Abstract
Background.
The impact of complete revascularization (CR) on angina-related health status (symptoms, function, quality-of-life) in chronic coronary disease (CCD) has not been well studied.
Objectives.
Among patients with CCD randomized to invasive (INV) vs. conservative (CON) management in the ISCHEMIA trial, we compared 1) the impact of anatomic and functional CR on health status compared with incomplete revascularization (ICR), and 2) the predicted impact of achieving CR in all INV patients compared with CON.
Methods.
Multivariable regression adjusting for patient characteristics was used to compare 12-month health status after independent core laboratory-defined CR vs. ICR in INV patients who underwent revascularization. Propensity-weighted modeling was then performed to estimate the treatment effect had CR or ICR been achieved in all INV patients, compared with CON.
Results.
Anatomic and functional CR were achieved in 43.3% and 57.8% of 1641 INV patients, respectively. Among revascularized patients, CR was associated with improved Seattle Angina Questionnaire Angina Frequency compared to ICR after adjustment for baseline differences. After modeling CR and ICR in all INV patients, patients with CR and ICR each had greater improvements in health status than CON, with better health status with CR than ICR. The projected benefits of CR were most pronounced in patients with baseline daily/weekly angina and not seen in those with no angina.
Conclusions.
Among patients with CCD in ISCHEMIA, health status improved more with CR compared with ICR or CON, particularly in those with frequent angina. Anatomic and functional CR provided comparable improvements in quality-of-life.
Keywords: Coronary artery disease, ischemia, revascularization, complete revascularization, quality-of-life
Condensed abstract
The impact of complete revascularization (CR) after percutaneous coronary intervention and bypass graft surgery compared with conservative management was examined in the ISCHEMIA trial. Anatomic and functional (ischemic) CR were achieved in 43.3% and 57.8% patients assigned to invasive management, respectively. After propensity weighted adjustment, patients with CR and ICR each had greater improvement in health status than CON, with additional advantage seen from CR compared with ICR (e.g., freedom from angina: anatomic CR vs ICR, OR 1.36 (1.05–1.70); functional CR vs ICR, OR 1.37 (1.11–1.68)), particularly in patients with daily/weekly angina at baseline.
Introduction
Randomized controlled trials have shown that, in patients with chronic coronary disease (CCD), the addition of revascularization to guideline-directed medical therapy (GDMT), i.e. an “invasive strategy” (INV), has no significant impact on mortality or overall major adverse cardiovascular events, but does improve angina-related health status (patients’ symptoms, function, quality-of life [QoL]) as compared with GDMT alone, i.e. a “conservative strategy” (CON)(1–7). However, the completeness of revascularization achieved in INV, both in clinical trials and practice, varies considerably due to underlying disease severity and operators’ skills. This variation may impact the observed outcomes of invasive treatment due to incomplete revascularization (ICR) resulting in less improvement in patients’ health status than that which could be achieved with more complete revascularization (CR).
Numerous observational studies have attempted to examine the effect of CR vs ICR on clinical outcomes in patients with coronary artery disease. However, these studies have been limited, because patients have often been pre-selected for revascularization candidacy; differences in the patient populations with CR versus ICR have not been adequately adjusted for; and the most contemporary revascularization techniques have not been included, resulting in variable conclusions(8). Furthermore, most of these studies have focused on clinical events and not QOL or other patient-reported outcomes.
In the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA), 5179 patients with CCD and at least moderate ischemia were randomized to an initial INV strategy (angiography and revascularization with PCI or CABG as appropriate per clinician discretion plus GDMT) versus an initial CON strategy (GDMT alone with angiography and revascularization reserved for medical therapy failure). With a median follow-up of 3.2 years, the differences between the groups in the rates of cardiovascular death or MI were not statistically different, but the INV group had significant improvements in angina-related health status, with minimal benefits in asymptomatic patients and larger benefits in those with baseline angina (6,7). An analysis of the completeness of revascularization in ISCHEMIA was pre-specified, and a comprehensive quantitative coronary angiography (QCA) methodology was developed to prospectively assess the completeness of both anatomic and functional (ischemic) revascularization (9). In this issue of the journal, Stone et al., have separately reported the effect of CR on major adverse cardiovascular events in the ISCHEMIA trial (Stone et al., 2022, In Review, JACC). The present report describes the impact of CR on angina-related health status. Collectively, these data provide a complete picture of the potential benefits of achieving CR in patients with CCD.
Methods
The design and primary results of the ISCHEMIA trial have been published (6,7,10). While functional CR (FCR) was encouraged by protocol, individual treating physicians determined the completeness of revascularization achieved. Shortly after enrollment began, the protocol was modified to exclude patients with prior CABG, because a large proportion were found to be unsuitable for revascularization. While the primary endpoint was a composite of cardiovascular death, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest, a major secondary outcome was angina-related health status(7). The protocol was approved by the institutional review board at New York University Grossman School of Medicine (the clinical coordinating center) and by the institutional review board and ethics committee at each participating site. The present study had 2 principal objectives: 1) To assess the frequency of anatomic CR (ACR) and FCR and their effects on angina-related health status outcomes in CCD patients treated with a planned revascularization; and 2) to compare the health status outcomes of the strategies of INV with CR vs. INV with ICR vs. CON among all patients.
Pre-specified definitions were developed for ACR and FCR that accounted for vessel size, stenosis severity and the myocardial distribution of ischemia as determined by QCA and review of operative reports after CABG. Categorization was performed by an independent angiographic core laboratory (Cardiovascular Research Foundation, NY, NY) blinded to clinical outcomes including health status. A complete description of the classification of ACR and FCR was published previously (9). Briefly, ACR was defined as revascularization of all vessels and side branches with a QCA reference vessel diameter (RVD) ≥2.0 mm and diameter stenosis (DS) ≥50%. FCR was defined as revascularization of all stenotic vessels with RVD ≥2.0 mm with significant lesions as determined by localization of ischemia using intracoronary hemodynamics based on pressure wire assessment, non-invasive ischemia imaging, electrocardiographic stress testing or QCA angiographic DS ≥70%.
Analytic cohorts:
Specific analysis cohorts were comprised to meet each of the study objectives, as outlined in Figure 1. Few patients with prior CABG were enrolled in the ISCHEMIA trial, and these were excluded from all present analyses given core laboratory analytic challenges. Patients were also excluded due to administrative errors in angina-related health status form completion at 5 sites. For INV group assessment of CR, patients were excluded if angiographic images or operative reports necessary for core laboratory assessment were absent or incomplete.
Figure 1. Study patient flow for comparison of patients randomized to invasive management.
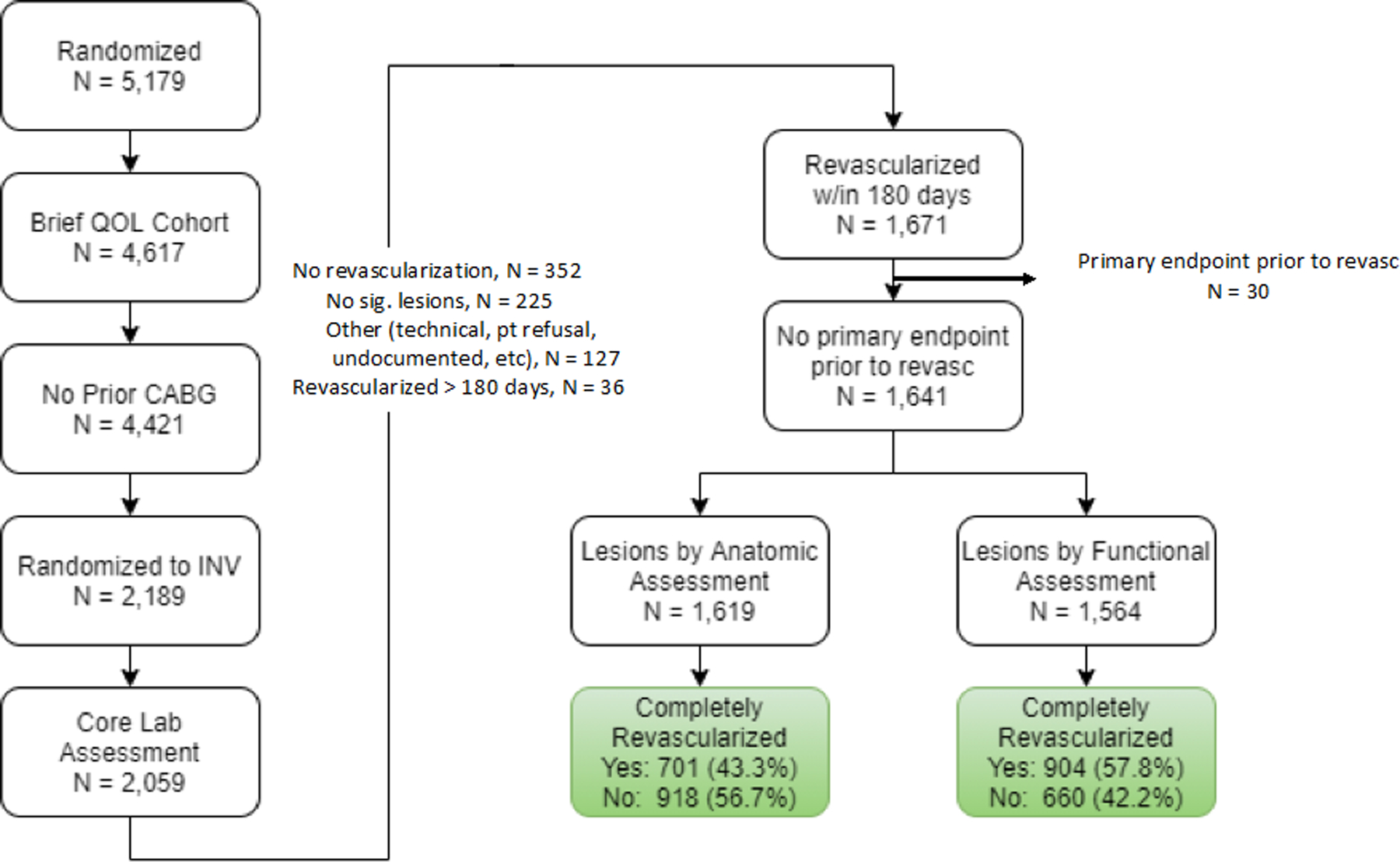
By following the sequence of boxes as indicated by the arrows, one can see how patients were selected to be evaluated for the comparison of complete and incomplete revascularization in patients randomized into the invasive arm of the ISCHEMIA trial. QOL = quality of life. CABG = coronary artery bypass grafting. INV = management.
The Objective 1 cohort included all INV patients in whom revascularization with PCI (including planned staged procedures), CABG or a hybrid approach (planned PCI plus CABG) was performed within 6 months of randomization and prior to a primary endpoint event, and in whom at least one qualifying lesion was present that met the pre-specified anatomic or ischemic criteria for revascularization. Thus, for Objective 1, patients who did not undergo revascularization were excluded from the analyses. The Objective 2 cohort included all INV patients in whom CR vs ICR could be assessed, and all CON patients. Some INV patients who were not revascularized because no qualifying anatomically or functionally significant lesions were found on angiography were included in the cohort, as they were deemed to be adequately vascularized at baseline. Similarly, INV patients with qualifying anatomic or functional lesions who did not receive revascularization within 6 months were treated as incompletely revascularized.
