Abstract
In the immunocompetent host, visceral leishmaniasis (VL) is a fatal disease if untreated. In immunosuppressed patients, VL is an opportunistic infection for which there is no effective treatment for relapses. Here we report on the long-term activity of orally administered hexadecylphosphocholine (HDPC) against established Leishmania infantum infection in BALB/c mice. HDPC is a synthetic phospholipid with antiproliferative properties that has been extensively studied for its cancerostatic activity. Its short-term leishmanicidal effects in mice recently infected with viscerotropic Leishmania species have been previously reported. First, we show that 5 days of oral therapy with HDPC (20 mg/kg of body weight/day) led to amastigote suppression in the liver and the spleen of 94 and 78%, respectively (versus 85 and 55% suppression by meglumine antimonate in the liver and spleen, respectively), in mice infected 6 weeks before treatment and examined 3 days after the end of treatment. These results demonstrate the short-term efficacy of HDPC against an established Leishmania infection. Next, the long-term efficacy of HDPC was examined. In HDPC-treated mice both the hepatic and splenic amastigote loads were significantly reduced (at least 89%) 10, 31, and 52 days after the end of the treatment. In the treated mice, the increase of the splenic load was significantly slower than that in the untreated mice, demonstrating that the HDPC-exerted inhibition of Leishmania growth persisted for at least 7 to 8 weeks. Orally administered HDPC—the safe doses and side effects of which are at least partially known—appears to be a promising candidate for the treatment of VL.
The viscerotropic Leishmania species L. donovani and L. infantum (L. chagasi) may cause asymptomatic infections, but clinically manifested human visceral leishmaniasis (VL) is a fatal disease if untreated (33). Major epidemics of VL have been reported in recent years in Brazil (24), Sudan (56), and Bangladesh and India (1). An increasing number of cases of VL are being diagnosed in human immunodeficiency virus-positive patients, and VL has been designated an opportunistic infection in the immunocompromised host (2).
Pentavalent antimonials remain, in most cases, an efficient antileishmanial treatment, but they have to be administered parenterally and may produce impairment of various vital functions, although such impairment is usually reversible in immunocompetent patients (3). In individuals coinfected with human immunodeficiency virus the antimonial drugs are inefficient and toxic (17, 30, 34, 53). Moreover, the emergence of antimony-unresponsive strains has been registered in India (43, 44, 52), and increasing numbers of therapeutic failures have been reported (5, 19). Amphotericin B has been shown to be effective in immunocompetent individuals (5, 13–15, 43, 45), but some formulations resulted in serious side effects (15, 16, 43). In immunosuppressed patients, a high-dose liposomal amphotericin B regimen appeared to be safe and useful but was ineffective in the treatment of relapses (27, 39). There is no efficient therapy for leishmaniasis in dogs, the main reservoir of L. infantum in Mediterranean areas. Therefore, in spite of recent progress in chemotherapy for VL (22), the discovery of new drug regimens is important. New formulations of old drugs (10, 31) and potential new antileishmanial compounds (8, 25, 47) have been tested in experimental in vivo models and in in vitro studies.
In recent studies, orally administered hexadecylphosphocholine (HDPC) (miltefosine) has shown promising activity against viscerotropic Leishmania species in BALB/c mice (12, 26). HDPC is a synthetic glycerol-free phospholipid analog which has been widely studied for its antiproliferative and cancerostatic properties. It is used as a topical agent (11) and has been tested by the oral route in phase I and II clinical antitumor trials (35, 46, 49), which have provided valuable information on human tolerance for the drug. Clinical trials of miltefosine in the treatment of VL in India are being planned by the World Health Organization. In the present study we tested three therapeutic schemes using the L. infantum-infected BALB/c mouse model: i) short-term efficacy against a recent infection, ii) short-term efficacy against an established infection, and iii) long-term efficacy against an established infection. The experimental protocols and the precise timing of the treatments are summarized in Table 1. In all these conditions HDPC proved to be nontoxic and highly efficient.
TABLE 1.
Assessment of HDPC efficacy against L. infantum infection in BALB/c mice: experimental protocolsa
| Protocol | Treatment group (no. of mice) | Treatment period (dpi)b | Day(s) of amastigote burden evaluation (dpi) |
|---|---|---|---|
| 1 | HDPC (11) | D7–D11 | D14 |
| MEGAN (10) | |||
| NTC (10) | |||
| 2 | HDPC (10) | D42–D46 | D49 |
| MEGAN (10) | |||
| NTC (10) | |||
| 3 | HDPC (11)c | D28–D32 | D42, D63, D84 |
| NTC (11)c | D42, D63, D84 |
Mice were intravenously inoculated with 108 stationary-phase L. infantum promastigotes/mouse. The day of infection is termed D0. Mice were randomly assigned to groups of 10 or 11 mice and were treated for 5 days with HDPC (20 mg/kg of body weight/day, oral administration) or MEGAN (200 mg/kg of body weight, subcutaneous injection) or were left untreated.
dpi, day post infection.
