Abstract
Interstitial lung disease (ILD) is an adverse event associated with gemcitabine administration. Gemcitabine plus nab‐paclitaxel, which is now a first‐line chemotherapy regimen for pancreatic cancer (PC), may increase the risk of ILD; however, large‐scale clinical data on this are limited. Thus, this study aimed to elucidate the incidence and risk factors of ILD in patients with PC receiving gemcitabine plus nab‐paclitaxel. Through the Diagnosis Procedure Combination database, a Japanese nationwide inpatient database with outpatient data, we identified consecutive patients with PC who received gemcitabine‐based chemotherapy between July 2010 and March 2019 at 205 hospitals. Competing‐risk analysis was used to examine the cumulative incidence and risk factors of ILD. Among the 6163 patients who received gemcitabine plus nab‐paclitaxel, we documented 168 patients (2.7%) who developed ILD with cumulative incidence rates (95% confidence intervals [CIs]) of 2.0% (1.6%–2.4%), 2.7% (2.2%–3.1%), and 3.1% (2.6%–3.6%) at 3, 6, and 12 months, respectively. Compared with patients with PC who received gemcitabine monotherapy, those who received gemcitabine plus nab‐paclitaxel had an adjusted subdistribution hazard ratio (SHR) for ILD of 1.93 (95% CI: 1.51–2.47). Older age was associated with a high risk of ILD in patients receiving gemcitabine plus nab‐paclitaxel (adjusted SHR comparing ≥75 to ≤74 years, 1.61; 95% CI: 1.16–2.24). In conclusion, this study demonstrated the clinical course of gemcitabine plus nab‐paclitaxel‐associated ILD in patients with PC. When gemcitabine plus nab‐paclitaxel is administered to elderly patients with PC, symptoms associated with ILD must be monitored.
Keywords: gemcitabine, interstitial lung disease, nab‐paclitaxel, pancreatic cancer
This study demonstrated the clinical course of gemcitabine plus nab‐paclitaxel‐associated interstitial lung disease (ILD) in patients with pancreatic cancer (PC). When gemcitabine plus nab‐paclitaxel is administered for elderly patients with PC, symptoms associated with ILD must be monitored.
Abbreviations
- BMI
body mass index
- CCI
Charlson Comorbidity Index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- DPC
Diagnosis Procedure Combination
- ICD‐10
the International Classification of Diseases and Related Health Problems 10th Revision
- ILD
interstitial lung disease
- PC
pancreatic cancer
- SHR
subdistribution hazard ratio
1. INTRODUCTION
Gemcitabine is a standard chemotherapeutic agent with a tolerable toxic property and an effective anti‐tumor effect on PC. 1 , 2 , 3 However, pulmonary toxicity is one of its recognized adverse events, which potentially results in substantial morbidity and mortality. 4 , 5 The clinical presentations of pulmonary toxicity that were associated with gemcitabine range from mild self‐limiting symptoms (e.g., cough, dyspnea) to severe potentially fatal pulmonary disorders requiring intensive in‐hospital care (e.g., acute respiratory distress syndrome). 6 Severe pulmonary toxicity that is associated with gemcitabine typically develops as ILD based on inflammatory and fibrotic reactions in the parenchymal lung alveolar regions.
The anti‐tumor effects of gemcitabine‐based chemotherapy regimens, which consist of gemcitabine combined with cytotoxic or molecular‐targeted agents, on PC have been further investigated. 7 , 8 Since a clinical randomized trial demonstrated that gemcitabine plus nab‐paclitaxel has better tumor‐suppressing properties compared with gemcitabine monotherapy in patients with unresectable PC, 9 gemcitabine plus nab‐paclitaxel has been administered as one of the first‐line regimens for this population. 9 , 10 , 11 , 12 Nab‐paclitaxel is a solvent‐free formulation of the chemotherapeutic agent, paclitaxel, that was initially developed to mitigate the toxicity associated with the solvent used and potentially enhance anti‐tumor effects. 6 , 13 However, nab‐paclitaxel administration may predispose the patients to the risk of ILD with a reported incidence rate of approximately 2%–10%. 14 , 15 , 16 , 17 Due to the lack of large‐scale data on the head‐to‐head comparison between gemcitabine with and without nab‐paclitaxel, whether the addition of nab‐paclitaxel to gemcitabine elevates the risk of ILD is unknown. A large sample of cases is required to evaluate the incidence and risk factors of the relatively rare adverse event. 14 , 15 , 16 , 17
Therefore, to address these clinical questions, we utilized a nationwide administrative database with outpatient data in Japan and conducted a retrospective population‐based cohort study to compare the incidence of ILD between patients with PC who were administered with gemcitabine plus nab‐paclitaxel and other gemcitabine‐based chemotherapy regimens. We additionally examined the risk factors for ILD in patients with PC who were administered gemcitabine plus nab‐paclitaxel.
2. METHODS
2.1. Study population
The DPC database, which is a nationwide administrative database of inpatient care in Japan that includes data on admission/discharge abstracts and administrative claims, was utilized. 18 , 19 , 20 Data on approximately 7 million inpatients were collected from >1000 hospitals throughout Japan in 2020. The data on inpatient care included main diagnoses, comorbidities identified at admission, and complications observed during hospitalizations, which were recorded according to the ICD‐10 codes. The database also contains detailed clinical information on the Union for International Cancer Control TNM classification for cases with newly diagnosed cancer, smoking status based on the Brinkman index (number of cigarettes per day multiplied by smoking years), Barthel Index for the activities of daily living (0–100 points with higher scores indicating higher activity levels), 21 medications, interventional/surgical procedures (indexed by the Japanese original codes), and discharge status, including in‐hospital mortality. Data on outpatients have been collected since 2010 from approximately 350 hospitals that also participated in inpatient data collection. Data on outpatient care included dates of outpatient clinic visits, procedures, and medications including intravenous and oral chemotherapeutic agents.
