Abstract
Aromatic dicationic compounds, such as pentamidine, have potent antimicrobial activities. Clinical use of these compounds has been restricted, however, by their toxicity and limited oral activity. A novel approach, using amidoxime derivatives as prodrugs, has recently been proposed to overcome these limitations. Although results were presented for amidoxime derivatives of only one diamidine, pentamidine, the authors in the original proposal claimed that amidoxime derivatives would work as effective prodrugs for all pharmacologically active diamidines. Nine novel amidoxime derivatives were synthesized and tested in the present study for activity against Pneumocystis carinii in corticosteroid-suppressed rats. Only three of the nine compounds had significant oral anti-Pneumocystis activity. The bisbenzamidoxime derivatives of three direct pentamidine analogs had excellent oral and intravenous activities and reduced acute host toxicity. These compounds are not likely candidates for future drug development, however, because they have chronic toxic effects and the active amidine compounds have multiple sites susceptible to oxidative metabolism, which complicates their pharmacology and toxicology. Novel diamidoximes from three other structural classes, containing different groups linking the cationic moieties, lacked significant oral or intravenous anti-Pneumocystis activity, even though the corresponding diamidines were very active intravenously. Both active and inactive amidoximes were readily metabolized to the corresponding amidines by cell-free liver homogenates. Thus, the amidoxime prodrug approach may provide a strategy to exploit the potent antimicrobial and other pharmacological activities of selected, but certainly not all, aromatic diamidines.
Aromatic dicationic compounds, including bisbenzamidines and dicationically substituted bisbenzimidazoles and carbazoles, have excellent experimental anti-Pneumocystis activities (14, 40, 46, 48, 50, 51) and are also active against other microbial pathogens, including protozoan parasites (2–5, 10, 38, 41, 43, 44), fungi (45), and some viruses (25–27, 49, 53). Aromatic dications also possess other pharmacological properties, including antiinflammatory and anticoagulant activities (29–37). Two problems hindering development of these compounds as new drugs, however, are limited oral bioavailability and toxicity (24, 38, 48, 51).
Recent studies of pentamidine metabolism (7–9, 21–23) have led to a novel approach to overcome the limited oral bioavailability and acute toxicity. Aromatic diamidoximes are hypothesized to be orally bioavailable prodrugs that are readily reduced by drug-metabolizing enzymes to the active aromatic amidines (19, 21, 22), resulting in excellent antimicrobial activity with reduced acute host toxicity.
Amidoximes were first shown by Lamb and White to be active against experimental African trypanosomiasis (42) and then later were shown to be active against other microorganisms (1, 17, 18, 28). Although activities were often reported for both amidoximes and corresponding amidines, no mention was made in these early publications that metabolic activation was required for in vivo activity of the amidoximes. Moreover, no systematic studies were performed to determine which analogs were orally active and if the amidoxime derivatives had increased oral activity compared to the amidines. Thus, the concept of amidoximes as prodrugs of amidines was not raised in earlier studies.
The hypothesis that amidoximes might be useful prodrugs resulted from research examining the metabolism of pentamidine (6–9, 21, 22). Two primary oxidative metabolites identified were the mono- and diamidoximes, formed by N-hydroxylation of pentamidine. Although the diamidoxime derivative of pentamidine has little or no activity against three protozoan parasites in vitro, both the mono- and diamidoximes were active against African trypanosomes and Leishmania spp. when given to experimental animals subcutaneously (19, 21–23, 39). The diamidoxime given orally to rats was absorbed from the gut and converted to pentamidine, a reaction subsequently shown to be catalyzed by an oxygen-independent hepatic reductase activity (21, 22). These observations led to the proposal that amidoxime derivatives, in general, are effective, orally absorbed prodrugs for all pharmacologically active amidine-containing compounds (19). However, the only amidoximes tested were derivatives of pentamidine. We recently demonstrated that two novel amidoximes of 2,5-bis[4-amidinophenyl]furan were highly active orally and intravenously (13). Moreover, Weller and coworkers demonstrated that amidoximes of potent monoamidine fibrinogen receptor antagonists greatly enhanced their oral bioavailability (54).
With this promising background, we began to synthesize potential amidoxime prodrugs of our most active, least toxic diamidines. Results presented here, however, demonstrate that amidoximes are not effective prodrugs for all aromatic dicationic compounds. The nature of the linker between the two amidoxime moieties plays a key role in determining if a particular diamidoxime has oral anti-Pneumocystis activity. Diamidoxime derivatives of the very promising bisbenzimidazole and carbazole classes of dications, and bisbenzamidoximes that contain additional nitrogen atoms in the aliphatic linkers, had little or no anti-Pneumocystis activity, even though the parent diamidines had excellent intravenous activity. Variability in activity does not appear to be caused by differences in enzymatic reductase activity, since both active and inactive diamidoximes were metabolized by cell-free liver homogenates.
MATERIALS AND METHODS
Synthesis of compounds.
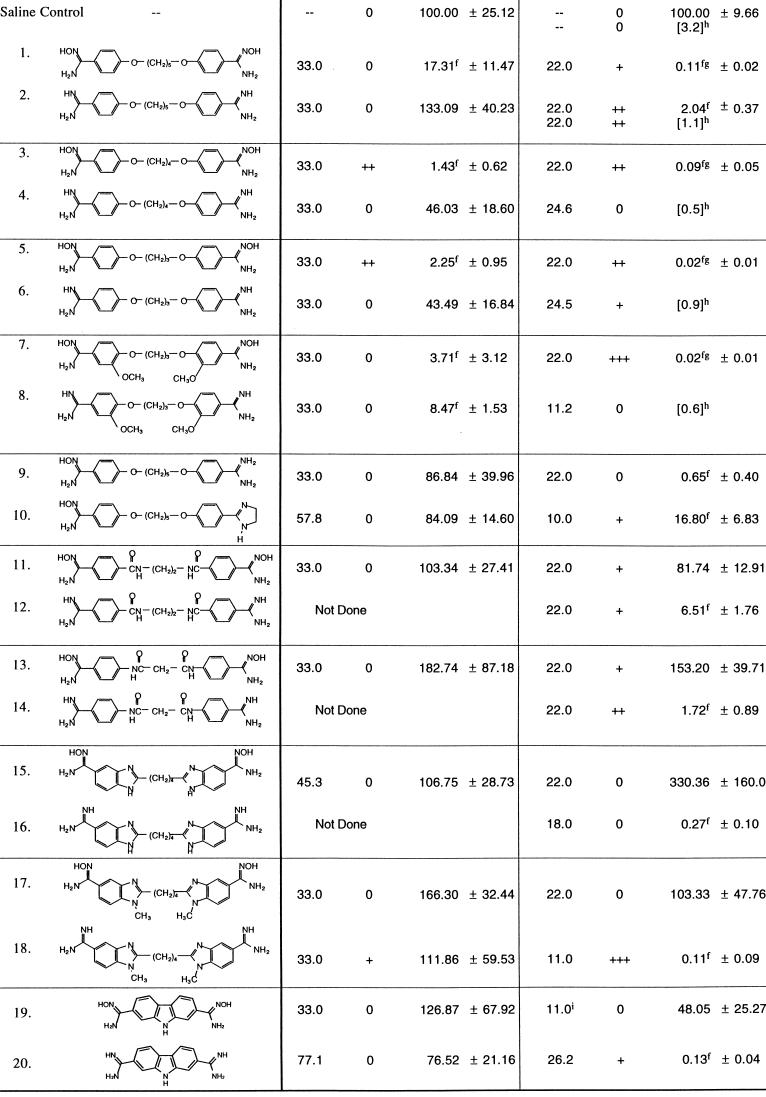
The known diamidine compounds tested in this study were synthesized in our laboratory by previously described methods (7, 40, 48, 51). The novel diamidines 12, 14, 18, and 19 (Table 1) were prepared by similar methods. The known di- and monoamidoximes (compounds 1 and 9 [Table 1]) of pentamidine were prepared according to the procedures of Clement and Raether (23). All novel amidoximes were prepared by similar procedures, with the exception of compound 19, which was prepared by a Pinner synthesis from the corresponding dinitriles. Each compound was characterized by high-performance liquid chromatography (HPLC), elemental analysis, high-resolution fast atom bombardment mass spectrometry, and proton magnetic resonance. Structures of test compounds are shown in Table 1, and the melting points and elemental analyses for the novel compounds are given in Table 2.
