Abstract
Testosterone therapy prompts the development of male secondary sexual characteristics coupled with numerous physiological changes; however, the effect of prolonged androgen exposure on transgender men's fertility remains to be fully elucidated. Multiple clinical consensuses advise assisted reproduction before hormone treatment and state that fertility preservation following androgen therapy entails the suspension of testosterone administration. Although the desire for reproduction among transgender men is prevalent, the discontinuation of gender-affirming hormone therapy poses a major challenge due to the anxiety, unease, and gender dysphoria that follow androgen withdrawal. The present investigation aimed to explore the feasibility and outcomes of oocyte retrieval in adult transgender men undergoing testosterone administration before or during fertility preservation. Seven case reports, four cohort studies, and two cross-sectional studies were identified following a systematic literature search on the PubMed/Ovid MEDLINE, Scopus, and ScienceDirect databases. The findings gathered in this review disclose the viability of oocyte retrieval after prolonged androgen exposure and suggest the absence of a direct relationship between the duration of testosterone suspension and fertility preservation outcomes. Although the reports are limited, recent evidence shows that continuous testosterone administration and the use of aromatase inhibitors during ovarian stimulation could potentially reduce the distressing effects of hormonal ovulation induction. New approaches to fertility preservation in transgender men must be further explored to ensure interventions aligned both with the reproductive desire and avoidance of gender dysphoria exacerbation that follow hormone therapy suspension.
Keywords: fertility preservation, gender-affirming hormone therapy, oocyte retrieval, testosterone, transgender
Introduction
Gender-affirming care for transgender men demands a multidisciplinary approach with interventions that promote masculinization aligned to the patient's desire and gender identity. In transgender men, hormone therapy aims to reach serum testosterone concentrations within the average range of cisgender males.1,2 Thereby, hormonal treatment aids in the development of male secondary sexual characteristics by prompting clitoral enlargement, facial hair growth, patterns of androgenic alopecia, seborrhea, and skeletal muscle mass increase with body fat redistribution.3 Moreover, testosterone administration induces physiological changes associated with a deepening of the voice pitch,4 increased sexual desire,5 and in the long term, menstrual cycle cessation.6 To prevent any regression of formerly achieved virilization, and therefore triggering the distress associated with gender dysphoria, hormonal treatment should be lifelong maintained and its interruption should be avoided whenever possible.7
In addition to gender-affirming hormone therapy, gender transitioning can be undergone concomitantly to surgical procedures such as a hysterectomy and bilateral oophorectomy, among others.8 In the latter scenario, transgender men experience an irreversible loss of their natural reproductive capacity.9,10 For this reason, the Endocrine Society1 and the Ethics Committee of the American Society for Reproductive Medicine11 have issued several guidelines advising fertility counseling before commencing surgical and hormonal interventions. Nonetheless, when considering assisted reproduction while on testosterone therapy, the optimal duration of hormone treatment suspension remains unknown.12
Pregnancy reports in transgender men13 provide suggestive evidence of preserved reproductive capacity; however, the effect of androgen therapy on fertility is still somewhat unclear.14 Amenorrhea and anovulation are reached around 6 months following hormonal therapy initiation.15 Yet, according to Ahmad and Leinung,6 individual variability is high and the relationship between the time for menses cessation and the administered testosterone dose is very slight.
Histopathological findings revealed that both the cortical distribution16,17 and histomorphology18 of ovarian follicles do not exhibit unusual characteristics following prolonged androgen exposure. Nevertheless, oocytes retrieved from transgender men on hormone treatment have shown poor in vitro maturation.19 Although gender-affirming hormone therapy neither appears to alter nor induces the loss of primordial follicles in the ovarian cortex,20 Moravek21 states that in the absence of further evidence, the current recommendation for fertility preservation in transgender men dictates the discontinuation of testosterone before ovarian stimulation.
The reproductive desire among transgender men is prevalent,10 yet the necessity for androgen therapy suspension still poses a considerable challenge.22 The findings published by Persky et al.23 point out the low number of transgender men willing to delay hormone therapy over the chance of fertility preservation. Coherently, it is well-known that testosterone withdrawal in transgender men exacerbates gender dysphoria inducing anxiety, unease, and potentially self-injurious behaviors.24,25 Hence, this study develops a review of the literature addressing fertility preservation in adult transgender men undergoing continuous testosterone administration before or during assisted reproductive therapies. As such, the present investigation aims to elucidate the existence of a relationship between prolonged exogenous androgen exposure and oocyte retrieval outcomes, as well as the necessity of testosterone suspension for fertility preservation in transgender men.
Materials and Methods
Search strategy
To assess the outcomes of assisted reproductive technologies in transgender men undergoing androgen treatment, the literature search focused on studies addressing fertility preservation in the following three scenarios: (a) discontinuation of testosterone following prolonged (>12 months) administration, (b) continuous use of testosterone during fertility therapy, and (c) comparison of fertility outcomes between transgender men on hormone treatment and transgender men without exogenous testosterone administration. No ethics committee review was required for this study.
The systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).26 PubMed/Ovid MEDLINE, ScienceDirect, and Scopus databases were consulted, compiling studies published up to October 1, 2021. The search strategy was constructed based on keywords and indexed terms as follows: (“Transgender persons” OR “Transgender men” OR “Transgender man” OR “Female-to-male transgender”) AND (“Hormone therapy” OR “Testosterone” OR “Androgen*” OR “Hormone replacement therapy” OR “Gender-affirming hormone therapy”) AND (“Ovarian stimulation” OR “Oocyte* retrieval” OR “Oocyte* cryopreservation” OR “Fertility preservation”).
Eligibility criteria
The studies eligible for inclusion were those addressing fertility preservation in transgender men undergoing testosterone administration before or during oocyte retrieval. Case reports, cohort studies, and cross-sectional studies written in English and published in peer-reviewed academic journals were considered for inclusion.
