Abstract
FLOT1, a scaffold protein of lipid rafts, is involved in several biological processes, including lipid raft protein-dependent or clathrin-independent endocytosis, and the formation of hippocampal synapses, amongst others. Increasing evidence has shown that FLOT1 can function as both a cancer promoter and cancer suppressor dependent on the type of cancer. FLOT1 can affect the occurrence and development of several types of cancer by affecting epithelial-mesenchymal transition, proliferation of cancer cells, and relevant signaling pathways, and is regulated by long intergenic non-coding RNAs or microRNAs. In the nervous system, overexpression or abnormally low expression of FLOT1 may lead to the occurrence of neurological diseases, such as Alzheimer's disease, Parkinson's disease, major depressive disorder and other diseases. Additionally, it is also associated with dilated cardiomyopathy, pathogenic microbial infection, diabetes-related diseases, and gynecological diseases, amongst other diseases. In the present review, the structure and localization of FLOT1, as well as the physiological processes it is involved in are reviewed, and then the upstream and downstream regulation of FLOT1 in human disease, particularly in different types of cancer and neurological diseases are discussed, with a focus on potentially targeting FLOT1 for the clinical treatment of several diseases.
Keywords: flotillin protein 1, lipid raft, endocytosis, tumorigenesis, regulation
1. Introduction
Lipid rafts are specialized domains rich in cholesterol and sphingolipids in the cell membranes that serve as physical platforms for a range of molecules to adjust the processes of various signal-transducing molecules (1,2). Flotillin proteins (FLOTs) are the primary proteins isolated from lipid rafts. They are highly conserved proteins associated with cell membranes and are part of the protein family that includes a Stomatin-Prohibitin-Flotillin-HflK/C (SPFH) domain. The Flotillin proteins (also known as the Reggie family) consist of two homologous isoforms, Flotillin-1(FlOT1)/Reggie-2 and Flotillin-2(FLOT2)/Reggie-1, which share 50% of the same amino acid sequences, and both of which physically interact with each other to form oligomeric and/or heterodimeric complexes (3,4). Additionally, both FLOTs have been used as markers of lipid rafts, and initiate receptor kinase signaling (5). FLOTs are plasma membrane (PM)-associated proteins in lymphocytes, neurons, and other cell types, as well as serving as scaffold proteins in non-vacuolar lipid raft microdomains (6).
FLOT1 is composed of 428 amino acids, and has a flotillin domain and a prohibition homology domain (PHB domain) (2). It is generally expressed in nearly all cell types (2), and is primarily localized at the PM, but is also present in Golgi, lysosomes, phagosomes, nuclei, endocytic compartments (7,8), as well as in early endosomes and extracellular vehicles (EVs) (9,10). Of note, the palmitoylation and phosphorylation of FLOT1 can alter its subcellular localization (11,12), and aberrant modification of FLOT1 is involved in promoting the progression of cervical cancer (CC) (13). Additionally, FLOT1 is a membrane protein that can be endocytosed from the PM to the intracellular compartments (14). In lipid rafts, FLOT1 is related to the formation of discrete planar microdomains (1). Naturally, FLOT1 has several raft-related functions, such as promoting the endocytosis of dopamine (DA) transporter (DAT) (15), glial glutamate transporter (EAAT2) (16), insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) (17), muscarinic type 3 receptor (M3R) (7), PrPC (6), certain glycosylphosphatidylinositol (GPI)-anchored proteins and certain proteins that participate in signal transduction and intracellular transport (15,18). Additionally, FLOT1 can also mediate clathrin-independent endocytosis (CIE) and the formation of hippocampal synapses (2,17). Moreover, FLOT1 is a marker of exosomes (19), participating in membrane trafficking. However, the abnormal expression of FLOT1 can lead to abnormal endocytosis, which induces certain neurodegenerative diseases, such as Parkinson's disease (PD) (16), and transmissible spongiform encephalopathy (TSEs) (6).
In the present review, the role of FLOT1 in human diseases by summarizing its structure, localization, physiological function, and mechanisms that contribute to human diseases including cancers, neurological diseases, dilated cardiomyopathy, pathogenic microbial infection, diabetes-related diseases, gynecological diseases and other diseases.
2. Structure and localization of FLOT1
The FLOT1 gene, present in chromosome 6, contains 13 exons and encodes a protein consisting of 427 residues. The FLOT1 gene can be silenced by the system of clustered regularly interspaced short palindromic repeats (CRISPR)-associated sequence 9 (CRISPR/Cas9), and altered splicing products can also produce abnormal protein products (20). FLOT1 and FLOT2 belong to the SPFH protein superfamily; they have a common N-terminal SPFH domain without clear understanding of its corresponding function. The C-terminus of FLOT1 and FLOT2 are longer than other SPFH proteins as well as being longer than the flotillin domain. The flotillin domain is characterized by the presence of glutamate-rich and alanine-rich repeat sequences, which are expected to form three coiled-coil stretches (21). FLOT1 also has a highly conserved PHB domain, which spans amino acids 1–154 and the flotillin domain spans amino acids 190–363 (22). FLOT1 and FLOT2 can interact with each other to form oligomeric and/or heterodimeric complexes (4). It has been shown that proteasome degradation occurs in FLOT1 in the absence of FLOT2; therefore stable FLOT1 protein expression requires the presence of FLOT2. However, the membrane association of FLOT1 is stronger than that of FLOT2, likely given the second hydrophobic stretch in the SPFH domain (21).