TABLE 1.
Anti-Pneumocystis activities of novel amidoximes and corresponding amidines
| Compound no. | Structure | Oral dosinga
|
i.v. dosingb
|
||||
|---|---|---|---|---|---|---|---|
| μmol/kg | Toxicitye | % Saline controlc ± SE | μmol/kg | Toxicitye | % Saline controld ± SE | ||
 | |||||||
Each compound was given by gavage to at least six rats once daily for 14 days. One rat dosed with compound 3, one dosed with compound 5, and one dosed with compound 18 died (on days 13, 7, and 11, respectively) during treatment.
Each compound was given via tail vein injection to at least six rats once daily for 14 days. Three rats died during treatment with compound 7, and three died during treatment with compound 18. i.v., intravenous.
Cysts per gram of lung were 37.7 × 106 for the oral saline control group (n = 6).
Cysts per gram of lung were 44.7 × 106 (n = 65) for the i.v. saline control group and 0.9 × 106 (n = 64) for the i.v. pentamidine group.
Toxicity scores are subjective evaluations of overt toxicity in dexamethasone-immunosuppressed rats. Scores of 0 indicate no observable deleterious effects from dosing, whereas +++, the highest score assigned to these compounds, indicates death of approximately 50% of the group. See the text for a more complete description.
Significantly different from appropriate saline control group; P < 0.05 (Student’s t test).
Significantly different from i.v. pentamidine group; P < 0.05 (Student’s t test).
Numbers in brackets are histologic scores determined subjectively from cysts detected in stained lung sections. Scores range from the lowest possible score of 0.5 to the highest of 4.0. Data reprinted from the Journal of Medicinal Chemistry (50).
Tested at a lower dose due to insufficient quantities available.
TABLE 2.
Physical data of novel amidines and amidoximes
| Compound no. | Formula | Melting point (°C) | Mass (M+ H) calculated/found | Elemental analysis (calculated/found [%])
|
||
|---|---|---|---|---|---|---|
| C | H | N | ||||
| 3 | C18H22N4O4 · 2C4H4O4 | 154 | 359.1719/359.1762 | NDa | ND | ND |
| 5 | C17H20N4O4 · 2C4H4O4 | 158–159 | ND | 52.08/52.15 | 4.90/4.95 | 9.72/9.68 |
| 7 | C27H32N4O14 · H2O | 134 | 405.1774/405.1795 | 49.53/49.54 | 5.24/5.31 | 8.56/8.40 |
| 10 | C21H26N4O3 · HCl · O.8H2O | 196–198 | ND | 58.21/57.99 | 6.65/6.55 | 12.93/13.29 |
| 11 | C18H20N6O4 · 2C4H4O4 | 207–208 | 385.1624/385.1604 | 50.65/50.51 | 4.58/4.68 | 13.63/13.38 |
| 12 | C18H20N6O2 · 2HCl · 0.4H2O | >300 | 353.1725/353.1752 | 49.99/50.24 | 5.31/5.54 | 19.43/19.09 |
| 13 | C17H18N6O4 · 2C4H4O4 | 159–161 | 371.1467/371.1461 | 49.84/49.67 | 4.35/4.44 | 13.95/14.20 |
| 14 | C17H18N6O2 · 2HCl | >300 | 339.1569/339.1552 | 49.65/49.74 | 4.90/4.95 | 20.43/20.33 |
| 15 | C20H22N8O2 · 2HCl | 284–287 | 407.1957/407.1956 | 59.10/58.88 | 5.46/5.57 | 27.57/27.35 |
| 17 | C22H26N8O2 · 2C4H4O4 | 145–147 | 435.2256/435.2245 | 54.05/53.93 | 5.14/5.34 | 16.81/16.70 |
| 18 | C22H26N8 · 2HCl · 1.7H2O | 280–282 | 403.2358/403.2369 | 52.22/52.30 | 6.25/6.15 | 22.14/21.99 |
| 19 | C14H13N5O2 · 2C4H4O4 | >300 | 284.1148/284.1141 | 51.27/50.51 | 4.11/4.14 | 13.59/12.50 |
ND, not done.
Anti-Pneumocystis activity.
The induction and treatment of Pneumocystis pneumonia in the rat model were carried out according to published methods (40, 50, 51). Adult male Sprague-Dawley rats, barrier raised, not certified virus free, and weighing 150 to 200 g, were obtained from Hilltop Laboratories (Scottdale, Pa.). The individually caged animals were begun immediately upon arrival on an immunosuppressive regimen consisting of a low-protein (8%) diet (Zeigler Brothers, Gardner, Pa.) and drinking water containing tetracycline (0.5 mg/ml) and dexamethasone (1.0 μg/ml). This regimen was continued for the next 8 weeks, with animals monitored daily and weighed weekly. At the beginning of the 7th week, animals were divided into groups of at least six animals per group and the test compounds were administered daily for 14 days either orally by gavage or intravenously by tail vein injection. Compounds were routinely tested orally at 33 μmol kg of body weight−1 day−1 and intravenously at 22 μmol kg of body weight−1 day−1. Saline- and pentamidine-treated groups were included as negative and positive controls, respectively.
All animals were sacrificed at the end of the 8th week by chloroform inhalation. The left lung was excised, placed in cold Hanks balanced salts minus Ca2+ and Mg2+ (HBSS−), then weighed, ground through a no. 60 wire mesh screen, and suspended 1:10 (wt/vol) in 10 mM β-mercaptoethanol in HBSS−. Slides were prepared by spotting 5 μl of lung homogenate and were allowed to air dry. Slides were treated with acid and stained with cresyl violet (11), and the cysts were counted by using a blinded protocol. The number of cysts per gram of original lung tissue was calculated, and the values for groups were reported as percentages of values for saline-treated controls (51).
In vivo toxicities of test compounds.