Variability in testosterone administration regimen and dosage was accepted as long as it was initiated before fertility therapy. The duration of exogenous testosterone exposure was considered to be prolonged for intervals greater than 12 months. The relevant outcomes extracted from the research articles were those of oocyte retrieval after ovarian stimulation or ovarian tissue cryopreservation. Additional reports such as in vitro fertilization (IVF) results, and oocyte maturation rate and viability were also analyzed. Different ovarian stimulation protocols were accepted.
Studies addressing adolescent populations were excluded as well as those focused exclusively on transgender men without prior testosterone administration or with reports of surgical interventions that could lead to reproductive capacity loss.
Study selection
Records were screened independently and each eligible article was then reviewed, discussed, and approved by both researchers. After the selection of the studies included, the following data were extracted: (a) study population, (b) study type and design, (c) the duration of testosterone therapy before fertility treatment, (d) fertility preservation technique/protocol, and (e) relevant outcomes regarding oocyte retrieval.
Results
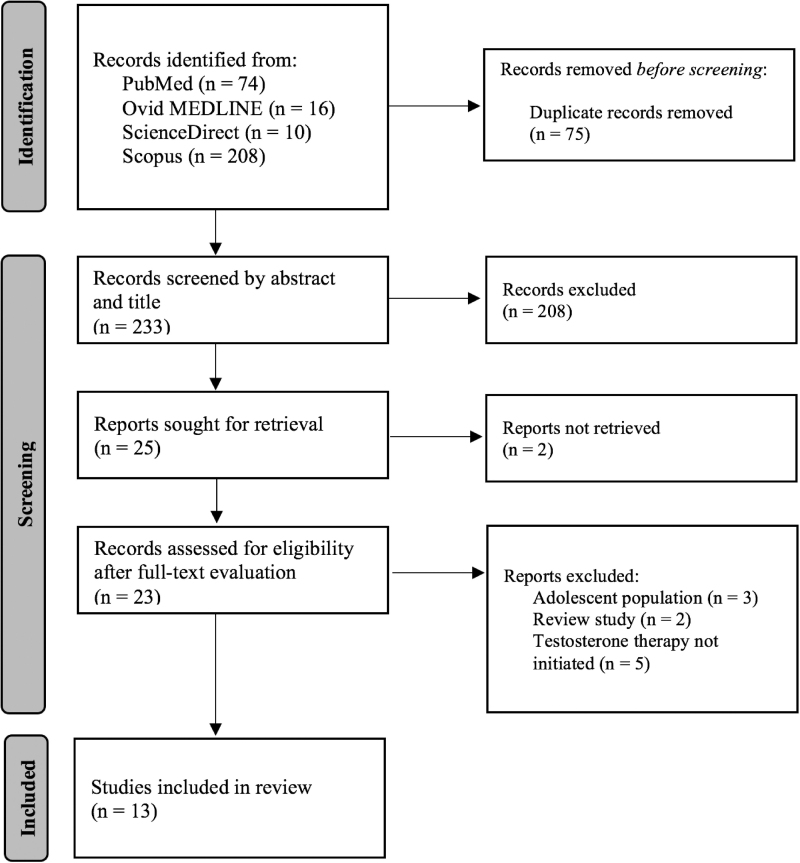
A total of 308 studies resulted from the initial search in the PubMed/Ovid MEDLINE, ScienceDirect, and Scopus databases. Following duplicate removal, and article screening based on title and abstract, 25 studies were selected. Out of this selection, 23 were further assessed by full-text evaluation. At last, seven case reports, four cohort studies (three retrospective and one prospective), and two cross-sectional studies were included in the review (Fig. 1). The characteristics of the selected studies are presented in Table 1.19,27–38
FIG. 1.
PRISMA flow diagram of the search strategy. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Fertility Preservation Outcomes in Transgender Men After/During Prolonged Testosterone Therapy
| Reference | Study type and design | Population | Testosterone therapy duration | Ovarian stimulation protocol/fertility preservation technique | Oocyte retrieval outcomes |
|---|---|---|---|---|---|
| Discontinuation of testosterone therapy for fertility preservation | |||||
| Gidoni et al.27 | Case report | Transgender man (age, 37 years). Transvaginal ultrasound revealed a 5 mm endometrial thickening, reduced ovarian size, and low follicular reserve. |
Testosterone enanthate administration (250 mg) every 16 days for 168 consecutive months before fertility therapy. | Two cycles of ovarian stimulation with oocyte retrieval for IVF and subsequent embryo cryopreservation. Testosterone suspension for 4 months. GnRH agonist protocol for ovarian stimulation. |
First cycle: Retrieval of 12 MII oocytes, out of which 7 were fertilized, and 4 of these were cryopreserved. Second cycle: Retrieval of 15 MII oocytes, out of which 14 were fertilized, and 12 of these were cryopreserved. |
| Insogna et al.28 | Case report | Transgender man (age, 21 years). Patient with 14 months of amenorrhea after initiation of hormone therapy. Serum AMH levels of 1.06 ng/mL while on testosterone therapy. |
Testosterone administration for 24 consecutive months before fertility therapy. | Two cycles of ovarian stimulation with oocyte retrieval for cryopreservation. Testosterone suspension for ∼3 months. First cycle: Administration of rhFSH (225 IU) and hMG (225 IU). A GnRH antagonist was administered on day 8, followed by hCG (10,000 IU) injection and oocyte retrieval on day 10. Second cycle: Administration of diluted leuprolide acetate (10 IU twice daily), rhFSH (225 IU), and hMG (225 IU). On day 11, hCG (10,000 IU) was administered and oocytes were retrieved. |
First cycle: Retrieval of 11 oocytes, out of which 10 were cryopreserved, and 6 of these were mature (MII). Second cycle: Retrieval of 14 oocytes, out of which 6 were mature (MII). |
| Broughton and Omurtag29 | Case report | Transgender man (age, 30 years). Patient with amenorrhea at fertility therapy initiation. Ultrasound revealed normal uterus and AFC of 25. | Testosterone administration for 26 consecutive months before fertility therapy. | Ovarian stimulation with oocyte retrieval for IVF and subsequent embryo transfer into the uterus of his cisgender partner. Testosterone discontinuation for a more than 3-month period. Fertility preservation protocol: Day 1: Administration of oral contraceptive for 2 weeks. Subsequent administration of GnRH antagonist. Day 8: hCG administration and oocyte retrieval. |
Retrieval of 16 oocytes. Of these, 13 were mature oocytes (MII), and 7 of these were successfully fertilized. |
| Resende et al.30 | Case report | Transgender man (age, 34 years). Patient with amenorrhea at fertility therapy initiation. | Testosterone administration (250 mg) for 24 consecutive months before fertility therapy. | Ovarian stimulation with oocyte retrieval for IVF and subsequent embryo transfer into the uterus of his cisgender partner. Testosterone was discontinued until menses resumption. The exact duration of androgen suspension is not stated. Fertility preservation protocol: Day 1: Administration of folic acid, and rhFSH with rhLH in 2:1 ratio. Day 3: Increase of rhFSH dose (150 IU) and administration of hMG (75 IU). Day 8: Pituitary blockade by administration of cetrotide (1.5 mg daily). Day 10: Increase of rhFSH dose (225 IU) with the same hMG dosage (75 IU). Day 12: hCG administration and oocyte retrieval. |