Health Status Outcomes.
To quantify angina-specific health status (symptoms, function, and QoL) among participants who underwent randomization, surveys were administered before randomization, at months 1.5, 3, and 6, and every 6 months thereafter until trial termination. The surveys included the 7-item Seattle Angina Questionnaire (SAQ), the Rose Dyspnea Scale, and the EuroQol-5 Dimensions (EQ5D) Visual Analogue Scale (11–13). The 7-item SAQ was the primary outcome for the health status assessments and has been shown to be highly valid, reliable, and sensitive to clinical change (11). The SAQ captures the frequency of angina (SAQ Angina Frequency score) and the disease-specific effect of angina on patients’ physical function (SAQ Physical Limitation score) and QoL (Quality of Life score) over the previous 4 weeks; these scores are averaged to obtain the SAQ Summary score, an overall measure of patients’ disease-specific health status. SAQ scores range from 0 to 100, with higher scores indicating less frequent angina, better function, and better QoL (14). SAQ Angina Frequency scores of 0 to 30, 31 to 60, 61 to 99, and 100 have been shown to validly reflect angina that occurs daily, weekly, several times per month (“monthly”), and no angina (freedom from angina), respectively, as assessed with daily diaries (15). The Rose Dyspnea Scale has four items indicating whether patients experience breathlessness with different activities (scores range from 0 to 4, with higher scores indicating dyspnea with milder activities). The prespecified primary endpoint of this substudy was SAQ Angina Frequency at 12 months, reflecting a time for the benefits of both INV and CON strategies to have been achieved and stable (7).
Statistical Analyses.
Baseline clinical, angiographic, and procedural characteristics were compared across patient groups. Categorical variables were summarized as percentages and were compared using chi-square tests. Continuous variables were summarized as means with standard deviations (SDs) or medians with interquartile ranges (IQRs) and were compared using Wilcoxon rank-sum tests. Parallel analyses were performed for ACR and FCR and for each health status endpoint.
For Objective 1, analyses were performed comparing INV patients in whom CR versus ICR was achieved within 6 months of randomization. Unadjusted health status scores at baseline, 1.5, 3, 6 and 12 months were compared using Wilcoxon rank-sum tests. Proportional odds models for 1-year outcomes were used to compare patients with CR and ICR, adjusting for patient, clinical and angiographic variables, including baseline health status score, age, sex, geographic region, hypertension, diabetes, smoking status, prior MI, heart failure, cerebrovascular disease/prior stroke, peripheral arterial disease, prior PCI, left ventricular ejection fraction, body mass index (BMI), glomerular filtration rate (GFR), New York Heart Association (NYHA) class, stress imaging modality, degree of ischemia on stress test, number of diseased vessels, Duke jeopardy score, SYNTAX score, number of chronic total occlusion (CTO) lesions, presence of calcification or tortuosity, use of intravascular ultrasound (IVUS), fractional flow reserve (FFR), total number of anatomic and ischemic lesions, left main disease, proximal left anterior descending artery (LAD) disease, and the initial mode of revascularization (PCI or CABG). Results are expressed as odds ratios of better health status with CR vs. ICR.
For Objective 2, we compared three groups of patients: (1) INV patients with CR; (2) INV patients with ICR; and (3) CON patients. Two stages of propensity weighting were used to balance the three groups. The first-stage propensity weights compared CON vs. INV patients and incorporated the fact that some patients randomized to INV treatment did not receive angiography (N=130, Figure 3). These weights were obtained from a multinomial model of CON versus INV with angiography vs. INV without angiography on patient demographic, clinical, stress testing/CCTA factors and baseline health status scores, as described above. The second stage of propensity weighting accounted for differences between completely and incompletely revascularized patients, among those with significant lesions found on angiography. This propensity model included all covariates from the first model, as well as angiographic characteristics listed above. Patients with no significant lesions were assigned a second-stage weight of 1. These weights were then multiplied by the first stage weights. All weights were calculated as the reciprocal of the probability of being the given group. The final resulting weights thus provided estimates of outcomes if all patients in ISCHEMIA were treated (1) invasively with CR, (2) invasively with ICR, or (3) conservatively. Differences in 1-year health status outcomes were estimated using propensity-weighted linear models (logistic regression for the binary outcome of freedom from angina). Confidence intervals were obtained using bootstrapped standard errors.
Figure 3. Study patient flow for comparison of patients randomized to invasive vs. conservative management.
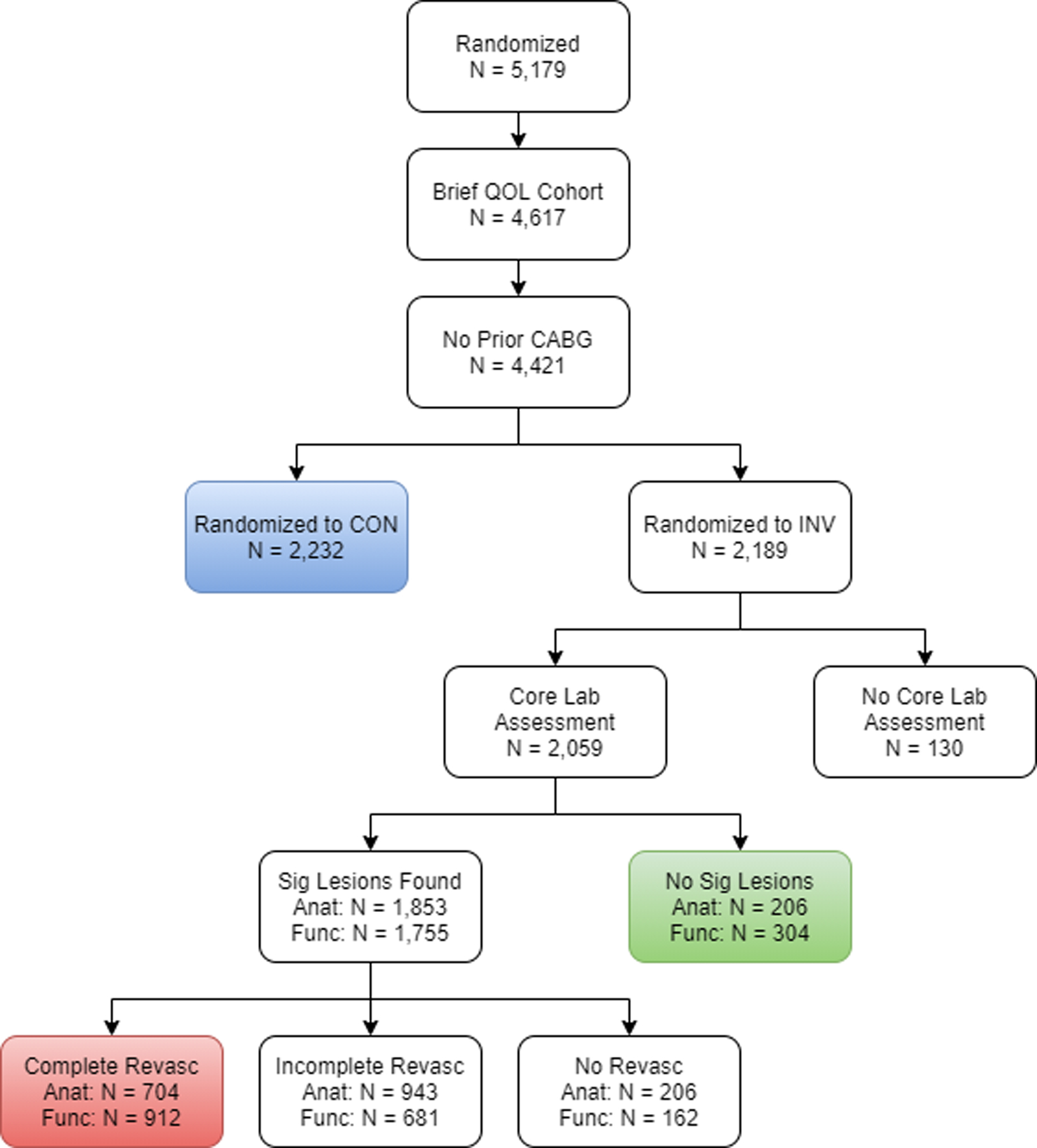
By following the sequence of boxes as indicated by the arrows, one can see how patients were selected for the comparison of invasively managed (INV) patents achieving complete revascularization and incomplete revascularization, versus the conservatively managed (CON) patients, from among all patients randomized into the ISCHEMIA trial. QOL = quality of life. CABG = coronary artery bypass grafting. INV = management. QOL = quality of life. CABG = coronary artery bypass grafting. CON = conservative strategy.
Of the 4,421 patients without prior CABG that formed the basis for both objectives, 1-year health status outcomes were missing in 8% (N=348: 218 missed follow-up assessments, 13 study withdrawals, 65 lost to follow-up, 50 deaths, and 2 due to study termination). Multiple imputation using chained equations was used to account for missing 1-year scores (16). The imputation model included all patient characteristics described above and all health status scores from baseline through 1 year.
In all regression and propensity models, continuous covariate effects were fit using restricted cubic splines to accommodate nonlinear associations. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary NC) and R version 3.6.3 (17).
Results
Of the 5179 patients with CCD and at least moderate ischemia in the ISCHEMIA trial, 4421 had no prior CABG and available health status assessments and formed the cohort for both objectives (Figure 1).
Objective 1 – Comparison of INV treatment with CR or ICR.
Of these 4421 patients, 2189 were randomized to INV. After exclusion of patients without core lab angiographic analysis (n = 130), without revascularization within 180 days (revascularization after 180 days, n = 36; no significant lesions, n = 225; no revascularization for other reasons, n = 127), or with a clinical endpoint preceding revascularization (n = 30), 1641 patients were evaluable for the assessment of the frequency of ACR and FCR in INV-assigned patients and their impact on health status outcomes (Figure 1). Of these, 1619 had QCA-defined anatomic lesions, with 701 (43.3%) achieving ACR. Among 1564 patients with QCA-defined functional lesions, 904 (57.8%) achieved FCR (Figure 1). Among the smaller population of patients with functional lesions, 99.8% had anatomic lesions.
Baseline clinical, angiographic and treatment characteristics of INV patients with vs without ACR and FCR are shown in Table 1 and Supplemental Tables 1 and 2. In univariate analysis, both ACR and FCR patients differed from their ICR counterparts by being more likely to be female, having better left ventricular function, having a lower BMI, and being less likely to have hypertension. Baseline health status measures were similar between CR and ICR (SAQ Angina Frequency score: anatomic CR vs ICR: 79.5 ± 19.7 vs 79.8 ± 20.8, p=0.33; functional CR vs ICR: 79.9 ± 20.2 vs 79.5 ± 20.4, p=0.84). On angiography, ACR and FCR patients had less complex coronary disease compared with ICR patients, as measured by the number of diseased vessels, the number of lesions, the Duke Jeopardy Score, the SYNTAX Score, and the number of chronic total occlusions (CTOs). (Table 1).
Table 1.