Preliminary evaluations of relative toxicities of test compounds were performed in two ways. First, the dexamethasone-immunosuppressed test rats were closely observed throughout the 14 days of intravenous or oral administration for overt toxic responses. Animals were observed closely for a 10- to 15-min period following injection of the test drug each day for signs of acute toxicity, including the hypotensive response (paling of eyes and paws, dyspnea, lethargy, and decreased body temperature) elicited by intravenous pentamidine at its effective dose. Their overall health and general well-being were observed and recorded on a daily basis for the remainder of the experiment. Excessive weight loss (more than a twofold loss compared to the saline controls over the 2-week dosing period) was considered a key indicator of declining health due to drug toxicity. At necropsy, the liver, spleen, kidneys, and pancreas were removed from each animal and examined for gross pathology. Subjective scores (40, 51) of toxicity associated with multiple dosing were assigned to each compound (Table 1) and are discussed further in Results and Discussion.
The second method used to evaluate relative toxicities of test compounds was to perform preliminary dose escalation studies with rats that were not immunosuppressed by dexamethasone treatment. Adult male Sprague-Dawley rats, barrier raised, not certified virus free, and weighing 300 to 450 g at the time of testing were obtained from Hilltop Laboratories. The individually caged animals were given water and standard rat chow (Agway, Syracuse, N.Y.) ad libitum. Each animal was injected via the tail vein with one dose of test compound. Each animal was closely observed for 15 min postinjection, especially for signs of hypotension elicited by pentamidine, as described above, and was monitored again at 30 min, 60 min, and 24 h postinjection.
In vitro metabolism.
In vitro metabolism of diamidoximes by rat liver homogenate 9,000 × g supernatants, postmitochondrial 105,000 × g supernatants, or microsomal fractions was performed as previously described (8, 9). Briefly, adult male barrier-raised Sprague Dawley rats (Hilltop Laboratories) were allowed free access to rat chow (22.5% protein, 5.5% fat, and 4.5% fiber, with essential vitamins and minerals; Agway) and tap water. Rats were euthanized by decapitation, and the livers were removed immediately, rinsed with 50 mM potassium phosphate buffer (pH 7.4), and placed on ice. All subsequent steps were performed at 4°C. The livers were minced and homogenized, and 9,000 × g supernatants, 105,000 × g supernatants, or 105,000 × g microsomal pellets were prepared as described elsewhere (6). Each fraction was assayed for protein content (15) and stored at −80°C. Fractions from rat kidneys, lungs, hearts, and brains were prepared in a similar fashion, as previously described (8, 9).
Reaction mixtures for all cell-free homogenates consisted of 1.5 ml of 50 mM potassium phosphate buffer (pH 7.4), 0.5 ml of cofactor solution (2 mg of NADPH/ml, 1.6 mg of MgCl2/ml, 1.04 mg of glucose-6-phosphate/ml, and 2 U of glucose-6-phosphate dehydrogenase/ml in 50 mM potassium phosphate buffer [pH 7.4]), 0.5 ml of tissue homogenate, and 0.5 ml of the appropriate substrate at concentrations shown in the figure legends. Reactions were started by adding substrate; then mixtures were incubated at 37°C in a shaking water bath for times shown in the figure legends. Reactions were terminated by extraction over C18 cartridges, and extracts were assayed as described below.
Metabolic experiments with intact cultured cells were routinely performed with the BRL 3A hepatocyte line, obtained from the Lineberger Tissue Culture Facility of the University of North Carolina at Chapel Hill. Cells were cultured in Costar (Cambridge, Mass.) 25-cm2 tissue culture flasks at 37°C under a moist atmosphere of 5% CO2 and 95% air in Ham’s F-12 medium (Gibco BRL, Gaithersburg, Md.) containing 5% fetal bovine serum (HyClone Laboratories, Inc., Logan, Utah). Confluent cultures were treated with 0.25% trypsin solution (Sigma Chemical Co., St. Louis, Mo.), and then approximately 2 × 105 cells/well were subcultured into Costar 6-well tissue culture chambers and the cells were allowed to grow to confluency. Fresh medium (10 ml) was added to each culture well, and incubations were started by adding 100 μl of amidoxime stock solution, prepared in sterile water, to a final concentration of 10 μM. Cell cultures were incubated at 37°C under a moist atmosphere of 5% CO2 and 95% air for 24 h, and then aliquots of culture supernatants were extracted and assayed for metabolites as described below. Similar metabolic experiments were performed with cultured J774 A.1 mouse monocyte-macrophage cells cultured in Dulbecco modified Eagle F-12 medium containing 10% fetal bovine serum and H9c2 rat heart myoblast cells cultured in Dulbecco modified Eagle H medium containing 10% fetal bovine serum.
Samples were extracted by solid-phase extraction and were assayed by HPLC by methods similar to those previously described (8, 9). Briefly, samples spiked with 2,5-bis[4-(N-isopropylamidino)phenyl]furan dihydrochloride as the internal standard were extracted over activated C18 Bond Elut cartridges (Varian Associates, Sunnydale, Calif.), washed with water, and eluted first with 100% acetonitrile and then with 75% acetonitrile–25% water containing 15 mM triethylamine and 35 mM acetic acid. The diamidoxime substrates elute in the 100% acetonitrile phase, while mono- and diamidine products elute in the acetonitrile-water mix containing triethylamine and acetic acid. Eluates were evaporated to dryness at 40°C under a gentle stream of nitrogen and were resuspended in 10% acetonitrile in HPLC-grade water.
Compounds were resolved by using a Hewlett-Packard (Avondale, Pa.) model 1090 HPLC equipped with an HP 1050 variable wavelength detector, a 4.6- by 250-mm Zorbax RX diisopropyl C8 column (Mac-Mod, Chadd’s Ford, Pa.) maintained at 40°C, and a Vectra 486/66U computer with HP ChemStation software. The mobile phase consisted of 15 mM triethylamine and 35 mM acetic acid in HPLC-grade water for pump A, and 15 mM triethylamine and 35 mM acetic acid in 75% aqueous acetonitrile in water for pump B. The solvent flow rate was 1.5 ml/min, and the solvent gradient ran from 0% B to 25% B at 22 min, to 40% B at 25 min, and then to 90% B at 35 min, followed by a 5-min recycle period. All solvents and reagents used for the assays were HPLC grade. For quantitative experiments, amounts of metabolites formed were calculated, by using peak area ratios of authentic standard to internal standard, from standard addition curves generated by spiking standards into tissue homogenates or tissue culture medium.
RESULTS AND DISCUSSION
Anti-Pneumocystis activities of bisbenzamidoximes. A major limitation of aromatic dicationic compounds as antimicrobial agents has been their lack of oral activity. Results from the present study demonstrate that four bisbenzamidoximes, diamidoxime derivatives of pentamidine and three novel direct pentamidine analogs, have excellent oral anti-Pneumocystis activities in the immunosuppressed-rat model of disease. Compound structures and anti-Pneumocystis activities of these bisbenzamidoximes and their corresponding bisbenzamidines are given in Table 1.
In agreement with previous results (19), the diamidoxime derivative (compound 1) of pentamidine had significant anti-Pneumocystis activity when given orally by gavage at 33 μmol kg−1 day−1, compared to the oral saline control group (Table 1). The novel bisbenzamidoxime compounds 3, 5, and 7 were even more active, with the lowest cyst scores reported to date from our laboratories for orally administered aromatic dicationic compounds (40, 48, 51).