Three groups of 11 mice were used.
MATERIALS AND METHODS
Drugs.
HDPC, as miltefosine, was a gift from Asta Medica (Frankfurt, Germany); meglumine antimonate (MEGAN) (Glucantime; 85 mg of antimony/ml) was purchased from Rhône-Poulenc-Rorer, Paris, France.
Parasites and infection.
L. infantum MON1 (MHOM/FR/94/LPN101), isolated from a patient with VL, was maintained by serial passages in Syrian hamsters. The promastigote form was cultured under usual conditions (38, 42), and after three in vitro passages, stationary-phase cells from 7-day-old cultures were used at a concentration of 2.5 × 107 promastigotes/ml to infect BALB/c mice. For infection the parasites were washed twice by sedimentation at 2,500 × g at 4°C for 15 min each time, resuspended in 0.9% NaCl at 2 × 108 cells/ml, and injected into caudal vein at 108 promastigotes/mouse. Hereafter the day of infection with L. infantum is termed day 0 (D0).
Mice and treatment.
Five-week-old female BALB/c mice, purchased from Iffa Credo (Arbresle, France) and maintained in a positive-pressure chamber (Flufrance) in our animal facility, were used for experimentation at 6 weeks of age. After the injection of Leishmania, the mice were randomly split into groups of 10 or 11 animals and were subsequently treated for 5 days, starting on D7, D42, or D28 (Table 1), with either HDPC (20 mg/kg of body weight/day administered orally through a soft feeding tube [Marquat; Genie Biomedical] in 0.5 ml of H2O) or MEGAN (200 mg/kg of body weight/day administered by subcutaneous injection in 0.5 ml of H2O) or were left untreated (nontreated control [NTC] group). Pursuant to the schedules of the experimental protocols presented in Table 1, mice were anesthetized with sodium thiopental (50 μg/g of body weight) and sacrificed. Livers and spleens were taken under sterile conditions and weighed. In the series of experiments corresponding to protocol 3, mice maintained in the same cage were randomly assigned to the HDPC and NTC groups to avoid any bias which could arise from the caging.
Evaluation of Leishmania infection.
The assessment of the amastigote burden was carried out by blinded microscopic enumeration with Giemsa-stained liver and spleen touch prints by two independent experienced parasitologists. The parasite load was expressed in Leishman Donovan units (LDU, number of amastigotes per 1,000 nucleated cells × organ weight [in grams] × 2 × 105), according to Stauber’s formula (40). The percent efficacy was calculated as [1 − (mean amastigote load in treated mice/mean amastigote load in NTC)] × 100.
Statistics.
Due to the low number of animals per group (10 or 11 mice), statistical analyses were performed by using a nonparametric test, i.e., the Kruskal-Wallis multiple comparison z-value test. When two effects were analyzed, two-way analysis of variance (ANOVA) was applied, followed by the Newman-Keuls test.
RESULTS
Short-term efficacy against recent infection.
In a first series of experiments we checked that the capacity of orally administered HDPC to induce L. donovani (12, 26) and L. infantum (26) amastigote suppression in BALB/c mice could be reproduced with the strain of L. infantum used in our laboratory. The treatment was initiated on D7 and was carried out for 5 consecutive days, and 3 days after the end of the treatment (D14) mice were examined (Table 1). No adverse effects were registered throughout the trial. No significant differences in the body, liver, and spleen weights were detected between the groups. The mean (± standard deviation [SD]) liver amastigote burden (expressed in millions of LDU) on D14 was 158.9 ± 41.7 for the NTC group (n = 10) compared to 0.52 ± 0.66 for the MEGAN-treated group (n = 10) and 0.13 ± 0.24 for the HDPC-treated group (n = 11). The amastigote suppression efficacy of MEGAN was 99.7% and that of HDPC was 99.9% (Kruskal-Wallis test, α < 0.001) in these experimental conditions.
Short-term efficacy against established infection.
