Abstract
GS 4071 is a potent carbocyclic transition-state analog inhibitor of influenza virus neuraminidase with activity against both influenza A and B viruses in vitro. GS 4116, the guanidino analog of GS 4071, is a 10-fold more potent inhibitor of influenza virus replication in tissue culture than GS 4071. In this study we determined the oral bioavailabilities of GS 4071, GS 4116, and their respective ethyl ester prodrugs in rats. Both parent compounds and the prodrug of the guanidino analog exhibited poor oral bioavailability (2 to 4%) and low peak concentrations in plasma (Cmaxs; Cmax <0.06 μg/ml). In contrast, GS 4104, the ethyl ester prodrug of GS 4071, exhibited good oral bioavailability (35%) as GS 4071 and high Cmaxs of GS 4071 (Cmax = 0.47 μg/ml) which are 150 times the concentration necessary to inhibit influenza virus neuraminidase activity by 90%. The bioavailability of GS 4104 as GS 4071 was also determined in mice (30%), ferrets (11%), and dogs (73%). The plasma of all four species exhibited high, sustained concentrations of GS 4071 such that at 12 h postdosing the concentrations of GS 4071 in plasma exceeded those necessary to inhibit influenza virus neuraminidase activity by 90%. These results demonstrate that GS 4104 is an orally bioavailable prodrug of GS 4071 in animals and that it has the potential to be an oral agent for the prevention and treatment of influenza A and B virus infections in humans.
Influenza virus infections, which have plagued humans throughout history (14), continue to be a serious health concern in terms of both morbidity and mortality (1). Current options for the control of influenza virus infections have limitations. Vaccines provide only partial protection due to their underutilization and the variability in the antigenic determinants of the surface glycoproteins (5, 29). Amantadine and rimantadine, the only antiviral agents approved for use for the treatment of influenza A virus infections, are not active against influenza B viruses, and virulent strains resistant to these agents develop quickly in the clinical setting (2, 7). Thus, there remains a need to identify new antiviral agents that can be used to prevent and treat influenza virus infections.
Recently, there has been a great deal of interest in the influenza virus neuraminidase (sialidase) as a potential antiviral target. This enzyme, which is expressed on the surfaces of influenza A and B viruses, hydrolyzes terminal sialic acid residues from glycoproteins, glycolipids, and oligosaccharides. The influenza virus neuraminidase is thought to be required for the elution of newly synthesized virions from infected cells and thus is essential for virus replication (17, 21, 22). In addition, the neuraminidase may facilitate movement of the virus through the mucus of the respiratory tract (4, 13).
Several sialic acid-based neuraminidase inhibitors have been shown to inhibit influenza A and B virus replication in vitro (22, 32, 35). Zanamivir (GG167) (Fig. 1), the most potent of these sialic acid-based inhibitors, has demonstrated efficacy in animal models of influenza virus infection (26, 27, 32, 35) and in studies with humans (9, 10), and it is under clinical development for the treatment of influenza A and B virus infections. However, due to its poor oral bioavailability, zanamivir is applied topically to the respiratory tract as an intranasal spray or inhalant (9, 10, 26, 27).
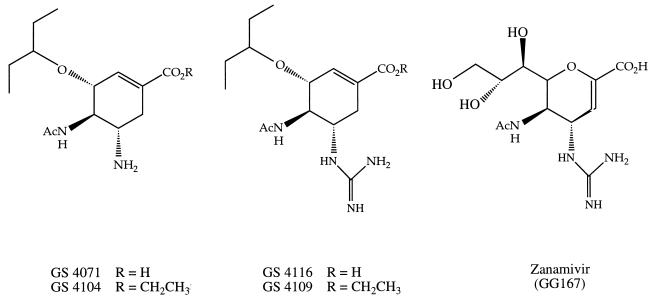
FIG. 1.
Structures of GS 4071, GS 4104, GS 4116, GS 4109, and zanamivir (GG167). Ac, acetyl.
In an attempt to identify potentially orally bioavailable influenza virus neuraminidase inhibitors, we have designed and synthesized a series of carbocyclic transition-state analog inhibitors of the influenza virus neuraminidases in which lipophilic side chains replace the polar glycerol moiety of the sialic acid-based inhibitors (12). GS 4071 (Fig. 1), the lead candidate from this series, is comparable to zanamivir in terms of its ability to inhibit influenza virus neuraminidase activity (Ki, ∼1 nM) and virus replication when tested in vitro (12, 20). However, because GS 4071 lacks the polar guanidino and glycerol groups present in zanamivir, we postulated that GS 4071 or a prodrug of GS 4071 might be orally bioavailable.
In this study we have investigated the oral bioavailability of GS 4071 and, for comparison, its more potent guanidino analog, GS 4116 (12, 19) (Fig. 1). On the basis of the observation that esterification of carboxylic groups has increased the oral bioavailabilities of many compounds (30), we have also determined the bioavailabilities of GS 4071 and GS 4116 following oral administration of their ethyl ester prodrugs.
MATERIALS AND METHODS
Compounds and reagents.