Retrieval of 16 oocytes. Of these, 13 were mature oocytes (MII) and successfully fertilized. |
| Cho et al.31 | Case report | Transgender man (age, 28 years). Transabdominal ultrasound revealed normal ovaries and uterus. Serum AMH levels of 2.50 ng/mL while on testosterone therapy. |
Testosterone administration (0.6 mg weekly) for 36 consecutive months before fertility therapy. | Ovarian stimulation with oocyte retrieval for cryopreservation. Testosterone suspension for 24 days with oral administration of letrozole (7.5 mg) from the beginning until 7 days after oocyte retrieval. Fertility preservation protocol: Day 1: Testosterone suspension. Day 5: Daily administration of ganirelix acetate (0.25 mg). Day 7: Subcutaneous administration of rhFSH (300 IU) and hMG (150 IU) for 13 consecutive days. Day 14: Administration of hCG (5000 IU), triptorelin (175 IU daily), and rhFSH (300 IU) when follicles reached 17 mm. Transvaginal oocyte retrieval was carried out 36 h after hCG injection. |
Retrieval of 13 oocytes, out of which 11 were mature (MII) and subsequently vitrified. |
| Leung et al.32 | Retrospective cohort study | 26 transgender men (mean age, 28.3±6.7 years), of whom 16 reported testosterone administration before fertility counseling. Mean serum AMH levels of 3.4±1.9 ng/mL. |
Mean duration of 43.9±31.0 months on testosterone therapy within the 16 transgender men undergoing hormone treatment. | Ovarian stimulation for oocyte retrieval and ovarian tissue cryopreservation with subsequent IVF. Testosterone suspension for 4.5±3.5 months (range 1–12 months). The ovarian stimulation protocol is not described. |
Retrieval of 18.6±9.3 oocytes, of which 77.0%±23.3% were mature (MII). |
| Fertility preservation undergoing testosterone therapy | |||||
| Lierman et al.19 | Cross-sectional study | 16 transgender men (mean age, 24.30±6.15 years). The study population was divided into three groups: (1) 4 subjects aged <20 years. (2) 9 subjects aged 20–30 years. (3) 3 subjects aged >30 years. |
Mean duration of 13.4±5.25 months on testosterone therapy at the moment of the oophohysterectomy. | Hysterectomy and bilateral oophorectomy for ovarian tissue cryopreservation and in vitro oocyte maturation. Protocol without suspension of testosterone therapy. |
Mean retrieval of 42.5 COCs for all individuals (n=16). (1) Subjects aged <20: 61.3±76.2 oocytes retrieved. (2) Subjects aged 20–30: 41.0±35.7 oocytes retrieved. (3) Subjects aged >30: 22.0±5.0 oocytes retrieved. |
| Gale et al.33 | Case report | Transgender man (age, 20 years). Transabdominal ultrasound revealed normal ovaries and uterus, with an AFC >40. Serum AMH levels of 19.9 ng/mL while on testosterone therapy. |
Testosterone administration (25 mg weekly) for 18 consecutive months before fertility therapy. | Ovarian stimulation with oocyte retrieval for cryopreservation. Protocol without suspension of testosterone therapy. Fertility preservation protocol: Day 1: Administration of cetrorelix with rhFSH (175 IU daily) and rhLH (75 IU daily). Day 9: Administration of leuprolide acetate (3 mg) and initiation of four doses of cabergoline (0.5 mg every third day). Transvaginal oocyte retrieval 36 h after leuprolide injection. |
Retrieval of 25 COCs. Of these, 22 MII oocytes were successfully cryopreserved. |
| Greenwald et al.34 | Case report | Transgender man (age, 33 years). Hysterosonography and serum AMH levels while on testosterone revealed an ovarian reserve above normal for age. | Testosterone administration (50 mg weekly) for 120 consecutive months before fertility therapy. | Ovarian stimulation with oocyte retrieval for reciprocal IVF. Protocol without suspension of testosterone therapy. Fertility preservation protocol: Day 1: Daily subcutaneous administration of rhFSH (300 IU), hMG (150 IU), and ganirelix acetate. Day 14: Induction of oocyte maturation by subcutaneous injection of leuprolide acetate (40 IU) and hCG (1000 IU). Transvaginal oocyte retrieval 36 h after leuprolide and hCG injection. |
Retrieval of 20 oocytes, of which 16 were mature (MII), and 13 were successfully fertilized. Subsequent to IVF, 5 embryos progressed to blastocysts, and 4 exhibited aneuploidy. |
| De Roo et al.35 | Prospective cohort study | 40 transgender men (mean age, 24.30±6.15 years). Subjects exhibited amenorrhea for an average of 70.59±31.89 weeks. Mean serum AMH levels of 4.66±3.84 ng/mL while on testosterone therapy. |
Mean duration of 13.39±6.11 months on testosterone therapy at the moment of the oophohysterectomy. | Hysterectomy and bilateral oophorectomy for ovarian tissue cryopreservation and in vitro oocyte maturation. Protocol without suspension of testosterone therapy. |
From the 40 transgender men, an average of 37.51±33.58 oocytes were retrieved. |
| Lierman et al.36 | Cross-sectional study | 83 transgender men (mean age, 20.0 [min: 17.6; max: 38.4] years). Serum AMH levels of 2.8 ng/mL while on testosterone therapy. |