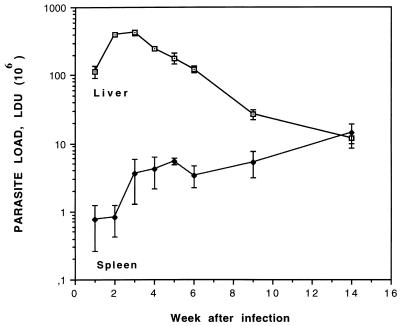
In the next series of experiments we examined the capacity of HDPC to suppress L. infantum amastigotes in an established infection. Figure 1 shows a typical time course of VL in BALB/c mice (9, 32, 38, 51). At 6 weeks after promastigote inoculation, i.e., at the end of the acute phase of the disease, the parasite load in the liver is decreasing but still high and in the spleen it is well detectable and increasing. The treatments (HDPC and MEGAN) were thus started on D42, and mice were examined 3 days after the end of the 5-day treatment. Table 2 shows the average body weights, the relative liver and spleen weights, and the average hepatic and splenic amastigote burdens for the treated and untreated mice on D49. The parasite load in the liver was significantly higher in the NTC group than in the HDPC-treated (z value = 4.291) or the MEGAN-treated (z value = 3.039) mice, whereas in the spleen only HDPC was significantly effective (z value = 3.283) (the z value for the MEGAN-treated group was 1.494). No wasting was detected during the course of the disease in L. infantum-infected BALB/c mice, and as previously reported for VL in mice due to L. infantum infection (38), hepatomegaly was not detected either, in contrast to L. infantum infection in humans, dogs, and hamsters. The degree of splenomegaly was notable, i.e., 3.3-fold normal spleen weight (which is on average 100 [± 10] mg), and there was no significant difference between the experimental groups (α = 0.6), indicating that 3 days after the end of HDPC uptake, inflammation was still present in spite of the suppression of amastigotes.
FIG. 1.
Course of L. infantum infection in BALB/c mice. Mice were inoculated with 108 stationary-phase promastigotes/mouse via the caudal vein, and parasite loads were evaluated on liver and spleen touch prints at the indicated time points after injection. Visceral proliferation of amastigotes was quantified in LDU (defined in Materials and Methods). Results are from a representative infection experiment, and the values are means ± SD for three mice at each time point. Patterns were similar in seven experiments.
TABLE 2.
Short-term efficacy of HDPC against established L. infantum infection in BALB/c micea
| Treatment group | Body wt (g) | Relative wt (%)b
|
Amastigote load, LDU (106) (% efficacy)c
|
||
|---|---|---|---|---|---|
| Liver | Spleen | Liver | Spleen | ||
| HDPC | 23.2 ± 0.8 | 5.6 ± 0.4 | 1.4 ± 0.2 | 8.8 ± 9.6 (93.8) | 2.2 ± 1.8 (77.9) |
| MEGAN | 24.2 ± 0.9 | 5.8 ± 0.3 | 1.4 ± 0.2 | 21.7 ± 20.5 (84.8) | 4.5 ± 2.3 (54.7) |
| NTC | 24.6 ± 0.6 | 6.0 ± 0.5 | 1.4 ± 0.2 | 142.2 ± 75.7 | 9.8 ± 6.0 |
Mice, intravenously inoculated with 108 stationary-phase L. infantum promastigotes/mouse on D0, were treated for 5 days, starting D42, with HDPC (20 mg/kg of body weight/day, oral administration) or MEGAN (200 mg/kg of body weight/day, subcutaneous injection) or were left untreated. Mice were examined 3 days later (D49). Data are means ± SD for 10 mice in each group.
Organ weight/body weight.
LDU, number of amastigotes per 1,000 nucleated cells × organ weight (in grams) × 2 × 105. Percent efficacy, [1 − (mean amastigote load in treated mice/mean amastigote load in control mice)] × 100.
Long-term efficacy against established infection.
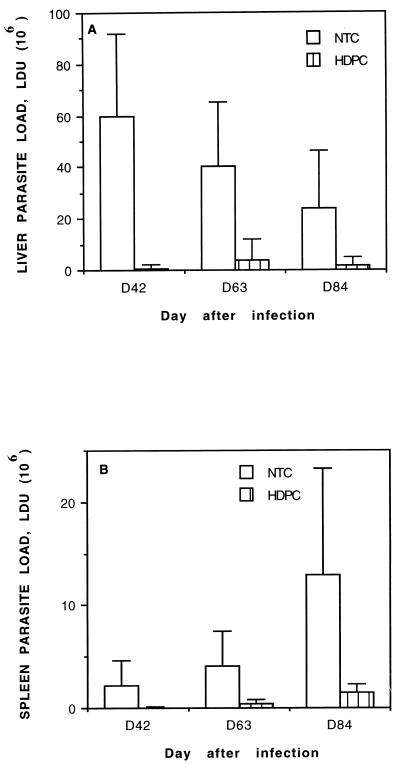
Our next aim was to determine for how long after the end of the treatment HDPC remained effective against the amastigote burden. Mice infected as described above were left untreated or were treated orally with HDPC for 5 consecutive days starting on D28 and were then examined after prolonged time periods. Figure 2 shows liver (Fig. 2A) and spleen (Fig. 2B) amastigote loads on D42, D63, and D84 for NTC and HDPC-treated mice (corresponding, respectively, to 10, 31, and 52 days after the end of the treatment). Between D42 and D84, the parasite burden of L. infantum-infected untreated mice decreased in the liver and increased steadily in the spleen (Fig. 1). In HDPC-treated mice the hepatic and splenic amastigote loads (Fig. 2 and Table 3) were suppressed by 98.8% on D42 and remained significantly lower (around 90% suppression) than in the untreated controls throughout the duration of the experiment. Two-factor (time and treatment group effect, α < 0.001) ANOVA indicated that even if the splenic parasite loads increased in both the HDPC-treated and untreated mice (time effect, α < 0.001), the increase was less important on D84 in HDPC-treated mice than in the control group (time-treatment interaction effect, α = 0.02). The data concerning body and organ weights were analyzed by two-way ANOVA and Newman-Keuls test and are summarized in Table 3. A moderate hepatomegaly was found which was slightly but significantly higher at each time point for the NTC mice (treatment effect, α < 0.001) than for the HDPC-treated mice. Splenomegaly was apparent and much more patent in the NTC group at all times (α < 0.001); moreover, a time-treatment interaction (α < 0.001) was evident, indicating that the increase of spleen weights was much less important in HDPC-treated mice than in the NTC mice.