The bioavailabilities of the following five compounds (Fig. 1) were determined in this study: GS 4071; GS 4104, an ethyl ester prodrug of GS 4071; GS 4116, the guanidino analog of GS 4071; GS 4109, an ethyl ester prodrug of GS 4116; and zanamivir (GG167). These compounds were synthesized at Gilead Sciences by previously published procedures (12, 33). Rat plasma and pooled human plasma were purchased from Pel-Freez Biologicals (Rogers, Ark.) and George King Bio-medicals (Overland Park, Kans.), respectively. Influenza A/PR/8/34 (H1N1) virus was from the American Type Culture Collection (Rockville, Md.). The fluorescent neuraminidase substrate 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MUN) and all other chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.) unless indicated otherwise. Microfluor “W” white, flat-bottom, 96-well plates were purchased from Dynatech Laboratories (Chantilly, Va.).
Quantitative neuraminidase assay to determine inhibitor concentration.
Neuraminidase activity was determined in an enzymatic assay with purified influenza A/PR/8/34 (H1N1) virus as the source of viral neuraminidase. The virus was grown in embryonated hen eggs and was purified from allantoic fluid with a sucrose gradient (15). The purified virus was harvested, resuspended in 10 mM Tris (pH 7.5) containing 0.1 M NaCl and 60% (vol/vol) glycerol, and stored at −70°C until use.
The fluorescent substrate MUN was used to measure the enzymatic activity of the viral neuraminidase (20, 23). Briefly, 10 μl of virus, diluted in 2× assay buffer (66 mM MES [2-(N-morpholino)ethanesulfonic acid; pH 6.5] containing 8 mM CaCl2), was mixed with an equal volume of water or animal plasma containing inhibitor, and the mixture was preincubated at room temperature for 30 min. The enzymatic reaction was initiated with the addition of 80 μl of assay buffer containing 12.5 μM MUN. After an 8-min incubation at 37°C, the reaction was terminated with the addition of 150 μl of 0.014 N NaOH in 83% ethanol. The stopped reaction mixtures were immediately transferred to a 96-well, white, flat-bottom plate, and the relative amount of the fluorescent product 4-methylumbelliferone produced in each sample was determined with a LS 50B luminescence spectrometer (Perkin-Elmer Limited, Beaconsfield, United Kingdom) with an excitation wavelength of 360 nm and an emission wavelength of 448 nm and with the slit widths set at 2.5 nm. Fluorescent values for uninhibited reactions were generally between 40 and 60 fluorescent units and were within the linear range of the assay.
The concentration of inhibitor in a sample was determined on the basis of the amount of fluorescent product formed in a reaction mixture containing the sample. The fluorescence intensity of a duplicate reaction with no inhibitor was used to determine the maximum (100%) neuraminidase activity, and that of a reaction containing substrate alone was used to determine the background values. Standard curves were constructed by plotting the percent neuraminidase activity relative to the activity of an uninhibited control versus the inhibitor concentration and were fitted with the four-parameter hyperbolic function f(x) = (a − d)/[(1 + (x/c)b] + d, where f(x) is the percentage of uninhibited neuraminidase activity; a is 100%, the value obtained from an uninhibited reaction; d is the background neuraminidase activity of a reaction containing substrate alone (generally <2%); x is the inhibitor concentration; b is the slope coefficient; and c is an inhibitor concentration approximately equal to the inhibitor concentration required to reduce neuraminidase activity by 50% (IC50) when d ≪ a. The concentration of inhibitor in a sample was then determined by solving for x with SigmaPlot software (Jandel Corp., San Rafael, Calif.). The sensitivity of this assay depends on the IC50 of the compound being tested; for GS 4071, GS 4116, and zanamivir the limit of detection was approximately 5 nM, or 0.0015 μg/ml. The error for this method was 5% on the basis of the results of experiments with known amounts of the three neuraminidase inhibitors.
Conversion of the prodrug to parent compound by plasma esterase activity.
The ethyl ester prodrugs GS 4104 and GS 4109 were incubated at a concentration of 50 μM in the presence or absence of plasma for 30 min at 37°C. The amount of parent compound generated during the incubation period was then determined by the quantitative neuraminidase assay described above, and the extent of conversion observed during the 30-min incubation was taken as a relative measure of the stability of the prodrug in plasma. No attempt was made to further characterize the in vitro conversion of the prodrugs to their respective parent compounds.
Pharmacokinetic studies.
Studies with animals were conducted in accordance with guidelines set forth in the Guide for the Care and Use of Laboratory Animals (20a). In rat studies, GS 4071, its ethyl ester prodrug GS 4104, GS 4116, its ethyl ester prodrug GS 4109, and zanamivir were each administered to four Sprague-Dawley rats (age, 8 to 10 weeks) as a single intravenous (i.v.) dose (10 mg/kg of body weight or a single oral dose (10 mg-eq/kg) of compound by gavage. The oral doses are presented as milligram equivalents per kilogram to indicate that the dose of compound given by this route has been corrected to ensure delivery of the same amount (moles) of compound delivered in the i.v. dose. This is important when parent compound is given by the i.v. route and the prodrug, which has a different molecular weight, is given by the oral route. In dog studies, a single 5-mg/kg i.v. dose of GS 4071 was administered to five beagle dogs (average weight, 7.9 kg). After a 1-week washout period, the same animals received a 5-mg-eq/kg oral dose of GS 4104. In other studies, groups of four mice (age, 8 to 10 weeks) or three ferrets (average weight, 1.4 kg) received either a single i.v. dose (10 or 1 mg/kg, respectively) of GS 4071 or a single oral dose (10 or 5 mg-eq/kg, respectively) of GS 4104 by gavage. All compounds were administered as aqueous solutions in 0.9% sodium chloride.