Baseline characteristics of invasively-managed patients with complete and incomplete revascularization.
| Anatomic CR | P-Value | Functional CR | P-Value | |||
|---|---|---|---|---|---|---|
| CR n = 701 |
ICR n = 918 |
CR n = 904 |
ICR n = 660 |
|||
| Age | 63.4 ± 9.7 | 64.1 ± 9.2 | 0.147 | 63.6 ± 9.5 | 64.1 ± 9.3 | 0.403 |
| Female | 181 (25.8%) | 174 (19.0%) | <0.001 | 208 (23.0%) | 125 (18.9%) | 0.052 |
| Hypertension | 509 (72.9%) | 721 (78.7%) | 0.006 | 671 (74.5%) | 523 (79.5%) | 0.021 |
| Diabetes | 295 (42.1%) | 367 (40.0%) | 0.393 | 370 (40.9%) | 264 (40.0%) | 0.711 |
| Prior MI | 125 (17.9%) | 197 (21.5%) | 0.074 | 161 (17.8%) | 146 (22.2%) | 0.034 |
| Peripheral vascular disease | 31 (4.4%) | 46 (5.0%) | 0.582 | 34 (3.8%) | 42 (6.4%) | 0.017 |
| Ejection fraction | 61.0 ± 8.0 | 60.0 ± 8.1 | 0.005 | 60.9 ± 8.0 | 59.7 ± 8.0 | 0.001 |
| Body mass index | 28.3 ± 4.8 | 29.0 ± 4.8 | 0.003 | 28.3 ± 4.6 | 29.2 ± 5.0 | 0.001 |
| SAQ Summary Score | 72.1 ± 18.8 | 73.3 ± 19.0 | 0.158 | 72.6 ± 19.0 | 73.2 ± 18.7 | 0.624 |
| SAQ Angina Frequency Score | 79.5 ± 19.7 | 79.8 ± 20.8 | 0.334 | 79.9 ± 20.2 | 79.5 ± 20.4 | 0.838 |
| Rose Dyspnea Scale | 1.2 ± 1.3 | 1.2 ± 1.3 | 0.293 | 1.2 ± 1.3 | 1.2 ± 1.3 | 0.327 |
| # of vessels ≥70% (CCTA) | <0.001 | <0.001 | ||||
| 0 | 48 (8.7%) | 25 (3.6%) | 48 (6.8%) | 16 (3.2%) | ||
| 1 | 171 (31.1%) | 178 (25.5%) | 220 (31.1%) | 122 (24.1%) | ||
| 2 | 82 (14.9%) | 144 (20.7%) | 111 (15.7%) | 110 (21.7%) | ||
| 3 | 39 (7.1%) | 114 (16.4%) | 61 (8.6%) | 92 (18.2%) | ||
| SYNTAX score | <0.001 | <0.001 | ||||
| <23 | 577 (82.3%) | 499 (54.4%) | 697 (77.1%) | 329 (49.8%) | ||
| 23 to <33 | 99 (14.1%) | 250 (27.2%) | 159 (17.6%) | 186 (28.2%) | ||
| ≥33 | 25 (3.6%) | 169 (18.4%) | 48 (5.3%) | 145 (22.0%) | ||
| Duke Jeopardy Score | < 0.001 | < 0.001 | ||||
| 1 | 45 (6.4%) | 24 (2.6%) | 40 (4.4%) | 10 (1.5%) | ||
| 2 | 159 (22.7%) | 117 (12.7%) | 196 (21.7%) | 71 (10.8%) | ||
| 3 | 166 (23.7%) | 174 (19.0%) | 200 (22.1%) | 127 (19.2%) | ||
| 4 | 162 (23.1%) | 217 (23.6%) | 221 (24.4%) | 148 (22.4%) | ||
| 5 | 113 (16.1%) | 183 (19.9%) | 158 (17.5%) | 135 (20.5%) | ||
| 6 | 36 (5.1%) | 135 (14.7%) | 56 (6.2%) | 114 (17.3%) | ||
| 7 | 20 (2.9%) | 68 (7.4%) | 33 (3.7%) | 55 (8.3%) | ||
| Number of anatomic lesions | < 0.001 | < 0.001 | ||||
| 1 | 343 (48.9%) | 45 (4.9%) | ||||
| 2 | 214 (30.5%) | 212 (23.1%) | 508 (56.2%) | 92 (13.9%) | ||
| 3 | 84 (12.0%) | 253 (27.6%) | 253 (28.0%) | 208 (31.5%) | ||
| 4 | 48 (6.8%) | 172 (18.7%) | 97 (10.7%) | 165 (25.0%) | ||
| 5 | 10 (1.4%) | 129 (14.1%) | 40 (4.4%) | 116 (17.6%) | ||
| 6 | 1 (0.1%) | 58 (6.3%) | 4 (0.4%) | 49 (7.4%) | ||
| 7 | 0 (0.0%) | 33 (3.6%) | 1 (0.1%) | 21 (3.2%) | ||
| 8 | 1 (0.1%) | 9 (1.0%) | 0 (0.0%) | 6 (0.9%) | ||
| 9 | 0 (0.0%) | 5 (0.5%) | 1 (0.1%) | 2 (0.3%) | ||
| 10 | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 1 (0.2%) | ||
| 11 | 0 (0.0%) | 1 (0.1%) | ||||
| Number of CTOs | <0.001 | <0.001 | ||||
| 0 | 475 (67.9%) | 411 (44.8%) | 587 (65.0%) | 249 (37.7%) | ||
| 1 | 194 (27.7%) | 401 (43.7%) | 275 (30.5%) | 315 (47.7%) | ||
| 2 | 30 (4.3%) | 87 (9.5%) | 39 (4.3%) | 78 (11.8%) | ||
| ≥3 | 1 (0.1%) | 18 (2.1%) | 2 (0.2%) | 17 (2.8%) | ||
CR = complete revascularization; ICR = incomplete revascularization. MI = myocardial infarction. SAQ = Seattle Angina Questionnaire. CCTA = coronary computed tomographic angiography. CTO = chronic total occlusion. PCI = percutaneous coronary intervention. CABG = coronary artery bypass grafting.
After revascularization, unadjusted 12-month QoL scores were similar between CR and ICR patients (Supplemental Tables 3 and 4). However, after adjustment for baseline clinical and angiographic characteristics and initial revascularization method, odds ratios favored better health status with ACR compared with ICR, particularly for the SAQ Angina Frequency (SAQ Angina Frequency: OR 1.37 (1.00–1.86), SAQ Summary Score: OR 1.08 (0.85–1.36) and Rose Dyspnea Score: OR 1.30 (0.98–1.73)). FCR had a similar effect (OR 1.35 (1.00–1.82), SAQ Summary Score (OR 1.24 (1.00–1.55) and Rose Dyspnea Score (OR 1.34 (1.03–1.74); Figure 2)).
Figure 2. Health status outcomes in invasively managed and revascularized patients.
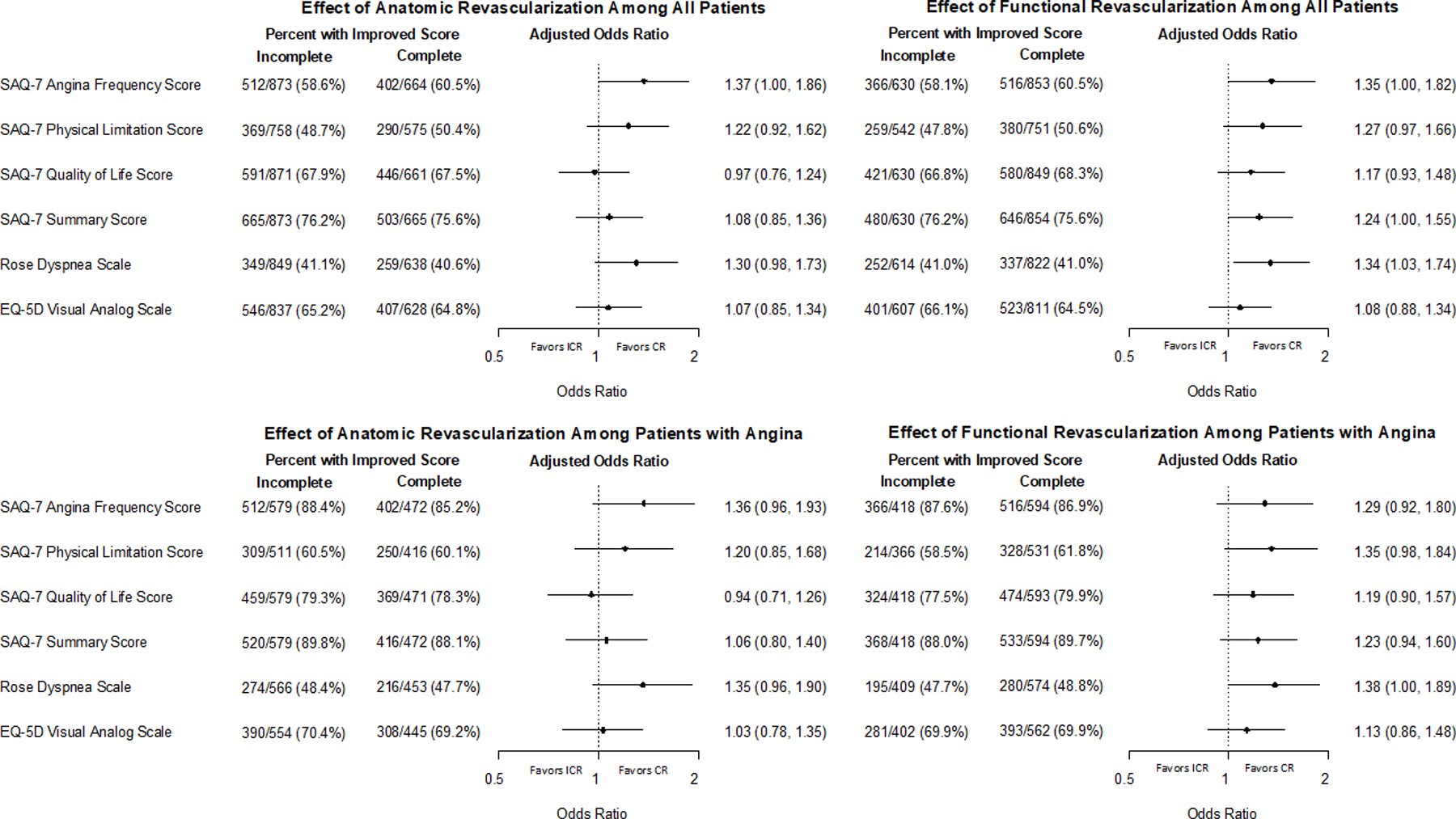
Patients achieving complete revascularization (CR) and incomplete revascularization (ICR) with improved health status score at one year are compared, using proportional odds models to adjust for patient, clinical and angiographic variables. Results are expressed as percent of patients with improved health status (unadjusted) as well as odds ratios of better health status with CR vs. ICR (adjusted). SAQ = Seattle Angina Questionnaire. EQ-5D = EuroQoL 5 Dimensions.
Stratification by patients’ frequency of angina at baseline (daily/weekly angina, monthly angina, or no angina) showed that patients with daily/weekly angina had the greatest benefit from ACR as compared with ICR, with adjusted ORs of improved SAQ Angina Frequency of 2.15 (1.31–3.53)) and SAQ Summary Score of 1.60 (1.05–2.44); Table 2). Similar benefits were observed for FCR vs ICR (SAQ Angina Frequency OR 2.06 (1.23–3.45), SAQ Summary Score OR 1.54 (1.03–2.32)). The benefits associated with CR vs ICR were less and not significant in patients with monthly or no angina (Table 2). No interaction between CR and the amount of baseline ischemia or number of CTOs was noted (Table 2).
