Key words: Intermittent fasting, Alternate-day fasting, Time-restricted eating, Weight loss, Bone health, Bone turnover markers, Bone mass
Abstract
Intermittent fasting (IF) is a promising strategy for weight loss and improving metabolic health, but its effects on bone health are less clear. This review aims to summarise and critically evaluate the preclinical and clinical evidence on IF regimens (the 5:2 diet, alternate-day fasting (ADF) and time-restricted eating (TRE)/time-restricted feeding and bone health outcomes. Animal studies have utilised IF alongside other dietary practices known to elicit detrimental effects on bone health and/or in models mimicking specific conditions; thus, findings from these studies are difficult to apply to humans. While limited in scope, observational studies suggest a link between some IF practices (e.g. breakfast omission) and compromised bone health, although lack of control for confounding factors makes these data difficult to interpret. Interventional studies suggest that TRE regimens practised up to 6 months do not adversely affect bone outcomes and may even slightly protect against bone loss during modest weight loss (< 5 % of baseline body weight). Most studies on ADF have shown no adverse effects on bone outcomes, while no studies on the ‘5–2’ diet have reported bone outcomes. Available interventional studies are limited by their short duration, small and diverse population samples, assessment of total body bone mass exclusively (by dual-energy X-ray absorptiometry) and inadequate control of factors that may affect bone outcomes, making the interpretation of existing data challenging. Further research is required to better characterise bone responses to various IF approaches using well-controlled protocols of sufficient duration, adequately powered to assess changes in bone outcomes and designed to include clinically relevant bone assessments.
Fasting has been practised for centuries during religious events (e.g. Ramadan), and historical data also exist on fasting during hunger strikes, famine and therapeutic fasts for the treatment of morbid obesity(1). However, short-term fasting has been modernised in the past 10–15 years as an unconventional approach to weight loss and improving metabolic health, with the terms ‘intermittent fasting’ and the ‘5:2 diet’ being popularised in the UK following the release of Michael Mosely’s 2013 book (‘the Fast Diet’). Today, intermittent fasting (IF) is a common method of reducing energy consumption, with 1 in 4 of American adults surveyed in a recent poll reporting that they consider using or have tried IF(2) and publications related to IF increasing exponentially every year over the past decade (2011:19 publications, 2021:375 publications; source: PubmedR, accessed 18th August 2022). There is evidence that IF can lead to weight loss and can elicit positive health-related outcomes, such as improved insulin sensitivity, blood lipid profile and lower blood pressure(3–6). What sets IF apart from traditional diets involving daily energy restriction is that IF involves either complete or substantial energy restriction within defined temporal windows and permits adequate or ad libitum eating outside of these windows. Despite IF being shown to have a variety of health benefits, there are limited data related to the impact of IF on other bodily systems including the skeletal system.
Weight loss achieved by continuous energy restriction alone (i.e. mild to severe energy restrictions with or without micronutrient supplementation) and/or in combination with exercise has been shown to reduce bone mass and negatively affect bone microstructure(7–12). Several mechanisms have been proposed to explain these effects including mechanical unloading, nutrient deficiencies and endocrine changes(13–15). It remains uncertain whether IF is a dietary intervention with similarly undesirable bone effects or if specific characteristics of the IF schedules may have positive effects on bone and counteract/prevent the bone changes seen with conventional weight loss approaches. For example, IF is suggested to affect metabolism by repeatedly alternating fixed periods of prolonged fasting (i.e. catabolism) with shortened periods of eating (i.e. anabolism) and/or by synchronising eating behaviours to endogenous circadian rhythms(3,16,17). Better metabolic control(18,19) and circadian alignments(20) are considered beneficial for skeletal health. Interestingly, the net effects of the different characteristics and effects of IF interventions on bone heath are poorly understood.
The purpose of this review is to (i) summarise and critically evaluate the recent evidence from preclinical and clinical (epidemiological and interventional) studies on IF regimens on bone health outcomes in adults, (ii) provide insights into potential mechanisms that may mediate/explain available findings and (iii) identify limitations and knowledge gaps of current research, with the goal to provide directions for future research. Hence, it is envisaged that this review will guide the design of future research in this area and will help practitioners/individuals who aim to follow these regimens reach informed decisions.
Definition of IF regimens
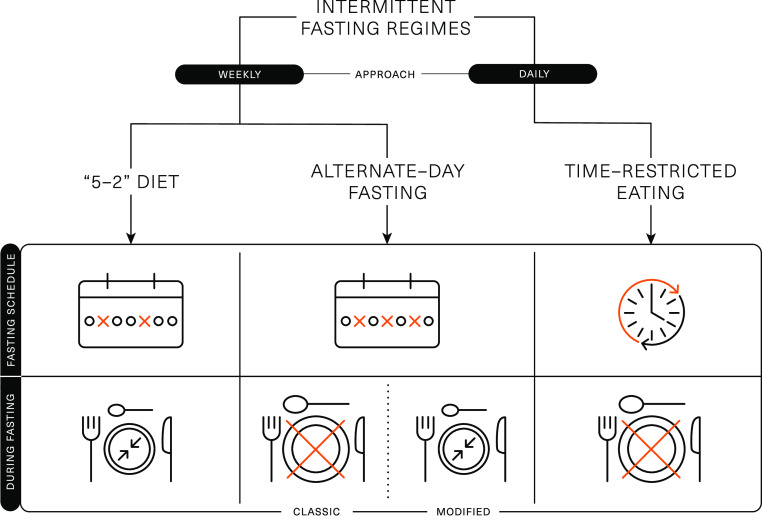
Several different methods are encompassed under the broad term of IF, which represents a challenge with interpreting data from the literature. The 5:2 diet, alternate-day fasting (ADF), alternate-day modified fasting and time-restricted eating (TRE)/time-restricted feeding are the most adopted and researched types of IF regimens (Fig. 1).
Fig. 1.
Schematic representation of the different intermittent fasting regimens.
The ‘5:2’ diet is framed around a 7-d rolling period (typically a week), wherein severe energy restriction (consuming ∼25 % of energy requirements) is imposed on 2 d of the week and ad libitum eating is permitted on the remaining 5 d. This method of IF has been shown to acutely reduce energy intake(21,22) and lead to 4–7 % weight loss over 8–52 weeks(3,4,23–26). Weight loss and body compositional changes resulting from ‘5:2’ dieting are broadly comparable to continuous daily energy restrictions and elicit similar improvements in metabolic health(4,23,27). There is some evidence that the effects of ‘5:2’ dieting are more pronounced if the 2 d of severe energy restriction are undertaken consecutively. This approach was shown to achieve greater improvements in insulin sensitivity and post-prandial lipaemia, compared with daily energy restriction resulting in similar weight loss and fat mass reductions(23,28). Some of the metabolic adaptations related to IF are thought to be due to prolonged periods of complete fasting; however, periods of fasting are curtailed on the ‘5:2’ diet due to the permission of a small meal on ‘fasting’ days, so the duration of uninterrupted fasting is usually unknown.
ADF involves a day of fasting alternated with a day of adequate or ad libitum eating. While ADF has been shown to achieve weight loss(29,30), day-long periods of complete fasting on alternate days have been shown to reduce lean mass to a greater extent than traditional energy restriction(30) and have been associated with lack of adherence(31). Due to these potential negative effects on body composition and compliance challenges, alternate-day modified fasting was devised as a hybrid of ADF and the 5:2 diet, which permits some energetic intake (usually 25 % of energy requirements; ∼2092 kJ or 500 kcal) on restricted (or ‘fast’) days. Alternate-day modified fasting has been shown to achieve 4–8 % weight loss over 8–52 weeks(3,6,29,32–34), which is comparable to the weight loss achieved by traditional energy restriction applied daily. There is also some evidence that alternate-day modified fasting can improve markers of metabolic health, including insulin sensitivity and blood lipids(6,35).
