Key Points
Question
For individuals undergoing medication treatment for opioid use disorder, can those who will return to opioid use be predicted at the individual level?
Findings
In this decision analytical model using a harmonized data set of 2199 adults with opioid use disorder, incorporating treatment entry characteristics and urine drug screen results in the first 3 weeks of treatment improved model performance in predicting return to use.
Meaning
These findings suggest that clinicians can use a web-based predictive model to stratify return-to-use risk when delivering care for patients with opioid use disorder.
This decision analytical model uses a harmonized data set to develop an individual-level prediction tool to assess the risk of return to use in patients treated for opioid use disorder.
Abstract
Importance
No existing model allows clinicians to predict whether patients might return to opioid use in the early stages of treatment for opioid use disorder.
Objective
To develop an individual-level prediction tool for risk of return to use in opioid use disorder.
Design, Setting, and Participants
This decision analytical model used predictive modeling with individual-level data harmonized in June 1, 2019, to October 1, 2022, from 3 multicenter, pragmatic, randomized clinical trials of at least 12 weeks’ duration within the National Institute on Drug Abuse Clinical Trials Network (CTN) performed between 2006 and 2016. The clinical trials covered a variety of treatment settings, including federally licensed treatment sites, physician practices, and inpatient treatment facilities. All 3 trials enrolled adult participants older than 18 years, with broad pragmatic inclusion and few exclusion criteria except for major medical and unstable psychiatric comorbidities.
Intervention
All participants received 1 of 3 medications for opioid use disorder: methadone, buprenorphine, or extended-release naltrexone.
Main Outcomes and Measures
Predictive models were developed for return to use, which was defined as 4 consecutive weeks of urine drug screen (UDS) results either missing or positive for nonprescribed opioids by week 12 of treatment.
Results
The overall sample included 2199 trial participants (mean [SD] age, 35.3 [10.7] years; 728 women [33.1%] and 1471 men [66.9%]). The final model based on 4 predictors at treatment entry (heroin use days, morphine- and cocaine-positive UDS results, and heroin injection in the past 30 days) yielded an area under the receiver operating characteristic curve (AUROC) of 0.67 (95% CI, 0.62-0.71). Adding UDS in the first 3 treatment weeks improved model performance (AUROC, 0.82; 95% CI, 0.78-0.85). A simplified score (CTN-0094 OUD Return-to-Use Risk Score) provided good clinical risk stratification wherein patients with weekly opioid-negative UDS results in the 3 weeks after treatment initiation had a 13% risk of return to use compared with 85% for those with 3 weeks of opioid-positive or missing UDS results (AUROC, 0.80; 95% CI, 0.76-0.84).
Conclusions and Relevance
The prediction model described in this study may be a universal risk measure for return to opioid use by treatment week 3. Interventions to prevent return to regular use should focus on this critical early treatment period.
Introduction
Medication for opioid use disorder (MOUD) has evolved from being delivered only in specialized practices in regulated settings to becoming a common intervention delivered more widely across a range of health care settings.1,2 The medications methadone, buprenorphine, and extended-release injection naltrexone (XR-NTX) are effective,3 but many patients return to opioid use during treatment.4 Practicing physicians would benefit from knowing at the outset whether a patient was deemed to be at a higher risk to return to opioid use.
Many risk scores are in use for other chronic conditions. The CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age, sex category) score is used to predict risks of stroke in atrial fibrillation5; the TIMI (thrombolysis in myocardial infarction) score is used to predict mortality in myocardial infarction6; and the Memorial Sloan Kettering Cancer Center cancer decision models are used to predict life expectancy for a variety of cancers.7 These scores are part of practice guidelines and provide a foundation for adaptive treatment strategies.8 Such individual-level risk prediction is not available for opioid use disorder (OUD) treatment, even though pragmatic clinical strategies could be considered, including more intensive psychotherapy programs, optimizing medication doses, or switching medications.
This study reports on prediction tools for risk of return to opioid use (commonly referred to as relapse). Previous studies have reported cohort-based differences in return-to-use risks from small heterogenous samples,9 and none created a quantitative, individual-level assessment. Leveraging a new Common Data Model–based harmonization effort through the National Institute of Drug Abuse Clinical Trials Network (CTN), we combined 3 studies (CTN-0027, Starting Treatment With Agonist Replacement Therapies [START]10; CTN-0030, Prescription Opioid Addiction Treatment Study [POATS]11; and CTN-0051, Extended-Release Naltrexone vs Buprenorphine for Opioid Treatment [X:BOT]12) into a single longitudinal data set. We hypothesized that a broadly applicable model could be built with predictors collectible from routine care,13 including demographic characteristics, self-reported drug use data (though collected more informally in clinical practice compared with the timeline follow-back [TLFB] used in clinical trial settings),14 and urine drug screen (UDS). We also aimed to develop a clinically tractable predictive rule disseminated with a web-based risk calculator.
Methods
Modeling Data Sources
In this decision analytical model, the trial protocols provided by the CTN dissemination library15 were used as a data cleaning reference. The START study recruited participants during 2006-2009 from 8 federally licensed opioid treatment programs. POATS recruited participants during 2006-2009 from 10 outpatient academic, public, and commercial nonprofit treatment facilities. The X:BOT study recruited participants during 2014-2016 from 8 sites with inpatient and outpatient services. The EMMES Corporation provided deidentified, limited investigators’ data sets. We performed the data analysis from October 1, 2019, to November 1, 2022. This study followed the Consolidate Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline, and the analysis protocol was determined by the New York State Psychiatric Institute institutional review board to be not human participant research and waived the need for informed consent. Subsequent harmonization and modeling were verified by 2 independent teams of statisticians, coded, and shown to be consistent across 2 sites.
Study Population
The studies enrolled individuals who met criteria for the DSM-IV-TR definition of opioid dependence (START, POATS) or the DSM-5 definition of OUD (X:BOT). All 3 trials enrolled adult participants older than 18 years, with broad pragmatic inclusion and few exclusion criteria. START was most inclusive, whereas POATS was the most restrictive, with a cohort composed of only participants who primarily used prescription opioids, excluding individuals with predominant or exclusive heroin use. The X:BOT study only excluded individuals who, at the time of randomization, received methadone treatment.
Trial Designs
The START study randomized individuals to open-label methadone or buprenorphine treatment over 24 weeks. Buprenorphine dose ranged from 2 to 8 mg at treatment initiation to 32 mg maximum, with a mean daily dose of 22 mg. Methadone dose had a 30-mg initial maximum with no specific ultimate maximum, with a daily mean dose of 93 mg.
POATS had 2 phases. In phase 1, individuals started receiving buprenorphine and were randomly assigned to 2 types of psychotherapy programs: standard medical management (SMM), which was a manual-based brief intervention; or SMM plus opioid dependence counseling, which provided additional sessions with specialist counselors. Buprenorphine dose was decreased to 0 mg during weeks 3 to 4. By week 12, approximately 7% of participants had maintained abstinence or near abstinence from opioids, and the rest were offered entry into phase 2. In phase 2, participants were randomized again to SMM alone or SMM plus opioid dependence counseling and followed up to 24 weeks with buprenorphine maintenance. For the harmonization process, UDS data from entry into the phase 2 study were lined up, week by week, to the start of the other 2 trials for 2 reasons. Phase 1 was a buprenorphine taper with a low success rate and is not directly comparable with the other 2 studies, and phase 2 had at least 12 weeks of weekly UDS data after buprenorphine induction, similar to the other 2 studies. Demographic characteristics and other drug use predictors were documented at the beginning of phase 1 to reflect patient characteristics at treatment entry.
