Abstract
Background
Muscimol’s quick onset and GABAergic properties make it a promising candidate for the treatment of pain. This systematic review and meta-analysis of preclinical studies aimed at summarizing the evidence regarding the efficacy of muscimol administration in the amelioration of nerve injury-related neuropathic pain.
Methods
Two independent researchers performed the screening process in Medline, Embase, Scopus and Web of Science extracting data were extracted into a checklist designed according to the PRISMA guideline. A standardized mean difference (SMD [95% confidence interval]) was calculated for each. To assess the heterogeneity between studies, I2 and chi-square tests were utilized. In the case of heterogeneity, meta-regression and subgroup analyses were performed to identify the potential source.
Results
Twenty-two articles met the inclusion criteria. Pooled data analysis showed that the administration of muscimol during the peak effect causes a significant reduction in mechanical allodynia (SMD = 1.78 [1.45–2.11]; P < 0.0001; I2 = 72.70%), mechanical hyperalgesia (SMD = 1.62 [1.28–1.96]; P < 0.0001; I2 = 40.66%), and thermal hyperalgesia (SMD = 2.59 [1.79–3.39]; P < 0.0001; I2 = 80.33%). This significant amendment of pain was observed at a declining rate from 15 minutes to at least 180 minutes post-treatment in mechanical allodynia and mechanical hyperalgesia, and up to 30 minutes in thermal hyperalgesia (P < 0 .0001).
Conclusions
Muscimol is effective in the amelioration of mechanical allodynia, mechanical hyperalgesia, and thermal hyperalgesia, exerting its analgesic effects 15 minutes after administration for up to at least 3 hours.
Keywords: Analgesia, Gamma-Aminobutyric Acid, Hyperalgesia, Meta-Analysis, Muscimol, Neuralgia, Pain, Peripheral Nerve Injuries, Spinal Cord Injuries
INTRODUCTION
Pain refers to the unpleasant emotional and sensory experience generated by noxious stimuli. It is a complex and multifaceted experience, and the most common symptomatic complaint in medicine [1]. The classifications of pain exhibit variations in scientific literature, leading to different estimates of prevalence, and treatment strategies [2–4]. Chronic pain is one of the most debilitating complications of trauma to the nervous system with a prevalence of around 68% in people with spinal cord injuries [5].
The International Association for the Study of Pain describes chronic neuropathic pain (NP) as “chronic pain caused by a lesion or disease of the somatosensory nervous system” [6,7]. NP is categorized into central and peripheral, with recent evidence suggesting that a majority of patients with traumatic nerve injuries are affected [8–10]. This type of pain is extremely hard to treat due to its complex and heterogeneous etiologies. NP is often severe and resistant to treatment, making management challenging for clinicians [11–13]. In light of that, it should be noted that the current management strategies express moderate efficacy, leading to low quality of life and high costs of care [14]. While acetaminophen, nonsteroidal anti-inflammatory drugs, and opioids have traditionally been the go-to medications for pain management, there has been an urgent need for safer and more effective alternatives mostly due to the side effects that limit their use, including the high potential of addiction and tolerance [15]. In recent years, remedies such as some derivatives of mushrooms have emerged as promising sources of analgesics. Muscimol, a compound found in the Amanita muscaria mushroom, has been identified as having analgesic properties because of its ability to activate gamma-aminobutyric acid (GABA) receptors [16].
GABAA receptors are ligand-gated ion channels that mediate the majority of inhibitory nerve transmission in the central nervous system. It is believed that by binding selectively to the GABAA receptors at the same site as GABA, muscimol increases GABA’s affinity for the receptor, which enhances neuronal inhibition and causes a subsequent reduction in pain sensation [17]. A hypothesis put forth was thatmuscimol demonstrated greater efficacy than GABA, producing approximately 120%–140% of GABA’s maximal efficacy [18].
Along with its analgesic properties, muscimol has been found to possess antioxidant and anti-inflammatory effects [19]. Moreover, another key advantage of muscimol as a potentialpain medication is its relatively short half-life. The effect of muscimol peaks around 3 hours after administration [20]. This demonstrates that muscimol is rapidly metabolized and excreted from the body, reducing the risk of accumulation and toxicity.
Muscimol’s quick onset and GABAergic properties make it a promising candidate for the management of pain. Its ability to selectively bind to specific GABAA receptor subtypes may also provide opportunities for the development of more targeted pain therapies with fewer side effects. Although muscimol has shown promising properties for alleviating pain, different studies have yielded variable results and conclusions, highlighting the need for a systematic review. The primary objective of this systematic review and meta-analysis is to gain a comprehensive understanding of muscimol’s potential as a treatment for alleviating nerve injury-related NP.
MATERIALS AND METHODS
1. Study design and search strategy
The present systematic review and meta-analysis aimed at summarizing the evidence regarding the efficacy of muscimol administration in the amelioration of nerve injury-related NP. For this purpose, the keywords related to muscimol, pain, and nerve injury were selected from a comprehensive search in the MeSH database of Medline, Emtree of Embase, and recommendations from experts in the field. The keywords were assembled in a search strategy designed exclusively for each database with appropriate tags and Boolean operators. An extensive search was conducted in Medline, Embase, Scopus, and Web of Science by May 1, 2023, to find related articles. Also, a manual search in the grey literature (Google and Google Scholar) was directed to avoid missing any articles. Table 1 presents our search strategies in each database.
Table 1.
Quality assessment of the included articles
| Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Overall | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dias | 2016 | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Low |
| Gwak | 2016 | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Low |
| Hama | 2012 | Unclear | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Low |
| Hosseini | 2014 | Yes | Yes | No | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Low |
| Hosseini | 2020 | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Low |
| Hwang | 1997 | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Low |
| Jeon | 2006 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Low |
| Jiang | 2014 | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Low |
| LaGraize | 2007 | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Low |
| Lee | 2010 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Low |
| Lee | 2015 | Unclear | Yes | Unclear | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Low |
| Moon | 2016 | Unclear | Yes | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Low |
| Moon | 2017 | Unclear | Yes | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | Low |
| Nasirinezhad | 2019 | Unclear | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes | Yes | Low |
| Pedersen | 2007 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Low |
| Rashid | 2002 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Low |
| Rode | 2005 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Low |
| Sadeghi | 2021 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Low |
| Seno | 2018 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Low |
| Wei | 2009 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Low |
| Yowtak | 2013 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Low |
| Zarrindast | 2001 | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Low |
1. Was the allocation sequence adequately generated and applied?
2. Were the groups similar at baseline or were they adjusted for confounders in the analysis?
3. Was the allocation adequately concealed?
4. Were the animals randomly housed during the experiment?
5. Were the caregivers and/or investigators blinded from knowledge which intervention each animal received during the experiment?
6. Were animals selected at random for outcome assessment?
7. Was the outcome assessor blinded?
8. Were incomplete outcome data adequately addressed?
9. Are reports of the study free of selective outcome reporting?
10. Was the study apparently free of other problems that could result in high risk of bias?
2. Selection criteria
PICO in this study was defined as Population (P) being animals with nerve injury-associated NP, Intervention (I) being the administration of muscimol, Comparison (C) being made with a control group, and Outcomes (O) being alterations in different scales of NP measurements. Review studies, studies without a traumatic nerve injury induction method, studies evaluating chemically-induced or inflammatory pain, studies not reporting a desired outcome, studies that did not use muscimol, studies without a valid control group, studies without an immediate post-intervention follow-up evaluation, and abstracts were excluded.