N-methyl-d-aspartate receptors (NMDARs) are glutamate receptors that regulate the transmission of excitatory synaptic potentials in the brain, which are primarily composed of NR2A and NR2B subunits. NR2B can bind to both FLOT1 and FLOT2, while NR2A interacts directly with FLOT1 only. NR2A and NR2B both interact with FLOT1 or FLOT2 at different subcellular localizations via the PHB domain. In addition, the interaction between NMDARs and FLOT1 seems to be stronger than that with FLOT2 (22).
3. PTMs of FLOT1
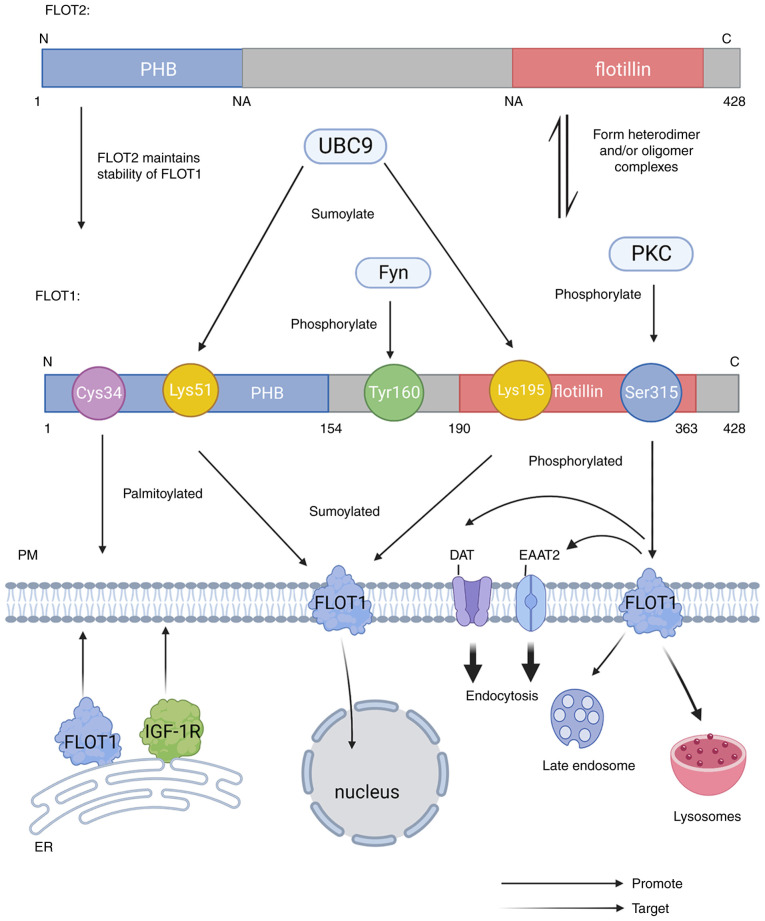
Palmitoylation, sumoylation and phosphorylation of proteins are reversible post-translational modifications (PTMs). FLOT1 is palmitoylated at Cys34, and sumolylated by UBC9 at Lys15 or Lys195. In addition, FLOT1 can be phosphorylated by protein kinase C (PKC) at Ser315 and by Fyn, and a type of Src kinase at Tyr160 (5,12,15). Palmitoylation and phosphorylation play important roles in protein subcellular localization (Fig. 1).
Figure 1.
The Structure of FLOT1 and FLOT2: The C-terminus of FLOT1 and FLOT2 are longer than other SPFH proteins, includes a flotillin domain, and the N-terminus also has a highly conserved PHB domain. The PHB domain of FLOT1 spans amino acids 1–154 and the flotillin domain encompasses amino acids 190–363. FLOT1 and FLOT2 interact with each other to form heterodimeric and/or oligomeric complexes. In addition, FLOT1 requires the presence of FLOT2 to be stable at the protein level. FLOT1 can be sumolylated by UBC9 at Lys15 and Lys195, and phosphorylated by PKC at Ser315 and by Fyn in Tyr160. FLOT, flotillin protein; PHB, prohibition homology; PKC, protein kinase C; IGF-1R, insulin-like growth factor-1 receptor; DAT, dopamine transporter; EAAT2, glial glutamate transporter.