For X:BOT, individuals were initially screened at inpatient sites and underwent pragmatic opioid withdrawal procedures and outpatient treatment over 24 weeks. Extended-release injection naltrexone was scheduled to be administered every 28 days with a fixed dose of 380 mg; missed doses resulted in a rechallenge dose of oral naltrexone to ascertain no active opioid use prior to reinitiating XR-NTX. Buprenorphine was initiated at 4 mg and dosed up to 24 mg.
Outcomes
Return to use was defined by 4 consecutive opioid use weeks, similar to the definition in X:BOT. An opioid use week was defined by either a UDS positive for any nonprescribed opioid or a UDS that was missing (ie, the participant either did not show for their scheduled study visit or refused to provide a urine sample) between weeks 3 and 12 after randomization.
Statistical Analysis
Harmonization of Predictors
The collection of case report forms (>2000 variable columns) was surveyed to generate the final set of predictors using 3 criteria: (1) conceptually equivalent data existed in all 3 studies (eg, UDS in all 3 studies), (2) more than 100 participants had the predictor recorded in all studies, and (3) the predictor had some clinical plausibility for predicting treatment outcome. The predictor domains included demographic characteristics (age, sex, and race and ethnicity), TLFB of drug use in the 28 days prior to treatment entry, UDS prior to randomization, UDS in the first 3 weeks after starting medication, withdrawal symptoms, risky behaviors (eg, sharing of injection equipment), chronic pain, medical and psychiatric diagnoses, vital signs, Addiction Severity Index individual items, and treatment group assignment (5 treatment groups, including START: buprenorphine; START: methadone; POATS: buprenorphine; X:BOT: XR-NTX; and X:BOT: buprenorphine) (Table 1). Race and ethnicity, which were self-reported by individual participants and recorded by study staff, were included as part of the standard survey of demographic characteristics for clinical trials for OUD studies.
Table 1. Predictors of Return to Use by Week 12 After Randomization Using the Entire Sample.
| Predictor | No. (%) | Association with return to usea | |||
|---|---|---|---|---|---|
| Total (N = 2199)b | Returned to use (n = 1096) | Not returned to use (n = 1103) | Test | P valuec | |
| Sex | |||||
| Male | 1471 (66.9) | 755 (68.9) | 716 (64.9) | χ21 = 2.2 | .14 |
| Female | 728 (33.1) | 341 (31.1) | 387 (35.1) | ||
| Age (range, 18-67 y), mean (SD), y | 35.3 (10.7) | 35.7 (10.4) | 35.0 (10.6) | z = −0.53 | .60 |
| Race | |||||
| African American | 175 (7.8) | 99 (9.0) | 76 (6.9) | χ22 = 15.4 | <.001d |
| White | 1653 (70.4) | 769 (70.2) | 884 (80.1) | ||
| Othere | 371 (14.7) | 228 (20.8) | 143 (13.0) | ||
| Hispanic ethnicity | 323 (14.7) | 182 (16.6) | 141 (12.8) | χ21 = 2.5 | .11 |
| Self-reported substance use in past 28 d (range, 0-28 d) | |||||
| Alcohol | 2.1 (5.4) | 1.7 (4.7) | 2.4 (6.1) | z = −2.30 | .02 |
| Alcohol intoxication | 1.4 (4.8) | 1.1 (4.0) | 1.7 (5.3) | z = −2.14 | .03 |
| Cannabis | 3.6 (8.1) | 3.2 (7.7) | 4.1 (8.5) | z = −1.47 | .14 |
| Cocaine | 2.7 (6.3) | 3.1 (6.6) | 2.4 (5.8) | z = 1.60 | .11 |
| Amphetamine | 0.7 (3.1) | 0.8 (3.4) | 0.6 (2.7) | z = 2.09 | .04 |
| Heroin | 18.5 (11.9) | 21.5 (10.4) | 15.7 (12.6) | z = 6.68 | <.001d |
| Methadone | 1.5 (5.0) | 1.3 (4.8) | 1.6 (5.1) | z = −1.14 | .26 |
| Prescription opioids | 6.9 (10.9) | 4.5 (9.4) | 9.2 (11.8) | z = −4.64 | <.001d |
| Prescription benzodiazepine | 1.2 (4.0) | 1.0 (3.9) | 1.2 (4.2) | z = 0.45 | .65 |
| Urine drug screen | |||||
| Amphetamine | |||||
| Negative | 2100 (95.5) | 1034 (94.3) | 1066 (96.7) | χ21 = 3.8 | .051 |
| Positive | 98 (4.5) | 61 (5.6) | 37 (3.3) | ||
| Benzodiazepine | |||||
| Negative | 1709 (77.7) | 851 (77.7) | 858 (77.8) | χ21 = 1.7 | .19 |
| Positive | 490 (22.3) | 245 (22.3) | 245 (22.2) | ||
| Methadone | |||||
| Negative | 1759 (80.0) | 863 (78.7) | 896 (81.2) | χ21 = 0.7 | .40 |
| Positive | 440 (20.0) | 233 (21.3) | 207 (18.8) | ||
| Oxycodone | |||||
| Negative | 1768 (80.4) | 933 (85.1) | 835 (75.7) | χ21 = 4.7 | .03 |
| Positive | 430 (19.6) | 162 (14.8) | 268 (24.3) | ||
| Cocaine | |||||
| Negative | 1667 (75.8) | 755 (68.9) | 912 (82.7) | χ21 = 25.0 | <.001d |
| Positive | 532 (24.2) | 341 (31.1) | 191 (17.3) | ||
| Methamphetamine | |||||
| Negative | 2069 (94.1) | 1007 (91.9) | 1062 (96.3) | χ21 = 11.2 | <.001d |
| Positive | 129 (5.9) | 89 (8.1) | 40 (3.6) | ||
| Morphine | |||||
| Negative | 804 (36.6) | 293 (26.7) | 511 (46.3) | χ21 = 40.0 | <.001d |
| Positive | 1395 (63.4) | 803 (73.3) | 592 (53.7) | ||
| Cannabis | |||||
| Negative | 1731 (78.7) | 862 (78.7) | 869 (78.8) | χ21 = 0.1 | .76 |
| Positive | 468 (21.3) | 234 (21.3) | 234 (21.2) | ||
| Opioid withdrawal level (range, 1-4), mean (SD)f | 2.6 (0.7) | 2.6 (0.7) | 2.6 (0.7) | z = −0.58 | .56 |