TRE is a daily approach to IF and is based around prescribed fasting and eating windows within each day. This approach restricts food intake to short daily windows (4–12 h), thereby extending the overnight fast to at least 12 h(3,16). TRE can be achieved by skipping a meal (breakfast or dinner) or shifting the time of meals (e.g. delaying breakfast and/or advancing dinner)(36,37). Evidence for breakfast omission is mixed, with some studies associating regular breakfast omission with a higher BMI and an increased risk of chronic diseases(38,39). However, short-term empirical studies indicate breakfast omission may be an effective way to reduce daily food intake(40–42). There is some evidence that evening fasting can improve a range of health outcomes(43), even in the absence of weight loss(44). This may be related to several metabolic markers displaying circadian variation that ameliorate in the morning and decline towards the evening. As such, when the fasting period is implemented may profoundly influence the effects of TRE.
Assessment of bone health and fragility
Bone mass or density, turnover, structure and strength can be evaluated (or estimated) using direct bone measurement or surrogate endpoints or biomarkers. For a detailed description of the available techniques, the reader is directed in previous reviews(45–47). In brief, dual-energy X-ray absorptiometry (DXA) is the most frequently used technique to determine bone mineral content (BMC) and areal bone mineral density. To date, DXA remains the gold standard for diagnosing and monitoring osteoporosis as it has been shown to be linearly associated with fracture risk(48,49). Peripheral quantitative computed tomography is a three-dimensional technique that can be used to assess volumetric bone mineral density and bone geometry at appendicular skeletal sites(46). It allows the distinction between trabecular and cortical bone and provides measures of total and cortical bone area, cortical thickness and estimates of bone strength. These instruments are mostly used for research purposes and can be further combined with other techniques to estimate bone mechanical characteristics. The assessment of fracture risk is the ultimate outcome in bone research. Nevertheless, incident fractures are commonly evaluated in observational studies but rarely in clinical trials(46,47). This is because of the large sample sizes required and the long periods of follow-up needed to capture their development/manifestation.
Alternatively, bone turnover markers (BTMs) are surrogate markers that allow the determination of changes in bone formation and bone resorption rates and may help to monitor the effects of shorter-term interventions(45,46). BTMs reflect acute changes in bone metabolic activity and require a shorter period of assessment than a serial collection of BMD/BMC. BTMs are classified as indices of bone resorption or formation. The most widely used bone resorption markers are products of type I collagen breakdown generated during bone resorption (C-terminal cross-linking telopeptide of type I collagen (CTX), NTX, pyridinium cross-links) or indicators of osteoclast activity (tartrate-resistant acid phosphatase). Bone formation markers include products of post-translational processing of type I collagen molecules (procollagen type I N propeptide (P1NP), procollagen type I C propeptide (P1CP)), matrix proteins (osteocalcin) or enzymes (bone-specific alkaline phosphatase) released in the circulation from osteoblasts during their activity of bone matrix synthesis. This is particularly useful given the majority of the studies that have assessed the effects of IF on bone health are currently of short duration (< 6 months).
Search strategy
A literature search was conducted using MEDLINE database until the 30th of September 2022. We included animal studies, human observational (cross-sectional and longitudinal) and interventional studies in adult populations. Relevant studies were selected using a combination of keywords for skeletal health outcomes (bone, bone mineral density, osteoporosis, fracture, bone turnover or bone remodelling) and IF, alternate-day, ‘5:2’ diet, time-restricting eating or feeding as explanatory variables. Additional studies were identified by a manual search of bibliographic references in original papers and reviews.
Studies affecting the effects of intermittent fasting on bone health
Animal studies
The animal studies that have investigated the effects of IF on bone health tend to utilise IF in addition to other practices (e.g. high-fat or ketogenic diet)(50,51) that are known to elicit negative effects on bone health(52,53) and/or in models of specific conditions (e.g. Alzheimer’s disease-induced oestrogen deficient rats(51), glucocorticoid-induced osteoporosis animal model(54)).
One study investigated the effects of a ketogenic diet with or without ADF for 12 weeks in rats(50). ADF while consuming a ketogenic diet inhibited osteoclast proliferation and osteogenic differentiation compared with a daily ketogenic diet (without ADF), but this did not translate in differences in bone structure. In the same study, compared with a control group consuming a standard diet, the ADF ketogenic diet caused a decrease in bone strength and impaired parameters of cancellous (lower trabecular total mineral density, bone volume/total volume, trabecular number and separation) and cortical bone (lower total area, bone area and cortical thickness), although the same adverse effects were also shown in rats consuming a daily ketogenic diet. Furthermore, the control group had lower levels of bone resorption markers and higher levels of bone formation markers than both the daily ketogenic diet and ADF ketogenic diet groups. While IF may contribute to the negative effects on bone characteristics, it is impossible to isolate the effect of the ketogenic diet, which appears to have largely driven the negative bone effects shown in this study.
Time-restricted feeding (3 h of feeding per day for 4 weeks) alongside a high-fat diet (dietary fat provided ∼46 % of total energy) was found to reduce femoral BMD compared with an ad libitum high-fat eating group, in Alzheimer’s disease-induced oestrogen deficient rats(51). The reason for time-restricted feeding having a negative effect on bone may relate to the high-fat composition of the diet(55). A greater amount of fat deposition, in the high-fat diet, could cause a reduction in osteoblasts as a result of bone marrow mesenchymal cells differentiation favouring adipogenesis ultimately having a negative effect on bone mass(56). Ultimately, this study found that time-restricted feeding was not able to protect bone against the negative effects of a high-fat diet.
Animal studies assessing the effects of IF on bone health are useful in providing mechanistic insights. However, combining IF with other dietary practices expected to elicit negative effects on bone may have confounded these findings. In addition, variation in the type of IF adopted and the study implementation in models mimicking specific medical conditions indicate caution when interpreting the effects of IF on bone characteristics. This underpins the need for further animal work to provide mechanistic insights into IF scenarios more applicable to humans.
Observational studies
Observational studies investigating IF and bone health are largely lacking. To our knowledge, in the only available cross-sectional analysis, no differences were seen in total body BMC or lumber spine BMD among healthy adults who were following ADF over periods ≥ 6 months and healthy controls with no history of performing ADF(57). Cross-sectional studies have a number of issues when assessing bone health and IF. Their design only allows for a discrete assessment of bone health to be made, and therefore it is impossible to establish a cause–effect relationship. It is also very rare for cross-sectional studies to control/monitor confounding factors, such as exercise status and the specific IF protocols.
Currently, the long-term implication of IF on bone health is best inferred using data from studies assessing breakfast omission. In a cross-sectional analysis, young women (aged 19–25 years old) who skipped breakfast ≥ 3 times per week had lower hip BMD compared with those who consumed breakfast daily(58). Furthermore, in a longitudinal study with a 3-year follow-up, young men who reported skipping breakfast (classified as breakfast consumed less often than every day) had higher odds of experiencing bone loss at the lumbar spine in comparison with men consuming breakfast daily, while no significant associations were seen for bone loss at other sites (i.e. hip) or in women(59). Interestingly, breakfast omission has also been associated with various unhealthy lifestyle factors, such as smoking and increased alcohol consumption(42), which also tended to be higher among those who lost bone(59). Taken together, while epidemiological evidence associates breakfast omission with bone loss, it is likely that indirect factors, such as unhealthy lifestyle choices, confound the direct associations made.
Interventional studies
Human trials exploring the effects of IF regimens on bone outcomes have only begun to emerge over the past 5 years. We identified eight randomised controlled trials (RCTs), which investigated the effects of IF on bone mass or BTMs in individuals with/without obesity and/or other metabolic disturbances (Table 1). These studies utilised ADF (n 3) or TRE (n 5) approaches, but we did not find any studies on the ‘5–2’ diet.
Table 1.