Mean duration of 20.75 months on testosterone therapy at the moment of the oophohysterectomy. | Hysterectomy and bilateral oophorectomy for ovarian tissue cryopreservation and in vitro oocyte maturation. Protocol without suspension of testosterone therapy. |
Mean retrieval of 23.0±15.8 COCs. |
| Comparison of fertility outcomes among transgender men | |||||
| Amir et al.37 | Retrospective cohort study | 12 transgender men divided into two groups: (1) 6 transgender men with no history of hormone treatment (mean age, 23.3±4.0 years). (2) 6 transgender men undergoing testosterone therapy (mean age, 30.3±3.8 years). |
Among the 6 individuals on hormone treatment, a mean duration of 7.0±55.3 months on testosterone therapy was reported before fertility therapy. | Ovarian stimulation with oocyte retrieval for cryopreservation and IVF. Mean testosterone suspension for 9.3±5.6 months. Fertility preservation protocol: Daily administration of rhFSH from the third day of menses resumption. GnRH antagonist administration: initiation of therapy with cetrorelix acetate (0.25 mg) from the time E2 levels reached >450 pg/mL and a follicular diameter ≥12 mm was achieved. GnRH agonist administration: Following cetrorelix acetate, triptorelin (0.2 mg/day) was administered at the time follicular diameter >18 mm was evident for at least three follicles. Transvaginal oocyte retrieval was carried out 36 h after triptorelin injection. |
From the group of 6 transgender men on testosterone therapy, 22±9.94 oocytes were retrieved on average. The number of oocytes retrieved did not reveal a significant difference between the two groups (p=0.651). |
| Adeleye et al.38 | Retrospective cohort study | 13 transgender men (mean age, 22.4 years) divided into two groups: (1) 6 transgender men with no history of hormone treatment (2) 7 transgender men undergoing testosterone therapy |
Among the 7 individuals on hormone treatment, a mean duration of 46 months on testosterone therapy was reported before fertility therapy. | Ovarian stimulation with oocyte retrieval for cryopreservation. Mean testosterone suspension for 6 months. Ten ovarian stimulation cycles with GnRH antagonist protocol and average duration of 10 days (range 8–14 days). |
From the group of 7 transgender men on testosterone therapy, an average of 12 mature oocytes (MII) were retrieved and exhibited a maturation rate of 92%. The number of oocytes retrieved did not reveal a significant difference between the two groups (p=0.148). |
AFC, antral follicular count; AMH, anti-Müllerian hormone; COCs, cumulus–oocyte complexes; E2, estradiol; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin; hMG, human menotropin; IVF, in vitro fertilization; MII, metaphase II; rhFSH, recombinant human follicle-stimulating hormone; rhLH, recombinant human luteinizing hormone.
Fertility outcomes following hormone treatment discontinuation
In 2013, Gidoni et al.27 reported the case of a 37-year-old transgender man with a 14-year history of testosterone enanthate administration. He wished to proceed with oocyte retrieval for further IVF with a sperm sample provided by his male cisgender partner. Following testosterone suspension for 4 months and two cycles of ovarian stimulation with a gonadotropin-releasing hormone (GnRH) agonist protocol, 12 and 15 metaphase II (MII) oocytes resulted from the first and second ovulation inductions, respectively. Despite the successful retrieval, the patient exhibited dysphoria resulting from virilization regression due to the hormone-induced ovarian stimulation.
Similarly, Insogna et al.28 presented the case of a 21-year-old transgender man who had been on testosterone therapy for 24 months. Hormone treatment was discontinued for an ∼3-month interval. During the first cycle of ovarian stimulation, 11 oocytes (6 MII oocytes) were retrieved, followed by the extraction of 14 oocytes (6 MII oocytes) in the second cycle. As such, the findings of both studies show that the response to ovarian stimulation in transgender men is preserved despite prolonged androgen exposure.
Fertility outcomes in patients who underwent a single ovarian stimulation cycle revealed a similar trend. Broughton and Omurtag29 described the case of a 30-year-old transgender man on testosterone depot administration for 26 consecutive months. After androgen therapy suspension over a period of ∼3 months, he underwent a cycle of ovarian stimulation, which resulted in successful fertility preservation. Likewise, Resende et al.30 reported an effective oocyte retrieval in the case of a 34-year-old transgender man with a history of testosterone ester administration for 24 consecutive months before fertility therapy. In this case, hormone treatment was interrupted until menses resumption; however, the duration of this suspension was not stated.
At last, Cho et al.31 presented the case of a 28-year-old transgender man on testosterone administration for 36 months and further discontinuation for 24 days. Aiming to prevent the regression of formerly achieved virilization, letrozole was administered during ovarian stimulation, which resulted in the successful retrieval of 13 oocytes (11 MII oocytes).