3. Data collection and quality assessment
The results of the systematic search were integrated into the Endnote 20.0 software and duplicate records were removed. In the initial screening process, two independent researchers screened the titles and abstracts of all obtained articles. If an article was considered potentially relevant, the full text was attained and all full texts were reviewed in the secondary screening process. By implementing the inclusion criteria, the final included articles were selected. If an article’s full text was unavailable, we contacted the corresponding author at least twice by email. If an article was in a language other than English, it was translated by a researcher fluent in both languages. The data from the included articles were extracted into a checklist designed based on the PRISMA guideline. Data included information regarding the study’s first author, year of publication, studied animals’ characteristics, nerve injury method, time interval to muscimol administration, muscimol dose, route of muscimol administration, assessment timelines, assessment sites for pain detection, and the outcome tests.
The quality of the included studies was evaluated based on the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE)’s risk of bias assessment tool. This tool evaluates the overall methodology and potential risk of bias in pre-clinical studies by answering the questions in 10 major domains. In general, the adequate generation, blinding, and application of the allocation sequence, the blinding of the research conductors, caregivers, and outcome assessors, the avoidance of selective outcome reporting, and random housing and outcome assessments are investigated. In the case of a disagreement, the dispute was resolved through discussions with a third researcher.
4. Certainty of evidence
The certainty of the evidence was assessed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework [21].
5. Statistical analyses
Statistical analyses were done using STATA 17.0 (StataCorp LLC). The included studies were classified based on the reported outcome. A standardized mean difference (SMD) with a 95% confidence interval (95% CI) was calculated for each sample and they were pooled to calculate an overall effect size. If a study used a scale in which a higher efficacy was observed with a lower score on the index scale, the absolute SMD value was inserted into the analysis. It should be noted that meta-analysis was only performed if data were reported by at least three separate analyses. A Galbraith plot was used to assess outlier studies. If we observed an outlier in a reported outcome, we did not include the data in the pooled analysis. A random or fixed effect model was chosen based on the presence or absence of heterogeneity. To assess the heterogeneity between studies, I2 and chi-square tests were utilized. In the case of heterogeneity, meta-regression was performed to identify the potential source. Additionally, publication bias was reported with a Funnel Plot using Egger’s test.
RESULTS
1. Study characteristics and flow
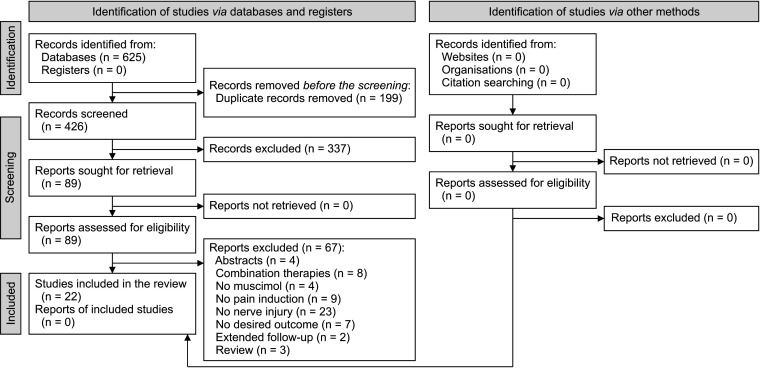
Finally, the data from 22 articles were included in the present meta-analysis (Fig. 1) [22–43]. Nineteen articles employed rats and 3 articles used mice. Nine studies used the chronic compression injury model and 5 studies carried out sciatic nerve injury/sciatic nerve ligation (SNL) for pain induction. Pain induction was established by spinal cord injury (SCI) in 6 studies. One study used a caudal trunk nerve cut to cause pain and another study induced pain during two separate experiments of SNL and SNL + SCI models.
Fig. 1.
PRISMA flow diagram of the article selection process.
The administered doses in the included studies ranged from 0.1 ng to 450,000 ng. In 12 studies, the administered dose was less than or equal to 100 ng in at least one experiment. Noticeably, the range of administered doses varied greatly. Therefore, the dose was entered into the analysis in the logarithm of 10. The method of administration was intrathecal in 12 studies, inside the brain nuclei in 7 studies, intraperitoneal in 2 studies, subcutaneous in one, and intraplantar in one. Mechanical allodynia was the investigated outcome in 21 studies, mechanical hyperalgesia in 5 studies, and thermal hyperalgesia in 2 studies. Table 2 shows a summary of the included articles.
Table 2.
Characteristics of the included studies
| Study | Animal species, sex, weight (g) | Model | Injury to muscimol administration (days) | Dose (ng) | Administration site | Treatment to pain | Pain type | N Treateda |
N Non-treated |
