Abstract
Objective
This study aimed to determine the frequency, type, and correlates of a broad spectrum of sleep disorders in adults with COVID-19 up to 32 months after infection.
Methods
We conducted a national online survey (Jun 2021–Dec 2022), gathering information on COVID-19 diagnosis, acute disease course, and the subsequent development of sleep disorders from 1507 respondents (mean age 44.5 ± 13.1 years, 64.1% women).
Results
81.3% (1223) reported at least one sleep difficulty that either worsened or first appeared with COVID-19. Females reported a higher number of symptoms (2.03 ± 1.44 versus 1.72 ± 1.43 in men, p < 0.0001). Most common were insomnia symptoms (59.4%), followed by night sweats (38.4%), hypersomnolence (33.3%), vivid dreams or nightmares (26.4%), restless leg syndrome (RLS) (22.8%), and sleep-related breathing disorders (11.1%). All symptoms were associated with a more severe acute disease. A mild decreasing trend in the persistence of sleep symptoms with a longer latency since infection was observed, with 66.7% reporting at least half of their symptoms present at 3–5 months after acute infection, compared to 64.9% at 6–8 months, and 62.4% at 9–11 months (p = 0.0427). However, among those after 12 or more months, over half of the symptoms persisted in 69.5%. The frequency of vivid dreams and nightmares increased in association with COVID-19 in 32.9% (p < 0.001). 9.4% (141) reported new-onset or increased parasomnic manifestations after the infection.
Conclusions
Our research shows that sleep disturbances are a common and persistent manifestation of COVID-19 that affects a large proportion of the population and deserves careful monitoring.
Keywords: Nightmare, Parasomnia, Dream-enactment behaviour, SARS-CoV-2, Long COVID, Prevalence, Post-COVID syndrome
1. Introduction
Soon after the start of the COVID-19 pandemic, reports emerged indicating the neurotropic potential of this primarily respiratory virus and its association with a wide range of neurological symptoms [[1], [2], [3], [4], [5], [6]]. Among the most frequently reported neurological manifestations were sleep disorders such as insomnia, excessive daytime sleepiness, sleep-related breathing disorders, circadian rhythm disturbances, and others [[7], [8], [9]].
While several studies have established a direct link between COVID-19 infection and sleep disorders, they also recognise the significant impact of the pandemic and lockdown measures on sleep quality and daily regime [8,10]. The effects of social isolation, financial stress, limited healthcare access, and significant changes in daily routine during the first waves of the pandemic may have contributed to the increased prevalence of these issues [9,10]. As a result, it is challenging to distinguish the direct impact of COVID-19 infection on sleep quality from that of other external factors.
We are currently witnessing an increase in reports of so called long COVID. While the definitions vary, a widely recognised definition by the WHO defines long COVID as a continuation or development of new symptoms 3 months after initial infection, with these symptoms lasting for at least 2 months with no other explanation. It may affect various organ systems; however, one of the most common manifestations include neurological symptoms and in particular sleep disorders [11,12].
Our study aims to evaluate the prevalence of long-term persistent sleep disturbances in individuals who had been diagnosed with COVID-19 more than a year ago in a large and diverse sample of the Czech population. By collecting data over an extended period of time outside of a lockdown or other strict anti-pandemic measures, we aimed to gain an insight into the type, frequency, and persistence of sleep disruptions associated with COVID-19 while reducing the influence of external factors such as anti-pandemic measures.
2. Methods
An online survey was conducted in the Czech Republic over a period of 32 months (from June 1, 2021 to December 31, 2022), i.e., it took place outside of a lockdown with no current anti-pandemic measures, minimising the influence of these factors on daily routine and sleep habits. The survey was published primarily through digital media, including social media (Facebook, Instagram) and various media outlets. Articles published between August 2021 and November 2022 were disseminated by various publishers based on a press release by the Czech News Agency (Opletalova 5/7, 111 44 Prague 1, Czech Republic).
The survey was intended for adults and was made available in the Czech language. The questionnaire was designed specifically for this purpose, using the open-source JavaScript library survey. JS (Devsoft Baltic OÜ, surveyjs. io), and was hosted on a private domain (www.neurocovid.cz). Information about the study, data processing, and ethical committee approval (Neurocovid study, approved by the Ethical Committee of the National Institute of Mental Health, Klecany, Czech Republic, December 16, 2020, No. 205/20) were provided on the homepage. Prior to starting the survey, participants need to agree to the conditions of the survey and data processing. It was designed to provide an optimal user experience on desktop computers, tablets, and mobile devices, and included standard checkbox, matrix, and scale tools, as well as fields for comments. To maximise the relevance of questions asked to each individual, the survey progressed based on their answers. For example, participants who reported having experienced a particular symptom would be asked about the duration of it, while participants who did not report this symptom would proceed directly to the next questions of the survey.