Table 2.
Adjusted 12-month health status outcomes in invasively managed and revascularized patients.
| Baseline Angina | None N=521 |
Monthly N=740 |
Daily/Weekly N=378 |
Interaction P-value |
|---|---|---|---|---|
| Effect of anatomic CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 1.55 (0.85, 2.83) | 1.04 (0.71, 1.53) | 2.15 (1.31, 3.53) | 0.04 |
| SAQ-7 Summary Score | 1.15 (0.79, 1.67) | 0.90 (0.67, 1.20) | 1.60 (1.05, 2.44) | 0.05 |
| Quality of Life | 0.99 (0.66, 1.48) | 0.78 (0.57, 1.07) | 1.39 (0.90, 2.16) | 0.06 |
| Physical Function | 1.03 (0.64, 1.65) | 1.08 (0.76, 1.53) | 1.72 (1.07, 2.76) | 0.18 |
| Rose Dyspnea Score | 1.28 (0.80, 2.06) | 1.21 (0.84, 1.72) | 1.79 (1.11, 2.89) | 0.35 |
| EQ-5D | 1.19 (0.83, 1.71) | 0.89 (0.66, 1.19) | 1.37 (0.90, 2.07) | 0.15 |
| Effect of functional CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 1.15 (0.64, 2.04) | 1.14 (0.77, 1.67) | 2.06 (1.23, 3.45) | 0.11 |
| SAQ-7 Summary Score | 1.13 (0.78, 1.63) | 1.17 (0.86, 1.58) | 1.54 (1.03, 2.32) | 0.43 |
| Quality of Life | 1.08 (0.73, 1.58) | 1.09 (0.79, 1.49) | 1.43 (0.94, 2.17) | 0.48 |
| Physical Function | 1.03 (0.66, 1.60) | 1.32 (0.90, 1.93) | 1.48 (0.93, 2.35) | 0.48 |
| Rose Dyspnea Score | 1.25 (0.80, 1.95) | 1.21 (0.85, 1.74) | 1.63 (0.99, 2.70) | 0.57 |
| EQ-5D | 0.95 (0.67, 1.34) | 1.00 (0.75, 1.35) | 1.48 (0.99, 2.21) | 0.17 |
| Degree of Ischemia |
None-Mild
N=160 |
Moderate
N=575 |
Severe
N=906 |
|
| Effect of anatomic CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 0.96 (0.46, 2.01) | 1.64 (1.04, 2.59) | 1.31 (0.88, 1.96) | 0.43 |
| SAQ-7 Summary Score | 0.98 (0.52, 1.82) | 1.51 (1.06, 2.15) | 0.90 (0.68, 1.19) | 0.04 |
| Quality of Life | 0.99 (0.52, 1.87) | 1.07 (0.74, 1.56) | 0.92 (0.68, 1.23) | 0.77 |
| Physical Function | 0.99 (0.47, 2.09) | 1.73 (1.14, 2.61) | 0.97 (0.69, 1.36) | 0.07 |
| Rose Dyspnea Score | 1.79 (0.90, 3.55) | 1.38 (0.91, 2.10) | 1.21 (0.85, 1.70) | 0.55 |
| EQ-5D | 1.52 (0.83, 2.79) | 1.15 (0.81, 1.62) | 0.98 (0.74, 1.30) | 0.35 |
| Effect of functional CR vs ICR | ||||
| SAQ-7 Summary Score | 1.22 (0.62, 2.43) | 1.66 (1.18, 2.33) | 1.03 (0.78, 1.35) | 0.07 |
| SAQ-7 Angina Frequency Score | 1.13 (0.50, 2.57) | 1.47 (0.94, 2.29) | 1.28 (0.87, 1.89) | 0.81 |
| Quality of Life | 1.10 (0.54, 2.22) | 1.27 (0.88, 1.81) | 1.14 (0.85, 1.52) | 0.87 |
| Physical Function | 1.43 (0.66, 3.07) | 1.85 (1.24, 2.76) | 0.96 (0.68, 1.35) | 0.02 |
| Rose Dyspnea Score | 2.32 (1.07, 5.02) | 1.25 (0.84, 1.85) | 1.28 (0.90, 1.81) | 0.33 |
| EQ-5D | 1.37 (0.71, 2.65) | 1.12 (0.81, 1.55) | 1.02 (0.77, 1.34) | 0.68 |
| Number of CTOs |
0
N=976 |
1
N=560 |
≥2
N=105 |
|
| Effect of anatomic CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 1.22 (0.85, 1.76) | 1.83 (1.10, 3.04) | 1.16 (0.32, 4.26) | 0.38 |
| SAQ-7 Summary Score | 1.01 (0.76, 1.34) | 1.34 (0.93, 1.91) | 0.58 (0.23, 1.49) | 0.17 |
| Quality of Life | 0.90 (0.67, 1.21) | 1.19 (0.81, 1.75) | 0.84 (0.31, 2.25) | 0.45 |
| Physical Function | 1.17 (0.83, 1.62) | 1.42 (0.90, 2.23) | 0.48 (0.17, 1.37) | 0.17 |
| Rose Dyspnea Score | 1.20 (0.84, 1.70) | 1.63 (1.04, 2.54) | 0.78 (0.24, 2.58) | 0.34 |
| EQ-5D Visual Analog Scale | 0.94 (0.72, 1.23) | 1.37 (0.97, 1.95) | 0.91 (0.30, 2.74) | 0.19 |
| Effect of functional CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 1.05 (0.71, 1.54) | 1.81 (1.15, 2.85) | 1.77 (0.48, 6.54) | 0.16 |
| SAQ-7 Summary Score | 1.10 (0.82, 1.47) | 1.49 (1.07, 2.07) | 0.91 (0.38, 2.14) | 0.28 |
| Quality of Life | 1.06 (0.78, 1.42) | 1.42 (1.01, 1.99) | 1.12 (0.46, 2.69) | 0.40 |
| Physical Function | 1.21 (0.86, 1.70) | 1.58 (1.06, 2.38) | 0.56 (0.20, 1.55) | 0.15 |
| Rose Dyspnea Score | 1.08 (0.76, 1.53) | 1.82 (1.23, 2.71) | 1.14 (0.39, 3.35) | 0.12 |
| EQ-5D Visual Analog Scale | 0.87 (0.65, 1.16) | 1.43 (1.05, 1.95) | 1.11 (0.44, 2.81) | 0.06 |
CR = complete revascularization; ICR = incomplete revascularization. SAQ=Seattle Angina Questionniare. EQ-5D = EuroQoL 5 Dimensions. Data are presented as odds ratio of likelihood of improvement with complete revascularization compared with incomplete revascularization. Results were adjusted for the following covariates: baseline health status score, age, sex, geographic region, hypertension, diabetes, smoking status, prior MI, heart failure, cerebrovascular disease/prior stroke, peripheral arterial disease, prior PCI, left ventricular ejection fraction, body mass index (BMI), glomerular filtration rate (GFR), New York Heart Association (NYHA) class, stress imaging modality, degree of ischemia on stress test, number of diseased vessels, Duke jeopardy score, SYNTAX score, number of chronic total occlusion (CTO) lesions, presence of calcification or tortuosity, use of intravascular ultrasound (IVUS), fractional flow reserve (FFR), total number of anatomic and ischemic lesions, left main disease, proximal left anterior descending artery (LAD) disease, and the initial mode of revascularization (PCI or CABG).
Objective 2 – Comparing Predicted Outcomes of INV treatment with CR and ICR as compared with CON Treatment Strategies.
To better estimate the potential of CR vs ICR as management strategies compared with a CON strategy in patients with CCD, we estimated the effects had all evaluable ISCHEMIA patients undergone; 1) INV with CR; 2) INV with ICR; or 3) CON (Figure 3). Comparison of baseline, angiographic and treatment characteristics are shown in Supplemental Table 5. After propensity weighted adjustment of the CR and ICR patients to match the overall population, patients achieving both CR and ICR, whether defined anatomically or functionally, had greater improvement in SAQ Angina Frequency Scores at 12 months than those managed conservatively (ACR vs CON: difference = 4.1 (3.2, 5.2); FCR vs CON: difference = 4.5 (3.4, 5.4); anatomic ICR vs CON: difference = 3.3 (2.4, 4.3); functional ICR vs CON: difference = 2.8 (1.8, 3.8). Similar improvements were present for the SAQ Quality-of-Life, Physical Function and Summary Scores, as well as the Rose Dyspnea and EQ5D Visual Analog Scales. (Figure 4, Table 3). Improvements compared with CON were greater with CR than with ICR, particularly with regards to freedom from angina: anatomic CR vs ICR OR 1.36 (1.05–1.70) and functional CR vs ICR OR 1.37 (1.11–1.68) (Table 3).
Figure 4. Adjusted health status outcomes in patients randomized to conservative vs. invasive management.