Adult patients (≥20 years old) who were admitted to any of the hospitals that participated in the outpatient data collection with a principal diagnosis of PC (ICD‐10 codes: C25.0–25.3 and C25.7–25.9) were identified through the DPC database. We included patients who started receiving gemcitabine plus nab‐paclitaxel or other gemcitabine‐based chemotherapy regimens for PC between July 1, 2010 and March 31, 2019. We excluded patients with multiple cancer types or who were receiving other gemcitabine combination regimens (i.e., gemcitabine plus S‐1 or gemcitabine plus erlotinib). In addition, we excluded patients having concomitant ILD at the time of gemcitabine‐based chemotherapy initiation.
This study was approved by the institutional review board of The University of Tokyo (Tokyo, Japan; approval number, #3501‐(5) [May 19, 2021]). The requirement for patients' written informed consent was waived because of the anonymity of the used data.
2.2. Ascertainment of ILD cases
The primary outcome of this study was the development of ILD following gemcitabine‐based chemotherapy that required hospitalization. ILD was defined according to the following ICD‐10 codes: J70.2 (acute drug‐induced interstitial lung disorders), J70.3 (chronic drug‐induced interstitial lung disorders), J70.4 (drug‐induced interstitial lung disorders, unspecified), J84.1 (other interstitial pulmonary diseases with fibrosis), and J84.9 (interstitial pulmonary disease, unspecified). Patients who were hospitalized for ILD were identified based on information on the ICD‐10 codes at the index and all subsequent admissions.
2.3. Definitions of other variables
Each ICD‐10 code of comorbidity was converted to a score, and the sum was used to calculate the CCI based on Quan's algorithm. 22 BMI was calculated and classified into three categories (<25, 25–30, and >30 kg/m2). The ICD‐10 codes were used to define COPD (J43), chronic lower respiratory tract infection (J40–J42 and J44), chronic renal failure (N18), liver cirrhosis (K74), and arrhythmia (I490–I495, I498, and I499).
2.4. Statistical analysis
Time to ILD was defined as the period from the initiation of gemcitabine‐based chemotherapy to hospitalization due to ILD, administration of subsequent chemotherapy, last follow‐up, or death, whichever came first. When ILD occurred during the initial hospitalization, the date of ILD development was defined as the date of discharge during the corresponding hospitalization. When ILD was documented in subsequent hospitalizations, the date of ILD development was defined as the date of admission due to ILD. Cumulative incidences of ILD were calculated using a competing‐risk analysis, in which the initiation of a subsequent chemotherapy regimen and death were treated as competing‐risk events and were compared using Gray's test. 23 Through the competing risks proportional hazard regression model with adjustment for potential confounders, 24 we calculated SHRs and 95% CIs for risk of ILD development comparing gemcitabine plus nab‐paclitaxel to other gemcitabine‐based chemotherapy regimens. Univariable analysis was conducted for each of the following variables: age (≤74 vs. ≥75 years), sex (female vs. male), the Barthel Index (≤55 vs. 60–95 vs. 100), chronic renal failure (absent vs. present), liver cirrhosis (absent vs. present), arrhythmia (absent vs. present), COPD (absent vs. present), chronic lower respiratory tract infection (absent vs. present), CCI (≤2 vs. 3–5 vs. ≥6), BMI (<25 kg/m2 vs. 25–30 kg/m2 vs. >30 kg/m2), smoking status (never smoker vs. past/current smoker), and distant metastasis (absent vs. present). Potential risk factors with p < 0.20 in univariable analyses were adjusted for in multivariable models. In a secondary analysis, the same set of covariates were examined as potential risk factors for ILD among patients receiving gemcitabine plus nab‐paclitaxel. Using the logistic regression model with adjustment for potential confounders, we examined risk factors for in‐hospital mortality among patients undergoing ILD and calculated odds ratios (ORs) and 95% CIs.
R statistical software (version 4.2.1) and cmprsk package (R Development Core Team; http://www.r‐project.org) were used to compute cumulative incidence rates and 95% CI at specific time points and conduct Gray's test. All other statistical analyses were performed using Stata statistical software, version 15 (StataCorp LLC, College Station, Texas, USA).
3. RESULTS
We identified 6163, 9061, 2337, and 227 patients who received gemcitabine plus nab‐paclitaxel, gemcitabine monotherapy, gemcitabine plus S‐1, and gemcitabine plus erlotinib, respectively, at 205 hospitals (Figure 1). Table 1 summarizes the clinical characteristics of patients with PC according to chemotherapy regimens.
FIGURE 1.
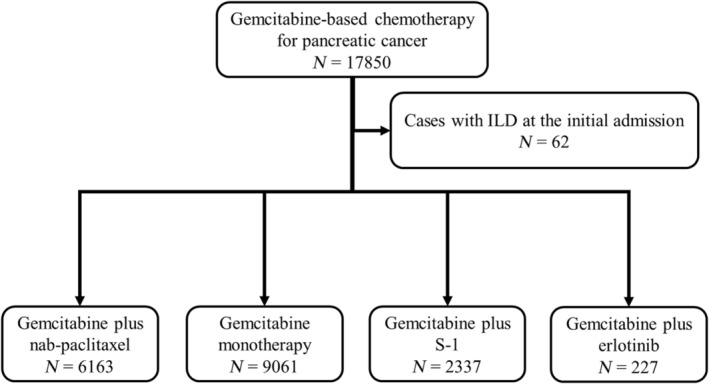
Flowchart of the selection of patients with pancreatic cancer receiving gemcitabine plus nab‐paclitaxel or other gemcitabine‐based chemotherapy regimens.