FIG. 2.
Long-term efficacy of HDPC against full-blown L. infantum infection in BALB/c mice. Mice, intravenously inoculated with 108 stationary-phase L. infantum promastigotes/mouse on D0, were treated orally for 5 days, starting on D28, with HDPC (20 mg/kg of body weight/day) or were left untreated. The effects on amastigote burden in the liver (A) and spleen (B) were examined 10, 31, and 52 days after the end of the treatment (D42, D63, and D84, respectively). Parasite loads were assessed by blinded microscopic enumeration with Giemsa-stained liver and spleen touch prints. Results are expressed in LDU (defined in Materials and Methods), and values are means ± SD for 11 mice in each group and at each time point.
TABLE 3.
Long-term efficacy of HDPC against established L. infantum infection in BALB/c micea
| Treatment group | Day of evaluation | Body wt (g) | Relative wt (%)b
|
% Efficacyc
|
||
|---|---|---|---|---|---|---|
| Liver | Spleen | Liver | Spleen | |||
| HDPC | D42 | 23.2 ± 1.0 | 4.7 ± 0.6 | 0.5 ± 0.1 | 98.9 | 98.8 |
| D63 | 22.4 ± 0.7 | 5.6 ± 0.4 | 0.6 ± 0.1 | 90.4 | 91.8 | |
| D84 | 25.4 ± 0.6 | 4.9 ± 0.4 | 0.6 ± 0.1 | 92.5 | 88.8 | |
| NTC | D42 | 23.5 ± 1.0 | 5.2 ± 0.4 | 0.8 ± 0.2 | ||
| D63 | 22.1 ± 0.7 | 6.1 ± 0.4 | 1.3 ± 0.3 | |||
| D84 | 25.0 ± 0.5 | 5.4 ± 0.5 | 2.0 ± 0.4 | |||
Mice, intravenously inoculated with 108 stationary-phase L. infantum promastigotes/mouse on D0 and treated for 5 days (starting D28) with HDPC (20 mg/kg of body weight/day, oral administration) or left untreated, were examined on D42, D63, and D84 (10, 31, and 52 days, respectively, after the end of the treatment). Values are means ± SD for 10 mice per group.
Organ weight/body weight.
[1 − (mean amastigote load in treated mice/mean amastigote load in NTC)] × 100. The parasite load data for the HDPC-treated and control mice are shown in Fig. 2.
DISCUSSION
In this paper we report on a remarkable long-term in vivo activity of orally administered HDPC against established L. infantum infection in BALB/c mice.
The data from previous studies (12, 26) showed some dependence of the optimal dose of HDPC on the particular species of Leishmania and, within a species, on the particular isolate. Therefore, we first demonstrated that HDPC at 20 mg/kg of body weight/day showed a high short-term efficacy against recent infection with the strain of L. infantum used in our laboratory. For the sake of completeness, since a direct extrapolation of the drug doses from mice to humans is unreliable, it should be noted that in cancerology the recommended therapeutic dose of HDPC in humans has been established to be 2 to 3 mg/kg of body weight/day (35, 36, 49).
We next demonstrated the short-term efficacy of HDPC against a Leishmania infection which evolved for 6 weeks prior to drug administration. This phase of murine leishmaniasis corresponds to the end of the acute phase of the disease (9) and is characterized by a spontaneous decrease of the hepatic and a steady increase of the splenic parasite burdens (9, 32, 38, 51). In spite of the notable reduction of the amastigote load in the spleen, the splenomegaly was as important in HDPC-treated mice as in control groups, suggesting that there was no direct activity of HDPC on inflammation.