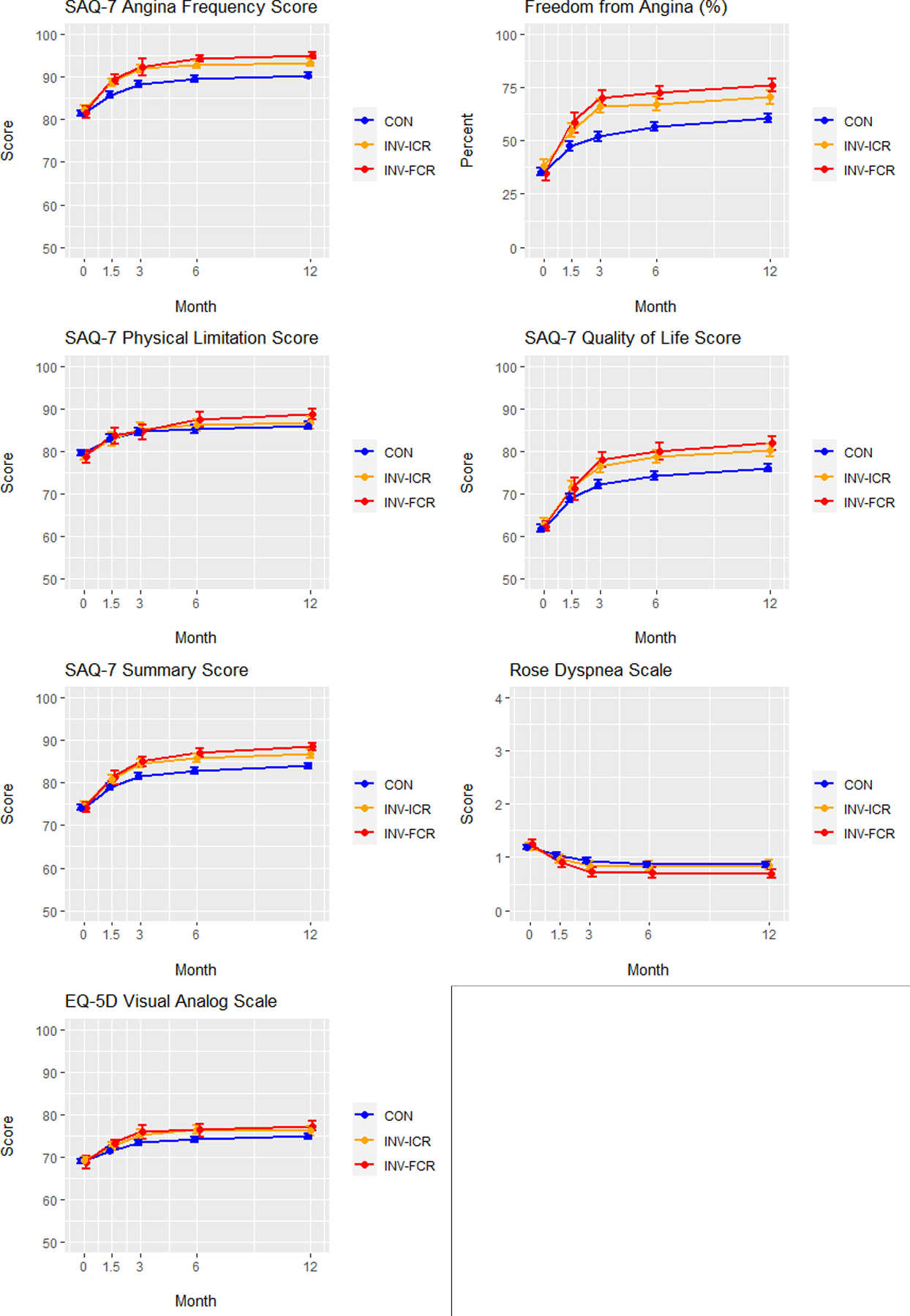
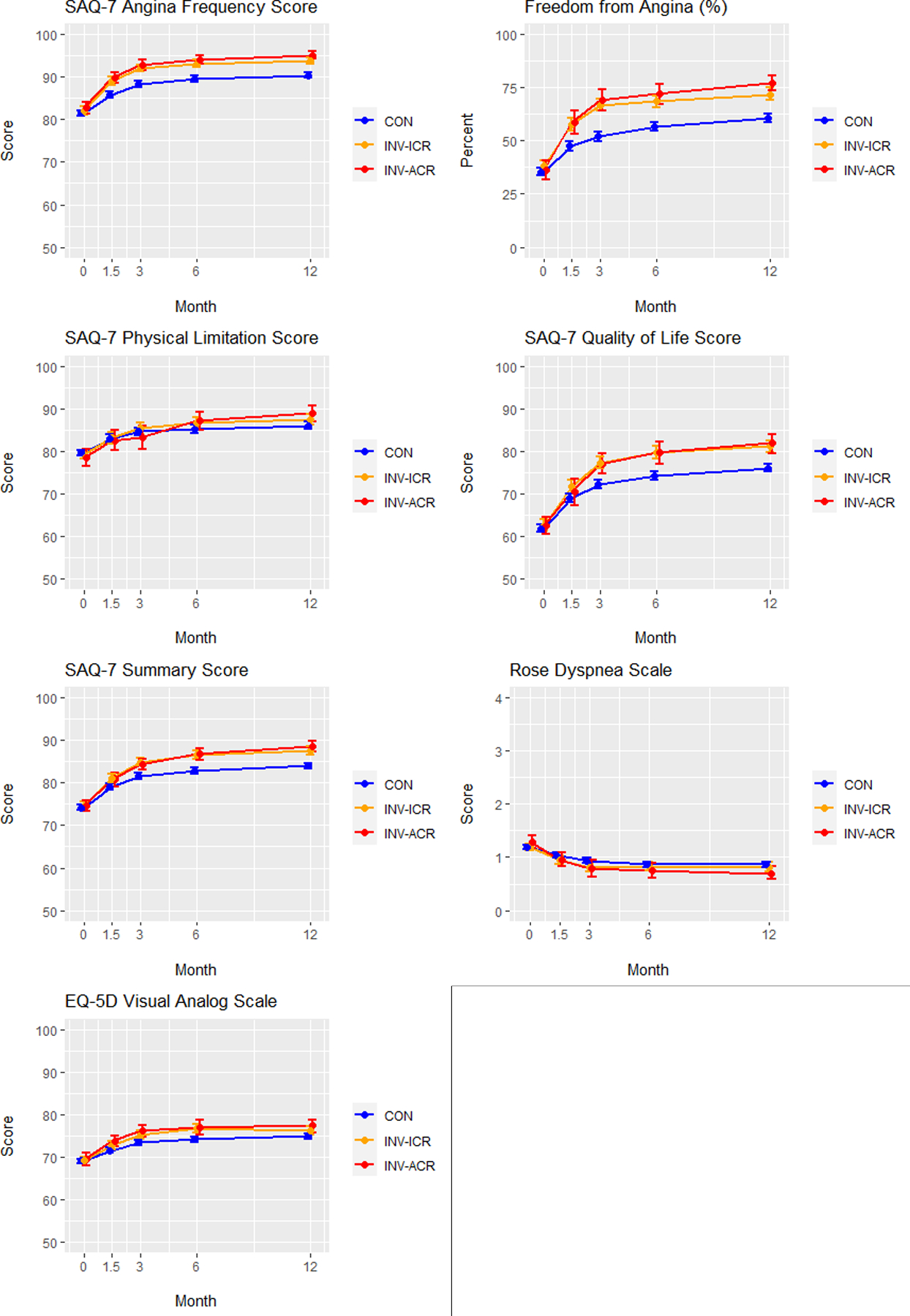
Adjusted Seattle Angina Questionnaire (SAQ)-7 scores, Rose Dyspnea Scale scores and EuroQoL 5 Dimensions (EQ5D) scores are depicted over time (0, 1.5, 3, 6 and 12 months) for conservatively managed (CON) patients (blue), invasively managed patients achieving incomplete revascularization (ICR) (orange) and invasively managed patients achieving complete revascularization (CR) (red). FCR = functional complete revascularization. ACR = anatomic complete revascularization.
A. Functional complete revascularization. B. Anatomic complete revascularization.
SAQ = Seattle Angina Questionnaire. EQ-5D = EuroQoL 5 Dimensions. Values plotted are estimated mean scores (percent for freedom from angina) and 95% confidence intervals
Table 3.
Difference in propensity-weighted 12-month health status score of invasively- vs conservatively-managed patients.
| Functional CR | CR vs. Conservative Management | ICR vs. Conservative Management | CR vs. ICR Management |
|---|---|---|---|
| ΔSAQ-7 Angina Frequency Score | 4.5 (3.4, 5.4) | 2.8 (1.8, 3.8) | 1.7 (0.6, 2.6) |
| Freedom from angina (OR) | 2.2 (1.8, 2.6) | 1.6 (1.3, 1.9) | 1.4 (1.1, 1.7) |
| ΔSAQ-7 Summary Score | 4.6 (3.4, 5.7) | 2.4 (1.3, 3.5) | 2.2 (0.9, 3.3) |
| ΔSAQ-7 Quality of Life Score | 5.9 (4.0, 7.6) | 3.8 (2.1, 5.4) | 2.1 (0.2, 3.8) |
| ΔSAQ-7 Physical Limitation Score | 3.2 (1.8, 4.7) | 0.6 (−1.0, 2.2) | 2.6 (0.9, 4.2) |
| ΔRose Dyspnea Scale | −0.2 (−0.3, −0.1) | 0.0 (−0.1, 0.1) | −0.2 (−0.3, −0.1) |
| ΔEQ-5D Visual Analog Scale | 2.7 (0.9, 4.1) | 1.6 (0.4, 2.7) | 1.1 (−0.8, 2.6) |
| Anatomical CR | CR vs. Conservative Management | ICR vs. Conservative Management | CR vs. ICR Management |
| ΔSAQ-7 Angina Frequency Score | 4.1 (3.2, 5.2) | 3.3 (2.4, 4.3) | 0.9 (0.0, 1.8) |
| Freedom from angina (OR) | 2.2 (1.8, 2.8) | 1.6 (1.4, 2.0) | 1.4 (1.1, 1.7) |
| ΔSAQ-7 Summary Score | 4.8 (3.7, 6.0) | 3.6 (2.5, 4.7) | 1.2 (0.0, 2.3) |
| ΔSAQ-7 Quality of Life Score | 5.7 (3.9, 7.7) | 5.1 (3.6, 6.6) | 0.6 (−1.4, 2.6) |
| ΔSAQ-7 Physical Limitation Score | 3.6 (2.0, 5.3) | 2.4 (1.1, 3.9) | 1.2 (−0.4, 3.0) |
| ΔRose Dyspnea Scale | −0.2 (−0.3, −0.1) | 0.0 (−0.1, 0.1) | −0.2 (−0.3, −0.1) |
| ΔEQ-5D Visual Analog Scale | 2.7 (1.2, 4.2) | 1.4 (0.4, 2.5) | 1.3 (−0.2, 2.7) |
Data are median (Q1, Q3) for all comparisons except OR (95% CI) for Freedom of Angina. CR = complete revascularization; ICR = incomplete revascularization. SAQ = Seattle Angina Questionnaire. OR = odds ratio. EQ-5D = EuroQoL 5 Dimensions.
When modeled over the entire population randomized to INV treatment, stratification of patients by baseline angina frequency revealed that the most incremental benefit of CR over ICR occurred in patients with daily/weekly angina (Figure 5; Supplemental Figure 2; Table 4), particularly with functional revascularization (FCR vs ICR: difference in SAQ Angina Frequency = 4.3 (1.1–7.4), difference in SAQ Summary Score = 4.2 (1.4–7.0); ACR vs ICR: difference in SAQ Angina Frequency = 3.1 (−0.1–6.2), difference in SAQ Summary Score = 2.0 (−0.8–4.8)) (Figure 5, Figure S2, Table 4). No incremental benefit of CR over ICR was seen in patients with no angina, and little incremental benefit was seen in patients with monthly angina (functional CR vs ICR: difference in SAQ Summary Score = 1.8 (0.0–3.7). No interaction between the benefit from INV-CR and the degree of ischemia was observed.
Figure 5. Adjusted health status outcomes in patients stratified by baseline angina level.