| Nicotine dependence (range, 0-10)g | 3.8 (2.6) | 3.8 (2.6) | 3.7 (2.7) | z = 0.39 | .69 |
| Substance use disorder in the past year | |||||
| Alcohol | |||||
| No | 1709 (77.7) | 870 (79.4) | 839 (76.1) | χ22 = 6.7 | .04 |
| Yes | 460 (20.9) | 209 (19.1) | 251 (22.8) | ||
| Missing | 30 (1.4) | 17 (1.5) | 13 (1.2) | ||
| Amphetamine | |||||
| No | 1909 (86.8) | 939 (85.7) | 970 (87.9) | χ22 = 1.9 | .38 |
| Yes | 258 (11.7) | 138 (12.6) | 120 (10.9) | ||
| Missing | 32 (1.5) | 19 (1.7) | 13 (1.2) | ||
| Cannabis | |||||
| No | 1727 (78.5) | 868 (79.2) | 859 (77.9) | χ22 = 0.8 | .66 |
| Yes | 440 (20.0) | 209 (19.1) | 231 (20.9) | ||
| Missing | 32 (1.5) | 19 (1.7) | 13 (1.2) | ||
| Cocaine | |||||
| No | 1564 (71.1) | 749 (68.3) | 815 (73.9) | χ22 = 1.2 | .54 |
| Yes | 603 (27.4) | 328 (29.9) | 275 (24.9) | ||
| Missing | 32 (1.5) | 19 (1.7) | 13 (1.2) | ||
| Sedatives | |||||
| No | 1797 (81.7) | 906 (82.7) | 891 (80.8) | χ22 = 0.6 | .75 |
| Yes | 370 (16.8) | 171 (15.6) | 199 (18.0) | ||
| Missing | 32 (1.5) | 19 (1.7) | 13 (1.2) | ||
| Risky behavior | |||||
| Heroin injection past 30 d | |||||
| No | 942 (42.8) | 362 (33.0) | 580 (52.6) | χ22 = 30.6 | <.001d |
| Yes | 1255 (57.1) | 733 (66.9) | 522 (47.3) | ||
| Shared needle | |||||
| No | 1903 (86.5) | 947 (86.4) | 956 (86.7) | χ22 = 1.2 | .55 |
| Yes | 277 (12.6) | 140 (12.8) | 137 (12.4) | ||
| Missing | 19 (0.9) | 9 (0.8) | 10 (0.9) | ||
| Homosexual behavior | |||||
| No | 2113 (96.1) | 1061 (96.8) | 1052 (95.4) | χ22 = 4.5 | .11 |
| Yes | 56 (2.5) | 21 (1.9) | 35 (3.2) | ||
| Missing | 30 (1.4) | 14 (1.3) | 16 (1.4) | ||
| Not monogamous relationship | |||||
| No | 1925 (87.5) | 960 (87.6) | 965 (87.5) | χ22 = 0.8 | .68 |
| Yes | 244 (11.1) | 122 (11.1) | 122 (11.1) | ||
| Missing | 30 (1.4) | 14 (1.3) | 16 (1.4) | ||
| Pain (moderate or severe) | |||||
| No | 1073 (48.8) | 547 (49.9) | 526 (47.7) | χ22 = 2.0 | .37 |
| Yes | 1104 (50.2) | 534 (48.7) | 570 (51.7) | ||
| Missing | 22 (1.0) | 15 (1.4) | 7 (0.6) | ||
| Medical historyh | |||||
| Neurologic injury | |||||
| No | 1949 (88.6) | 992 (90.5) | 957 (86.8) | χ21 = 3.3 | .07 |
| Yes | 247 (11.2) | 102 (9.3) | 145 (13.1) | ||
| Epilepsy or seizure disorder | |||||
| No | 2101 (95.5) | 1050 (95.8) | 1051 (95.3) | χ21 = 0.1 | .78 |
| Yes | 98 (4.5) | 46 (4.2) | 52 (4.7) | ||
| Schizophrenia | |||||
| No | 2155 (98.0) | 1070 (97.6) | 1085 (98.4) | χ21 = 0.2 | .65 |
| Yes | 40 (1.8) | 24 (2.2) | 16 (1.5) | ||
| Bipolar disorder | |||||
| No | 1950 (88.7) | 970 (88.5) | 980 (88.9) | χ21 = 0.01 | .93 |
| Yes | 247 (11.2) | 125 (11.4) | 122 (11.1) | ||
| Anxiety or panic disorder | |||||
| No | 1436 (65.3) | 748 (68.3) | 688 (62.4) | χ21 = 4.7 | .03 |
| Yes | 762 (34.7) | 348 (31.7) | 414 (37.5) | ||
| Major depression | |||||
| No | 1538 (69.9) | 801 (73.1) | 737 (66.8) | χ21 = 7.3 | .007 |
| Yes | 661 (30.1) | 295 (26.9) | 366 (33.2) | ||
| Vital signsi | |||||
| Blood pressure | |||||
| Systolic (range, 76-187 mm Hg), mean (SD) | 121.2 (16.0) | 120.6 (15.9) | 121.9 (16.1) | z = −1.24 | .22 |
| Diastolic (range, 42-120 mm Hg), mean (SD) | 77.0 (11.1) | 76.5 (11.2) | 77.4 (11.0) | z = −2.44 | .02 |
| Weight (range, 80-362 lb), mean (SD) | 170.7 (38.7) | 169.5 (37.9) | 171.8 (39.3) | z = −0.79 | .43 |
| ASI-Lite | |||||
| Education | |||||
| Less median school graduate (<12 y) | 232 (10.6) | 86 (7.9) | 146 (13.2) | χ23 = 3.6 | .31 |
| Above median school graduate (12 y) | 437 (19.9) | 178 (16.2) | 259 (23.5) | ||
| College (13-22 y) | 424 (19.3) | 170 (15.5) | 254 (23.0) | ||
| Missing | 1106 (50.3) | 662 (60.4) | 444 (40.3) | ||
| Marital status | |||||
| Married | 183 (8.3) | 74 (6.8) | 109 (9.9) | χ23 = 3.0 | .39 |
| Widow, separated, or divorced | 248 (11.3) | 96 (8.8) | 152 (13.8) | ||
| Never married | 660 (30.0) | 264 (24.1) | 396 (35.9) | ||
| Missing | 1108 (50.4) | 662 (60.4) | 446 (40.4) | ||
| Unemployed past 3 y | |||||
| No | 839 (38.2) | 321 (29.3) | 518 (47.0) | χ22 = 2.6 | .28 |
| Yes | 253 (11.5) | 113 (10.3) | 140 (12.7) | ||
| Missing | 1107 (50.3) | 662 (60.4) | 445 (40.3) | ||
| Living with someone with alcohol problems | |||||
| No | 969 (44.1) | 389 (35.5) | 580 (52.6) | χ22 = 3.7 | .16 |
| Yes | 111 (5.0) | 39 (3.6) | 72 (6.5) | ||
| Missing | 1119 (50.9) | 668 (60.9) | 451 (40.9) | ||
| Living with someone with drug problems | |||||
| No | 860 (39.1) | 339 (30.9) | 521 (47.2) | χ22 = 1.8 | .41 |
| Yes | 220 (10.0) | 89 (8.1) | 131 (11.9) | ||
| Missing | 1119 (50.9) | 668 (61.0) | 451 (40.9) | ||
| Alcohol treatment lifetime | |||||
| No | 926 (42.1) | 374 (34.1) | 552 (50.1) | χ22 = 1.8 | .41 |
| Yes | 150 (6.8) | 56 (5.1) | 94 (8.5) | ||
| Missing | 1123 (51.1) | 666 (60.8) | 457 (41.4) | ||
| Drug treatment lifetime | |||||
| No | 267 (12.1) | 77 (7.0) | 190 (17.2) | χ22 = 4.9 | .09 |
| Yes | 818 (37.2) | 357 (32.6) | 461 (41.8) | ||
| Missing | 1114 (50.7) | 662 (60.4) | 452 (41.0) | ||