At predetermined time points up to 24 h postdosing, blood samples were collected via a jugular cannula or by venipuncture from the jugular or cephalic vein, placed into heparinized tubes, and processed to recover the plasma, which was then stored at −20°C. As an example of a representative sampling schedule, plasma samples were collected at 0.08, 0.25, 0.5, 0.75, 1, 2, 4, 6, 12, and 24 h after administration of the i.v. dose to the rats and at 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 12, and 24 h after administration of the oral dose to the rats. The concentrations of inhibitor in the rat, dog, and ferret plasma samples were determined by the quantitative neuraminidase assay described above. The concentration of inhibitor in the mouse plasma samples was determined by a fluorescence derivatization high-pressure liquid chromatography (HPLC) assay as described previously (6).
The plasma samples from animals receiving oral prodrug (GS 4104 or GS 4109) were assayed in two ways to determine the concentration of parent compound and total compound. One aliquot was diluted and assayed in buffer to detect parent compound. A second aliquot was diluted in rat plasma and was incubated at 37°C for 30 min to hydrolyze any remaining prodrug and allow the measurement of the total amount of compound present. In preliminary experiments it was determined that this procedure would convert all the remaining GS 4104 and GS 4109 to their respective parent compounds. Since the quantitative enzymatic assay is most sensitive at about the IC50 of each inhibitor, the samples were diluted until the neuraminidase activity fell between 30 and 70% of that of an unihibited reaction, i.e., near the IC50 of the parent compound. A standard curve for the parent compound was constructed each time that the plasma samples were assayed. Duplicate aliquots of a limited number of samples were assayed for GS 4071 and GS 4104 by both the quantitative neuraminidase assay and the fluorescence derivatization HPLC assay. The two quantitation methods gave comparable results for samples assayed by both methods, confirming that the quantitative enzymatic assay detects parent compound. This conclusion is also supported by the fact that we have not identified metabolites of GS 4104, other than GS 4071, which have neuraminidase inhibitory activity (30a).
Determination of pharmacokinetic parameters.
The area under the plasma concentration-versus-time curve (AUC) for the 24 h following administration of compound (AUC0–24), the terminal-phase half-life (t1/2), the maximum concentration of compound in plasma (Cmax), the time to Cmax (Tmax), and the mean residence time (MRT) were calculated by a noncompartmental method with PCNONLIN software (Statistical Consultants, Inc., Lexington, Ky.). The oral bioavailability (F) of the parent compound from prodrug or from oral administration of the parent compound was calculated from the AUC0–24 of the oral dose divided by the AUC0–24 of the i.v. dose of the parent compound. For example, the oral bioavailability of GS 4071 from orally administered GS 4104 was calculated as follows: F = [(AUCp.o.)/(dosep.o.)]/[(AUCi.v.)/(dosei.v.)], where AUCp.o. is the AUC of GS 4071 after oral (p.o.) administration of GS 4104, dosep.o. is the dose of GS 4104 in milligram equivalents of GS 4071/per kilogram, AUCi.v. is the AUC of GS 4071 after i.v. administration, and dosei.v. is the i.v. dose of GS 4071. Total clearance (CL) from plasma was calculated as dose/AUCi.v.. The volume of distribution at steady-state (VSS) of drug administered i.v. was calculated as CL × MRT.
RESULTS
Neuraminidase inhibitor prodrugs are readily hydrolyzed to parent compound in rat plasma.
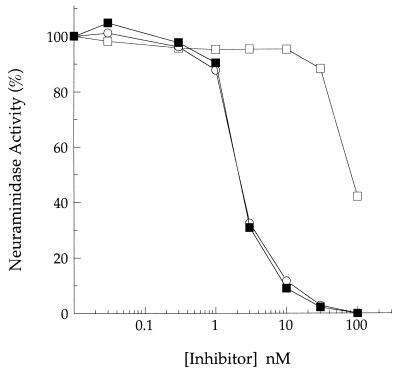
As shown in Fig. 2, GS 4071 is a potent inhibitor of influenza virus neuraminidase activity, with an IC50 and an IC90 of 2 and 10 nM, respectively, in an in vitro neuraminidase assay (12, 20). In contrast, GS 4104, the ethyl ester prodrug of GS 4071, exhibited poor activity in this assay, with an IC50 of approximately 100 nM (Fig. 2). GS 4109 is an ethyl ester prodrug of GS 4116, the guanidino derivative of GS 4071, and also was a poor inhibitor of influenza virus neuraminidase activity in the in vitro enzymatic assay, with no inhibitory activity detected at a concentration of 100 nM; for comparison, the IC50 and the IC90 of GS 4116 in this assay were 0.9 and 5 nM, respectively. The parent compounds and the prodrugs were stable under the assay conditions, with no change in potency observed for either of the compounds after 24 h at room temperature (data not shown).
FIG. 2.
GS 4104 is converted to GS 4071 by rat plasma esterase activity. Dose-dependent inhibition of influenza virus neuraminidase activity by GS 4104, the ethyl ester prodrug of GS 4071, after a 30-min incubation at 37°C in buffer (□) or rat plasma (▪). A dose-response curve for GS 4071, assayed in buffer, is shown for comparison (○).
