Abstract
We have investigated the contribution of drug accumulation and inhibition of heme polymerization to the in vitro activities of a series of antimalarial drugs. Only those compounds exhibiting structural relatedness to the quinolines inhibited heme polymerization. We could find no direct correlation between in vitro activity against chloroquine-susceptible or chloroquine-resistant isolates and either inhibition of heme polymerization or cellular drug accumulation for the drugs studied. However, in vitro activity against a chloroquine-susceptible isolate but not a chloroquine-resistant isolate showed a significant correlation with inhibition of heme polymerization when the activity was normalized for the extent of drug accumulation. The importance of these observations to the rational design of new quinoline-type drugs and the level of agreement of these conclusions with current views on quinoline drug action and resistance are discussed.
The malarial parasite Plasmodium falciparum digests a large proportion of its host cell hemoglobin during its erythrocytic cycle, presumably as a source of essential nutrients (24, 25, 28). Digestion is a complex process involving three proteases: one cysteine protease (falcipain) and two aspartic proteases (plasmepsins I and II) (22). Digestion is thought to be initiated by the action of plasmepsin I on native hemoglobin, leading to the release of iron II ferroprotoporphyrin IX (FPIX) (12). Free FPIX is a toxic substance, and parasites which are lacking in heme oxygenase are unable to detoxify free FPIX by metabolism. Instead, malarial parasites have evolved an autocatalytic detoxification process in which FPIX is oxidized to iron III FPIX (hematin), which is then polymerized, forming inert crystals of hemozoin or malaria pigment (8).
Following early observations that free FPIX was able to form complexes with nitrogenous bases such as pyridines and quinolines (6, 18, 23) it was hypothesized that the quinoline-containing antimalarial agents exerted their effects by forming toxic complexes with free FPIX released in situ (5). More recent work has shown that quinoline antimalarial agents are able to inhibit the spontaneous polymerization of hematin, suggesting a mechanism by which free FPIX or FPIX-chloroquine (CQ) complexes may concentrate in the food vacuole and kill the parasite (8, 26).
There is strong evidence that the accumulation of quinoline-containing drugs is influenced by the physicochemical properties of the drugs and the proton gradient which exists between the external environment and the intracellular parasite (2, 19). Such factors profoundly influence the local drug concentration in the food vacuole and therefore the ability of a drug to inhibit heme polymerization in situ. Indeed, studies carried out in this laboratory have clearly indicated that the extent of accumulation of quinoline-containing drugs is an important determinant of their activity (14, 15). In the present study, using a series of 27 antimalarial agents (14 quinolines and 13 nonquinolines), we have tested the relative contribution of drug accumulation and inhibition of heme polymerization activity to in vitro antiparasitic drug activity against CQ-resistant and -susceptible isolates of P. falciparum.
MATERIALS AND METHODS
Drugs used in the study.
CQ, amodiaquine (AQ), mepacrine (MEP), quinine (QN), quinidine (QND), cinchonine (CIN), cinchonidine (CIND), and primaquine (PQ) were purchased from Sigma, Dorset, United Kingdom. 7-Chloro-4-(4′-pyrollidino-1′-methylbutamino)quinoline (chloroquine-pyrollidinyl; CQ-PYROL), monodesethyl chloroquine (DesCQ), and bidesethyl chloroquine (BidesCQ) were obtained from The Walter Reed Army Institute, Washington, D.C. Monodesethyl amodiaquine (DesAQ), bidesethyl amodiaquine (BidesAQ), amopyroquine (AMOPYR), and tebuquine (TBQ) were obtained from Parke- Davis Limited, Hampshire, United Kingdom. Pyronaridine (PYR) was a gift from D. Warhurst, London School of Hygiene and Tropical Medicine. Halofantrine (HF) was from SmithKline Beecham; mefloquine (MQ) was obtained from Roche; and artemether (ARM), arteether (ART), and dihydroartemisinine (DHA) were provided by the World Health Organization. 4-[3′-[(Diethylamino)methyl]-4′-hyroxyanilino]pyridine (AQ-PYRID), 4-(diethylamino-1′-methylbutylamino)pyridine (CQ-PYRID), 7-chloro-4-(3′-t-butylaminomethyl- 4′-hydroxyanilino) quinoline (N-t-butyl amodiaquine; NTB-AQ), 4′-dehydroxytebuquine (4-deOH TBQ), 4′-dehydroxy-4′-fluorotebuquine (4F-TBQ), and 7-chloro-4-hydroxyanilinoquinoline (De-SC-AQ) were synthesized as reported previously (13, 20). The chemical structures of all the drugs are shown in Fig. 1.