We then examined the stability of the effects of HDPC against the infection. HDPC therapy was administered during the acute phase, and the mice were examined 10, 31, and 52 days after the end of the treatment. The amastigote loads were significantly lower in the spleens and livers of HDPC-treated animals than in those of the controls at all time points tested. Moreover, the progression of the splenomegaly and the increase of the splenic load were found to be significantly lower in HDPC-treated mice than in controls, demonstrating that parasite suppression and the inhibition of growth persisted for at least 7 to 8 weeks after the end of the HDPC uptake. Although our data do not provide a direct measure of the parasite killing (1 − [amastigote load at the end of the treatment/amastigote load before the start of the treatment]), they support the notion (26) that HDPC induces effective leishmanicidal activity.
The spleen is one of the major sites of Leishmania multiplication in the natural infection. In BALB/c mice, the splenic parasite burden is initially quite low, but it increases steadily for at least 3 months, and unlike the hepatic burden, it does not decline spontaneously without treatment. The splenic efficacy of HDPC should be emphasized, since until recently splenectomy was performed as the last recourse for cases of antimony-resistant leishmaniasis. The efficacy of HDPC in the spleen is compatible with the available data on its tissue distribution. In the rat (29, 37), a steady-state level of the drug was achieved in several organs, and maximal concentrations of HDPC were found in the spleen, the kidney, and the adrenal glands. The prolonged effect of HDPC on parasite growth suggests a low catabolism and/or slow clearance, resulting in a long biological half-life for the drug. To our knowledge there is no available in vivo data on these issues. In cultured tumor cells (41), HDPC was detectable 48 h after its removal from the medium.
The mechanism of antileishmanial activity of HDPC is unknown. Part of HDPC’s effects could be mediated by its stimulatory activity of the immune system. It was previously suggested (26) that HDPC could act as a costimulator for the interleukin 2-mediated activation of T cells (48). HDPC was shown to enhance gamma interferon, interleukin 3, and granulocyte-macrophage colony-stimulating factor (4, 23) expression and gamma interferon secretion in human mononuclear cells (23). More recently it was reported by several groups that HDPC had the capacity to induce macrophage activation and to stimulate NO release and tumor necrosis factor alpha production in human (18) and murine cells (54, 55). But a leishmanicidal activity against the promastigote form of L. infantum and L. donovani was also shown in in vitro assays (12, 26), and thus a direct killing of amastigotes cannot be excluded. This could be achieved by the interference of HDPC, whose uptake seems mediated via a receptor-independent endocytotic mechanism (20), with membrane lipid metabolism at various sites (7, 21, 50) and/or by its interference with signal transduction (6, 28) in host cells.
On the basis of these results and considered together with at least partial knowledge of its safe doses and side effects, and its administration by the oral route, HDPC appears to be a promising candidate for the treatment of human VL.
ACKNOWLEDGMENTS
This work was supported, in part, by grants from the Ministry of Education and Research and from the University of Nice.
We thank Georgette Pagliardini for technical and administrative assistance, Clothilde Roptin for all intravenous injections, and Gilbert Dabbene for taking care of our animal facility.
REFERENCES
- 1.Addy M, Nandy A. Ten years of kala-azar in West Bengal. Part I. Did post-kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull W H O. 1992;70:341–346. [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J, Gutierrez-Solar B, Pachon I, Calbacho E, Ramirez M, Valles R, Guillen J, Canavate C, Amela C. AIDS and Leishmania infantum. New approaches for a new epidemiological problem. Clin Dermatol. 1996;14:541–546. doi: 10.1016/0738-081x(96)00046-6. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, N., J. Jackson, G. Wortmann, and C. Oster. 1997. Sodium stibogluconate therapy for leishmaniasis: the experience of the american military 1989–1996, abstr. 374. Acta Parasitol. Turcica 21(Suppl. 1):177.
- 4.Beckers T, Voegeli R, Hilgard P. Molecular and cellular effects of hexadecylphosphocholine (Miltefosine) in human myeloid leukaemic cell lines. Eur J Cancer. 1994;30:2143–2150. doi: 10.1016/0959-8049(94)00438-b. [DOI] [PubMed] [Google Scholar]
- 5.Ben Said, M., A. Mili, F. Amri, and H. Kharrat. 1997. Resistance to N-methylglucamine in tunisian visceral leishmaniasis children, abstr. 395. Acta Parasitol. Turcica 21(Suppl. 1):184.
- 6.Bergmann J, Junghahn I, Brachwitz H, Langen P. Multiple effects of antitumor alkyl-lysophospholipid analogs on the cytosolic free Ca2+ concentration in a normal and a breast cancer cell line. Anticancer Res. 1994;14:1549–1556. [PubMed] [Google Scholar]
- 7.Berkovic D, Grunwald U, Menzel W, Unger C, Hiddemann W, Fleer E A. Effects of hexadecylphosphocholine on membrane phospholipid metabolism in human tumour cells. Eur J Cancer. 1995;31:2080–2085. doi: 10.1016/0959-8049(95)00350-9. [DOI] [PubMed] [Google Scholar]
- 8.Bhaduri, A., and K. R. Santhamma. 1997. Leishmanial fumarate reductase is a new target for drug development: studies with a synthetic Lapachol, abstr. 403. Acta Parasitol. Turcica 21(Suppl. 1):187.