Recent clinical trials (2017–to date) exploring the effects of IF regimens on outcomes of bone health
| Study | Study population | Duration | Intervention description | Weight changes | Main bone findings |
|---|---|---|---|---|---|
| ADF (n 3) | |||||
| Barnosky et al., 2017(60) | Overweight/obese adults aged 18–65 years, 3 groups (ADF, CER, control) ADF: n 21, mean ± sem age: 44 ± 2 years, BMI: 34 ± 1 kg/m2, 19 W/2 M CER: n 24, age: 44 ± 2 years, BMI: 34 ± 1 kg/m2, 20 W/4 M Control: n 17, age: 40 ± 3 years, BMI: 32 ± 1 kg/m2, 15W/2M |
6 months | ADF: 25 % DEI fast day, alternated with 125 % DEI feast day | –7·8 ± 1·2 % | Total body BMC and BMD remained unchanged in all groups. OC, BAP and CTX did not change in any group. No differences between premenopausal or postmenopausal women for any marker |
| CER: 75 % DEI every day | –8·8 ± 1·5 % | ||||
| Control: habitual intake | NS | ||||
| Stekovic et al., 2019(57) | Non-obese adults aged 35–65 years, 2 groups (ADF, control) ADF: n 29, median age (IQR): 48 (43–55) years, mean BMI (±sd): 25·5 ± 1·8 kg/m2, 17 W/12 M Control: n 28, age 51 (45–57) years, BMI: 25·4 ± 2·2 kg/m2, 17 W/11 M | 4 weeks | ADF: ad libitum eating every second-day, no kcal on the fast days | –3·5 ± 1·5 kg | BMD at the lumbar spine region (derived from total body scans) ↓ after ADF but not after control. Comparing Comparison of ΔBMDs of both groups did not yield significant differences. No within or between group differences were seen for total body BMC |
| Control: habitual diet | NS:–0·2 ± 1·1 kg | ||||
| Templeman et al., 2021(30) | Lean healthy adults aged 18–65 years, 3 groups (CER 75:75, ADF 0:150, ADF 0:200) CER 75:75: n 12, mean ± sd age: 45 ± 6 years, BMI: 24·0 ± 1·9 kg/m2, 7W/5M ADF 0:150: n 12, age: 42 ± 11 years, BMI: 23·9 ± 2·4 kg/m2, 5W/7M ADF 0:200: n 12, age: 41 ± 14 years, BMI: 23·6 ± 2·1 kg/m2, 9 W/3 M | 3 weeks | CER: 75 % DEI (75:75) | –1·9 ± 1·0 kg | No differences in plasma CTX pre- and post-intervention. No group differences in BMC or BMD |
| ADF with ER: 24-h fasting with 150 % DEI on alternate days (0:150) | –1·6 ± 1·1 kg | ||||
| ADF without ER: 24-h fasting 200 % DEI on alternate days (0:200) | NS: −0·5 ± 1·1 kg | ||||
| TRE (n 5) | |||||
| Martens et al., 2019(63 | Apparently healthy, non-obese adults, 2 crossed-over interventions (TRE, control) n 22, mean ± sem age: 67 ± 1 years, BMI: 24·7 ± 0·6 kg/m2, 12 W/10 M |
6 weeks | TRF: ad libitum 8-h eating window | NS | Total and regional BMD were not different between conditions |
| Control: habitual diet | NS | ||||
| Lowe et al., 2020(61) | Overweight and obese adults (in-person cohort), two groups (TRE, consistent meal timing (CMT)) TRE: n 25, mean ± sd age: 43 ± 12 years, BMI: 31·5 ± 4·5 kg/m2, 12 W/13 M CMT: n 25, age:44 ± 11 years, BMI: 31·3 ± 3·5 kg/m2, 10 W/15 M | 12 weeks | TRE: ad libitum 8-h eating window between 12.00 and 20.00 hours | –1·7 kg | A trend towards a ↑ in BMC in the TRE group (P = 0·09) but not in the CMT group. BMC changes did not differ between groups |
| CMT: Habitual diet consumed in 3 structured meals per day |
NS: 0·6 kg | ||||
| Lobene et al., 2021(62) | Overweight and obese adults, two groups (TRE, non-TRE) TRE: n 11, mean ± sem age: 47 ± 4 years, BMI: 33·8 ± 2·3 kg/m2, 9 W/2 M non-TRE: n 9, age: 44 ± 4 years, BMI:34·4 ± 2·6 kg/m2, 8 W/1 M | 12 weeks | TRE: ad libitum 8-h eating window | –3·7 ± 0·5 % | P1NP ↓ in both groups (time effect) with a trend towards a greater ↓ in the non-TRE group (time × group interaction, P = 0·07). Total body BMC ↑ in the TRE group and ↓ in the non-TRE group (time × group interaction, P = 0·02) |
| Non-TRE: habitual diet | NS | ||||
| Kotarsky et al., 2021(67) | Overweight/obese adults, two groups (TRE + ex, non-TRE + ex) TRE + ex: n 11, age: 45 ± 3 years, BMI: 29·8 ± 0·8 kg/m2, 9 W/2 M non-TRE + ex: n 10, age: 44 ± 2 years, BMI: 29·4 ± 0·8 kg/m2, 9 W/1 M | 8 weeks | TRE + ex : ad libitum 8-h eating window between 12.00 and 20.00 hours + exercise (aerobic + resistance) | 3·3 % | No significant time, group or time × group interaction for total body BMC or BMD |
| Non-TRE + ex: habitual diet + exercise (aerobic + resistance) | NS: 0·2 % | ||||
| Papageorgiou et al., 2022(64) | Individuals with at least one component of the metabolic syndrome, two groups (TRE, SDA) TRE: n 23, median (IQR) age: 47 (32, 57) years, BMI: 27·9 (25·5, 31·4) kg/m2, 18 W/5 M SDA: n 19, age: 45 (27, 50) years, BMI: 26·7 (23·8, 30·6) kg/m2, 14 W/5 M |
6 months | TRE: ad libitum 12-h eating window | –0·6 kg (median) |
Total cohort: no between-group differences (TRE v. SDA) in CTX, P1NP or total body BMC/BMD responses. Analysis by weight loss response: among weight loss responders, CTX tended to ↓ after TRE but ↑ after SDA (between-group differences P = 0·041), P1NP changes did not differ between groups. Total body BMC ↓ after SDA, but remained unchanged after TRE (between-group differences in weight loss responders P = 0·028). Among non-responders (< 0·6 kg weight loss), there were no between-group differences in bone outcomes |
| SDA: 10-min counselling for healthy eating + healthy eating brochure | NS | ||||
↓, indicates a decrease; ↑, indicates an increase; ADF, alternate-day fasting; BAP, bone-specific alkaline phosphatase; BMC, bone mineral content; BMD, bone mineral density; CER, continuous energy expenditure; CMT, consistent meal timing; CTX, β-carboxyterminal telopeptide of type I collagen; Δ, delta; DEI, dietary energy intake; ex, exercise; M, men; NS, non-significant; OC, osteocalcin; P1NP, procollagen type 1 N-terminal propeptide; SDA, standard dietary advice; TRE, time-restricted eating; W, women; IQR, inter-quartile range; .
The primary outcomes of the included studies were changes in body weight (n 3; Barnosky et al. (60); Lowe et al. (61); Kotarsky et al. (67)), changes in body composition (n 1; Templeman et al. (30)), changes in insulin sensitivity (n 1; Stekovic et al. (57)), changes in components of energy balance and post-prandial metabolism (n 1; Templeman et al. (30)), change in the metabolic syndrome components (n 1; Papageorgiou et al. (64)) and changes in endothelium-dependent dilation (n 1; Martens et al. (63)).
Alternate-day fasting
A study in healthy lean males and females compared the effects of ADF with net energy restriction (25 % energy deficit) with continuous energy restriction (matched 25 % energy deficit applied daily) and ADF without energy restriction over 3 weeks(30). Energy restriction, however implemented, resulted in similar weight loss (∼2 kg), which was greater than the weight loss observed after ADF without energy restriction. Interestingly, while weight loss after continuous energy restriction was largely achieved by reducing body fat mass, ADF led to lesser reductions in fat mass but also a trend towards a reduction in lean mass. No significant changes were seen in the plasma concentrations of the bone resorption marker CTX or total body BMD (assessed by DXA), which may be explained by the modest changes in body weight/body composition and the short duration of the study protocol.