FIG. 1.
Chemical structures of the compounds used in this study.
Parasite isolates and maintenance and preparation of isolates.
Four isolates of P. falciparum were used in this study. Isolates 3D7 and HB3 were CQ-susceptible isolates, and isolate K1 was a CQ-resistant isolate; these isolates were obtained from D. Walliker, University of Edinburgh, Edinburgh, United Kingdom. Isolate PH3, a CQ-resistant isolate, was obtained from M. Hommel, Liverpool School of Tropical Medicine, Liverpool, United Kingdom. Cultures, which were maintained by an adaptation of the method of Jensen and Trager (16), consisted of a 1 to 5% suspension of type O-positive erythrocytes in complete culture medium (RPMI 1640 medium supplemented with 10% human type AB serum, 25 mM HEPES buffer, and 23 mM NaHCO3). These cultures were gassed with an atmosphere of 93% N2, 3% CO2, and 4% O2. Cultures were synchronized by the method of Lambros and Vandenburg (17) before use.
Drug susceptibility assays.
Drug potency was assessed by an adaptation of the standard 48-h microdilution technique described by Desjardins et al. (7). Parasites were exposed to serial dilutions of drug over 48 h, and growth was assessed by comparing the level of incorporation of [G-3H]hypoxanthine by the parasites, at each drug concentration, with that of an appropriate control. Dose-response curves were then plotted by using the Grafit software package (Erithacus Software, Staines, United Kingdom). The 50% inhibitory concentrations (IC50s) were calculated graphically by interpolation of the dose-response curve. Assays were carried out with an inoculum size of 1 to minimize any inoculum effect associated with the IC50 of the drug. Results are given as the means of at least three separate experiments.
Measurement of drug accumulation and absolute drug activity using inoculum effect analysis.
The measured IC50 of a drug increases with increased inoculum size (where inoculum size = level of parasitemia × hematocrit) due to significant depletion of drug from the medium (10). This phenomenon is termed the “inoculum effect.” In this study, the potencies of the compounds studied were assessed at inoculum sizes ranging from 1 to 10 (fractional parasite volume, 0.0001 to 0.001). Over this range, the relationship between measured IC50 of the drug and inoculum size is linear. Extrapolation of this line to an inoculum size of zero provides a measure of absolute drug potency from the following equation (10): IC50 measured = IC50 absolute + (IC50 absolute × accumulation ratio × fractional volume of PRBCs), where PRBCs are parasitized erythrocytes. Furthermore, we have previously validated the use of this mathematical relationship for the determination of the cellular drug accumulation ratio (CAR) from the following equation (3): CAR = (IC50 measured − IC50 absolute)/(IC50 absolute × fractional volume of PRBCs), where CAR is the ratio of the amount of drug in the infected cell pellet to the amount of drug in a similar volume of medium. This approach allows the measurement of accumulation in the absence of radioactively labelled drug.
Heme polymerization assay.
The ability of the compounds studied to inhibit heme polymerization was assessed by a modification of the protocol reported by Dorn et al. (8). Incubations were performed in 1.5-ml Eppendorf tubes containing 50 μg of β-hematin, 140 μM 14C-hemin chloride (specific activity, 105 μCi μmol−1; University of Leeds Innovations Industrial Services Ltd., Leeds, United Kingdom), and test drug at the appropriate concentration in a 100-μl final volume of 500 mM sodium acetate buffer (pH 4.8). The samples were then incubated at 37°C overnight. Appropriate drug-free and radioactive background controls were included. Following incubation, unreacted β-hematin was removed by incubating the samples in 2% sodium dodecyl sulfate (1 ml in 0.1 M sodium bicarbonate buffer [pH 9.1]) at 37°C for 15 min. The samples were centrifuged (14,000 × g, 15 min) and the supernatant was removed, and the cell pellet was washed by subsequent incubation (37°C, 15 min) and centrifugation (14,000 × g, 15 min) steps with 1 ml of 0.1 M sodium bicarbonate buffer (pH 9.1) and then 1 ml of 50 mM Tris-HCl (pH 7.5). Finally, 100 μl of 0.1 M NaOH was added to resuspend the cell pellet, and the samples were transferred to liquid scintillation vials containing 100 μl of glacial acetic acid. Following the addition of 4 ml of Optiphase Safe liquid scintillant, samples were counted on a Rackbeta 1219 liquid scintillation counter.
