Abstract
Lysyl oxidase‐like 2 (LOXL2) is a matrix‐remodeling enzyme that has recently been identified as an important regulator of tumor progression and metastasis. This study discovered that LOXL2 expression in oral squamous cell carcinoma (OSCC) tissues was significantly associated with tumor clinical stage, lymph node metastasis and patients' overall survival time. LOXL2‐overexpressing human buccal SCC TW2.6 (TW2.6/LOXL2) and hypopharyngeal SCC FaDu (FaDu/LOXL2) cells exhibited enhanced migration, invasion, epithelial–mesenchymal transition (EMT), and cancer stem cell (CSC) phenotypes, independently of its enzymatic activity. Moreover, TW2.6/LOXL2 significantly increased tumor‐initiating frequency in SCID mice. We further demonstrated that LOXL2 increased the levels of interferon‐induced protein with tetratricopeptide repeats 1 (IFIT1) and IFIT3 in TW2.6/LOXL2 and FaDu/LOXL2 cells. We also identified IFIT1 and IFIT3 as key downstream components of LOXL2 action in migration, invasion, EMT, and CSC phenotypes in TW2.6 and FaDu cells. Furthermore, a significant positive correlation between LOXL2 expression and IFIT1 and IFIT3 overexpression in human OSCC tissues was observed. In addition, TW2.6/LOXL2 and FaDu/LOXL2 cells were 3.3‐ to 3.6‐fold more susceptible to the epidermal growth factor receptor (EGFR) inhibitor gefitinib than were their respective control cells. The antitumor effect of gefitinib on orthotopic TW2.6/LOXL2 xenograft tumor was fourfold higher than that on controls. Our results indicate that LOXL2 expression is a strong prognostic factor for OSCC and may be used as a marker to identify patients most likely to respond to EGFR‐targeted therapy.
Keywords: cancer stem cells, gefitinib, interferon‐induced protein with tetratricopeptide repeats 1, interferon‐induced protein with tetratricopeptide repeats 3, lysyl oxidase‐like 2
Lysyl oxidase‐like 2 (LOXL2) increased tumor‐initiating frequency in an oral cancer xenograft model. LOXL2 enhanced EGFR signaling, epithelial–mesenchymal transition (EMT), and stemness via IFIT1 and IFIT3. LOXL2 enhanced antitumor effects of gefitinib about fourfold on xenograft tumors.
Abbreviations
- CSC
cancer stem cell
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- HNSCC
head and neck squamous cell carcinoma
- IFIT1
interferon‐induced protein with tetratricopeptide repeats 1
- IFIT3
interferon‐induced protein with tetratricopeptide repeats 3
- LOX
lysyl oxidase
- LOXL2
lysyl oxidase‐like protein 2
- OSCC
oral squamous cell carcinomas
- SCID
severe combined immunodeficiency
1. INTRODUCTION
Head and neck squamous cell carcinomas (HNSCCs) arise from the mucosal epithelium in the oral cavity, pharynx, and larynx and are the sixth most common types of cancers worldwide. 1 Oral squamous cell carcinoma (OSCC) is the most common subgroup of HNSCC. The major risk factors for HNSCC are tobacco and alcohol use. However, significant geographic or cultural and/or habitual variations are noted in the incidence and risk factors for HNSCC. 1 , 2 , 3 Habitual areca nut (AN, Areca catechu) chewing is highly prevalent in the countries with the highest incidence of OSCC (e.g., India, Pakistan, Bangladesh, Sri Lanka, and Taiwan). 3 , 4 Although substantial progress has been made in detecting and treating HNSCC, approximately 50% of patients with HNSCC experience recurrence or metastasis and have a median survival time of less than 1 year. 5 , 6 The overall survival of patients with HNSCC has not improved significantly over the last 20 years, with 5‐year survival rates remaining at 50%–60%. 5 , 6 , 7 The leading cause of treatment failure is cancer cells’ resistance to treatment, which is thought to be driven by cancer stem cells (CSCs). 8
Lysyl oxidase‐like protein 2 (LOXL2) is a secreted amine oxidase of the lysyl oxidase (LOX) family, which consists of five members (LOX and LOXL1–4). 9 , 10 The primary function of these enzymes is to oxidatively deaminate peptidyl lysine and hydroxylysine into peptidyl allysine and hydroxyallysine, respectively, to induce intramolecular and intermolecular cross‐linking of collagen and elastin in the extracellular matrix (ECM). This activity leads to an increase in tissue tensile strength and contributes to the stabilization and remodeling of the ECM. Additionally, LOXs participate in transcription regulation, cell signaling transduction, and cell adhesion. 10 , 11 Aberrant expression of LOXs promotes tumor cell migration, invasion, metastasis, and malignant transformation in part due to modification of the ECM, which changes the tumor microenvironment in several tumor types. 12 A humanized IgG4 monoclonal antibody (mAb) that inhibits the enzymatic activity of LOXL2, simtuzumab, has recently entered a clinical trial for pancreatic adenocarcinoma but has exhibited unsatisfactory clinical outcomes thus far. 13
Apart from its involvement in ECM organization, intracellular LOXL2 serves as a key driver of epithelial–mesenchymal transition (EMT) by regulating EMT‐inducing transcription factors and epigenetically regulating EMT‐related genes. 9 Catalytic activity is not required for LOXL2‐mediated EMT induction. 14 Catalytic‐inactive LOXL2 mutants can collaborate with EMT regulator Snail to repress epithelial marker E‐cadherin expression to trigger EMT and can promote FAK/Src pathway activation to support EMT in kidney cells. 14 Furthermore, a study showed that overexpressed LOXL2 could not only bring about EMT but also produce CSC‐like phenotype in MCF‐7 cells such as increased tumor sphere formation capability and elevated aldehyde dehydrogenase and CD44 expression. 15 However, whether LOXL2 overexpression can increase tumor‐initiating frequency in HNSCC has not been reported previously.
Clinically, elevated LOXL2 expression has been detected in various human tumors, including breast, 16 cervical, 17 colon, 18 esophageal, 19 gastric, 20 laryngeal, 21 liver, 22 and lung 23 cancers. High LOXL2 expression is associated with short survival time and poor prognosis. A few studies have reported increased LOXL2 expression in OSCC with different clinical outcomes. Shieh et al. 24 reported that LOX and LOXL2 mRNA expression in OSCC samples was significantly higher than that in their matched noncancerous tissue samples. Saito et al. 25 discovered that the immunoreactivities of LOX and LOXL2 in stage III/IV OSCC tissues were significantly higher than those in healthy tissues. The differences in immunoreactivities were more pronounced in regional lymph node metastasis‐positive OSCCs. However, Bharti et al. 26 identified a significantly higher proportion of cases with strong LOXL2 cytoplasmic staining among patients with N0 and N1 disease than among those with N2 disease. No significant differences in LOXL2 staining were identified among the tissues of patients with different tumor stages and AN chewing habits. 26 To the best of our knowledge, no studies have reported the association between LOXL2 expression and survival time in patients with OSCC. In addition, the mechanisms by which LOXL2 affects HNSCC are not well understood.
2. MATERIALS AND METHODS
2.1. Patients and tissues
This study was approved by the Research Ethics Committee of the National Taiwan University Hospital (NTUH). The enrolment criterion was primary HNSCC without previous cancer treatment before surgical therapy. Since the primary approach to treat laryngeal and pharyngeal SCCs has been shifted to chemoradiotherapy to achieve organ preservation and the primary treatment for OSCC remains surgical, we retrieved formalin‐fixed paraffin‐embedded (FFPE) tissue samples from 98 OSCC patients at the Department of Oral Pathology. All the participating patients underwent surgical excision of the primary lesion at the Department of Oral and Maxillofacial Surgery at NTUH from 2015 to 2020 and provided written informed consent to use their resected tissues. The TNM status of the OSCCs at initial presentation was determined according to the staging system of the American Joint Committee on Cancer. 27 The patients' demographic and clinicopathological data and oral habits were recorded. Normal oral mucosa (NOM) tissues were collected from 28 healthy volunteers as controls.