TABLE 1.
Baseline characteristics of patients with pancreatic cancer according to chemotherapy regimens.
| Characteristic | Chemotherapy regimen | p‐value | |||
|---|---|---|---|---|---|
| Gemcitabine plus nab‐paclitaxel (N = 6163) | Gemcitabine monotherapy (N = 9061) | Gemcitabine plus S‐1 (N = 2337) | Gemcitabine plus erlotinib (N = 227) | ||
| Age, years | 67.4 (9.0) | 70.0 (9.4) | 65.7 (9.5) | 63.7 (8.6) | <0.01 |
| Age ≤74 years | 4826 (78%) | 5856 (65%) | 1918 (82%) | 206 (91%) | <0.01 |
| Age ≥75 years | 1337 (22%) | 3205 (35%) | 419 (18%) | 21 (9.3%) | |
| Sex | |||||
| Male | 3679 (60%) | 5071 (56%) | 1407 (60%) | 130 (57%) | <0.01 |
| Female | 2484 (40%) | 3990 (44%) | 930 (40%) | 97 (43%) | |
| Barthel Index | |||||
| 100 | 5795 (94%) | 8087 (89%) | 2176 (93%) | 216 (95%) | <0.01 |
| 60–95 | 285 (4.6%) | 709 (7.8%) | 115 (4.9%) | 9 (4.0%) | |
| 0–55 | 83 (1.3%) | 265 (2.9%) | 46 (2.0%) | 2 (0.9%) | |
| Chronic renal failure | |||||
| No | 6127 (99%) | 8947 (99%) | 2333 (99%) | 227 (100%) | <0.01 |
| Yes | 36 (0.6%) | 114 (1.3%) | 4 (0.2%) | 0 | |
| Liver cirrhosis | |||||
| No | 6111 (99%) | 8992 (99%) | 2318 (99%) | 225 (99%) | 0.95 |
| Yes | 52 (0.8%) | 69 (0.8%) | 19 (0.8%) | 2 (0.9%) | |
| Arrhythmia | |||||
| No | 6138 (99%) | 8992 (99%) | 2327 (99%) | 225 (99%) | 0.03 |
| Yes | 25 (0.4%) | 69 (0.8%) | 110 (0.4%) | 2 (0.9%) | |
| COPD | |||||
| No | 6151 (99%) | 9036 (99%) | 2335 (99%) | 226 (99%) | 0.28 |
| Yes | 12 (0.2%) | 25 (0.3%) | 2 (0.1%) | 1 (0.4%) | |
| Chronic lower respiratory tract infection | |||||
| No | 6077 (98.6%) | 8955 (99%) | 2309 (99%) | 227 (100%) | 0.21 |
| Yes | 86 (1.4%) | 106 (1.2%) | 28 (1.2%) | 0 | |
| Charlson Comorbidity Index a | |||||
| ≤2 | 3718 (60%) | 5278 (58%) | 1418 (61%) | 114 (50%) | <0.01 |
| 3–5 | 534 (8.7%) | 857 (9.5%) | 200 (8.6%) | 8 (3.5%) | |
| ≥6 | 1909 (31%) | 2923 (32%) | 719 (31%) | 105 (46%) | |
| Body mass index a | |||||
| <25 kg/m2 | 5343 (87%) | 7944 (89%) | 2058 (89%) | 190 (84%) | <0.01 |
| 25–30 kg/m2 | 687 (11%) | 903 (10%) | 227 (9.8%) | 30 (13%) | |
| >30 kg/m2 | 88 (1.4%) | 88 (1.0%) | 24 (1.0%) | 6 (2.7%) | |
| Smoking status a | |||||
| Current or past | 2225 (36%) | 2784 (34%) | 754 (37%) | 84 (40%) | <0.01 |
| Never | 3281 (64%) | 5299 (66%) | 1307 (63%) | 127 (60%) | |
| Distant metastasis | |||||
| No | 2488 (40%) | 3275 (36%) | 892 (38%) | 58 (26%) | <0.01 |
| Yes | 3675 (60%) | 5786 (64%) | 1445 (62%) | 169 (74%) | |
Note: Data are shown as n (%) or mean (standard deviation).
Patients with missing data were excluded.
Abbreviation: COPD, chronic obstructive pulmonary disease.
