Abstract
Coproporphyrin (CP)-I and CP-III are the markers of organic anion-transporting polypeptides’ (OATPs) activities, and they are porphyrin metabolites that originate from heme synthesis. Furthermore, CP-I and CP-III, which are OATP1B endogenous metabolites, have gradually attracted the attention of scientists and researchers in recent years. Previous studies have also observed CP-I and CP-III levels as clinical biomarkers for predicting OATP1B inhibition in drug–drug interaction studies. To establish an accurate ultra-high performance liquid chromatography–mass spectrometry method for the quantitation of CP-I and CP-III, we reviewed previous methodological publications and applied them to a clinical pharmacology study using a human urine matrix. We used 13.25 M formic acid as a working solution for internal standards (CP-I 15N4 and CP-III d8) to avoid isobaric interference. The calibration curve showed good linearity in the range of 1–100 ng/mL, with a correlation coefficient (R2) higher than 0.996 in each validation batch. Both the between-run and within-run assays achieved good precision and accuracy, and we found that both CP-I and CP-III were stable in the pre-study validation. The method exhibited suitable dilution integrity, allowing for the re-analysis of samples with concentrations exceeding the upper limit of quantification through dilution. Overall, the application of the described method in a clinical study revealed that it can be utilized effectively to monitor drug–drug interactions mediated by OATP1B.
Keywords: Organic Anion-Transporting Polypeptides, Coproporphyrin I, Coproporphyrin III, Liquid Chromatography
INTRODUCTION
Organic anion-transporting polypeptides (OATPs) are transporters that facilitate the uptake of numerous drugs and naturally occurring substances in the liver and are primarily located on the basolateral membrane of hepatocytes [1,2,3]. OATPs, with their high affinity, not only affect the absorption, elimination, and tissue distribution of drugs but also possess extensive substrate specificity, thereby change a crucial role in maintaining cell homeostasis of steroid hormones [2]. The OATP transporters can influence the levels of a drug in serum and tissue, as well as impact its efficacy and safety [2].
Porphyrins are 18π aromatic macrocyclic compounds [4] that display high chemical stability [5]. The porphyrin metabolites, coproporphyrin (CP)-I and CP-III, are biomarkers of the OATP1B transporters [6,7]. They are derived from heme synthesis and are excreted into the bile and urine of the body [6,8]. It has been reported that the urine CP-I and CP-III concentrations are 5–35 and 1–35 ng/mL, respectively, in a healthy human pooled urine sample [9]. A previous study assessed the utility of OATP1B endogenous biomarkers such as CP-I and CP-III using an OATP1B inhibitor (rifampicin) and a probe drug cocktail consisting of atorvastatin, pitavastatin, rosuvastatin, and valsartan [10]. Additionally, this study demonstrated a strong correlation between the endogenous biomarkers and probe drugs in terms of AUC and Cmax [10]. The findings provide support for utilizing OATP1B endogenous substrates in the evaluation of drug–drug interactions [10]. Furthermore, consistent with the previous studies, the drug–drug interactions involving CP-I and CP-III were observed, underscoring the clinical significance of these biomarkers in assessing OATP1B inhibition [10,11,12]. Thus, quantitation of CP-I and CP-III is important to utilize as an endogenous biomarker for drug transporters and evaluating the drug effects such as drug-drug interactions.
In this study, an ultra-high performance liquid chromatography–mass spectrometry (UHPLC–MS/MS) system was used due to its high accuracy and precision, and we developed a reliable LC–MS/MS method for the quantitation of CP-I and CP-III by referring to previous methodological publications [9]. This method was validated and applied to a clinical pharmacology study using a human urine matrix.
METHODS
Chemicals and reagents
CP-I dihydrochloride (85%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). CP-III dihydrochloride (95%), internal standard CP-I 15N4 (96%), and internal standard CP-III d8) 95%) were purchased from TRC Inc. (Toronto Research Chemicals, North York, Canada). Water, acetonitrile (ACN, 99.9%), and ethyl acetate (99.6%) of HPLC grade were purchased from J.T. Baker (Phillipsburg, NJ, USA), and formic acid (95%) was purchased from Sigma-Aldrich.
