Abstract
Surveys indicated that stroke classified among the leading cause of death as well as combined death and disability worldwide resulting in a great loss for the global economy. The present study aims to evaluate the neuroprotective potential of the biflavonoid amentoflavone (AMNT) in alleviating cerebral ischemia/reperfusion (IR) injury in rats, and to elucidate the possible underlying mechanism of an experimental condition with similar circumstances to stroke. Cerebral ischemia was achieved through left common carotid artery occlusion for 60 min, followed by blood flow restoration. Sham-operated control rats subjected to the same surgical process except for brain IR. Rats were orally administered AMNT/ or vehicle for three days’ prior surgical operation, and for another three days after left brain IR. Rats of all groups were assessed for neurological deficits 24 h following brain IR. Each group was divided into two subgroups one for the rotarod testing and biochemical assessment while the other subgroup to perform the activity cage testing, histopathological study, immunohistochemistry, and gene expression analysis. AMNT enhanced brain levels of GSH and CAT activities, suppressed neuroinflammation via reducing the inflammatory cytokines in the serum, and enhanced brain contents of TBK1 and IFNβ. AMNT downregulated TLR4-/NF-κB signaling pathway as a result of the HMGB1 suppression. Moreover, AMNT blocked apoptotic cell death by suppressing the NF-κB signaling pathway and reducing the activation of caspase-3. These findings revealed that AMNT attenuates I/R–induced cerebral injury possibly by regulating the HMGB1-mediated TLR4/NF-kB pathway. Thus, AMNT could provide potential preventive and therapeutic option for cerebral stroke.
Keywords: Common carotid artery occlusion, NF-κB, TBK1, Amentoflavone, Rat
1. Introduction
Recent estimate indicated that stroke classified as second leading cause of death and comes as the third cause of combined death and disability worldwide (Feigin et al., 2022). Stroke costs the global economy about $ 891 billion (Owolabi et al., 2022). The number of cases increased by 70% between 1990 and 2019 including 43% increase in death (Feigin et al., 2022). Stroke involves sudden blockage of blood vessels by either embolism or thrombus resulted in instant deprivation of glucose and oxygen supply to the cerebral tissue. Evidences indicated that inflammation and ischemic injury support pathogenic development of stroke although different other mechanisms may be involved (Lakhan et al., 2009).
The pathogenesis of stroke and other neurological conditions resulted from oxidative stress along with other factors. Oxidative stress is a condition characterized by disturbance of the physiological balance normally exists between oxidants and antioxidants in favor of the oxidant species causing potential cells and tissues damage. Oxidative stress resulted in the development of reactive oxygen species (ROS) and reactive nitrogen species (RNS) via several injury mechanisms including mitochondrial inhibition, reperfusion injury, Ca+2 overload and inflammation. These injury mechanisms end up with ischemic cell death (Coffey et al., 1990).
Cytokines are overexpressed in the brain as a result of stroke and other diseases. Cytokines are expressed in the brain by resident brain cells such as neurons, glia and multiple immune cells. Moreover, peripherally produced cytokines are also involved in brain inflammation. Consequently, the peripherally originated cytokines from NK cells, T lymphocytes, mononuclear phagocytes and polymorphonuclear leukocytes might have a crucial rule in the CNS inflammation (Ferrarese et al., 1999).
Amentoflavone (AMNT) is a biflavonoid also known as 3′, 8″-biapigenin isolated for the first time from the leaves of Selaginella rupestris, S. tamariscina then from Ginkgo biloba (Chakravarthy et al., 1981, Lobstein-Guth et al., 1988, Okigawa et al., 1971). AMNT exhibit multiple biological activities including antiviral, antibacterial, antifungal, anti-inflammatory, anti-arthritis, anti-oxidative, anti-angiogenesis, osteogenesis, radioprotection, antidiabetic, neuroprotection and antidepressant (Xiong et al., 2021). AMNT mechanisms of action include inhibition of degranulation and arachidonic acid release from neutrophils as well as suppression of cyclooxygenases, phospholipase A2 and inducible nitric oxide synthase (Bonesi et al., 2018).
Administration of AMNT by different routes using rats indicated that 90.7% of AMNT was circulated as conjugated metabolites after oral administration while in case of intravenous and intraperitoneal injection, 73.2% ± 6.29% and 70.2% ± 5.18% of AMNT was present as conjugated metabolites respectively. The T1/2 and Tmax of AMNT were 2.06 h ± 0.13 h and 1.13 h ± 0.44 h in normal rats respectively (Yu et al., 2017).
The current study was conducted to investigate the potential protective rule of AMNT against cerebral injury induced by ischemia/reperfusion in a unilateral common carotid artery occlusion (CCAO) rat model.
2. Materials and methods
2.1. Chemicals
Amentoflavone (AMNT) (powder form, 98% by HPLC) was obtained from Aktin Chemicals, Inc (Chengdu, China). Ketamine, xylazine, tween 80, Bovine Serum Albumin (BSA), 3,3diaminobenzidine (DAB), Tris Buffered Saline (TBS), Mayer’s hematoxylin, DPX and Triazole were purchased from Merck (Darmstadt, Germany).
2.2. Animals
Forty-eight adult male Wistar rats (180 and 200 g each), were provided by the Animal Facility at the National Research Centre, Giza, Egypt. The animals were housed under normal dark–light cycles with a controlled room temperature using standard food and water ad libitum feeding, in cages free from infectious rodent pathogens. Before beginning the experimental procedure, the animals were adapted to these conditions for one week. Experiments complied with the National Regulations of Animal Welfare and the Institutional Animal Ethical Committee (IAEC), (approval no. 1416072022).
2.3. Induction of brain I/R injury
The left common carotid artery occlusion described by (Das et al., 2018) was used to induce focal cerebral I/R injury in the experimental groups of rats. The animals were orally administered with AMNT/ or vehicle for three days’ prior under normal dark–light cycles with a controlled room temperature the surgical operation. After 60 min of the last dose of AMNT/ or vehicle rats were anesthetized by an intraperitoneal injection of ketamine at 50 mg/kg. Rats were then placed in the reclining position and their limbs were fixed by adhesive tape to the operating table. After a midline cervical incision, the left common carotid artery was accessed, and separated from the Vagus nerve with great care. Noninvasive arterial clamps were used to blockage the left common carotid artery inducing the targeted cerebral ischemia. The clamps were removed to restore the blood flow and allow reperfusion after 60 min of occlusion. Sham-operated control group of rats encountered the same surgical procedure except for brain IR. A lamp radiation was used to ensure rectal temperature is kept during the surgery at 37 ± 0.5 °C. A recovery period of four hours was allowed in individual cages without restrictions to food and water.
2.4. Experimental groups
The 48 rats were arranged aimlessly into four groups each containing 12 animals:
-
1.