In contrast, the corresponding diamidines were less active when given orally (Table 1). Pentamidine (compound 2) was completely inactive at the oral daily dose of 33 μmol kg−1 for 14 days. Cyst scres for diamidine compounds 4 and 6 were slightly, but not significantly, reduced compared to the oral saline controls. Cyst counts were more than 15-fold higher for these diamidines than for their corresponding diamidoximes, compounds 3 and 5 (Table 1). The only diamidine compound with good oral activity was compound 8. Its diamidoxime analog, compound 7, had a slightly lower mean cyst score.
Each of the bisbenzamidoxime compounds 1, 3, 5, and 7 also had excellent anti-Pneumocystis activity when given intravenously at 22 μmol kg−1 once daily for 14 days (Table 1). Mean cyst counts were greatly reduced compared to those for the saline controls and were also significantly lower than the mean count for the intravenous pentamidine (compound 2) control group. The diamidine compounds 4, 6, and 8 have previously been shown to have intravenous activity (40, 48; Table 1). Direct comparisons of intravenous anti-Pneumocystis activities of these diamidines to those of the corresponding diamidoximes, compounds 3, 5, and 7, cannot be made because the intravenous activities of diamidines were previously evaluated by a different cyst score method and at slightly different doses. Subjectively, however, diamidoximes do appear to compare favorably with the corresponding diamidines with regard to intravenous efficacy.
Anti-Pneumocystis activities of monobenzamidoximes.
Substitution of amidoxime moieties for both dicationic groups may be required for oral but not for intravenous anti-Pneumocystis activity. Two mono-substituted amidoximes were synthesized and tested (Table 1). Compound 9, the monoamidoxime analog of pentamidine, was significantly active at an intravenous dose of 22 μmol kg−1 day−1. The compound was inactive at an oral dose of 33 μmol kg−1 day−1, however. A second monoamidoxime, compound 10, which contains one amidoxime moiety and one imidazoline cationic group, was active intravenously at a dose of 10 μmol kg−1 day−1 but was not active when given orally at the high dose of 58 μmol kg−1 day−1. Thus, both cationic moieties apparently must be in the amidoxime form for oral uptake to occur.
Diamidoximes that lack activity against Pneumocystis.
Not all diamidoxime compounds have improved antimicrobial activity compared to the diamidine analogs, indicating that the prodrug approach will not work for all classes of aromatic dicationic compounds. The potent antitrypanosomal diamidine compound, diminazine (Berenil), has excellent in vivo activity against African trypanosomes and Leishmania; its diamidoxime derivative, however, was only marginally active when given subcutaneously (20). Diminazine has a triazine bridge connecting the benzamidino moieties. Our current results indicate that diamidoximes from three other classes of compounds lack oral and intravenous activity against Pneumocystis. These diamidoximes, like the diamidoxime of diminazine, also have nitrogen atoms in positions other than the amidoxime moieties and lack the ether oxygens in the bridge between aromatic groups.
(i) Bisbenzamidoximes that lack anti-Pneumocystis activity.
Two novel bisbenzamidine compounds (compound 12 and 14) (Table 1), each containing internal amide groups as part of the bridge linking the benzamidine moieties, were synthesized and tested for activity against Pneumocystis. The compounds were synthesized because they were hypothesized to have improved DNA binding properties, increased aqueous solubility, and reduced metabolism along the cleavage pathways that have been proposed to decrease the activity and increase the toxicity of pentamidine. Both diamidines had significant anti-Pneumocystis activity when administered at 22 μmol kg−1 day−1 intravenously. The diamidoxime derivatives (compounds 11 and 13) of each compound, however, were not active, either intravenously (22 μmol kg−1 day−1) or orally (33 μmol kg−1 day−1) (Table 1).
(ii) Bisbenzimidazole and carbazole diamidoximes that lack anti-Pneumocystis activity.
Dicationically substituted bisbenzimidazoles have excellent activity against Pneumocystis, with selected compounds showing marked improvements in toxicity and pharmacologic properties compared to pentamidine (51). However, oral bioavailability for these compounds also appears to be limited. To determine if the prodrug approach could be used for this class of aromatic dications, we synthesized and tested the diamidoxime derivative (compound 15) (Table 1) of one of our most active bisbenzimidazole diamidines (compound 16). Although the diamidine has been shown to have excellent anti-Pneumocystis activity at 18 μmol kg−1 day−1 intravenously, the diamidoxime was completely inactive intravenously and orally (Table 1). One other bisbenzimidazole diamidoxime, in which the benzimidazole nitrogen was substituted with a methyl group (compound 17) (Table 2), was synthesized and tested. The diamidine analog (compound 18) had excellent intravenous activity at 22 μmol kg−1 day−1. The diamidoxime, however, lacked significant intravenous and oral activity (Table 1).
Finally, a new series of aromatic dicationic compounds containing a carbazole nucleus linking the dications have been synthesized. Several members of this class, most notably compound 20 (Table 1), have been shown to have superb activity against Pneumocystis carinii and Cryptosporidium parvum in vivo and are also active against several other opportunistic pathogens in vitro (47). The diamidoxime 19 was synthesized as a potential prodrug of compound 20 to increase its oral bioavailability. However, compound 19 was inactive when given orally at 33 μmol kg−1 day−1 (Table 1).
Toxicity of bisbenzamidoximes.
A second major factor limiting development of aromatic dications as antimicrobial drugs has been toxicity. We report here preliminary observations of acute and subchronic toxicity in rats treated intravenously and orally with three bisbenzamidoximes active against Pneumocystis. Although the toxicity information presented is mainly anecdotal and should not be considered definitive, these evaluations do permit important preliminary comparisons of relative toxicities of compounds.
(i) Acute toxicity in nonimmunosuppressed rats.
A preliminary dose escalation study was performed with non-dexamethasone-treated rats to compare overt acute toxic responses elicited by diamidoxime compounds 1, 5, and 7 and their corresponding diamidines. Compound 3 was not available in sufficient quantity for evaluation of acute toxicity. Overt acute adverse reactions following single intravenous bolus injections were greatly reduced for the three bisbenzamidoximes compared to those for the bisbenzamidines, and no adverse reactions were observed after high single oral doses of the bisbenzamidoximes.
Normal rats injected over 30 s with 20 μmol of pentamidine (compound 2) kg−1 appeared hypotensive, with rapid paling of extremities, hypoactivity, and dyspnea, which progressed to slight cyanosis of extremities. Increased lacrimation and minor hind-leg ataxia were observed immediately before the onset of hypoactivity. Animals appeared to fully recover within 5 min. Animals injected with pentamidine at 40 μmol kg−1 had immediate severe hind-limb muscular contractions, increased salivation, dyspnea, initial paling of the extremities that progressed to marked cyanosis, and profound hypoactivity, with no movement for at least 5 min. All animals recovered approximately 20 min after injection. In contrast, the diamidoxime analog (compound 1) of pentamidine, given in the same manner, caused no observable adverse reactions from 20 to 60 μmol kg−1. Minor toxic responses, including barely observable hind-leg ataxia and slight hypoactivity, were observed at 80 μmol kg−1, with complete recovery within 5 min postinjection. Bolus injections above 120 μmol kg−1 produced severe dyspnea and profound hypoactivity.