Preparation of stock and working solutions
Stock solutions of CP-I dihydrochloride, CP-III dihydrochloride and CP-I 15N4, and CP-III d8 were accurately weighed and dissolved in dimethyl sulfoxide at concentrations of 1, 0.25, and 0.125 mg/mL, respectively. All stock solutions were stored at −20°C. The stock solutions of CP-I 15N4 and CP-III d8 were diluted to 1,000 ng/mL using 13.25 M formic acid, and a mixture of CP-I 15N4 and CP-III d8 was used as the internal standard solution. Furthermore, ACN was used to serially dilute the stock solutions of CP-I and CP-III as the working solution.
Preparation of calibration standards and quality control (QC) samples
Calibration standards were prepared at the following concentrations: 1, 2, 5, 10, 25, 50, 80, and 100 ng/mL. CP-I and CP-III were spiked at 8 concentrations by adding a 20 µL mixture of the CP-I and CP-III working solution into 380 µL of a 3-times charcoal-stripped urine sample. QCs were prepared at the following concentrations: 1 ng/mL (lower limit of quantification [LLOQ]), 3 ng/mL (low QC [LQC]), 20 ng/mL (medium QC [MQC]), and 75 ng/mL (high QC [HQC]) by adding a 350 µL mixture of the CP-I and CP-III working solution into 6,650 µL of the 3-times charcoal-stripped urine sample for 3 batches, with 5 replications in each batch for validation. Dilution QCs (DiQCs) were prepared at the following dilution factors: 10-, 50-fold by adding a 50 µL CP-I and CP-III mixture into 950 µL of the 3-times charcoal-stripped urine sample for 5 replications in one batch. A 3-times charcoal-stripped urine sample was prepared using this process. 0.8 g of charcoal per 45 mL of urine was mixed, and the mixture was shaken at 200 rpm overnight in an ice bath. The centrifugation process was continued until the charcoal no longer sank beneath the urine.
Sample preparation
We developed a method for quantifying CP-I and CP-III in urine by reviewing the previous methods [9]. Sample preparation was achieved by liquid–liquid extraction. A 10 µL mixture of CP-I 15N4 and CP-III d8 (IS, 500 ng/mL) was added to 200 µL of each sample and vortexed for 1 minute at room temperature. The mixtures were extracted with 1 mL of ethyl acetate, vortexed at room temperature for 1 minute, and then centrifuged at 14,000 rpm at 4°C for 10 minutes. After that, the centrifuged samples were stored at −80°C for 1 hour. Then, the unfrozen supernatants were transferred into new tubes and dried under nitrogen at 40°C. The samples were reconstituted in 100 µL of 1:1 ACN:water, and 2 µL of each sample was injected into the UHPLC–MS/MS system. All the samples were processed from amber vials and amber tubes in an ice bath during the experiment.
LC-MS/MS conditions
The samples were analyzed by an ACQUITY UPLC system (Waters Corporation, Milford, MA, USA) coupled to a Triple Quad 5500 mass spectrometer (AB Sciex, Redwood City, CA, USA). An ACQUITY UPLC BEH C18, 1.7 µm × 100 mm column (Waters Corporation) was connected to the UPLC system. For the mobile phase, we used water containing 0.1% formic acid (mobile phase A) and ACN with 0.1% formic acid (mobile phase B) under gradient conditions. The gradient elution started with 80% A at 0.5 minutes, reduced to 50% A at 6.5 minutes, and 2% A at 7.5 minutes, which was maintained up to 10 minutes. It then increased to 50% A at 11 minutes and 80% A at 13 minutes, allowing the system to re-equilibrate at 17 minutes. The flow rate was 0.6 mL/min, and the column temperature was maintained at 50°C. Analytes and internal standards were detected with the following settings: source temperature, 600°C; ion spray voltage, 4,500 V; curtain gas, 20; gas 1 (nebulizer gas), 40; gas 2 (auxiliary gas), 40; and CAD gas, 9.
The multiple reaction monitoring mode was considered under the positive ion mode. The monitored transitions were as follows: CP-I [655.132 → 596.1 (63 V)], CP-III [655.154 → 596.2 (63 V)], CP-I 15N4 [660.132 → 601.2 (61 V)], and CP-III d8 [664.203 → 603.3 (65 V)], as provided in Table 1.