A retrospective cohort study carried out by Leung et al.32 describes the outcomes of fertility preservation among 26 transgender men (28.3±6.7 years), of whom 16 reported previous testosterone administration for an average of 43.9±31.0 months. In the latter group, hormone therapy was discontinued for an interval of 4.5±3.5 months, and a total of 18.6±9.3 oocytes were retrieved. Moreover, ovarian stimulation outcomes in transgender men on hormone therapy and cisgender women were compared, revealing the absence of a significant difference in the number of oocytes retrieved between both groups (p>0.05).
Fertility preservation undergoing testosterone administration
A pioneering and innovative approach to fertility preservation is presented in recent studies where the authors reveal the feasibility of oocyte retrieval in transgender men without androgen treatment interruption. Gale et al.33 describe the case of a 20-year-old transgender man with an 18-month history of continuous testosterone use. An ovarian stimulation protocol without discontinuation of hormone therapy was performed for oocyte retrieval with subsequent cryopreservation and IVF. After ovulation induction concomitant to exogenous testosterone use, 25 cumulus–oocyte complexes (COCs) were retrieved. A similar study by Greenwald et al.34 shows successful outcomes of ovarian stimulation during continuous administration of testosterone. The authors reported the retrieval of 20 oocytes (16 MII oocytes) in a 33-year-old transgender man on testosterone for 120 months without suspension during fertility therapy.
Similarly, surgical ovary removal with tissue and oocyte cryopreservation outcomes discloses fertility preservation despite a prolonged and continuous exogenous androgen exposure. Lierman et al.19 studied a cohort of 16 transgender men (24.1±6.1 years) who underwent gender reassignment surgery with ovarian removal for cryopreservation and in vitro oocyte maturation. A hysterectomy with bilateral oophorectomy was performed without discontinuation of testosterone therapy and an average extraction of 42.5 COCs was achieved. Furthermore, De Roo et al.35 reported the results of oocyte retrieval in a group of 40 transgender men (24.30±6.15 years) on hormone treatment with administration of different presentations of testosterone for 13.39±6.11 months. Following a hysterectomy with bilateral oophorectomy while on continuous androgen therapy, an average of 37.51±33.58 COCs were retrieved.
At last, the research conducted by Lierman et al.36 showed similar outcomes. A group of 83 transgender men with testosterone ester administration for an average of 20.75 months proceeded with a hysterectomy and bilateral oophorectomy and further oocyte retrieval without discontinuation of hormone therapy. An average of 23.0±15.8 COCs were successfully retrieved. The aforementioned findings evidence the viability of oocyte retrieval without the interruption of testosterone therapy, both by ovarian stimulation and tissue cryopreservation.
Fertility outcomes in transgender men on and off hormone therapy
When comparing fertility preservation between transgender men on and off testosterone therapy, current evidence indicates the absence of a significant difference in the number of oocytes retrieved by ovarian stimulation. Amir et al.37 conducted a retrospective study in a cohort of 12 transgender men, of whom 6 reported no androgen use (23.3±4.0 years) and the other 6 reported testosterone administration (30.3±3.8 years). In the latter group, an average of 77.0±55.3 months of testosterone administration before fertility therapy was reported. Hormone treatment was suspended for 9.3±5.6 months, after which a cycle of ovarian stimulation with GnRH antagonists was initiated. An average of 22.0±9.9 oocytes were retrieved from the group of six transgender men on testosterone, which did not exhibit a significant difference from the group of transgender men without androgen administration (p>0.05).
Lastly, Adeleye et al.38 studied a cohort of 13 transgender men, of which 7 reported testosterone cypionate administration for an average of 46 months before fertility preservation. After ovarian stimulation and androgen therapy suspension for 6 months, an average of 12 MII oocytes were retrieved from the group of 7 transgender men on hormone therapy. The analysis following the removal of outlier values showed that the mean number of oocytes retrieved from the group of transgender men on hormone therapy did not differ significantly from the group of transgender men without testosterone administration (p>0.05).
Discussion
The findings gathered in this review suggest preservation of fertility in transgender men on testosterone treatment and allow one to approach the relationship between masculinizing hormone therapy and reproductive capacity. As such, we evidenced that obtaining COCs from surgically removed ovarian tissue appears to be viable after exogenous androgen exposure.19,35,36 Moreover, recent studies suggest that ovarian stimulation results in successful oocyte retrieval following long-term hormone therapy (>12 months)27–32,37,38 or under continuous testosterone administration during ovulation induction.33,34 Hence, fertility preservation in transgender men does not appear to be impaired by exogenous androgen administration despite prolonged exposure. Likewise, the duration of hormone treatment withdrawal does not seem to be correlated with the number of oocytes retrieved; still, no certain conclusion can be drawn given the high variance in testosterone therapy regimen and ovarian reserve.
Masculinizing hormone therapy effect on reproductive physiology
The morphophysiological adaptations that result from testosterone administration prompt the development of male secondary sexual characteristics, and while hormone therapy achieves notorious virilization, the effect of exogenous androgens on the female reproductive system has not yet been fully elucidated. When compared with pre- and postmenopausal cisgender women, transgender men on hormone treatment exhibit a significant reduction of vaginal epithelium proliferation, a decrease in intracellular glycogen deposits, and a lower expression of α and β estrogen receptors (ER-α and ER-β).39 Uterine findings are rather contradictory as studies have revealed both an atrophic40 and proliferative endometrium.41
Likewise, conflicting results regarding testosterone's effect on ovarian tissue have been documented. Histological findings in transgender men's ovaries reveal the presence of multiple cysts that resemble the morphology of polycystic ovaries,42 while transvaginal imaging reports have revealed no polycystic morphology following exogenous androgen exposure.43 Although histomorphological changes are still inconclusive, ovarian cortex hyperplasia and stromal luteinization bear a resemblance to the findings in patients with polycystic ovarian syndrome.44 According to Moravek et al.,14 the effect of androgen administration on the female reproductive organs remains controversial, and the absence of a standard testosterone dosing regimen protocol makes it difficult to extrapolate the findings reported thus far.