|---|---|---|---|---|---|---|---|---|---|
| Dias and Prado [39] | Rat, Wistar, M, 140–160 | SNL, SNL and SCI | 2, 7 | 300 | Intrathecal | 15, 30, 60, 90 | Mechanical allodynia | 5 | 5 |
| Gwak et al. [40] | Rat, SD, M, 200–250 | SCI | 21 | 1,000 | Intrathecal | 30, 120, 180 | Mechanical allodynia | 5 | 5 |
| Hama and Sagen [22] | Rat, SD, M, 100–150 | SCI | 21 | 0.1, 0.3, 1, 3 | Intrathecal | 30, 60, 90, 120 | Mechanical allodynia | 7 | 7 |
| Hosseini et al. [23] | Rat, Wistar, M, 140–160 | SCI | 21 | 10, 100, 1,000 | Intrathecal | 15, 60, 180 | Thermal hyperalgesia, mechanical hyperalgesia, mechanical allodynia | 10 | 10 |
| Hosseini et al. [24] | Rat, Wistar, M, 140–160 | SCI | 24 | 10 | Intrathecal | 15, 60 | Mechanical allodynia, mechanical hyperalgesia | 10 | 10 |
| Hwang and Yaksh [41] | Rat, SD, M, 120–150 | SNL | 7 | 100, 300, 1,000 | Intrathecal | 15, 30, 45, 60, 120, 180 | Mechanical allodynia | 5-6 | 5-6 |
| Jeon et al. [25] | Rat, SD, N/R, 200–250 | CCI | 14 | 100, 300, 1,000, 1.71, 3.42, 1,711 | Intrathecal, Intraplantar | 15, 30, 45, 60, 75, 90, 105, 120 | Mechanical allodynia | 6 | 6 |
| Jiang et al. [31] | Rat, SD, M, 150–180 | SNL | 7 | 25 | Intra-CeA | 30 | Mechanical allodynia | 14 | 14 |
| LaGraize and Fuchs [42] | Rat, SD, M, 3–4 mo | SNL | 3 | 1, 100, 500 | Intra rostral anterior cingulate cortex | 35 | Mechanical allodynia | 8 | 10 |
| Lee et al. [26] | Rat, SD, M, 150–200 | Caudal trunk nerve cut | 14 | 1,000 | Intrathecal | 30 | Mechanical allodynia | 7 | 5 |
| Lee et al. [27] | Rat, SD, M, 180–200 | CCI | 7 | 570 | Intrathecal | 30, 60, 90, 120, 180 | Mechanical allodynia | 8 | 10 |
| Moon et al. [28] | Rat, SD, M, 250–300 | SCI | 14 | 570, 1,140, 1,700 | ZI | 60 | Mechanical hyperalgesia | 10 | 10 |
| Moon and Park [29] | Rat, SD, M, 250–300 | CCI | 10 | 285, 2,830 | ZI | 120 | Mechanical allodynia | 10 | 10 |
| Nasirinezhad et al. [43] | Rat, Wistar, M, 140–160 | SCI | 24 | 10, 100, 1,000 | Intrathecal | 15, 60, 180 | Mechanical hyperalgesia, thermal hyperalgesia, mechanical allodynia | 8 | 8 |
| Pedersen et al. [30] | Rat, SD, M, 180–200 | CCI | 14 | 20, 50 | CeA | 30 | Mechanical allodynia, mechanical hyperalgesia | 8 | 6 |
| Rashid and Ueda [32] | Mice, ddY, M, 25–30 | CCI | 0 | 3.42, 11.4, 34.2 | Intrathecal | 60 | Mechanical allodynia | 6 | 6 |
| Rode et al. [33] | Rat, SD, M, 250 | SNI | 0 | Sub-cutaneous | 30, 60, 90, 120, 150 | Mechanical allodynia, mechanical hyperalgesia | 6 | 6 | |
| Sadeghi et al. [34] | Rat, Wistar, M, 200–250 | CCI | 14 | 112,500, 225,000, 450,000 | Intra-peritoneal | 30 | Thermal hyperalgesia, mechanical allodynia | 8 | 8 |
| Seno et al. [35] | Rat, Wistar, M, 180–200 | CCI | 14 | 500 | CeA, BLA | 30 | Mechanical hyperalgesia, mechanical allodynia | 8 | 5 |
| Wei et al. [36] | Rat, Hannover-Wistar, M, 180–250 | CCI | 4 | 100, 300 | Hypothalamus A11 | 15, 30, 60 | Mechanical allodynia, mechanical hyperalgesia, thermal hyperalgesia | 5-6 | 6 |
| Yowtak et al. [37] | Mice, GAD67-EGFP, M, N/R | CCI | 4 | 50, 100 | Intrathecal | 30, 60, 90, 120 | Mechanical allodynia | 8 | 8 |
| Zarrindast and Mahmoudi [38] | Mice, albino NMRI, M, 20–25 | SNI | 14 | 10,000, 20,000, 40,000 | Intra-peritoneal | 75 | Thermal hyperalgesia | 8 | 8 |
SNL: sciatic nerve ligation, SCI: spinal cord injury, CCI: chronic compression injury, SNI: sciatic nerve injury, CeA: central nuclei of the amygdala, ZI: zona incerta, BLA: basolateral nuclei of the amygdala, N/R: not recorded.
aThe number of animals was reported as number of animals per each treated group. Some studies had several experimental groups according to dose of muscimol, site of administration and model of pain induction.
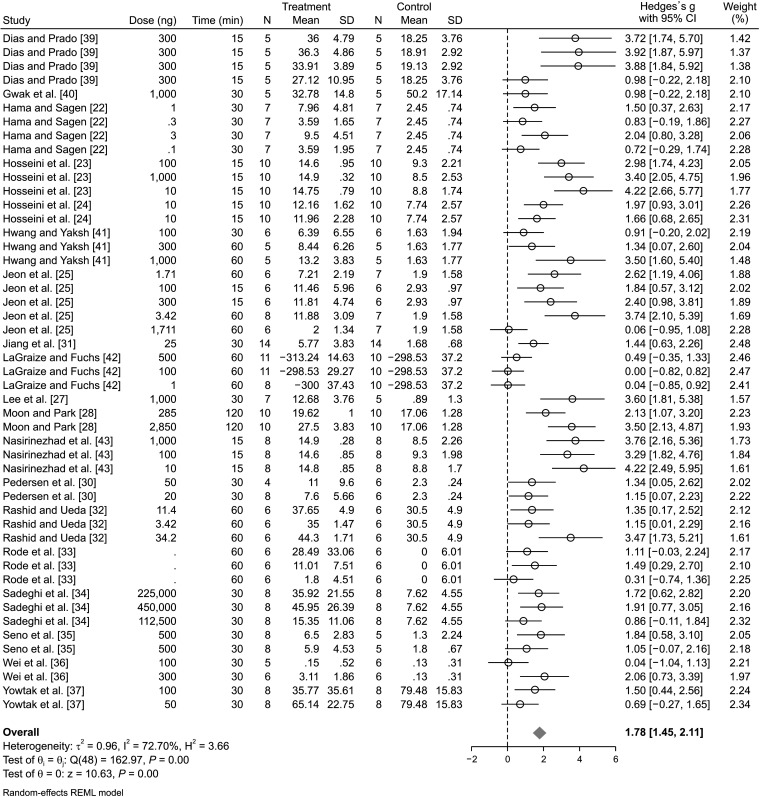
2. The effect of muscimol administration on mechanical allodynia
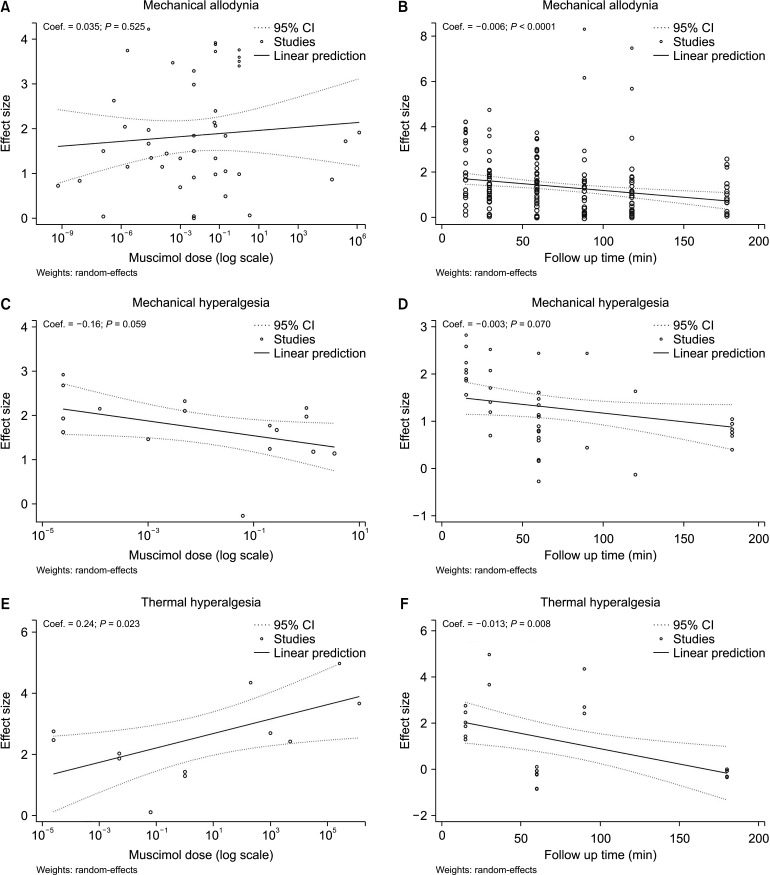
Data from 20 studies evaluated mechanical allodynia. Galbraith's plot demonstrated that 2 experiments were outliers. Therefore, Lee et al. [27] was omitted from the pooled analysis and data from 19 studies comprising 49 separate experiments were included in the present analysis. Pooled data analysis showed that the administration of muscimol during the peak effect caused a significant reduction in mechanical allodynia (SMD = 1.78; 95% CI: 1.45, 2.11, P < 0.0001; I2 = 72.70%) (Fig. 2). Subgroup analysis was performed to find the origin of the observed moderate heterogeneity. The analysis showed that the administration of muscimol ameliorated pain with a central origin (SMD = 2.47; 95% CI: 1.82, 3.11; P < 0.0001; I2 = 72.59%) and with a peripheral origin (SMD = 1.47; 95% CI: 1.13, 1.82; P < 0.0001; I2 = 66.05%). It was