- 9.Bradley D J. Genetics of susceptibility and resistance in the vertebrate host. In: Peters W, Killick-Kendrick R, editors. The leishmaniasis in biology and medicine. London, United Kingdom: Academic Press; 1987. pp. 551–577. [Google Scholar]
- 10.Carter, K. C., A. B. Mullen, and A. J. Baillie. 1997. Treatment with a vesicular formulation of sodium stibogluconate (SSG) and immunity to homologous challenge with Leishmania donovani, abstr. 401. Acta Parasitol. Turcica 21(Suppl. 1):187.
- 11.Clive S, Léonard R C F. Miltefosine in recurrent cutaneous breast cancer. Lancet. 1996;349:621–622. doi: 10.1016/S0140-6736(05)61570-X. [DOI] [PubMed] [Google Scholar]
- 12.Croft S L, Snowden D, Yardley V. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J Antimicrob Chemother. 1996;38:1041–1047. doi: 10.1093/jac/38.6.1041. [DOI] [PubMed] [Google Scholar]
- 13.Davidson R N, di Martino L, Gradoni L, Giacchino R, Russo R, Gaeta G B, Pempinello R, Scotti S, Raimondi F, Cascio A. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: a multicentre trial. Q J Med. 1994;87:75–81. [PubMed] [Google Scholar]
- 14.Davidson R N, di Martino L, Gradoni L, Giacchino R, Gaeta G B, Pempinello F, Scotti S, Cascio A, Castagnola E, Maisto A. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome) Clin Infect Dis. 1996;22:938–943. doi: 10.1093/clinids/22.6.938. [DOI] [PubMed] [Google Scholar]
- 15.Dietze R, Milan E P, Berman J D, Grogl M, Falqueto A, Feitosa T F, Luz K G, Suassuna F A B, Marinho L A C, Ksionski G. Treatment of Brazilian kala-azar with a short course of Amphocil® (amphotericin B cholesterol dispersion) Clin Infect Dis. 1993;17:981–986. doi: 10.1093/clinids/17.6.981. [DOI] [PubMed] [Google Scholar]
- 16.Dietze, R., S. M. S. Fagundes, E. F. Brito, E. P. Milan, T. F. Feitosa, F. A. B. Suassuna, G. Fonschiffrey, G. Ksionski, and J. Dember. Treatment of kala-azar in Brazil with Amphocil® (amphotericin B cholesterol dispersion) for 5 days. Trans. R. Soc. Trop. Med. Hyg. 89:309–311. [DOI] [PubMed]
- 17.Domingo P, Ferrer S, Kolle L, Munoz C, Rodriguez P. Acute pancreatitis associated with sodium stibogluconate treatment in a patient with human immunodeficiency virus. Arch Intern Med. 1996;156:1029–1032. doi: 10.1001/archinte.156.9.1029. [DOI] [PubMed] [Google Scholar]
- 18.Eue I, Zeisig R, Arndt D. Alkylphosphocholine-induced production of nitric oxide and tumor necrosis factor alpha by U937 cells. J Cancer Res Clin Oncol. 1995;121:350–356. doi: 10.1007/BF01225687. [DOI] [PubMed] [Google Scholar]
- 19.Faraut-Gambarelli, F., R. Piarroux, M. Deniau, B. Giusiano, P. Marty, M. Michel, B. Faugere, and H. Dumon. 1997. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate, abstr. 408. Acta Parasitol. Turcica 21(Suppl. 1):188. [DOI] [PMC free article] [PubMed]
- 20.Fleer E A, Berkovic D, Eibl H, Unger C. Investigations on the cellular uptake of hexadecylphosphocholine. Lipids. 1993;28:731–736. doi: 10.1007/BF02535995. [DOI] [PubMed] [Google Scholar]
- 21.Geilen C C, Wieder T, Haase A, Reutter W, Morre D M, Morre D J. Uptake, subcellular distribution and metabolism of the phospholipid analogue hexadecylphosphocholine in MDCK cells. Biochim Biophys Acta. 1994;1211:14–22. doi: 10.1016/0005-2760(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 22.Gradoni L. Chemotherapy of leishmaniasis and trypanosomiasis. Curr Opin Infect Dis. 1996;9:435–438. [Google Scholar]
- 23.Hochhuth C H, Vehmeyer K, Eibl H, Unger C. Hexadecylphosphocholine induces interferon-gamma secretion and expression of GM-CSF mRNA in human mononuclear cells. Cell Immunol. 1992;141:161–168. doi: 10.1016/0008-8749(92)90135-c. [DOI] [PubMed] [Google Scholar]
- 24.Jeromino S M B, Oliveira R M, Mackay S, Costa R M, Sweet J, Nascimento E T, Luz K G, Fernandes M Z, Jernigan J, Pearson R D. An urban outbreak of visceral leishmaniasis in Natal, Brazil. Trans R Soc Trop Med Hyg. 1994;88:386–388. doi: 10.1016/0035-9203(94)90393-x. [DOI] [PubMed] [Google Scholar]
- 25.Kharazmi, A., M. Chen, L. Zhai, S. F. Nielsen, and S. B. Christensen. 1997. Discovery and development of oxygenated Chalcones A5 potential novel antileishmanial agents, abstr. 371. Acta Parasitol. Turcica 21(Suppl. 1):176.