2.1. Survey structure
The following data were collected.
2.1.1. Demographic variables
Age, gender, highest level of education, place of residence, body mass index (BMI), and current or past smoking habits.
2.1.2. COVID-19 testing
Test type and test result, date of positive test, reason for getting tested. Participants who had never been infected by COVID-19 based on the information provided were excluded from the study.
2.1.3. Information about the acute phase of the disease
Presence of common symptoms (runny nose, sore throat, fever, cough, shortness of breath, nausea/vomiting, diarrhoea, muscle and joint ache, fatigue, loss of smell and taste). Participants who had symptoms were asked to indicate when they first appeared, while those without symptoms were asked to state the date of their first positive test (MM/YYYY). We further enquired about the severity of the acute phase of the infection and the need for hospitalisation, with four options (asymptomatic/mild, symptomatic without hospitalisation, hospitalisation on a general ward, hospitalisation in ICU).
2.1.4. Sleep disturbances
The primary focus of the questionnaire was to further investigate sleep disturbances potentially linked to COVID-19. The initial question was posed as a matrix with a list of symptoms and four possible answers for each (“I've never had this issue”; “I had this issue prior to COVID-19, it did not change”; “I had this issue prior to COVID-19, it got worse after the infection”; “After COVID-19, I developed this issue for the first time”). Next, we asked about the presence of insomnia symptoms (“Difficulty initiating or maintaining sleep”), hypersomnolence (“Excessive daytime sleepiness or falling asleep suddenly during the day”), restless legs syndrome (RLS) (“Unpleasant sensation in the legs when falling asleep, forcing you to move” - formulation equivalent to the RLS1Q), sleep apnoea symptoms (“Loud and explosive snoring or pauses in breathing during sleep”), night sweats (“Excessive sweating at night”), nightmares or vivid dreams, and sleepwalking. If the participant indicated that at least one of the symptoms got worse or first appeared after having had COVID-19, they were asked follow-up questions: 1) “Which of the symptoms persist at this time?“; 2) “For the symptoms that do not persist anymore, how long did they last for?“; 3) “How severe of an impact do these symptoms have your quality of life?” (Scale 1–10).
2.1.5. Information related to circadian rhythm
We enquired about changes in the duration of sleep (“I sleep much less than before COVID-19”, “I sleep much more than before COVID-19”, “No change”) and the timing of sleep (“I fall asleep much earlier than before COVID-19”, “I fall asleep much later than before COVID-19”, “No change”).
2.1.6. Dreaming and parasomnia symptoms
Dreaming and possible signs of parasomnias were evaluated in more detail through a series of questions: 1) “How often did you have vivid dreams before COVID 19?“; 2) “How often do you have vivid dreams after COVID-19?” (“Several times per week”, “Max. 1 × per week”, “Max. 1 × per month”, “Max 1 × year”, “Never”); 4) “How often do these dreams have the character of nightmares (a dream that is scary, stressful, and that you remember)?” (“Very often”, “From time to time”, “I don't have nightmares”); 5) “Does this problem persist?” (“Yes”, “Yes, but less”, “No”).
To assess symptoms of possible parasomnias, a multiple-choice question was used, enquiring about the presence of several symptoms in the previous 12 months. The Czech translation of the RBDQ1 was included in the question [13]. “Did you experience or were you told about any of the following behaviour in the last few years?” “Talking or screaming in your sleep”, “Enacting your dreams (moving your arms, gesturing, or kicking in your sleep)”, “Getting injured in your sleep” “Waking up screaming or crying but not remembering the dream or the event in the morning”, “I'm not sure”.
If at least one of the symptoms was checked, we asked about the changes in frequency relating to COVID-19 (“No change”; “Symptom got less frequent after COVID-19”; “Symptom is more frequent after COVID-19”; “Symptom appeared after COVID-19 for the first time”).
2.1.7. Mental status
One multiple choice question and a comment field were used to gather information on mental condition, specifically “Have you been experiencing any of the following after having had COVID-19 (in connection with the disease itself)?“, with the possible answers being “Feeling sad, depressed or hopeless” (referred to as “Depression” further in the text), “Feeling anxious or nervous” (referred to as “Anxiety” further in the text); and “Other changes in mood (please describe)”.