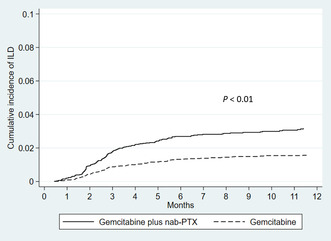
ILD was observed in 168 (2.7%) patients receiving gemcitabine plus nab‐paclitaxel with a median time to ILD of 2.7 months (interquartile range, 1.8–4.9 months) and in 133 (1.5%) patients receiving gemcitabine monotherapy with a median time to ILD of 2.8 months (interquartile range, 1.8–4.9 months). In patients receiving gemcitabine plus nab‐paclitaxel, the cumulative incidence rates of ILD were 2.0% (95% CI: 1.6–2.4), 2.7% (95% CI: 2.2–3.1) and 3.1% (95% CI: 2.6–3.6) at 3, 6, and 12 months, respectively, compared with 0.9% (95% CI: 0.8–1.2), 1.4% (95% CI: 1.1–1.7), and 1.6% (95% CI: 1.3–1.9), respectively, in patients receiving gemcitabine monotherapy (p < 0.01, Figure 2). Table 2 summarizes the characteristics of patients who were hospitalized due to ILD and treated during hospitalization. Mechanical ventilation was administered in 14 patients (8.3%) receiving gemcitabine plus nab‐paclitaxel and 12 patients (9.0%) receiving gemcitabine monotherapy. In patients receiving gemcitabine plus nab‐paclitaxel, most patients received steroid therapy (82%): orally in 29 patients (17%), intravenously in 24 patients (14%), and both in 84 patients (50%). The in‐hospital mortality with ILD was 21% and 32% in patients receiving gemcitabine plus nab‐paclitaxel and gemcitabine monotherapy, respectively. In the total study population, the incidence of ILD with an in‐hospital death was 0.5%. In an analysis of risk factors for in‐hospital mortality among patients with ILD, low levels of the Barthel Index and high levels of CCI, but not the chemotherapy regimens, were independent risk factors (Table 3).
FIGURE 2.
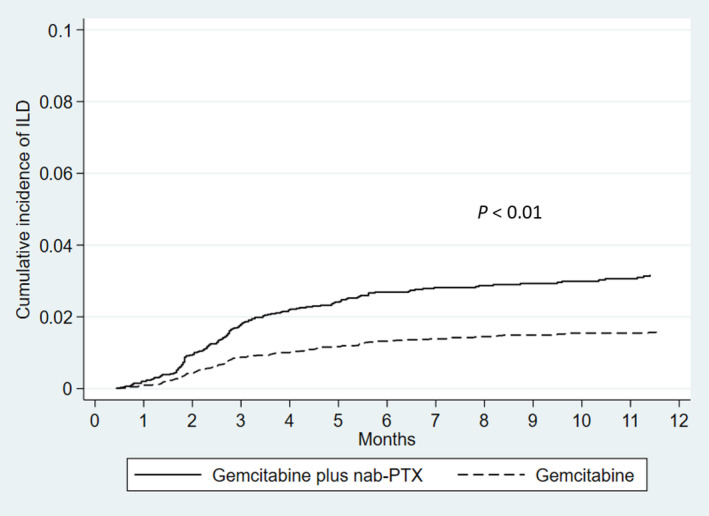
Cumulative incidence rates of interstitial lung disease (ILD) among patients with pancreatic cancer by chemotherapy regimens (gemcitabine plus nab‐paclitaxel vs. gemcitabine monotherapy). PTX, paclitaxel.
TABLE 2.
Characteristics and treatment modalities of patients who developed interstitial lung disease associated with gemcitabine‐based chemotherapy.
| Chemotherapy regimen | p‐value | ||||
|---|---|---|---|---|---|
| Gemcitabine plus nab‐paclitaxel (N = 168) | Gemcitabine monotherapy (N = 133) | Gemcitabine plus S‐1 (N = 24) | Gemcitabine plus erlotinib (N = 8) | ||
| Demographic | |||||
| Age, years | 70 (8.0) | 72 (8.6) | 69 (9.2) | 64 (7.3) | 0.05 |
| Sex | |||||
| Male | 109 (65%) | 83 (62%) | 16 (67%) | 8 (100) | 0.19 |
| Female | 59 (35%) | 50 (38%) | 8 (33%) | 0 | |
| Treatment | |||||
| Oxygen therapy | 110 (66%) | 103 (77%) | 17 (71%) | 5 (63%) | 0.15 |
| Mechanical ventilation | 14 (8.3%) | 12 (9.0%) | 1 (4.2%) | 0 | 0.71 |
| Steroid therapy, any type | 137 (82%) | 119 (90%) | 20 (83%) | 6 (75%) | 0.24 |
| Oral | 29 (17%) | 15 (11%) | 4 (17%) | 0 | |
| Intravenous | 24 (14%) | 30 (23%) | 3 (13%) | 1 (13%) | |
| Both | 84 (50%) | 74 (56%) | 13 (54%) | 5 (63%) | <0.01 |
| Intravenous diuretic agent | 44 (26%) | 52 (39%) | 12 (50%) | 6 (75%) | |
Note: Data are shown as n (%) or mean (standard deviation).
TABLE 3.
Univariable and multivariable logistic regression analyses to assess the risk factors for in‐hospital death among patients with interstitial lung disease.