- 26.Kuhlencord A, Maniera T, Eibl H, Unger C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob Agents Chemother. 1992;36:1630–1634. doi: 10.1128/aac.36.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laguna F, Torre-Cisneros J, Moreno V, Villanueva J L, Valencia E. Efficacy of intermittent liposomal amphotericin B in the treatment of visceral leishmaniasis in patients infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:711–712. doi: 10.1093/clinids/21.3.711. [DOI] [PubMed] [Google Scholar]
- 28.Maly K, Uberall F, Schubert C, Kindler E, Stekar J, Brachwitz H, Grunicke H H. Interference of new alkylphospholipid analogues with mitogenic signal transduction. Anti-Cancer Drug Des. 1995;10:411–425. [PubMed] [Google Scholar]
- 29.Marschner N, Kotting J, Eibl H, Unger C. Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother Pharmacol. 1992;31:18–22. doi: 10.1007/BF00695989. [DOI] [PubMed] [Google Scholar]
- 30.McBride M O, Linney M, Davidson R N, Weber J N. Pancreatic necrosis following treatment of leishmaniasis with sodium stibogluconate. Clin Infect Dis. 1995;21:710. doi: 10.1093/clinids/21.3.710. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 31.Mullen, A., A. J. Baillie, O’Grady, and K. C. Carter. 1997. Comparison of the efficacy of a non-ionic surfactant vesicular formulation of sodium stibogluconate (SSG) in a hamster model of visceral leishmaniasis, abstr. 402. Acta Parasitol. Turcica 21(Suppl. 1):186.
- 32.Murray H W, Masur H, Keithly J S. Cell-mediated immune response in experimental visceral leishmaniasis. Correlation between resistance to L. donovani and lymphokine-generating capacity. J Immunol. 1982;129:344–349. [PubMed] [Google Scholar]
- 33.Pearson R D, de Queiroz Sousa A. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1995;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Peters B S, Fish D, Golden R, Evans D A, Bryceson A D M, Pinching A J. Visceral leishmaniasis in HIV infection and AIDS: clinical features and response to therapy. Q J Med. 1990;77:1101–1111. doi: 10.1093/qjmed/77.2.1101. [DOI] [PubMed] [Google Scholar]
- 35.Planting A S, Stoter G, Verweij J. Phase II study of daily oral miltefosine (hexadecylphosphocholine) in advanced colorectal cancer. Eur J Cancer. 1993;29:518–519. doi: 10.1016/s0959-8049(05)80142-x. [DOI] [PubMed] [Google Scholar]
- 36.Pronk L C, Planting A S, Oosterom R, Drogendijk T E, Stoter G, Verweij J. Increases in leucocyte and platelet counts induced by the alkylphospholipid hexadecylphosphocholine. Eur J Cancer. 1994;30:1019–1022. doi: 10.1016/0959-8049(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 37.Reitz R C, Kotting J, Unger C, Eibl H. Comparison of the tissue distribution of hexadecylphosphocholine and erucylphosphocholine. Prog Exp Tumor Res. 1992;34:143–152. doi: 10.1159/000420839. [DOI] [PubMed] [Google Scholar]
- 38.Rousseau D, Le Fichoux Y, Stien X, Suffia I, Ferrua B, Kubar J. Progression of visceral leishmaniasis due to Leishmania infantum in BALB/c mice is markedly slowed by prior infection with Trichinella spiralis. Infect Immun. 1997;65:4978–4983. doi: 10.1128/iai.65.12.4978-4983.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo R, Nigro L C, Minniti S, Montineri A, Gradoni L, Caldeira L, Davidson R N. Visceral leishmaniasis in HIV infected patients: treatment with high dose liposomal amphotericin B (AmBisome) J Infect. 1996;32:133–137. doi: 10.1016/s0163-4453(96)91343-2. [DOI] [PubMed] [Google Scholar]
- 40.Stauber L A. Host resistance to the Khartoum strain of Leishmania donovani. Rice Inst Pamphlets. 1958;45:80–83. [Google Scholar]
- 41.Steelant W F, Bruyneel E A, Mareel M M, Van den Eeckhout E G. Capillary gas chromatography of hexadecylphosphocholine in Caco-2T cells and cell culture media. Anal Biochem. 1995;227:246–250. doi: 10.1006/abio.1995.1277. [DOI] [PubMed] [Google Scholar]
- 42.Suffia I, Quaranta J F, Eulalio M C, Ferrua B, Marty P, Le Fichoux Y, Kubar J. Human T-cell activation by 14- and 18-kilodalton nuclear proteins of Leishmania infantum. Infect Immun. 1995;63:3765–3771. doi: 10.1128/iai.63.10.3765-3771.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundar S, Murray H W. Cure of antimony-unresponsive Indian visceral leishmaniasis with amphotericin B lipid complex. J Infect Dis. 1996;173:762–765. doi: 10.1093/infdis/173.3.762. [DOI] [PubMed] [Google Scholar]
- 44.Sundar, S., G. Horwith, and H. W. Murray. 1997. Cure of antimony-refractory Indian kala-azar with short-course, low-dose intravenous Amphotericin B Lipid Complex (AbLC) therapy, abstr. 375. Acta Parasitol. Turcica 21(Suppl. 1):177.