also found that the site of administration was not the source of heterogeneity. Moreover, different routes of administration including intrathecal (SMD = 2.18; 95% CI: 1.74, 2.62; P < 0.0001; I2 = 68.46%), intracerebral (SMD = 1.18; 95% CI: 0.61, 1.75; P < 0.0001; I2 = 71.94%), and systemic (SMD = 1.43; 95% CI: 0.75, 2.11; P < 0.0001; I2 = 67.80%) were all significantly effective in the amelioration of mechanical allodynia (Table 3). We conducted a meta-regression analysis to investigate the effect of the administered muscimol dose on its effectiveness in the amendment of mechanical allodynia. Meta-regression showed that the increase in dose had no significant effect on the efficacy of muscimol in the amelioration of this type of pain (Coef. = 0.035; 95% CI: –0.73, 0.14; P = 0.525). In other words, the evidence demonstrates that muscimol ameliorated mechanical allodynia in all reported doses (Fig. 3A). As an additional analysis, the effect of follow-up time on the efficacy of muscimol in mechanical allodynia was investigated. This analysis showed that mechanical allodynia was significantly improved 15 minutes after the treatment (SMD = 2.13, 95% CI: 1.58, 2.68; P < 0.0001; I2 = 75.02%) and lasted for up to 180 minutes (SMD = 1.00, 95% CI: 0.63, 1.37; P < 0.0001; I2 = 45.44%) (Table 3). Yet, the effectiveness of muscimol in mechanical allodynia decreases over time (Coef. = –0.006; 95% CI: –0.009, –0.003; P < 0.0001) (Fig. 3B). Since the amount of heterogeneity in some classes was reduced by performing this subgroup analysis, it seems that the cause of the observed heterogeneity was due to the difference in the follow-up time.
Fig. 2.
The effect of muscimol administration on nerve injury-related mechanical allodynia in observed peak effect time. SD: standard deviation, 95% CI: 95% confidence interval.
Table 3.
Subgroup analysis for the effect of muscimol on nerve injury-related neuropathic pain
| Subgroups | n experiments | SMD | 95% CI | P value | I2 (%) | P value | |
|---|---|---|---|---|---|---|---|
| Mechanical allodynia | |||||||
| Time of follow-up (min) | |||||||
| 15 | 22 | 2.13 | 1.58, 2.68 | 0.000 | 75.02 | < 0.0001 | |
| 30 | 33 | 1.44 | 1.15, 1.73 | 0.000 | 48.63 | < 0.0001 | |
| 60 | 46 | 1.49 | 1.20, 1.78 | 0.000 | 64.47 | < 0.0001 | |
| 90 | 26 | 1.03 | 0.63, 1.42 | 0.000 | 66.79 | < 0.0001 | |
| 120 | 28 | 0.99 | 0.62, 1.36 | 0.000 | 67.05 | < 0.0001 | |
| 180 | 15 | 1.00 | 0.63, 1.37 | 0.000 | 45.44 | 0.024 | |
| Pain origin | |||||||
| Central | 15 | 2.47 | 1.82, 3.11 | 0.000 | 72.59 | < 0.0001 | |
| Peripheral | 34 | 1.47 | 1.13, 1.82 | 0.000 | 66.05 | < 0.0001 | |
| Administration site | |||||||
| Intrathecal | 28 | 2.18 | 1.74, 2.62 | 0.000 | 68.46 | < 0.0001 | |
| Intracerebral | 12 | 1.18 | 0.61, 1.75 | 0.000 | 71.94 | < 0.0001 | |
| Systemic | 9 | 1.43 | 0.75, 2.11 | 0.000 | 67.80 | 0.003 | |
| Mechanical hyperalgesia | |||||||
| Time of follow-up (min) | |||||||
| 15 | 8 | 2.07 | 1.68, 2.46 | 0.000 | 0.00 | 0.850 | |
| 30 | 6 | 1.50 | 1.01, 1.99 | 0.000 | 1.23 | 0.389 | |
| 60 | 14 | 0.87 | 0.57, 1.16 | 0.000 | 23.95 | 0.118 | |
| 90 | 2 | Lack of sufficient data | |||||
| 120 | 2 | Lack of sufficient data | |||||
| 180 | 8 | 1.15 | 0.64, 1.66 | 0.000 | 49.55 | 0.050 | |
| Pain origin | |||||||
| Central | 11 | 1.79 | 1.47, 2.10 | 0.000 | 0.00 | 0.466 | |
| Peripheral | 7 | 1.27 | 0.58, 1.95 | 0.000 | 57.51 | 0.028 | |
| Administration site | |||||||
| Intrathecal | 8 | 2.07 | 1.68, 2.46 | 0.000 | 0.00 | 0.850 | |
| Intracerebral | 8 | 1.21 | 0.74, 1.67 | 0.000 | 35.25 | 0.142 | |
| Systemic | 2 | Lack of sufficient data | |||||
| Thermal hyperalgesia | |||||||
| Time of follow-up (min) | |||||||
| 15 | 6 | 1.87 | 1.43, 2.32 | 0.000 | 0.00 | 0.416 | |
| 30 | 3 | 4.74 | 3.32, 6.17 | 0.000 | 39.21 | 0.195 | |
| 60 | 7 | –0.31 | –0.66, 0.03 | 0.077 | 0.00 | 0.700 | |
| 90 | 2 | Lack of sufficient data | |||||
| 180 | 6 | –0.13 | –0.49, 0.23 | 0.478 | 0.00 | 0.989 | |
| Pain origin | |||||||
| Central | 6 | 1.87 | 1.43, 2.32 | 0.000 | 0.00 | 0.416 | |
| Peripheral | 7 | 3.33 | 1.87, 4.80 | 0.000 | 84.89 | < 0.0001 | |
| Administration site | |||||||
| Intrathecal | 6 | 1.87 | 1.43, 2.32 | 0.000 | 0.00 | 0.416 | |
| Intracerebral | 1 | Lack of sufficient data | |||||
| Systemic | 6 | 3.82 | 2.75, 4.90 | 0.000 | 61.01 | 0.029 | |
SMD: standardized mean difference, 95% CI: 95% confidence interval.
Fig. 3.
Meta-regression for the assessment of dose-effect and follow-up duration on muscimol efficacy in nerve injury-related mechanical allodynia (A, B), mechanical hyperalgesia (C, D), and thermal hyperalgesia (E, F). 95% CI: 95% confidence interval.