The studies compiled in the present review show that following prolonged testosterone exposure, primordial follicle viability is maintained, as well as the ovulatory response after hormonal stimulation. As such, evidence suggests that a deleterious effect of exogenous androgens on both ovarian reserve and function could be revisited as a rationale for gender-affirming hormone treatment discontinuation when considering fertility preservation therapies in transgender men.
Furthermore, the assessment of the reproductive capacity becomes challenging due to the physiological changes that arise from masculinizing hormone therapy. The use of hormonal fertility markers drawn from cisgender women turns out to be conflicting when interpreting these results in transgender men.45 Anti-Müllerian hormone (AMH), for instance, is used to evaluate the ovarian reserve46 in both cisgender and transgender patients.45 Nonetheless, the variation in serum AMH levels among transgender men on gender-affirming hormone therapy has been understudied, and current reports show conflicting results. Both a significant decrease47 and a minimum change48 on serum AMH concentrations have been reported in response to prolonged testosterone administration. In the studies gathered in this review, serum AMH levels measured while on testosterone therapy appeared to be highly variable, yet they do not seem to exhibit a direct relationship with the duration of androgen exposure or hormone treatment discontinuation.
Recent evidence published by Greene et al.45 shows that transgender men undergoing gender-affirming hormone therapy exhibit slightly higher AMH concentrations when compared with cisgender women. Thus, fertility evaluation in transgender men based on serum AMH levels still requires more studies to determine a reference range allowing a more accurate assessment of the ovarian reserve in patients undergoing gender-affirming hormone therapy.
Assisted reproduction in transgender men on hormone treatment
Fertility preservation options in transgender men depend on the moment during gender transitioning in which the patient chooses to undergo assisted reproduction. Before surgical interventions that involve a bilateral oophorectomy, ovarian stimulation induces follicular development and prompts ovulation allowing oocyte retrieval for further cryopreservation or IVF.49 As such, ovarian stimulation protocols comprise GnRH analogs and gonadotropin administration that result in an increase of estrogen production, which concomitantly to testosterone therapy cessation leads to a regression of virilization that might trigger gender dysphoria. The results from the study conducted by Gidoni et al.27 disclose the feasibility of oocyte retrieval after ovarian stimulation following 168 months of testosterone administration; however, the suspension of androgen treatment and the consequent dysphoria exhibited by the patient point out the considerable limitations associated with this therapy.
Although an elevation of serum estradiol levels following ovarian stimulation is imminent, Cho et al.31 reported the use of letrozole while undergoing assisted reproduction. Similarly, a case report of fertility preservation with letrozole administration in an adolescent transgender man, described by Martin et al.,50 reveals an effective oocyte retrieval with minimal morphological changes and minor elevation of serum estradiol levels. Fertility preservation outcomes described by these authors suggest that the use of aromatase inhibitors could reduce the masculinization regression without significantly affecting the results of ovarian stimulation. Further longitudinal studies are still required to ensure with a greater degree of certainty the feasibility of this therapeutic approach in assisted reproduction protocols.
In the last few years, two case reports of transgender men on testosterone therapy while undergoing ovarian stimulation have been published. Gale et al.33 and Greenwald et al.34 disclose a successful oocyte retrieval following hormonal ovulation induction without cessation of gender-affirming hormone therapy. Still, at present, no studies different from case reports have explored fertility preservation concomitantly to exogenous androgen administration. Thus, the results reported by these authors constitute a groundbreaking approach in transgender medicine that raises questions about the necessity and effectiveness of hormone treatment suspension during assisted reproductive therapies. Hence, although the studies are limited, these findings call for a thorough assessment of the possibility of fertility preservation without testosterone therapy suspension, which could convey a novel strategy to minimize the distress associated with androgen withdrawal in transgender men.
At last, when comparing ovarian stimulation outcomes in transgender men on and off hormone therapy, no significant difference in the number of oocytes retrieved was observed based on the findings of Amir et al.37 (six transgender men on hormone therapy vs. six transgender men off hormone therapy [p=0.651]) and Adeleye et al.38 (seven transgender men on hormone therapy vs. six transgender men off hormone therapy [p=0.148]). In addition, the number of oocytes obtained after ovarian stimulation does not appear to vary significantly between transgender men on hormone treatment and cisgender women (p=0.716).37 These studies suggest minimal effects of exogenous androgen exposure on the reproductive capacity of transgender men. Nonetheless, more studies are required and additional investigations are encouraged to further explore the difference in oocyte retrieval outcomes between transgender men on testosterone, and both transgender men off testosterone and cisgender women.
Likewise, several variables relevant to fertility preservation need further exploration and are still subject to future research. For instance, reports of viability and maturation rates of oocytes retrieved from transgender men on hormone therapy are crucial but limited as only two investigations have been documented, which reveal no alteration in oocyte meiotic spindles19 but a low in vitro developmental capacity.36
Limitations
The scarcity of longitudinal studies addressing fertility preservation in transgender men on hormone therapy conveys one of the main limitations of this review. As such, while the gathered evidence serves to explore the relationship between prolonged exogenous androgen exposure and oocyte retrieval outcomes, 7 out of 13 studies included were case reports, hindering the extrapolation of these findings to a wider population given the high variance among each individual. Moreover, it is worth mentioning the heterogeneity in the regimen of testosterone administration as well as in fertility parameters such as the antral follicular count, serum AMH levels, and ovaries' morphological features that could diminish ovarian reserve and thus oocyte retrieval.