Sham group: Rats were exposed to the same surgical procedure but excluding the left common carotid artery blockage and treated with the vehicle (2% Tween 80 in sterile saline) for three days prior to sham operation.
-
2.
I/R Control group: Rats were subjected to left brain ischemia and treated with the vehicle for three days prior to brain IR.
-
3.
I/R + AMNT-20 group: Rats were subjected to ischemia and treated with AMNT at 20 mg/kg for three days prior to brain IR.
-
4.
I/R + AMNT-40 group: Rats were subjected to ischemia and treated with AMNT at 40 mg/kg for three days prior to brain IR.
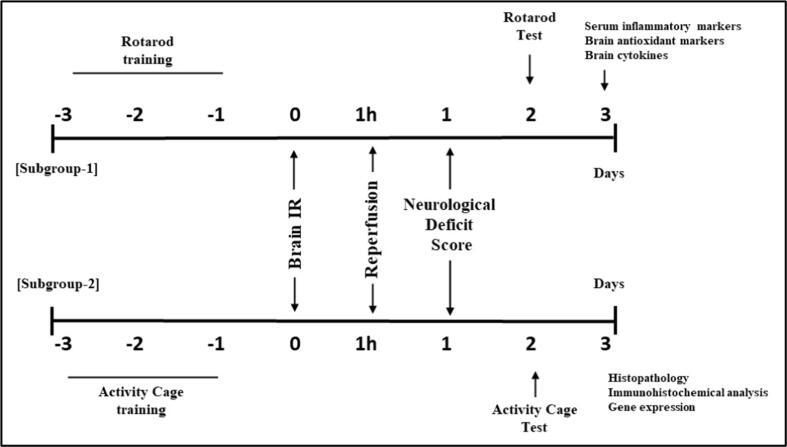
Two doses of AMNT were used based on Su et al. (2019). The vehicle and AMNT were given orally for three days to rats prior to brain IR and at the onset of reperfusion. Administration was continued for additional three days after left brain IR. All rats were assessed for neurological deficits 24 h following brain IR. Animals from each group were divided into two subgroups each of 6 rats. The first subgroup was used to perform the rotarod test and biochemical assessment, while the second subgroup was used to perform the activity cage test, histopathological analysis, immunohistochemistry, and gene expression analysis (Fig. 1).
Fig. 1.
Experimental design.
2.5. Effect on neurological deficits
Animal neurological deficit was evaluated following the reported method (Longa et al., 1989). Neurological status was scored on a four-point scale: 0 = no observable deficits; 1 = failure to extend right forepaw fully indicating a mild focal neurologic deficit; 2 = spontaneous circling to the right reflecting a moderate focal neurologic deficit; 3 = falling to the right as a result of severe focal deficit; and 4 = no spontaneous activity and depressed level of consciousness as a result of severe focal deficit. Rats scoring 0 or 4 were excluded from the study while those scoring 1–3 points met the inclusion criteria for the experiment (Abdel-Rahman et al., 2020). In our procedures none of the animals were excluded based on this criteria.
2.6. Behavioral evaluation
2.6.1. Effect on motor coordination and balance using rotarod
Motor coordination and balance of the rats was evaluated two days after brain IR using rotarod apparatus (Model No. 7750; Ugo Basile) as described earlier (Vijitruth et al., 2006). Each animal was trained on the apparatus using accelerated velocity level from 4 to 40 rpm three days prior to focal cerebral I/R injury induction. On the test day rats were individually placed in the apparatus and subjected to the testing within 5 min. The observer remained ignorant concerning the experimental groups. Each rat was subjected to the experiment for three times with 10 min interval between them. The latency to fall was recorded with a maximum time of 300 s in each trial. The mean value was determined for each rat. Passive rotation, going with the rod without walking was considered as a fall (Abdel-Rahman et al., 2020).
2.6.2. Effect on spontaneous motor activity using activity cage
The spontaneous motor activity of rats was determined two days after brain IR using a grid floor activity cage (Model No. 7430, Ugo-Basile, Comerio, Italy). Rat’s movements resulted in infrared beams interruption that recorded automatically. The activity cage software converts the beam-interruption information to provide horizontal movements counts. Animals were introduced into the activity cage for a 5 min session and the basal activity counts were recorded three days before the induction of focal cerebral I/R injury. The instrument floor was cleaned using 70% (v/v) alcohol solution in distilled water between sessions to exclude any olfactory cues. On the test day, each rat was re-exposed to the activity cage for a 5 min session and the final horizontal movements counts were obtained (Ogaly et al., 2022).
2.7. Blood samplings
Blood samples were collected two hours after the last dose. Animals were anesthetized by intraperitoneal injection with 75 mg/Kg ketamine and 5 mg/ kg xylazine. Blood samples were collected through the retro-orbital venous plexus using sampling tubes. Immediately after collection blood samples were subjected to centrifugation for 15 min at 3500 rpm and 4 ± 2 °C to separate serum.
2.8. Effect on serum inflammatory biomarkers
Cytokines known to mediate inflammation (inflammatory cytokines) such as (IL-1β, IL-6 and TNF-ɑ,) were estimated in the collected serum using commercial ELISA kits according to their manufacturer's guidance.
2.9. Brain tissue samplings
After blood sampling, anesthetized animals were decapitated and their left-brain hemispheres were quickly dissected on ice plate. Rat brains of subgroup-1 were used for evaluation of the antioxidant markers (MDA, GSH and CAT) and cytokines (TBK1, NF-κB and IFNβ) and those of subgroup-2 were used for histopathological, immunohistochemical and gene expression evaluations.
2.10. Preparation of tissue homogenates
Fresh brain tissues were collected and washed with refrigerated saline. A 10% (w/v) of brain tissue homogenates were prepared in 0.1 M phosphate buffer (pH 7.4). Using a cooling centrifuge (2 k15; Sigma/Laborzentrifugen) the homogenates were centrifuged at 10,000 rpm for 15 min. The resulting supernatants were collected, instantly frozen in liquid nitrogen and stored at −80 °C for further investigation.
2.11. Effect on brain inflammatory cytokines
Nuclear factor kappa- B (NF-κB), TANK-binding kinase-1 (TBK1), and Interferon beta (IFNβ) levels were determined in brain homogenate using commercial ELISA kits.
2.12. Effect on brain antioxidant markers
2.12.1. Malondialdehyde (MDA) content
Brain MDA content was determined according to the reported method (Begoña Ruiz-Larrea et al., 1994).
2.12.2. Reduced glutathione (GSH) content
Brain GSH content was determined following the literature method (Ellman, 1959).
2.12.3. Catalase (CAT) content
Brain CAT content was determined in accordance with the described procedure (Chance & Maehly, 1955).
