J, Lambre C R, Pilatte Y. Initial characterization of human thymocyte sialidase activity: evidence that this enzymatic system is not altered during the course of T-cell maturation. Int J Biochem. 1994;26:769–776. doi: 10.1016/0020-711x(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 11.Hayden F, Cote K, Douglas R G., Jr Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob Agents Chemother. 1980;17:865–870. doi: 10.1128/aac.17.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden F G, Hay H J. Emergence and transmission of influenza A viruses resistant to amantadine and rimantidine. Curr Top Microbiol Immunol. 1992;176:119–130. doi: 10.1007/978-3-642-77011-1_8. [DOI] [PubMed] [Google Scholar]
- 13.Hayden F G, Lobo M, Treanor J J, Miller M, Mills R G. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy Program Addendum. Washington, D.C: American Society for Microbiology; 1997. Efficacy and tolerability of oral GS 4104 for early treatment of experimental influenza in humans, abstr LB-26; p. 7. [Google Scholar]
- 14.Hayden F G, Osterhaus A D M E, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 15.Hayden F G, Rollins B S, Madren L K. Anti-influenza virus activity of the neuraminidase inhibitor 4-guanidino-Neu5Ac2en in cell culture and in human respiratory epithelium. Antivir Res. 1994;25:123–131. doi: 10.1016/0166-3542(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 16.Hayden F G, Treanor J J, Betts A F, Lobo M, Esinhart J D, Hussey E K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996;275:295–299. [PubMed] [Google Scholar]
- 17.Huffman J H, Sidwell R W, Barnard D L, Morrison A, Otto M J, Hill C L, Schinazi R F. Influenza virus-inhibitory effects of a series of germanium- and silicon-centred polyoxometalates. Antivir Chem Chemother. 1997;8:75–83. [Google Scholar]
- 17a.Jakemen, K. J., and C. Sweet. Unpublished observations.
- 18.Kim C U, Lew W, Williams M A, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen M S, Mendel D B, Tai C Y, Laver W G, Stevens R C. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 19.Klenk H-D, Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–280. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Escarpe P A, Eisenburg E J, Cundy K C, Sweet C, Jakeman K J, Merson J, Lew W, Williams M, Zhang L, Kim C U, Bischofberger N, Chen M S, Mendel D B. Identification of GS 4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS 4071. Antimicrob Agents Chemother. 1998;42:647–653. doi: 10.1128/aac.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Eichelberger M C, Compans R W, Air G M. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lui K J, Kendal A P. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77:712–716. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palese P, Compans R W. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol. 1976;33:159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 24.Palese P, Tobita K, Ueda M, Compans R W. Characterization of temperature sensitive influenza virus mutants. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 25.Penn C R, Barnett J M, Bethell R C, Fenton R, Gearing K L, Healy N, Jowett A J. Selection of influenza virus with reduced sensitivity in vitro to the neuraminidase inhibitor GG167 (4-guanidino-Neu5Ac2en): changes in haemagglutinin may compensate for loss of neuraminidase activity. In: Brown L E, Hampson A W, Webster R G, editors. Options for the control of influenza III. Amsterdam, The Netherlands: Elsevier; 1996. pp. 735–740. [Google Scholar]
- 26.Potier M, Mameli L, Bélisle M, Dallaire L, Melançon S B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 27.Quon C Y, Mai K, Patil G, Stampfli H F. Species differences in the stereoselective hydrolysis of esmol by blood esterases. Drug Metab Dispos. 1988;16:425–428. [PubMed] [Google Scholar]
- 28.Raut S, Hurd J, Cureton R J, Blandford G, Heath R B. The pathogenesis of infections of the mouse caused by virulent and avirulent variants of an influenza virus. J Med Microbiol. 1975;8:127–136. doi: 10.1099/00222615-8-1-127. [DOI] [PubMed] [Google Scholar]
- 29.Reuman P D, Keely S, Schiff G M. Assessment of signs of influenza illness in the ferret model. J Virol Methods. 1989;24:27–34. doi: 10.1016/0166-0934(89)90004-9. [DOI] [PubMed] [Google Scholar]