These results are in accordance with a 6-month RCT which compared the effects of ADF (25 % energy deficit), continuous energy restriction (25 % energy deficit applied daily) or participants’ habitual diet (control group) in individuals with overweight or obesity(60). Although participants achieved significant weight loss after ADF (–7·8 (sd 1·2) %) and continuous energy restriction (–8·8 (sd 1·5) %) compared with controls, no significant changes were reported for total body BMC or BMD (by DXA) in any of the groups. Circulating levels of surrogate markers of bone formation (osteocalcin, bone alkaline phosphatase) and bone resorption (CTX) also remained unchanged in all groups.
In another RCT, non-obese adults were randomly allocated to either an ADF group or a control group maintaining their habitual diet(57). After 4 weeks, the ADF group reduced their energy intake by ∼37 %, body weight by 3·5 % and trunk fat by 15 %. Although total body BMC (by DXA) was not affected in either group, BMD at the level of the lumbar spine (extracted from regional analysis of total body DXA scans) decreased on average by 0·9 % in the ADF group compared with smaller BMD reductions (0·5 %) in the control group. Notably, this decrease was only significant when analysed within the ADF group, while between-group comparison of BMD changes was not significantly different. Postmenopausal women typically experience 1 % BMD loss per year, while BMD has been shown to significantly decrease by ∼1 % over longer-term periods of milder continuous energy restrictions(7,10,11). Thus, the magnitude of BMD reductions (57) within such a short timeframe, if continued, could be concerning for longer-term bone health and lifelong fragility fracture risk.
Time-restricted eating
Two RCT investigated the effects of TRE (8-h eating window) v. control (habitual diet) for 12 weeks in adults with overweight/obesity(61,62). Lowe et al. found that the TRE group decreased their body mass from baseline by a small amount (–1·7 kg) tended to have an increase in total body BMC, while no changes were observed for body weight or bone mass in the control group(61). Lobene et al. reported reductions in the bone formation marker P1NP when pooling data from the TRE and control groups, with a trend towards a greater P1NP reduction in the control group, and no changes in other markers of bone metabolism (NTX or PTH)(62). The BTM results from Lobene et al. may indicate a protective response of TRE and were, to some extent, further supported by small changes in bone mass, with total body BMC decreasing in the control group but increasing in the TRE group.
Using a similar TRE protocol (16-h fasting with 8-h ad libitum eating), a cross-over RCT explored the feasibility and safety of a 6-week TRE intervention (v. control-habitual diet) in middle-aged and older individuals(63). This study found that TRE had no impact on participants’ body mass, lean mass, total body or regional BMD (by total body DXA). The absence of significant changes in these bone parameters may be explained by the modest changes in lifestyle factors (i.e. participants reduced their eating window by ∼4 h but did not change their energetic intake or dietary quality) or may reflect the short follow-up of the study which was likely insufficient to detect small BMD changes.
In line with these findings, a longer-term (6 months) TRE intervention (employing a 12-h ad libitum eating window) had no unfavourable effects on bone metabolism (BTMs and bone-related hormones) or bone loss (total body BMC/BMD by DXA) compared with the provision of standard dietary advice for healthy eating, in individuals with at least one component of the metabolic syndrome(64). Additional sub-analysis in participants who lost weight with either TRE or standard dietary advice (based on median body weight changes: ≥ 0·6 kg weight loss) found that those who lost weight by following SDA experienced a modest loss of total body BMC, which was supported by small, albeit non-significant increases in bone resorption (CTX). By contrast, when weight loss was achieved by TRE, BMC was preserved with CTX concentrations tending to decrease. These findings suggest a possible benefit of TRE on bone health during weight loss, although it should be noted that results reflect bone responses to a milder TRE intervention allowing a longer eating window than typically employed (12 h), and high inter-participant variation in body weight responses.
Exercise, particularly resistance exercise, has previously been shown to mitigate the undesirable effects of weight loss on bone and muscle(9,65,66). Therefore, Kotarsky et al. compared the effects of 8 weeks of TRE (eating window between 12.00 and 20.00 hours) in combination with an aerobic and resistance exercise programme (TRE+ex), compared with a habitual diet with the same exercise programme (control+ex), in adults with overweight and obesity(67). Both interventions induced significant energy deficits (TRE+ex: ∼300 kcal/d, control+ex: ∼250 kcal/d), but TRE+ex reduced total body mass (3·3 % v. 0·2 %) and fat mass (9·0 % v. 3·3 %) to a greater extent than control+ex. LBM tended to increase due to exercise, with no differences between groups. These changes in body weight and body composition were not accompanied by changes in total body BMC or BMD.
Despite the increasing number of interventions reporting bone outcomes, it is challenging to draw conclusions from the current studies. Overall, studies suggest that TRE regimens practised for relatively short periods (up to 6 months) do not appear to adversely affect bone outcomes and may even slightly protect bone when weight loss occurs. Similarly, most studies on ADF have shown no adverse effects on bone outcomes, while studies on the effects of the ‘5:2’ diet on bone are lacking. Current understanding is limited by the following factors, indicating caution when interpreting the results of existing studies and underpinning the importance of future research.
Short duration: Current trials had short durations (ranging from 3 weeks to 6 months). Trial duration affects body weight, metabolic and skeletal effects. For example, longer trial durations (> 12 weeks) may be required with some methods of IF to observe clinically significant weight loss (≥ 5 % weight loss from baseline)(3). The effects of dietary interventions on bone mass also need sufficient time to present(7). This is because a complete cycle of bone remodelling takes 4–6 months; thus, studies are proposed to have a duration of ≥ 6 months to allow the detection of clinically meaningful changes in bone structure (by DXA or other imaging modalities)(7). The very few studies that have assessed BTM responses to IF interventions suggest no changes after ADF(60) or some favourable effects of TRE(62,64), with these findings indicating no major bone breakdown at least in the short/medium term.
Bone assessments: It is of note that all available studies have reported total body and/or regional BMC/BMD based on total body DXA assessments but have not performed scans at clinically relevant sites, that is, the hip or the lumbar spine, or evaluations of bone microstructure and fracture risk.
Lack of power to detect changes/differences in bone outcomes: None of the available studies has assessed bone parameters as a priori outcomes (Table 1). Conversely, since most of the available trials had small sample sizes, it is likely that these studies were not powered to detect differences in bone parameters assessed as secondary outcome measures.
Population at high risk for bone fragility: Overall, existing studies have been conducted in small mixed population samples, with the effects of IF regimens on bone outcomes in groups at high risk for bone fragility (i.e. elderly, postmenopausal women) remaining largely understudied. One study found that TRE was not associated with bone loss in middle-aged and older individuals, although the TRE intervention implemented did not result in pronounced lifestyle changes and had a short duration (6 weeks) (for a detailed description, see Table 1). Notably, it has been suggested that IF may not be appropriate for some population groups including children and adolescents, pregnant or breast-feeding women, individuals with a history of eating disorders and/or already low BMI/underweight and patients with specific medical conditions (e.g. diabetes treated with certain medications). The bone health of these subgroups requires special attention, and IF practices may, in theory, exacerbate/result in nutritional deficiencies and interact with growth/development or drugs with further implications for their bone health.
Dietary intervention characteristics: The magnitude of the energy deficit elicited, the dietary composition of the intervention arms and the control of related lifestyle behaviours such as physical activity and sleeping patterns may all impact bone health parameters; nevertheless thus far, they are often poorly controlled and/or reported. For example, TRE regimens place emphasis on the duration and/or the timing of the eating window within a 24-h cycle and often disregard energetic intake or the quality of the diet consumed over the eating windows, which could introduce considerable variability in the bone responses. Similarly, the control arms are commonly instructed to follow their habitual diet which may significantly differ among individuals.