| Unstable living arrangement past 3 y | |||||
| No | 1046 (47.6) | 414 (37.8) | 632 (57.3) | χ22 = 1.6 | .44 |
| Yes | 46 (2.1) | 20 (1.8) | 26 (2.4) | ||
| Missing | 1107 (50.3) | 662 (60.4) | 445 (40.3) | ||
| Physical or sexual abuse | |||||
| No | 627 (28.5) | 258 (23.5) | 369 (33.5) | χ22 = 5.8 | .054 |
| Yes | 459 (20.9) | 172 (15.7) | 287 (26.0) | ||
| Missing | 1113 (50.7) | 666 (60.8) | 447 (40.5) | ||
| Days of serious family conflict past 30 d | |||||
| 0 | 698 (31.7) | 291 (26.5) | 407 (36.9) | χ23 = 5.4 | .15 |
| 1-10 | 240 (10.9) | 88 (8.0) | 152 (13.8) | ||
| 11-30 | 143 (6.5) | 51 (4.7) | 92 (8.3) | ||
| Missing | 1118 (50.8) | 666 (60.8) | 452 (41.0) | ||
| Serious thought of suicide | |||||
| No | 820 (37.3) | 325 (29.6) | 495 (44.9) | χ22 = 3.0 | .22 |
| Yes | 272 (12.4) | 108 (9.9) | 164 (14.9) | ||
| Missing | 1107 (50.3) | 663 (60.5) | 444 (40.2) | ||
| Treatment | |||||
| CTN-0027 buprenorphine | 740 (33.7) | 467 (42.6) | 273 (24.8) | χ24 = 135.1 | <.001d |
| CTN-0027 methadone | 529 (24.1) | 282 (25.7) | 247 (22.4) | ||
| CTN-0030 buprenorphine | 360 (16.4) | 102 (9.3) | 258 (23.4) | ||
| CTN-0051 buprenorphine | 287 (13.1) | 105 (9.6) | 182 (16.5) | ||
| CTN-0051 XR-NTX | 283 (12.9) | 140 (12.8) | 143 (13.0) | ||
Abbreviations: ASI-Lite, Addiction Severity Index Lite; CTN, Clinical Trials Network; XR-NTX, extended-release naltrexone.
Five treatment groups were controlled in the bivariate models.
Smaller than total sample size reflected some participants had missing values and were excluded.
Predictors were assessed at treatment entry.
Indicates Bonferroni correction of the listed P value at original α = .05 (69 tests total).
Other race included American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, other (including Iranian, Middle Eastern), more than 1 race, and unknown.
By Clinical Opiate Withdrawal Scale or Subjective Opiate Withdrawal Scale, with 1 indicating a little and 4 extremely. Data missing for 11 participants.
By Fagerström Test for Nicotine Dependence, with 1 indicating low dependence and 10 high dependence. Data missing for 8 participants.
Zero to 4 participants were missing information.
Four participants were missing information.
Model Construction, Testing, and Dissemination
The harmonized data were divided into a training set (1650 [75%]) and a testing set (549 [25%]). The testing set was randomly selected, stratifying for treatment group assignment (5 treatment groups) and set aside during model development. Predictive performance was constructed using the area under the receiver operating characteristic curve (AUROC) on the testing set. Least absolute shrinkage and selection operator (LASSO) regression was used for variable selection, which automatically conducted internal cross-validation in choosing tuning parameters.16 To correct for bias introduced by penalization in LASSO variable selection, the final calibrated risk score was constructed by refitting a logistic regression model with variables selected by LASSO. If more than 15 participants had missing information for a particular variable, a dummy value was created (“missing”). If fewer than 15 participants had missing values, they were removed prior to the LASSO procedure (37 [1.7%] from the full sample of predictors). The web-based portal http://www.oudriskscore.org was developed using the R Shiny package, version 1.7.0 or greater using elements of the flexdashboard, knitr, tidyverse, shinyvalidate, and caret packages. The statistical analysis was performed using R, version 4.3.1 software (R Foundation for Statistical Computing). Further details and access to technical support are provided in the eMethods in Supplement 1.
Results
Predictor Characteristics
The sample included 2199 participants across the 3 studies (mean [SD] age, 35.3 [10.7] years; 728 female [33.1%] and 1471 male [66.9%]; and 175 African American [7.8%], 323 Hispanic [14.7%], 1653 White [70.4%], and 371 other race and ethnicity, including American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, other [including Iranian, Middle Eastern], more than 1 race, and uknown [14.7%]). Univariable characteristics of the predictors are shown in Table 1. African American and other race; more self-reported days of heroin use; fewer self-reported days of prescription opioid use; UDS results positive for cocaine, methamphetamine, and morphine; and positive heroin injection status in the past 30 days were associated with return to use. Positive UDS results for nonprescribed opioids (oxycodone, morphine [indicating heroin and other opioids with morphine as a metabolite], nonprescribed methadone, and nonprescribed buprenorphine) or missing UDS in any of the first 3 weeks of treatment were associated with return to use (Table 2).
Table 2. Urine Drug Screen (UDS) in the First 3 Weeks as Predictors of Return to Use.