Mean disintegrations per minute are expressed as percent inhibition relative to the amount of hemozoin formation in a drug-free control. Dose-response curves were plotted with the Grafit software package (Erithacus Software). IC50s were calculated graphically by interpolation of the dose-response curve. Results are given as the means of at least three separate experiments.
RESULTS
The heme polymerization inhibitory potentials and antimalarial activities (at an inoculum size of 1) of the drugs against each of the four parasite isolates are presented in Table 1. Ignoring the data obtained for the sesquiterpene lactones ARM, ART, and DHA and the 8-aminoquinoline PQ (which are considered to have independent mechanisms of action), the drugs tested showed greater than 2,000- and 5,000-fold ranges in their antimalarial activities (IC50) against the CQ-susceptible and the CQ-resistant isolates, respectively. The equivalent ranges for the 14 quinoline compounds were 300-fold against the CQ-susceptible isolates and 200-fold against the CQ-resistant isolates. In contrast, there was only a 10-fold difference in the heme polymerization inhibitory activities of the compounds studied. No heme polymerization inhibitory activity could be measured for the sesquiterpene lactones ARM, ART, and DHA or the 8-aminoquinoline PQ within the range of drug solubility.
TABLE 1.
IC50s for heme polymerization and antimalarial drug potencies
| Drug | IC50 (μM) for heme polymerization | Antimalarial drug potency (IC50 [nM] at an inoculum size of 1)
|
|||
|---|---|---|---|---|---|
| 3D7 | HB3 | K1 | PH3 | ||
| AQ | 15.1 | 7.8 | 8.5 | 18.5 | 13.8 |
| CQ | 24.4 | 14.0 | 18.5 | 192.1 | 158.8 |
| DesAQ | 42.8 | 6.9 | 6.5 | 38.8 | 33.4 |
| BidesAQ | 72.9 | 21.3 | 18.6 | 166.0 | 119.8 |
| DesCQ | 44.8 | 18.9 | 18.3 | 718.5 | 645.1 |
| BidesCQ | 58.3 | 55.3 | 47.2 | 1,338.6 | 1,256.3 |
| AMOPYR | 29.5 | 5.3 | 4.7 | 11.5 | 9.8 |
| PYR | 64.4 | 5.7 | 6.2 | 9.1 | 10.1 |
| MEP | 41.0 | 12.9 | 17.8 | 43.3 | 32.6 |
| TBQ | 52.7 | 9.5 | 8.2 | 13.1 | 11.6 |
| 4-deOH TBQ | 50.6 | 42.1 | 57.2 | 69.3 | 75.2 |
| 4F-TBQ | 40.5 | 60.7 | 53.7 | 68.7 | 61.3 |
| NTB-AQ | 31.6 | 5.5 | 4.8 | 9.1 | 10.5 |
| CQ-PYROL | 43.6 | 19.5 | 17.3 | 89.6 | 76.3 |
| AQ-PYRID | 72.5 | 682.3 | 794.1 | 1,337.5 | 1,298.0 |
| CQ-PYRID | 91.6 | 11,660.5 | 10,224.5 | 15,119.4 | 14,612.3 |
| De-SC-AQ | 19.0 | 1,398.1 | 1,546.8 | 2,264.7 | 2,139.3 |
| QN | 64.8 | 34.2 | 36.8 | 81.2 | 74.3 |
| QND | 24.0 | 21.5 | 23.9 | 50.6 | 43.6 |
| CIN | 22.6 | 20.6 | 26.7 | 56.2 | 40.5 |
| CIND | 27.2 | 40.1 | 35.1 | 90.6 | 75.4 |
| HF | 184.5 | 5.8 | 5.2 | 2.8 | 3.2 |
| MQ | 46.9 | 23.4 | 18.2 | 9.4 | 11.2 |
| ARM | >1,000 | 2.3 | 1.6 | 1.2 | 2.2 |
| ART | >1,000 | 4.3 | 3.4 | 5.8 | 4.2 |
| DHA | >1,000 | 0.1 | 0.3 | 0.2 | 0.4 |
| PQ | >1,000 | NDa | ND | ND | ND |
ND, not determined.