| N | Events (%) | Univariable | Multivariable b | |||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p | Odds ratio | 95% CI | p | |||
| Chemotherapy regimen | ||||||||
| Gemcitabine monotherapy | 133 | 43 (32%) | 1 | Referent | 1 | Referent | ||
| Gemcitabine plus nab‐paclitaxel | 168 | 36 (21%) | 0.57 | 0.34–0.96 | 0.03 | 0.70 | 0.39–1.24 | 0.22 |
| Gemcitabine plus S‐1 | 24 | 9 (38%) | 1.25 | 0.51–3.12 | 0.62 | 2.00 | 0.73–5.53 | 0.18 |
| Gemcitabine plus erlotinib | 8 | 2 (25%) | 0.70 | 0.14–3.57 | 0.67 | 0.56 | 0.08–3.81 | 0.56 |
| Age, ≤74 years | 219 | 58 (27%) | 1 | Referent | ||||
| ≥75 years | 114 | 32 (28%) | 1.08 | 0.65–1.80 | 0.78 | |||
| Sex, female | 117 | 35 (30%) | 1 | Referent | ||||
| Male | 216 | 55 (26%) | 0.80 | 0.49–1.32 | 0.38 | |||
| Barthel Index, 100 | 222 | 47 (21%) | 1 | 1 | Referent | |||
| 60–95 | 61 | 16 (26%) | 1.32 | 0.69–2.55 | 0.40 | 1.36 | 0.68–2.75 | 0.39 |
| 0–55 | 50 | 27 (54%) | 4.37 | 2.30–8.31 | <0.01 | 5.27 | 2.61–10.7 | <0.01 |
| Chronic renal failure, No | 329 | 87 (26%) | 1 | Referent | 1 | Referent | ||
| Yes | 4 | 3 (75%) | 8.34 | 0.86–81.3 | 0.07 | 8.30 | 0.70–98.9 | 0.09 |
| Liver cirrhosis, No | 329 | 89 (27%) | 1 | Referent | ||||
| Yes | 4 | 1 (25%) | 0.90 | 0.09–8.75 | 0.93 | |||
| Arrhythmia, No | 333 | 90 (27%) | 1 | Referent | ||||
| Yes | 0 | 0 | NA | NA | NA | |||
| COPD, No | 328 | 88 (27%) | 1 | |||||
| Yes | 5 | 2 (40%) | 1.82 | 0.30–11.1 | 0.52 | |||
| Chronic lower respiratory tract infection, No | 320 | 87 (27%) | 1 | |||||
| Yes | 13 | 3 (23%) | 0.80 | 0.22–2.99 | 0.74 | |||
| CCI, ≤2 a | 169 | 37 (22%) | 1 | Referent | 1 | Referent | ||
| 3–5 | 36 | 9 (25%) | 1.19 | 0.51–2.75 | 0.69 | 1.02 | 0.40–2.63 | 0.96 |
| ≥6 | 124 | 43 (35%) | 1.89 | 1.13–3.18 | 0.02 | 1.90 | 1.06–3.39 | 0.03 |
| BMI <25 kg/m2 a | 303 | 81 (27%) | 1 | Referent | ||||
| 25–30 kg/m2 | 23 | 5 (22%) | 0.76 | 0.27–2.12 | 0.60 | |||
| >30 kg/m2 | 0 | 0 | NA | NA | NA | |||
| Smoking status, Never a | 163 | 51 (31%) | 1 | Referent | 1 | Referent | ||
| Ever | 142 | 35 (25%) | 0.72 | 0.43–1.19 | 0.20 | 0.82 | 0.47–1.41 | 0.47 |
| Distant metastasis, No | 240 | 63 (26%) | 1 | Referent | ||||
| Yes | 93 | 27 (29%) | 1.15 | 0.68–1.96 | 0.61 | |||
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; NA, not available.
Patients with missing data were excluded.
The variables with p < 0.20 in the univariable analyses were entered in a multivariable model.
In the multivariable competing‐risk regression analysis (Table 4), patients receiving gemcitabine plus nab‐paclitaxel were at a higher risk of ILD compared with patients receiving gemcitabine monotherapy or gemcitabine plus S‐1 with adjusted SHRs of 1.93 (95% CI: 1.51–2.47) and 2.81 (95% CI: 1.83–4.31), respectively. Erlotinib has been recognized as a risk factor for the development of ILD 8 and, therefore, we compared the risks of ILD between gemcitabine plus nab‐paclitaxel and gemcitabine plus erlotinib (Table 4 and Figure S1). No statistically significant difference was observed between the groups although the limited number of patients receiving gemcitabine plus erlotinib precluded a robust statistical assessment in this subgroup. In an analysis of risk factors for ILD in patients receiving gemcitabine plus nab‐paclitaxel, older age was an independent risk factor for ILD with an adjusted SHR comparing ≥75 years to ≤74 years of 1.61 (95% CI: 1.16–2.24; Table 5). The adjusted SHR per 10‐year increase in age was 1.44 (95% CI: 1.19–1.75). The number of patients with COPD or chronic lower respiratory tract who underwent ILD due to gemcitabine plus nab‐paclitaxel was limited, thereby precluding a robust statistical analysis. Among patients receiving gemcitabine monotherapy, smoking status was an independent risk factor for ILD with an adjusted SHR comparing past/current smokers to never smokers of 1.51 (95% CI: 1.00–2.29; Table S1).
TABLE 4.
Univariable and multivariable competing risks regression analyses to assess the risk of interstitial lung disease associated with gemcitabine plus nab‐paclitaxel compared with gemcitabine monotherapy, gemcitabine plus S‐1, or gemcitabine plus erlotinib.
| Chemotherapy regimen | N | Events (%) | Univariable | Multivariable a | ||||
|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | p‐value | SHR | 95% CI | p‐value | |||
| Gemcitabine monotherapy | 9061 | 133 (1.5%) | 1 | Referent | ||||
| Gemcitabine plus nab‐paclitaxel | 6163 | 168 (2.7%) | 1.93 | 1.54–2.42 | <0.01 | 1.93 | 1.51–2.47 | <0.01 |
| Gemcitabine plus S‐1 | 2337 | 24 (1.0%) | 1 | Referent | ||||
| Gemcitabine plus nab‐paclitaxel | 6163 | 168 (2.7%) | 2.97 | 1.94–4.55 | <0.01 | 2.81 | 1.83–4.31 | <0.01 |
| Gemcitabine plus erlotinib | 227 | 8 (3.5%) | 1 | Referent | ||||
| Gemcitabine plus nab‐paclitaxel | 6163 | 168 (2.7%) | 0.77 | 0.38–1.55 | 0.46 | 0.69 | 0.34–1.40 | 0.30 |
Abbreviations: CI, confidence interval; SHR, subdistribution hazard ratio.