We also gathered information about neurological symptoms including cognitive disorders, the number of COVID-19 infections (reinfections) and vaccination; however, for the purpose of this article, these data will not be included.
2.2. Exclusion criteria
Exclusion criteria were: 1) individuals under 18 years of age; 2) those who indicated unrealistic information about the timing of the COVID-19 infection, i.e., prior to March 2020, which is when testing became available in the Czech Republic; 3) and those who were not tested positive for COVID-19.
2.3. Statistical analysis
Statistical analysis was performed with R software. For descriptive statistics, percentages, mean, median, range, and standard deviation (SD) were calculated.
Chi-square analyses were conducted to examine the association between non-numerical variables, such as gender, disease severity, and sleep symptoms. The Mann-Whitney U Test and unpaired T-test were used to assess associations with numerical variables, such as age, time elapsed from acute infection, and number of sleep symptoms reported. In all instances, values of p < 0.05 were considered statistically significant.
3. Results
3.1. Demographics
A total of 1674 individuals filled out the survey (only complete responses were recorded). Out of these, 1507 participants who filled out the survey between June 1, 2021 and December 31, 2022, were included in the statistical analysis. In total, 64.1% (966) were women. Mean age was 44.5 ± 13.1 years. Mean BMI was 26.3 ± 5.3. The participants were residents of the Czech Republic (97.88%, n = 1475), with minor representation of other countries, i.e., Slovakia (1.39%, n = 21). A total of 0.73% stated their country of residence was elsewhere (Germany, Austria, Hungary, USA, Canada). Three participants did not specify.
In terms of education, 45.65% (688) indicated having a university degree (Bachelor's, Master's, or higher), 39.68% (598) had a higher education (13 years), 12.81% (193) a vocational education (12–13 years), and 1.86% (28) a basic education (9 years).
3.2. COVID-19 disease – acute phase
A total of 32.1% (483) individuals indicated having a very mild or asymptomatic course of the acute phase of the disease, 62.0% (934) stated they had significant difficulties but did not require hospital admission, 4.4% (67) were admitted to a general ward, and 1.5% (23) required ICU care or ECMO.
A more severe course with the need of hospitalisation was associated with higher age. Participants with a mild to moderate course were 44.0 ± 12.80 years old compared to 52.93 ± 14.75 for those who required hospitalisation (p < 0.001).
3.3. Sleep disturbances
There was a relatively homogenous distribution between individuals who had any sleep symptoms prior to COVID-19 (47.4%), and those who did not (52.4%). Of the total of 1507 respondents, 81.3% (1223) indicated that at least one sleep symptom either got worse or appeared anew after having had COVID-19 (see Table 1).
Table 1.
Sleep disturbances before and after COVID-19.
| Change after COVID-19 |
|||||
|---|---|---|---|---|---|
| Sleep disturbances prior to COVID-19 | No change (no symptom got worse or appeared anew) | At least one symptom got worse, no new symptoms | At least one new symptom | TOTAL | |
| NO | n | 207 | – | 584 | 791 |
| % (row total) | 26.2% | – | 73.8% | ||
| YES | n | 77 | 193 | 446 | 716 |
| % (row total) | 10.8% | 27.0% | 62.3% | ||
| TOTAL | n | 284 | 193 | 1030 | 1507 |
| % (row total) | 18.8% | 12.8% | 68.3% | ||
We found that individuals who had pre-existing sleep symptoms were more likely to develop a higher number of new sleep symptoms (p < 0.0001), and that all of those were more likely to persist for a long period of time.
In general, if a symptom appeared anew, it would be less likely to persist over an extended period of time (3 months or more) compared to if it got worse with COVID-19. This was true for insomnia, where 55.3% (315) of those who first developed this symptom after COVID-19 indicated it was persisting for 3 or more months, compared to 69.1% (251) of those who indicated it got worse (p < 0.001). In the case of nightmares, it was 62.4% (174) versus 77.3% (92), p < 0.001. For hypersomnolence, 60.7% (263) versus 78.6% (81), p < 0.001, for RLS 61.0% (161) versus 72.3% (70), p = 0.054, and for night sweating 61.0% (280) versus 71.7% (99), p < 0.022.
Fig. 1 shows the frequency of individual sleep disturbances in relation to COVID-19.
Fig. 1.
Sleep disturbances linked to COVID-19.