There was no correlation between the inhibition of heme polymerization and antiparasitic activity (measured at an inoculum size of 1) whether these were considered against each parasite isolate alone or against all isolates (all r values for correlations between the IC50 for heme polymerization and the IC50 of the antiparasitic drug at an inoculum size of 1 against isolates 3D7, HB3, K1, and PH3 were <0.25; P, >0.28).
Table 2 presents the results obtained from inoculum effect experiments involving a more limited series of quinoline compounds. There was a 50-fold range in the absolute antimalarial activities (IC50s) and an 80-fold range in the CARs of the drugs tested against CQ-susceptible isolate 3D7. The values for CQ-resistant isolate K1 were 20- and 10-fold, respectively. Again, there was no obvious correlation between inhibition of heme polymerization and the absolute IC50 of the drug for either the 3D7 or the K1 isolate (r, <0.21; P, >0.65 in each case). Similarly, there was no significant correlation between the inhibition of heme polymerization and CAR for either the 3D7 or the K1 isolate (r, <0.53; P, >0.23 in each case).
TABLE 2.
Absolute antimalarial drug potencies and CARs derived from inoculum effect analysis and accumulation-normalized absolute IC50s of drugsa
| Drug | Absolute antimalarial drug potency (IC50 [nM])
|
CAR
|
Accumulation-normalized IC50 of the drug (arbitrary units)
|
|||
|---|---|---|---|---|---|---|
| 3D7 | K1 | 3D7 | K1 | 3D7 | K1 | |
| AQ | 2.4 | 16.8 | 8,955 | 3,653 | 0.27 | 4.18 |
| CQ | 5.8 | 83.6 | 5,768 | 1,346 | 0.42 | 7.66 |
| DesAQ | 3.26 | 36.9 | 19,250 | 3,758 | 0.79 | 9.44 |
| NTB-AQ | 1.67 | 7.21 | 47,171 | 14,694 | 0.99 | 7.21 |
| AMOPYR | 0.84 | 4.57 | 48,173 | 12,994 | 0.51 | 4.04 |
| TBQ | 1.22 | 4.3 | 79,456 | 14,584 | 1.22 | 4.27 |
| 4F-TBQ | 49.4 | 65.4 | 905 | 1,495 | 0.56 | 6.65 |
The accumulation-normalized absolute IC50s of various drugs (for isolates 3D7 and K1) were calculated by normalizing the CAR for each drug studied to that for the drug with the highest CAR for each isolate. These normalized levels of drug accumulation have then been multiplied by the corresponding absolute IC50 of each compound.
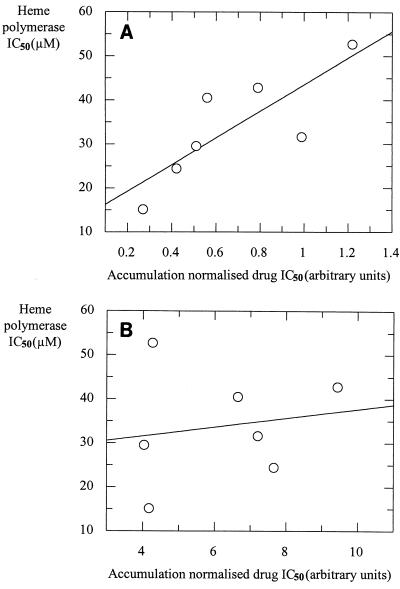
It has been shown previously (14) that drug accumulation plays a pivotal role in the antimalarial potencies of quinolines. To account for the role of drug accumulation in the potencies of antimalarial drugs, we have normalized the absolute IC50 for CAR (measured by inoculum effect analysis) for each compound. For isolates 3D7 and K1 the CAR for each drug studied has been normalized to that of the drug with the highest CAR for each isolate. These normalized levels of drug accumulation have then been multiplied by the corresponding absolute IC50 of each compound. These calculated accumulation-normalized IC50s (Table 2) have been correlated with the IC50 of heme polymerase (Fig. 2A and B). There is a significant correlation between the accumulation-normalized IC50 and the heme polymerase IC50 for the CQ-susceptible isolate (Fig. 2A; r = 0.81; P = 0.026) but not the CQ-resistant isolate (Fig. 2B; r = 0.17; P = 0.72).