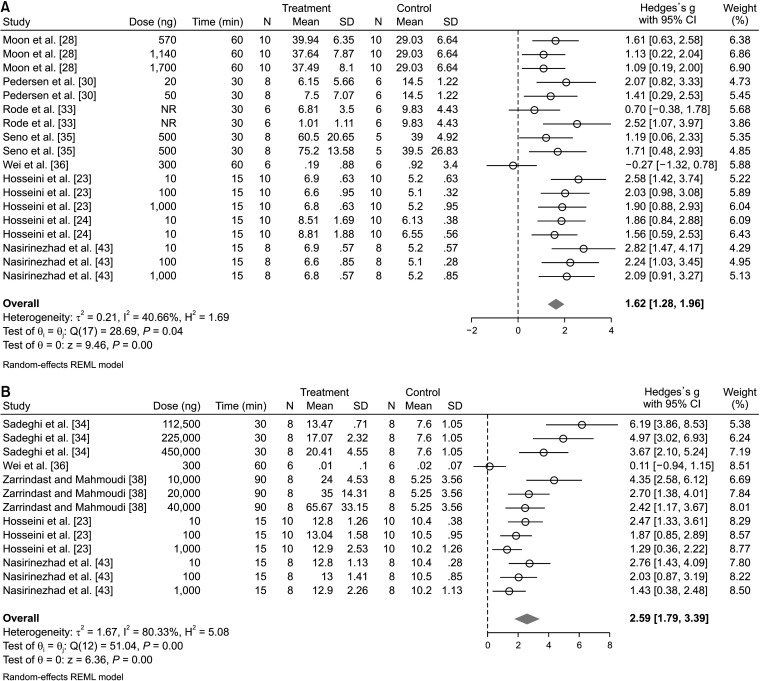
3. The effect of muscimol administration on mechanical hyperalgesia
In the assessment of the efficacy of muscimol in mechanical hyperalgesia, data from 8 articles and 18 separate analyses were included. Pooled analysis showed that muscimol significantly reduced mechanical hyperalgesia (SMD = 1.62; 95% CI: 1.28, 1.96; P < 0.0001; I2 = 40.66%) (Fig. 4A). Subgroup analysis showed that the administration of muscimol was effective both in pain with a central origin (SMD = 1.79; 95% CI: 1.47, 2.10; P < 0.0001; I2 = 0.00%) and in pain with a peripheral origin (SMD = 1.27; 95% CI: 0.58, 1.95; P < 0.0001; I2 = 57.51%). It was also found that the route of administration was not the source of heterogeneity. Both the intrathecal administration of muscimol (SMD = 2.07; 95% CI: 1.68, 2.46; P < 0.0001; I2 = 0.00%) and the intracerebral administration (SMD = 1.21; 95% CI: 0.74, 1.67; P < 0.0001; I2 = 35.25%) significantly improved mechanical hyperalgesia (Table 3). To investigate the effect of the administered muscimol dose on its efficacy in mechanical hyperalgesia, meta-regression was performed. Meta-regression showed that an increase in the administered dose had no meaningful effect on the effectiveness of muscimol in the amelioration of mechanical hyperalgesia (Coef. = –0.16; 95% CI: –0.33, 0.006; P = 0.059). In other words, the evidence shows that muscimol improved mechanical hyperalgesia in all reported doses (Fig. 3C). Moreover, the effect of follow-up time on the effectiveness of muscimol in mechanical hyperalgesia was investigated. This analysis showed that mechanical hyperalgesia was improved 15 minutes (SMD = 2.07, 95% CI: 1.68, 2.46; P < 0.0001; I2 = 0.00%) and up to 180 minutes after the administration of muscimol (SMD = 1.15, 95% CI: 0.64, 1.66; P < 0.0001; I2 = 49.55%) (Table 3). Although the effectiveness of muscimol decreases over time, this decrease was not statistically significant (Coef. = –0.003; 95% CI: –0.008, 0.003; P = 0.070) (Fig. 3D).
Fig. 4.
The effect of muscimol on nerve injury-related mechanical hyperalgesia (A) and thermal hyperalgesia (B) in the observed peak effect time. SD: standard deviation, 95% CI: 95% confidence interval.
4. The effect of muscimol administration on thermal hyperalgesia
Regarding the effect of muscimol on thermal hyperalgesia, data from 5 articles and 13 separate experiments were included. Pooled data analysis with high heterogeneity showed that muscimol significantly reduced thermal hyperalgesia (SMD = 2.59; 95% CI: 1.79, 3.39; P < 0.0001; I2 = 80.33%) (Fig. 4B). Subgroup analysis demonstrated that the administration of muscimol was effective both in pain of a central origin (SMD = 1.87; 95% CI: 1.43, 2.32; P < 0.0001; I2 = 0.00%) and in pain of a peripheral origin (SMD = 3.33; 95% CI: 1.87, 4.80; P < 0.0001; I2 = 84.89%). Since the amount of heterogeneity in pain with a central origin was equal to zero, the origin of pain may be one of the main causes of heterogeneity. Additionally, it was also found that the site of administration may also be a source of heterogeneity. Intrathecal muscimol administration (SMD = 1.87; 95% CI: 1.43, 2.32; P < 0.0001; I2 = 0.00%) and systemic administration (SMD = 3.82; 95% CI: 2.75, 4.90; P < 0.0001; I2 = 61.01%) were both effective in the amelioration of thermal hyperalgesia (Table 3). To investigate the effect of the administered dose on its efficacy in the improvement of thermal hyperalgesia, meta-regression was performed. Meta-regression showed that the efficacy of muscimol in thermal hyperalgesia increased with the dose (Coef. = 0.24; 95% CI: 0.033, 0.440; P = 0.023). In other words, the evidence shows that muscimol improved thermal hyperalgesia in all reported doses and this effect was dose-dependent (Fig. 3E). Moreover, the effect of follow-up time on the effectiveness of muscimol in thermal hyperalgesia was investigated. This analysis showed that thermal hyperalgesia was improved 15 minutes after the administration of muscimol (SMD = 1.87, 95% CI: 1.43, 2.32; P < 0.0001; I2 = 0.00%) and 30 minutes post-treatment (SMD = 4.74; 95% CI: 3.32, 6.17; P < 0.0001; I2 = 39.21%). Nevertheless, the administration of muscimol had no meaningful effect on the improvement of thermal hyperalgesia from 60 to 180 minutes post-treatment (Table 3). In other words, the effectiveness of muscimol on thermal hyperalgesia decreased over time (Coef. = –0.013; 95% CI: –0.023, –0.003; P = 0.008) (Fig. 3F).
5. Quality control and certainty of evidence
We evaluated the methodology and the overall risk of bias in our included pre-clinical studies according to SYRCLE’s risk of bias tool. The allocation sequence was adequately generated and applied in only two articles. All included articles used animals similar at baseline. The allocation concealment was clearly disclosed in 4 articles, and in one article no concealment was reported. Random housing during the experiment was reported in 4 articles. Investigators and outcome assessors were blinded in 4 and 8 articles, respectively. A random selection for outcome assessment was unclear in all articles, except for one in which no randomization was observed. There was no incomplete outcome data or other factors that could potentially cause bias. Conclusively, the overall quality of the included articles was considered low (Table 1).
In the assessment of the certainty of evidence based on the GRADE framework, the level of evidence was downrated one grade due to the serious risk of bias for all included outcomes. The overall level of evidence was considered moderate (Table 4).
Table 4.