Mechanistic perspectives
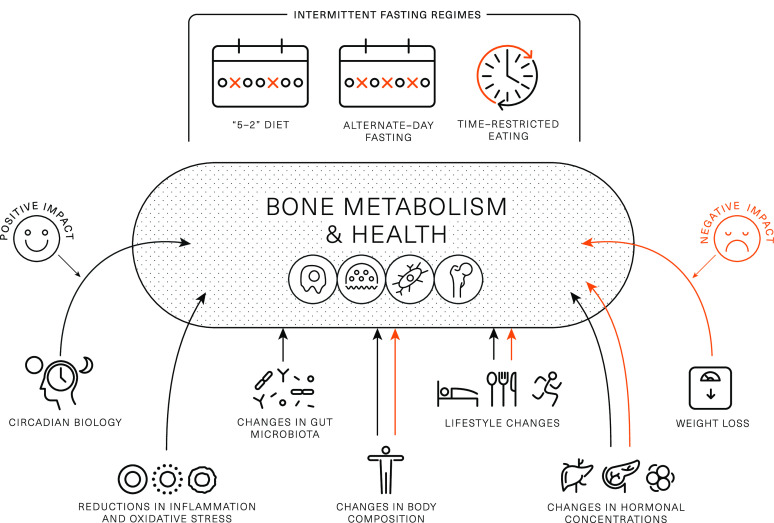
From a skeletal health perspective, IF interventions are very interesting as they involve behaviour/lifestyle changes and induce metabolic changes (e.g. realignment with circadian rhythm, changes in body weight, body composition, endocrine profile and gut microbiome) that theoretically may have positive, neutral or negative effects on bone health outcomes and their net effects are uncertain (Fig. 2).
Fig. 2.
Theoretical framework on intermittent fasting characteristics and induced changes that may positively or negatively affect bone metabolism and health. Black arrows indicate positive impact, while red arrows indicate negative impact.
Components of energy balance and weight loss
IF typically leads to a reduction in energy intake(5,6,21,34,40,42,68), which is likely the reason for weight loss, and current evidence suggests this is one of the primary mechanisms responsible for improvements in metabolic health(3). Short-/medium-term continuous energy restriction (ranging in duration from a few days to 2–3 months) has been shown to promote bone breakdown (as assessed by BTMs)(7,69–71), and longer-term (≥ 6 moths) continuous energy restriction is often accompanied by bone loss(7,72). Thus, it is possible that IF causes a negative effect on bone health via a reduction in energy intake and accompanying weight loss. From the available literature, it is challenging to separate the effects of IF from those of weight loss. In Papageorgiou et al. (64), participants who lost weight by following standard dietary advice experienced small reductions in BMC, which were not seen in those who lost weight after a TRE intervention. In contrast, in another RCT in which ADF led to a significant reduction in energetic intake, and rapid weight loss, a reduction in lumbar spine BMD was reported(57). Given these conflicting results, further studies appropriately designed to differentiate the effects of weight loss from other IF characteristics are needed.
In addition to reductions in energy intake and resulting weight loss, some IF interventions may reduce physical activity levels, and this could elicit an independent-detrimental effect on bone health. For example, skipping breakfast for 6 week resulted in a reduction in daily physical activity energy expenditure of approximately 450 kcal/d(40), with similar findings reported with other methods of severe energy restriction(22). Moreover, Templeman et al. observed a reduction in physical activity energy expenditure of approximately 100 kcal/d during 3 weeks of ADF, while no such reductions were observed when the same energy deficit was induced through daily energy restriction(30). These studies indicate that IF may independently reduce physical activity to a greater extent than other methods of energy restriction, which may indirectly confer negative effects to bone health via a reduction in mechanical loading(73). Conversely, interventions that have combined IF with exercise have been proved feasible and suggest that participants are able to perform moderate- to high-intensity endurance or resistance exercise during extended periods of fasting(32,67,74). As such, individuals should be encouraged to engage in their exercise routines or new programmes for maximising metabolic and musculoskeletal benefits.
Changes in body composition
Muscle, fat and bone are biomechanically and molecularly interacting tissues(14,75). From a biomechanical perspective, it is well established that during locomotion and systematic exercise skeletal muscle applies forces on bone that stimulate high-magnitude strains which induce adaptations of bone mass, structure and strength. Furthermore, muscle and fat as contributors to body weight offer mechanical stimuli for increasing bone mass to support a higher body weight, while absolute reductions in muscle/fat and the resulting mechanical unloading have been proposed to partially explain the effects of weight loss on bone health(13–15). The interactions between the three tissues at molecular level appear to involve (i) molecules produced by muscle (myokines such as IL6 and IL15, irisin) or fat (adipokines such as leptin and adiponectin) which act on bone, (ii) molecules secreted by bone (e.g. osteocalcin) with action on muscles/fat and iii) local/systemic endocrine factors (e.g. sex steroids) with effects on multiple tissues(14,15,75).
Weight loss through energetic restriction derives largely from reductions in fat mass (accounting for ∼75 % of the weight lost) and to a less extent from fat-free mass loss (approximately the rest 25 %)(76). The effects of IF regimens on body composition are still debated. Some reviews on this topic suggest reductions or no changes in fat mass and lean mass(36) and a similar ratio of fat mass to lean mass loss (75–25 %) as conventional energy restrictions(3). Conversely, some well-controlled studies have reported greater contributions of muscle mass loss to the total amount of weight loss(30,61), raising the question whether IF interventions are safe for population groups at risk for osteoporosis (postmenopausal women, elderly and individuals with metabolic diseases) and skeletal injuries (e.g. athletes). To date, the contributions of body composition changes to changes in bone outcomes remain uncertain.
Endocrine factors
Changes in body mass and composition cause changes in several tonic hormones implicated in bone metabolism and health. For example, insulin and leptin are known to have anabolic effects on bone; nevertheless, the influence of resistance/sensitivity to their actions on bone remains unclear. For example, obesity is associated with high insulin and leptin concentrations, which are thought to contribute to the higher BMD values seen in individuals with overweight/obesity(15,77). Nevertheless, hyperglycaemia, excess insulin levels and insulin resistance (i.e. in type 2 diabetes) are purported to be associated with low bone turnover, impaired bone microstructure and bone matrix quality and thus, increased fracture risk(19,77). Conversely, weight loss enhances insulin and leptin resistance but reduces their absolute concentrations; these changes appear to be associated with weight-induced bone loss(13,15). Available research demonstrates that IF interventions result in reductions in fasting blood glucose and insulin, improvements in insulin sensitivity and decreases in leptin levels(78); however, the impact of these changes on bone outcomes remains unexplored.
Insulin-like growth factor-1 (IGF-1) is another important anabolic factor for bone(79), with current studies suggesting no changes or decreases in IGF-1 circulating levels in response to energetic and/or protein restrictions over periods 6–24 months(10,80–82). IF studies have reported mixed results on IGF-1 responses. For example, an intervention of TRE (8-h eating window) in conjunction with resistance training resulted in decreases in testosterone and IGF-1 levels; nevertheless, these changes were not accompanied by unfavourable changes in body composition or compromises of muscle strength at least over the timeframe of the study. In contrast, in an ADF intervention, IGF-1 was unaltered in the ADF and the control groups but increased after continuous energy restriction, with no changes seen in bone mass in any of the three intervention groups(60).
Many of the endocrine changes discussed are likely due to energy/macronutrient restriction and weight loss, rather than IF specifically. However, a distinguishing feature of IF is the frequent metabolic shift that occurs, owing to the switch between the prolonged catabolic fasted state and shortened anabolic periods of feeding. Prolonging the catabolic state stimulates lipid turnover more than traditional daily energy restriction, causing a proportional increase in lipid metabolism and a reciprocal reduction in the carbohydrate metabolism(28). There have been several benefits to metabolic health proposed in relation to this switch, but a consequence is an acute period of post-prandial insulin resistance in response to the first meal consumed after breaking the fast(28,40,83,84). Acutely elevated insulin concentrations have been shown to suppress concentrations of CTX and osteocalcin(85).