2.2. Immunostaining
Immunostaining on FFPE specimens was performed as described previously. 28 Sections from patients were stained with rabbit anti‐LOXL2 polyclonal antibody (Genetex), mouse anti‐interferon‐induced protein with tetratricopeptide repeats 1 (IFIT1) mAb (Genetex), or rabbit anti‐IFIT3 polyclonal antibody (Genetex). The evaluation of immunostaining was performed by two of the authors (H.‐H. K. and S.‐J. C.), who were blinded to the clinical outcomes of the patients. The labeling indices (LIs) of LOXL2 expression in each specimen were evaluated according to the percentage of immunostaining‐positive cells to the total number of counted cancer or epithelial cells. The sections with an interobserver variation of more than 10% were rechecked to achieve agreement in all cases.
2.3. Cell culture
Six human HNSCC cell lines, Ca9‐22 (gingival SCC), FaDu (hypopharyngeal SCC), HSC‐3 (tongue SCC), OECM‐1 (gingival SCC), SAS (tongue SCC), and TW2.6 (buccal SCC) were used in the present study. The cells were cultured as previously described. 29 All the tissue culture agents were obtained from Invitrogen.
2.4. Plasmids, transient transfection, and stably transfected clone selection
Transfection of HNSCC cells with plasmids (pCMV‐LOXL2, pCMV vector [OriGene Technologies]; pLKO vector, pLKO‐shIFIT‐1, pLKO‐shIFIT‐3 [RNAi core, Academia Sinica]) or small interfering (si)RNAs against LOXL2, Snail, IFIT1, and IFIT3 (Santa Cruz Biotechnology) was performed with Lipofectamine (Invitrogen) according to the manufacturer's instructions, as previously described. 29 The resistant clones were selected and cultured. Western blot analysis was used to verify the delivery of the plasmid DNA or siRNAs. To avoid off‐target effects of siRNA, another set of LOXL2 and Snail siRNAs was purchased from Thermo Fisher Scientific Inc.
2.5. Other materials and methods
Other materials and methods can be found in the Supporting Information as Appendix S1.
3. RESULTS
3.1. LOXL2 was negatively associated with survival in patients with OSCC
LOXL2 expression in the OSCC and NOM specimens was examined using an anti‐LOXL2 antibody on the FFPE sections. Typical staining patterns for LOXL2 are presented in Figure 1A. The LOXL2 LIs for the OSCC samples (61.1 ± 27) were significantly higher than those for the NOM samples (10.2 ± 9.1, p < 0.001, Figure 1B). The relationships between LOXL2 expression and the clinicopathological parameters of the 98 OSCC patients are shown in Table 1. There was no correlation between LOXL2 expression and patients' age, sex, cancer location, OSCC histological differentiation, smoking, or alcohol drinking habits (p > 0.05). However, a higher LOXL2 LI was significantly associated with larger tumor size (p = 0.005), lymph node metastasis (p = 0.006), and advanced clinical stage (p = 0.003). A significant positive correlation was also discovered between the LOXL2 LI and AN chewing habit (p = 0.001). However, there was no significant difference in TNM stage between AN users and nonusers (data not shown). We further used Kaplan–Meier analysis to validate the correlation between LOXL2 LI and postoperative survival of the OSCC patients. Figure 1C showed patients with high LOXL2 expression (LIs > 61%) had significantly reduced overall survival time compared with those who had LOXL2 LIs ≤61% (p = 0.037, log‐rank test).
FIGURE 1.
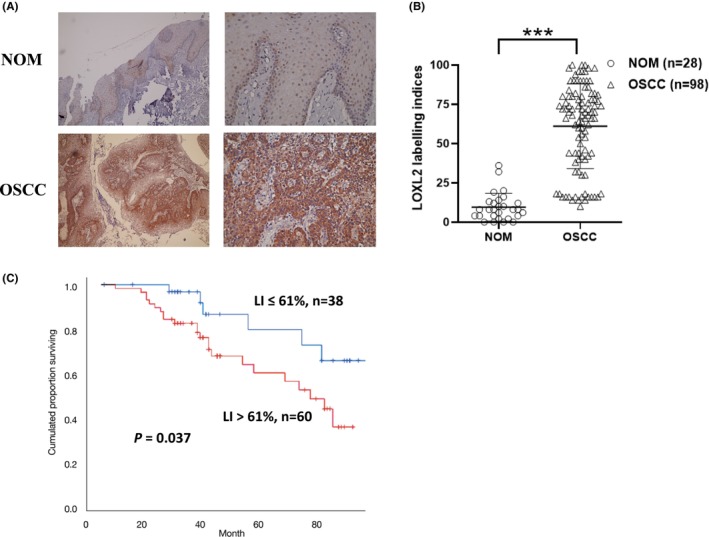
LOXL2 is overexpressed in OSCC and is associated with prognosis of OSCC. (A) Representative micrographs of LOXL2 immunostaining. (B) The LOXL2 LIs for the OSCC samples were significantly higher than those for the NOM samples. (C) Kaplan–Meier survival analysis revealed that patients with LOXL2 LIs > 61% had significantly shorter overall survival time than those with LOXL2 LIs ≤ 61% (p = 0.037, log‐rank test). ***p < 0.001. LIs, labeling indices; LOXL2, lysyl oxidase‐like protein 2; NOM, normal oral mucosa; OSCC, oral squamous cell carcinoma.
TABLE 1.
Correlation between the LOXL2 LIs in OSCC samples and clinicopathological parameters of OSCC patients.
| Mean LOXL2 LI ± SD (%) | p‐Value | |
|---|---|---|
| Patients' age (year) | ||
| ≦50 (n = 55) | 57 ± 28 | 0.344 |
| >50 (n = 43) | 63 ± 26 | |
| Patients' sex | ||
| Men (n = 81) | 60 ± 27 | 0.271 |
| Women (n = 17) | 68 ± 24 | |
| Cancer location | ||
| Buccal mucosa (n = 52) | 60 ± 27 | 0.910 |
| Tongue (n = 29) | 60 ± 29 | |
| Other oral mucosal sites (n = 24) | 63 ± 19 | |
| T classification | ||
| T1 + T2 (n = 67) | 56 ± 27 | 0.005* |
| T3 + T4 (n = 31) | 72 ± 23 | |
| N classification | ||
| N0 (n = 76) | 57 ± 27 | 0.006* |
| N1 + N2 + N3 (n = 22) | 75 ± 22 | |
| Clinical staging | ||
| Stage 1 + 2 (n = 55) | 54 ± 27 | 0.003* |
| Stage 3 + 4 (n = 43) | 70 ± 23 | |
| Histology of OSCC | ||
| Well differentiated (n = 58) | 62 ± 27 | 0.632 |
| Moderately and poorly differentiated (n = 40) | 61 ± 27 | |
| Oral habits | ||
| Alcohol drinking | ||
| Without (n = 37) | 67 ± 20 | 0.110 |
| With (n = 61) | 58 ± 30 | |
| Areca nut chewing | ||
| Without (n = 26) | 47 ± 24 | 0.001* |
| With (n = 72) | 66 ± 26 | |
| Cigarette smoking | ||
| Without (n = 27) | 63 ± 22 | 0.642 |
| With (n = 71) | 60 ± 28 | |
Abbreviations: LIs, labeling indices; OSCC, oral squamous cell carcinoma.
Statistical significance (p < 0.05).