| Variable | No. (%) | Association with return to usea | |||
|---|---|---|---|---|---|
| Total (N = 2199) | Returned to use (n = 1096) | Not returned to use (n = 1103) | χ22 | P valueb | |
| Opioids first week | |||||
| Negative | 789 (35.9) | 212 (19.3) | 577 (52.3) | 197.9 | <.001 |
| Positive | 606 (27.6) | 377 (34.4) | 229 (20.8) | ||
| Missing | 804 (36.6) | 507 (46.3) | 297 (26.9) | ||
| Opioids second week | |||||
| Negative | 961 (43.7) | 227 (20.7) | 734 (66.5) | 367.5 | <.001 |
| Positive | 650 (29.6) | 433 (39.5) | 217 (19.7) | ||
| Missing | 588 (26.7) | 436 (39.8) | 152 (13.8) | ||
| Opioids third week | |||||
| Negative | 981 (44.6) | 206 (18.8) | 775 (70.3) | 464.0 | <.001 |
| Positive | 562 (25.6) | 387 (35.3) | 175 (15.9) | ||
| Missing | 656 (29.8) | 503 (45.9) | 153 (13.9) | ||
| Oxycodone first week | |||||
| Negative | 1312 (59.7) | 541 (49.4) | 771 (69.9) | 91.3 | <.001 |
| Positive | 83 (3.8) | 48 (4.4) | 35 (3.2) | ||
| Missing | 804 (36.6) | 507 (46.3) | 297 (26.9) | ||
| Oxycodone second week | |||||
| Negative | 1490 (67.8) | 593 (54.1) | 897 (81.3) | 173.2 | <.001 |
| Positive | 121 (5.5) | 67 (6.1) | 54 (4.9) | ||
| Missing | 588 (26.7) | 436 (39.8) | 152 (13.8) | ||
| Oxycodone third week | |||||
| Negative | 1436 (65.3) | 533 (48.6) | 903 (81.9) | 235.0 | <.001 |
| Positive | 107 (4.9) | 60 (5.5) | 47 (4.3) | ||
| Missing | 656 (29.8) | 503 (45.9) | 153 (13.9) | ||
| Heroin first week | |||||
| Negative | 948 (43.1) | 294 (26.8) | 654 (59.3) | 172.6 | <.001 |
| Positive | 447 (20.3) | 295 (26.9) | 152 (13.8) | ||
| Missing | 804 (36.6) | 507 (46.3) | 297 (26.9) | ||
| Heroin second week | |||||
| Negative | 1084 (49.3) | 285 (26.0) | 799 (72.4) | 358.3 | <.001 |
| Positive | 527 (24.0) | 375 (34.2) | 152 (13.8) | ||
| Missing | 588 (26.7) | 436 (39.8) | 152 (13.8) | ||
| Heroin third week | |||||
| Negative | 1084 (49.3) | 256 (23.4) | 828 (75.1) | 454.5 | <.001 |
| Positive | 459 (20.9) | 337 (30.7) | 122 (11.1) | ||
| Missing | 656 (29.8) | 503 (45.9) | 153 (13.9) | ||
| Methadone first week | |||||
| Negativec | 1281 (58.3) | 523 (47.7) | 758 (68.7) | 78.6 | <.001 |
| Positive | 113 (5.1) | 65 (5.9) | 48 (4.4) | ||
| Missing | 805 (36.6) | 508 (46.4) | 297 (26.9) | ||
| Methadone second week | |||||
| Negative | 1536 (69.8) | 614 (56.0) | 922 (83.6) | 160.0 | <.001 |
| Positive | 74 (3.4) | 46 (4.2) | 28 (2.5) | ||
| Missing | 589 (26.8) | 436 (39.8) | 153 (13.9) | ||
| Methadone third week | |||||
| Negative | 1501 (68.3) | 566 (51.6) | 935 (84.8) | 217.7 | <.001 |
| Positive | 42 (1.9) | 27 (2.5) | 15 (1.4) | ||
| Missing | 656 (29.8) | 503 (45.9) | 153 (13.9) | ||
| Buprenorphine first week | |||||
| Negatived | 363 (16.5) | 132 (12.0) | 231 (20.9) | 15.0 | <.001 |
| Positive | 26 (1.2) | 14 (1.3) | 12 (1.1) | ||
| Missing | 1810 (82.3) | 950 (86.7) | 860 (78.0) | ||
| Buprenorphine second week | |||||
| Negative | 393 (17.9) | 118 (10.8) | 275 (24.9) | 75.1 | <.001 |
| Positive | 6 (0.3) | 1 (0.1) | 5 (0.5) | ||
| Missing | 1800 (81.9) | 977 (89.1) | 823 (74.6) | ||
| Buprenorphine third week | |||||
| Negative | 393 (17.9) | 104 (9.5) | 289 (26.2) | 111.4 | <.001 |
| Positive | 8 (0.4) | 3 (0.3) | 5 (0.4) | ||
| Missing | 1798 (81.8) | 989 (90.2) | 809 (73.4) | ||
Five treatment groups were controlled in the bivariate models.
All tests listed indicated statistical significance after Bonferroni correction of the listed P values at original α = .05 (15 total tests).
Methadone-positive results were defined as a positive UDS for nonprescribed methadone. In the Clinical Trials Network (CTN)-0027 Starting Treatment With Agonist Replacement Therapies methadone treatment group, this variable was coded negative.
Buprenorphine-positive results were defined as a positive UDS result for nonprescribed buprenorphine. CTN-0027 did not have a UDS for buprenorphine, which explained many missing entries for this variable.
Predictive Models With Predictors at Treatment Entry
The LASSO procedure consistently selected 4 predictors at treatment entry (Table 3, model 1), including days of heroin use in the past month, morphine-positive UDS results, any injection heroin use in the past 30 days, and cocaine-positive UDS results, and did not select any interaction terms. We refitted a logistic regression model (Table 3, model A) as the final model at treatment entry for the testing set, yielding a testing set performance AUROC of 0.67 (95% CI, 0.62-0.71). Separately including treatment group assignment did not improve model performance.
Table 3. Performance of Predictive Models for Opioid Return to Use.
| LASSO model, coefficienta | Logistic models on training set, OR (95% CI)b | ||||
|---|---|---|---|---|---|
| (1) Treatment entry predictors only | (2) Treatment entry predictors and opioid use first 3 wk | (A) Treatment entry variables | (B) Opioid use first 3 wk | (C) Treatment entry variables, opioid use first 3 wk | |
| Treatment entry | |||||
| Heroin use (0-28 d) | 0.0223 | 0.0151 | 1.02 (1.01-1.04) | NA | 1.02 (1.00-1.03) |
| Positive UDS result for morphine | 0.2645 | NA | 1.54 (1.19-2.00) | NA | 1.03 (0.76-1.38) |
| Heroin injection | 0.0505 | NA | 1.28 (0.98-1.67) | NA | 1.13 (0.82-1.56) |
| Positive UDS result for cocaine | 0.2763 | 0.0687 | 1.64 (1.26-2.14) | NA | 1.25 (0.91-1.71) |
| Week 1: negative (reference) | |||||
| Positive | NA | 0.4894 | NA | 3.72 (2.59-5.36) | 3.34 (2.30-4.83) |
| Missing | NA | 0.5202 | NA | 3.81 (2.71-5.37) | 3.50 (2.47-4.95) |
| Week 2: negative (reference) | |||||
| Positive | NA | 0.8396 | NA | 3.58 (2.57-4.99) | 3.34 (2.39-4.66) |
| Missing | NA | 1.2467 | NA | 7.18 (5.04-10.21) | 6.87 (4.83-9.79) |
| Week 3: negative (reference) | |||||
| Positive | NA | 1.0226 | NA | 4.36 (3.11-6.09) | 4.13 (2.94-5.81) |
| Missing | NA | 1.4181 | NA | 8.31 (5.94-11.63) | 7.72 (5.51-10.83) |
| Log likelihood (df) | NA | NA | −1062.10 (6) | −781.52 (9) | −772.18 (13) |
| Likelihood ratio test with model C | NA | NA | χ27 = 579.84 | χ211 = 18.68 | NA |
| χ2 P value | NA | NA | <.001 | .002 | NA |
| AUROC (95% CI) on testing setsc | 0.67 (0.62-0.71) | 0.81 (0.77-0.85) | 0.68 (0.63-0.72) | 0.82 (0.78-0.85) | 0.82 (0.78-0.85) |
Abbreviations: AUROC, area under the receiver operating characteristic curve; LASSO, least absolute shrinkage and selection operator; NA, not applicable; OR, odds ratio; UDS, urine drug screen.