The certainty of evidence based on the GRADE framework
| Outcome | Number of experiments | Risk of bias | Imprecision | Inconsistency | Indirectness | Publication bias | Judgment | Level of evidence |
|---|---|---|---|---|---|---|---|---|
| Mechanical allodynia | 49 | Serious | No serious imprecision | No serious inconsistencya | No serious indirectness | No publication bias | Level of evidence was downrated one grade due to possible risk of bias | Moderate |
| Mechanical hyperalgesia | 18 | Serious | No serious imprecision | No serious inconsistencya | No serious indirectness | No publication bias | Level of evidence was downrated one grade due to possible risk of bias | Moderate |
| Thermal hyperalgesia | 13 | Serious | No serious imprecision | No serious inconsistencya | No serious indirectness | No publication bias | Level of evidence was downrated one grade due to possible risk of bias | Moderate |
GRADE: Grading of Recommendations Assessment, Development, and Evaluation.
aThere is no serious inconsistency since the sources of heterogeneity were identified.
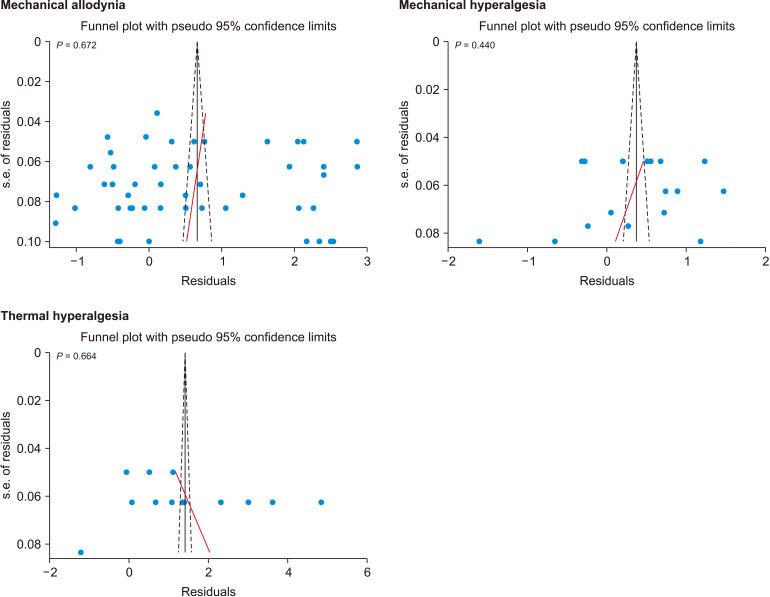
6. Publication bias
Egger’s test demonstrated that there was no publication bias in the reports of mechanical allodynia (P = 0.672), mechanical hyperalgesia (P = 0.440), and thermal hyperalgesia (P = 0.664) (Fig. 5).
Fig. 5.
Publication bias for assessment of muscimol on nerve injury-related neuropathic pain.
DISCUSSION
Our findings indicate that muscimol, an agonist for the GABAA receptor, was able to significantly alleviate pain in its peak effect, determined by the amelioration of behavioral responses to stimuli for mechanical allodynia, mechanical hyperalgesia, and thermal hyperalgesia. Although this efficacy is dose-independent in mechanical allodynia and mechanical hyperalgesia, the observed effect increases with dose in the evaluation of thermal hyperalgesia.
The underlying mechanisms of pain are fundamentally different from one another and their precise pathways are yet unknown [44]. It is believed that pain following nerve injury mostly incorporates the peripheral activation of previously non-nociceptive neurons into nociceptors, alterations in neuronal excitability in pain pathways, inflammation, axonal loss due to injury, and various subsequent dysfunctions of the supraspinal regions that are responsible for pain perception [45,46]. GABAergic neurons are important mediators of pain and therefore, dysregulation in their signaling has been shown to play a pivotal role in the development of pain [47].
Muscimol, a GABAA receptor agonist, exerts its analgesic effects through multiple pathways. For instance, this mushroom-derivative compound exhibits antioxidant properties that potentially halt the reactive oxygen species in inflammatory cascades of the injured tissue [48]. Moreover, current evidence suggests that muscimol improves the plasticity in the posterior horn of the spinal cord as the central terminal of the afferent pain pathways, which is affected by both central and peripheral nerve injuries [49,50].
In this literature review, we demonstrated that as a relatively short-acting GABA analog [51], muscimol begins to exert its analgesic effects 15 minutes after administration. While decreasing in efficacy, this effect lasts for up to 180 minutes in the improvement of mechanical allodynia and hyperalgesia. Conversely, muscimol seems to be effective in thermal hyperalgesia only from 15 to 30 minutes post-treatment. These findings are in alignment with previous evidence which suggests a different response to muscimol in thermal hyperalgesia than allodynia. Conspicuously, Sadeghi et al. [34] concluded that thermal hyperalgesia is more sensitive to muscimol than allodynia, while Hosseini et al. [24] disclosed that muscimol is effective in mechanical allodynia and hyperalgesia, but not effective in thermal hyperalgesia.
Moreover, we demonstrated that different routes of muscimol administration are all effective in the amelioration of both the peripheral and central origins of pain, which could be due to muscimol’s ability to cross the blood-brain barrier through an active transport system, and the vast distribution of its receptors throughout the nervous system [52–54]. However, it should be considered that this broad dispersion of the target receptors holds a liability for potential adverse effects [55].
A limitation of our study is the broad differences in the administered doses of muscimol. Although different routes of administration require different administration doses for optimal efficacy, this study demonstrated the need for a more comprehensive approach for selecting the muscimol administration route and dose in future prospective studies.
Also, it has recently been argued that due to the lack of concordance between guidelines for conducting preclinical studies and guidelines for their quality assessment, some domains might be at high risk of bias, solely due to the fact that the authors did not document them in their articles, even though those recommendations might have been followed during the experiment [56]. The overall level of evidence was downrated only due to the serious risk of bias. Therefore, the advancement of research into clinical trials could be taken into consideration.
Moreover, although chronic NP is most often considered to be simultaneous and non-evoked in humans, evoked pain perception is the target of research in most preclinical studies [57]. Therefore, the findings of our study should be interpreted in terms of the potential efficacy of muscimol in the symptomatic management of NP for future clinical research [58].
Conclusively, muscimol is effective in the amelioration of mechanical allodynia, mechanical hyperalgesia, and thermal hyperalgesia. Muscimol exerts its analgesic effects 15 minutes after administration, and this effect is observed for up to at least 3 hours post-administration.
Funding Statement
FUNDING This study was supported by the Men's Health and Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Footnotes
DATA AVAILABILITY
The datasets supporting the finding of this study are available from the corresponding author upon reasonable request.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Hamzah Adel Ramawad: Data gathering, Manuscript drafting, Critical revision; Parsa Paridari: Data gathering, Manuscript drafting, Critical revision; Sajjad Jabermoradi: Data gathering, Manuscript drafting, Critical revision; Pantea Gharin: Data gathering, Critical revision; Amirmohammad Toloui: Data analysis, Manuscript drafting, Critical revision; Saeed Safari: Study design, Data analysis, Critical revision; Mahmoud Yousefifard: Study design, Data analysis, Manuscript drafting, Critical revision.