Similar results were observed for diamidoximes 5 and 7 compared to their respective diamidine analogs (data not shown). The diamidine compound 6 did appear less acutely toxic than pentamidine, while the diamidine compound 8 was slightly more acutely toxic. Finally, no overt acute toxic responses were seen when animals were given any of the test compounds per os, including single oral doses as high as 160 μmol kg−1 for each of the diamidoxime compounds 1, 5, and 7.
(ii) Subchronic toxicity in corticosteroid-immunosuppressed rats.
Although the bisbenzamidoxime compounds 1, 5, and 7 appear to cause less overt acute toxicity following single intravenous injections, the diamidoximes do retain substantial overt toxicity in the dexamethasone-suppressed rats treated with test compounds for 14 days. Subjective scores of multiple dosing toxicity were assigned and are included in Table 1. Pentamidine given at its intravenous therapeutic dose of 22 μmol kg−1 day−1 has been assigned a subjective toxicity score of ++, primarily because of its hypotensive response but also because it can cause inflammation of the tail with multiple injections. The four bisbenzamidoximes (compounds 1, 3, 5, and 7) tested were assigned scores of + to +++ when given intravenously. Each bisbenzamidoxime caused substantial inflammation of the tail. The tails of most animals in each group became reddened and swollen after 3 to 5 days of injections; then many became severely necrotic before the end of the 14-day experiment. The response to compound 3 was particularly severe. In addition, diamidoxime compound 7 caused deaths late in the treatment regime. Three of six animals treated intravenously with compound 7 at 22 μmol kg−1 died late (days 11 and 12) during treatment. Animals that died became very pale 2 to 3 days before death, and internal organs were blanched and grossly necrotic upon necropsy. Finally, one animal treated orally at 33 μmol kg−1 day−1 with diamidoxime compound 3 and one treated orally with compound 5 died during the experiment. No other adverse reactions were detected in orally treated animals, and the deaths may not have been treatment related.
Metabolism of amidoximes. (i) Metabolism of bisbenzamidoximes active against Pneumocystis.
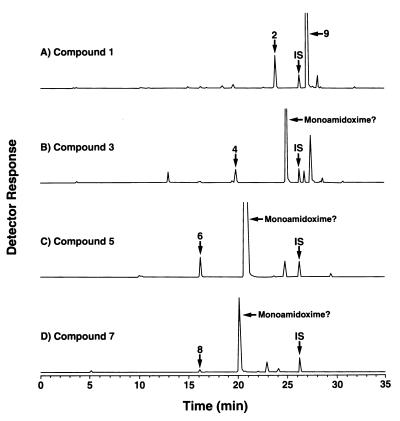
Data presented in Fig. 1A confirm previous observations that rat liver homogenates reduce the diamidoxime (compound 1) of pentamidine, forming the monoamidine-monoamidoxime (compound 9) and smaller quantities of the diamidine, pentamidine (compound 2). The metabolites have previously been identified by mass spectrometry (16, 52) and were confirmed in the present study by coelution with authentic standards.
FIG. 1.
Metabolism of bisbenzamidoximes (compound 1, 3, 5, or 7) by rat liver homogenate 9,000 × g supernatant fraction. Homogenates containing 167 μM diamidoxime as substrate plus cofactor solution were incubated for 30 min at 37°C, then assayed by HPLC as described in Materials and Methods. Compound structures are shown in Table 1. IS, internal standard.
Three other bisbenzamidoximes (compounds 3, 5, and 7) with good oral anti-Pneumocystis activity appear to be metabolized similarly. The smaller peaks in Fig. 1B through D coeluted exactly with the authentic diamidine standard compounds 4, 6, and 8, respectively. Although synthetic standards were not available for the monoamidine-monoamidoxime derivatives of compounds 3, 5, and 7, the relative retention times of the chromatographic peaks are entirely consistent with those predicted for the monoamidoxime derivatives. Most monoamidine-monoamidoxime derivatives have been difficult to synthesize and to analyze by mass spectrometric methods developed to characterize pentamidine metabolites (7, 16, 52). Positive identification of these putative metabolites must await successful development of new synthetic and analytical approaches.
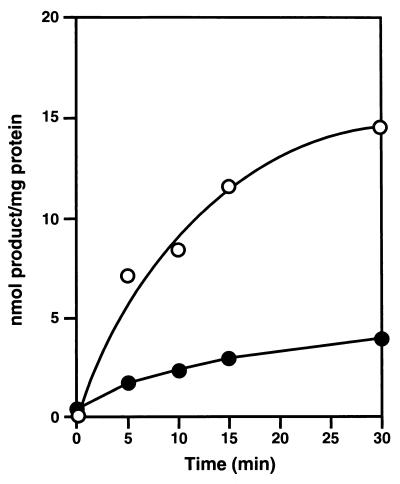
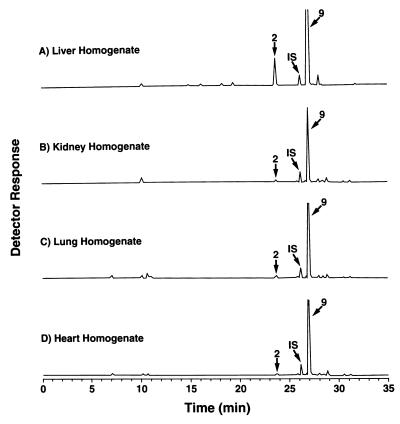
Although oxidative N-hydroxylation of amidines to form amidoximes is known to be catalyzed by specific cytochromes P-450 (6, 22), reduction of amidoximes back to amidines has been reported to be catalyzed by a non-cytochrome P-450-dependent reductase activity (22). Data presented in Fig. 2 and 3 are consistent with these observations (22). First, reductase activity was present in the postmitochondrial microsomal fraction, as reported; however, activity was even higher in the postmitochondrial supernatant fraction (Fig. 2). Second, reductase activity was detected in cell extracts from liver, as reported (22), but activity is also present in homogenates from rat kidneys, lung, heart, and brain (Fig. 3). Finally, intact cells of the established liver cell line BRL 3A (Fig. 4A), the heart cell line H9c2, and the macrophage cell line J774 A.1 (data not shown) all absorbed and metabolized the diamidoxime compound 1.
FIG. 2.
Time course of reduction of bisbenzamidoxime compound 1 to its monoamidoxime-monoamidine product (compound 9) by rat liver microsomes (•) and the postmitochondrial supernatant fraction (○). Reaction conditions and the HPLC assay were as described for Fig. 1 and in Materials and Methods.
FIG. 3.
Metabolism of the bisbenzamidoxime compound 1 by 9,000 × g supernatants from homogenates of rat liver, kidney, lung, and heart. Incubation conditions were as described for Figure 1. Compound structures are shown in Table 1.
FIG. 4.