Model coefficients were derived from the training set only. Predictive performance of the models was estimated with the AUROC using the testing set. Thirty-seven participants were excluded for the following reasons: missing any information on timeline follow-back (TLFB), UDS, heroin injection, withdrawal, nicotine dependence, and physical and mental condition.
Three participants were excluded for missing information on TLFB or heroin injection; treatment site was treated as a random effect in the logistic regression model.
Training AUROC and details of fitting the LASSO model are provided in the eMethods in Supplement 1. Effects of UDSs missing or positive were assumed additive across weeks, including 1 additional parameter for all 3 weeks with negative results.
Predictive Model Using UDS Results in the First 3 Weeks
We next constructed a LASSO model using both predictors at treatment entry and UDS data in the first 3 weeks of treatment. All 3 weeks were selected (Table 3, model 2). These predictors were refitted and calibrated using standard logistic regression (Table 3, models B and C). Model B (with only the first 3 weeks of UDS as predictors) and model C (combining the first 3 weeks of UDS and treatment entry predictors) had equivalent predictive performance on the testing data set (both AUROC, 0.82; 95% CI, 0.78-0.85). Therefore, the simpler model B was chosen as the final disseminated model.
We performed several sensitivity analyses. Including the treatment group assignment and the interaction of treatment group by predictor did not improve predictive performance (eTable 2 in Supplement 1). The model predicting probability of return to use corresponded well with the actual proportion of return to use in the testing data set, indicating a well-calibrated model (Figure; eTable 3 in Supplement 1). Performance of the models B and C did not change when the outcomes were calculated using both UDS and TLFB data or when UDS data were imputed using TLFB (ie, any reported use or missing visit was counted as a use week). Performance also did not change with the inclusion of UDS of nonopioid substances, such as cocaine and methamphetamine, in the first 3 weeks.
Figure. Estimated Probability of Return to Use by Patterns of Urine Drug Screen (UDS) Results for Any Nonprescribed Opioid in the First 3 Weeks of Treatment.
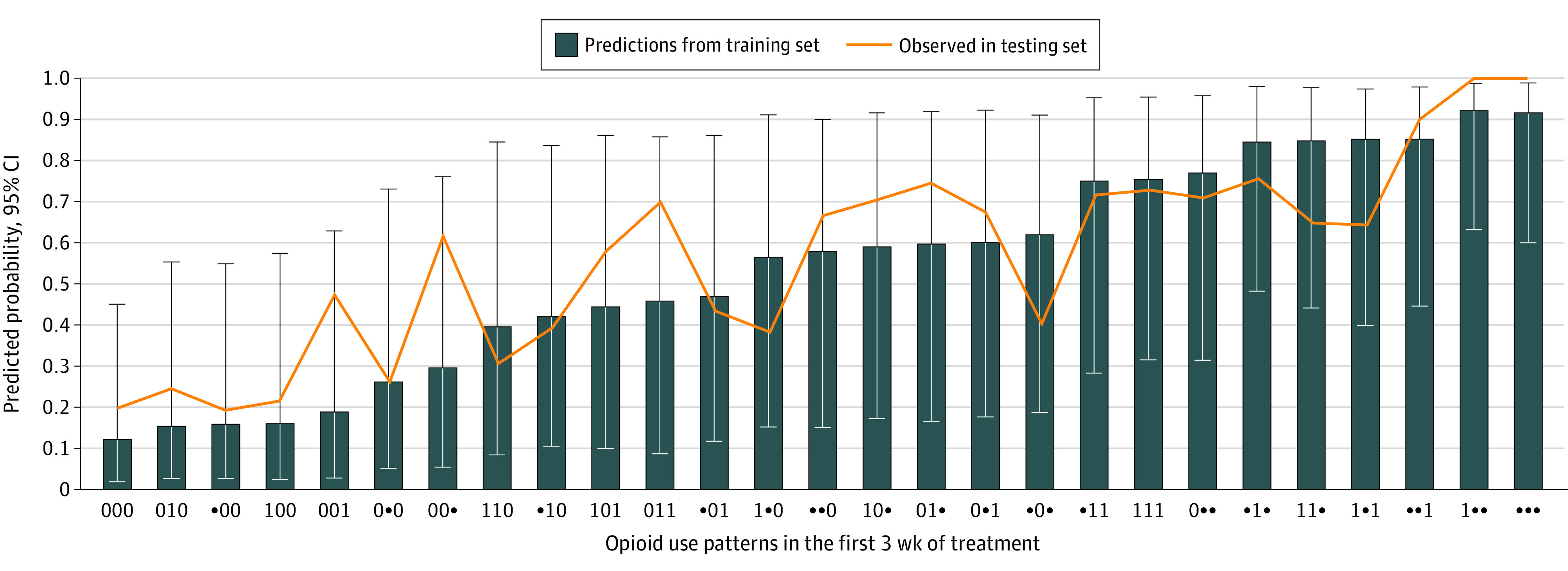
Training set values are in ascending order of probability of return to opioid use. The correlation coefficient of the training and testing sets was 0.84.
Aiming at straightforward clinical applicability, we constructed the CTN-94 OUD Return-to-Use Risk Score (CORRS) based on a single metric: the total number of weeks in the first 3 weeks in which a patient had a UDS positive for any nonprescribed opioids or missing (range, 0-3) (Table 4). If a patient had 0 missing or opioid-positive UDS results in the first 3 weeks, the predicted return-to-use probability was 0.13 (range, 0.02-0.47). The risk of return to use increased monotonically as CORRS increased. When 3 of 3 UDSs were either missing or positive for opioids, the probability of return to use at week 12 was 0.85 (range, 0.43-0.97). The testing set AUROC for this simplified risk score was 0.80 (95% CI, 0.76-0.84).
Table 4. Clinical Trials Network-0094 Opioid Use Disorder Return-to-Use Risk Scorea.
| No. of weeks using opioids in the first 3 wkb | OR (95% CI) | Predicted risk of return to use on training data (95% CI) | Observed returned to use on testing set, % (No. of total) |
|---|---|---|---|
| 0 | 1 [Reference] | 0.13 (0.02-0.47) | 20 (21 of 105) |
| 1 | 1.91 (1.26-2.92) | 0.22 (0.04-0.65) | 29 (45 of 156) |
| 2 | 7.52 (4.89-11.58) | 0.53 (0.13-0.89) | 52 (48 of 93) |
| 3 | 38.47 (25.29-58.52) | 0.85 (0.43-0.97) | 80 (156 of 194) |
Predicted risk on training set and observed risk on testing set, N = 2199.
Indicated by opioid-positive or missing urine drug screen results. Four participants with missing information on heroin use and injection were excluded, and treatment site was treated as a random effect in the logistic regression model.