Overall, female gender was associated with a higher number of new or worsened sleep problems regarding COVID-19. The average number of sleep symptoms reported by women was 2.03 ± 1.44 versus 1.72 ± 1.43 in men (p < 0.0001). Specifically, we found an association with female gender for hypersomnolence (p = 0.0003), vivid dreams and nightmares (p = 0.0125), and excessive sweating during sleep (p = 0.0406).
Higher age was associated with an increased prevalence of insomnia (mean age 45.0 ± 12.7 versus 43.9 ± 13.7 years, p = 0.0286), RLS (mean age 46.4 ± 12.8 versus 44.0 ± 13.2 years, p = 0.0003), snoring or possible signs of sleep apnoea (mean age 47.4 ± 11.7 versus 44.2 ± 13.3 years, p = 0.0002), and excessive sweating during sleep (mean age 45.8 ± 12.9 versus 43.7 ± 13.2 years, p = 0.0001).
All the sleep disturbances we asked about (Fig. 1) were associated with a more severe course of the acute phase of the disease (p < 0.002).
Participants were divided into groups based on how much time had elapsed between filling out the survey and either the acute phase of the disease or their positive test result (in the case of asymptomatic individuals). Within the respondents, 7.4% (112) had the disease 2 months or less before taking the survey, 25.8% (389) 3–5 months before, 30.5% (460) 6–8 months before, 22.8% (343) 9–11 months before, and 13.47% (203) 12 months or more before the survey, with the longest time span being 32 months.
The long time span of our study allowed us to evaluate how the sleep issues change over time. We compared how many symptoms persisted in relation to the time that elapsed since the acute disease or positive test. The results show a mild trend of the number of sleep symptoms diminishing over time, with a peak in the last time period (12 or more months elapsed), see Fig. 2.
Fig. 2.
Persistence of sleep symptoms according to time elapsed from COVID-19 diagnosis/positive test.
When observing the sleep symptoms individually, a similar trend was present for insomnia (initiating and/or maintaining sleep), excessive sweating during sleep, sleep apnoea, hypersomnolence, and RLS. In comparison, vivid dreams or nightmares were more likely to decrease with time; for those with 2 or less months from the acute infection, they persisted in 75.8%, after 3–5 months in 68.8%, after 6–8 in 62.5%, after 9–11 months in 57.6% (p < 0.0427). However, a similar peak was observed in the last group with 12 months or more since the acute disease, where 77.8% indicated the issue persisted.
3.4. Quality of life
We found insomnia to be the main influencing factor in the perceived severity of the sleep symptoms. Participants who had new or worsened insomnia after COVID-19 reported a significantly higher impact the sleep problems had on their daily life (6.25 ± 2.29 out of 10) compared to those who did not have insomnia but had other symptoms (4.75 ± 2.54 out of 10), p < 0.001. However, whether insomnia appeared anew or got worse did not make a significant difference.
3.5. Circadian rhythm changes
From the total number of respondents, 29.6% (446) participants did not notice a change in either the duration or timing of sleep after having had COVID-19.40.6% (612) experienced shorter sleep duration after having had the disease. A majority of this group (51.3%, n = 314) did not change their bedtime, whereas 28.4% (174) went to bed later than before, and 20.3% (124) moved their bedtime to earlier hours. A total of 23.3% (612) reported sleeping more after having had COVID-19, mainly indicating they go to bed earlier than before (55.3% [194]). For 31.1% (109), bedtime remained the same, and 13.7% (48) reported going to bed later.
3.6. Dreaming and parasomnia symptoms
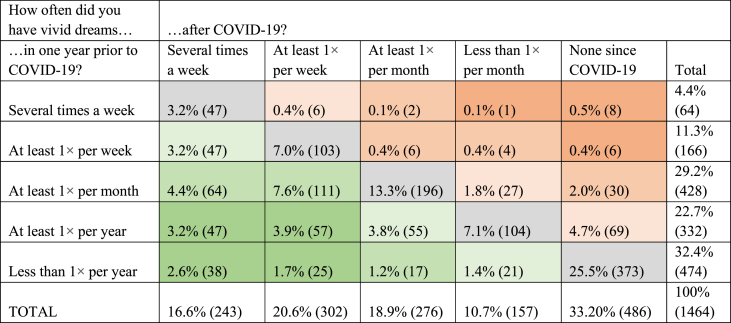
From the total sample, 26.4% (389) participants indicated that vivid dreams or nightmares appeared as a significant symptom of COVID-19. When asked about changes in dream frequency, 32.9% (482) of the respondents indicated an increase in the frequency of vivid dreams after having had COVID-19, whereas 56.2% (823) did not report any change, and 10.9% (159) reported having fewer vivid dreams. The last group consisted mostly of individuals who filled out the questionnaire soon after infection, and who had few vivid dreams before the infection as well (details are shown in Table 2).