FIG. 2.
Correlation of heme polymerase IC50 and accumulation-normalized absolute drug IC50 (calculated for isolates 3D7 and K1 by normalizing the CAR for each drug studied to that for the drug with the highest CAR for each isolate). These normalized levels of drug accumulation have then been multiplied by the corresponding absolute IC50 of each compound for isolates 3D7 (r = 0.81; P = 0.026) (A) and K1 (r = 0.17; P = 0.72) (B).
DISCUSSION
Considerable experimental evidence suggests that inhibition of heme polymerization is central to the mechanism of action of the quinoline-containing antimalarial agents (9). However, recent evidence from this laboratory indicates that the extent of drug accumulation at the site of heme polymerization is also a regulator of antimalarial activity (4, 14). Earlier studies investigating the simple relationship between activity and inhibition of heme polymerization with a limited number of drug substrates have produced contradictory findings (21, 26). In the present study we have undertaken a detailed analysis of the antimalarial activities and heme polymerization inhibitory activities of 27 antimalarial drugs. In addition, we have extended our investigations to consider the relative contribution of drug accumulation to the inhibition of heme polymerization and in vitro antiparasitic activities of drugs against CQ-resistant and -susceptible isolates of P. falciparum. Although CAR represents total parasite accumulation, all available evidence suggests that most of this occurs at the level of the acid food vacuole. This assumption is based on the acidic pH of this organelle and the vacuolar generation of sites where the drug binds to heme.
Within the range of solubility achievable, no heme polymerization inhibitory activity could be measured for the sesquiterpene lactones ART, ARM, and DHA or for the 8-aminoquinoline PQ. These results are unsurprising, given that these compounds have mechanisms of action that are distinct from those of the other classes of antimalarial drugs studied.
The present study failed to demonstrate any clear relationship between antiparasitic activity and inhibition of heme polymerization for any of the isolates studied. This was true even if the 4-aminoquinolines are considered in isolation. It is clear that a number of compounds, such as CQ-PYRID, AQ-PYRID, and De-SC-AQ, have very limited activity against either CQ-susceptible or CQ-resistant isolates of P. falciparum yet are potent inhibitors of heme polymerization (Table 1). Therefore, although the polymerization inhibition assay identifies compounds whose chemistry lends itself well to inhibition of heme polymerization, this does not in itself guarantee antimalarial activity.
The reason for such a discrepancy is the requirement for these antimalarial drugs to accumulate to very high concentrations within the parasite. The commonly used antimalarial agents CQ (11) and AQ (15) accumulate to high concentrations within the malaria parasite, and this ability to accumulate to such high concentrations is fundamental to their ability to inhibit parasite growth. Previous studies from this laboratory have also indicated that the levels of accumulation of a series of 4-aminoquinoline antimalarial agents is related to their ability to inhibit parasite growth (14). CQ and AQ are able to accumulate to micromolar concentrations within the whole parasite and most probably to millimolar concentrations within the acid food vacuole of the parasite (the site of hemoglobin digestion and heme polymerization) (1, 15). Interestingly, as can be seen from Table 1, the majority of the compounds studied here inhibit heme polymerization over a narrow range of micromolar concentrations. We argue, therefore, that the principal difference between the quinolines which show good antiparasitic activity in vitro and those that do not is a reflection of their ability to accumulate within the parasite rather than their ability to inhibit polymerization. This argument is supported by the correlation between the accumulation-normalized antimalarial activity and heme polymerization observed with CQ-susceptible isolate 3D7 (Fig. 2A). We have demonstrated recently that resistant isolates have a mechanism which reduces the local concentration of CQ at the heme binding site (4, 27). This results in a high proportion of the total CQ uptake into resistant isolates being nonspecific or nonpharmacologically active. Thus, total drug accumulation is not well correlated with activity in resistant isolates for drugs which are recognized by the verapamil-sensitive resistance mechanism, including CQ, AQ, and DesAQ. This explains the lack of correlation of normalized antimalarial activity with heme polymerization observed with isolate K1, which is highly CQ resistant (Fig. 2B).