Table 1. Precursor and product ions of CP-I, CP-III, CP-I 15N4, and CP-III d8 in positive ion mode.
| Compound name | Q1 Mass (Da) | Q3 Mass (Da) | Time (msec) | DP (volts) | EP (volts) | CE (volts) | CXP (volts) |
|---|---|---|---|---|---|---|---|
| CP-I | 655.132 | 596.1 | 150 | 171 | 10 | 63 | 32 |
| CP-III | 655.154 | 596.2 | 150 | 91 | 10 | 63 | 26 |
| CP-I 15N4 | 660.132 | 601.2 | 150 | 71 | 10 | 61 | 6 |
| CP-III d8 | 664.203 | 603.3 | 150 | 171 | 10 | 65 | 0 |
CP-I, coproporphyrin-I; CP-III, coproporphyrin-III; DP, declustering potential; EP, entrance potential; CE, collision energy; CXP, collision cell exit potential.
Bioanalytical method validation
We included linearity, precision and accuracy, carry-over, recovery, dilution integrity, and short-term stability in both the human urine sample and working solution to validate this study. Additionally, processed sample stability and re-injection stability for partial validations were included, which referred to the bioanalytical methods validation guideline [13]. The calibration curve was plotted relatively by calculating the ratio of the analyte signal to the internal standard using 1/x2 as a weight factor. The correlation coefficient (R2) was measured to evaluate the linearity of the calibration curve of each batch. The between-run precision and accuracy of this method were assessed by analyzing 3 batches of 8-point calibration levels and 5 replications of the QC samples in each batch. The within-run precision and accuracy of this method were assessed by analyzing 5 replications of 4 different levels of the QC samples (LLOQ, LQC, MQC, and HQC). The dilution integrity was assessed by analyzing 5 replications of 2 different levels of high-QC-level samples (10- and 50-fold).
For the recovery, the peak areas of the samples extracted and spiked from 3 different levels of QC (LQC, MQC, and HQC) were compared. The recovery results were calculated by the ratio of the mean peak area of the extracted QC to that of the spiked QC. Additionally, to assess carry-over, it is necessary to analyze blank samples after the upper limit of quantification (ULOQ). Thus, the carry-over was evaluated by calculating the ratio of the blank signal to the LLOQ signal, which had to be less than 20% of the analyte response in the blank samples compared to the LLOQ [13]. The short-term stability was assessed using both the human urine sample and working solution over 4 hours. Additionally, processed sample and re-injection stabilities were assessed using human urine over 36 and 12 hours, respectively. Moreover, all the samples were stored at 4°C.
Urine sample collection
This study used the part of the samples collected from the phase 1 clinical trial (Institutional Review Board No. B-2110-715-001), which was conducted at the Seoul National University Bundang Hospital, Seoul, South Korea (ClinicalTrials.gov identifier: NCT05575297). Twenty-two human urine samples obtained from 11 healthy subjects were analyzed in this method. The sampling times for these samples ranged from -1 day 0–12 hours to -1 d 12–24 hours in the baseline. The urine samples were stored at −80°C before the analysis.
RESULTS
Linearity
The range of 1–100 ng/mL calibration curves for CP-I and CP-III were constructed on 3 validation days. An 8-point calibration curve achieved good linearity with a R2 (0.9962–0.9992) in both CP-I and CP-III. The average slope was 0.03743 with a relative standard deviation of 0.2% in CP-I, and it was 0.08713 with a relative standard deviation of 0.8% in CP-III (data not shown).
Precision and accuracy
The between-run precision and accuracy of the calibration standards were successful, with the precision below 6.901% and accuracy ranging from 91.27% to 105.7% for CP-I. Similarly, for CP-III, the precision was below 8.675% and the accuracy ranged from 92.17% to 103% (data not shown). Additionally, the between-run precision and accuracy of the QC samples were successful, with the precision below 9.899% and accuracy ranging from 94.35% to 109.1% for CP-I. For CP-III, the precision was below 9.912% and the accuracy ranged from 87.81% to 106.7%. Moreover, the within-run precision and accuracy of the QC samples were also successful, with the precision below 6.696% and the accuracy ranging from 97.98% to 112.6% for CP-I. For CP-III, the precision was below 5.747% and the accuracy ranged from 90.74% to 110.6%. The precision and accuracy data for the partial validations of between-run and within-run calibration standards and QC data are summarized in Table 2.