Univariable analysis was conducted by entering each of the following variables: age, sex, Barthel Index, chronic renal failure, liver cirrhosis, arrhythmia, COPD, chronic lung infection, Charlson Comorbidity Index, body mass index, smoking status, and distant metastasis. In addition to the chemotherapy regimen, the variables with p < 0.20 in the univariable analyses (age, sex, COPD, Charlson Comorbidity Index, smoking status, and distant metastasis for the comparison with gemcitabine monotherapy; age, sex, and Barthel Index for the comparison with gemcitabine plus S‐1; and age and smoking status for the comparison with gemcitabine plus erlotinib) were entered in a multivariable model.
TABLE 5.
Univariable and multivariable competing risks regression analyses to assess the risk factors for interstitial lung disease among patients receiving gemcitabine plus nab‐paclitaxel.
| N | Events (%) | Univariable | Multivariable b | |||||
|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | p‐value | SHR | 95% CI | p‐value | |||
| Age, ≤74 years | 4826 | 118 (2.4%) | 1 | Referent | 1 | Referent | ||
| ≥75 years | 1337 | 50 (3.7%) | 1.58 | 1.14–2.20 | <0.01 | 1.61 | 1.16–2.24 | <0.01 |
| Sex, female | 2484 | 59 (2.4%) | 1 | Referent | 1 | Referent | ||
| Male | 3679 | 109 (3.0%) | 1.27 | 0.92–1.74 | 0.14 | 1.30 | 0.95–1.78 | 0.11 |
| Barthel Index, 100 | 5795 | 159 (2.7%) | 1 | |||||
| 60–95 | 285 | 5 (1.8%) | 0.68 | 0.28–1.66 | 0.39 | |||
| 0–55 | 83 | 4 (4.8%) | 1.85 | 0.68–5.01 | 0.23 | |||
| Chronic renal failure, No | 6127 | 168 (2.7%) | 1 | Referent | ||||
| Yes | 36 | 0 | NA | NA | NA | |||
| Liver cirrhosis, No | 6111 | 166 (2.7%) | 1 | Referent | ||||
| Yes | 52 | 2 (3.8%) | 1.28 | 0.31–5.20 | 0.73 | |||
| Arrhythmia, No | 6138 | 168 (2.7%) | 1 | Referent | ||||
| Yes | 25 | 0 | NA | NA | NA | |||
| COPD, No | 6151 | 168 (2.7%) | 1 | |||||
| Yes | 12 | 0 | NA | NA | NA | |||
| Chronic lower respiratory tract infection, No | 6077 | 167 (2.7%) | 1 | |||||
| Yes | 86 | 1 (1.2%) | 0.41 | 0.06–2.99 | 0.38 | |||
| CCI, ≤2 a | 3718 | 99 (2.7%) | 1 | Referent | ||||
| 3–5 | 534 | 12 (2.2%) | 0.85 | 0.47–1.53 | 0.58 | |||
| ≥ 6 | 1909 | 57 (3.0%) | 1.16 | 0.84–1.61 | 0.37 | |||
| BMI <25 kg/m2 a | 5343 | 146 (2.7%) | 1 | Referent | ||||
| 25–30 kg/m2 | 687 | 20 (2.9%) | 1.08 | 0.68–1.73 | 0.73 | |||
| > 30 kg/m2 | 88 | 2 (2.3%) | 0.86 | 0.21–3.43 | 0.83 | |||
| Smoking status, Never a | 2225 | 83 (3.7%) | 1 | Referent | ||||
| Ever | 3281 | 65 (2.0%) | 1.19 | 0.86–1.65 | 0.29 | |||
| Distant metastasis, No | 2488 | 65 (2.6%) | 1 | Referent | ||||
| Yes | 3675 | 103 (2.8%) | 1.05 | 0.77–1.43 | 0.75 | |||
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; NA, not available; SHR, subdistribution hazard ratio.
Patients with missing data were excluded.
The variables with p < 0.20 in the univariable analyses were entered in a multivariable model.
4. DISCUSSION
In this large longitudinal cohort study based on a nationwide database, we characterized the clinical course of patients with PC who developed ILD following gemcitabine plus nab‐paclitaxel administration with substantial rates of incidence and in‐hospital mortality. Compared with patients receiving gemcitabine monotherapy, patients receiving gemcitabine plus nab‐paclitaxel were at a higher risk of ILD, in which older age was the only risk factor. Our population‐based data would help to increase the awareness of oncologists on gemcitabine plus nab‐paclitaxel‐associated ILD and stratify patients who are indicated for this chemotherapy regimen in terms of the ILD risk.
The current study suggests that the addition of nab‐paclitaxel may elevate the risk of gemcitabine‐associated ILD, which has been well recognized in clinical oncology. 18 Nab‐paclitaxel has several potential advantages over conventional solvent‐based paclitaxel including increasing the intratumor concentration of paclitaxel and decreasing adverse events (e.g., allergic reactions). 25 , 26 , 27 , 28 In the studies of patients with any cancer receiving nab‐paclitaxel, the incidence of ILD was reported to be 6%–8%. 14 , 15 , 16 , 29 , 30 Based on our data implicating the additive effects of gemcitabine and nab‐paclitaxel on ILD development, clinical oncologists should be aware of this potentially lethal adverse event and pay attention to the early symptoms of ILD (e.g., dyspnea and cough) and chest radiographic changes (typically, interstitial pulmonary infiltrates) when administrating the combination therapy. In this study, the median time to ILD development was approximately 2–3 months after the initiation of gemcitabine with or without nab‐paclitaxel; therefore, particular attention should be paid to patients during this period.