REFERENCES
- 1.Chen J, Kandle PF, Murray IV, Fitzgerald LA, Sehdev JS. Physiology, pain. StatPearls [Internet] StatPearls Publishing; 2023. Available at: https://www.ncbi.nlm.nih.gov/books/NBK539789/#article-26536.s1 . [PubMed] [Google Scholar]
- 2.Lee S, Zhao X, Hatch M, Chun S, Chang E. Central neuropathic pain in spinal cord injury. Crit Rev Phys Rehabil Med. 2013;25:159–72. doi: 10.1615/CritRevPhysRehabilMed.2013007944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norouzkhani N, Chaghian Arani R, Mehrabi H, Bagheri Toolaroud P, Ghorbani Vajargah P, Mollaei A, et al. Effect of virtual reality-based interventions on pain during wound care in burn patients; a systematic review and meta-analysis. Arch Acad Emerg Med. 2022;10:e84. doi: 10.22037/aaem.v10i1.1756.e1072ab378ed403eaaa9ba6b3ca3d017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farahmand Rad R, Zolfaghari Sadrabad A, Jafari M, Ghilian M. Efficacy of sumatriptan/placebo versus sumatriptan/propofol combination in acute migraine; a randomized clinical trial. Arch Acad Emerg Med. 2022;10:e27. doi: 10.22037/aaem.v10i1.1510.5ff51f38a2224882bc9c01d2351fdbd2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt C, Moman R, Peterson A, Wilson R, Covington S, Mustafa R, et al. Prevalence of chronic pain after spinal cord injury: a systematic review and meta-analysis. Reg Anesth Pain Med. 2021;46:328–36. doi: 10.1136/rapm-2020-101960. [DOI] [PubMed] [Google Scholar]
- 6.Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice ASC, et al. A new definition of neuropathic pain. Pain. 2011;152:2204–5. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG), author The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160:53–9. doi: 10.1097/j.pain.0000000000001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miclescu A, Straatmann A, Gkatziani P, Butler S, Karlsten R, Gordh T. Chronic neuropathic pain after traumatic peripheral nerve injuries in the upper extremity: prevalence, demographic and surgical determinants, impact on health and on pain medication. Scand J Pain. 2019;20:95–108. doi: 10.1515/sjpain-2019-0111. [DOI] [PubMed] [Google Scholar]
- 9.Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: a systematic review and meta-analysis. Eur J Pain. 2017;21:29–44. doi: 10.1002/ejp.905. [DOI] [PubMed] [Google Scholar]
- 10.Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal Cord. 2001;39:256–62. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- 11.Deng Y, Luo L, Hu Y, Fang K, Liu J. Clinical practice guidelines for the management of neuropathic pain: a systematic review. BMC Anesthesiol. 2016;16:12. doi: 10.1186/s12871-015-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, et al. A comprehensive algorithm for management of neuropathic pain. Pain Med. 2019;20(Suppl 1):S2–S12. doi: 10.1093/pm/pnz075. Erratum in: Pain Med 2023; 24: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernetti A, Agostini F, de Sire A, Mangone M, Tognolo L, Di Cesare A, et al. Neuropathic pain and rehabilitation: a systematic review of international guidelines. Diagnostics (Basel) 2021;11:74. doi: 10.3390/diagnostics11010074.6729c3e30fc94693b41efd430c69e1a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:2058738419838383. doi: 10.1177/2058738419838383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azizkhani R, Shahnazari Sani M, Heydari F, Saber M, Mousavi S. Topical lidocaine plus diclofenac as a local anesthetic agent in central venous catheterization; a randomized controlled clinical trial. Arch Acad Emerg Med. 2021;9:e63. doi: 10.22037/aaem.v9i1.1389.cb19b220d9224ce98741eea99dd7268c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinton T, Johnston GAR. GABA, the major inhibitory neurotransmitter in the brain. Reference Module in Biomedical Sciences. Elsevier; 2018. [DOI] [Google Scholar]
- 17.Duncan BR. Volatile anesthetics and O2 activate TASK-1/3 by weak H-bonding with X-gate uncharged Arg-245: the major molecular mechanism for carotid body hypoxic sensitivity and further insights into fighter pilot +Gz-induced LOC. OSF Preprints [Preprint] doi: 10.31219/osf.io/y7z6e. doi: 10.31219/osf.io/y7z6e. [DOI] [Google Scholar]
- 18.Johnston GA. Muscimol as an ionotropic GABA receptor agonist. Neurochem Res. 2014;39:1942–7. doi: 10.1007/s11064-014-1245-y. [DOI] [PubMed] [Google Scholar]
- 19.Li YH, Hsu DZ, Liu CT, Chandrasekaran VRM, Liu MY. The protective effect of muscimol against systemic inflammatory response in endotoxemic mice is independent of GABAergic and cholinergic receptors. Can J Physiol Pharmacol. 2022;100:665–78. doi: 10.1139/cjpp-2021-0682. [DOI] [PubMed] [Google Scholar]
- 20.Hollister LE. New class of hallucinogens: GABA-enhancing agents. Drug Dev Res. 1990;21:253–6. doi: 10.1002/ddr.430210311. [DOI] [Google Scholar]
- 21.Wei D, Tang K, Wang Q, Estill J, Yao L, Wang X, et al. The use of GRADE approach in systematic reviews of animal studies. J Evid Based Med. 2016;9:98–104. doi: 10.1111/jebm.12198. [DOI] [PubMed] [Google Scholar]
- 22.Hama A, Sagen J. Combinations of intrathecal gamma-amino-butyrate receptor agonists and N-methyl-d-aspartate receptor antagonists in rats with neuropathic spinal cord injury pain. Eur J Pharmacol. 2012;683:101–8. doi: 10.1016/j.ejphar.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosseini M, Karami Z, Janzadenh A, Jameie SB, Haji Mashhadi Z, Yousefifard M, et al. The effect of intrathecal administration of muscimol on modulation of neuropathic pain symptoms resulting from spinal cord injury; an experimental study. Emerg (Tehran) 2014;2:151–7.4ee21a8dfbaa4a3c8d65e82aed0b62be [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseini M, Karami Z, Yousefifard M, Janzadeh A, Zamani E, Nasirinezhad F. Simultaneous intrathecal injection of muscimol and endomorphin-1 alleviates neuropathic pain in rat model of spinal cord injury. Brain Behav. 2020;10:e01576. doi: 10.1002/brb3.1576.428fb3158c4744ccbeed3359cffc6c4d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon YH, Yoon DM, Nam TS, Leem JW, Paik GS. Spinal and peripheral GABA-A and B receptor agonists for the alleviation of mechanical hypersensitivity following compressive nerve injury in the rat. Korean J Pain. 2006;19:22–32. doi: 10.3344/kjp.2006.19.1.22. [DOI] [Google Scholar]