Metabolism of bisbenzamidoximes by intact BRL 3A hepatocytes in vitro. Cells cultured in Ham’s F-12 medium containing 5% fetal bovine serum and 10 μM diamidoxime substrate were incubated for 24 h at 37°C under 5% CO2. The extracellular medium was extracted and assayed by HPLC as described for Fig. 1. Compound structures are shown in Table 1.
Diamidoxime prodrugs effective against extracellular parasites, such as Pneumocystis, apparently must enter host cells and be chemically reduced back to the active amidine, then released extracellularly and taken up by the infectious organism. This process of cell uptake, metabolism, and release occurs readily in intact BRL 3A hepatocytes cultured in vitro (Fig. 4). Each of the bisbenzamidoximes 1, 3, 5, and 7 added to BRL 3A cultures was metabolized predominantly to the corresponding diamidine, then released into the culture supernatants. For each chromatogram of Fig. 4, the peak labeled as a diamidine coeluted exactly with the authentic standard of that diamidine.
(ii) Metabolism of diamidoximes that lack anti-Pneumocystis activity.
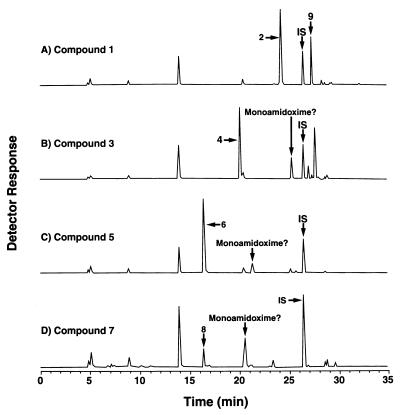
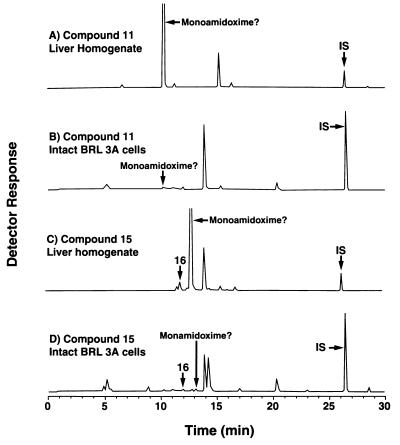
The novel bisbenzamidoxime compounds 11 and 13, the bisbenzimidazole diamidoximes 15 and 17, and the carbazole compound 19 had little or no activity orally or intravenously against Pneumocystis, even though the corresponding diamidines were very active intravenously (Table 1). Lack of activity is not associated with inability to be metabolized in vitro. Incubation of diamidoxime 15 with rat liver homogenates produced two new peaks, one that coeluted with the authentic standard for diamidine 16 and a larger peak with a relative retention time consistent with that predicted for the corresponding monoamidoxime (Fig. 5C). Similar results were obtained with diamidoximes 11 (Fig. 5A), 13, 17, and 19 (data not shown).
FIG. 5.
Metabolism of diamidoximes that lack activity against Pneumocystis. (A and C) Metabolism by rat liver homogenate supernatant fractions as described for Fig. 1; (B and D) metabolism by intact BRL 3A hepatocytes as described for Fig. 4. Compound structures are shown in Table 1.
Preliminary data with intact cultured cells suggest that one possible hypothesis for the absence of anti-Pneumocystis activity may be reduced cellular uptake and metabolism of the inactive diamidoximes. In contrast to the results presented above for the active bisbenzamidoximes 1, 3, 5, and 7 (Fig. 4), the inactive diamidoximes 11 (Fig. 5B) and 15 (Fig. 5D) were not readily metabolized by intact BRL 3A hepatocytes in culture. Little or no detectable diamidine or putative monoamidine-monoamidoxime products were present in the culture medium.
Structural features causing diamidoximes 11, 13, 15, 17, and 19 to have reduced anti-Pneumocystis activity have not been determined. Inactive diamidoximes have two obvious structural features that differ from those of active diamidoximes. Inactive compounds all contain additional nitrogen atoms in positions other than the amidoxime moieties, and they lack ether oxygens linking the alkane chains to the aromatic rings. The additional nitrogen atoms of inactive compounds 11 and 13 are part of the internal amide groups; reversing the order of the CO and NH groups had no effect on activity (Table 1). The additional nitrogens of compound 15 are part of the benzimidazole groups; N-methyl substitution (compound 17) did not improve activity (Table 1). A diamidoxime from the exciting new carbazole class of dicationic compounds (47) was also shown to be inactive against P. carinii orally and was not metabolized by intact cells. Compounds from this series contain one additional nitrogen in the carbazole nucleus. Inactive compounds 11 and 13 have oxygens in the bridge between the benzamidoxime groups, although they are carbonyl and not ether oxygens. Compounds 15, 17, and 19 entirely lack oxygens in the bridge. Diamidoxime derivatives from one other class of aromatic dications, the very promising furan series (12–14), have recently been shown to have anti-Pneumocystis activity orally and intravenously (13). The link between the benzamidoxime moieties and the furan bridge of these compounds is via carbon-carbon bonds, although an ether oxygen is present in the furan ring of the bridge. Further research using structurally diverse compounds to examine cellular transport, metabolism, and subsequent release is in progress in an effort to understand and predict why some amidoxime derivatives are not active.
The present study thus provides additional evidence that the amidoxime prodrug approach should work to improve oral activity for some diamidines. The diamidoxime of pentamidine is indeed an orally effective anti-Pneumocystis agent, and three novel bisbenzamidoxime analogs of pentamidine were even more effective than the pentamidine derivative. Moreover, acute overt toxicity following single bolus intravenous injections of these bisbenzamidoximes was greatly reduced compared to that of the bisbenzamidines. The bisbenzamidoximes, however, are not likely candidates for future drug development because the parent compounds have significant toxicity (40, 48), and they retain multiple sites subject to cytochrome P-450-mediated oxidative metabolism. Finally, the present results demonstrate that the diamidoxime prodrug approach will work for some but not all classes of antimicrobially active aromatic dications. Novel diamidoximes of two bisbenzamidines, two bisbenzimidazole diamidines, and one carbazole diamidine were not active against Pneumocystis intravenously or orally, even though the parent compounds were very active. Although all diamidoximes examined could be metabolized by tissue homogenates, the inactive compounds were not metabolized to the diamidines by intact cultured cells, suggesting that lack of activity may be associated with reduced cellular uptake and reduced subsequent metabolism by intact cells. Current efforts to develop effective new prodrugs focus on development of predictive experimental systems to evaluate uptake, metabolism, and subsequent antimicrobial activity in vitro.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health NCDDG-OI award no. 2-U19-AI33363, National Institutes of Health STTR award no. 1-R41-AI40518, and Pharm-Eco Laboratories, Inc., Lexington, Mass.
We thank Joe Craft for technical assistance in preparing one of the amidoximes.