Treatment Group Assignment and Treatment-by-Predictor Interactions
The predictive performance of models with treatment group assignment did not improve (eTable 2 in Supplement 1). The LASSO procedure did not select either treatment group assignment or the interaction terms as significant predictors. Sensitivity testing with additional models did not yield improvement for positive UDS results for nonprescribed opioids in the first 3 weeks and treatment group assignment interactions (AUROC, 0.82; 95% CI, 0.78-0.85) or for the sum of all positive UDS results in the first 3 weeks and treatment group assignment interactions (AUROC, 0.82; 95% CI, 0.78-0.85). Finally, the boosted tree algorithm (XGboost open-source software) that automatically detects possible subgroup interactions showed no improvement at treatment entry (AUROC, 0.69; 95% CI, 0.64-0.73) or predictors at week 3 (AUROC, 0.82; 95% CI, 0.78-0.85).
Discussion
In this decision analytical model, we harmonized data from 3 large, pragmatic, clinical trials of community-based medication treatment for OUD, including drug treatment sites, physician practices, and inpatient treatment facilities, and built predictive models that estimated risk of return to opioid use by week 12. A small number of predictors at treatment entry were informative, replicating a number of previous subgroup analyses.17 The most informative predictor was UDS results in the first 3 weeks. Participants who provided 3 negative urine tests had a low risk of return to use (13%), whereas those who had 3 out of 3 positive or missing tests had a high risk (85%). The individual-level prediction models had an overall out-of-sample AUROC of 0.82, which is considerably better than chance, and performed similarly well across the different treatment groups. Clinicians might use the OUD risk score calculator disseminated online (eMethods in Supplement 1) or the simplified CORRS (Table 4) offline.
Early achievement of remission is well known to predict ongoing treatment success. Adding to existing knowledge, our results quantify this association by indicating how likely a patient is to return to opioid use. This finding might be universal: Individuals across different treatment settings, whether predominantly using prescription opioids or heroin and who were randomized to any medication, would have low return-to-use risk if they are able to sustain abstinence early. Second, replicating previous findings, the models selected treatment entry predictors that reflect OUD severity.18 Demographic predictors showed small, inconsistent associations: African American and other (non-White) race were associated with a higher risk, but the association was not selected by the LASSO procedure. It is possible that these sociodemographic correlates of treatment response19 are highly treatment context–specific or share variance with other predictors, such as those that reflect severity. However, none of the Addiction Severity Index subcategories were selected as predictors. Therefore, more straightforward behavioral queries of addiction severity, such as days of use and self-reported intravenous use, might be sufficient and less cumbersome.
We chose our outcome (return to use) for clinicians to target high-risk patients as soon as possible during treatment. For buprenorphine, an obvious approach would be to increase and achieve a higher dose quickly. Recent computational studies showed that such dosing strategies can be optimized to achieve improved outcomes.20 Another approach is to switch to an extended-release injection formulation. For treatment with methadone, doses cannot be increased too quickly given safety considerations, but a higher target dose might be considered. For XR-NTX, additional oral naltrexone or increased dosing frequency might be considered. Poor initial response should also prompt an evaluation for potential drivers, such as psychosocial stress, social determinants of health, or co-occurring psychiatric disorders, and corresponding interventions and adjunctive medications, such as clonidine,21 lofexidine,22 and in some specific cases, mood stabilizers.23 In addition, our model suggests a quantitative clinical decision algorithm. If a patient misses the first couple of visits, clinicians can implement behavioral interventions, such as engaging significant others24 or involving peer navigators,25 to work with the patient based on an individualized risk threshold. Finally, consideration may be given to switching medications. Future trials could be designed to test these interventions by adaptively randomizing higher-risk patients.26 A sizable proportion showed early treatment dropout and would benefit from interventions that target retention, such as contingency management and intensive psychosocial interventions.27 One interesting finding was that the difference in predicted risk of return to use between 0 and 1 opioid-positive UDS result was smaller than the differences between 1 and 2. Therefore, the threshold for applying additional intervention could be set above 1 positive UDS result. Other derived binary outcomes, such as clinic attendance and sustained abstinence, could be considered in future studies, although they have less uniformity in definition in the literature and show more outcome imbalance; when only a small fraction had any given outcome, training algorithms for predictive models can become unstable.
Several other important practical considerations follow from our study results. First, performing UDSs for the first 3 weeks of treatment, even in primary care, would be useful in aiding personalized treatment decisions. These initial data points should be fully covered by insurance and not be associated with copays. Additional sensitivity analysis indicated that in practice, clinicians would not have to collect a urine sample at every visit but could infer an opioid-positive UDS result if the patient reported any opioid use in the prior week. Random UDSs (rather than sequenced UDSs) could be used as a verification. Our model could be broadly used in many clinical settings as part of a clinical decision support system in an electronic health record. A clinician who surveyed the drug use pattern at the 1-month follow-up appointment would receive, as the electronic health record output, a numeric risk estimate and can then adjust treatment according to clinical capacity and feasibility. Clinicians could also gather a rough estimate of elevated risk at treatment entry with an understanding that the precision of the prediction would improve as early UDS data become available. Our data suggest that by week 3, clinicians can forecast higher risks accurately without treatment entry data. Potentially, other context-specific social determinants of health, such as poverty, legal problems, and housing instability, that were not uniformly collected in the studies we harmonized could be incorporated into flexible predictive models to achieve more sophisticated clinical decision support.
Strengths and Limitations
This study has several strengths. It included a large sample, harmonized through the multiinstitutional CTN infrastructure. Consistent instruments were collected across all 3 studies, and the combination of several studies with diverse cohorts in both the training and testing sets enhanced model generalizability. Furthermore, predictors at treatment entry and UDS data were commonly collected and are practical and interpretable in clinical practice.
The study also had several limitations. An important limitation is that patients who sign up for a clinical trial may not be fully representative of those seeking treatment in the community.28 In addition, the 3 data sets came from distinct patient populations,29 and although the model results were similar across all 3, replication in other populations would be necessary to determine generalizability. The harmonizable study data covered only 12 weeks, a necessary first stage of achieving sustained abstinence. Future sequential models with longer follow-up could estimate timing and probability of return to use over a longer period. Two of the studies enrolled patients in 2006 (START and POAT), and while much of today’s MOUD treatment population could be captured by this harmonized data set, the rise of fentanyl use was not. Future studies are needed in contemporary populations with prevalent use of fentanyl or other high-potency synthetic opioid agonists.30 As with all individual-level predictive models, there might be substantial pitfalls due to algorithmic bias,31 as well as issues relative to the underrepresentation of demographic groups that might affect predictive fairness.29 Finally, using a variety of binary outcomes derived from UDSs (eg, full abstinence, longer or shorter duration of consecutive missing or positive results) could produce a family of predictive models and benefit from a generative modeling approach. Future studies will examine these aspects in further detail.