Table 2. Between-run and within-run precision and accuracy of the QC samples for CP-I and CP-III in human urine.
| Compound name | Levels | Nominal concentration (ng/mL) | Between-run (n = 3) | Within-run (n = 5) | ||||
|---|---|---|---|---|---|---|---|---|
| Observed concentration (ng/mL) | Precision (%) | Mean accuracy (%) | Observed concentration (ng/mL) | Precision (%) | Mean accuracy (%) | |||
| CP-I | LLOQ | 1 | 1.05 ± 0.104 | 9.899 | 105.0 | 1.072 ± 0.054 | 5.084 | 107.2 |
| LQC | 3 | 2.831 ± 0.117 | 4.118 | 94.35 | 2.940 ± 0.197 | 6.696 | 97.98 | |
| MQC | 20 | 21.80 ± 0.841 | 3.856 | 109.1 | 22.48 ± 0.698 | 3.104 | 112.6 | |
| HQC | 75 | 81.24 ± 0.139 | 0.171 | 108.3 | 81.32 ± 5.338 | 6.564 | 108.2 | |
| CP-III | LLOQ | 1 | 1.067 ± 0.065 | 6.126 | 106.7 | 1.052 ± 0.034 | 3.251 | 105.2 |
| LQC | 3 | 2.669 ± 0.078 | 2.907 | 87.81 | 2.724 ± 0.105 | 3.855 | 90.74 | |
| MQC | 20 | 19.81 ± 1.964 | 9.912 | 99.18 | 22.08 ± 0.942 | 4.265 | 110.6 | |
| HQC | 75 | 75.35 ± 2.792 | 3.705 | 100.53 | 78.56 ± 4.515 | 5.747 | 104.8 | |
QC samples in the 3-times charcoal-stripped urine at concentrations of 1, 3, 20, and 75 ng/mL were replicated 5 times for each batch. All the samples were run in 3 batches.
QC, quality control; CP-I, coproporphyrin-I; CP-III, coproporphyrin-III; LLOQ, lower limit of quantification; LQC, low quality control; MQC, mid quality control; HQC, high quality control.
Carry-over and recovery
Carry-over was evaluated during validation to detect blank samples after the ULOQ. We calculated the peak area ratio by dividing the blank signal by the LLOQ signal; the peak area ratio in the blank samples should not exceed 20% of the analyte response [13]. The peak area of the LLOQ was 4,470 for CP-I and 9,620 for CP-III (Fig. 1A). On the other hand, the peak area of the blank sample injected immediately after the ULOQ was 772 for CP-I and 2,130 for CP-III (Fig. 1B). Furthermore, the peak area ratios (%) for CP-I and CP-III were 15.64 ± 0.503 and 27.51 ± 7.763, respectively, for the blank sample injected right after the ULOQ (Table 3). Therefore, we observed a carry-over effect on the blank sample. We implemented a solution for this issue by injecting 50% ACN and washing twice after the highest sample concentrations. We applied this method, and as a result, the peak area of the blank sample injected after the ULOQ and 2-time wash decreased significantly (Fig. 1C). Additionally, the CP-I peak area ratio (%) was 1.61 ± 0.559 and the CP-III peak area ratio (%) was 4.34 ± 2.512 (Table 3). Thus, washing twice with 50% ACN after the highest levels of spiked samples ensured that there was no observed carry-over effect in the human urine samples.
Figure 1. Peak areas of LLOQ, blank 1, and blank 2 for CP-I and CP-III in human urine. (A) Peak areas of LLOQ in CP-I and CP-III, (B) peak area of blank 1, and (C) peak area of blank 2. Blank 1 was injected right after the ULOQ, and blank 2 was injected after the ULOQ and 2 washes.
LLOQ, lower limit of quantification; ULOQ, upper limit of quantification; CP-I, coproporphyrin I; CP-III, coproporphyrin III.
