Abstract
Key Clinical Message
Schistosomus reflexus (SR), an unusual congenital defect in calves, can be diagnosed grossly by exposed viscera and curved spine with hindquarters twisted up toward the head. SR is typically associated with dystocia and fetal deaths in cows. Hence, cattle breeding programs must be aware of these anomalies to avoid losses from abnormal, non‐viable calves.
Abstract
Schistosomus reflexus (SR) is a rare and fatal congenital malformation in bovines from autosomal recessive disorders. We report a typical case of SR in a non‐viable calf after the cesarean section of a crossbred Friesian cow. It was characterized by the inversion of the spinal column and a pronounced ventral curvature causing the cranium to be positioned near the sacrum and tail, along with exposed thoracic and abdominal viscera, limb ankylosis, and tongue protrusion. The postoperative management of the cow, along with the outcome, is also described here.
Keywords: cesarean delivery, congenital defects, dead fetus, Schistosomus reflexus
A typical case of Schistosomus reflexus (SR) in a non‐viable calf has been reported after the cesarean section of a crossbred Friesian cow. Diagnosis is based on the gross evidence of exposed viscera (due to definite abdominal wall defects), ankylosed limbs, a notable curved vertebral column leading the head to be near the sacrum and tail, and protrusion of the tongue. The postoperative care and follow‐up of the cow have been described further.

1. INTRODUCTION
Schistosomus reflexus (SR) is an abnormal and exceptional type of fetal monstrosity in ruminants due to the fatal hereditary and developmental defects that cause fetal death. 1 , 2 Fetal monstrosities are the consequences of developmental anomalies of the ovum, embryo, or fetus leading to great structural distortion. 3 This often leads to dystocia in the dam. 4 , 5 , 6 SR is frequently reported in cattle, although goats, sheep, and other species may have this defect. 7 , 8 Livestock industries and cattle farms occasionally face economic losses due to the occurrences of SR that cause a reduction of viable offspring and thereby milk production loss, infertility, prolonged inter‐calving interval, and management costs for dystocia correction. 9 The actual reason for this malformation is not clearly identified yet; however, genetic factors (i.e., recessive genes either from the dam or sire, or both) affecting embryonic development, mutation, chromosomal abnormalities, exogenous elements (i.e., environmental and infectious agents), and teratogenic effects are thought to be the predisposing factors. 2 , 10 , 11
The affected fetuses usually are not viable or do not survive after birth. SR is grossly diagnosed by evidence of visceral exposure and spinal inversion. 8 The word “schistosomus” implies exposed abdominal (usually) and thoracic (occasionally) viscera, and “reflexus” signifies a marked angulation of the vertebral column that leads the tail to lie close to the head. 12 , 13 In most cases, assisted delivery is required. Per‐vaginal traction (following lubrication and caudal epidural anesthesia) is helpful for the expulsion of fetuses in small‐sized SR monsters, whereas surgical approaches, that is, fetotomy or cesarean section (CS) are usually recommended to deal with cases of fully grown SR monsters. 14 , 15 , 16
In this study, we report a field case of dystocia in a crossbred Friesian cow and characterize SR in a dead calf after the CS of the cow.
2. CASE PRESENTATION
2.1. Case history and physical examination
A crossbred Friesian cow of 7 years old and 351.47 kg body weight (BW) with a body condition score of around 3.5 was presented to a local veterinary hospital with the complaint of marked dystocia. Previously, the cow was served with artificial insemination (AI) from a local bull station. The earlier breeding history included stillbirth at first calving (parity). Prior to the presentation, the cow was assisted by non‐professional personnel, but they failed to manage the dystocia and referred the case to the hospital for professional vet service. There was no history of other treatment or medical therapies at any stage of pregnancy and dystocia of the cow. At the presentation, the animal was in the second parity with prolonged gestation and had non‐productive straining for parturition. There was no external protrusion of fetal parts through the vagina. Clinical examination and per‐vaginal palpation revealed an open cervix and anterior presentation of the fetus with forelimbs and deviated head near the pelvic canal. Moreover, there was severe obstruction from abnormal fetal orientation, and manual traction for the expulsion of the fetus through the birth canal was unsuccessful. Based on the overall condition of the cow, a CS was eventually considered with the consent of the owner to manage the case.