Conclusions
Through this decision analytical model, we developed a simple, clinically applicable individual-level return-to-use prediction method for people with OUD receiving treatment with buprenorphine, methadone, or XR-NTX. Future studies will aim to use this method to stratify individuals at high risk for return to use to evaluate novel therapeutic options and potentially improve on MOUD treatment outcomes.
eMethods
eTable 1. UDS Results for Nonopioid Substances in the First 3 Weeks After Randomization as Predictors of Return to Use Using the Entire Sample (N = 2199)
eTable 2. Performance of Predictive Models for Opioid Return To Use, Including Treatment Group Assignment as a Predictor
eTable 3. Predicted Probability of Return to Opioid Use by Opioid Use in the First 3 Weeks in the Logistic Model
eReferences
Data Sharing Statement
References
- 1.Korthuis PT, McCarty D, Weimer M, et al. Primary care–based models for the treatment of opioid use disorder: a scoping review. Ann Intern Med. 2017;166(4):268-278. doi: 10.7326/M16-2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakeman SE, Barnett ML. Primary care and the opioid-overdose crisis—buprenorphine myths and realities. N Engl J Med. 2018;379(1):1-4. doi: 10.1056/NEJMp1802741 [DOI] [PubMed] [Google Scholar]
- 3.Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatry. 2020;87(1):82-88. doi: 10.1016/j.biopsych.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 4.Hser YI, Evans E, Grella C, Ling W, Anglin D. Long-term course of opioid addiction. Harv Rev Psychiatry. 2015;23(2):76-89. doi: 10.1097/HRP.0000000000000052 [DOI] [PubMed] [Google Scholar]
- 5.Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GYH. Assessment of the CHA2DS2-VASc score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA. 2015;314(10):1030-1038. doi: 10.1001/jama.2015.10725 [DOI] [PubMed] [Google Scholar]
- 6.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835-842. doi: 10.1001/jama.284.7.835 [DOI] [PubMed] [Google Scholar]
- 7.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245-254. doi: 10.1056/NEJMoa1301851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med. 2016;375(1):65-74. doi: 10.1056/NEJMra1510061 [DOI] [PubMed] [Google Scholar]
- 9.Ciraulo DA, Piechniczek-Buczek J, Iscan EN. Outcome predictors in substance use disorders. Psychiatr Clin North Am. 2003;26(2):381-409. doi: 10.1016/S0193-953X(02)00106-5 [DOI] [PubMed] [Google Scholar]
- 10.Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi: 10.1016/j.drugalcdep.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss RD, Potter JS, Provost SE, et al. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemp Clin Trials. 2010;31(2):189-199. doi: 10.1016/j.cct.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606 [DOI] [PubMed] [Google Scholar]
- 14.Sobell LC, Sobell MB. Timeline follow-back. In: Litten RZ, Allen JP, eds. Measuring Alcohol Consumption. Springer; 1992:41-72. doi: 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- 15.Clinical Trials Network dissemination library. National Drug Abuse Treatment . Accessed February 20, 2023. http://ctndisseminationlibrary.org
- 16.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol. 1996;58(1):267-288. doi: 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- 17.Nunes EV Jr, Scodes JM, Pavlicova M, et al. Sublingual buprenorphine-naloxone compared with injection naltrexone for opioid use disorder: potential utility of patient characteristics in guiding choice of treatment. Am J Psychiatry. 2021;178(7):660-671. doi: 10.1176/appi.ajp.2020.20060816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunes EV, Pavlicova M, Hu MC, et al. Baseline matters: the importance of covariation for baseline severity in the analysis of clinical trials. Am J Drug Alcohol Abuse. 2011;37(5):446-452. doi: 10.3109/00952990.2011.596980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. 2018;108(2):182-186. doi: 10.2105/AJPH.2017.304187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudolph KE, Williams NT, Goodwin ATS, et al. Buprenorphine & methadone dosing strategies to reduce risk of relapse in the treatment of opioid use disorder. Drug Alcohol Depend. 2022;239:109609. doi: 10.1016/j.drugalcdep.2022.109609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowalczyk WJ, Phillips KA, Jobes ML, et al. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a randomized controlled trial with ecological momentary assessment. Am J Psychiatry. 2015;172(8):760-767. doi: 10.1176/appi.ajp.2014.14081014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorodetzky CW, Walsh SL, Martin PR, Saxon AJ, Gullo KL, Biswas K. A phase III, randomized, multi-center, double blind, placebo controlled study of safety and efficacy of lofexidine for relief of symptoms in individuals undergoing inpatient opioid withdrawal. Drug Alcohol Depend. 2017;176:79-88. doi: 10.1016/j.drugalcdep.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 23.Maremmani I, Pacini M, Lamanna F, et al. Mood stabilizers in the treatment of substance use disorders. CNS Spectr. 2010;15(2):95-109. doi: 10.1017/S1092852900027346 [DOI] [PubMed] [Google Scholar]
- 24.Sullivan MA, Bisaga A, Pavlicova M, et al. A randomized trial comparing extended-release injectable suspension and oral naltrexone, both combined with behavioral therapy, for the treatment of opioid use disorder. Am J Psychiatry. 2019;176(2):129-137. doi: 10.1176/appi.ajp.2018.17070732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galanter M, Dermatis H, Glickman L, et al. Network therapy: decreased secondary opioid use during buprenorphine maintenance. J Subst Abuse Treat. 2004;26(4):313-318. doi: 10.1016/j.jsat.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 26.Shulman M, Weiss R, Rotrosen J, Novo P, Costello E, Nunes EV. Prior National Drug Abuse Treatment Clinical Trials Network (CTN) opioid use disorder trials as background and rationale for NIDA CTN-0100 “optimizing retention, duration and discontinuation strategies for opioid use disorder pharmacotherapy (RDD)”. Addict Sci Clin Pract. 2021;16(1):15. doi: 10.1186/s13722-021-00223-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan B, Gean E, Arkhipova-Jenkins I, et al. Retention strategies for medications for opioid use disorder in adults: a rapid evidence review. J Addict Med. 2021;15(1):74-84. doi: 10.1097/ADM.0000000000000739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susukida R, Crum RM, Stuart EA, Ebnesajjad C, Mojtabai R. Assessing sample representativeness in randomized controlled trials: application to the National Institute of Drug Abuse Clinical Trials Network. Addiction. 2016;111(7):1226-1234. doi: 10.1111/add.13327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudolph KE, Russell M, Luo SX, Rotrosen J, Nunes EV. Under-representation of key demographic groups in opioid use disorder trials. Drug Alcohol Depend Rep. 2022;4:100084. doi: 10.1016/j.dadr.2022.100084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariani JJ, Mahony A, Iqbal MN, Luo SX, Naqvi NH, Levin FR. Case series: rapid induction onto long acting buprenorphine injection for high potency synthetic opioid users. Am J Addict. 2020;29(4):345-348. doi: 10.1111/ajad.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kordzadeh N, Ghasemaghaei M. Algorithmic bias: review, synthesis, and future research directions. Eur J Inf Syst. 2022;31(3):388-409. doi: 10.1080/0960085X.2021.1927212 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. UDS Results for Nonopioid Substances in the First 3 Weeks After Randomization as Predictors of Return to Use Using the Entire Sample (N = 2199)
eTable 2. Performance of Predictive Models for Opioid Return To Use, Including Treatment Group Assignment as a Predictor
eTable 3. Predicted Probability of Return to Opioid Use by Opioid Use in the First 3 Weeks in the Logistic Model
eReferences
Data Sharing Statement