Ethyl ester prodrugs were chosen because, once absorbed, they are readily converted to the parent compound by esterase activity in plasma and tissues (30). To test whether plasma esterase activity could convert the neuraminidase inhibitor prodrugs to the parent compounds, the prodrugs were incubated with rat plasma at 37°C for 30 min prior to assaying their inhibitory activities in the enzymatic assay. As indicated in Fig. 2, the inhibitory activity of GS 4104 was indistinguishable from that of the parent compound following incubation with rat plasma, indicating that the esterase activity in rat plasma can efficiently convert the prodrug to GS 4071. GS 4104 is rapidly hydrolyzed to the parent compound, as indicated by the observation that full conversion occurred in less than 5 min at 37°C. Similar results were obtained for the prodrug of GS 4116 (data not shown). The presence of rat plasma at a final concentration of 10% of the total reaction volume did not affect the inhibitory activity of GS 4071, GS 4116, or zanamivir as indicated by the fact that the IC50s of these compounds were identical when rat plasma was present or absent from the reaction mixture (data not shown).
In contrast to the rapid and complete conversion of the prodrugs to their respective parent compounds in rat plasma, the prodrugs were relatively stable in human, ferret, and dog plasma. In human and ferret plasma, 15 and 10% of the prodrug was converted to the parent compound, respectively, during a 30-min incubation at 37°C. Essentially none of the prodrug was converted to the parent compound during a 30-min incubation in dog plasma. None of the animal plasmas had an effect on the activity of either parent compound or zanamivir in the enzymatic assay when the plasma was present at a level of 10% of the final reaction volume.
GS 4104 is orally bioavailable in rats.
To determine the oral bioavailabilities of GS 4071, GS 4116, and their respective ethyl ester prodrugs, GS 4104 and GS 4109, the four compounds were administered to rats by the i.v. and oral routes. The oral bioavailability of zanamivir was also determined for comparison. A summary of the values of the pharmacokinetic parameters determined from experiments described in this section is provided in Table 1.
TABLE 1.
Values of pharmacokinetic parameters for neuraminidase inhibitors administered as 10 mg-eq/kg doses to ratsa
| Compound | Route | AUC0–24 (mg · h/liter) | Cmax (μg/ml) | tmax (h) | CL (liters/h/kg) | t1/2 (h)b | VSS (liters/kg) | F as parent compound (%) |
|---|---|---|---|---|---|---|---|---|
| GS 4071 | i.v. | 8.4 ± 1.4 | 1.5 ± 0.3 | 1.6 ± 0.4 | 1.3 ± 0.6 | 100 | ||
| Oral | 0.3 ± 0.1 | 0.03 ± 0.00 | 4.0 ± 1.6 | 10.6 ± 5.5 | 4.3 ± 1.6 | |||
| GS 4104 | i.v. | 6.6 ± 1.2 | 1.8 ± 0.3 | 6.2 ± 2.3 | 3.1 ± 0.9 | 79 ± 14 | ||
| Oral | 3.0 ± 0.9 | 0.47 ± 0.13 | 1.6 ± 1.6 | 7.0 ± 0.6 | 35 ± 11 | |||
| GS 4116 | i.v. | 9.0 ± 1.7 | 1.3 ± 0.2 | 5.7 ± 0.8 | 1.1 ± 0.3 | 100 | ||
| Oral | 0.4 ± 0.1 | 0.06 ± 0.01 | 1.9 ± 1.5 | 20.1 ± 7.0 | 4.0 ± 1.0 | |||
| GS 4109 | i.v. | 9.2 ± 0.9 | 1.2 ± 0.1 | 6.0 ± 1.0 | 1.6 ± 0.3 | 102 ± 10 | ||
| Oral | 0.2 ± 0.1 | 0.03 ± 0.00 | 0.8 ± 0.8 | 18.0 ± 5.3 | 2.1 ± 1.1 | |||
| Zanamivir (GG167) | i.v. | 5.5 ± 1.5 | 1.9 ± 0.6 | 1.1 ± 0.3 | 0.8 ± 0.1 | 100 | ||
| Oral | 0.2 ± 0.2 | 0.06 ± 0.01 | 1.2 ± 0.7 | 1.8 ± 0.6 | 3.7 ± 2.3 |
Values are means ± standard deviations for four animals on the basis of the levels of parent compound in plasma.
The terminal t1/2 was estimated on the basis of the values at 6 and 12 h for the samples from animals given drug i.v. and the values at 12 and 24 h for the samples from animals given drug orally (or values obtained at 4 and 12 h for orally administered zanamivir).
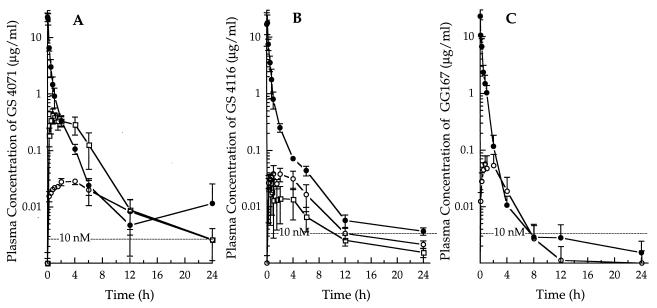
Following the i.v. administration of GS 4071 to rats, the concentrations of GS 4071 in plasma declined in a multiexponential manner (Fig. 3A), with an apparent elimination t1/2 of 1.6 h. The CL of GS 4071 was 1.47 liters/h/kg and the VSS was 1.26 liters/kg. Following oral administration of a 10-mg/kg dose of GS 4071 to rats, the Cmax of GS 4071 was 0.03 μg/ml (105 nM) at 4.0 h postdosing (Fig. 3A). The F of GS 4071, based on a comparison of the AUC of GS 4071 for the oral and i.v. doses, was 4.3%.