REFERENCES
- 1.Andrewes C H, King H, Walker J. Experimental chemotherapy of typhus. Anti-rickettsial action of p-sulphonamidobenzamidine and related compounds. Proc R Soc Lond Ser B. 1946;133:20–62. doi: 10.1098/rspb.1946.0002. [DOI] [PubMed] [Google Scholar]
- 2.Apted F I C. Present status of chemotherapy and chemoprophylaxis of human trypanosomiasis in the Eastern hemisphere. Pharmacol Ther. 1980;11:391–413. doi: 10.1016/0163-7258(80)90035-2. [DOI] [PubMed] [Google Scholar]
- 3.Bell C A, Cory M, Fairley T A, Hall J E, Tidwell R R. Structure-activity relationships of pentamidine analogs against Giardia lamblia and correlation of antigiardial activity with DNA-binding affinity. Antimicrob Agents Chemother. 1991;35:1099–1107. doi: 10.1128/aac.35.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell C A, Dykstra C C, Naiman N A, Cory M, Fairley T A, Tidwell R R. Structure-activity studies of dicationically substituted bisbenzimidazoles against Giardia lamblia: correlation of antigiardial activity with DNA binding affinity and giardial topoisomerase II inhibition. Antimicrob Agents Chemother. 1993;37:2668–2673. doi: 10.1128/aac.37.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell C A, Hall J E, Kyle D E, Grogl M, Ohemeng K A, Allen M A, Tidwell R R. Structure-activity relationships of analogs of pentamidine against Plasmodium falciparum and Leishmania mexicana amazonensis. Antimicrob Agents Chemother. 1990;34:1381–1386. doi: 10.1128/aac.34.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger B J. Ph.D. dissertation. Chapel Hill: University of North Carolina; 1992. [Google Scholar]
- 7.Berger B J, Lombardy R J, Marbury G D, Bell C A, Dykstra C C, Hall J E, Tidwell R R. Metabolic N-hydroxylation of pentamidine in vitro. Antimicrob Agents Chemother. 1990;34:1678–1684. doi: 10.1128/aac.34.9.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger B J, Naiman N A, Hall J E, Peggins J, Brewer T G, Tidwell R R. Primary and secondary metabolism of pentamidine by rats. Antimicrob Agents Chemother. 1992;36:1825–1831. doi: 10.1128/aac.36.9.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger B J, Reddy V V, Le S T, Lombardy R J, Hall J E, Tidwell R R. Hydroxylation of pentamidine by rat liver microsomes. J Pharmacol Exp Ther. 1991;256:883–889. [PubMed] [Google Scholar]
- 10.Blagburn B L, Sundermann C A, Lindsay D S, Hall J E, Tidwell R R. Inhibition of Cryptosporidium parvum in neonatal Hsd:(ICR)BR Swiss mice by polyether ionophores and aromatic amidines. Antimicrob Agents Chemother. 1991;35:1520–1523. doi: 10.1128/aac.35.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowling M C, Smith I M, Wescott S L. A rapid staining procedure for Pneumocystis carinii. Am J Med Technol. 1973;39(7):267–268. [PubMed] [Google Scholar]
- 12.Boykin, D. W. 1996. Unpublished data.
- 13.Boykin D W, Kumar A, Hall J E, Bender B C, Tidwell R R. Anti-Pneumocystis activity of bis-amidoximes and bis-O-alkylamidoximes prodrugs. Bioorg Med Chem Lett. 1996;6:3017–3020. [Google Scholar]
- 14.Boykin D W, Kumar A, Spychala J, Zhou M, Lombardy R J, Wilson W D, Dykstra C C, Jones S K, Hall J E, Tidwell R R, Laughton C, Neidle S. Dicationic diarylfurans as anti-Pneumocystis carinii agents. J Med Chem. 1995;38:912–916. doi: 10.1021/jm00006a009. [DOI] [PubMed] [Google Scholar]
- 15.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Bronner U, Ericsson O, Nordin J, Wikstrom I, Abdi Y A, Hall J E, Tidwell R R, Gustafsson L L. Metabolism is an important route of pentamidine elimination in the rat: disposition of 14C-pentamidine and identification of metabolites in urine using liquid chromatography-tandem mass spectrometry. Pharmacol Toxicol. 1995;77:114–120. doi: 10.1111/j.1600-0773.1995.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 17.Buu-Hoi N P, Welsch M, Xuong N D, Thang K U. Une nouvelle famille de composes tuberculostatiques: les amidoximes. Experientia. 1954;10:169. doi: 10.1007/BF02157193. [DOI] [PubMed] [Google Scholar]
- 18.Chabrier P, Maillard G, Quevauviller A. Nouvelles recherches sur les rapports entre structure chimique, activite antibacterienne, antifungique et toxicite, dans la serie des esters de l’acide dithiocarbamique N-disubstitue. Ann Pharm Fr. 1956;14:720–728. [PubMed] [Google Scholar]
- 19.Clement, B. June 1994. German patent DE 4321444A1.
- 20.Clement B, Immel M, Raether W. Metabolic N-hydroxylation of diminazine in vitro. Arzneim-Forsch. 1992;42:1497–1504. [PubMed] [Google Scholar]
- 21.Clement B, Immel M, Terlinden R, Wingen F. Reduction of amidoxime derivatives to pentamidine in vivo. Arch Pharm. 1992;325:61–62. doi: 10.1002/ardp.19923250114. [DOI] [PubMed] [Google Scholar]
- 22.Clement B, Jung F. N-hydroxylation of the antiprotozoal drug pentamidine catalyzed by rabbit liver cytochrome P-450 2C3 or human liver microsomes, microsomal retroreduction, and further oxidative transformation of the formed amidoximes. Drug Metab Dispos. 1994;22:486–497. [PubMed] [Google Scholar]
- 23.Clement B, Raether W. Amidoximes of pentamidine: synthesis, trypanocidal and leishmaniacidal activity. Arzneim-Forsch. 1985;35:1009–1014. doi: 10.1002/chin.198544129. [DOI] [PubMed] [Google Scholar]
- 24.Drake S, Lampasona V, Nicks H L, Schwarzmann S W. Pentamidine isethionate in the treatment of Pneumocystis carinii pneumonia. Clin Pharm. 1985;4:507–516. [PubMed] [Google Scholar]
- 25.Dubovi E J, Geratz J D, Shaver S R, Tidwell R R. Inhibition of respiratory syncytial virus-host cell interaction by mono- and diamidines. Antimicrob Agents Chemother. 1981;19:649–656. doi: 10.1128/aac.19.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubovi E J, Geratz J D, Tidwell R R. Inhibition of respiratory syncytial virus by bis(5-amidino-2-benzimidazolyl)methane. Virology. 1980;103:502–504. doi: 10.1016/0042-6822(80)90207-x. [DOI] [PubMed] [Google Scholar]
- 27.Dykstra, C. C. 1996. Unpublished data.