3.2. LOXL2 induced proliferation, migration, and EMT of HNSCC cells, independently of its catalytic activity
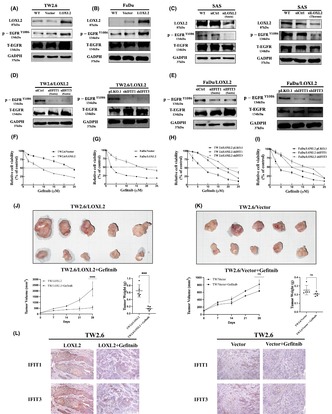
To examine the functional importance of LOXL2 in oral cancer cells, we screened the expression level of LOXL2 in six HNSCC cell lines (Figure 2A) and determined that LOXL2 expression was high in the OECM‐1, HSC3, and SAS cell lines and low in the TW2.6, FaDu, and Ca9‐22 cell lines. We selected TW2.6 and FaDu as parental cell lines to produce lines with stable LOXL2 overexpression and SAS as a parental line to produce line with LOXL2 knockdown. Stable clones of the LOXL2‐overexpressing cell lines (TW2.6/LOXL2 and FaDu/LOXL2) exhibited higher cell proliferation, migration, and invasion abilities than did the pCMV vector clones (Figure 2B,C). Transient transfection of LOXL2 siRNA into the SAS cells consistently inhibited the proliferation, migration, and invasion abilities of the SAS cells (Figure 2D). EMT is a crucial process in promoting cancer cell migration and metastasis in epithelium‐derived carcinoma. 30 We therefore examined the expression of EMT markers in TW2.6/LOXL2 and FaDu/LOXL2 cells. Figure 2E,F showed increased N‐cadherin, vimentin, and Snail expression and decreased E‐cadherin levels in the TW2.6 and FaDu LOXL2 transfectants. The suppression of LOXL2 in the SAS cells consistently increased E‐cadherin levels and decreased N‐cadherin, vimentin, and Snail levels (Figure 2G). Snail knockdown in the LOXL2 transfectants resulted in the suppression of LOXL2‐mediated migration and invasion (Figure 2H) and a switch from N‐cadherin to E‐cadherin (Figure 2I). We next used a selective LOXL2 inhibitor (2‐Chloropyridin‐4‐yl) methanamine hydrochloride (LOXL2‐IN‐1) 31 , 32 , 33 to investigate whether the catalytic activity of LOXL2 is required for migration, invasion, and EMT of HNSCC cell. As shown in Figure 2J‐L, 2.5 μM LOXL2‐IN‐1 (concentration that inhibited the catalytic activity of LOXL2 to the levels of wild‐type control (data not shown) did not affect the migration, invasion, and EMT of TW2.6/LOXL2 and FaDu/LOXL2 cells.
FIGURE 2.
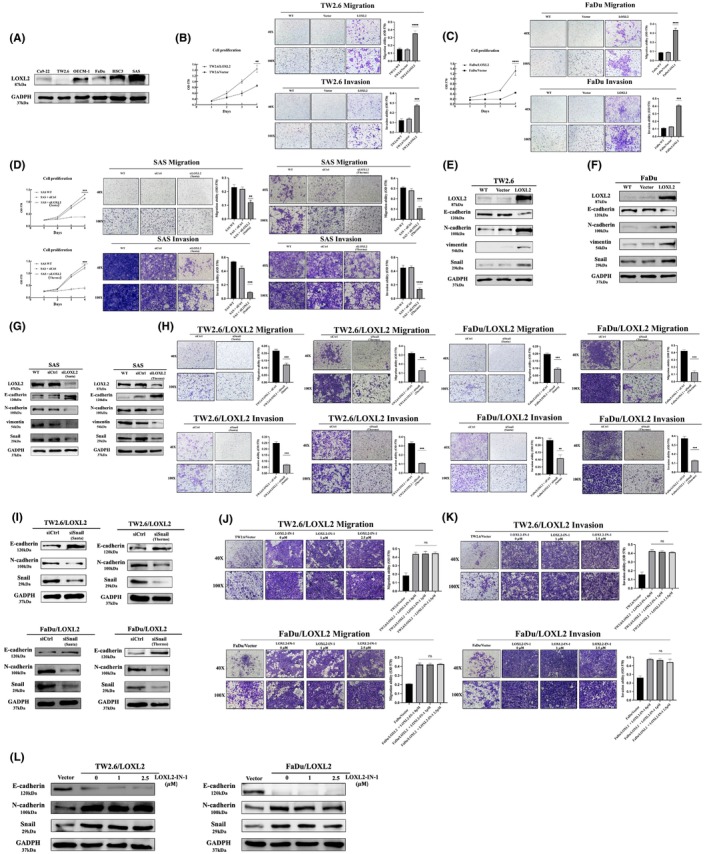
LOXL2 induced cell proliferation, migration, invasion, and EMT marker expression in HNSCC cells. (A) Western blot analysis of LOXL2 expression in six HNSCC cell lines. (B–D) Proliferation, migration, and invasion assays of TW2.6/LOXL2 (B), FaDu/LOXL2 (C), and SAS cells transfected with two individual LOXL2 siRNAs (SAS/siLOXL2) (D). (E–G) Western blot analysis of E‐cadherin, N‐cadherin, vimentin, and Snail in TW2.6/LOXL2 (E), FaDu/LOXL2 (F), and SAS/siLOXL2 (G) cells. (H,I) Migration and invasion abilities (H) and E‐cadherin, N‐cadherin, and Snail expression (I) of TW2.6/LOXL2 and FaDu/LOXL2 cells transfected with two individual Snail siRNAs. (J–L) LOXL2 inhibitor (LOXL2‐IN‐1) did not affect migration (J), invasion (K), and E‐cadherin, N‐cadherin, and Snail expression (L) of TW2.6/LOXL2 and FaDu/LOXL2 cells. **p < 0.01, ***p < 0.001, ****p < 0.0001. EMT, epithelial–mesenchymal transition; HNSCC, head and neck squamous cell carcinoma; LOXL2, lysyl oxidase‐like protein 2.
3.3. LOXL2 upregulates stemness marker protein expression and promotes spheroid formation
The Snail signaling pathway has been implicated in the development of CSC characteristics in OSCC. 34 We reasoned that LOXL2 may enhance cellular stemness characteristics in HNSCC. Western blotting demonstrated upregulation of stem cell‐related proteins Oct4, Sox2, and Nanog in the TW2.6/LOXL2 (Figure 3A) and FaDu/LOXL2 (Figure 3B) cells. Ectopic LOXL2 expression also enhanced the spheroid‐forming ability (Figure 3D,E). Consistently, knockdown of LOXL2 in SAS sphere cells reduced the Oct4, Sox2, and Nanog expression (Figure 3C) and the spheroid‐forming ability (Figure 3F). Moreover, Snail knockdown suppressed LOXL2‐mediated CSC‐like phenotypes in TW2.6/LOXL2 and FaDu/LOXL2 cells, as evidenced by decreased Oct4, Sox2, and Nanog expression (Figure 3G,H) and spheroid‐forming ability (Figure 3I,J). These results suggest that LOXL2 induces CSC‐like phenotypes through the Snail signaling pathway in HNSCC cells. Furthermore, 2.5 μM LOXL2‐IN‐1 did not affect the spheroid‐forming ability of TW2.6/LOXL2 (Figure 3K) and FaDu/LOXL2 cells (Figure 3L).
FIGURE 3.