FIG. 3.
Concentration-time profiles of influenza virus neuraminidase inhibitors in rat plasma. (A) Concentration of GS 4071 following i.v. (•) or oral (○) administration of a 10-mg/kg dose of GS 4071 or oral administration of a 10 mg-eq/kg-dose of the prodrug GS 4104 (□) (datum points represent means ± standard deviations for four animals). (B) Concentration of GS 4116 following i.v. (•) or oral (○) administration of a 10-mg/kg dose of GS 4116 or oral administration of a 10-mg-eq/kg dose of the prodrug GS 4109 (□) (datum points represent the means ± standard deviations for four animals). (C) Concentration of zanamivir following i.v. (•) or oral (○) administration of a single 10-mg/kg dose (datum points represent the means ± standard deviations for three animals). A horizontal line indicating a concentration in plasma of 10 nM, the IC90 of GS 4071 in the influenza virus neuraminidase enzymatic assay (Fig. 2), is shown for reference.
The plasma concentration-versus-time profile of the parent compound following the i.v. administration of GS 4104 was similar to that following the administration of the parent compound (data not shown), as were most of the pharmacokinetic parameters. However, VSS was much greater for GS 4104 (3.05 liters/kg) than for the parent compound (1.26 liters/kg). The larger VSS for GS 4104 may indicate that it is distributed outside the vascular space more efficiently than the parent compound. Following oral administration of GS 4104, the Cmax (0.52 μg/ml; 1,830 nM) and F (35%) of the parent compound were approximately 10-fold higher than those observed following the oral administration of GS 4071 itself. The concentration of parent compound in plasma 12 h after the oral administration of GS 4104 was 0.009 μg/ml (32 nM), which is three times the concentration required to inhibit 90% of the enzymatic activity in the in vitro assay (see above). No prodrug was detected in any of the samples.
Figure 3B indicates the plasma concentration-versus-time profile for GS 4116, the guanidino analog of GS 4071, following the i.v. or oral administration of GS 4116 or the oral administration of the prodrug GS 4109. The results obtained for the parent compound are similar to those obtained for GS 4071, with GS 4116 having an F of 4.0%. However, in contrast to what was observed for GS 4104, the bioavailability of GS 4116 from its oral prodrug was poor (2.1%). Again, no prodrug was detected in any of the samples.
The plasma concentration-versus-time profiles for zanamivir administered by the i.v. and oral routes are presented in Fig. 3C and are consistent with the results obtained following the i.v. or oral administration of zanamivir to mice (26). The F of zanamivir (3.7%) was comparable to those of GS 4071 and GS 4116 (Table 1).
GS 4104 is detected in the plasma of dogs and ferrets.
To ensure that the bioavailability of the parent compound from orally administered GS 4104 was not specific to rats, similar experiments were performed with mice, dogs, and ferrets. Mice and ferrets were included because they are used in animal models of influenza virus infection, and dogs were chosen because they are often used as a nonrodent species in preclinical studies. A comparison of the values of the pharmacokinetic parameters determined for the four species are summarized in Table 2.
TABLE 2.
Values of pharmacokinetic parameters for GS 4071 following i.v. administration of GS 4071 or oral administration of GS 4104 to animalsa
| Species | Compound | Dose (mg-eq/kg), route of administration | AUC0–24 (mg · h/liter) | Cmax (μg/ml) | Tmax (h) | CL (liters/h/kg) | t1/2 (h) | VSS (liters/kg) | F (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mouse (n = 3) | GS 4071 | 10, i.v. | 13 | 0.77 | NDb | 3.9 | |||
| GS 4104 | 10, oral | 3.9 | 1.2 | 1.0 | ND | 30 | |||
| Rat (n = 4) | GS 4071 | 10, i.v. | 8.4 | 1.5 | 1.6 | 1.3 | |||
| GS 4104 | 10, oral | 3.0 | 0.47 | 1.6 | 7.0 | 35 | |||
| Ferret (n = 3) | GS 4071 | 1, i.v. | 2.2 | 0.33 | 0.6 | 0.28 | |||
| GS 4104 | 5, oral | 1.2 | 0.20 | 2.0 | 5.7 | 11 | |||
| Dog (n = 5) | GS 4071 | 5, i.v. | 18 | 0.32 | 1.8 | 0.56 | |||
| GS 4104 | 5, oral | 11 | 1.2 | 3.8 | 3.7 | 73 |
Values are means and are based on the levels of GS 4071 in plasma.
ND, not determined.
Compared to the rat, the plasma concentration-versus-time profile for the parent compound in mice administered an oral dose of GS 4104 shows a comparable Tmax and a comparable rate of decline from the Cmax (data not shown). Mice were also similar to rats in that GS 4104 was not detected in any of the samples. The bioavailability of GS 4071 from orally administered GS 4104 in mice was 30%.