2.2. CS for fetal expulsion
Prior to the surgery, the animal was stabilized with intravenous (IV) 2000 mL of 0.9% NaCl (normal saline) solution. To execute CS, at first, paravertebral anesthesia along with an inverted L block in the left paralumbar fossa was performed with 35 (15 + 20) mL of 2% lidocaine hydrochloride (Jasocaine®, Jayson Pharmaceuticals Ltd.). After proper desensitization and aseptic preparations, the animal was conscientiously restrained and then approached in the right lateral recumbency, and a vertical (dorsoventral) incision (about 30 cm long) was made on the left paralumbar fossa. Next, an exploratory laparotomy was carried out, and the voluminous uterus was located. A large incision was made along the greater curvature of the uterus to extract the fetus. During fetal expulsion by manual traction, the uterus was carefully grasped to prevent the spillage of uterine contents in the abdomen. After the removal of the fetus along with its contents, the uterus was flushed with normal saline, and the uterine incision was approximated with double layers of continuous inverting sutures (i.e., lembert) using polyglactin 910 of size 1 (Vicryl™, Ethicon, J & J Medical Devices Companies). Then, routine closure of the abdomen was done, which involved layer‐by‐layer closures of the peritoneum and muscles with simple continuous sutures using chromic catgut of size 2 (Trugut™, Sutures India Pvt. Ltd.). Before abdominal closure, a combination of penicillin and streptomycin (StreptoPen®, Renata Ltd.) was used as a solution to pour on the incised abdominal wound to prevent infection. Finally, the skin was approximated with horizontal mattress sutures using nylon thread of size 1 (ETHILON*, Ethicon, J & J Medical Devices Companies). Medicated gauze was then applied externally over the sutured wound, and the cow was able to stand within 10 min of surgery (Figure 1).
FIGURE 1.

The cow immediately after CS.
2.3. Clinical diagnosis of SR
The removed fetus was a dead, malformed, monster calf of 29.5 kg with undefined sex. It was found with visceral eventration, characterized by the absence of thoracic and abdominopelvic walls along with exposed heart and lungs (hypoplastic), diaphragmatic hypoplasia, costal aplasia (no ribs and associated cartilages), and underdeveloped and exposed gastrointestinal organs with an abnormal presentation; in addition, ankylosis of all the limbs (similar to arthrogryposis), limbs positioning near the skull, shortened and inverted (ventrally convex) spine/vertebral column indicating severe dorsiflexion, dwarf and contracted tail and sacrum close to the head, and protruded tongue (Figures 2, 3, 4) were noted. Based on these gross findings, it was diagnosed as a case of SR. Postmortem was not performed, and the carcass was disposed of in accordance with the carcass disposal protocols of the hospital.
FIGURE 2.

Dead calf presented with SR: (a) ankylosed limbs, (b) exposed viscera due to lack of body cavity walls, (c) protruded tongue, (d) eye, (e) head, (f) ear, (g) liver, (h) traction rope, (i) small intestine, and (j) large intestine.
FIGURE 3.

Abnormal visceral organs and defective appendicular skeleton: (a) heart, (b) hypoplasia of right lung, (c) reticulum, (d) abomasum, (e) premature rumen (yellow circle), (f) right forelimb, (g) left forelimb, (h) right hindlimb, and (i) left hindlimb.
FIGURE 4.
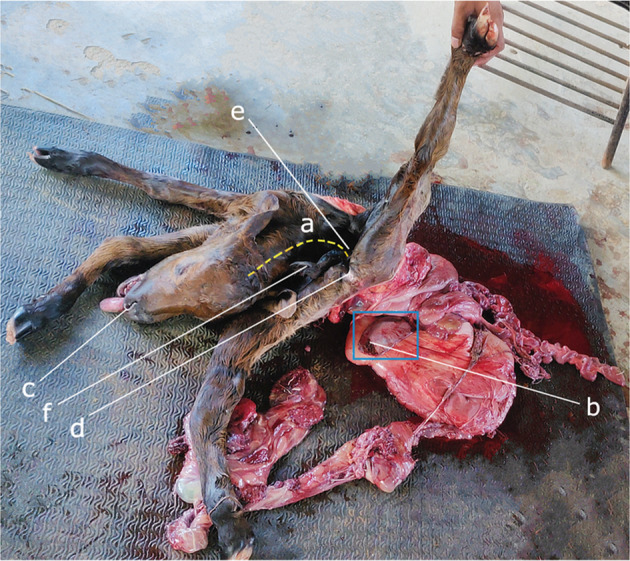
Short and inverted spine and other visible body parts: (a) ventral curvature of the vertebral column, (b) spleen (blue rectangle), (c) nostril, (d) anal orifice, (e) sacrum, and (f) short and contracted tail.