Table 3. Carry over of blank samples for CP-I and CP-III in human urine.
| Compound name | Samples | Peak area ratio (%) |
|---|---|---|
| CP-I | Blank sample injected right after the ULOQ | 15.64 ± 0.503 |
| Blank sample injected after the ULOQ and washing twice | 1.61 ± 0.559 | |
| CP-III | Blank sample injected right after the ULOQ | 27.51 ± 7.763 |
| Blank sample injected after the ULOQ and washing twice | 4.34 ± 2.512 |
The peak area ratio (%) was calculated by dividing the peak area of the blank by the peak area of the lower limit of quantification.
CP-I, coproporphyrin-I; CP-III, coproporphyrin-III; ULOQ, upper limit of quantification.
The recovery was observed in the 3-times charcoal-stripped urine sample in the LQC at 3 ng/mL, MQC at 20 ng/mL, and HQC at 75 ng/mL. The recovery results were evaluated by calculating the ratio of the mean peak area of the extracted QC samples to that of the spiked QC samples. The recovery for CP-I was 52% in the LQC, 69.74% in the MQC, and 56.29% in the HQC. For CP-III, the recovery was 43.43% in the LQC, 65.64% in the MQC, and 55.75% in the HQC. The mean recovery was 59.35% for CP-I and 54.94% for CP-III (data not shown). Although the recovery was not good enough, reproducibility seemed feasible.
Dilution integrity
We obtained high QC levels with each of the 2 different human urine samples by adding 150 ng/mL of the CP-I and CP-III mixture. These samples were diluted with the 3-times charcoal-stripped urine using 2 dilution factors (10- and 50-fold) to cover the calibration curve range as much as possible. The within-run precision and accuracy of DiQC samples were successful, with a precision below 3.137% and an accuracy ranging from 86.82% to 96.74% for CP-I (Table 4). For CP-III, the precision was below 6.54%, and the accuracy ranged from 94.94% to 99.12% (Table 4). These data show that samples with a concentration greater than the ULOQ could be re-analyzed by dilution.
Table 4. Within-run precision and accuracy of DiQC samples for CP-I and CP-III in human urine.
| Compound name | Dilution factor | Within-run (n = 5) | ||
|---|---|---|---|---|
| Observed concentration (ng/mL) | Precision (%) | Mean accuracy (%) | ||
| CP-I | 10 | 145.4 ± 4.561 | 3.137 | 96.74 |
| 50 | 130.4 ± 2.074 | 1.590 | 86.82 | |
| CP-III | 10 | 148.8 ± 9.731 | 6.540 | 99.12 |
| 50 | 142.4 ± 6.229 | 4.374 | 94.94 | |
The DiQC samples in 3-times charcoal-stripped urine at dilution factors of 10- and 50-fold were replicated 5 times for each batch.
DiQC, dilution quality control; CP-I, coproporphyrin-I; CP-III, coproporphyrin-III.
Stability
It was found that both CP-I and CP-III were stable in the short term and after re-injection. The processed samples were also stable for 36 hours in 4°C autosamplers. Previous studies indicated that CP-I and CP-III are light sensitive [9], and these data show that using amber vials and tubes could protect CP-I and CP-III from light (Table 5).
Table 5. Processed sample stability of CP-I and CP-III in human urine at 4°C.
| Compound name | Levels | Storage period (hr) | Observed concentration (ng/mL) | Percent difference from baseline (%) |
|---|---|---|---|---|
| CP-I | LQC | 0 | 2.708 ± 0.124 | |
| 36 | 2.895 ± 0.205 | 6.675 | ||
| HQC | 0 | 81.46 ± 1.996 | ||
| 36 | 81.48 ± 2.223 | 0.018 | ||
| CP-III | LQC | 0 | 2.580 ± 0.027 | |
| 36 | 2.983 ± 0.090 | 14.472 | ||
| HQC | 0 | 73.96 ± 2.702 | ||
| 36 | 84.375 ± 1.987 | 13.156 |
CP-I, coproporphyrin-I; CP-III, coproporphyrin-III; LQC, low quality control; HQC, high quality control.