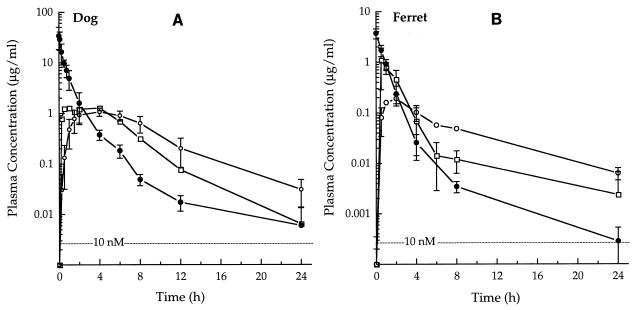
The plasma concentration-versus-time profile for the parent compound in dogs orally given the prodrug was different from those observed in mice and rats (Fig. 4) in that the concentrations of the parent compound in plasma were sustained for a longer period in dogs than in rats or mice. Dogs also differed from rats and mice in that substantial amounts of GS 4104 were detected in the plasma samples; this was especially true during the first 4 h postdosing, during which time GS 4104 was the predominant species. The F of the parent compound from GS 4104 in dogs was 73%.
FIG. 4.
Concentration-time profiles of GS 4071 and the prodrug GS 4104 in dog and ferret plasma. (A) Concentration of GS 4071 (•) in dog plasma following administration of an i.v. dose (5 mg/kg) of GS 4071 or the concentration of GS 4071 (○) and GS 4104 (□) in dog plasma following administration of an oral dose (5 mg-eq/kg) of the prodrug GS 4104 (datum points represent the means ± standard deviations for five animals). (B) Concentration of GS 4071 (•) in ferret plasma following administration of an i.v. dose (1 mg/kg) of GS 4071 or the concentration of GS 4071 (○) and GS 4104 (□) in ferret plasma following administration of an oral dose (5 mg-eq/kg) of the prodrug GS 4104 (datum points represent the means ± standard deviations for three animals). A horizontal line indicating a concentration in plasma of 10 nM, the IC90 of GS 4071 in the influenza virus neuraminidase enzymatic assay (Fig. 2), is shown for reference.
Among the four animal species tested, ferrets had the lowest bioavailability of parent compound (11%) from orally administered GS 4104. In ferrets the rate of elimination of GS 4071 derived from oral prodrug was similar to that seen in dogs (Fig. 4). Ferrets were also similar to dogs in that intact GS 4104 was present in their plasma samples. However, whereas the peak concentrations of GS 4071 and prodrug in plasma were not dramatically different in dogs, the concentration of prodrug in the ferret plasma samples obtained during the first 2 to 3 h postdosing was substantially greater than that of GS 4071.
DISCUSSION
The influenza viruses are well understood but poorly controlled human pathogens which are responsible for approximately 30,000 deaths each year in the United States alone (18). The demonstration that zanamivir, a potent and selective inhibitor of the influenza virus neuraminidases in vitro, is effective both in animal models of influenza infection and in challenge studies with humans (10, 26, 27, 32, 35) indicates that the influenza virus neuraminidase is a valid antiviral target. However, due to its poor oral bioavailability and rapid elimination, zanamivir is administered topically to the respiratory tract as a nasal spray or inhalant. We have identified GS 4071, a member of a series of novel carbocyclic transition-state analog inhibitors of the influenza virus neuraminidases, as a lead compound with the potential to maintain potency while being orally bioavailable (12).
Although GS 4071 is more lipophilic than zanamivir, with an amino group replacing the guanidino group of zanamivir and a lipophilic 3-pentyloxy group replacing the polar glycerol group, GS 4071 itself exhibited poor oral bioavailability in rats. However, the parent compound was bioavailable (F = 11 to 73%) in rats, mice, dogs, and ferrets following the oral administration of GS 4104, the ethyl ester prodrug of GS 4071. Ester prodrugs have been used to increase the bioavailability of carboxylic acid-containing compounds (30). Once absorbed, these prodrugs are readily hydrolyzed by a variety of esterases present in the blood and tissues of many species and the parent compound is released (16, 24). Notably, esterification of the carboxylic group of GS 4116, the more polar guanidino analog of GS 4071, did not increase its bioavailability.
The results presented in this report indicate that oral administration of GS 4104 led to higher and more sustained concentrations of the active form of the drug in plasma than did oral administration of GS 4071 itself. This was particularly evident in dogs and ferrets, the species with detectable levels of intact prodrug in their plasma. The observation that the apparent terminal t1/2 of GS 4071 following oral administration to rats (10.6 h) was significantly longer than that following intravenous dosing (1.6 h) indicates that the elimination of GS 4071 is rate limited by slow oral absorption of the polar parent molecule (flip-flop kinetics). In the case of the prodrug GS 4104, i.v. and oral administration to rats gave similar apparent terminal t1/2s for the parent (6.2 and 7.0 h, respectively). These data suggest that elimination of the parent compound after administration of prodrug is rate limited by conversion to GS 4071 rather than by absorption. While the stability of the prodrug in plasma would be expected to affect its rate of conversion to GS 4071, the ability of prodrug to aid delivery of the compound to extravascular tissues may also play a role in maintaining the concentrations of GS 4071 in plasma. For example, it has recently been demonstrated that GS 4104 binds selectively to the lung tissue of rats (6), and slow release of prodrug from this or other tissue compartments may also contribute to the longer t1/2 of the parent compound observed after the administration of prodrug.