2.4. Postoperative care and follow‐up
Postoperatively, the cow was provided with IV 3000 mL of 5% dextrose in normal saline. The other supportive medications included intramuscular ceftriaxone at the rate of 15 mg/kg BW (Renacef®, Renata Ltd.) twice daily for 3 days followed by once daily for the next 7 days, amoxicillin at the rate of 10 mg/kg BW (Moxilin Vet, ACME Laboratories Ltd.) for 5 days, flunixin meglumine at the rate of 2 mg/kg BW (Lega Vet, ACME Laboratories Ltd.) for 3 days, pheniramine maleate at the rate of 1 mg/kg BW (Asta‐Vet, ACME Laboratories Ltd.) for 3 days, and oxytocin at the rate of 20 IU/cow (Oxcin‐10, Techno Drugs Ltd.) once at a time. Additionally, slow IV 250 mL of calcium preparation (Cofacalcium, Coophavet‐Dopharma, Saint‐Herblon) was supplied once daily for 2 days. After 3 days of surgery, ivermectin at the rate of 0.2 mg/kg BW (Vermic, Techno Drugs Ltd.) was administered subcutaneously to avoid the risk of myiasis. Apart from these, periodic dressing of the surgical site was done with 10% povidone‐iodine (Viodin® 10% Solution, Square Pharmaceuticals Ltd.). On the 10th postoperative day, the skin sutures were removed, and the cow was apparently healthy without any complications. However, a decrease in milk production (around 7 liters/day) was noted. After 3 months of surgery, the owner decided not to breed the cow again and thereafter did the culling.
3. DISCUSSION
Congenital anomalies are found in almost all breeds of cattle, which are often inherited and considered to be economically important. 17 Various types of fetal malformations are associated with dystocia in cattle. 18 Fetal monstrosity is relatively common in cattle and buffaloes and can further be categorized into distorted and celosomian monstrosities of which SR and perosomus elumbis are the most common examples. 19 , 20
SR is occasionally reported in Hostein‐Friesian, Jersey, Hariana, and Sahiwal breeds. 4 , 5 , 16 , 20 , 21 , 22 In this study, a true SR was documented in a non‐viable calf after dystocia correction in a crossbred Friesian cow. The cases that include both visceral exposure and spinal inversion are denominated as true SR. 2 , 8 The diagnosis of the present case is in accordance with several previous reports. 5 , 20 , 22 , 23 The defect mainly includes a complete failure in the closure of the ventral body wall along with a malformed vertebral column and skeletal system. Although congenital schistocoelia is documented in several species, the reflexus feature is found in a limited number of species. This study includes detailed and gross clinical features of SR in a dead calf, which is rarely reported. Although a reliable pedigree analysis is not available here, a short reproduction history of the dam is included. To the best of our knowledge, pedigree analysis in cattle regarding SR is very limited and has yet to be investigated.
The probable cause of this defect might be genetic aberrations that can affect the post‐gastrulation embryo involving the intermediate mesoderm. An earlier study showed that mutations of the Murine genes were responsible for severe anomalies in ventral body walls and visceral organs. 2 SR was detected not only in crossbreeding but also in inbreeding programs, where common ancestors were repeatedly associated with the occurrence of SR pedigrees; it was stated that the ancestors shared by the affected calves were different from those shared by the unaffected calves. 21 It is believed that the genetic defects involved in SR are due to recessive traits that can be evaluated by molecular analysis, that is, SNP‐based genetic mapping. 24
Usually, the sires with a previous history of SR in the progeny are not recommended to be used in the breeding of cows. 20 , 25 In this study, the cow was reported with two consecutive unsuccessful breeding as both times the calves had been abnormal and non‐viable. Thus, dams other than sires might also be responsible for the occurrence of SR in the offspring. In addition, during AI, certain recessive factors carried by semen can cause SR in the offspring.