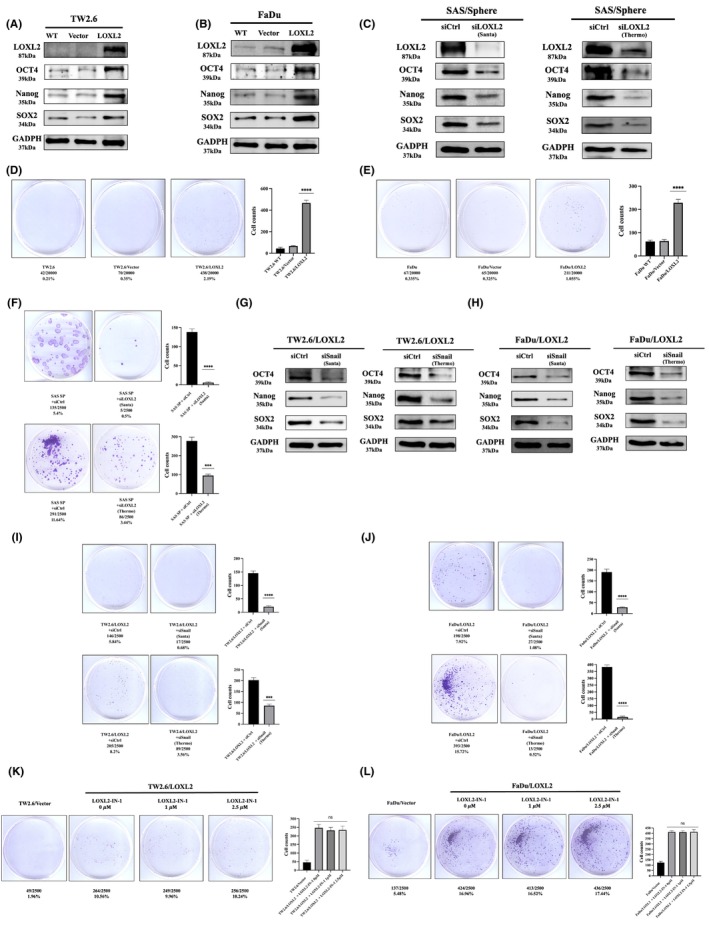
LOXL2 upregulates stemness marker protein expression and promotes spheroid formation. (A–C) Western blot analysis of Oct4, Sox2, and Nanog in TW2.6/LOXL2 (A), FaDu/LOXL2 (B), and SAS sphere cells (C) receiving two individual LOXL2 siRNAs (SAS‐sp/siLOXL2). (D–F) The sphere‐forming ability of TW2.6/LOXL2 (D), FaDu/LOXL2 (E), and SAS‐sp/siLOXL2 (F) cells. (G,H) Western blot analysis of Oct4, Sox2, and Nanog in TW2.6/LOXL2 (G) and FaDu/LOXL2 (H) cells transfected with two individual Snail siRNAs. (I,J) The sphere‐forming ability of TW2.6/LOXL2 (I) and FaDu/LOXL2 (J) cells transfected with two individual Snail siRNAs. (K,L) LOXL2 inhibitor (LOXL2‐IN‐1) did not affect the sphere‐forming ability of TW2.6/LOXL2 (K) and FaDu/LOXL2 cells (L). ****p < 0.0001. LOXL2, lysyl oxidase‐like protein 2.
3.4. LOXL2 overexpression increased tumor initiation in HNSCC in vivo
We next evaluated the in vivo tumor initiation potential of limiting dilutions (1 × 105, 1 × 104 and 1 × 103 cells) of TW2.6/LOXL2 and TW2.6/vector cells subcutaneously injected into the buccal mucosa of SCID mice. Figure 4A showed TW2.6/LOXL2 cells were 25‐fold more efficient in tumor initiation than vector cells (p < 0.0001). Overexpression of LOXL2 also increased the volume (Figure 4B) and weight (Figure 4C) of the formed tumors in all conditions compared with TW2.6/vector cells. Immunohistochemical examination of TW2.6/LOXL2 and TW2.6/vector xenografts showed decreased E‐cadherin expression and increased N‐cadherin, Snail (Figure 4D), Oct4, Sox2, and Nanog levels (Figure 4E) in the TW2.6/LOXL2 xenografts.
FIGURE 4.
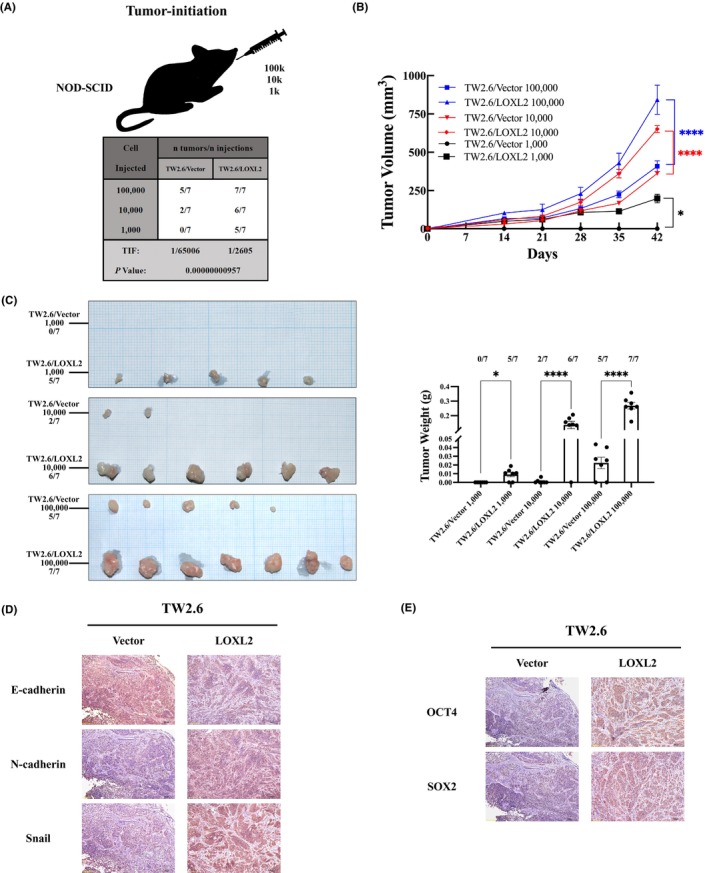
LOXL2 overexpression increased tumor initiation in OSCC in vivo. SCID mice were orthotopically (buccal mucosa) injected with limiting dilutions (1 × 105, 1 × 104 and 1 × 103 cells) of TW2.6/LOXL2 and TW2.6/vector cells (n = 7 for each group). Mice were sacrificed at day 42 after injection. (A) Overexpression of LOXL2 increased the TIF at day 42. The volume (B) and weight (C) of TW2.6/LOXL2 xenografts were larger than those of TW2.6/vector. *p < 0.05, ****p < 0.0001. (D,E) Sections of xenografts were subjected to immunostaining with anti‐human (D) LOXL2, E‐cadherin, N‐cadherin, Snail, (E) Oct4, Sox2, and Nanog. LOXL2, lysyl oxidase‐like protein 2; TIF, tumor‐initiating frequency.
3.5. IFIT1 and IFIT3 are involved in LOXL2‐mediated migration, invasion, EMT, and CSC‐like phenotypes
We next investigated the downstream targets of LOXL2‐induced EMT‐ and CSC‐like properties in HNSCC cells. A cDNA microarray analysis of the TW2.6/LOXL2, FaDu/LOXL2, and control cells was conducted. Among the top 10 most upregulated genes in both the TW2.6/LOXL2 and FaDu/LOXL2 cells, four (IFIT1, CXCL10, IFIT3, and CXCL11) are known to induce EMT in cancer cells. The IFIT1 gene was the highest upregulated gene in TW2.6/LOXL2 and FaDu/LOXL2 cells (Figure 5A). Studies have indicated that IFIT1 and IFIT3 are interdependent. 35 IFIT3 gene was also upregulated in the TW2.6/LOXL2 and FaDu/LOXL2 cells (Figure 5A). These results suggest that IFIT1 and IFIT3 might be downstream targets of LOXL2‐mediated migration, invasion, and EMT of HNSCC cells. Western blot analysis confirmed that both IFIT1 and IFIT3 are upregulated in TW2.6/LOXL2 and FaDu/LOXL2 cells (Figure 5B). LOXL2 knockdown in the SAS cells inhibited IFIT1 and IFIT3 expression (Figure 5B). Knockdown of IFIT1 or IFIT3 with either siRNA or shRNA in TW2.6/LOXL2 and FaDu/LOXL2 cells decreased migration and invasion (Figure 5C,D), N‐cadherin, vimentin, Snail (Figure 5E,F), Oct4, Sox2, and Nanog expression (Figure 5G,H) and spheroid‐forming ability (Figure 5I,J) in both cell lines.
FIGURE 5.