Table 2.
Changes in frequency of vivid dreams - before and after COVID-19.
A total of 16.3% of those who had vivid dreams at least once per year stated that they frequently experienced them as nightmares, whereas 38.29% indicated that this was the case sometimes, and 45.4% did not experience nightmares. We found that those who had a more makeable increase in dream frequency after COVID-19 were also more likely to have nightmares (p < 0.001). Out of the 1507 respondents, 27.1% (401) participants indicated that they had at least one possible symptom of parasomnia in the last few years, whereas 20.0% (301) were not sure. Specifically, talking or screaming during sleep was reported by 11.5% (173), gesturing, kicking or similar limb movements in sleep were present in 9.5% (143), 5.1% (77) had scary dreams, woke up screaming at night but did not remember the event in the morning, and 1.1% (16) indicated they were injured during sleep.
The follow-up question pertaining to the development of these issues in relation to COVID-19 was answered by 302 participants. Out of these, 39.4% (119) did not notice any change in relationship to COVID-19, for 33.4% (101), these symptoms became more frequent after having had the disease, 13.2% (40) experienced them for the first time after COVID-19, and 13.9% (42) were not sure.
We found that increased frequency of vivid dreams, nightmares, and dream enactment behaviour were strongly associated with a severe course of the acute phase of the disease (p < 0.001). A significant increase in vivid dream frequency was noticed by 25.9% of participants who had an asymptomatic or very mild course of the acute disease, compared to 35.6% of those with a more severe course, and 41.9% of those who needed hospitalisation. In the case of nightmares, 43.0% of those with asymptomatic course indicated they had nightmares at least sometimes. For a moderate course, it was 59.0%, and for a severe course 64.9%.
Age was of significance for dream frequency prior to COVID-19, where younger participants (18–39 years) were more likely to report a higher dream frequency (p < 0.001). However, we did not determine any association between age and increased dream frequency after COVID-19 or the presence of nightmares. Dream enactment behaviour was negatively associated with age, with 23.9% (137) of participants between 18 and 39 years reporting this issue compared to 15.1% (124) and 15.0% (14) in the age groups 40–64 years and 65+ years, respectively (p < 0.001). No association with gender was found.
3.7. COVID-19, sleep, and mental status
In the total sample of this study, 59.4% (895) reported experiencing some form of mental changes in connection to COVID-19. Specifically, 28.1% (423) indicated having anxiety; 9.4% (142) reported feeling depressed; and 21.9% (330) experienced both.
The presence of mental status changes was strongly associated with a higher number of new or worsened sleep problems due to COVID-19. Participants with anxiety or depression reported a higher mean count of sleep problems (2.23 ± 1.41) compared to those without psychological changes (1.60 ± 1.38; Z = −6.6552, p < 0.0001). Analysis of individual sleep disturbances revealed a strong association between anxiety and insomnia (p < 0.0001). A total of 56.3% (171 out of 304) of participants with isolated insomnia after COVID-19 reported anxiety, compared to 39.1% (146 out of 373) of those without insomnia. Similar associations were found for isolated excessive sweating during sleep (p = 0.0060) and isolated vivid dreams or nightmares (p = 0.0214). No association with anxiety or depression was found for isolated RLS, snoring or problems breathing, or hypersomnolence.
Depressive experiences were not associated with any of the individual symptoms, but rather with the total number of sleep disturbances that developed or worsened in relation to COVID-19. The mean count of new or worsened sleep problems in those with isolated depression was 2.18 ± 1.42 versus 1.80 ± 1.44 in those without mental status changes (Z = −3.5474, p < 0.0001).
Psychological changes were strongly associated with female gender. A total of 63.8% (616) female participants indicated they had experienced anxiety and/or depression in relation to COVID-19, compared to 51.6% (277) of males (p < 0.001).
Severe acute course of the disease with hospitalisation was associated with the presence of isolated anxiety (p = 0.041); however, no association was found depression or mental status changes in general.