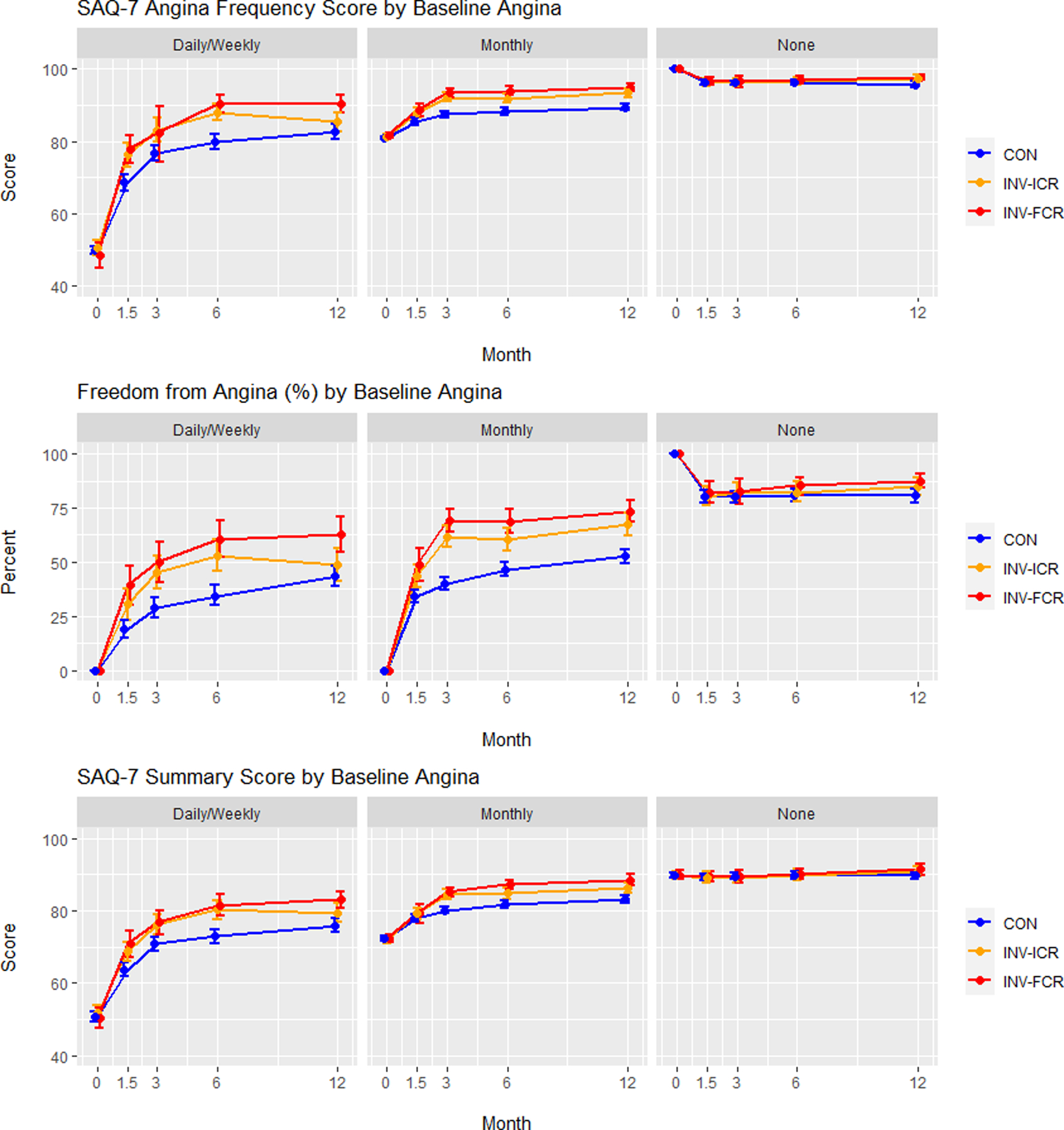
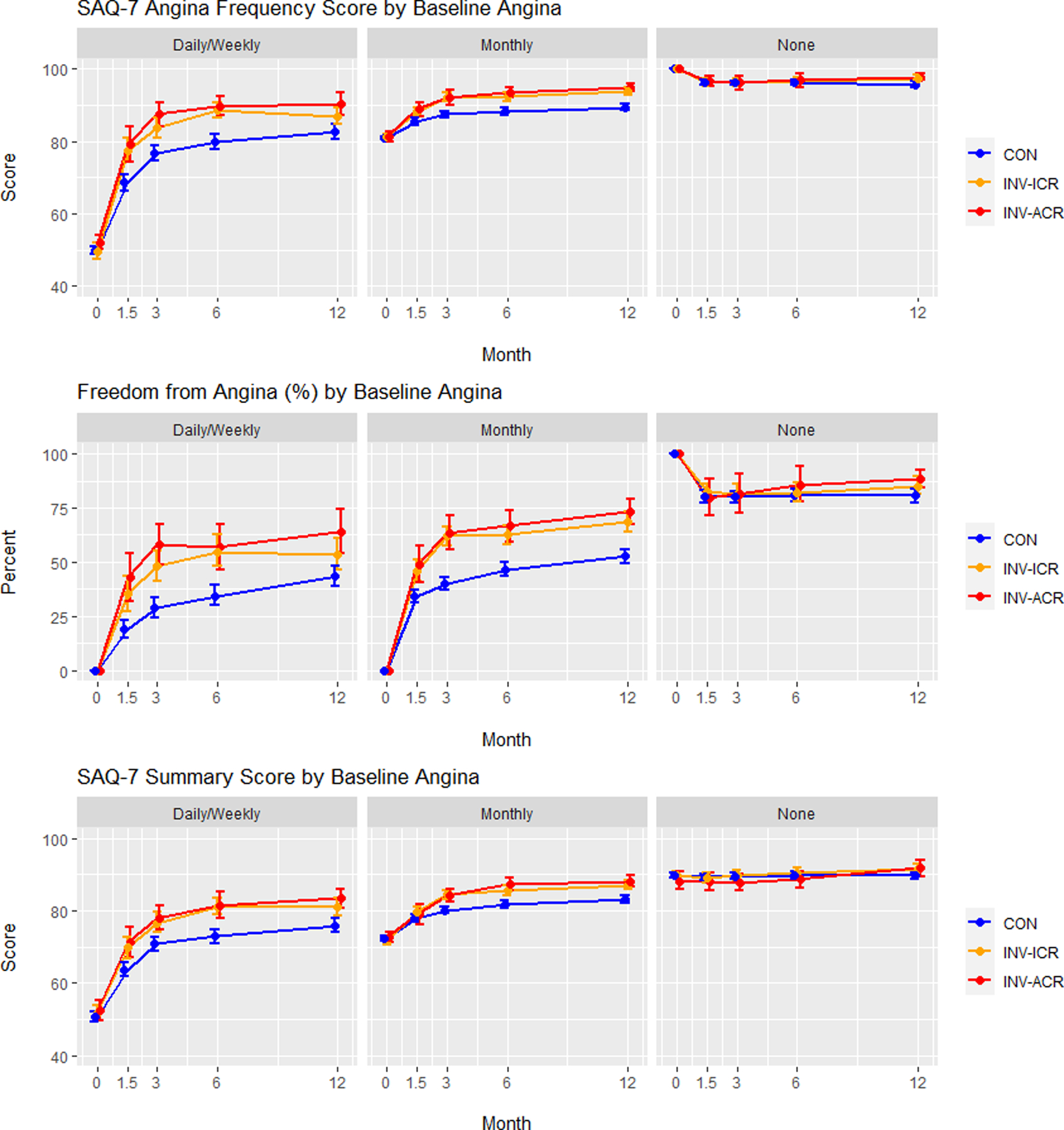
Adjusted Seattle Angina Questionnaire (SAQ)-7 Angina Frequency scores, Freedom from Angina (Angina Frequency = 100), and Summary Score are depicted over time (0, 1.5, 3, 6 and 12 months) for conservatively managed (CON) patients (blue), invasively managed patients achieving incomplete revascularization (ICR) (orange) and invasively managed patients achieving complete revascularization (CR) (red). Patients are stratified into groups based on baseline angina levels: Daily/weekly, monthly and none. FCR = functional complete revascularization. ACR = anatomic complete revascularization.
A. Functional complete revascularization. B. Anatomic complete revascularization.
SAQ = Seattle Angina Questionnaire. EQ-5D = EuroQoL 5 Dimensions. Values plotted are estimated mean scores (percent for freedom from angina) and 95% confidence intervals
Table 4.
Difference in propensity-weighted 12-month health status score of invasively-managed patients, stratified by angina and ischemia.
| Baseline Angina | None | Monthly | Daily/Weekly | Interaction P-value |
|---|---|---|---|---|
| Effect of anatomic CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 0.2 (−0.7, 1.0) | 1.3 (−0.1, 2.7) | 3.1 (−0.1, 6.2) | 0.13 |
| Freedom from angina (OR) | 1.10 (1.63, 0.74) | 1.25 (1.81, 0.87) | 1.51 (0.97,2.34) | 0.61 |
| SAQ-7 Summary Score | 1.6 (−0.4, 3.6) | 0.9 (−0.9, 2.8) | 2.0 (−0.8, 4.8) | 0.80 |
| Quality of Life | 1.8 (−1.7, 5.3) | −0.2 (−3.2, 2.8) | 2.9 (−1.6, 7.3) | 0.43 |
| Physical Function | 1.9 (−0.9, 4.7) | 2.0 (−0.3, 4.2) | 1.4 (−2.0, 4.8) | 0.96 |
| Rose Dyspnea Score | 0.0 (−0.2, 0.2) | −0.3 (−0.4, −0.1) | −0.2 (−0.5, 0.1) | 0.05 |
| EQ-5D | 1.4 (−1.2, 3.9) | 0.9 (−1.3, 3.2) | 0.4 (−2.2, 3.0) | 0.88 |
| Effect of functional CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 0.3 (−0.5, 1.1) | 1.2 (−0.2, 2.7) | 4.3 (1.1, 7.4) | 0.03 |
| Freedom from angina (OR) | 1.12 (1.56, 0.80) | 1.35 (1.87, 0.98) | 1.87 (1.22,2.84) | 0.15 |
| SAQ-7 Summary Score | 0.2 (−1.3, 1.7) | 1.8 (0.0, 3.7) | 4.2 (1.4, 7.0) | 0.03 |
| Quality of Life | 1.0 (−1.8, 3.8) | 2.2 (−0.7, 5.0) | 3.0 (−0.8, 6.9) | 0.72 |
| Physical Function | −0.3 (−2.8, 2.3) | 3.8 (1.2, 6.4) | 2.8 (−0.5, 6.1) | 0.07 |
| Rose Dyspnea Score | −0.1 (−0.2, 0.1) | −0.2 (−0.4, −0.1) | −0.3 (−0.6, −0.1) | 0.07 |
| EQ-5D | −0.3 (−2.1, 1.4) | 1.8 (−1.2, 4.9) | 2.2 (−0.4, 4.8) | 0.23 |
| Degree of Ischemia | None-Mild | Moderate | Severe | |
| Effect of anatomic CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 0.5 (−2.3, 3.3) | 1.8 (0.3, 3.3) | 1.2 (−0.1, 2.4) | 0.69 |
| Freedom from angina (OR) | 0.94 (1.59, 0.55) | 1.49 (2.18, 1.02) | 1.38 (2.08, 0.92) | 0.36 |
| SAQ-7 Summary Score | 1.8 (−1.2, 4.7) | 2.9 (0.9, 5.0) | 0.3 (−1.4, 2.0) | 0.14 |
| Quality of Life | 1.6 (−3.2, 6.5) | 2.8 (−0.5, 6.1) | −0.2 (−3.1, 2.7) | 0.40 |
| Physical Function | 2.3 (−1.4, 6.0) | 4.4 (1.2, 7.6) | 0.0 (−2.3, 2.3) | 0.10 |
| Rose Dyspnea Score | −0.3 (−0.6, 0.0) | −0.1 (−0.3, 0.0) | −0.2 (−0.3, 0.0) | 0.64 |
| EQ-5D | 3.3 (−0.6, 7.3) | −0.4 (−3.0, 2.3) | 1.4 (−0.4, 3.2) | 0.31 |
| Effect of functional CR vs ICR | ||||
| SAQ-7 Angina Frequency Score | 0.2 (−0.7, 1.0) | 1.3 (−0.4, 3.0) | 1.9 (0.7, 3.2) | 0.47 |
| Freedom from angina (OR) | 1.10 (1.63, 0.74) | 1.32 (1.88, 0.93) | 1.57 (2.20, 1.13) | 0.52 |
| SAQ-7 Summary Score | 1.6 (−0.4, 3.6) | 2.1 (0.3, 4.0) | 1.5 (−0.3, 3.3) | 0.85 |
| Quality of Life | 1.8 (−1.7, 5.3) | 1.1 (−1.8, 4.0) | 2.8 (0.0, 5.5) | 0.64 |
| Physical Function | 1.9 (−0.9, 4.7) | 3.2 (0.9, 5.6) | 0.7 (−1.7, 3.2) | 0.20 |
| Rose Dyspnea Score | 0.0 (−0.2, 0.2) | −0.1 (−0.3, 0.0) | −0.2 (−0.4, −0.1) | 0.66 |
| EQ-5D | 1.4 (−1.2, 3.9) | 0.3 (−1.8, 2.3) | 1.6 (−1.3, 4.5) | 0.78 |
Data are median (Q1, Q3) for all comparisons except OR (95% CI) for Freedom of Angina. CR = complete revascularization; ICR = incomplete revascularization. SAQ = Seattle Angina Questionnaire. OR = odds ratio. EQ-5D = EuroQoL 5 Dimensions.