Conclusions
Strategies to improve fertility preservation and assisted reproduction in transgender men need to be sought. The findings gathered in this review reveal a viable oocyte retrieval following prolonged androgen exposure and suggest the absence of a direct relationship between the duration of testosterone suspension and fertility preservation outcomes. While the evidence is limited, continuous testosterone administration or the use of aromatase inhibitors during ovarian stimulation could represent novel approaches to lessen the distressing effects associated with hormonal ovulation induction. Furthermore, additional studies are still required to determine serum AMH reference values that could allow the assessment of ovarian reserve in transgender men undergoing hormone treatment.
At last, the present review sheds light on the necessity of reappraising testosterone therapy's effect on fertility preservation to ensure that interventions aligned with both the reproductive desire and the avoidance of gender dysphoria and virilization regression that follow hormone therapy suspension in transgender men.
Acknowledgment
The authors thank Nancy E. Parrado for her assistance with English language revision and article proofreading.
Abbreviations Used
- AFC
antral follicular count
- AMH
anti-Müllerian hormone
- COCs
cumulus–oocyte complexes
- E2
estradiol
- ER-α and ER-β
α and β estrogen receptors
- GnRH
gonadotropin-releasing hormone
- hCG
human chorionic gonadotropin
- hMG
human menotropin
- IVF
in vitro fertilization
- MII
metaphase II
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- rhFSH
recombinant human follicle-stimulating hormone
- rhLH
recombinant human luteinizing hormone
Authors' Contributions
J.A.B. and I.M. contributed equally to the conceptualization, methodology, and literature review. J.A.B. drafted the original article. I.M. provided supervision and revised the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
Cite this article as: Barrero JA, Mockus I (2023) Preservation of fertility in transgender men on long-term testosterone therapy: a systematic review of oocyte retrieval outcomes during and after exogenous androgen exposure, Transgender Health 8:5, 408–419, DOI: 10.1089/trgh.2022.0023.
References
- 1. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–3903. [DOI] [PubMed] [Google Scholar]
- 2. Irwig MS. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017;5:301–311. [DOI] [PubMed] [Google Scholar]
- 3. Wierckx K, Van Caenegem E, Schreiner T, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European Network for the Investigation of Gender Incongruence. J Sex Med. 2014;11:1999–2011. [DOI] [PubMed] [Google Scholar]
- 4. Irwig MS, Childs K, Hancock AB. Effects of testosterone on the transgender male voice. Andrology. 2017;5:107–112. [DOI] [PubMed] [Google Scholar]
- 5. Gooren LJ. Management of female-to-male transgender persons: medical and surgical management, life expectancy. Curr Opin Endocrinol Diabetes Obes. 2014;21:233–238. [DOI] [PubMed] [Google Scholar]
- 6. Ahmad S, Leinung M. The response of the menstrual cycle to initiation of hormonal therapy in transgender men. Transgend Health. 2017;2:176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. T'Sjoen G, Arcelus J, Gooren L, et al. Endocrinology of transgender medicine. Endocr Rev. 2019;40:97–117. [DOI] [PubMed] [Google Scholar]
- 8. Al-Tamimi M, Pigot GL, Elfering L, et al. Genital gender-affirming surgery in transgender men in The Netherlands from 1989 to 2018: the evolution of surgical care. Plast Reconstr Surg. 2020;145:153e–161e. [DOI] [PubMed] [Google Scholar]
- 9. Gooren LJ, Giltay EJ, Bunck MC. Long-term treatment of transsexuals with cross-sex hormones: extensive personal experience. J Clin Endocrinol Metab. 2008;93:19–25. [DOI] [PubMed] [Google Scholar]
- 10. Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27:483–487. [DOI] [PubMed] [Google Scholar]
- 11. Ethics Committee of the American Society for Reproductive Medicine. Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104:1111–1115. [DOI] [PubMed] [Google Scholar]
- 12. Blakemore JK, Quinn GP, Fino ME. A discussion of options, outcomes, and future recommendations for fertility preservation for transmasculine individuals. Urol Clin North Am. 2019;46:495–503. [DOI] [PubMed] [Google Scholar]
- 13. Light AD, Obedin-Maliver J, Sevelius JM, et al. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124:1120–1127. [DOI] [PubMed] [Google Scholar]
- 14. Moravek MB, Kinnear HM, George J, et al. Impact of exogenous testosterone on reproduction in transgender men. Endocrinology. 2020;161:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deutsch MB, Bhakri V, Kubicek K. Effects of cross-sex hormone treatment on transgender women and men. Obstet Gynecol. 2015;125:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borrás A, Manau MD, Fabregues F, et al. Endocrinological and ovarian histological investigations in assigned female at birth transgender people undergoing testosterone therapy. Reprod Biomed Online. 2021;43:289–297. [DOI] [PubMed] [Google Scholar]
- 17. Grimstad FW, Fowler KG, New EP, et al. Ovarian histopathology in transmasculine persons on testosterone: a multicenter case series. J Sex Med. 2020;17:1807–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marschalek J, Pietrowski D, Dekan S, et al. Markers of vitality in ovaries of transmen after long-term androgen treatment: a prospective cohort study. Mol Med. 2020;26:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lierman S, Tilleman K, Braeckmans K, et al. Fertility preservation for trans men: frozen-thawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. J Assist Reprod Genet. 2017;34:1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van den Broecke R, Van der Elst J, Liu J, et al. The female-to-male transsexual patient: a source of human ovarian cortical tissue for experimental use. Hum Reprod. 2001;16:145–147. [DOI] [PubMed] [Google Scholar]
- 21. Moravek MB. Gender-affirming hormone therapy for transgender men. Clin Obstet Gynecol. 2018;61:687–704. [DOI] [PubMed] [Google Scholar]