4. Discussion
The main aim of our study was to evaluate the type, frequency, and persistence of various sleep symptoms related to COVID-19 up to one year after acute infection/positive testing in a homogenous Caucasian population. To our knowledge, our sample of 1507 participants from a single country, collected by one tertiary sleep centre, is the largest sample yet. Our study took place outside of strict anti-pandemic measures, minimising their effect on our findings. The centralised character of the study and a relatively homogenous sample eliminates potential discrepancies caused by a varying degree of both anti-pandemic measures and pandemic severity, which were reported to be a limiting factor in international studies published to-date [14].
Sleep disorders are a common manifestation of COVID-19 infection and a frequent component of post-acute COVID syndrome (PACS) also known as long COVID [7,15,16]. The reported prevalence of sleep disorders among COVID-19 patients varies significantly, ranging from 26.0% to 74.8%, as per different studies and meta-analyses [[6], [7], [8],17]. Our finding that 62.3% of individuals reported at least one newly developed sleep symptom in relation to COVID-19 falls within this range. The variation in prevalence reported across the literature may likely be attributed to differences in study design, study populations, specific symptoms included, and timing of the research. Anti-pandemic measures and lockdowns have been recognised as a potential confounding factor, significantly contributing to an increase in sleep disturbances such as insomnia or nightmares [9,18].
We found that post-COVID sleep disturbances in general as well as all the individual symptoms were associated with a more severe acute course of the disease. Pre-existing sleep disorders were also found to be a risk factor for both the number and duration of sleep disturbances after COVID-19. Both of these findings confirm previous reports in the literature [8,9,19,20].
Most of the research published to-date only mentions post-COVID sleep disorders in general. Only one study providing details on the prevalence of individual sleep symptoms after COVID-19 has been found [16]. Similar to our findings, Davis et al. reported insomnia as the most prevalent sleep disorder during or up to 7 months after COVID-19 (60%), followed by night sweats (41%), vivid dreams (33%), nightmares (26%), RLS (18%), and sleep apnoea (10%).
Furthermore, our aim was also to evaluate gender differences in post-COVID sleep disorders. The results showed that the female gender was associated with a higher number of new or worsened sleep symptoms after COVID-19.
Literature findings differ in this regard. Some studies indicate that females are more at risk of developing long COVID [2,17,22]. The meta-analysis by Alimoradi et al. shows that the female gender was a risk factor for occurrence of sleep disorders both in COVID-19 patients and the general population during the pandemic [23]. On the contrary, Deng et al. report no gender differences [19], and Jahrami et al. report the male gender as having a higher prevalence of sleep disorders in patients with COVID-19 (β = 3.6, p = 0.001) as well lower sleep quality indicated by the Pittsburgh Sleep Quality Index (p = 0.04) [17].
In terms of specific sleep symptoms, we found excessive daytime sleepiness, vivid dreams and nightmares, and night sweats to be more common in females. Gender association of individual sleep symptoms in COVID-19 patients has scarcely been discussed. Similar to our results, the study by Liguori et al. found that women were more likely than men to report daytime sleepiness (47.72% [21] versus 22.03% [13], p = 0.0061), whereas for sleep impairment, gender did not play a role [6]. However, the study sample was rather small, with 103 participants. Data were collected during hospitalisation with acute COVID-19; therefore, it is not indicative of the prevalence or gender association of these symptoms from a long-term point of view.
Another interesting finding of our study was that female participants were also significantly more likely to report anxiety or depression after COVID-19. Although we did not collect detailed information on the onset or duration of the mental health issues, a bidirectional relationship between sleep and mental changes has been well documented [17,24]. Our results seem to confirm this, showing a significant association between the number of sleep disorders and both anxiety and depression.
Several theories may explain the differences in gender association reported in the literature. In studies conducted during the first lockdown and first pandemic wave, the different stress-coping strategies applied by both genders may have been an influencing factor. Salfi et al. report that women were particularly affected by sleep and mood disorders at the start of lockdown, while men reported an increase of these issues after several weeks, with the gender gap decreasing [22,23]. Regional and cultural differences may have also played a role. The metanalysis by Cénat et al. suggests that while Western and Middle Eastern studies reported an association between mental health problems and female gender during the first wave of the pandemic, Chinese studies did not [25].
Similar to the study by Liu et al. [26], we found a negative association between increased dream-enactment behaviour in individuals after COVID-19 and age. While dream-enactment behaviour traditionally tends to be perceived as a manifestation of rapid eye movement sleep behaviour disorder (RBD), the high presence of this symptom in a young population of post-COVID patients points to other potential causes, including post-traumatic stress disorder (PTSD) or nREM parasomnias (non-REM parasomnias) with an RBD-like manifestation [10,24,26]. Clinical examination, polysomnography monitoring as well as a long-term follow-up of these patients would be necessary to better understand this phenomenon and its causes.