Discussion
In the ISCHEMIA trial--the largest, most contemporary, randomized controlled strategy trial of patients with CCD and moderate or severe ischemia—an INV management strategy resulted in greater improvement in disease-specific health status (including angina symptoms, physical function and disease specific QoL) than a CON management strategy, but the influence of the completeness of revascularization on these outcomes had not been assessed (7). The present pre-specified analysis shows that CR was only achieved in ~50% (ACR: 43.3%, FCR: 57.8%) of patients assigned to the INV strategy. After adjustment for differences in baseline, angiographic and procedural characteristics, CR was associated with greater health status improvement than ICR. When modeled in the entire trial population, INV-assigned patients with both CR and ICR had greater health status gains than CON-assigned patients. For most measures, the health status gains after FCR and ACR were similar. In those patients with daily or weekly angina at baseline, the estimated benefits of CR were greater than ICR, both of which were better than CON. In contrast, there were no differences in health status between CR or ICR and CON in asymptomatic patients, and little evidence of substantial advantage of CR or ICR, as compared with CON, in those with monthly angina. Collectively, these data suggest little difference between INV and CON strategies on health status at 12 months in patients without angina or with only monthly angina, regardless as to whether CR is achieved. However, in patients with more frequent angina, CR has the potential to confer better health, and efforts to provide more complete revascularization to improve these patients’ symptoms, function and quality of life may be important. (Central Illustration). Finally, these data overall support a patho-mechanistic approach to the treatment of angina in CCD.
Central Illustration. Health Status associated with Complete and Incomplete Revascularization in the ISCHEMIA Trial.
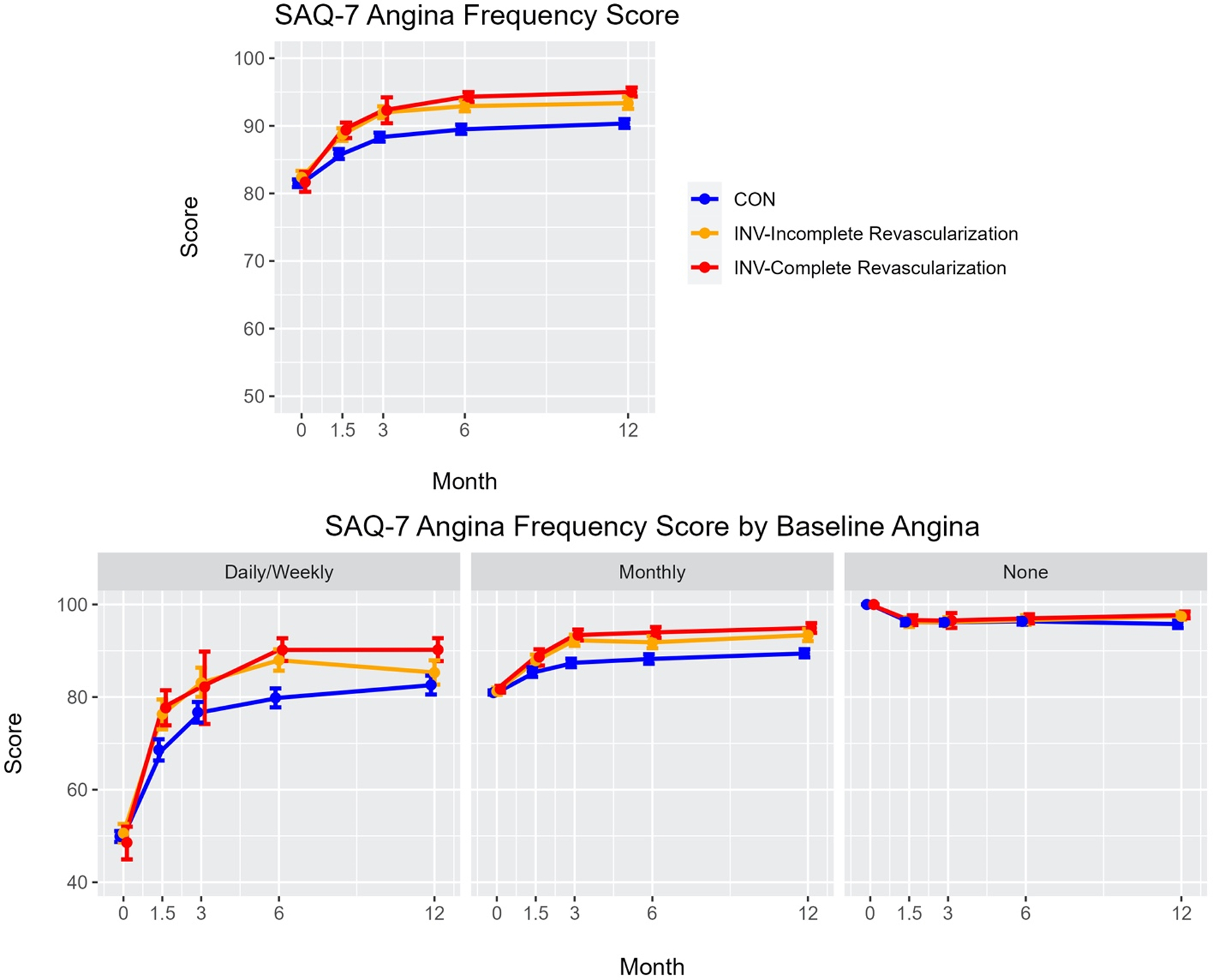
Top Panel: Adjusted Seattle Angina Questionnaire (SAQ)-7 Angina Frequency scores over time (0, 1.5, 3, 6 and 12 months) in ISCHEMIA patients undergoing conservative management (CON), invasive management (INV) achieving incomplete revascularization, and INV achieving complete revascularization.
Bottom Panel: Adjusted SAQ-7 Angina Frequency scores over time (0, 1.5, 3, 6 and 12 months) in ISCHEMIA patients stratified for baseline angina levels (daily/weekly, monthly or no angina) undergoing conservative management (CON), invasive management (INV) achieving incomplete revascularization and INV achieving complete revascularization.
Values plotted are estimated mean scores and 95% confidence intervals
Despite the ISCHEMIA protocol’s encouragement of the use of CABG and advanced CTO PCI techniques, FCR was achieved in only 57.8% of participants, while ACR was achieved in only 43.3% of participants. While these CR rates seem similar to the 50.5% CR rate reported in a meta-analysis of 35 older studies of completeness of revascularization, some differences between ISCHEMIA and these prior studies should be noted (17). The prior studies primarily included patients pre-selected for their anatomic revascularization potential (18), whereas in ISCHEMIA, invasive angiography—and therefore feasibility of revascularization--was only determined after randomization. In addition, unlike most prior studies, this ISCHEMIA analysis determined CR by using a very detailed and algorithmic core laboratory-based approach. Notwithstanding the different methodologies used to assess CR and differences in patient populations, the comparison of ISCHEMIA with the prior meta-analysis suggests the rate of achieving CR (~50%) has been relatively stable over time.
In general terms, functional lesions are a subgroup of anatomic lesions, representing those in whom ischemia has been demonstrated (or is likely). As ischemia underlies angina and anginal-equivalent symptoms in most patients, it is not surprising that CR based on anatomic and functional criteria each resulted in health status improvements at 12 months in our study. However, anatomic CR may provide benefits beyond FCR in providing a greater reduction in cardiovascular death or MI, as separately shown by Stone et al. (Stone et al., 2022, In Review, JACC) Presumably this discordance is due to the potential for cardiovascular events to arise from non-obstructive plaques that may not be ischemic at baseline (19,20).
The reasons that CR is not always achieved in the invasive management of CCD are multifactorial. Independent predictors of ICR in ISCHEMIA included diabetes, BMI, number of diseased vessels and lesions, higher SYNTAX score, and more CTOs, all of which are markers for more complex CAD, and the use of CABG as a revascularization technique (Stone et al., 2022, In Review, JACC). These factors suggest that the achievement of CR is dependent on patient comorbidities and coronary artery disease complexity, patient and physician selection for PCI vs. CABG (a decision that may reflect local technical expertise, as well as logistical and convenience issues and patient preferences), and the perceived importance of CR vs ICR. Critically, this analysis informs the last factor—and therefore the overall revascularization decision-making process—by providing a deeper understanding of the type and magnitude of benefit of CR with regards to angina-related health status.
Few prior studies have assessed the effect of CR vs ICR on health status. A small single-center study of 210 patients with CR or ICR after CABG showed greater improvement in non-disease specific SF-36 scores in patients achieving CR, although these results were not adjusted for baseline differences (21). In the COMPLETE study, patients with ST-segment elevation MI and multivessel disease randomized to CR (PCI of non-culprit lesions as well as the culprit lesion) had greater improvements in SAQ scores at 6 months and 3 years and a 3.2% absolute increase in freedom from angina (87.5% vs 84.3%, p=0.01) compared with patients randomized to intentional ICR (PCI of the culprit lesion only), despite approximately 50% of patients having no baseline angina (22). The larger ISCHEMIA trial extends these findings to a broader population of patients with CCD, finding a larger improvement in health status with CR in the most symptomatic patients.
The lack of impact of the number of CTOs present on the benefit of CR suggests that revascularization of such lesions has a similar impact on QoL improvement as non-CTO lesions, a fact that should be recognized when developing an overall revascularization plan designed to optimize health status. Successful revascularization of CTOs may be greater with CABG than PCI, although the success rates for CTO PCI are high with advanced techniques at centers of excellence (23). Both registries and randomized controlled trials have shown more angina relief with CTO PCI than medical therapy alone (20, 24,25). The extent to which myocardial viability influences the improvement in health status achieved after revascularization of CTOs (or non-CTOs) after PCI or CABG was not assessed in ISCHEMIA. The benefits of pursuing CR in patients with complex CTO(s) warrant further evaluation.
The findings from this pre-specified sub-study of the ISCHEMIA trial should be interpreted in the context of the following potential limitations. First, it was not possible to randomize participants to CR vs ICR and despite the use of multivariable adjustment, the potential for residual confounding remains. Accordingly, causality cannot be assumed. Second, the lack of a sham group in ISCHEMIA introduces the possibility of a placebo effect when comparing CR vs. ICR, and the INV and CON groups. However, the impact of a placebo effect is diminished by 1) the fact that this technical issue may not be known by some patients, and 2) the fact that the benefit in health status overall in the ISCHEMIA trial was comparable to that of the sham-controlled ORBITA trial (26). Thirdly, there was no adjustment for multiple testing. Finally, the complex modeling to compare ICR and CR strategies with CON involved a number of assumptions that might account for some of the observed differences, and therefore definitive conclusions on treatment effect should not be drawn from this type of analysis.