2.13. Histopathological examination of brain tissue
Brain left hemispheres was preserved using 10% neutral-buffered formalin for 24 h, then washed with tap water prior to light microscopy staining. Samples were dehydrated using serial dilutions of alcohol followed by clearance in xylene and then embedded in paraffin wax using hot air oven at 56 °C for 6 h. Paraffin wax tissue blocks were sectioned by microtome adjusted to 4–6 µm thickness. The obtained sections were placed on glass slides and deparaffinized. Sections were stained using Hematoxylin and Eosin stain for routine histological examination (Bancroft & Gamble, 2008).
2.14. Immunohistochemical examination of Caspase-3
Brain samples of all the groups were deparaffinized then rehydrated. The antigen recovery was performed using 10 mM citrate buffer of pH 6.0 as described earlier (Ogaly et al., 2022). Rabbit polyclonal anti-Caspase-3 Antibody (Elabscience, Cat# E-AB-63602, Dil.1:50) was added to the brain tissue specimens and incubated overnight in humid place. The primary antisera were excluded with 1 mg/mL BSA (Sigma) as negative control slides. The tissue sections were thoroughly washed with Tris buffer saline to get rid of the unconjugated antibodies. Then sections were incubated for 10 min with a biotinylated goat anti rabbit and mouse antibody (Vectastain ABC-HRP kit, Vector laboratories) followed by thorough washing with TBS. The DAB (3,3diaminobenzidine) solution was then added to the sections prior to counterstaining with Mayer’s hematoxylin and mounting with DPX. The photographed pictures of the sections were analyzed using ImageJ soft wear. The percentage of positive immune-staining cells was estimated from the results of 10 microscopic fields/group.
2.15. Real-time PCR analysis of TLR4 and HMGB1 genes
The expression levels of TLR4 and HMGB1mRNA in the brain tissues were analyzed using RT-PCR after 24 h of the cerebral ischemia reperfusion. Total RNA purification from homogenized brain tissues was achieved using Triazol Reagent method (Invitrogen) as described by the manufacturer. The DNA removal, reverse transcription and PCR amplification reactions were carried out using the One-Step SuperScript IV RT-PCR kit (Thermo Fisher Scientific) and specific oligonucleotide primers listed in Table 1. During PCR amplification, levels of mRNA expression of the target genes were analyzed by the real-time quantitative method using SYBR green fluorescence, and the GAPDH gene was used as an internal control gene for normalization of variation in the transcription levels of target genes. For data analysis, the relative expression of TLR4 and HMGB1 mRNA were calculated using cycle threshold (Ct) for the target genes and GAPDH by the ΔΔCt method (Livak & Schmittgen, 2001).
Table 1.
Primers sequences used in Real-time PCR.
| Gene | Upstream primer (5′-3′) | Downstream primer (5′-3′) | Accession # |
|---|---|---|---|
| TLR4 | CCGTCACCACATACTGCCTTTA | GCAGTTTGGACTATTGAAATACGAAA | NM_001109668.1 |
| HMGB1 | CCTAAGAAGCCGAGAGGCAA | AAGTTGACAGAAGCATCCGGG | NM_001409387.1 |
| GAPDH | CCTCGTCTCATAGACAAGATGGT | GGGTAGAGTCATACTGGAACATG | NM_001394060.2 |
2.16. Statistical analysis
All the obtained results were demonstrated as mean ± SEM. Statistical evaluation was performed using one-way analysis of variance (one-way ANOVA) followed by Tukey's test to evaluate the intergroup variability by using Graph Prism®. The probability levels of equal or less than 0.05 was considered statistically significant.
3. Results
3.1. Effect on neurological deficits
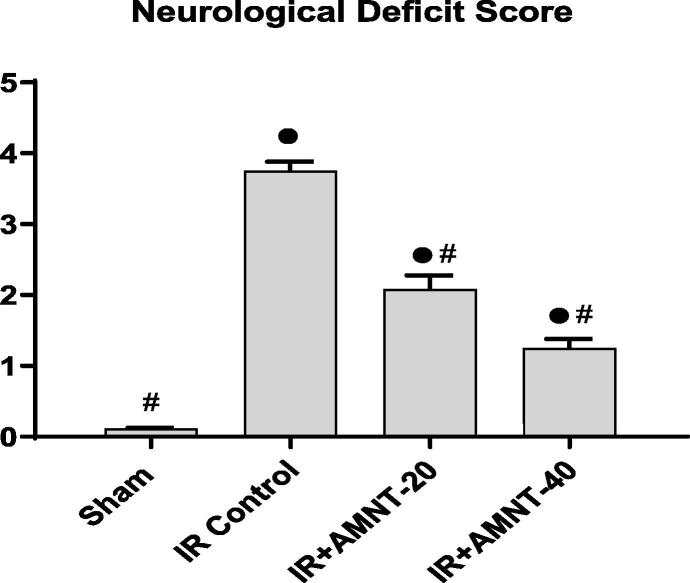
After reperfusion for one day the neurological deficit score was recorded by the neurological function testing for each group. Rats of the sham control group showed no neurological deficits. On the other hand, the neurological deficit score of the IR control group was significantly higher when compared with the sham group (Fig. 2).
Fig. 2.
Neurological deficit score. Data expressed as Mean ± SEM (n = 12). ● Significantly different from the values of sham group at p ≤ 0.05. # Significantly different from the values of IR control group at p ≤ 0.05.
As shown in Fig. 2, AMNT treatments (20 and 40 mg/kg) significantly attenuated the neurological deficit score in rats after IR injury as matched to the IR control rats. Notably, rats treated with the AMNT‐40 showed a more significant improvement in their neurological deficit score compared to AMNT-20 group.
3.2. Behavioral evaluation
3.2.1. Effect on motor coordination and balance using rotarod
Motor coordination activity was evaluated by the rotarod assay, as shown in Table 2. In the rotarod test, IR control rats expressed the lowest performance in the testing by showing a decreased latency to fall off the rotarod. The administration of AMNT improved the Rotarod performance significantly (P less than 0.05) in the treated groups as compared to the IR control rats. Interestingly, rats treated with the large dose of AMNT (40 mg/kg) expressed better improvement in the motor function in the rotarod test when compared to rats exposed to the lower dose of AMNT.
Table 2.
Effect of Amentoflavone on motor coordination using Rotarod test and locomotor activity using Activity cage test in IR rats.
| Group |
Falling latency time (sec) |
Locomotor activity (counts/5 min) |
||
|---|---|---|---|---|
| Basal | After IR | Basal | After IR | |
| Sham | 271.5 ± 7.46 | 278.3 ± 6.65 | 58.6 ± 2.54 | 55.8 ± 2.89 |
| IR Control | 266.8 ± 3.32 | 116.2 ± 5.69● | 54.0 ± 3.83 | 17.8 ± 1.01● |
| IR + AMNT-20 | 264.3 ± 8.97 | 169.7 ± 7.77●# | 51.5 ± 3.38 | 29.5 ± 1.06●# |
| IR + AMNT-40 | 267.7 ± 13.81 | 216.7 ± 11.26●#φ | 52.5 ± 3.10 | 43.7 ± 1.12●#φ |
Values are expressed as Mean ± SEM of six animals in each group.