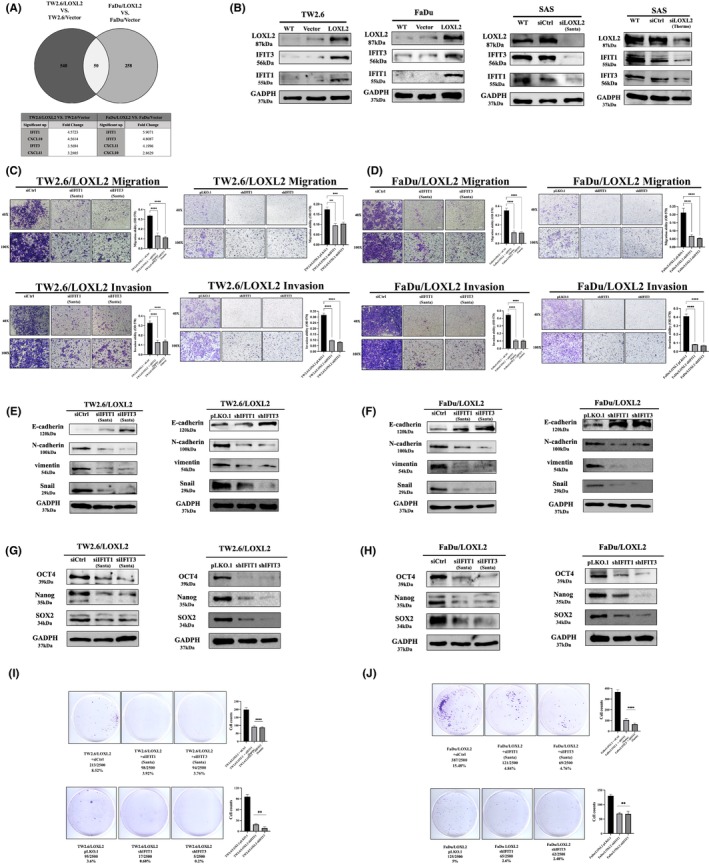
IFIT1 and IFIT3 are downstream targets of LOXL2‐mediated migration, invasion, and EMT in HNSCC cells. (A). Schematic representation of the strategy for mining the downstream targets of LOXL2 (upper panel). Four genes are known to induce EMT among the top 10 most upregulated genes (lower panel). (B) Western blot analysis of IFIT1 and IFIT3 levels in TW2.6/LOXL2, FaDu/LOXL2, and SAS/siLOXL2 cells. (C,D) Migration and invasion abilities of TW2.6/LOXL2 transfected with siIFIT1, siIFIT3, shIFIT1, or shIFIT3 (C) and FaDu/LOXL2 transfected with siIFIT1, siIFIT3, shIFIT1, or shIFIT3 (D). (E,F) Western blot analysis of E‐cadherin, N‐cadherin, vimentin, and Snail in TW2.6/LOXL2 (E) and FaDu/LOXL2 (F) transfected with siIFIT1, siIFIT3, shIFIT1, or shIFIT3. G‐H. Western blot analysis of Oct4, Sox2, and Nanog in TW2.6/LOXL2 (G) and FaDu/LOXL2 (H) transfected with siIFIT1, siIFIT3, shIFIT1, or shIFIT3. (I,J) The sphere‐forming ability of TW2.6/LOXL2 (I) and FaDu/LOXL2 (J) cells transfected with siIFIT1, siIFIT3, shIFIT1, or shIFIT3. **p < 0.01, ***p < 0.001, ****p < 0.0001. EMT, epithelial–mesenchymal transition; HNSCC, head and neck squamous cell carcinoma; LOXL2, lysyl oxidase‐like protein 2.
3.6. IFIT1 and IFIT3 expression was positively correlated with LOXL2 expression in OSCCs
We further examined the expression of IFIT1 and IFIT3 in 40 OSCC and 27 NOM specimens using anti‐IFIT1 and anti‐IFIT3 antibodies to investigate the link between LOXL2 and IFIT1 and IFIT3 expression (Figure 6A). The IFIT1 and IFIT3 LIs of the OSCC samples (59 ± 33 and 71 ± 28, respectively) were significantly higher than those of the NOM samples (2.3 ± 1.4 and 2.8 ± 2.5, respectively; both p < 0.001; Figure 6B). The relationships between LOXL2 expression and the clinicopathological parameters of the 40 OSCC patients are shown in Table 2. There was no correlation between IFIT1/IFIT3 expression and patients' age, sex, cancer location, OSCC histological differentiation, smoking, or alcohol drinking habits (p > 0.05). However, higher IFIT1/IFIT3 LIs were significantly associated with large tumor size (p < 0.001 and p < 0.001, respectively), lymph node metastasis (p = 0.014 and p = 0.01, respectively), and advanced clinical stage (p < 0.001 and p = 0.005, respectively). The OSCC specimens with high LOXL2 levels also had high IFIT1 and IFIT3 levels, whereas those with low LOXL2 levels had weak or negative IFIT1 and IFIT3 levels. Positive correlations between LOXL2 and IFIT1, LOXL2 and IFIT3, and IFIT1 and IFIT3 (r = 0.512, p < 0.001, Figure 6C; r = 0.566, p < 0.001, Figure 6D; r = 0.707, p < 0.001, Figure 6E; respectively) were observed in the OSCC tissues.
FIGURE 6.
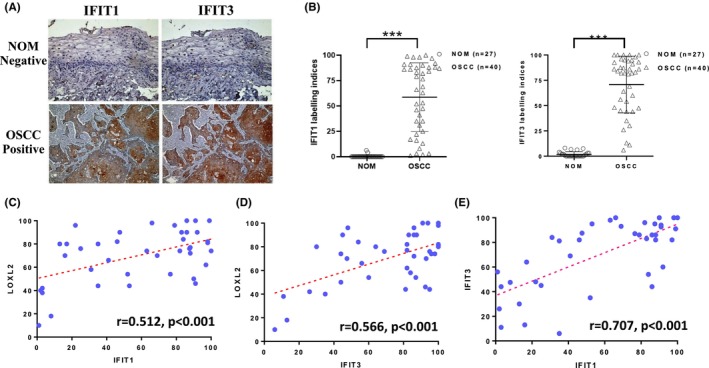
IFIT1 and IFIT3 expression was positively correlated with LOXL2 expression in OSCCs. (A) Representative micrographs of IFIT1 and IFIT3 immunostaining. (B) The mean IFIT1 and IFIT3 LIs of the OSCC samples were significantly higher than those of NOM. (C–E) Correlation between IFIT1/LOXL2 (C), IFIT3/LOXL2 (D), and IFIT1/IFIT3 (E) in OSCC specimens. ***p < 0.001. r, correlation coefficients. LIs, labeling indices; LOXL2, lysyl oxidase‐like protein 2; NOM, normal oral mucosa; OSCC, oral squamous cell carcinoma
TABLE 2.
Correlation between the IFIT1/3 LIs in OSCC samples and clinicopathological parameters of OSCC patients.
| Clinical parameters | Mean IFIT1 LI ± SD (%) | p‐Value | Mean IFIT3 LI ± SD (%) | p‐Value |
|---|---|---|---|---|
| Patients' age (year) | ||||
| ≤50 (n = 19) | 55 ± 30 | 0.838 | 68 ± 26 | 0.795 |
| >50 (n = 21) | 60 ± 23 | 75 ± 16 | ||
| Patients' sex | ||||
| Female (n = 7) | 57 ± 24 | 0.470 | 63 ± 21 | 0.260 |
| Male (n = 33) | 64 ± 22 | 72 ± 18 | ||
| Cancer location | ||||
| Buccal mucosa (n = 20) | 64 ± 21 | 0.952 | 63 ± 24 | 0.089 |
| Tongue (n = 13) | 69 ± 26 | 79 ± 18 | ||
| Other sites (n = 7) | 54 ± 20 | 72 ± 6 | ||
| T classification | ||||
| T1 + T2 (n = 23) | 43 ± 30 | <0.001* | 58 ± 28 | <0.001* |
| T3 + T4 (n = 17) | 87 ± 10 | 87 ± 16 | ||
| N classification | ||||
| N0 (n = 22) | 56 ± 27 | 0.014* | 61 ± 27 | 0.010* |
| N1 + N2 + N3 (n = 18) | 75 ± 22 | 83 ± 23 | ||
| Clinical staging | ||||
| Stage1 + 2 (n = 11) | 43 ± 26 | <0.001* | 55 ± 29 | 0.005* |
| Stage3 + 4 (n = 29) | 78 ± 21 | 80 ± 21 | ||
| Histology of OSCC | ||||
| Well differentiated (n = 29) | 56 ± 20 | 0.509 | 68 ± 22 | 0.447 |
| Moderately and poorly differentiated (n = 11) | 61 ± 18 | 74 ± 17 | ||
| Oral habits | ||||
| Alcohol drinking | ||||
| Without (n = 10) | 54 ± 19 | 0.440 | 66 ± 20 | 0.203 |
| With (n = 30) | 61 ± 21 | 75 ± 17 | ||
| Areca nut chewing | ||||
| Without (n = 9) | 43 ± 24 | 0.012* | 56 ± 23 | 0.025* |
| With (n = 31) | 69 ± 25 | 76 ± 21 | ||
| Cigarette smoking | ||||
| Without (n = 8) | 54 ± 16 | 0.483 | 68 ± 20 | 0.391 |
| With (n = 32) | 61 ± 21 | 74 ± 17 | ||
Abbreviations: LIs, labeling indices; OSCC, oral squamous cell carcinoma.