- 26.Lee J, Back SK, Lim EJ, Cho GC, Kim MA, Kim HJ, et al. Are spinal GABAergic elements related to the manifestation of neuropathic pain in rat? Korean J Physiol Pharmacol. 2010;14:59–69. doi: 10.4196/kjpp.2010.14.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MC, Nam TS, Jung SJ, Gwak YS, Leem JW. Modulation of spinal GABAergic inhibition and mechanical hypersensitivity following chronic compression of dorsal root ganglion in the rat. Neural Plast. 2015;2015:924728. doi: 10.1155/2015/924728.ccf3fe1741d145a0b49f4cd16d46895d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon HC, Lee YJ, Cho CB, Park YS. Suppressed GABAergic signaling in the zona incerta causes neuropathic pain in a thoracic hemisection spinal cord injury rat model. Neurosci Lett. 2016;632:55–61. doi: 10.1016/j.neulet.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Moon HC, Park YS. Reduced GABAergic neuronal activity in zona incerta causes neuropathic pain in a rat sciatic nerve chronic constriction injury model. J Pain Res. 2017;10:1125–34. doi: 10.2147/JPR.S131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen LH, Scheel-Krüger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain. 2007;127:17–26. doi: 10.1016/j.pain.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, Fang D, Kong LY, Jin ZR, Cai J, Kang XJ, et al. Sensitization of neurons in the central nucleus of the amygdala via the decreased GABAergic inhibition contributes to the development of neuropathic pain-related anxiety-like behaviors in rats. Mol Brain. 2014;7:72. doi: 10.1186/s13041-014-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashid MH, Ueda H. Neuropathy-specific analgesic action of intrathecal nicotinic agonists and its spinal GABA-mediated mechanism. Brain Res. 2002;953:53–62. doi: 10.1016/S0006-8993(02)03270-5. [DOI] [PubMed] [Google Scholar]
- 33.Rode F, Jensen DG, Blackburn-Munro G, Bjerrum OJ. Centrally-mediated antinociceptive actions of GABA(A) receptor agonists in the rat spared nerve injury model of neuropathic pain. Eur J Pharmacol. 2005;516:131–8. doi: 10.1016/j.ejphar.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi M, Manaheji H, Zaringhalam J, Haghparast A, Nazemi S, Bahari Z, et al. Evaluation of the GABAA receptor expression and the effects of muscimol on the activity of wide dynamic range neurons following chronic constriction injury of sciatic nerve in rats. Basic Clin Neurosci. 2021;12:651–66. doi: 10.32598/bcn.2021.1726.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seno MDJ, Assis DV, Gouveia F, Antunes GF, Kuroki M, Oliveira CC, et al. The critical role of amygdala subnuclei in nociceptive and depressive-like behaviors in peripheral neuropathy. Sci Rep. 2018;8:13608. doi: 10.1038/s41598-018-31962-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei H, Viisanen H, Pertovaara A. Descending modulation of neuropathic hypersensitivity by dopamine D2 receptors in or adjacent to the hypothalamic A11 cell group. Pharmacol Res. 2009;59:355–63. doi: 10.1016/j.phrs.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Yowtak J, Wang J, Kim HY, Lu Y, Chung K, Chung JM. Effect of antioxidant treatment on spinal GABA neurons in a neuropathic pain model in the mouse. Pain. 2013;154:2469–76. doi: 10.1016/j.pain.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarrindast MR, Mahmoudi M. GABA mechanisms and antinociception in mice with ligated sciatic nerve. Pharmacol Toxicol. 2001;89:79–84. doi: 10.1034/j.1600-0773.2001.d01-139.x. [DOI] [PubMed] [Google Scholar]
- 39.Dias QM, Prado WA. The lesion of dorsolateral funiculus changes the antiallodynic effect of the intrathecal muscimol and baclofen in distinct phases of neuropathic pain induced by spinal nerve ligation in rats. Brain Res Bull. 2016;124:103–15. doi: 10.1016/j.brainresbull.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Gwak YS, Tan HY, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma. 2006;23:1111–24. doi: 10.1089/neu.2006.23.1111. [DOI] [PubMed] [Google Scholar]
- 41.Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/S0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- 42.LaGraize SC, Fuchs PN. GABAA but not GABAB receptors in the rostral anterior cingulate cortex selectively modulate pain-induced escape/avoidance behavior. Exp Neurol. 2007;204:182–94. doi: 10.1016/j.expneurol.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasirinezhad F, Hosseini M, Karami Z, Janzadeh A, Yousefifard M. Comparative efficacy of GABAA and GABAB receptor agonists in pain alleviation in a spinal cord injury model of neuropathic pain. Neurophysiology. 2019;51:322–31. doi: 10.1007/s11062-020-09826-9. [DOI] [Google Scholar]
- 44.Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol. 2011;24:400–7. doi: 10.1097/ACO.0b013e32834871df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kocot-Kępska M, Zajączkowska R, Mika J, Wordliczek J, Dobrogowski J, Przeklasa-Muszyńska A. Peripheral mechanisms of neuropathic pain-the role of neuronal and non-neuronal interactions and their implications for topical treatment of neuropathic pain. Pharmaceuticals (Basel) 2021;14:77. doi: 10.3390/ph14020077.13132af34c0b4771b9b1957ec68ef060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/S1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- 48.Kondeva-Burdina M, Voynova M, Shkondrov A, Aluani D, Tzankova V, Krasteva I. Effects of Amanita muscaria extract on different in vitro neurotoxicity models at sub-cellular and cellular levels. Food Chem Toxicol. 2019;132:110687. doi: 10.1016/j.fct.2019.110687. [DOI] [PubMed] [Google Scholar]
- 49.Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain. 2003;105:347–53. doi: 10.1016/S0304-3959(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 50.Terayama R, Uchibe K. Reorganization of synaptic inputs to spinal dorsal horn neurons in neuropathic pain. Int J Neurosci. 2022;132:1210–6. doi: 10.1080/00207454.2021.1873980. [DOI] [PubMed] [Google Scholar]
- 51.Disorbo A, Wilson GN, Bacik S, Hoxha Z, Biada JM, Mickley GA. Time-dependent retrograde amnesic effects of muscimol on conditioned taste aversion extinction. Pharmacol Biochem Behav. 2009;92:319–26. doi: 10.1016/j.pbb.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puschner B. In: Veterinary toxicology. Gupta RC, editor. Academic Press; 2007. Mushroom toxins; pp. 915–25. [DOI] [Google Scholar]
- 53.Waldvogel HJ, Baer K, Faull RLM. In: GABA and sleep: molecular, functional and clinical aspects. Monti JM, Pandi-Perumal SR, Möhler H, editors. Springer Basel; 2010. Distribution of GABAA receptor subunits in the human brain; pp. 73–93. [DOI] [Google Scholar]
- 54.Ochoa-de la Paz LD, Gulias-Cañizo R, Ruíz-Leyja ED, Sánchez-Castillo H, Parodí J. The role of GABA neurotransmitter in the human central nervous system, physiology, and pathophysiology. Rev Mex Neuroci. 2021;22:67–76. doi: 10.24875/RMN.20000050. [DOI] [Google Scholar]
- 55.Moss MJ, Hendrickson RG. Toxicity of muscimol and ibotenic acid containing mushrooms reported to a regional poison control center from 2002-2016. Clin Toxicol (Phila) 2019;57:99–103. doi: 10.1080/15563650.2018.1497169. [DOI] [PubMed] [Google Scholar]
- 56.Ahmadzadeh K, Roshdi Dizaji S, Yousefifard M. Lack of concordance between reporting guidelines and risk of bias assessments of preclinical studies: a call for integrated recommendations. Int J Surg. 2023;109:2557–8. doi: 10.1097/JS9.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics. 2018;15:635–53. doi: 10.1007/s13311-018-0633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gray P. Acute neuropathic pain: diagnosis and treatment. Curr Opin Anaesthesiol. 2008;21:590–5. doi: 10.1097/ACO.0b013e32830c900c. [DOI] [PubMed] [Google Scholar]