Treatment of drug‐induced ILD is usually initiated by supportive management including sufficient physical rest and oxygen supplementation. Steroid therapy has been the standard care for patients with moderate to severe ILD, particularly for those with underlying comorbidities and who are unamenable to initial supportive care. 31 , 32 In the current study, 82% of patients with ILD following gemcitabine plus nab‐paclitaxel received intravenous or oral steroid therapy. Given the high in‐hospital mortality rate of patients with gemcitabine plus nab‐paclitaxel‐associated ILD, chemotherapy should be discontinued immediately when symptoms suggestive of ILD development are observed at the clinic, and other chemotherapy regimens should be considered as an alternative subsequent treatment. During hospitalizations associated with ILD, patients should be monitored prudently to avoid delays in administering appropriate intensive treatment options. In addition to that, our analyses suggest that comorbid conditions and physical activity levels may result in fatal outcomes associated with ILD. These findings support the importance of a comprehensive assessment of patients' general conditions in the risk stratification of patients presenting with ILD. 33
Risk factors for gemcitabine plus nab‐paclitaxel‐associated ILD have not yet been fully investigated. In prior studies of patients with PC receiving gemcitabine plus nab‐paclitaxel, the ABO blood group or concomitant use of Chinese herbal medications appeared to be associated with the risk of ILD. 14 , 15 However, those studies were limited by their single‐institutional study design and statistical power. The large comprehensive dataset used in this study allowed us to identify risk factors with adjustment for a spectrum of potential confounders. In the current study, a 10‐year increase in age translated into an approximately 1.4‐fold increased risk of ILD. Older patients might have underlying pulmonary disorders, thereby potentially increasing the risk of drug‐induced inflammatory reactions in the lung and resultant ILD. 34 , 35 Given these findings, the risk of ILD should be recognized, particularly when gemcitabine plus nab‐paclitaxel is administered to elderly patients.
This study has several limitations. In our multivariable models, we adjusted for multiple potential confounding factors (e.g., smoking, arrhythmia). 36 , 37 However, due to the nature of the database used, we could not access some important clinical information, including the results of laboratory tests and imaging studies. Consequently, the ascertainment of ILD cases was based solely on the ICD‐10 codes. Nonetheless, those ICD‐10 codes were assigned by treating physicians at the participating hospitals. Another limitation was that data at the referred hospital were not available for analysis when a patient was transferred to another hospital. Despite these limitations, a major strength of the current study was the use of large‐scale data from many hospitals throughout Japan, which not only enabled us to document the clinical outcomes of patients with gemcitabine plus nab‐paclitaxel‐associated ILD with robust statistical estimates, but also potentially ensured the generalizability of our findings.
In conclusion, our large cohort study demonstrated that a substantial incidence rate of ILD was observed in patients with PC who were administered gemcitabine plus nab‐paclitaxel. Despite the increased anti‐tumor effects of gemcitabine plus nab‐paclitaxel for unresectable PC, this combination chemotherapy may increase the risk of ILD compared with gemcitabine monotherapy. Our risk factor analysis suggests that elderly patients who receive gemcitabine plus nab‐paclitaxel must be carefully monitored. These findings imply that treatment indications must be assessed considering the trade‐off of survival benefits and risk of ILD.
FUNDING INFORMATION
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007 and 22AA2003 to H.Y.); and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907 to H.Y.). T.H. received grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP19K08362 and JP22H02841) and Takeda Science Foundation. Y.N. received grants from Taiho Pharmaceutical. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
This study was approved by the institutional review board of The University of Tokyo (Tokyo, Japan; approval number, #3501‐(5) [May 19, 2021]).
Informed Consent: The requirement for patients' written informed consent was waived because of the anonymity of the used data.
Registry and the Registration No. of the study: N/A.
Animal Studies: N/A.
Supporting information
Figure S1.
Table S1.
ACKNOWLEDGMENTS
None.
Saito K, Michihata N, Hamada T, et al. Gemcitabine plus nab‐paclitaxel for pancreatic cancer and interstitial lung disease: A nationwide longitudinal study. Cancer Sci. 2023;114:3996‐4005. doi: 10.1111/cas.15910
Kei Saito, Nobuaki Michihata, and Tsuyoshi Hamada contributed equally as co‐first authors.
Hideo Yasunaga and Mitsuhiro Fujishiro contributed equally as co‐last authors.