In conclusion, the antimalarial activities of quinoline-type drugs are a function of both the ability of the drug to interfere with the polymerization process and the ability of the drug to accumulate to pharmacologically relevant concentrations at the site of drug action. The heme polymerase assay is useful as a screening assay for agents whose chemistries lend themselves to the inhibition of heme polymerization, but it is clearly not a predictor of antimalarial activity in vitro. The development of novel aminoquinolines with improved pharmacological characteristics will require an understanding of both those features of the molecule essential for interaction with the polymerization process and those features which control the rate and extent of drug accumulation at the intraparasitic site of drug action.
ACKNOWLEDGMENTS
This work was supported by a research program grant from The Wellcome Trust. S.A.W. is in receipt of a Wellcome Trust Research Leave Fellowship.
REFERENCES
- 1.Bray P G, Boulter M K, Ritchie G Y, Ward S A. Relationship of global chloroquine transport and reversal of chloroquine resistance in P. falciparum. Mol Biochem Parasitol. 1994;63:87–94. doi: 10.1016/0166-6851(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Bray P G, Hawley S R, Mungthin M, Ward S A. Physicochemical properties correlated with drug resistance and the reversal of drug resistance in Plasmodium falciparum. Mol Pharmacol. 1996;50:1559–1566. [PubMed] [Google Scholar]
- 3.Bray P G, Hawley S R, Ward S A. 4-Aminoquinoline resistance of Plasmodium falciparum: insights from the study of amodiaquine uptake. Mol Pharmacol. 1996;50:1551–1558. [PubMed] [Google Scholar]
- 4.Bray, P. G., M. Mungthin, R. Ridley, and S. A. Ward. Submitted for publication.
- 5.Chou A C, Fitch C D. Haemolysis of mouse erythrocytes by ferriprotoporphyrin IX and chloroquine. Chemotherapeutic implications. J Clin Invest. 1980;66:856–858. doi: 10.1172/JCI109925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S N, Phifer K O, Yielding K L. Complex formation between chloroquine and ferrihaemic acid in vitro and its effect on the antimalarial action of chloroquine. Nature. 1964;202:805–806. doi: 10.1038/202805a0. [DOI] [PubMed] [Google Scholar]
- 7.Desjardins R E, Canfield J, Haynes D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorn A, Stoffel R, Matile H, Bubendorf A, Ridley R. Malarial haemozoin/β-haematin supports the polymerisation of haem in the absence of protein. Nature. 1995;374:269–271. doi: 10.1038/374269a0. [DOI] [PubMed] [Google Scholar]
- 9.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance. Int J Parasitol. 1997;27:231–240. doi: 10.1016/s0020-7519(96)00152-x. [DOI] [PubMed] [Google Scholar]
- 10.Geary T G, Divo A D, Jensen J B, Zangwill M, Ginsburg H. Kinetic modelling of the response of Plasmodium falciparum to chloroquine and its experimental testing in vitro. Implications for mechanism of action and resistance to the drug. Biochem Pharmacol. 1990;40:685–691. doi: 10.1016/0006-2952(90)90302-2. [DOI] [PubMed] [Google Scholar]
- 11.Geary T G, Jensen J B, Ginsburg H. Uptake of 3H chloroquine by drug-sensitive and -resistant strains of the human malarial parasite Plasmodium falciparum. Biochem Pharmacol. 1986;35:3805–3812. doi: 10.1016/0006-2952(86)90668-4. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg D E, Slater A F G. The pathway of hemoglobin degradation in malaria parasites. Parasitol Today. 1992;8:280–283. doi: 10.1016/0169-4758(92)90146-s. [DOI] [PubMed] [Google Scholar]