Dystocia from SR is often reported in cattle due to abnormal fetal conformation/posture and fetopelvic disproportion that lead to birth canal obstruction at parturition. Historically, SR was found to be associated with about 1.3% of bovine dystocia cases. 6 The inheritance of certain autosomal recessive genes with incomplete penetrance in the generation results in embryonic defects. 2 , 13 The ancestral latent or recessive genes responsible for SR occasionally get prominent or dominant in the offspring and cause severe structural malformation and fatality, although the dams or sires (i.e., the ancestors) carrying those recessive genes are physically normal. An earlier investigation suggests that the SR fetuses may have two presentations; in one case, the exposed viscera protrude through the vulva, and the ankylosed limbs and head lie in the vagina, whereas, in another presentation, the ankylosed limbs are often enclosed in an inverted pouch of skin attached to a misshapen trunk that can be palpated through the vagina. 20 However, the aforementioned case was found with a different presentation during per‐vaginal palpation, which involved the fetal forelimbs, lying near the pelvic canal in an anterior presentation, along with a deviation of the fetal head.
This case was clinically managed with CS, which is consistent with the findings of others. 8 , 11 , 16 , 18 Previous literature showed that, among SR dystocia cases, about 57% of cases were treated by fetotomy, 26% by CS, and 3% by manual traction, and normal delivery was absent. 26 In fact, CS is recommended in those cases where per‐vaginal traction and fetotomy or embryotomy are not possible, similar to this case. Prompt surgical management is crucial in severe cases; otherwise, any delay can cause fetal emphysema followed by decomposition; and thereby, general toxemia can lead the animal to death. The anesthetic protocols applied in this case are correlated to those in other research. 8 , 11 , 16 Paravertebral anesthesia and inverted L block combinedly showed better desensitization over the surgical period without any anesthetic toxicity. During surgery, large laparotomic and uterine incisions were made for easy expulsion of the fetus without rupturing or tearing internal muscles and structures. The used suture materials and suture patterns are in arrangement with those in different studies. 27 , 28 Preoperative fluid therapy was considered to check dehydration and hypovolemia. Following surgery, parenteral dextrose saline was used to support the animal with energy and prevent hypoglycemia.
Postoperatively, the cow did not show any complications, which might be attributed to the effective surgical approaches and routine postoperative medications and management. The antibiotics (ceftriaxone, amoxicillin) were provided to prevent secondary infections, anti‐inflammatory (flunixin) and antihistamine (pheniramine) to check pain and tissue reactions, calcium for promoting muscle tonicity and strength, oxytocin for strengthening uterine muscle contractility and thereby uterine involution. Besides these, regular dressing with antiseptic solutions was done to prevent surgical site contamination.
The cow gradually recovered after the surgical intervention, but the daily milk yield was lower than normal, which might be due to the absence of physiological and hormonal stimuli for milk production and milk let‐down initiated usually by a calf. Apart from these, the surgery itself is a definite stress that temporarily reduces the milk yield. The cow further did not regain the full milk production capacity and was not used for breeding anymore and was subsequently culled by the owner after 90 days of surgery. Culling was done considering the economic point of view that involved the costs of rearing, feeding, and management versus poor production and reproduction performances.
4. CONCLUSIONS
Crossbred bovine calves are occasionally found with SR due to genetic abnormalities that are most probably because of autosomal recessive genes. Dystocia of the dam is commonly seen due to fetal monstrosity with abnormal skeleton, spine, and voluminous exposed viscera. The loss of offspring and overall management cause huge economic losses. Therefore, healthy cows, bulls, and bull‐semen (used in AI) with a good pedigree (i.e., genetic history) should be considered for breeding purposes to avoid this hereditary defect. This study describes the diagnosis and management of bovine SR, but further research should be prioritized in the pedigree and genetic analysis to detect the exact factors responsible for this defect in the progeny.
AUTHOR CONTRIBUTIONS
Mohammad Raguib Munif: Conceptualization; data curation; investigation; writing – original draft. Mohammad Musharraf Uddin Bhuiyan: Conceptualization; supervision; writing – review and editing. Mst. Sanjida Safawat: Data curation; investigation; writing – original draft. Md. Sabuj Rahman: Methodology; visualization.