Several gastrointestinal hormones (e.g. ghrelin, peptide YY, glucagon-like peptide 1 and peptide-P) have shown acute changes upon transitioning from a prolonged catabolic to an anabolic state(21,84). Differences in bone remodelling have been found when providing nutrients orally or intravenously, suggesting a mediating role of gastrointestinal hormones in bone turnover(86). Incretin hormones, such as lucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1), enhance insulin secretion(87), so they may influence bone health through insulin-mediated pathways(77).
Changes in dietary factors
A balanced diet with adequate intakes of certain nutrients (i.e. calcium (Ca) and proteins) and foods and food groups (e.g. dairy products, fruits and vegetables) is important for maximising and maintaining bone properties(88). Conversely, an unbalanced Western type diet typically high in processed foods, saturated fats, refined sugars and salt appears to compromise bone health through direct (e.g. salt-induced increases urinary Ca excretion) and indirect (chronic inflammation, contribution to obesity and associated metabolic diseases) mechanisms(89). Notably, individuals who follow IF regimens commonly place their focus on meal timing rather than food quantity or quality. Thus, IF interventions do not necessarily translate into a (bone) healthy diet. Several studies have reported changes in aspects of dietary quality during IF; nevertheless, these have not been characterised in relation to bone health. Research on macronutrient composition with ADF/the ‘5–2 diet’/TRE regimens does not support pronounced differences in carbohydrate, protein or fat intake (as % of energy intake) pre- and post-interventions (for a review, see(3)), although reductions in absolute amounts of macronutrients appear to contribute to the lower energy intakes reported. Yet, a pertinent question, especially for individuals at risk for bone loss and muscle wasting, is whether IF protocols offer opportunities for meeting protein recommendations(90). Current evidence supports additional musculoskeletal benefits from higher protein intakes (≥ 1·2 g/kg/d) for older individuals, while, to maximise protein synthesis, distribution of protein intake over waking hours and consumption of ≥ 2 meals per day with ∼0·4 g protein/kg are encouraged(91,92). Such recommendations appear somewhat discordant with IF protocols in which all energy content are consumed within a shortened period of time each day (in the case of TRE) or are severely restricted for periods > 24 h (in case of ADF and the 5:2 diet). Whether IF regimens result in changes in micronutrient intakes and specific food group intake with subsequent implications for bone health remains unknown. Hypothetically, if somebody habitually consumes a breakfast rich in dairy products and this meal is skipped as part of practising TRE, this person may miss the opportunity to consume adequate Ca intake. Conversely, positive eating behaviour changes such as reductions in late evening snacks and alcohol observed after TRE(37) may have favourable influences on bone health(93,94).
Circadian biology
Several lines of evidence suggest that bone is subjected to circadian variability (for a review, see(20)). Clock genes are expressed in bone cells, while clock gene knockout mice exhibit altered bone phenotypes. In line with this preclinical evidence, clinical studies have shown that bone-related hormones and BTM display circadian variations, while circadian rhythms disturbances such as working night shifts and/or sleeping disorders have been associated with impaired bone metabolism, reduced bone mass and increased fracture risk(20). Conversely, it remains largely unknown how alignment of mealtimes with circadian rhythms such as those achieved in TRE may impact bone health. Indirect evidence suggests that favourable changes in circadian biology as a result of TRE are linked to cardiometabolic benefits which may occur independently of weight loss(16,44) and which have been linked to improved bone outcomes in separate investigations(18,95). The direct links between measurable TRE-induced circadian changes and bone health outcomes require elucidation.
Changes in gut microbiome
Changes in gut microbiota during IF are important mediators of its metabolic benefits(96). Preclinical studies have shown that fasting periods induce a ‘gut rest’ which contributes to (i) improved gut barrier function (e.g. increased villi length and expression of tight junction proteins(97,98) and reductions in plasma levels of lipopolysaccharide(99)), (ii) enhanced gut microbial richness(100), (iii) enrichment of beneficial bacteria(97–99,101), (iv) alteration in microbial pathways involved in fuel utilisation (e.g. up-regulation of ketone body pathway), antioxidant signalling (enhancement of glutathione metabolism pathways) and low-grade inflammation (down-regulation of the lipopolysaccharide biosynthesis pathway)(99) and (v) changes in gut microbiota-associated metabolites (e.g. increases in faecal short-chain fatty acids (SCFAs))(97). There are limited studies in humans that have assessed such parameters, but of those few, some confirm some beneficial gut microbiota changes after TRE(102) or IF during Ramadan(103,104), while others have shown no significant alterations(105). Research suggests that the gut microbiome affects bone health through several mechanisms including the production of metabolites (e.g. SCFAs) that affect bone metabolism, the bioavailability of nutrients important for bone health (e.g. Ca), the regulation of the immune system and hormonal modulation(106,107). Given this emerging evidence of the gut–bone axis positive effects on gut microbiota may, in theory, positively affect the skeleton. Future animal and human studies need to address the complex interactions between different IF regimens, gut microbiota and bone health outcomes.
Conclusions
IF represents a promising dietary approach for weight loss and prevention/treatment of metabolic disorders; nevertheless, its effects on bone health have only recently started to be unravelled. While animal studies currently offer limited insights into scenarios pertinent to humans and epidemiological studies are largely lacking from this area of research, most available evidence comes from interventional studies that have reported bone outcomes. These suggest that TRE regimens practised up to 6 months do not adversely affect bone outcomes and may have small protective bone effects when modest weight loss is achieved (< 5 % of baseline body weight). Similarly, most current research on ADF has shown no adverse effects on bone outcomes, while no studies on the ‘5–2’ diet have assessed bone outcomes. Available studies are limited by their short duration (3 weeks to 6 months), their small and diverse population samples, assessment of bone mass exclusively by total body DXA and inadequate control of factors that may affect bone outcomes. Thus, the interpretation of existing findings is challenging, and further research is required to better characterise bone responses to various IF schedules using well-controlled protocols of longer duration, adequately powered to assess changes in bone outcomes and designed to include clinically relevant bone assessments (BMD at the hip/lumbar spine, bone microstructure and fracture risk).
Acknowledgements
None.
No funding to report for this work.
All authors conceived and designed this review, contributed to the writing and critical review of the manuscript and approved its final version.
The authors declare no conflicts of interest.
References
- 1. Johnstone A (2015) Fasting for weight loss: an effective strategy or latest dieting trend? Int J Obes 39, 727–733. [DOI] [PubMed] [Google Scholar]
- 2. YouGovAmerica (2020) Americans Say this Popular Diet is Effective and Inexpensive. https://today.yougov.com/topics/consumer/articles-reports/2020/02/24/most-effective-diet-intermittent-fasting-poll (accessed August 2022).