- 13.Hawley S R, Bray P G, O’Neill P M, Naisbitt D J, Park B K, Ward S A. Manipulation of the N-alkyl substituent in amodiaquine to overcome the verapamil-sensitive chloroquine resistance component. Antimicrob Agents Chemother. 1996;40:2345–2349. doi: 10.1128/aac.40.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley S R, Bray P G, O’Neill P M, Park B K, Ward S A. The role of drug accumulation in 4-aminoquinoline antimalarial potency—the influence of structural substitution and physicochemical properties. Biochem Pharmacol. 1996;52:723–733. doi: 10.1016/0006-2952(96)00354-1. [DOI] [PubMed] [Google Scholar]
- 15.Hawley S R, Bray P G, Park B K, Ward S A. Amodiaquine accumulation in Plasmodium falciparum as a possible explanation for its superior antimalarial activity over chloroquine. Mol Biochem Parasitol. 1996;80:15–25. doi: 10.1016/0166-6851(96)02655-2. [DOI] [PubMed] [Google Scholar]
- 16.Jensen J B, Trager W. Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle-jar method. J Parasitol. 1977;63:883–886. [PubMed] [Google Scholar]
- 17.Lambros C, Vandenburg J P. Synchronisation of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 18.Macomber P B, Sprinz H, Tousimis A J. Morphological effects of chloroquine on Plasmodium berghei in mice. Nature. 1967;214:937–939. doi: 10.1038/214937a0. [DOI] [PubMed] [Google Scholar]
- 19.Martiney J A, Cerami A, Slater A F G. Verapamil reversal of chloroquine resistance in the malaria parasite Plasmodium falciparum is specific for resistant parasites and independent of the weak base effect. J Biol Chem. 1995;270:22393–22398. doi: 10.1074/jbc.270.38.22393. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill P M, Willock D J, Hawley S R, Bray P G, Storr R C, Ward S A, Park B K. Synthesis, antimalarial activity, and molecular modeling of tebuquine analogues. J Med Chem. 1997;40:437–448. doi: 10.1021/jm960370r. [DOI] [PubMed] [Google Scholar]
- 21.Raynes K, Foley M, Tilley L, Deady L W. Novel bisquinoline antimalarials. Synthesis, antimalarial activity, and inhibition of haem polymerisation. Biochem Pharmacol. 1996;52:551–559. doi: 10.1016/0006-2952(96)00306-1. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal P J, Meshnick S R. Hemoglobin catabolism and iron utilisation by malaria parasites. Mol Biochem Parasitol. 1996;83:131–139. doi: 10.1016/s0166-6851(96)02763-6. [DOI] [PubMed] [Google Scholar]
- 23.Schueler F W, Cantrell W F. Antagonism of the antimalarial action of chloroquine by ferrihaemate and a hypothesis for the mechanism of chloroquine resistance. J Pharmacol Exp Ther. 1964;143:278–281. [PubMed] [Google Scholar]
- 24.Sherman I W. Amino acid metabolism and protein synthesis in malarial parasites. Bull W H O. 1977;55:265–276. [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman I W, Tanigoshi L. Incorporation of 14C-amino acids by malaria (Plasmodium lophurae) Int J Biochem. 1970;1:635–637. [PubMed] [Google Scholar]
- 26.Slater A F G, Cerami A. Inhibition by chloroquine of a novel haeme polymerase enzyme activity in malaria trophozoites. Nature. 1992;355:167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- 27.Ward S A, Bray P G, Hawley S R. Quinoline resistance mechanisms in Plasmodium falciparum: the debate goes on. Parasitology. 1997;114:S125–S136. [PubMed] [Google Scholar]
- 28.Zarchin S, Krugliak M, Ginsburg H. Digestion of the host erythrocyte by malarial parasites is the primary target for quinoline-containing antimalarials. Biochem Pharmacol. 1986;35:2435–2442. doi: 10.1016/0006-2952(86)90473-9. [DOI] [PubMed] [Google Scholar]