- 30.Ryan D M, Ticehurst J, Dempsey M H. GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is a potent inhibitor of influenza virus in ferrets. Antimicrob Agents Chemother. 1995;39:2583–2584. doi: 10.1128/aac.39.11.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan D M, Ticehurst J, Dempsey M H, Penn C R. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase) Antimicrob Agents Chemother. 1994;38:2270–2275. doi: 10.1128/aac.38.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Sidwell, R. W., J. H. Huffman, D. L. Barnard, K. W. Bailey, M.-H. Wong, A. Morrison, T. Syndergaard, and C. U. Kim. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral. Res., in press. [DOI] [PubMed]
- 32.Sidwell R W, Huffman J H, Call E W, Alaghamandan H, Cook P D, Robins R K. Activity of selenazofurin against influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1985;28:375–377. doi: 10.1128/aac.28.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidwell R W, Huffman J H, Call E W, et al. Effect of selenazofurin on influenza A and B virus infections of mice. Antivir Res. 1986;6:343–353. doi: 10.1016/0166-3542(86)90016-1. [DOI] [PubMed] [Google Scholar]
- 34.Smith P W, Sollis S L, Howes P D, Cherry P C, Cobley K N, Taylor H, Whittington A R, Scicinski J, Bethell R C, Taylor N, Skarzynski T, Cleasby A, Singh O, Wonacott A, Varghese J, Colman P. Novel inhibitors of influenza sialidases related to GG167 structure-activity, crystallographic and molecular dynamic studies with 4H-pyran-2-carboxylic acid 6-carboxamides. Bioorg Chem Med Chem Lett. 1996;6:2931–2936. [Google Scholar]
- 35.Sollis S L, Smith P W, Howes P D, Cherry P C, Bethell R C. Novel inhibitors of influenza sialidase related to GG167 synthesis of 4-amino and guanidino-4H-pyran-2-carboxylic acid-6-propylamides; selective inhibitors of influenza A virus sialidase. Bioorg Chem Med Chem Lett. 1996;6:1805–1808. [Google Scholar]
- 36.Taylor N R, von Itzstein M. Molecular modeling studies on ligand binding to sialidase from influenza virus and the mechanism of catalysis. J Med Chem. 1994;37:616–624. doi: 10.1021/jm00031a011. [DOI] [PubMed] [Google Scholar]
- 37.Thomas G P, Forsyth M, Penn C R, McCauley J W. Inhibition of the growth of influenza viruses in vitro by 4-guanidino-2,4-dideoxy-N-acetylneuraminic acid. Antivir Res. 1994;24:351–356. doi: 10.1016/0166-3542(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 38.Toms G L, Davies J A, Woodward C G, Sweet C, Smith H. The relation of pyrexia and nasal inflammatory response to virus levels in nasal washings of ferrets infected with influenza viruses of differing virulence. Br J Exp Pathol. 1977;58:444–458. [PMC free article] [PubMed] [Google Scholar]
- 39.Varghese J N, McKimm-Breschkin J L, Caldwell J B, Kortt A A, Colman P M. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins. 1992;14:327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- 40.von Itzstein M, Wu W-Y, Kok G B, Pegg M S, Cyason J C, Jin B, Phan T V, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 41.von Itzstein M, Wan Yang M, Jin B. The synthesis of 2,3-didehydro-2,4-dideoxy-4-guanidinyl-N-acetylneuraminic acid: a potent influenza virus sialidase inhibitor. Carbohydr Res. 1994;259:301–305. doi: 10.1016/0008-6215(94)84065-2. [DOI] [PubMed] [Google Scholar]
- 42.White C L, Janakiraman M N, Laver W G, Philippon C, Vasella A, Air G M, Luo M. A sialic acid-derived phosphonate analog inhibits different strains of influenza virus neuraminidase with different efficiencies. J Mol Biol. 1995;245:623–634. [PubMed] [Google Scholar]
- 43.Wood N D, Aitken M, Sharp S, Evison H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Tolerability and pharmacokinetics of the influenza neuraminidase inhibitor Ro 64-0802 (GS4071) following oral administration of the prodrug Ro 64-0796 (GS4104) to healthy male volunteers, Abstr. A-123; p. 25. [Google Scholar]
- 44.Woods J M, Bethell R C, Coates J A V, Healy N, Hiscox S A, Pearson B A, Ryan D M, Ticehurst J, Tilling J, Walcott S M, Penn C R. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993;37:1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyde P R, Couch R B, Mackler B F, Cate T R, Levy B M. Effects of low- and high-passage influenza virus infection in normal and nude mice. Infect Immun. 1977;15:221–229. doi: 10.1128/iai.15.1.221-229.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]