The ability of GS 4104, like several other basic lipophilic compounds containing a primary amino group (3, 25, 31), to accumulate in lung tissue may also affect its antiviral activity at the primary site of influenza virus infection and replication. Consistent with this hypothesis, Eisenberg et al. (6) have demonstrated that GS 4071 is present in bronchoalveolar lavage fluid following oral administration of the prodrug to rats. Of particular interest, the peak concentration of GS 4071 in the bronchoalveolar lavage fluid was similar to that in the plasma, but it declined more slowly compared to the rate of decline in plasma, indicating that the antiviral activity in lung tissue may be more persistent than is suggested by the plasma concentration-versus-time profile (6). Because these experiments were carried out with rats, which do not have detectable levels of circulating prodrug in plasma, we would anticipate that the accumulation of prodrug in lung tissue might be even more pronounced in animal species such as dogs or ferrets which have greater amounts of circulating prodrug. In this respect, it is noteworthy that prodrug is detected in human plasma samples following the oral administration of GS 4104 (34).
In summary, we have demonstrated that GS 4104 is an orally bioavailable prodrug of the potent influenza virus neuraminidase inhibitor GS 4071 in each of the four animal species tested. We have also demonstrated that in each of the four animal species, including ferrets, in which the lowest bioavailability of parent compound (11%) from orally administered GS 4104 was found, the concentrations of parent compound in plasma are well above those necessary to inhibit influenza virus neuraminidase activity, even at 12 h postdosing. These results are consistent with the subsequent demonstration that GS 4104 administered orally twice daily is effective in the ferret and mouse models of influenza virus infection (11, 20, 28). On the basis of the demonstrated efficacy of orally administered GS 4104 in animal models of influenza virus infection and preliminary animal toxicity data (20), we conclude that GS 4104 has the potential to be an oral agent for the prophylaxis and treatment of influenza A and B virus infections in humans. This conclusion is supported by the recent demonstrations (34) that the levels of GS 4071 in plasma following oral administration of GS 4104 to humans are similar to those reported here and that early treatment with oral GS 4104 is associated with significant clinical efficacy and antiviral effect in experimental influenza A virus infection in humans (8).
ACKNOWLEDGMENT
We thank Jay Toole for critical review of the manuscript.
REFERENCES
- 1.Assaad F, Bektimirov T, Esteves K L. Influenza—world experience. In: Stuart-Harris C H, editor. The molecular virology and epidemiology of influenza. London: Academic Press; 1984. pp. 5–38. [Google Scholar]
- 2.Belshe R B, Burke E, Newman F, Cerruti R L, Sim I S. Resistance of influenza A virus to amantidine and rimatidine: result of one decade of surveillance. J Infect Dis. 1989;159:430–435. doi: 10.1093/infdis/159.3.430. [DOI] [PubMed] [Google Scholar]
- 3.Bergogne-Berezin E. Pharmacokinetics of antibiotics in respiratory secretions. In: Pennington J, editor. Respiratory infections; diagnosis and management. New York, N.Y: Raven Press; 1988. pp. 608–631. [Google Scholar]
- 4.Burnet F M. Mucins and mucoids in relation to influenza virus action. Aust J Exp Biol Med Sci. 1948;26:381–387. doi: 10.1038/icb.1948.39. [DOI] [PubMed] [Google Scholar]
- 5.Couch A B. Advances in influenza virus vaccine research. Ann N Y Acad Sci. 1993;685:803–812. doi: 10.1111/j.1749-6632.1993.tb35946.x. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg E J, Bidgood A, Cundy K C. Penetration of GS4071, a novel influenza neuraminidase inhibitor, into rat bronchoalveolar lining fluid following oral administration of the prodrug GS4104. Antimicrob Agents Chemother. 1997;41:1949–1952. doi: 10.1128/aac.41.9.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayden F G, Hay H J. Emergence and transmission of influenza A viruses resistant to amantadine and rimantidine. Curr Top Microbiol Immunol. 1992;176:119–130. doi: 10.1007/978-3-642-77011-1_8. [DOI] [PubMed] [Google Scholar]
- 8.Hayden F G, Lobo M, Treanor J J, Miller M, Mills R G. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy Program Addendum. Washington, D.C: American Society for Microbiology; 1997. Efficacy and tolerability of oral GS 4104 for early treatment of experimental influenza in humans, abstr. LB-26; p. 7. [Google Scholar]
- 9.Hayden F G, Osterhaus A D M E, Treanor J J, Fleming D M, Aoki F Y, Nicholson K G, Bohnen A M, Hirst H M, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 10.Hayden F G, Treanor J J, Betts A F, Lobo M, Esinhart J D, Hussey E K. Safety and efficacy of the neuraminidase inhibitor GG167 in experimental human influenza. JAMA. 1996;275:295–299. [PubMed] [Google Scholar]
- 11.Kim C U, Bischofberger N, Williams M A, Lew W, Merson J, Sweet C. Efficacy of GS 4104 in ferrets infected with influenza A virus. Antivir Res. 1997;34:A74. . (Abstract 114.) [Google Scholar]