FUNDING INFORMATION
The authors received no financial support for the work, authorship, and/or publication of this paper.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflict of interest.
ETHICS STATEMENT
This work has been approved by the authority of the VTH of BAU.
CONSENT
Written informed consent was obtained from the animal owner to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
The authors greatly appreciate the support provided by the staff of the Veterinary Teaching Hospital (VTH) of Bangladesh Agricultural University (BAU) for the management of the case. A part of this paper with a few pictures has been published as a preprint (https://doi.org/10.22541/au.166609519.93313525/v1).
Munif MR, Bhuiyan MMU, Safawat MS, Rahman MS. Schistosomus reflexus dystocia in a crossbred dairy cow. Clin Case Rep. 2023;11:e8009. doi: 10.1002/ccr3.8009
DATA AVAILABILITY STATEMENT
All the data are included in the case report.
REFERENCES
- 1. Pandey AK, Kumar S, Gunwant P, Verma A, Phogat JB. Schistosomus reflexus monster fetus in bovine and its successful management. Res J Vet Pract. 2017;5(2):25‐27. doi: 10.17582/journal.rjvp/2017/5.2.25.27 [DOI] [Google Scholar]
- 2. Laughton KW, Fisher KRS, Halina WG, Partlow GD. Schistosomus reflexus syndrome: a heritable defect in ruminants. Anat Histol Embryol. 2005;34:312‐318. doi: 10.1111/j.1439-0264.2005.00624.x [DOI] [PubMed] [Google Scholar]
- 3. Vegad JL. Textbook of Veterinary General Pathology. 2nd ed. International Book Distribution Company; 2007:539‐545. https://swu.phinma.edu.ph/wp‐content/uploads/2021/05/A‐Textbook‐of‐Veterinary‐General‐Pathology‐2nd‐Edition‐by‐J.‐L.‐Vegad.pdf [Google Scholar]
- 4. Patel A, Yadav SS, Yadav D, Sonker V, Saxena A. Dystocia due to schistosoma reflexus in a Hariana cow. Int J Livest Res. 2015;5(4):122‐124. doi: 10.5455/ijlr.20150413085920 [DOI] [Google Scholar]
- 5. Varudharajan VM, Selvaraju S, Prakash K, Ravikumar D, Krishnan G, Kumar SK. Dystocia due to schistosomus reflexus Holstein Friesian fetal monster in a Gir heifer. Int J Curr Microbiol App Sci. 2019;8(7):1190‐1192. doi: 10.20546/ijcmas.2019.807.141 [DOI] [Google Scholar]
- 6. Knight RP. The occurrence of schistosomus reflexus in bovine dystocia. Aust Vet J. 1996;73(3):105‐107. doi: 10.1111/j.1751-0813.1996.tb09988.x [DOI] [PubMed] [Google Scholar]
- 7. Roberts SJ. Veterinary Obstetrics and Genital Diseases (Theriogenology). 2nd ed. CBC New Publishers and Distributors; 2004:300‐335 https://babel.hathitrust.org/cgi/pt?id=coo.31924002840605&seq=1 [Google Scholar]
- 8. Tsuma VT, Abuom TO . A case report of schistosomus reflexus in a lamb. Kenya Veterinarian. 2008;32(1):41‐43. doi: 10.4314/kenvet.v32i1.45298 [DOI] [Google Scholar]
- 9. Yadav HP, Shah N, Brijesh Kumar B, Saxena A. Dystocia due to schistosoma reflexus and its management through fetotomy: a case report. Indian J Vet Sci Biotechnol. 2017;13(1):91‐93. doi: 10.21887/ijvsbt.v13i01.8745 [DOI] [Google Scholar]
- 10. Ozsoy SY, Oto C, Haziroglu R. Schistosoma reflexus in a dog. Ankara Univ Vet Fak Derg. 2009;56:225‐226. [Google Scholar]
- 11. Azawi O, Ahmed O, Abass S. Schistosomus reflexus foetus in cross breed Iraqi cow: a case report. Iraqi J Vet Sci. 2012;26(2):103‐104. doi: 10.33899/IJVS.2012.67481 [DOI] [Google Scholar]
- 12. Roberts SJ. Veterinary Obstetrics and Genital Diseases. 3rd ed. Woodstock; 1986. doi: 10.1016/0093-691x(86)90160-3 [DOI] [Google Scholar]