- 28.Fuller A T, Tonkin I M, Walker J. Chemotherapeutic agents of the sulphone type. II. Sulphones related to benzamidine and benzylamine. J Chem Soc. 1945;1945:633–637. [Google Scholar]
- 29.Geratz J D, Cheng M C F, Tidwell R R. New aromatic diamidines with central alpha-oxyalkane or alpha, omega-dioxyalkane chains. Structure-activity relationships for the inhibition of trypsin, pancreatic kallikrein and thrombin and for the inhibition of the overall coagulation process. J Med Chem. 1975;18:477–481. doi: 10.1021/jm00239a007. [DOI] [PubMed] [Google Scholar]
- 30.Geratz J D, Cheng M C F, Tidwell R R. Novel bis(benzamidino) compounds with an aromatic central link. Inhibitors of thrombin, pancreatic kallikrein, trypsin and complement. J Med Chem. 1976;19:634–639. doi: 10.1021/jm00227a011. [DOI] [PubMed] [Google Scholar]
- 31.Geratz J D, Pryzwansky K B, Schwab J H, Anderle S K, Tidwell R R. Modulation of local and systemic responses in streptococcal cell wall-induced inflammation of the air pouch by two anti-inflammatory bis-benzimidazoles and by cyclosporine A. Am J Pathol. 1993;142:1227–1237. [PMC free article] [PubMed] [Google Scholar]
- 32.Geratz J D, Pryzwansky K B, Schwab J H, Anderle S K, Tidwell R R. Suppression of streptococcal cell wall-induced arthritis by a potent protease inhibitor, bis(5-amidino-2-benzimidazolyl)methane. Arthritis Rheum. 1988;31:1156–1164. doi: 10.1002/art.1780310911. [DOI] [PubMed] [Google Scholar]
- 33.Geratz J D, Shaver S R, Tidwell R R. Inhibitory effect of amidino-substituted heterocyclic compounds on the amidase activity of plasmin and high and low molecular weight urokinase and on urokinase-induced plasminogen activation. Thromb Res. 1981;24:73–83. doi: 10.1016/0049-3848(81)90033-5. [DOI] [PubMed] [Google Scholar]
- 34.Geratz J D, Stevens F M, Polakoski K L, Parrish R F, Tidwell R R. Amidino-substituted aromatic heterocycles as probes of the specificity pocket of trypsin-like proteases. Arch Biochem Biophys. 1979;197:551–559. doi: 10.1016/0003-9861(79)90279-0. [DOI] [PubMed] [Google Scholar]
- 35.Geratz J D, Tidwell R R, Brinkhous K M, Mohammad S F, Dann O, Loewe H. Specific inhibition of platelet agglutination and aggregation by aromatic amidino compounds. Thromb Haemostasis. 1978;39:411–425. [PubMed] [Google Scholar]
- 36.Geratz J D, Tidwell R R, Lombardy R J, Schwab J H, Anderle S K, Pryzwansky K B. Streptococcal cell wall-induced systemic disease. Beneficial effects of trans-bis(5-amidino-2-benzimidazolyl)ethene, a novel macrophage-directed antiinflammatory agent. Am J Pathol. 1991;139:921–931. [PMC free article] [PubMed] [Google Scholar]
- 37.Geratz J D, Tidwell R R, Schwab J H, Anderle S K, Pryzwansky K B. Sequential events in the pathogenesis of streptococcal cell wall-induced arthritis and their modulation by bis(5-amidino-2-benzimidazolyl)methane (BABIM) Am J Pathol. 1990;136:909–921. [PMC free article] [PubMed] [Google Scholar]
- 38.Goa K L, Campoli-Richards D M. Pentamidine isethionate. A review of its antiprotozoal activity, pharmacokinetic properties and therapeutic use in Pneumocystis carinii pneumonia. Drugs. 1987;33:242–258. doi: 10.2165/00003495-198733030-00002. [DOI] [PubMed] [Google Scholar]
- 39.Hall, J. E. 1996. Unpublished data.
- 40.Jones S K, Hall J E, Allen M A, Morrison S D, Ohemeng K A, Reddy V V, Geratz J D, Tidwell R R. Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990;34:1026–1030. doi: 10.1128/aac.34.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King H, Lourie E, Yorke W. Studies in chemotherapy. XIX. Further report on new trypanocidal substances. Ann Trop Med Parasitol. 1938;32:177–192. [Google Scholar]
- 42.Lamb I D, White A C. Some amidines and amidoximes with trypanocidal activity. J Chem Soc. 1939;1939:1253–1257. [Google Scholar]
- 43.Lindsay D S, Blagburn B L, Hall J E, Tidwell R R. Activity of pentamidine and pentamidine analogs against Toxoplasma gondii in cell cultures. Antimicrob Agents Chemother. 1991;35:1914–1916. doi: 10.1128/aac.35.9.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lourie E M, Yorke W. Studies in chemotherapy. XXI. The trypanocidal action of certain aromatic diamidines. Ann Trop Med Parasitol. 1939;33:289–304. [Google Scholar]
- 45.Perfect, J. R. 1996. Unpublished data.
- 46.Spychala J, Boykin D W, Wilson W D, Zhao M, Tidwell R R, Dykstra C C, Hall J E, Jones S K, Schinazi R F. Synthesis of dicationic diaryltriazines as nucleic acid binding agents. Eur J Med Chem. 1994;29:363–367. doi: 10.1016/0223-5234(94)90061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tidwell, R. R. 1996. Unpublished data.
- 48.Tidwell R R, Bell C A. Pentamidine and related compounds in the treatment of Pneumocystis carinii infection. In: Walzer P, editor. Pneumocystis carinii. 2nd ed. New York, N.Y: Marcel Dekker; 1993. pp. 561–583. [Google Scholar]
- 49.Tidwell R R, Geratz J D, Clyde W A, Jr, Rosenthal K U, Dubovi E J. Suppression of respiratory syncytial virus infection in cotton rats by bis(5-amidino-2-benzimidazolyl)methane. Antimicrob Agents Chemother. 1984;26:591–593. doi: 10.1128/aac.26.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tidwell R R, Jones S K, Geratz J D, Ohemeng K A, Cory M, Hall J E. Analogues of 1,5-bis(4-amidinophenoxy)pentane (pentamidine) in the treatment of experimental Pneumocystis carinii pneumonia. J Med Chem. 1990;33:1252–1257. doi: 10.1021/jm00166a026. [DOI] [PubMed] [Google Scholar]
- 51.Tidwell R R, Jones S K, Naiman N A, Berger L C, Brake W B, Dykstra C C, Hall J E. Activity of cationically substituted bis-benzimidazoles against experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1993;37:1713–1716. doi: 10.1128/aac.37.8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Breemen R B, Jiang O, Hall J E, Brewer T G, Tidwell R R. Fast atom bombardment tandem mass spectrometry of the anti-parasitic agent pentamidine and its oxygenated metabolites. Drug Metab Dispos. 1995;30:549–556. [Google Scholar]
- 53.Vonderfecht S L, Miskuff R L, BiWee S, Sato S, Tidwell R R, Geratz J D, Yolken R H. Protease inhibitors suppress the in vitro and in vivo replication of rotavirus. J Clin Invest. 1988;82:2011–2016. doi: 10.1172/JCI113821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weller T, Alig L, Beresini M, Blackburn B, Bunting S, Hadvary P, Muller M H, Knopp D, Levet-Trafit B, Lipari M T, Modi N B, Muller M, Refino C J, Schmitt M, Schonholzer P, Weiss S, Steiner B. Orally active fibrinogen receptor antagonists. 2. Amidoximes as prodrugs of amidines. J Med Chem. 1996;39:3139–3147. doi: 10.1021/jm9509298. [DOI] [PubMed] [Google Scholar]