● Significantly different from the values of sham group at p ≤ 0.05.
# Significantly different from the values of IR control group at p ≤ 0.05.
φ Significantly different from the values of IR + AMNT-20 group at p ≤ 0.05.
3.2.2. Effect on spontaneous motor activity using activity cage
The spontaneous motor activity of the rats in each group are presented in Table 2. Rats of all groups showed a similar basal locomotor activity. I/R control rats displayed significant decrease in the locomotor activity when compared with the sham-operated rats. AMNT at 20 mg/kg resulted in a marked increase in the locomotor activity of rats by approximately 1.65 folds, compared with the I/R group. The most significant improvement in the spontaneous motor activity of rats was recorded for the group treated with AMNT at 40 mg/kg.
3.3. Effect on serum inflammatory biomarkers
In Table 3 the serum levels of TNF-α, IL-1β, and IL-6 in IR control rats expressed dramatic enhancement which were 3.98-, 3.95-, and 5.18-fold higher than those of sham rats. AMNT treated at 20 and 40 mg/kg showed dose-dependent diminished accumulation of pro-inflammatory cytokines induced by I/R in comparison with IR control rats. In higher dose AMNT treated group, serum levels of TNF-α, IL-1β, and IL-6 were reduced to 30.44%, 29.34% and 25.35%, respectively of IR control rats.
Table 3.
Effect of Amentoflavone on serum inflammatory biomarkers of IR rats.
| Group |
TNF-α (pg/mL) |
IL-1β (pg/mL) |
IL-6 (pg/mL) |
|---|---|---|---|
| Sham | 28.1 ± 1.49 | 27.5 ± 1.10 | 16.5 ± 1.40 |
| IR Control | 112.0 ± 6.25● | 108.7 ± 4.37● | 85.6 ± 2.48● |
| IR + AMNT-20 | 65.9 ± 3.07●# | 70.0 ± 2.55●# | 52.6 ± 2.74●# |
| IR + AMNT-40 | 34.1 ± 2.05#φ | 31.9 ± 1.36#φ | 21.7 ± 1.95#φ |
Values are expressed as Mean ± SEM of six animals in each group.
● Significantly different from the values of sham group at p ≤ 0.05.
# Significantly different from the values of IR control group at p ≤ 0.05.
φ Significantly different from the values of IR + AMNT-20 group at p ≤ 0.05.
TNF-α: tumor necrosis factor alpha; IL-1β: interleukin −1 beta; IL-6: interleukin 6.
3.4. Effect on brain inflammatory cytokines
The brain inflammatory biomarkers were estimated in the sham and IR rats (Table 4). Significant decreases in the levels of brain inflammatory biomarkers; TBK1 and IFNβ in IR control rats in comparison with the sham group. On the contrary, I/R control rats showed increased level of NF-κB compared with the sham group. AMNT at 20 and 40 mg/kg significantly increased the levels of brain inflammatory biomarkers; TBK1 and IFNβ and diminished the brain level of NF-κB in a dose-dependent typical fashion (Table 4).
Table 4.
Effect of Amentoflavone on brain inflammatory cytokines of IR rats.
| Group |
TBK1 (mg/mg protein) |
NF-κB (ng/mg protein) |
IFNβ (pg/mg protein) |
|---|---|---|---|
| Sham | 0.5 ± 0.01 | 23.5 ± 1.48 | 74.8 ± 2.99 |
| IR Control | 0.1 ± 0.01● | 139.1 ± 2.99● | 17.9 ± 1.02● |
| IR + AMNT-20 | 0.2 ± 0.01●# | 65.6 ± 2.95●# | 46.7 ± 2.05●# |
| IR + AMNT-40 | 0.4 ± 0.01#φ | 31.8 ± 1.28#φ | 66.1 ± 2.70#φ |
Values are expressed as Mean ± SEM of six animals in each group.
● Significantly different from the values of sham group at p ≤ 0.05.
# Significantly different from the values of IR control group at p ≤ 0.05.
φ Significantly different from the values of IR + AMNT-20 group at p ≤ 0.05.
TBK1: TANK-binding kinase-1; NF-κB: Nuclear factor kappa- B; IFNβ: interferon beta.
3.5. Effect on brain antioxidant markers
Malondialdehyde (MDA) and glutathione (GSH) levels, and catalase (CAT) activity were quantified in brain homogenates as presented in Table 5. Massive increase in MDA level coinciding with massive decrease in GSH level, and activity of CAT were observed in IR control rats compared with the sham group. AMNT (20 mg/kg) treatment resulted in reduced MDA content and higher GSH content as compared to IR control rats. Interestingly, treatment with AMNT-40 decreased the brain MDA content, showed enhancement in the brain levels of GSH and CAT to just about their normal levels.
Table 5.
Effect of Amentoflavone on brain antioxidant markers of IR rats.
| Group |
MDA (nmol/mg protein) |
GSH (nmol/mg protein) |
CAT (mU/mg protein) |
|---|---|---|---|
| Sham | 0.4 ± 0.04 | 1.6 ± 0.11 | 2.9 ± 0.19 |
| IR Control | 1.6 ± 0.12● | 0.3 ± 0.02● | 1.1 ± 0.06● |
| IR + AMNT-20 | 1.0 ± 0.08●# | 0.9 ± 0.03●# | 1.7 ± 0.07●# |
| IR + AMNT-40 | 0.5 ± 0.04#φ | 1.3 ± 0.10#φ | 2.5 ± 0.10#φ |
Values are expressed as Mean ± SEM of six animals in each group.
●Significantly different from the values of sham group at p ≤ 0.05.
#Significantly different from the values of IR control group at p ≤ 0.05.
φSignificantly different from the values of IR + AMNT-20 group at p ≤ 0.05.
MDA: malondialdehyde; GSH: reduced glutathione; CAT: catalase.