- 12.Kim C U, Lew W, Williams M A, Liu H, Zhang L, Swaminathan S, Bischofberger N, Chen M S, Mendel D B, Tai C Y, Laver W G, Stevens R C. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc. 1997;119:681–690. doi: 10.1021/ja963036t. [DOI] [PubMed] [Google Scholar]
- 13.Klenk H-D, Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–280. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langmuir A D, Worthen T D, Solomon J, Ray C G, Peterson E. Thucydides syndrome. A new hypothesis for the cause of the plague of Athens. N Engl J Med. 1985;313:1027–1030. doi: 10.1056/NEJM198510173131618. [DOI] [PubMed] [Google Scholar]
- 15.Laver W G, Colman P M, Webster R G, Hinshaw V S, Air G M. Influenza virus neuraminidase with hemagglutinin activity. Virology. 1984;137:314–323. doi: 10.1016/0042-6822(84)90223-x. [DOI] [PubMed] [Google Scholar]
- 16.Leinweber F-J. Possible physiological roles of carboxylic ester hydrolases. Drug Metab Dispos. 1987;16:425–428. doi: 10.3109/03602538708994129. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Eichelberger M C, Compans R W, Air G M. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lui K J, Kendal A P. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77:712–716. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendel D B, Tai C Y, Escarpe P, Li W-X, Kim C U, Williams M A, Lew W, Zhang L, Bischofberger N, Huffman J H, Sidwell R W, Chen M S. GS 4071 is a potent and selective inhibitor of the growth and neuraminidase activity of influenza A and B viruses in vitro. Antivir Res. 1997;34:A73. . (Abstract 111.) [Google Scholar]
- 20.Mendel D B, Tai C Y, Escarpe P A, Li W, Sidwell R W, Huffman J H, Sweet C, Jakeman K J, Merson J, Lacy S A, Lew W, Williams M A, Zhang L, Chen M S, Bischofberger N, Kim C U. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.National Institutes of Health. Guide for the care and use of laboratory animals. NIH publication 86-23. Bethesda, Md: National Institutes of Health; 1986. [Google Scholar]
- 21.Palese P, Compans R W. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol. 1976;33:159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 22.Palese P, Tobita K, Ueda M, Compans R W. Characterization of temperature sensitive influenza virus mutants. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 23.Potier M, Mameli L, Bélisle M, Dallaire L, Melançon S B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-d-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- 24.Quon C Y, Mai K, Patil G, Stampfli H F. Species differences in the stereoselective hydrolysis of esmolol by blood esterases. Drug Metab Dispos. 1988;16:425–428. [PubMed] [Google Scholar]
- 25.Roerig D L, Kotrly K J, Dawson C A, Ahlf S B, Gualtieri J F, Kampine J P. First-pass uptake of verapamil, diazepam, and thiopental in the human lung. Anesth Analg. 1989;69:461–466. [PubMed] [Google Scholar]
- 26.Ryan D M, Ticehurst J, Dempsey M H. GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is a potent inhibitor of influenza virus in ferrets. Antimicrob Agents Chemother. 1995;39:2583–2584. doi: 10.1128/aac.39.11.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan D M, Ticehurst J, Dempsey M H, Penn C R. Inhibition of influenza virus replication in mice by GG167 (4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid) is consistent with extracellular activity of viral neuraminidase (sialidase) Antimicrob Agents Chemother. 1994;38:2270–2275. doi: 10.1128/aac.38.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidwell R W, Huffman J H, Barnard D L, Bailey K W, Wong M-H, Morrison A, Syndergaard T, Kim C U. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. 1997. Antiviral Res., in press. [DOI] [PubMed] [Google Scholar]
- 29.Smith F I, Palese P. Variation in influenza virus genes—Epidemiological, pathogenic, and evolutionary consequences. In: Krug R M, editor. The influenza viruses. New York, N.Y: Plenum; 1989. pp. 319–359. [Google Scholar]
- 30.Stella V J, Charman W N A, Naringrekar V H. Prodrugs: do they have advantages in clinical practice? Drugs. 1985;29:455–473. doi: 10.2165/00003495-198529050-00002. [DOI] [PubMed] [Google Scholar]
- 30a.Sweeny, D., et al. Unpublished results.
- 31.Taylor G. The absorption and metabolism of xenobiotics in the lung. Adv Drug Delivery Rev. 1990;5:37–61. [Google Scholar]
- 32.von Itzstein M, Wu W-Y, Kok G B, Pegg M S, Cyason J C, Jin B, Phan T V, Smythe M L, White H F, Oliver S W, Colman P M, Varghese J N, Ryan D M, Woods J M, Bethell R C, Hotham V J, Cameron J M, Penn C R. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 33.von Itzstein M, Wan Yang M, Jin B. The synthesis of 2,3-didehydro-2,4-dideoxy-4-guanidinyl-N-acetylneuraminic acid: a potent influenza virus sialidase inhibitor. Carbohydrate Res. 1994;259:301–305. doi: 10.1016/0008-6215(94)84065-2. [DOI] [PubMed] [Google Scholar]
- 34.Wood N D, Aitken M, Sharp S, Evison H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Tolerability and pharmacokinetics of the influenza neuraminidase inhibitor Ro 64-0802 (GS4071) following oral administration of the prodrug Ro 64-0796 (GS4104) to healthy male volunteers, abstr. A-123; p. 25. [Google Scholar]
- 35.Woods J M, Bethell R C, Coates J A V, Healy N, Hiscox S A, Pearson B A, Ryan D M, Ticehurst J, Tilling J, Walcott S M, Penn C R. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993;37:1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]