- 3. Varady KA, Cienfuegos S, Ezpeleta M, et al. (2022) Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Rev Endocrinol 18, 309–321. [DOI] [PubMed] [Google Scholar]
- 4. Schubel R, Nattenmuller J, Sookthai D, et al. (2018) Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 weeks: a randomized controlled trial. Am J Clin Nutr 108, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cienfuegos S, Gabel K, Kalam F, et al. (2020) Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab 32, 366–378.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trepanowski JF, Kroeger CM, Barnosky A, et al. (2018) Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: secondary analysis of a randomized controlled trial. Clin Nutr 37, 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zibellini J, Seimon RV, Lee CMY, et al. (2015) Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J Bone Miner Res 30, 2168–2178. [DOI] [PubMed] [Google Scholar]
- 8. Harper C, Pattinson AL, Fernando HA, et al. (2016) Effects of obesity treatments on bone mineral density, bone turnover and fracture risk in adults with overweight or obesity. Horm Mol Biol Clin Investig 28, 133–149. [DOI] [PubMed] [Google Scholar]
- 9. Villareal DT, Fontana L, Weiss EP, et al. (2006) Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 166, 2502–2510. [DOI] [PubMed] [Google Scholar]
- 10. Villareal DT, Shah K, Banks MR, et al. (2008) Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab 93, 2181–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz AV, Johnson KC, Kahn SE, et al. (2012) Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res 27, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen VFH, Molck AM, Dalgaard M, et al. (2021) Changes in bone mass associated with obesity and weight loss in humans: applicability of animal models. Bone 145, 115781. [DOI] [PubMed] [Google Scholar]
- 13. Papageorgiou M, Kerschan-Schindl K, Sathyapalan T, et al. (2020) Is weight loss harmful for skeletal health in obese older adults? Gerontology 66, 2–14. [DOI] [PubMed] [Google Scholar]
- 14. Iwaniec UT & Turner RT (2016) Influence of body weight on bone mass, architecture and turnover. J Endocrinol 230, R115–R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shapses SA & Sukumar D (2012) Bone metabolism in obesity and weight loss. Annu Rev Nutr 32, 287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manoogian ENC, Chow LS, Taub PR, et al. (2022) Time-restricted eating for the prevention and management of metabolic diseases. Endocr Rev 43, 405–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Cabo R & Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381, 2541–2551. [DOI] [PubMed] [Google Scholar]
- 18. Li CI, Liu CS, Lin WY, et al. (2015) Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan Diabetes Cohort Study. J Bone Miner Res 30, 1338–1346. [DOI] [PubMed] [Google Scholar]
- 19. Hofbauer LC, Busse B, Eastell R, et al. (2022) Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol 10, 207–220. [DOI] [PubMed] [Google Scholar]
- 20. Swanson CM, Kohrt WM, Buxton OM, et al. (2018) The importance of the circadian system & sleep for bone health. Metabolism 84, 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clayton DJ, Stensel DJ & James LJ (2016) Effect of breakfast omission on subjective appetite, metabolism, acylated ghrelin and GLP-17–36 during rest and exercise. Nutrition 32, 179–185. [DOI] [PubMed] [Google Scholar]
- 22. James R, James LJ & Clayton DJ (2020) Anticipation of 24 h severe energy restriction increases energy intake and reduces physical activity energy expenditure in the prior 24 h, in healthy males. Appetite 152, 104719. [DOI] [PubMed] [Google Scholar]
- 23. Harvie MN, Pegington M, Mattson MP, et al. (2011) The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes 35, 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harvie M, Wright C, Pegington M, et al. (2013) The effect of intermittent energy and carbohydrate restriction vs. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr 110, 1534–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carter S, Clifton PM & Keogh JB (2018) Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open 1, e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fitzgerald KC, Vizthum D, Henry-Barron B, et al. (2018) Effect of intermittent v. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult Scler Relat Disord 23, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carter S, Clifton PM & Keogh JB (2016) The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract 122, 106–112. [DOI] [PubMed] [Google Scholar]
- 28. Antoni R, Robertson TM, Robertson MD, et al. (2018) A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci 7, e22. [Google Scholar]
- 29. Catenacci VA, Pan Z, Ostendorf D, et al. (2016) A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 24, 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Templeman I, Smith HA, Chowdhury E, et al. (2021) A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med 13, eabd8034. [DOI] [PubMed] [Google Scholar]
- 31. Heilbronn LK, Smith SR, Martin CK, et al. (2005) Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr 81, 69–73. [DOI] [PubMed] [Google Scholar]
- 32. Bhutani S, Klempel MC, Kroeger CM, et al. (2013) Effect of exercising while fasting on eating behaviors and food intake. J Int Soc Sports Nutr 10, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cho Y, Hong N, Kim KW, et al. (2019) The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med 8, 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varady KA, Bhutani S, Klempel MC, et al. (2013) Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J 12, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parvaresh A, Razavi R, Abbasi B, et al. (2019) Modified alternate-day fasting v. calorie restriction in the treatment of patients with metabolic syndrome: a randomized clinical trial. Complement Ther Med 47, 102187. [DOI] [PubMed] [Google Scholar]
- 36. Kang J, Ratamess NA, Faigenbaum AD, et al. (2021) Effect of time-restricted feeding on anthropometric, metabolic, and fitness parameters: a systematic review. J Am Coll Nutr 41, 1–16. [DOI] [PubMed] [Google Scholar]
- 37. Gill S & Panda S (2015) A Smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 22, 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mekary RA, Giovannucci E, Willett WC, et al. (2012) Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr 95, 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Timlin MT & Pereira MA (2007) Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev 65, 268–281. [DOI] [PubMed] [Google Scholar]
- 40. Betts JA, Richardson JD, Chowdhury EA, et al. (2016) The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr 100, 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chowdhury EA, Richardson JD, Holman GD, et al. (2016) The causal role of breakfast in energy balance and health: a randomized controlled trial in obese adults. Am J Clin Nutr 103, 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clayton DJ, Barutcu A, Machin C, et al. (2015) Effect of breakfast omission on energy intake and evening exercise performance. Med Sci Sports Exerc 47, 2645–2652. [DOI] [PubMed] [Google Scholar]
- 43. Clayton DJ, Mode WJA & Slater T (2020) Optimising intermittent fasting: evaluating the behavioural and metabolic effects of extended morning and evening fasting. Nutr Bull 45, 444–455. [Google Scholar]
- 44. Sutton EF, Beyl R, Early KS, et al. (2018) Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metabolism 27, 1212–1221.e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vasikaran S, Cooper C, Eastell R, et al. (2011) International osteoporosis foundation and international federation of clinical chemistry and laboratory medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med 49, 1271–1274. [DOI] [PubMed] [Google Scholar]
- 46. Bailey RL, Sahni S, Chocano-Bedoya P, et al. (2019) Best practices for conducting observational research to assess the relation between nutrition and bone: an international working group summary. Adv Nutr 10, 391–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lewis JR, Voortman T & Ioannidis JP (2021) Evaluating and strengthening the evidence for nutritional bone research: ready to break new ground? J Bone Miner Res 36, 219–226. [DOI] [PubMed] [Google Scholar]
- 48. Johnell O, Kanis JA, Oden A, et al. (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20, 1185–1194. [DOI] [PubMed] [Google Scholar]
- 49. Fuggle NR, Curtis EM, Ward KA, et al. (2019) Fracture prediction, imaging and screening in osteoporosis. Nat Rev Endocrinol 15, 535–547. [DOI] [PubMed] [Google Scholar]
- 50. Xu X, Ding J, Wu X, et al. (2019) Bone microstructure and metabolism changes under the combined intervention of ketogenic diet with intermittent fasting: an in vivo study of rats. Exp Anim 68, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shin BK, Kang S, Kim DS, et al. (2018) Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer’s disease-induced estrogen deficient rats. Exp Biol Med 243, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Z, Zhang Z, Pei L, et al. (2022) How high-fat diet affects bone in mice: a systematic review and meta-analysis. Obes Rev 23, e13493. [DOI] [PubMed] [Google Scholar]
- 53. Merlotti D, Cosso R, Eller-Vainicher C, et al. (2021) Energy metabolism and ketogenic diets: what about the skeletal health? A narrative review and a prospective vision for planning clinical trials on this issue. Int J Mol Sci 22, 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alrowaili MG, Hussein AM, Eid EA, et al. (2021) Effect of intermittent fasting on glucose homeostasis and bone remodeling in glucocorticoid-induced osteoporosis rat model. J Bone Metab 28, 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Patsch JM, Kiefer FW, Varga P, et al. (2011) Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism 60, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nuttall ME & Gimble JM (2004) Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol 4, 290–294. [DOI] [PubMed] [Google Scholar]