Human urine samples analysis
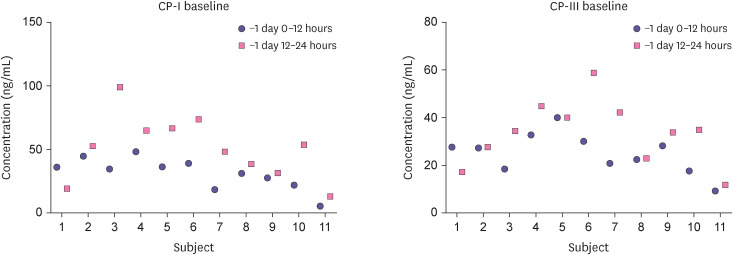
The concentrations of CP-I and CP-III in the human urine samples obtained from healthy subjects were measured using the validated quantitation method. The concentrations of CP-I and CP-III ranged from 5 to 95 ng/mL and 1 to 59 ng/mL, respectively (Fig. 2). In both CP-I and CP-III, the period of -1 day 12–24 hour showed a trend of increased concentration compared to -1 day 0–12 hour, but it was not statistically significant. Thus, this validated method can successfully cover the concentration range in the human urine samples and can be applied to further clinical studies.
Figure 2. Clinical application of the CP-I and CP-III quantitation method for illustrating the concentrations in the urine CP-I, CP-III levels of healthy volunteers before drug administration.
Baseline: before drug administration.
CP-I, coproporphyrin I; CP-III, coproporphyrin III.
DISCUSSION
In this study, we presented an accurate UHPLC–MS/MS method for CP-I and CP-III quantification. We used ethyl acetate and 1:1 ACN:water for sample preparation. Using ethyl acetate as an extraction solvent has the advantage of rapid nitrogen drying. However, one of the challenges of CP-I and CP-III quantitation is the isobaric interference in urine [9]. To avoid this for CP-I, CP-III, and internal standards (CP-I 15N4 and CP-III d8), we used 13.25 M formic acid as the working solution [9,14].
We proved that the calibration curve achieved good linearity and reproducibility in the range of 1–100 ng/mL with R2 ranging between 0.9962 and 0.9992. All between-run and within-run QCs showed satisfactory precision and accuracy, revealing the good sensitivity of the method. The precision and accuracy results of the QC samples indicated the suitability of the 3-times charcoal-stripped urine for CP-I and CP-III quantification. Furthermore, carry-over was evaluated during validation, which was inevitable. To address this issue, 50% ACN washing was performed twice after the highest levels of spiked samples were achieved. Therefore, no carry-over effect was observed on the blank peak. However, the carry-over signal of the blank samples after the second washing was found to be less than 20% of the analyte response compared to the LLOQ (1 ng/mL). Thus, it did not affect the results of accuracy and precision.
We used this validated quantitation method for the human urine samples obtained from the clinical trial studies at the Seoul National University Bundang Hospital. We successfully detected CP-I and CP-III in the human urine samples using this method. In a previous study, the CP-I and CP-III concentrations in control pooled human urine were 5–35 and 1–35 ng/mL, respectively [9]. However, CP-I and CP-III baseline concentration exceeded 35 ng/mL for some subjects in this study. The previous study utilized pooled urine purchased from BioIVT (Westbury, NY, USA), whereas our urine samples were collected individually, and the data were presented individually as well. Therefore, there might be differences in the CP-I and CP-III baseline concentration ranges depending on the method of urine sample collection.
In conclusion, the developed UHPLC-MS/MS method for the accurate quantitation of CP-I and CP-III has demonstrated its successful applicability in clinical research, particularly in the context of monitoring drug-drug interactions involving OATP1B. Its utilization holds promise for advancing our understanding of the pharmacokinetics, pharmacodynamics, and individualized treatment strategies in various disease settings, ultimately benefiting patient outcomes and therapeutic interventions.
ACKNOWLEDGMENTS
We thank all study individuals who contributed to this study.
Footnotes
Conflict of Interest: - Authors: Nothing to declare
- Reviewers: Nothing to declare
- Editors: Nothing to declare
Reviewer: This article was reviewed by peer experts who are not TCP editors.