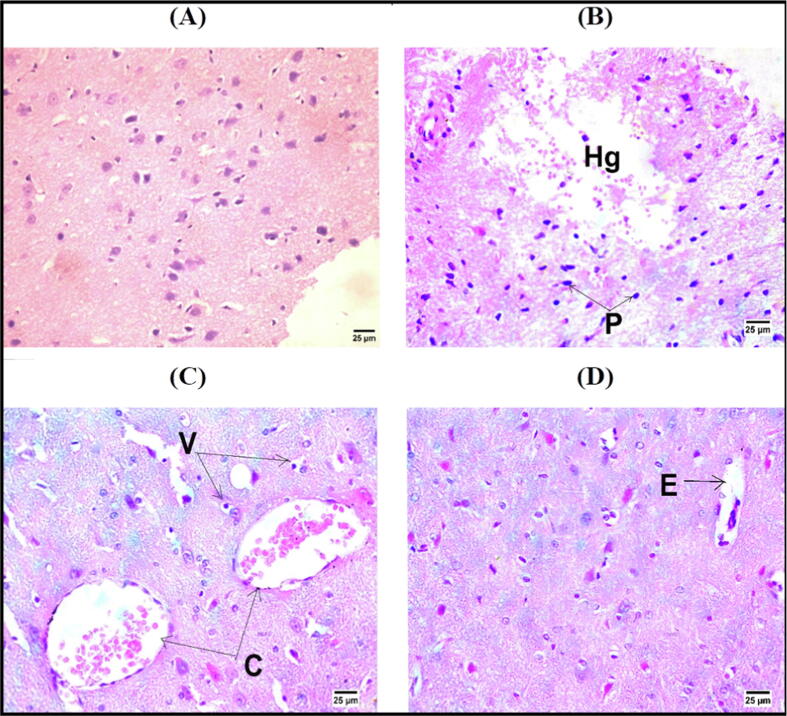
3.6. Histopathological examination of brain tissue
The brain tissues of normal control group exprssed normal histological architecture (Fig. 3A). In the IR control rats, there was marked cerebral hemorrhage with pyknotic nuclei of neurons (Fig. 3B). Nonetheless, brain sections of rats treated with AMNT-20 and AMNT-40 revealed congestion of cerebral blood vessels with vacuolar degeneration in neurons and perivascular edema (Fig. 3C&D).
Fig. 3.
Photomicrographs showing normal histological structure of brain of NC rats (A), cerebral hemorrhage [Hg] with pyknotic nuclei (P) of neurons in cerebrum of IR control rats (B), congestion of cerebral blood vessels [C] with vacuolar degeneration [V] in neurons of IR + AMNT-20 rats (C) and perivascular edema [E] in IR + AMNT-40 rats (D)(Hematoxylin and Eosin stain).
3.7. Immunohisochemical examination of Caspase-3
Fig. 4 shows the effect of AMNT-20 and AMNT-40 on Caspase-3 expression in the brain sections of I/R rats. Caspase-3 expression was dropped in the brain sections of sham-operated rats (Fig. 4A). Brain tissue sections of I/R control group disclosed increased expression of Caspase-3 immunopositive cells (Fig. 4B). Mild expression of Caspase-3 was observed in AMNT-40 -treated groups as shown in Fig. 4D.
Fig. 4.
Caspase-3 immuopositive cell was with dark brown cytoplasmic and nuclear color (arrows). (A) Sham group, (B) I/R Control group & (C) IR + AMNT-20 group showing high positive reaction for Caspase 3. (D) IR + AMNT-40 showing mild positive reaction for caspase 3 (IHC – peroxidase – DAB). (E) The bar chart represent caspase-3 immuopositive reaction. All values are expressed as mean ± SE (n = 6); ●P ≤ 0.05, statistically significant from the sham group. #P ≤ 0.05, statistically significant from IR control group.
3.8. Effect on TLR4 and HMGB1 gene expression
The expression of TLR4 mRNA in the brain tissue exposed to ischemic conditions of I/R model group, compared with sham group, expressed rising trend (P ≤ 0.05), while the expression of TLR4 in the ischemic brain tissues in the AMNT-20 and AMNT-40 treated groups revealed falling trend (P ≤ 0.05) (Fig. 5). Similarly, HMGB1 mRNA expression level tended to increase (P ≤ 0.05) in the brain of IR model group as compared to sham group. AMNT-20 and AMNT-40 administration significantly downregulated HMGB1 expression (P ≤ 0.05) (Fig. 5).
Fig. 5.
Effect of TLR4 and HMGB1 gene expression in rat brain tissues. Total RNA was purified and relative expression of TLR4 (A) and HMGB1 (B) was assessed by quantitative real-time PCR. Data are expressed as fold change over basal relative to GAPD gene, (mean ± SEM) (n = 6). ●P ≤ 0.05, statistically significant from the sham group. #P ≤ 0.05, statistically significant from IR control group.
4. Discussion
Cerebral circulation disorders resulted in a range of neurological and mental illnesses. Induction of cerebral transient hypoperfusion, in experimental animal models via unilateral left common carotid artery occlusion (CCAO) for specified period, followed by reperfusion, results in significant microvascular changes and neuronal function impairment. Brain hypoxia alters the intracellular chemical microenvironment by elevating the levels of calcium, lipoxygenase, lipid peroxidation, and cyclooxygenase (Das et al., 2018, Stummer et al., 1994).
So far, antioxidant substances, that are free radical scavengers, particularly those found in dietary sources such as flavonoids, have been extensively studied to limit ischemia/reperfusion injury. For instance, flavonoids have a wide range of biological activities including the prevention of gastrointestinal disorders, coronary heart diseases, and inflammation (Zhang et al., 2015). Amentoflavone, a bioflavonoid found in Ginkgo biloba possesses beneficial properties was used in the current investigation to explore its possible neuroprotective effects.
Cerebral transient hypoperfusion was achieved via the left common carotid artery occlusion (CCAO) for 1 h followed by reperfusion. This treatment resulted in clear neurological deficits in the I/R group, proving that the IR control was successfully raised. However, AMNT treatment led to an enhancement in the neurological function after ischemia/reperfusion in rats. Moreover, the present data demonstrated that the left CCAO for 1 h, followed by reperfusion led to a significant decrease in the falling latency time and the locomotor activity count in IR control group as compared to sham-operated rats, reflecting severe neurological and motor dysfunctions. These findings are fully complied with previous studies (Alexis et al., 1995, Szymankiewicz-Szukala et al., 2023). CCAO, as a stroke model, provided exciting information on the significant influence of motor cortex activity and efferent neurotransmission of the corticospinal pathways. Additionally, AMNT showed neuroprotective benefits by enhancing neurologic function, improving motor coordination, and increasing the locomotor activity. Our findings were supported by previous studies revealed that AMNT effectively protects against hypoxic-ischemic brain damage in rat brain by blocking several cellular processes that cause brain damage (Shin et al., 2006).
Proinflammatory cytokines have been shown to be putative mediators of brain damage following hypoxic-ischemic injury. For instance, the astrocytes and microglia produce early response cytokines including TNF-α and IL-1 (Calvert & Zhang, 2005). Furthermore, (Yang et al., 2013) mentioned that elevated systemic and cerebral expression of the cytokine interleukin 6 (IL-6) is part of the inflammatory response to traumatic brain injury. Systemic IL-6 also acts as an amplifier signal for the inflammatory response and motor coordination deficit. In accordance with the previous studies, serum inflammatory biomarkers (TNF-α, IL-1β, and IL-6) of IR rats significantly improved compared to sham operated rats. AMNT administration to IR rats resulted in significant fall in the serum TNF-α, IL-1β, and IL-6 levels when compared with the IR control group.