- 57. Stekovic S, Hofer SJ, Tripolt N, et al. (2019) Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metabolism 30, 462–476.e466. [DOI] [PubMed] [Google Scholar]
- 58. Kuroda T, Onoe Y, Yoshikata R, et al. (2013) Relationship between skipping breakfast and bone mineral density in young Japanese women. Asia Pac J Clin Nutr 22, 583–589. [DOI] [PubMed] [Google Scholar]
- 59. Nagata K, Yoshida M, Ishimoto Y, et al. (2014) Skipping breakfast and less exercise are risk factors for bone loss in young Japanese adults: a 3-year follow-up study. J Bone Miner Metab 32, 420–427. [DOI] [PubMed] [Google Scholar]
- 60. Barnosky A, Kroeger CM, Trepanowski JF, et al. (2017) Effect of alternate day fasting on markers of bone metabolism: an exploratory analysis of a 6-month randomized controlled trial. Nutr Healthy Aging 4, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lowe DA, Wu N, Rohdin-Bibby L, et al. (2020) Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity. JAMA Intern Med 180, 1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lobene AJ, Panda S, Mashek DG, et al. (2021) Time-restricted eating for 12 weeks does not adversely alter bone turnover in overweight adults. Nutrients 13, 1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martens CR, Rossman MJ, Mazzo MR, et al. (2020) Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience 42, 667–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Papageorgiou M, Biver E, Mareschal J, et al. (2022) The effects of time-restricted eating and weight loss on bone metabolism and health: a 6-month randomized controlled trial. Obesity 31, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Armamento-Villareal R, Sadler C, Napoli N, et al. (2012) Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res 27, 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Beavers KM, Walkup MP, Weaver AA, et al. (2018) Effect of exercise modality during weight loss on bone health in older adults with obesity and cardiovascular disease or metabolic syndrome: a randomized controlled trial. J Bone Miner Res 33, 2140–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kotarsky CJ, Johnson NR, Mahoney SJ, et al. (2021) Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep 2021, e14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gabel K, Hoddy KK, Haggerty N, et al. (2018) Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 4, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Papageorgiou M, Martin D, Colgan H, et al. (2018) Bone metabolic responses to low energy availability achieved by diet or exercise in active eumenorrheic women. Bone 114, 181–188. [DOI] [PubMed] [Google Scholar]
- 70. Grinspoon SK, Baum HB, Kim V, et al. (1995) Decreased bone formation and increased mineral dissolution during acute fasting in young women. J Clin Endocrinol Metab 80, 3628–3633. [DOI] [PubMed] [Google Scholar]
- 71. Papageorgiou M, Elliott-Sale KJ, Parsons A, et al. (2017) Effects of reduced energy availability on bone metabolism in women and men. Bone 105, 191–199. [DOI] [PubMed] [Google Scholar]
- 72. Soltani S, Hunter GR, Kazemi A, et al. (2016) The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 27, 2655–2671. [DOI] [PubMed] [Google Scholar]
- 73. Brooke-Wavell K, Skelton DA, Barker KL, et al. (2022) Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br J Sports Med 56, 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moro T, Tinsley G, Bianco A, et al. (2016) Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med 14, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ilich JZ, Kelly OJ, Inglis JE, et al. (2014) Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev 15, 51–60. [DOI] [PubMed] [Google Scholar]
- 76. Heymsfield SB, Gonzalez MC, Shen W, et al. (2014) Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev 15, 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Conte C, Epstein S & Napoli N (2018) Insulin resistance and bone: a biological partnership. Acta Diabetol 55, 305–314. [DOI] [PubMed] [Google Scholar]
- 78. Albosta M & Bakke J (2021) Intermittent fasting: is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin Diabetes Endocrinol 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mazziotti G, Lania AG & Canalis E (2022) Skeletal disorders associated with the growth hormone-insulin-like growth factor 1 axis. Nat Rev Endocrinol 18, 353–365. [DOI] [PubMed] [Google Scholar]
- 80. Fontana L, Weiss EP, Villareal DT, et al. (2008) Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fontana L, Villareal DT, Das SK, et al. (2016) Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell 15, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Villareal DT, Fontana L, Das SK, et al. (2016) Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res 31, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Clayton DJ, Biddle J, Maher T, et al. (2018) 24-h severe energy restriction impairs postprandial glycaemic control in young, lean males. Br J Nutr 120, 1107–1116. [DOI] [PubMed] [Google Scholar]
- 84. O’Connor KL, Scisco JL, Smith TJ, et al. (2016) Altered appetite-mediating hormone concentrations precede compensatory overeating after severe, short-term energy deprivation in healthy adults. J Nutr 146, 209–217. [DOI] [PubMed] [Google Scholar]
- 85. Ivaska KK, Heliovaara MK, Ebeling P, et al. (2015) The effects of acute hyperinsulinemia on bone metabolism. Endocr Connect 2015, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Westberg-Rasmussen S, Starup-Linde J, Hermansen K, et al. (2017) Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects. Bone 97, 261–266. [DOI] [PubMed] [Google Scholar]
- 87. Baggio LL & Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157. [DOI] [PubMed] [Google Scholar]
- 88. Rizzoli R, Biver E & Brennan-Speranza TC (2021) Nutritional intake and bone health. Lancet Diabetes Endocrinol 9, 606–621. [DOI] [PubMed] [Google Scholar]
- 89. Movassagh EZ & Vatanparast H (2017) Current evidence on the association of dietary patterns and bone health: a scoping review. Adv Nutr 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tinsley GM & Paoli A (2019) Time-restricted eating and age-related muscle loss. Aging (Albany NY) 11, 8741–8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Traylor DA, Gorissen SHM & Phillips SM (2018) Perspective: protein requirements and optimal intakes in aging: are we ready to recommend more than the recommended daily allowance? Adv Nutr 9, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rizzoli R, Biver E, Bonjour JP, et al. (2018) Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos Int 29, 1933–1948. [DOI] [PubMed] [Google Scholar]
- 93. Nguyen HH, Wu F, Oddy WH, et al. (2021) Associations between dietary patterns and osteoporosis-related outcomes in older adults: a longitudinal study. Eur J Clin Nutr 75, 792–800. [DOI] [PubMed] [Google Scholar]
- 94. Melaku YA, Gill TK, Adams R, et al. (2016) Association between dietary patterns and low bone mineral density among adults aged 50 years and above: findings from the North West Adelaide Health Study (NWAHS). Br J Nutr 116, 1437–1446. [DOI] [PubMed] [Google Scholar]
- 95. Wang B, Wang Z, Poundarik AA, et al. (2022) Unmasking fracture risk in type 2 diabetes: the association of longitudinal glycemic hemoglobin level and medications. J Clin Endocrinol Metab 2022, 107.e1390–e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mohr AE, Gumpricht E, Sears DD, et al. (2021) Recent advances and health implications of dietary fasting regimens on the gut microbiome. Am J Physiol Gastrointest Liver Physiol 320, G847–G863. [DOI] [PubMed] [Google Scholar]
- 97. Liu Z, Dai X, Zhang H, et al. (2020) Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun 11, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Beli E, Yan Y, Moldovan L, et al. (2018) Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes 67, 1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cignarella F, Cantoni C, Ghezzi L, et al. (2018) Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab 27, 1222–1235.e1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van der Merwe M, Sharma S, Caldwell JL, et al. (2020) Time of feeding alters obesity-associated parameters and gut bacterial communities, but not fungal populations, in C57BL/6 male mice. Curr Dev Nutr 4, nzz145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zheng X, Zhou K, Zhang Y, et al. (2018) Food withdrawal alters the gut microbiota and metabolome in mice. FASEB J 32, 4878–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zeb F, Wu X, Chen L, et al. (2020) Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr 123, 1216–1226. [DOI] [PubMed] [Google Scholar]
- 103. Ozkul C, Yalinay M & Karakan T (2020) Structural changes in gut microbiome after Ramadan fasting: a pilot study. Benef Microbes 11, 227–233. [DOI] [PubMed] [Google Scholar]
- 104. Su J, Wang Y, Zhang X, et al. (2021) Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Am J Clin Nutr 113, 1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gabel K, Marcell J, Cares K, et al. (2020) Effect of time restricted feeding on the gut microbiome in adults with obesity: a pilot study. Nutr Health 26, 79–85. [DOI] [PubMed] [Google Scholar]
- 106. Zaiss MM, Jones RM, Schett G, et al. (2019) The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest 129, 3018–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Papageorgiou M & Biver E (2021) Interactions of the microbiome with pharmacological and non-pharmacological approaches for the management of ageing-related musculoskeletal diseases. Ther Adv Musculoskelet Dis 13, 1759720X211009018. [DOI] [PMC free article] [PubMed] [Google Scholar]