Statistical significance (p < 0.05).
3.7. LOXL2 enhanced EGFR activation and the antitumor effects of gefitinib on HNSCC via IFIT1 and IFIT3
IFIT1 and IFIT3 has been shown to promote EGFR activation in OSCC cells and enhance the tumor‐suppressive effect of gefitinib, an EGFR inhibitor. 35 We therefore investigated whether LOXL2 promotes EGFR activation in HNSCC cells. Figure 7A,B showed the p‐EGFRY1086 levels in the TW2.6/LOXL2 and FaDu/LOXL2 cells were significantly higher than those in their respective control cells. Consistently, the suppression of LOXL2 in the SAS cells decreased the p‐EGFRY1086 level (Figure 7C). Knockdown of IFIT1 or IFIT3 with either siRNA or shRNA decreased the p‐EGFRY1086 level in the TW2.6/LOXL2 (Figure 7D) and FaDu/LOXL2 (Figure 7E) cells. Furthermore, Figure 7F,G showed TW2.6/LOXL2 and FaDu/LOXL2 cells were 3.3‐fold and 3.6‐fold, respectively, more susceptible to gefitinib than were their respective controls (IC50: 7.48 vs. 24.67 μM and 4.71 vs. 17.17 μM, respectively). IFIT1 and IFIT3 knockdown suppressed the LOXL2‐enhanced cytotoxic effects of gefitinib on both the TW2.6/LOXL2 (Figure 7H) and FaDu/LOXL2 cells (Figure 7I). The IC50 of gefitinib for the TW2.6/LOXL2/pLKO control, TW2.6/LOXL2/shIFIT1, TW2.6/LOXL2/shIFIT3, FaDu/LOXL2/pLKO control, FaDu/LOXL2/shIFIT1, and FaDu/LOXL2/shIFIT3 cells were 7.02, 15.09, 19.56, 4.306, 10.92, and 14.75 μM, respectively. These results suggest that LOXL2 increased EGFR activation and enhanced the effect of gefitinib via IFIT1 and IFIT3. We next examined the therapeutic effect of gefitinib against orthotopic xenograft tumor of TW2.6/LOXL2 and TW2.6/vector (Figure 7J,K). The mice bearing TW2.6/LOXL2 and TW2.6/vector xenografts were orally treated with gefitinib weekly at 200 mg/kg after the tumor size reached about 100 mm3 (day 0). At day 28, gefitinib suppressed the growth of the TW2.6/LOXL2 and TW2.6/vector xenografts by 76.25 (p < 0.001) and 18.96%, respectively, with no significant changes in animal body weights. Sections of xenografts were stained with IFIT1 and IFIT3 antibodies (Figure 7L). Results showed gefitinib suppressed IFIT1 and IFIT3 expression in the TW2.6/LOXL2 xenografts.
FIGURE 7.
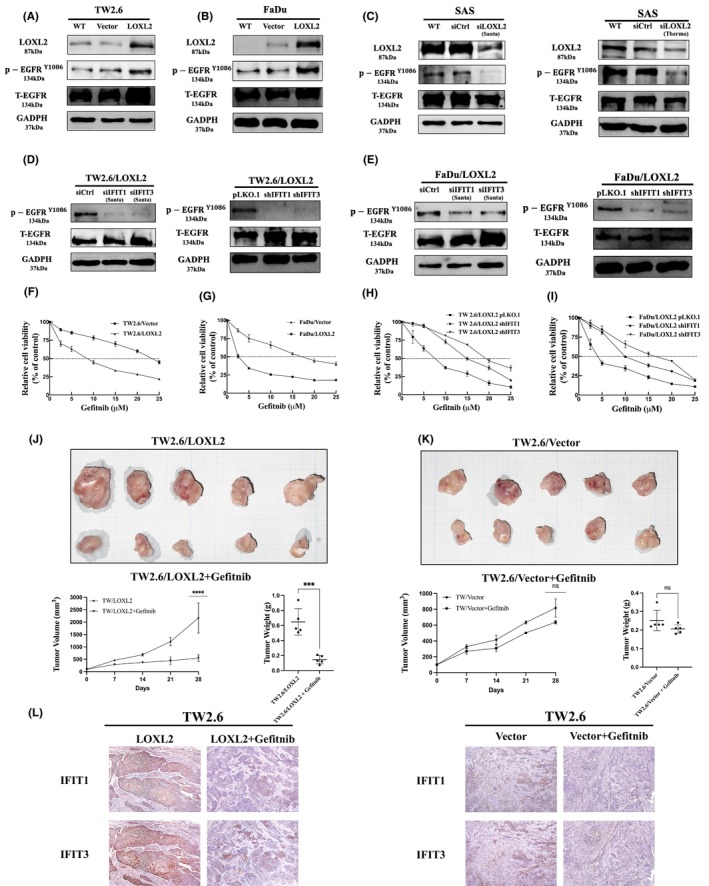
LOXL2 enhances EGFR activation and the antitumor effects of gefitinib on HNSCC. (A–C) Western blot analysis of p‐EGFRY1086 levels in TW2.6/LOXL2, FaDu/LOXL2, and SAS/siLOXL2. (D,E) Western blot analysis of p‐EGFRY1086 in TW2.6/LOXL2 (D) and FaDu/LOXL2 cells (E) transfected with siIFIT1, siIFIT3, shIFIT1, or shIFIT3 and their respective controls. (F–I) MTT assay of TW2.6/LOXL2, FaDu/LOXL2, TW2.6/LOXL2/shIFIT1, TW2.6/LOXL2/shIFIT3, FaDu/LOXL2/shIFIT1, FaDu /LOXL2/shIFIT3, and their respective control cells treated with various concentrations of gefitinib for 72 h. (J,K) LOXL2 increased antitumor effect of gefitinib. Orthotopic xenograft tumors derived from TW2.6/LOXL2 (J) and TW2.6/vector (K) were treated with gefitinib (200 mg/kg once a week for 4 weeks) by oral administration after the tumor size reached about 100 mm3 (day 0). At Day 28, mice were euthanized and the subcutaneous tumors were harvested, weighed, and photographed. The results shown are the means ± SD from seven mice. ***p < 0.001, ****p < 0.0001. (L) Sections of xenografts were subjected to immunostaining with mouse anti‐human IFIT1 monoclonal antibody and rabbit anti‐human IFIT3 polyclonal antibody. HNSCC, head and neck squamous cell carcinoma.
4. DISCUSSION
In this study, we demonstrated that LOXL2 levels in OSCC specimens are significantly associated with tumor size, nodal metastasis, clinical stage, and shorter overall survival. We have further for the first time demonstrated that LOXL2 increased tumor‐initiating frequency in an oral cancer xenograft model. Our results are comparable to those reported by Saito et al., 25 who determined that LOXL2 expression was significantly increased in stage III/IV OSCC; however, our results differ from those reported by Bharti et al., 26 who demonstrated that tissues from patients with N0 and N1 disease had stronger LOXL2 staining than those with N2 disease. These differences may be due to differences in the ethnicities or risk factors of the recruited patients. Nevertheless, our results suggest that LOXL2 expression may be used as an adjuvant marker of poor survival in patients with OSCC.