REFERENCES
- 1. Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S‐1, S‐1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640‐1648. [DOI] [PubMed] [Google Scholar]
- 2. Ueno H, Ikeda M, Ueno M, et al. Phase I/II study of nab‐paclitaxel plus gemcitabine for chemotherapy‐naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2016;77:595‐603. [DOI] [PubMed] [Google Scholar]
- 3. Portal A, Pernot S, Tougeron D, et al. Nab‐paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113:989‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barlési F, Villani P, Doddoli C, Gimenez C, Kleisbauer JP. Gemcitabine‐induced severe pulmonary toxicity. Fundam Clin Pharmacol. 2004;18:85‐91. [DOI] [PubMed] [Google Scholar]
- 5. Belknap SM, Kuzel TM, Yarnold PR, et al. Clinical features and correlates of gemcitabine‐associated lung injury: findings from the RADAR project. Cancer. 2006;106:2051‐2057. [DOI] [PubMed] [Google Scholar]
- 6. Kundranda MN, Niu J. Albumin‐bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015;9:3767‐3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakai Y, Isayama H, Sasaki T, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S‐1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer. 2012;106:1934‐1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2007;25:1960‐1966. [DOI] [PubMed] [Google Scholar]
- 9. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldstein D, El‐Maraghi RH, Hammel P, et al. Nab‐paclitaxel plus gemcitabine for metastatic pancreatic cancer: long‐term survival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413. [DOI] [PubMed] [Google Scholar]
- 11. Philip PA, Lacy J, Portales F, et al. Nab‐paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open‐label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285‐294. [DOI] [PubMed] [Google Scholar]
- 12. Perri G, Prakash L, Qiao W, et al. Response and survival associated with first‐line FOLFIRINOX vs gemcitabine and nab‐paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. JAMA Surg. 2020;155:832‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viúdez A, Ramírez N, Hernández‐García I, Carvalho FL, Vera R, Hidalgo M. Nab‐paclitaxel: a flattering facelift. Crit Rev Oncol. 2014;92:166‐180. [DOI] [PubMed] [Google Scholar]
- 14. Takeda T, Sasaki T, Fukuda K, et al. Risk factors for gemcitabine plus nab‐paclitaxel‐induced interstitial lung disease in pancreatic cancer patients. Int J Clin Oncol. 2021;26:543‐551. [DOI] [PubMed] [Google Scholar]
- 15. Ueda R, Yamamoto N, Hori Y, et al. Risk factors for interstitial lung disease induced by gemcitabine plus albumin‐bound paclitaxel therapy in pancreatic ductal adenocarcinoma patients. J Pharm Health Care Sci. 2022;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kashiwada T, Saito Y, Terasaki Y, et al. Interstitial lung disease associated with nanoparticle albumin‐bound paclitaxel treatment in patients with lung cancer. Jpn J Clin Oncol. 2019;49:165‐173. [DOI] [PubMed] [Google Scholar]
- 17. Yasuda Y, Nomizo T, Ozasa H, et al. Retrospective analysis of acute exacerbation of interstitial lung diseases with nanoparticle albumin‐bound paclitaxel in patients with advanced lung cancer with preexisting interstitial lung disease. Mol Clin Oncol. 2017;7:677‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamada T, Yasunaga H, Nakai Y, et al. Interstitial lung disease associated with gemcitabine: a Japanese retrospective cohort study. Respirology. 2016;21:338‐343. [DOI] [PubMed] [Google Scholar]
- 19. Katsuki R, Jo T, Yasunaga H, Kumazawa R, Uda K. Outcomes of laparoscopic versus open pancreatoduodenectomy: a nationwide retrospective cohort study. Surgery. 2021;169:1427‐1433. [DOI] [PubMed] [Google Scholar]
- 20. Okada A, Ono S, Yamaguchi S, et al. Association between nutritional guidance or ophthalmological examination and discontinuation of physician visits in patients with newly diagnosed diabetes: a retrospective cohort study using a nationwide database. J Diabetes Investig. 2021;12:1619‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61‐65. [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 23. Marubini E, Valsecchi MG. Analysing Survival Data from Clinical Trials and Observational Studies. John Wiley and Sons; 1995. [Google Scholar]
- 24. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 25. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin‐bound paclitaxel compared with polyethylated castor oil‐based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794‐7803. [DOI] [PubMed] [Google Scholar]
- 26. Shitara K, Takashima A, Fujitani K, et al. Nab‐paclitaxel versus solvent‐based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open‐label, randomised, non‐inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:277‐287. [DOI] [PubMed] [Google Scholar]
- 27. Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055‐2062. [DOI] [PubMed] [Google Scholar]
- 28. Stage TB, Bergmann TK, Kroetz DL. Clinical pharmacokinetics of paclitaxel monotherapy: an updated literature review. Clin Pharmacokinet. 2018;57:7‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyauchi E, Inoue A, Usui K, et al. Phase II study of modified carboplatin plus weekly nab‐paclitaxel in elderly patients with non‐small cell lung cancer: North Japan lung cancer study group trial 1301. Oncologist. 2017;22:640‐e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshimura N, Sawa K, Nakai T, et al. Phase II study of the modified weekly nab‐paclitaxel regimen in previously treated patients with advanced non‐small cell lung cancer. Am J Clin Oncol. 2021;44:613‐618. [DOI] [PubMed] [Google Scholar]
- 31. Podolanczuk AJ, Wong AW, Saito S, Lasky JA, Ryerson CJ, Eickelberg O. Update in interstitial lung disease 2020. Am J Respir Crit Care Med. 2021;203:1343‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conte P, Ascierto PA, Patelli G, et al. Drug‐induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. 2022;7:100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwarzkopf L, Witt S, Waelscher J, Polke M, Kreuter M. Associations between comorbidities, their treatment and survival in patients with interstitial lung diseases–a claims data analysis. Respir Res. 2018;19(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Margaritopoulos GA, Vasarmidi E, Jacob J, Wells AU, Antoniou KM. Smoking and interstitial lung diseases. Eur Respir Rev. 2015;24:428‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer. 2004;91(Suppl 2):S3‐S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bargagli E, Cameli P, Carleo A, et al. The effect of cigarette smoking on bronchoalveolar lavage protein profiles from patients with different interstitial lung diseases. Panminerva Med. 2020;62:109‐115. [DOI] [PubMed] [Google Scholar]
- 37. Skeoch S, Weatherley N, Swift AJ, et al. Drug‐induced interstitial lung disease: a systematic review. J Clin Med. 2018;7:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1.