- 22. Armuand G, Dhejne C, Olofsson JI, et al. Transgender men's experiences of fertility preservation: a qualitative study. Hum Reprod. 2017;32:383–390. [DOI] [PubMed] [Google Scholar]
- 23. Persky RW, Gruschow SM, Sinaii N, et al. Attitudes toward fertility preservation among transgender youth and their parents. J Adolesc Health. 2020;67:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Besse M, Lampe NM, Mann ES. Experiences with achieving pregnancy and giving birth among transgender men: a narrative literature review. Yale J Biol Med. 2020;93:517–528. [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng PJ, Pastuszak AW, Myers JB, et al. Fertility concerns of the transgender patient. Transl Androl Urol. 2019;8:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, et al. Reprint-preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys Ther. 2009;89:873–880. [PubMed] [Google Scholar]
- 27. Gidoni YS, Raziel A, Strassburger D, et al. Can we preserve fertility in a female to male trangender after a long term testosterone treatment—case report. Fertil Steril. 2013;100:S169–S170. [Google Scholar]
- 28. Insogna IG, Ginsburg E, Srouji S. Fertility preservation for adolescent transgender male patients: a case series. J Adolesc Health. 2020;66:750–753. [DOI] [PubMed] [Google Scholar]
- 29. Broughton D, Omurtag K. Care of the transgender or gender-nonconforming patient undergoing in vitro fertilization. Int J Transgend. 2017;18:372–375. [Google Scholar]
- 30. Resende SS, Kussumoto VH, Arima FHC, et al. A transgender man, a cisgender woman, and assisted reproductive technologies: a Brazilian case report. JBRA Assist Reprod. 2020;24:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cho K, Harjee R, Roberts J, et al. Fertility preservation in a transgender man without prolonged discontinuation of testosterone: a case report and literature review. F S Rep. 2020;1:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leung A, Sakkas D, Pang S, et al. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil Steril. 2019;112:858–865. [DOI] [PubMed] [Google Scholar]
- 33. Gale J, Magee B, Forsyth-Greig A, et al. Oocyte cryopreservation in a transgender man on long-term testosterone therapy: a case report. F S Rep. 2021;2:249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Greenwald P, Dubois B, Lekovich J, et al. Successful in vitro fertilization in a cisgender female carrier using oocytes retrieved from a transgender man maintained on testosterone. AACE Clin Case Rep. 2021;8:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Roo C, Lierman S, Tilleman K, et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online. 2017;34:557–566. [DOI] [PubMed] [Google Scholar]
- 36. Lierman S, Tolpe A, De Croo I, et al. Low feasibility of in vitro matured oocytes originating from cumulus complexes found during ovarian tissue preparation at the moment of gender confirmation surgery and during testosterone treatment for fertility preservation in transgender men. Fertil Steril. 2021;116:1068–1076. [DOI] [PubMed] [Google Scholar]
- 37. Amir H, Yaish I, Samara N, et al. Ovarian stimulation outcomes among transgender men compared with fertile cisgender women. J Assist Reprod Genet. 2020;37:2463–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adeleye AJ, Cedars MI, Smith J, et al. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet. 2019;36:2155–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baldassarre M, Giannone FA, Foschini MP, et al. Effects of long-term high dose testosterone administration on vaginal epithelium structure and estrogen receptor-α and -β expression of young women. Int J Impot Res. 2013;25:172–177. [DOI] [PubMed] [Google Scholar]
- 40. Perrone AM, Cerpolini S, Maria Salfi NC, et al. Effect of long-term testosterone administration on the endometrium of female-to-male (FtM) transsexuals. J Sex Med. 2009;6:3193–3200. [DOI] [PubMed] [Google Scholar]
- 41. Hawkins M, Deutsch MB, Obedin-Maliver J, et al. Endometrial findings among transgender and gender nonbinary people using testosterone at the time of gender-affirming hysterectomy. Fertil Steril. 2021;115:1312–1317. [DOI] [PubMed] [Google Scholar]
- 42. Loverro G, Resta L, Dellino M, et al. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55:686–691. [DOI] [PubMed] [Google Scholar]
- 43. Caanen MR, Schouten NE, Kuijper EAM, et al. Effects of long-term exogenous testosterone administration on ovarian morphology, determined by transvaginal (3D) ultrasound in female-to-male transsexuals. Hum Reprod. 2017;32:1457–1464. [DOI] [PubMed] [Google Scholar]
- 44. Ikeda K, Baba T, Noguchi H, et al. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28:453–461. [DOI] [PubMed] [Google Scholar]
- 45. Greene DN, Schmidt RL, Winston-McPherson G, et al. Reproductive endocrinology reference intervals for transgender men on stable hormone therapy. J Appl Lab Med. 2021;6:41–50. [DOI] [PubMed] [Google Scholar]
- 46. Oh SR, Choe SY, Cho YJ. Clinical application of serum anti-müllerian hormone in women. Clin Exp Reprod Med. 2019;46:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caanen MR, Soleman RS, Kuijper EAM, et al. Antimüllerian hormone levels decrease in female-to-male transsexuals using testosterone as cross-sex therapy. Fertil Steril. 2015;103:1340–1345. [DOI] [PubMed] [Google Scholar]
- 48. Taub RL, Ellis SA, Neal-Perry G, et al. The effect of testosterone on ovulatory function in transmasculine individuals. Am J Obstet Gynecol. 2020;223:229..e1–229.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blough K, Mansfield C, Kondapalli LA. Seamless integration of clinical care and research in an innovative fertility preservation program: the Colorado Oncofertility Program model. J Cancer Surviv. 2014;8:533–538. [DOI] [PubMed] [Google Scholar]
- 50. Martin CE, Lewis C, Omurtag K. Successful oocyte cryopreservation using letrozole as an adjunct to stimulation in a transgender adolescent after GnRH agonist suppression. Fertil Steril. 2021;116:522–527. [DOI] [PubMed] [Google Scholar]