In OSCC, Saito et al. 25 indicated that LOX/LOXL2‐catalyzed collagen cross‐links created a stiff microenvironment that could be conducive to cancer cell invasion and metastasis. Mahjour et al. 36 suggested that extracellular LOXL2 secreted by oral tumor cells oxidized lysine residues on platelet‐derived growth factor receptor beta in proximal fibroblasts to promote proliferation and the tendency toward fibrosis, thereby contributing to cancer development. However, Shieh et al. 24 demonstrated that LOX overexpression reduced the migration and invasion of OSCC cells. Zou et al. 37 determined that LOXL2‐ΔLO, a nonenzymatic form of LOXL2, promoted the migration of esophageal SCC cells more strongly than did wild‐type LOXL2. Almacellas‐Rabaiget et al. 38 showed LOXL2 increased cell migration, invasion, and lung metastasis in rhabdomyosarcoma, independently of its catalytic activity. The present study found LOXL2 promoted the migration, invasion, EMT, and CSC‐like phenotypes in HNSCC cells, independently of its catalytic activity. Furthermore, LOXL2 induces CSC‐like phenotypes through the Snail signaling pathway. These results highlight the need for developing another strategy not focused on the enzymatic activity of LOXL2.
Previous studies showed LOXL2 can collaborate with Snail to repress E‐cadherin expression to trigger EMT. 14 , 39 Cuevas et al. 40 further demonstrated that LOXL2 drives EMT via XBP1‐mediated upregulation of Snail. However, LOXL2 did not significantly increase the expression of the XBP1 target genes EDEM1 and DNAJB9 in our cDNA microarray analysis (data not shown). Through bioinformatics mining and functional experimentation, we for the first time identified IFIT1 and IFIT3 as key downstream targets of LOXL2. IFIT1 and IFIT3 are products of interferon‐stimulating genes, which are involved in innate immunity, antiviral immune responses, and inflammatory responses. 35 Recent studies discovered that IFIT proteins participate in the progression of some types of cancer, including oral cancer. 35 Overexpression of IFIT1 or IFIT3 induce OSCC cell invasion by promoting EMT. The present study found knockdown of IFIT1 or IFIT3 in LOXL2‐overexpressing cells decreased Snail expression and LOXL2‐mediated migration, invasion, EMT, and CSC phenotypes in these cells. IFIT1 and IFIT3 are upstream molecules of Snail. We also identified a positive correlation between LOXL2 and IFIT1 and IFIT3 in our clinical samples. LOXL2 may be a key factor regulating IFIT1 and IFIT3 expression. To our knowledge, this study is the first to demonstrate that IFIT1 and IFIT3 are involved in LOXL2‐mediated OSCC migration, invasion, EMT, and CSC‐like phenotypes. Our results also suggest, for the first time, that IFIT1 and IFIT3 are invo1ved in the acquisition of CSC phenotypes. However, the mechanisms by which LOXL2 increases the levels of IFIT1 and IFIT3 in OSCC cells remain unknown. Additional studies are currently underway to explore the detailed mechanisms underlying LOXL2‐mediated IFIT1 and IFIT3 expression and IFIT1‐ and IFIT3‐mediated EMT.
A previous study demonstrated that IFIT1 or IFIT3 overexpression enhanced EGFR activation in OSCC cells. 35 EGFR is a transmembrane receptor tyrosine kinase belonging to the ErbB family. The binding of a ligand to EGFR causes the autophosphorylation of the EGFR and activation of downstream signaling pathways. 41 Complete activation of EGFR or termination of its signaling depends on its internalization into endosomes, from which it can be recycled to the membrane or further sorted into lysosomes, where it undergoes degradation. 41 IFIT1 and IFIT3 enhanced the EGFR endocytic recycling process by interacting with annexin‐2. 35 EGFR is overexpressed in many epithelial cancers, and researchers have therefore developed anti‐EGFR therapies including mAbs and small‐molecule tyrosine kinase inhibitors (TKIs). 42 Although EGFR is overexpressed in >80% of HNSCCs, the addition of an EGFR‐targeting mAb to radiation or chemoradiation therapy has shown limited success. 42 Several mechanisms have been reported to contribute to the resistance of EGFR‐targeted mAb therapies in HNSCC including EMT. 43 Because EGFR‐targeted therapies are expensive and only marginally effective, it is necessary to identify patients most likely to benefit from these treatments. In this study, we found LOXL2 enhanced EGFR activation and the antitumor effects of gefitinib on TW2.6 and FaDu cells via IFIT1 and IFIT3. The antitumor effect of gefitinib on orthotopic TW2.6/LOXL2 xenograft tumor was fourfold higher than that on controls. Our results are comparable to those reported by Pidugu et al. that IFIT1 and IFIT3 contribute to the antitumor effect of gefitinib via enhancing p‐EGFR recycling in OSCCs. 35 However, we also found gefitinib suppressed IFIT1 and IFIT3 expression in the TW2.6/LOXL2 xenografts. EGFR signaling may be also required for the LOXL2‐induced IFIT1 and IFIT3 expression. A previous study reported that LOXL2 overexpression in normal human mammary epithelial cells induced oncogenic transformation and cancer progression by activating ErbB2. 44 Additional studies are needed to explore the exact mechanisms underlying LOXL2‐enhanced EGFR signaling and the progression of HNSCCs.
In conclusion, this study demonstrated LOXL2 levels are significantly associated with tumor size, nodal metastasis, clinical stage, and shorter overall survival. HNSCC cells with LOXL2 overexpression were more efficient in tumor initiation in SCID mice. LOXL2 knockdown inhibits cell migration, invasion, EMT, and CSC phenotypes. We have also for the first time revealed IFIT1 and IFIT3 as being key downstream components of LOXL2 action in migration, invasion, EMT, and CSC phenotypes in HNSCC cells. Moreover, LOXL2 enhances antitumor effects of gefitinib. LOXL2 may be used as a marker to identify the patients most likely to respond to EGFR‐targeted therapy. Our findings may have important clinical implications for the treatment of patients with oral cancer.
AUTHOR CONTRIBUTIONS
Yi‐Jie Lu: design, data acquisition, analysis of in vitro and animal studies, and revision of the manuscript. Yi‐Ting Deng, Mark Yen‐Ping Kuo, Shih‐Jung Cheng: conception, design, data analysis, interpretation, draft, and revision of the manuscript. Hui‐Hsin Ko, Hsin‐Hui Peng: design, data acquisition, analysis of clinicopathological studies, and revision of the manuscript. Hsiang‐Chieh Le: bioinformatics. All authors gave final approval and agree to be accountable for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: This study was approved by the Research Ethics Committee of the NTUH and carried out according to the Declaration of Helsinki.
Informed Consent: Written informed consent was obtained from patients.
Registry and the Registration No. of the study/trial: 201706120RIN.
Animal Studies: All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the College of Medicine at National Taiwan University (reference number: 20170376).
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
This study was supported by the research grant MOST 107‐2314‐B‐002‐085‐MY3 and MOST 110‐2314‐B‐002‐103‐MY3 from the Ministry of Science and Technology, Taipei, Taiwan.
Lu Y‐J, Deng Y‐T, Ko H‐H, et al. Lysyl oxidase‐like 2 promotes stemness and enhances antitumor effects of gefitinib in head and neck cancer via IFIT1 and IFIT3. Cancer Sci. 2023;114:3957‐3971. doi: 10.1111/cas.15912
Contributor Information
Mark Yen‐Ping Kuo, Email: oddie@ntu.edu.tw.
Shih‐Jung Cheng, Email: sjcheng56@ntu.edu.tw.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are available from the corresponding authors on reasonable request.