- 45.Thakur C P, Pandey A K, Sinha G P, Roy S, Behbehani K, Olliaro P. Comparison of three treatment regimens with liposomal amphotericin B (AmBisome®) for visceral leishmaniasis in India: a randomized dose finding study. Trans R Soc Trop Med Hyg. 1996;90:319–322. doi: 10.1016/s0035-9203(96)90271-0. [DOI] [PubMed] [Google Scholar]
- 46.Theischen M, Bornfeld N, Becher R, Kellner U, Wessing A. Hexadecylphosphocholine may produce reversible functional defects of the retinal pigment epithelium. Ger J Ophthalmol. 1993;2:113–115. [PubMed] [Google Scholar]
- 47.Torres-Santos, E. C., D. L. Moreira, M. A. C. Kaplan, and B. Rossi-Bergmann. 1997. A chalcone isolated from the plant Piper aduncum is selectively toxic for Leishmania, abstr. 390. Acta Parasitol. Turcica 21(Suppl. 1):177–178.
- 48.Vehmeyer K, Scheurich P, Eibl H, Unger C. Hexadecylphosphocholine-mediated enhancement of T-cell response to interleukin-2. Cell Immunol. 1991;137:232–238. doi: 10.1016/0008-8749(91)90072-j. [DOI] [PubMed] [Google Scholar]
- 49.Verweij J, Planting A, Van der Burg M, Stoter G. A dose-finding study of miltefosine (hexadecylphosphocholine) in patients with metastatic solid tumours. J Cancer Res Clin Oncol. 1992;118:606–608. doi: 10.1007/BF01211805. [DOI] [PubMed] [Google Scholar]
- 50.Wieder T, Haase A, Geilen C C, Orfanos C E. The effect of two synthetic phospholipids on cell proliferation and phosphatidylcholine biosynthesis in Madin-Darby canine kidney cells. Lipids. 1995;30:389–393. doi: 10.1007/BF02536296. [DOI] [PubMed] [Google Scholar]
- 51.Wilson M E, Sandor M, Blum A M, Young B M, Metwali A, Elliott D, Lynch R G, Weinstock J V. Local suppression of IFNγ in hepatic granulomas correlates with tissue-specific replication of Leishmania chagasi. J Immunol. 1996;156:2231–2239. [PubMed] [Google Scholar]
- 52.World Health Organization. Antimonials: large-scale failure in leishmaniasis “alarming.”. Trop Dis Res News. 1990;34:1. , 7. [Google Scholar]
- 53.World Health Organization. AIDS, leishmaniasis dangers of clash highlighted. Trop Dis Res News. 1991;36:1. , 11. [Google Scholar]
- 54.Zeisig R, Eue I, Kosch M, Fichtner I, Arndt D. Preparation and properties of sterically stabilized hexadecylphosphocholine (miltefosine)-liposomes and influence of this modification on macrophage activation. Biochim Biophys Acta. 1996;1283:177–184. doi: 10.1016/0005-2736(96)00090-9. [DOI] [PubMed] [Google Scholar]
- 55.Zeisig R, Rudolf M, Eue I, Arndt D. Influence of hexadecylphosphocholine on the release of tumor necrosis factor and nitroxide from peritoneal macrophages in vitro. J Cancer Res Clin Oncol. 1995;121:69–75. doi: 10.1007/BF01202215. [DOI] [PubMed] [Google Scholar]
- 56.Zijlstra E E, El-Hassan A M, Ismael A, Ghalib H W. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1994;51:826–836. doi: 10.4269/ajtmh.1994.51.826. [DOI] [PubMed] [Google Scholar]