Consistent with our findings, (Zhang et al., 2015), and (Rong et al., 2019) revealed that AMNT possesses neuroprotective effect and prevents the occurrence of seizures via reducing the inflammatory cytokines (IL-18, IL-1β, and TNF-α) and diminished the damaging effect and apoptosis within hippocampal neurons via inhibiting NF-κB signaling pathway. Further, AMNT reduced the production of proinflammatory mediators such as cyclo-oxygenase-2, IL-1β, TNF-α in microglial BV-2 cells and inducible nitric oxide synthase (Shin et al., 2006).
As a result of I/R injury, apoptosis, necrosis, and impairment of microvascular function, are provoked. The high metabolic rate of neurons in the brain render it susceptible to oxidative stress with consequent elevated production of ROS. The low brain contents of catalase, superoxide dismutase, and glutathione peroxidase limits the ability of the brain to eliminate the superoxide anion and hydrogen peroxide rendering the brain tissue enormously vulnerable to the effects of ROS under oxidative stress (Althurwi et al., 2022, Pietta, 2000). The polyunsaturated fatty acids abundant in then membrane lipids of the brain are liable to free radical-induced peroxidation and are one of the most likely causes of cell damage in the brain (Raefsky et al., 2018). Consistent with previous studies, the obtained results revealed that brain levels of GSH and CAT were significantly declined while the brain contents of MDA were significantly raised in I/R control rats as compared to sham-operated group. AMNT administration significantly improved the brain antioxidant markers GSH, CAT and decreased MDA levels. The obtained results indicate a better protection from oxidative stress‑induced inflammatory response owed to cerebral IR. AMNT from Ginkgo biloba leaves was found to express more powerful anti-cytotoxic properties than other structurally comparable bioflavonoids on SH-SY5Y cells when exposed to oxidative stress supporting our results of its antioxidant effect (Woo et al., 2005).
Interestingly, neurodegeneration and neuroinflammation have been linked to decreased TANK-Binding-Kinase 1 (TBK1) pathway activity. TBK1 is a key player in the interaction between selective autophagy and inflammatory interferon (IFN) signaling. Autophagy is a homeostatic process in which unhealthy intracellular substances are destroyed and the nutrients are recycled. Nutrient deficiency causes bulk autophagy also known as autophagosomal degradation of cytosolic proteins, whereas selective autophagy includes the targeted destruction of damaged organelles (Herhaus, 2021). Interferon‐β (IFNβ), a cytokine with immunomodulatory properties. (Kuo et al., 2016) verified that the anti‐inflammatory action of IFN-β supports protection against ischemic stroke. On the other hand, nuclear factor kappa- B (NF-κB), plays a major role in the stimulation of inducible nitric oxide synthase and other inflammatory reactions. Functional NF-κB complexes are found in all types of brain cells, including astrocytes, microglia, and oligodendrocytes, like in the nervous system. It has also been observed that receptor-linked signal transduction pathways in neurons and glial cells, such as those triggered TNF-α and Fas ligand, eventually lead to NF-κB activation (Bruce et al., 1996, O’Neill and Kaltschmidt, 1997).
Supporting the induction of neuroinflammation in IR rats, the brain inflammatory biomarkers TBK1 and IFNβ were significantly decreased, compared with sham operated rats, while NF-κB level was significantly increased. The beneficial effect of AMNT administration in IR rats was demonstrated as improvement of brain inflammatory cytokines TBK1 and IFNβ with significant decrease in the level of NF-κB as compared to IR control rats.
In accordance with our results, former research has shown that AMNT can impede the capacity of NF-κB -mediated inducible nitric oxide synthase, which produces nitric oxide (NO), to diminish the generation of NO and thus suppress the inflammatory response (Woo et al., 2005).
Notably, neuroinflammation, following cerebral ischemia resulted in the deposition of inflammatory cells and other coactivators in the ischemic brain tissue from native brain cells (activated microglia/macrophages, astrocytes), as well as from the invading immune cells (leukocytes), which then triggers inflammatory damage (Shukla et al., 2017).
In this study, cerebrum of IR control rats showed hemorrhage with pyknotic nuclei of neurons. These observations are further supported by neurological deficit scores and findings of the biochemical analysis of brain tissues of IR rats. Nevertheless, brain sections of rats treated with AMNT, showed mild congestion of cerebral blood vessels with some vacuolar degeneration in neurons and perivascular edema.
Caspase-3 is the most predominant cysteine aspartate found in the mature rat brain, and is essential for both neuronal growth and in other pathological circumstances like hypoxic brain damage. Immunoblotting and immunohistochemistry are used to identify active caspase-3 subunits in ischemic tissues (Althurwi et al., 2022). A significant increase in the caspase-3 proteins expression was detected by immunostaining in brain sections evoked by cerebral ischemia/reperfusion injury (Abdel-Rahman et al., 2020). In the current study, the I/R control group revealed a significant increase in caspase-3 immunopositive cells, appearing with dark brown cytoplasmic and nuclear color. AMNT-treated groups showed showing mild positive reactions for caspase-3. Previous studies showed that AMNT inhibits caspase-3 activation as shown by DNA fragmentation and the appearance of the active form (p18) of caspase 3 in the brain ipsilateral to carotid ligation (Shin et al., 2006).
The pro-inflammatory cytokine HMGB1 is a highly conserved non-histone DNA binding protein belonging to the damage-associated-molecular-pattern proteins (DAMP) family (Kang et al., 2014, Singh et al., 2016). HMGB1 has been reported to be synthesized by almost all cell types and to be associated with the pathogenesis of various diseases including neurological disorders (Andersson & Tracey, 2011). HMGB1 is localized intranuclear in the normal brain cells. Upon activation in inflammatory conditions such as ischemic stroke, HMGB1 undergoes cytoplasmic translocation and then releases into the extracellular space and binds to Toll-like receptors (TLRs), or receptors advanced glycation end products (RAGE). The formed complex activates various cellular inflammatory pathways such as NF-κB pathway with excessive expression of inflammatory cytokines (such as TNF-α and IL-1), and subsequently shift the microglia to a pro-inflammatory phenotype (Singh et al., 2016). Accumulating evidence showed that HMGB1/TLR4 activation is a key contributor for ischemic brain injuries (Shah et al., 2019). Therefore, suppressing HMGB1/TLR4 axis has been reported to be an efficient mechanism to manage the neuroinflammation in cerebral ischemia (Xu et al., 2023, Zhao et al., 2018).