In terms of considering sleep disturbances as a part of long COVID, our study demonstrated that with more time elapsing since COVID-19 diagnosis, the number of persisting sleep symptoms gradually decreased. However, we observed a surprising increase in all symptoms after twelve or more months since the acute infection. Alzueta et al. reported similar findings in their online international study of post-COVID sleep health using the Regulatory Satisfaction Alertness Timing Efficiency Duration (RUSATED) questionnaire (1001 participants, 1–436 days since COVID-19 diagnosis), suggesting that a longer time elapsing from acute infection may be associated with poorer sleep health [27]. The meta-analysis by Alkodayami et al. further supports this finding, in which sleep disturbances (a grouping term used for insomnia, daytime sleepiness, sleep difficulties, and/or sleep disorders) were reported by 24% at a 3- to <6-month follow-up compared to 30% at a >12-month follow-up [28]. To our knowledge, no study has previously evaluated the development of individual sleep symptoms related to COVID-19 for more than 6 months.
Finally, it is worth noting that in patients with long COVID, lasting 12 or more months, the chronic character of their condition and its impact on their mental health may present a significant stress factor, which may in turn affect their sleep quality and contribute to exacerbation of sleep disturbances [[29], [30], [31]]. Our findings as well as many previous studies show that mental health issues such as anxiety and depression are strongly associated with sleep disturbances, and there is a bidirectional relationship between these two groups of symptoms. Therefore, extra caution should be taken when treating patients presenting with either of these complaints, as they may require more comprehensive and targeted care to address the underlying causes of their sleep problems [8,32].
Further research and long-term follow-up are needed to gain a deeper understanding of the development of sleep disturbances in post-COVID patients over time and to distinguish the differences between viral lineages.
Several factors may have influenced our results. Selection bias related to a decreased interest in taking part in the study from asymptomatic participants with a long time since diagnosis may have contributed to the higher prevalence found within the last group. It is also likely that the viral lineage contracted by the participants played a role. The first four groups (0–11 months elapsed) completed the survey between June 1, 2021, and December 31, 2022. Based on the time of acute disease onset, we estimated that 12.5% of participants were infected with one of the Omicron lineages. The last group (12 months or more elapsed) completed the questionnaire prior to January 2022, when Omicron was not yet present in the Czech Republic and were most likely affected by the Alpha or Delta mutation, both of which were associated with a more severe course, higher hospitalisation rates, and potentially higher tendency to develop long COVID [33,34].
5. Conclusion
Sleep disturbances are one of the most common neurological manifestations of COVID-19 and can present as a broad spectrum of symptoms. Our study shows that these issues can persists for months to years. While prospective studies are needed to evaluate how these symptoms will change over time, it is obvious they affect a large group of the population. Previous exposure to COVID-19 should therefore be considered as a potential contributing factor in patients with a sudden onset or rapid progression of sleep disorders after the start of the pandemic and should become an essential part of the patient's history in sleep medicine practice or even in first-contact health care providers.
Funding
This work was supported by the Charles University Grant Agency (GA UK) [project number: 318522, name: “Neuro-covid: Long-term evaluation of sleep symptoms related to Covid-19 infection”, submitted under the Third Faculty of Medicine, Charles University, Ruská 87, 100 00 Prague Czech Republic].
Ethics approval
The project was approved by the Ethical Committee of the National Institute of Mental Health, Klecany, Czech Republic (Neurocovid study, approval No. 205/20, approved on 16 December 2020).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Tereza Dvořáková, Email: tereza.dvorakova@nudz.cz.
Radana Měrková, Email: radana.merkova@nudz.cz.
Jitka Bušková, Email: jitka.buskova@nudz.cz.