In conclusion, in the ISCHEMIA trial, CR was only achieved in ~50% patients with CCD undergoing revascularization. However, the present analysis suggests that the likelihood of safely achieving CR is an important consideration when developing a revascularization plan for patients with CCD, as achieving CR was associated with a greater improvement in QoL, particularly in patients with more frequent angina at baseline.
Supplementary Material
Clinical Perspectives:
Competency in Medical Knowledge: Complete revascularization should be considered, when it can be safely performed, in order to provide the best angina-related health status.
Competency in Patient Care: The patient with angina should be made aware of the potential for complete revascularization, and the strategies needed to accomplish this, because complete revascularization may afford the best chance for future angina relief.
Translational Outlook 1: Complete revascularization is only achieved in clinical practice in approximately 50% of patients. The reasons why complete revascularization is not performed need to be assessed and quantitated in order to focus future efforts on increasing the rate of complete revascularization achievement.
Translational Outlook 2: Randomized studies of complete vs incomplete revascularization could be performed to confirm complete revascularization’s additional benefit on quality-of-life.
Sources of Funding:
NIH grants U01HL105907, U01HL105462, U01HL105561, U01HL105565, T32 HL079896
Other Support:
This project was supported in part by Clinical Translational Science Award Nos. 11UL1 TR001445 and UL1 TR002243 from the National Center for Advancing Translational Sciences and by grants from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Devices or medications were provided by Abbott Vascular (previously St. Jude Medical, Inc); Medtronic, Inc.; Phillips (previously Volcano Corporation); and Omron Healthcare, Inc.; medications provided by Amgen Inc; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Espero Pharmaceuticals; Merck, Sharp & Dohme Corp. and Sunovion Pharmaceuticals
Disclosures
Dr. Mavromatis reports grants from National Heart, Lung, and Blood Institute; grants from NHLBI (CV Inflammation Reduction Trial and GMCSF in PAD-3 Trial), grants from CSL Behring, St Jude’s Medical, Medtronic, DalCor Pharmaceuticals, AstraZeneca, Novartis, Regeneron, and Member of American College of Cardiology and Society of Cardiovascular Angiography and Interventions.
Dr. Ali has institutional grant support from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems Inc, Medtronic Inc, National Institute of Health, Opsens Medical, Philips, Teleflex. Consulting fees from Astra Zeneca, Philips, Shockwave. Equity in Elucid, Spectrawave, Shockwave, VitalConnect.
Dr. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, Abbott; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore, Amgen, Adona Medical, Millennia Biopharma; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, Xenter. Dr. Stone’s daughter is an employee at IQVIA. Institutional disclosure: Dr. Stone’s employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Shockwave, Vascular Dynamics, Pulnovo and V-wave.
Dr. Bangalore reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Abbott Vascular; personal fees from Biotronik, Pfizer, Amgen, and Reata outside the submitted work.
Dr. Genereux reports Abbott Vascular: Consultant, advisor, speaker Fees; Abiomed: Consultant, advisor, speaker fees; BioTrace Medical: Consultant, advisor, speaker Fees; Boston Scientific: Consultant; CARANX Medical: Consultant; Cardiovascular System Inc.: Consultant, PI Eclipse Trial; Edwards LifeSciences: Consultant, advisor, speaker fees, proctor, institutional research grant, PI EARLY-TAVR trial, PI PROGRESS trial; GE Healthcare: Consultant; iRythm Technologies: Consultant; Medtronic: Consultant, advisor, speaker fees; Opsens: Consultant; Pi-Cardia: Equity, consultant; Puzzle Medical: Equity, consultant; Saranas: Equity, consultant; Shockwave: Consultant, speaker fees; Siemens: Consultant; Soundbite Medical Inc.: Equity, consultant; Teleflex: Consultant; 4C Medical: Consultant, PI Feasibility study.
Dr. Goodman reports grants and personal fees from National Heart, Lung, and Blood Institute, during the conduct of the study; grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants and personal fees from Bayer, personal fees from Boehringer Ingelheim/Eli Lilly, grants and personal fees from Bristol Myers Squibb/Pfizer, grants and personal fees from CSL Behring/PERFUSE, grants and personal fees from Daiichi-Sankyo/American Regent/DCRI, personal fees from Esperion/C5, grants and personal fees from Ferring, personal fees from GlaxoSmithKline, personal fees from HLS Therapeutics, personal fees from Merck, grants and personal fees from Novartis, personal fees from NovoNordisk, grants and personal fees from Regeneron/Sanofi, personal fees from Servier, outside the submitted work; .
Dr. Alexander reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Dr. Chen reports grants from NHLBI during the conduct of the study.
Dr. Boden reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Abbvie, grants from Amarin, grants from Amgen, personal fees from Amgen, personal fees from Cleveland Clinic Clinical Coordinating Center, personal fees from Janssen, outside the submitted work.
Dr. Reynolds reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study, she receives support from Abbott Vascular (donation of optical coherence tomography catheters for an unrelated research study) and Biotelemetry Inc (donation of telemetry monitors for an unrelated research study)
Dr. Maron reports grants from NHLBI during the conduct of the study.
Dr. Hochman is PI for the ISCHEMIA trial for which, in addition to support by NHLBI grant, devices and medications were provided by Medtronic, Inc., Abbott Vascular, Inc (formerly St. Jude Medical, Inc.), Royal Philips NV (formerly Volcano Corporation), Arbor Pharmaceuticals, LLC, AstraZeneca Pharmaceuticals, LP, Merck Sharp & Dohme Corp., Omron Healthcare, Inc, Sunovion Pharmaceuticals, Inc, Espero BioPharma, and Amgen Inc, and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP.
Dr. Spertus discloses providing consultative services on patient-reported outcomes and evidence valuation to Alnylam, AstraZeneca, Bayer, Merck, Janssen, Bristol Meyers Squibb, Edwards, Kineksia, 4DT Medical, Terumo, Imbria, and United Healthcare. He holds research grants from Bristol Meyers Squibb, Abbott Vascular and Janssen. He owns the copyright to the Seattle Angina Questionnaire, Kansas City Cardiomyopathy Questionnaire, and Peripheral Artery Questionnaire and serves on the Board of Directors for Blue Cross Blue Shield of Kansas City.
Drs. Jones, Mathew, Mark, O’Brien, Dressler, Bhargava, and Uxa, and Ms. Rhodes and Horst have nothing to report.
Abbreviations
- ACR
anatomic complete revascularization
- CCD
chronic coronary disease
- CABG
coronary artery bypass grafting
- CON
conservative treatment strategy
- CR
complete revascularization
- FCR
functional complete revascularization
- ICR
incomplete revascularization
- INV
invasive treatment strategy
- PCI
percutaneous coronary intervention
- QCA
quantitative coronary angiography
Footnotes
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Non-Author Collaborators: A list of non-author collaborators for indexing is included in the supplement.
Tweet: In ISCHEMIA, invasive management with complete revascularization associated with improved quality-of-life compared to both conservative management and invasive management with incomplete revascularization.
References
- 1.Boden WE, O’Rourke RA, Teo KK et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub WS, Spertus JA, Kolm P et al. Effect of PCI on Quality of Life in Patients with Stable Coronary Disease. N Engl J Med 2008;359:677–687. [DOI] [PubMed] [Google Scholar]
- 3.Group BDS, Frye RL, August P et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks MM, Chung S-C, Helmy T et al. Health Status After Treatment for Coronary Artery Disease and Type 2 Diabetes Mellitus in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial. Circulation 2010;122:1690–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bruyne B, Pijls NH, Kalesan B et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 6.Maron DJ, Hochman JS, Reynolds HR et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spertus JA, Jones PG, Maron DJ et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N Engl J Med 2020;382:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaba P, Gersh BJ, Ali ZA, Moses JW, Stone GW. Complete versus incomplete coronary revascularization: definitions, assessment and outcomes. Nat Rev Cardiol 2021;18:155–168. [DOI] [PubMed] [Google Scholar]
- 9.Ali ZA, Horst J, Gaba P et al. Standardizing the Definition and Analysis Methodology for Complete Coronary Artery Revascularization. J Am Heart Assoc 2021;10:e020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maron DJ, Hochman JS, O’Brien SM et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes 2014;7:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:1–188. [PubMed] [Google Scholar]
- 13.EuroQol G EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 14.Thomas M, Jones PG, Arnold SV, Spertus JA. Interpretation of the Seattle Angina Questionnaire as an Outcome Measure in Clinical Trials and Clinical Care: A Review. JAMA Cardiol 2021;6:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold SV, Kosiborod M, Li Y et al. Comparison of the Seattle Angina Questionnaire With Daily Angina Diary in the TERISA Clinical Trial. Circ Cardiovasc Qual Outcomes 2014;7:844–50. [DOI] [PubMed] [Google Scholar]
- 16.van Buuren SG-O, Karin. MICE: Multivariate Imputation by Chained Equations in R. J Stat Soft 2011;45:1–67. [Google Scholar]
- 17.R Core Team. A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria, 2022. https://www.r-project.org/ [Google Scholar]
- 18.Garcia S, Sandoval Y, Roukoz H et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol 2013;62:1421–31. [DOI] [PubMed] [Google Scholar]
- 19.Kedhi E, Berta B, Roleder T et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J 2021;42:4671–4679. [DOI] [PubMed] [Google Scholar]
- 20.Erlinge D, Maehara A, Ben-Yehuda O et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet 2021;397:985–995. [DOI] [PubMed] [Google Scholar]
- 21.Chen N, Zhang JY, Yang SZ, Li YD. Impact of complete and incomplete revascularization on short- and long-term quality of life in patients with multivessel coronary artery disease. Eur Rev Med Pharmacol Sci 2016;20:4581–4585. [PubMed] [Google Scholar]
- 22.Mehta SR, Wang J, Wood DA et al. Complete Revascularization vs Culprit Lesion-Only Percutaneous Coronary Intervention for Angina-Related Quality of Life in Patients With ST-Segment Elevation Myocardial Infarction: Results From the COMPLETE Randomized Clinical Trial. JAMA Cardiol 2022;7(11):1091–1099. doi: 10.1001/jamacardio.2022.3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajti P, Karmpaliotis D, Alaswad K et al. The Hybrid Approach to Chronic Total Occlusion Percutaneous Coronary Intervention: Update From the PROGRESS CTO Registry. JACC Cardiovasc Interv 2018;11:1325–1335. [DOI] [PubMed] [Google Scholar]
- 24.Khariton Y, Airhart S, Salisbury AC et al. Health Status Benefits of Successful Chronic Total Occlusion Revascularization Across the Spectrum of Left Ventricular Function: Insights From the OPEN-CTO Registry. JACC Cardiovasc Interv 2018;11:2276–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner GS, Martin-Yuste V, Hildick-Smith D et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J 2018;39:2484–2493. [DOI] [PubMed] [Google Scholar]
- 26.Al-Lamee R, Thompson D, Dehbi HM et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