- Conceptualization: Jang Y, Chung JY, Cho JY, Hwang S.
- Formal analysis: Jang Y.
- Investigation: Jang Y, Kang J, Hwang S.
- Methodology: Jang Y, Kang J.
- Validation and visualization: Jang Y.
- Supervision: Kang J, Cho JY.
- Writing - original draft: Jang Y.
- Writing - review & editing: Kang J, Hwang S, Chung JY, Cho JY.
References
- 1.Stieger B, Hagenbuch B. Organic anion-transporting polypeptides. Curr Top Membr. 2014;73:205–232. doi: 10.1016/B978-0-12-800223-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oswald S. Organic Anion Transporting Polypeptide (OATP) transporter expression, localization and function in the human intestine. Pharmacol Ther. 2019;195:39–53. doi: 10.1016/j.pharmthera.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 3.König J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000;278:G156–G164. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- 4.Hiroto S, Miyake Y, Shinokubo H. Synthesis and functionalization of porphyrins through organometallic methodologies. Chem Rev. 2017;117:2910–3043. doi: 10.1021/acs.chemrev.6b00427. [DOI] [PubMed] [Google Scholar]
- 5.Gu J, Peng Y, Zhou T, Ma J, Pang H, Yamauchi Y. Porphyrin-based framework materials for energy conversion. Nano Res Energy. 2022;1:e9120009 [Google Scholar]
- 6.Shen H, Dai J, Liu T, Cheng Y, Chen W, Freeden C, et al. Coproporphyrins I and III as functional markers of OATP1B activity: in vitro and in vivo evaluation in preclinical species. J Pharmacol Exp Ther. 2016;357:382–393. doi: 10.1124/jpet.116.232066. [DOI] [PubMed] [Google Scholar]
- 7.Phillips JD. Heme biosynthesis and the porphyrias. Mol Genet Metab. 2019;128:164–177. doi: 10.1016/j.ymgme.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuvonen M, Tornio A, Hirvensalo P, Backman JT, Niemi M. Performance of plasma coproporphyrin I and III as OATP1B1 biomarkers in humans. Clin Pharmacol Ther. 2021;110:1622–1632. doi: 10.1002/cpt.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramanathan R, King-Ahmad AJ, Holliman CL, Rodrigues AD. A highly selective and sensitive LC-MS/HRMS assay for quantifying coproporphyrins as organic anion-transporting peptide biomarkers. Bioanalysis. 2017;9:1787–1806. doi: 10.4155/bio-2017-0181. [DOI] [PubMed] [Google Scholar]
- 10.Mori D, Kimoto E, Rago B, Kondo Y, King-Ahmad A, Ramanathan R, et al. Dose-dependent inhibition of OATP1B by rifampicin in healthy volunteers: comprehensive evaluation of candidate biomarkers and OATP1B probe drugs. Clin Pharmacol Ther. 2020;107:1004–1013. doi: 10.1002/cpt.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalluri HV, Kikuchi R, Coppola S, Schmidt J, Mohamed MF, Bow DA, et al. Coproporphyrin I can serve as an endogenous biomarker for OATP1B1 inhibition: assessment using a Glecaprevir/Pibrentasvir clinical study. Clin Transl Sci. 2021;14:373–381. doi: 10.1111/cts.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yee SW, Giacomini MM, Shen H, Humphreys WG, Horng H, Brian W, et al. Organic anion transporter polypeptide 1B1 polymorphism modulates the extent of drug-drug interaction and associated biomarker levels in healthy volunteers. Clin Transl Sci. 2019;12:388–399. doi: 10.1111/cts.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. Bioanalytical method validation, guidance for industry [Internet] 2018. [Accessed May 22, 2018]. Available from: https://www.fda.gov/media/70858/download .
- 14.Kandoussi H, Zeng J, Shah K, Paterson P, Santockyte R, Kadiyala P, et al. UHPLC-MS/MS bioanalysis of human plasma coproporphyrins as potential biomarkers for organic anion-transporting polypeptide-mediated drug interactions. Bioanalysis. 2018;10:633–644. doi: 10.4155/bio-2017-0246. [DOI] [PubMed] [Google Scholar]