References
- 1.Di Carlo D.T., Montemurro N., Petrella G., Siciliano G., Ceravolo R., Perrini P. Exploring the clinical association between neurological symptoms and COVID-19 pandemic outbreak: a systematic review of current literature. J Neurol. 2021;268(5):1561–1569. doi: 10.1007/s00415-020-09978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ousseiran Z.H., Fares Y., Chamoun W.T. Neurological manifestations of COVID-19: a systematic review and detailed comprehension. Int J Neurosci. 2021;27:1–16. doi: 10.1080/00207454.2021.1973000. Published online September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfì A., Bernabei R., Landi F. For the gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liguori C., Pierantozzi M., Spanetta M., et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun. 2020;88:11–16. doi: 10.1016/j.bbi.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alimoradi Z., Broström A., Tsang H.W.H., et al. Sleep problems during COVID-19 pandemic and its' association to psychological distress: a systematic review and meta-analysis. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Partinen M., Holzinger B., Morin C.M., et al. Sleep and daytime problems during the COVID-19 pandemic and effects of coronavirus infection, confinement and financial suffering: a multinational survey using a harmonised questionnaire. BMJ Open. 2021;11(12) doi: 10.1136/bmjopen-2021-050672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto J., van Zeller M., Amorim P., et al. Sleep quality in times of COVID-19 pandemic. Sleep Med. 2020;74:81–85. doi: 10.1016/j.sleep.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbarialiabad H., Taghrir M.H., Abdollahi A., et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49(6):1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . 2022. Post COVID-19 condition (long COVID). World health organization europe.https://www.who.int/europe/news-room/fact-sheets/item/post-COVID-19-condition Published December 7. [Google Scholar]
- 13.Postuma R.B., Arnulf I., Hogl B., et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov. Disord. Off. J. Mov. Disord. Soc. 2012;27(7):913–916. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fränkl E., Scarpelli S., Nadorff M.R., et al. How our dreams changed during the COVID-19 pandemic: effects and correlates of dream recall frequency - a multinational study on 19,355 adults. Nat Sci Sleep. 2021;13:1573–1591. doi: 10.2147/NSS.S324142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis H.E., Assaf G.S., McCorkell L., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahrami H., BaHammam A.S., Bragazzi N.L., Saif Z., Faris M., Vitiello M.V. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2021;17(2):299–313. doi: 10.5664/jcsm.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musse F.C.C., Castro L. de S., Sousa K.M.M., et al. Mental violence: the COVID-19 nightmare. Front Psychiatr. 2020;11 doi: 10.3389/fpsyt.2020.579289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng J., Zhou F., Hou W., et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2021;1486(1):90–111. doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merikanto I., Dauvilliers Y., Chung F., et al. Sleep symptoms are essential features of long-COVID - comparing healthy controls with COVID-19 cases of different severity in the international COVID sleep study (ICOSS-II) J Sleep Res. 2023;32(1) doi: 10.1111/jsr.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asadi-Pooya A.A., Akbari A., Emami A., et al. Risk factors associated with long COVID syndrome: a retrospective study. Iran J Med Sci. 2021;46(6):428–436. doi: 10.30476/ijms.2021.92080.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alimoradi Z., Gozal D., Tsang H.W.H., et al. Gender-specific estimates of sleep problems during the COVID-19 pandemic: systematic review and meta-analysis. J Sleep Res. 2022;31(1) doi: 10.1111/jsr.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neculicioiu V., Colosi I., Costache C., Sevastre-Berghian A., Clichici S. Time to sleep? - a review of the impact of the COVID-19 pandemic on sleep and mental health. Int J Environ Res Publ Health. 2022;19:3497. doi: 10.3390/ijerph19063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cénat J.M., Blais-Rochette C., Kokou-Kpolou C.K., et al. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatr Res. 2021;295 doi: 10.1016/j.psychres.2020.113599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Partinen E., Chan N.Y., et al. Dream-enactment behaviours during the COVID-19 pandemic: an international COVID-19 sleep study. J Sleep Res. 2023;32(1) doi: 10.1111/jsr.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alzueta E., Perrin P.B., Yuksel D., et al. An international study of post-COVID sleep health. Sleep Health. 2022;8(6):684–690. doi: 10.1016/j.sleh.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alkodaymi M.S., Omrani O.A., Fawzy N.A., et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022;28(5):657–666. doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salfi F., Amicucci G., Corigliano D., et al. Two years after lockdown: longitudinal trajectories of sleep disturbances and mental health over the COVID-19 pandemic, and the effects of age, gender and chronotype. J Sleep Res. 2022;1 doi: 10.1111/jsr.13767. Published online November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam M.H.B., Wing Y.K., Yu M.W.M., et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 31.Han Q., Zheng B., Daines L., Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathog. Basel Switz. 2022;11(2) doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang H., Tu S., Sheng J., Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. 2019;23(4):2324–2332. doi: 10.1111/jcmm.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.COG-CZ - Česká Republika. Linie SARS-CoV-2 Detekované v Česku. COG-CZ - Česká Republika https://virus.img.cas.cz/lineages..
- 34.Nyberg T., Ferguson N.M., Nash S.G., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet Lond Engl. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.