REFERENCES
- 1. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scully C, Bedi R. Ethnicity and oral cancer. Lancet Oncol. 2000;1:37‐42. [DOI] [PubMed] [Google Scholar]
- 3. Miranda‐Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551. [DOI] [PubMed] [Google Scholar]
- 4. Guha N, Warnakulasuriya S, Vlaanderen J, Straif K. Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta‐analysis with implications for cancer control. Int J Cancer. 2014;135:1433‐1443. [DOI] [PubMed] [Google Scholar]
- 5. Jerjes W, Upile T, Petrie A, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1‐T2 oral squamous cell carcinoma patients. Head Neck Oncol. 2010;2:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ionna F, Bossi P, Guida A, et al. Recurrent/metastatic squamous cell carcinoma of the head and neck: a big and intriguing challenge which may be resolved by integrated treatments combining locoregional and systemic therapies. Cancers (Basel). 2021;13:2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu AJ, Choi JS, Swanson MS, et al. Association of race/ethnicity, stage, and survival in oral cavity squamous cell carcinoma: a SEER study. OTO Open. 2019;3:2473974X19891126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawson DA, Bhakta NR, Kessenbrock K, et al. Single‐cell analysis reveals a stem‐cell program in human metastatic breast cancer cells. Nature. 2015;526:131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wen B, Xu L‐Y, Li E‐M. LOXL2 in cancer: regulation, downstream effectors and novel roles. Biochim Biophys Acta Rev Cancer. 2020;1874:188435. [DOI] [PubMed] [Google Scholar]
- 10. Ye M, Song Y, Pan S, Chu M, Wang Z‐W, Zhu X. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol Ther. 2020;215:107633. [DOI] [PubMed] [Google Scholar]
- 11. Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;12:540‐552. [DOI] [PubMed] [Google Scholar]
- 12. Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benson AB III, Wainberg ZA, Hecht JR, et al. A phase II randomized, double‐blind, placebo‐controlled study of simtuzumab or placebo in combination with gemcitabine for the first‐line treatment of pancreatic adenocarcinoma. Oncologist. 2017;22:241‐e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cuevas EP, Moreno‐Bueno G, Canesin G, Santos V, Portillo F, Cano A. LOXL2 catalytically inactive mutants mediate epithelial‐to‐mesenchymal transition. Biol Open. 2014;3:129‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weidenfeld K, Schif‐Zuck S, Abu‐Tayeh H, et al. Dormant tumor cells expressing LOXL2 acquire a stem‐like phenotype mediating their transition to proliferative growth. Oncotarget. 2016;7:71362‐71377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barker HE, Chang J, Cox TR, et al. LOXL2‐mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao C, Lin S, Zhi W, et al. LOXL2 expression status is correlated with molecular characterizations of cervical carcinoma and associated with poor cancer survival via epithelial‐mesenchymal transition (EMT) phenotype. Front Oncol. 2020;10:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui X, Wang G, Shen W, Huang Z, He H, Cui L. Lysyl oxidase‐like 2 is highly expressed in colorectal cancer cells and promotes the development of colorectal cancer. Oncol Rep. 2018;40:932‐942. [DOI] [PubMed] [Google Scholar]
- 19. Li T‐Y, Xu L‐Y, Wu Z‐Y, et al. Reduced nuclear and ectopic cytoplasmic expression of lysyl oxidase–like 2 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Hum Pathol. 2012;43:1068‐1076. [DOI] [PubMed] [Google Scholar]
- 20. Peng L, Ran Y‐L, Hu H, et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660‐1669. [DOI] [PubMed] [Google Scholar]
- 21. Peinado H, Moreno‐Bueno G, Hardisson D, et al. Lysyl oxidase–like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541‐4550. [DOI] [PubMed] [Google Scholar]
- 22. Choi J, Chung T, Rhee H, et al. Increased expression of the matrix‐modifying enzyme lysyl oxidase‐like 2 in aggressive hepatocellular carcinoma with poor prognosis. Gut Liver. 2019;13:83‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhan P, Lv XJ, Ji YN, Xie H, Yu LK. Increased lysyl oxidase‐like 2 associates with a poor prognosis in non‐small cell lung cancer. Clin Respir J. 2018;12:712‐720. [DOI] [PubMed] [Google Scholar]
- 24. Shieh T‐M, Lin S‐C, Liu C‐J, Chang S‐S, Ku T‐H, Chang K‐W. Association of expression aberrances and genetic polymorphisms of lysyl oxidase with areca‐associated oral tumorigenesis. Clin Cancer Res. 2007;13:4378‐4385. [DOI] [PubMed] [Google Scholar]
- 25. Saito T, Uzawa K, Terajima M, et al. Aberrant collagen cross‐linking in human oral squamous cell carcinoma. J Dent Res. 2019;98:517‐525. [DOI] [PubMed] [Google Scholar]
- 26. Bharti A, Urs AB, Kumar P. Significance of HIF‐1α expression and LOXL‐2 localization in progression of Oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2021;22:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Joint Committee on Cancer . Cancer Staging Manual. 8th ed. Springer International Publishing; 2021. [Google Scholar]
- 28. Ko HH, Cheng SL, Lee JJ, Chen HM, Kuo MYP, Cheng SJ. Expression of AKR1B10 as an independent marker for poor prognosis in human oral squamous cell carcinoma. Head Neck. 2017;39:1327‐1332. [DOI] [PubMed] [Google Scholar]
- 29. Yang C‐N, Deng Y‐T, Tang J‐Y, et al. MicroRNA‐29b regulates migration in oral squamous cell carcinoma and its clinical significance. Oral Oncol. 2015;51:170‐177. [DOI] [PubMed] [Google Scholar]
- 30. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395‐412. [DOI] [PubMed] [Google Scholar]
- 31. Hutchinson JH, Rowbottom MW, Lonergan D, et al. Small molecule lysyl oxidase‐like 2 (LOXL2) inhibitors: the identification of an inhibitor selective for LOXL2 over LOX. ACS Med Chem Lett. 2017;8:423‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fan Z, Zheng W, Li H, et al. LOXL2 upregulates hypoxia‐inducible factor‐1α signaling through snail‐FBP1 axis in hepatocellular carcinoma cells. Oncol Rep. 2020;43:1641‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Y, Wu Y, Yang Y, et al. Lysyl oxidase‐like 2 inhibitor rescues D‐galactose‐induced skeletal muscle fibrosis. Aging Cell. 2022;21:e13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu L‐F, Hu Y, Yang C‐C, et al. Snail overexpression induces an epithelial to mesenchymal transition and cancer stem cell‐like properties in SCC9 cells. Lab Invest. 2012;92:744‐752. [DOI] [PubMed] [Google Scholar]
- 35. Pidugu VK, Wu M‐M, Yen A‐H, et al. IFIT1 and IFIT3 promote oral squamous cell carcinoma metastasis and contribute to the anti‐tumor effect of gefitinib via enhancing p‐EGFR recycling. Oncogene. 2019;38:3232‐3247. [DOI] [PubMed] [Google Scholar]
- 36. Mahjour F, Dambal V, Shrestha N, et al. Mechanism for oral tumor cell lysyl oxidase like‐2 in cancer development: synergy with PDGF‐AB. Oncogenesis. 2019;8:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zou H, Wen B, Li R‐L, et al. Lysyl oxidase‐like 2 promotes esophageal squamous cell carcinoma cell migration independent of catalytic activity. Int J Biochem Cell Biol. 2020;125:105795. [DOI] [PubMed] [Google Scholar]
- 38. Almacellas‐Rabaiget O, Monaco P, Huertas‐Martinez J, et al. LOXL2 promotes oncogenic progression in alveolar rhabdomyosarcoma independently of its catalytic activity. Cancer Lett. 2020;474:1‐14. [DOI] [PubMed] [Google Scholar]
- 39. Peinado H, Iglesias‐de DC, la Cruz M, Olmeda D, et al. A molecular role for lysyl oxidase‐like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446‐3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cuevas EP, Eraso P, Mazón MJ, et al. LOXL2 drives epithelial‐mesenchymal transition via activation of IRE1‐XBP1 signalling pathway. Sci Rep. 2017;7:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Madshus IH, Stang E. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J Cell Sci. 2009;122:3433‐3439. [DOI] [PubMed] [Google Scholar]
- 42. Tian Y, Lin J, Tian Y, et al. Efficacy and safety of anti‐EGFR agents administered concurrently with standard therapies for patients with head and neck squamous cell carcinoma: a systematic review and meta‐analysis of randomized controlled trials. Int J Cancer. 2018;142:2198‐2206. [DOI] [PubMed] [Google Scholar]
- 43. Yamaoka T, Ohba M, Ohmori T. Molecular‐targeted therapies for epidermal growth factor receptor and its resistance mechanisms. Int J Mol Sci. 2017;18:2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang J, Nicolau MM, Cox TR, et al. LOXL2 induces aberrant acinar morphogenesis via ErbB2 signaling. Breast Cancer Res. 2013;15:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
All data generated or analyzed during this study are available from the corresponding authors on reasonable request.