Our obtained results showed a significant elevation in the mRNA expression of HMGB1 and TLR4 in brain tissues. These findings come in line with previous studies (Singh et al., 2016, Xu et al., 2023). Interestingly, AMNT supplementation obviously downregulated HMGB1 and TLR4 expression, demonstrating that inhibiting HMGB1/TLR4 axis is likely to contribute to the anti-inflammatory and the brain function improving effects of AMNT. These results were consistent with the previous studies where AMNT alleviated lipopolysaccharide-induced neuroinflammation by regulating the TLR4/ NF-κB pathway (Rong et al., 2022). These findings add more support to the accumulating evidences for the beneficial effects of Ginko biloba containing AMNT for the brain (Lobstein-Guth et al., 1988, Stough et al., 2011).
5. Conclusion
The current study provided an evidence that AMNT has neuroprotective benefits during cerebral IR injury in rats, possibly by targeting the HMGB1-mediated TLR4/NF-κB signaling pathway. These findings may provide a new therapeutic target for developing treatments for cerebral ischemic damage. However, further studies are required to explore other possible mechanisms of AMNT.
Funding
The project finically supported by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through the funding number (IF-PSAU-2022/03/22877).
CRediT authorship contribution statement
Abdulaziz S. Saeedan: Funding acquisition, Project administration, Resources, Visualization, Data curation, Writing – review & editing. Rehab F. Abdel-Rahman: Conceptualization, Methodology, Investigation, Writing – original draft. Gamal A. Soliman: Conceptualization, Resources, Methodology, Writing – review & editing. Hanan A. Ogaly: Methodology, Investigation, Writing – original draft. Maged S. Abdel-Kader: Conceptualization, Resources, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Our thanks to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for supporting the current work via funding number (IF-PSAU-2022/03/22877).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Rahman R.F., El Awdan S.A., Hegazy R.R., Mansour D.F., Ogaly H.A., Abdelbaset M. Neuroprotective effect of Crocus sativus against cerebral ischemia in rats. Metab. Brain Dis. 2020;35(3) doi: 10.1007/s11011-019-00505-1. [DOI] [PubMed] [Google Scholar]
- Alexis N.E., Dietrich W.D., Green E.J., Prado R., Watson B.D. Nonocclusive common carotid artery thrombosis in the rat results in reversible sensorimotor and cognitive behavioral deficits. Stroke. 1995;26(12) doi: 10.1161/01.STR.26.12.2338. [DOI] [PubMed] [Google Scholar]
- Althurwi H.N., Abdel-Rahman R.F., Soliman G.A., Ogaly H.A., Alkholifi F.K., Abd-Elsalam R.M., Alqasoumi S.I., Abdel-Kader M.S. Protective effect of beta-carotene against myeloperoxidase- mediated oxidative stress and inflammation in rat ischemic brain injury. Antioxidants. 2022;11(12) doi: 10.3390/antiox11122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U., Tracey K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011;29 doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft J.D., Gamble M. Theory and practice of histological techniques. Elsevier Health Sci. 2008 [Google Scholar]
- Begoña Ruiz-Larrea M., Ma Leal A., Liza M., Lacort M., de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59(6) doi: 10.1016/0039-128X(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Bonesi M., Loizzo M.R., Menichini F., Tundis R. Flavonoids in treating psoriasis. Immunity and Inflammation in Health and Disease: Emerging Roles of Nutraceuticals and Functional Foods in Immune Support. 2018;281–294 doi: 10.1016/B978-0-12-805417-8.00023-8. [DOI] [Google Scholar]
- Bruce A.J., Boling W., Kindy M.S., Peschon J., Kraemer P.J., Carpenter M.K., Holtsberg F.W., Mattson M.P. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat. Med. 1996;2(7) doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Calvert J.W., Zhang J.H. Pathophysiology of an hypoxic-ischemic insult during the perinatal period. Neurological Research. 2005;27(3) doi: 10.1179/016164105X25216. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B.K., Rao Y.V., Gambhir S.S., Gode K.D. Isolation of amentoflavone from selaginella rupestris and its pharmacological activity on central nervous System, smooth muscles and isolated frog heart preparations. Planta Med. 1981;43(1) doi: 10.1055/s-2007-971475. [DOI] [PubMed] [Google Scholar]
- Chance B., Maehly A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955;2(C) doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Coffey C.E., Figiel G.S., Weiner R.D., Saunders W.B. Caffeine augmentation of ECT. Am. J. Psychiatry. 1990;147(5):579–585. doi: 10.1176/ajp.147.5.579. https://api.semanticscholar.org/CorpusID:25837066 [DOI] [PubMed] [Google Scholar]
- Das K., Yendigeri S., Patil B., Bagoji I., Reddy R., Bagali S., Biradar M., Saha S. Subchronic hypoxia pretreatment on brain pathophysiology in unilateral common carotid artery occluded albino rats. Indian J. Pharmacol. 2018;50(4) doi: 10.4103/ijp.IJP_312_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Feigin V.L., Brainin M., Norrving B., Martins S., Sacco R.L., Hacke W., Fisher M., Pandian J., Lindsay P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke. 2022;17(1) doi: 10.1177/17474930211065917. [DOI] [PubMed] [Google Scholar]
- Ferrarese C., Mascarucci P., Zoia C., Cavarretta R., Frigo M., Begni B., Sarinella F., Frattola L., De Simoni M.G. Increased cytokine release from peripheral blood cells after acute stroke. J. Cereb. Blood Flow Metab. 1999;19 doi: 10.1097/00004647-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Herhaus L. TBK1 (TANK-binding kinase 1)-mediated regulation of autophagy in health and disease. Matrix Biol. 2021;100–101 doi: 10.1016/j.matbio.2021.01.004. [DOI] [PubMed] [Google Scholar]
- Kang R., Chen R., Zhang Q., Hou W., Wu S., Cao L., Huang J., Yu Y., Fan X.G., Yan Z., Sun X., Wang H., Wang Q., Tsung A., Billiar T.R., Zeh H.J., Lotze M.T., Tang D. HMGB1 in health and disease. Mol. Aspects Med. 2014;40:1–116. doi: 10.1016/J.MAM.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo P.C., Scofield B.A., Yu I.C., Chang F.L., Ganea D., Yen J.H. Interferon-β modulates inflammatory response in cerebral ischemia. J. Am. Heart Assoc. 2016;5(1) doi: 10.1161/JAHA.115.002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan S.E., Kirchgessner A., Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J. Transl. Med. 2009;7 doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4) doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lobstein-Guth A., Briancon-Scheid F., Victoire C., Haag-Berrurier M., Anton R. Isolation of Amentoflavone from Ginkgo biloba. Planta Med. 1988;54(06):555–556. doi: 10.1055/s-2006-962549. [DOI] [PubMed] [Google Scholar]
- Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1) doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- O’Neill L.A.J., Kaltschmidt C. NF-kB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20(6):252–258. doi: 10.1016/S0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Ogaly H.A., Abdel-Rahman R.F., Mohamed M.A.E., Ahmed-Farid O.A., Khattab M.S., Abd-Elsalam R.M. Thymol ameliorated neurotoxicity and cognitive deterioration in a thioacetamide-induced hepatic encephalopathy rat model; involvement of the BDNF/CREB signaling pathway. Food Funct. 2022;13(11) doi: 10.1039/d1fo04292k. [DOI] [PubMed] [Google Scholar]
- Okigawa M., Hwa C.W., Kawano N., Rahman W. Biflavones in Selaginella species. Phytochemistry. 1971;10(12) doi: 10.1016/S0031-9422(00)97392-8. [DOI] [Google Scholar]
- Owolabi M.O., Thrift A.G., Mahal A., Ishida M., Martins S., Johnson W.D., Pandian J., Abd-Allah F., Yaria J., Phan H.T., Roth G., Gall S.L., Beare R., Phan T.G., Mikulik R., Akinyemi R.O., Norrving B., Brainin M., Feigin V.L., Zhang P. Primary stroke prevention worldwide: translating evidence into action. The Lancet Public Health. 2022;7(1):e74–e85. doi: 10.1016/S2468-2667(21)00230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietta P.G. Flavonoids as antioxidants. In. J. Nat. Prod. 2000;Vol. 63(7:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Raefsky S.M., Furman R., Milne G., Pollock E., Axelsen P., Mattson M.P., Shchepinov M.S. Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid β-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging. 2018;66 doi: 10.1016/j.neurobiolaging.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S., Wan D., Fan Y., Liu S., Sun K., Huo J., Zhang P., Li X., Xie X., Wang F., Sun T. Amentoflavone affects epileptogenesis and exerts neuroprotective effects by inhibiting NLRP3 inflammasome. Front. Pharmacol. 2019;10(JULY) doi: 10.3389/fphar.2019.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S., Yang C., Wang F., Wu Y., Sun K., Sun T., Wu Z. Amentoflavone Exerts Anti-Neuroinflammatory Effects by Inhibiting TLR4/MyD88/NF- κ B and Activating Nrf2/HO-1 Pathway in Lipopolysaccharide-Induced BV2 Microglia. Mediators Inflamm. 2022;2022 doi: 10.1155/2022/5184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B.S., Burt K.G., Jacobsen T., Fernandes T.D., Alipui D.O., Weber K.T., Levine M., Chavan S.S., Yang H., Tracey K.J., Chahine N.O. High mobility group box-1 induces pro-inflammatory signaling in human nucleus pulposus cells via toll-like receptor 4-dependent pathway. J. Orthop. Res. 2019;37(1):220–231. doi: 10.1002/jor.24154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D.H., Bae Y.C., Kim-Han J.S., Lee J.H., Choi I.Y., Son K.H., Kang S.S., Kim W.K., Han B.H. Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. J. Neurochem. 2006;96(2):561–572. doi: 10.1111/j.1471-4159.2005.03582.x. [DOI] [PubMed] [Google Scholar]
- Shukla V., Shakya A.K., Perez-Pinzon M.A., Dave K.R. Cerebral ischemic damage in diabetes: an inflammatory perspective. In. J. Neuroinflammation. 2017;14(1) doi: 10.1186/s12974-016-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Singh V., Roth S., Veltkamp R., Liesz A. HMGB1 as a key mediator of immune mechanisms in ischemic stroke. Antioxid. Redox Signal. 2016;24(12):635–651. doi: 10.1089/ars.2015.6397. [DOI] [PubMed] [Google Scholar]
- Stough C., Silberstein R.B., Pipingas A., Song J., Camfield D.A., Nathan P.J. Examining brain-cognition effects of ginkgo biloba extract: Brain activation in the left temporal and left prefrontal cortex in an object working memory task. Evid. Based Complement. Alternat. Med. 2011;2011 doi: 10.1155/2011/164139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummer W., Weber K., Tranmer B., Baethmann A., Kempski O. Reduced mortality and brain damage after locomotor activity in gerbil forebrain ischemia. Stroke. 1994;25(9) doi: 10.1161/01.STR.25.9.1862. [DOI] [PubMed] [Google Scholar]
- Su C., Yang C., Gong M., Ke Y., Yuan P., Wang X., Li M., Zheng X., Feng W. Antidiabetic activity and potential mechanism of amentoflavone in diabetic mice. Molecules. 2019;24(11):2184. doi: 10.3390/molecules24112184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymankiewicz-Szukala A., Huber J., Czarnecki P., Wiertel-Krawczuk A., Dąbrowski M. Temporary occlusion of common carotid arteries does not evoke total inhibition in the activity of corticospinal tract neurons in experimental conditions. Biomedicines. 2023;11 doi: 10.3390/biomedicines11051287. https://api.semanticscholar.org/CorpusID:258388029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijitruth R., Liu M., Choi D.Y., Nguyen X.V., Hunter R.L., Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J. Neuroinflammation. 2006;3 doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo E.R., Lee J.Y., Cho I.J., Kim S.G., Kang K.W. Amentoflavone inhibits the induction of nitric oxide synthase by inhibiting NF-κB activation in macrophages. Pharmacol. Res. 2005;51(6):539–546. doi: 10.1016/J.PHRS.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Xiong X., Tang N., Lai X., Zhang J., Wen W., Li X., Li A., Wu Y., Liu Z. Insights into amentoflavone: a natural multifunctional biflavonoid. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.768708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhang J., Gao F., Cheng W., Zhang Y., Wei C., Zhang S., Gao X. Engeletin alleviates cerebral ischemia reperfusion-induced neuroinflammation via the HMGB1/TLR4/NF-κB network. J. Cell Mol. Med. 2023;27(12):1653–1663. doi: 10.1111/jcmm.17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.H., Gangidine M., Pritts T.A., Goodman M.D., Lentsch A.B. Interleukin 6 mediates neuroinflammation and motor coordination deficits after mild traumatic brain injury and brief hypoxia in mice. Shock. 2013;40(6):471–475. doi: 10.1097/SHK.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Yan H., Zhang L., Shan M., Chen P., Ding A., Li S.F. A review on the phytochemistry, pharmacology, and pharmacokinetics of amentoflavone, a naturally-occurring biflavonoid. Molecules. 2017;22(2):299. doi: 10.3390/molecules22020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Sun T., Niu J.G., He Z.Q., Liu Y., Wang F. Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen. Res. 2015;10(7) doi: 10.4103/1673-5374.160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Chen Z., Xie L.J., Liu G.F. Suppression of TLR4/NF-κB signaling pathway improves cerebral ischemia-reperfusion injury in rats. Mol. Neurobiol. 2018;55(5) doi: 10.1007/s12035-017-0552-0. [DOI] [PubMed] [Google Scholar]