- 13. Noakes DE, Parkinson TJ, England GCW, Arthur GH. Arthur's Veterinary Reproduction and Obstetrics. 8th ed. Elsevier; 2002:129‐212. https://www.vet‐ebooks.com/arthurs‐veterinary‐reproduction‐obstetrics‐8th‐edition‐pdf [Google Scholar]
- 14. Kalita D, Bhuyan D, Mukit A, Islam S. Dystocia due to schistosomus reflexus in a goat. Indian J Anim Reprod. 2004;25(1):76‐77. [Google Scholar]
- 15. Ahuja AK, Singh H, Singh AK. Fetotomy of schistosoma reflexus and brachygnathist buffalo calf: a case report. Res J Chem Environ Sci. 2017;5(4):142‐144. [Google Scholar]
- 16. Sheetal SK, Patil AD, Sahatpure SK, Gahlod BM, Akhre SB. Management of dystocia due to schistosoma reflexus in a cross‐bred cow. Vet Sci Res. 2018;3(3):000161. https://medwinpublishers.com/OAJVSR/OAJVSR16000161.pdf [Google Scholar]
- 17. Whitlock BK. Heritable birth defects of cattle. Applied Reproductive Strategies Conference Proceedings Nashville, TN. 2010. 146–153 pp. https://www.yumpu.com/en/document/view/53428636/heritable‐birth‐defects‐in‐cattle‐applied‐reproductive‐#google_vignette
- 18. Youngquist RS, Threlfall WR. Current Therapy in Large Animal Thereogenology. 2nd ed. Saunders‐Philadelphia; 2007:313‐468. https://evolve.elsevier.com/cs/product/9780721693231?role=student [Google Scholar]
- 19. Srivastava KK, Sharma AK, Ahlawat SPS, Maithy SK. Schistosomus reflexus perosomus elumbis in Holstein Friesian cow. Indian J Anim Reprod. 1998;19(1):75. [Google Scholar]
- 20. Sitali MC, Mwaanga ES, Zulu VC, Mwanza AM. Schistosomus reflexus from a Holstein–Friesian cow – case report. Theriogenology Insight. 2014;4(2):65‐70. doi: 10.5958/2277-3371.2014.00735.9 [DOI] [Google Scholar]
- 21. Citek J. Pedigree analysis of Czech Holstein calves with schistosoma reflexum. Acta Vet Scand. 2012;54(1):22. doi: 10.1186/1751-0147-54-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarath T, Pugazharasi C, Arunmozhi N, Sureshkumar R, Jayaganthan P. Dystocia due to schistosomus reflexus fetal monster in a Jersey crossbred cow. Ind J Vet Anim Sci Res. 2021;50(6):91‐93. [Google Scholar]
- 23. Kumar A, Singh G, Arjun V, Hariom H, Jain V, Chandolia R. Dystocia due to schistosomus reflexus monster in a Sahiwal cow: a case report. Int J Livest Res. 2020;10(1):84‐87. [Google Scholar]
- 24. Charlier C, Coppieters W, Rollin F, et al. Highly effective SNP‐based association mapping and management of recessive defects in livestock. Nat Genet. 2008;40:449‐454. doi: 10.1038/ng.96 [DOI] [PubMed] [Google Scholar]
- 25. Noakes DE. Veterinary Reproduction and Obstetrics. Vol 2001. 8th ed. WB Saunders; 2001:212‐344. https://pdfkeys.com/download/2537297‐Veterinary%20Reproduction%20And%20Obstetrics.pdf [Google Scholar]
- 26. Newman KD. Bovine C‐section in the field. Vet Clin Food Anim. 2008;2008(24):273‐293. doi: 10.1016/j.cvfa.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 27. Fesseha H, Negash G, Gebrekidan B. Caesarean operation in cow due to prolonged pregnancy. Vet Med Open J. 2020;5(1):9‐13. doi: 10.17140/VMOJ-5-141 [DOI] [Google Scholar]
- 28. Adugna SA, Kitessa JD, Feyissa CT, Adem SA. Review on a cesarean section in the cow: its incision approaches, relative advantage, and disadvantages. Vet Med Sci. 2022;8(4):1626‐1631. doi: 10.1002/vms3.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are included in the case report.