Palmitoylation of FLOT1
FLOT1 is palmitoylated at Cys34, a conserved cysteine residue in the PHB domain, and the palmitoylation of FLOT1 is indispensable for PKC-triggered endocytosis of DAT (15). In addition, the palmitoylation of FLOT1 in the endoplasmic reticulum (ER) is indispensable for FLOT1 targeting to the PM with IGF-1R, which changes the subcellular localization of FLOT1 (11). One study showed that desmoglin 2 (Dsg2) function can be eliminated by mutated forms (Dsg2cacs) that fail to be palmitoylated, resulting in reduced subcellular localization of FLOT1 or other membrane raft proteins, which is essential for the transport of early endosomal and membrane raft proteins, thereby modulating the release of EVs (10).
Phosphorylation of FLOT1
Ser315 of FLOT1 can be phosphorylated by activated PKC, and this phosphorylation can promote the endocytosis of DAT and EAAT2 (15). A study showed that the redistribution of FLOT1 from the PM to lysosomes and late endosomes was induced by the expression of an active form of Fyn, which is relevant to the phosphorylation of FLOT1 by Src kinase. The mutation of Tyr160 in FLOT1 to phenylalanine prevents Fyn-induced FLOT1 internalization (12). In addition, Tyr160 of FLOT1 is phosphorylated by Src kinase, and following this phosphorylation, FLOT1 is endocytosed into late endosomes after stimulation by epidermal growth factor (EGF) (23).
Sumoylation of FLOT1
As ubiquitin-associated proteins, small ubiquitin-associated modifiers (SUMOs) can regulate protein function by covalently conjugating to lysine residues in a large number of proteins (24). Upregulated E2 conjugating enzyme UBC9 can mediate the sumoylation of FLOT1 at Lys51 and Lys195 with small ubiquitin-like modifier (SUMO)-2/3 modification. The sumoylation of FLOT1 can trigger its nuclear heterotopic, which is related to the occurrence of prostate cancer (PCa) (5). In addition, one study has shown that the sumoylation of FLOT1 participates in modulating synaptic plasticity (25) (Table I).
Table I.
Regulators of FLOT1.
| First author/s, year | Regulation | Regulator | Mode of regulation | (Refs.) |
|---|---|---|---|---|
| Liu et al, 2019 | Translational regulation | HOTAIR | The upregulation of HOTAUR promotes the expression of FLOT1 in HCC. | (42) |
| Cai et al, 2021 | A1BG-AS1 | The upregulation of A1BG-AS1 promotes the expression of FLOT1 in BC. | (44) | |
| Lv et al, 2020 | TUG1 | The upregulation of TUG1 promotes the expression of FLOT1 in ccRCC. | (45) | |
| Wang et al, 2022 | FAM201A | The upregulation of FAM201A promotes the expression of FLOT1 in CC. | (59) | |
| Li et al, 2021 | SNHG6 | The upregulation of SNHG6 promotes the expression of FLOT1 in Malignant glioma. | (60) | |
| Liu et al, 2019, | miR-214-3p, | The upregulation of miR-214-3p and miR-6809-5p | (42,61) | |
| Yang et al, 2019 | miR-6809-5p | inhibits the expression of FLOT1 in HCC. | ||
| Li et al, 2013, | miR-485-5p, | The upregulation of miR-485-5p and miR-124 | (44,62) | |
| Cai et al, 2021 | miR-124 | inhibits the expression of FLOT1 in BC. | ||
| Yang et al, 2015, | miR-31-5p, | The upregulation of miR-31-5p, miR-506 and | (45,56) | |
| Lv et al, 2020 | miR-506, miR-124-3p | miR-124-3p inhibits the expression of FLOT1 in ccRCC. | ||
| Kan et al, 2020, | miR-1271-5p, | The upregulation of miR-1271-5p and miR-1294 | (43,59) | |
| Wang et al, 2022 | miR-1294 | inhibits the expression of FLOT1 in CC. | ||
| Gong et al, 2013 | miR-138 | The upregulation of miR-138 inhibits the expression of FLOT1 in ESCC. | (63) | |
| Jang et al, 2019 | Post- | UBC9 | UBC9 sumolylates FLOT1 at Lys15 and Lys195. | (5) |
| Cremona et al, 2011 | translational modification | PKC | PKC phosphorylates FLOT1 at Ser315 | (15) |
| Riento et al, 2009 | Fyn | Fyn phosphorylates FLOT1 in Tyr160. | (12) |
4. Physiological effects of FLOT1
Lipid raft protein-dependent or clathrin-independent endocytosis
FLOT1 is a pivotal regulator of CIE pathways (22). The cargoes of the FLOT1-dependent pathway involved in CIE regulation are principally certain GPI-anchored proteins, such as PrPC, cholera toxin B, CD59, and Thy1, which positionally colocalize with FLOT1 at the PM microdomains (6,26). FLOT1 and FLOT2 can form homo- and hetero-oligomers, and the latter is necessary for their endocytosis (23). Endocytosis is a major regulatory factor in the transmission of constitutive signals from the cell surface to the cytoplasm and nucleus (17), maintaining cell homeostasis, nutrient absorption, drug transport, and receptor signaling regulation (7,27).
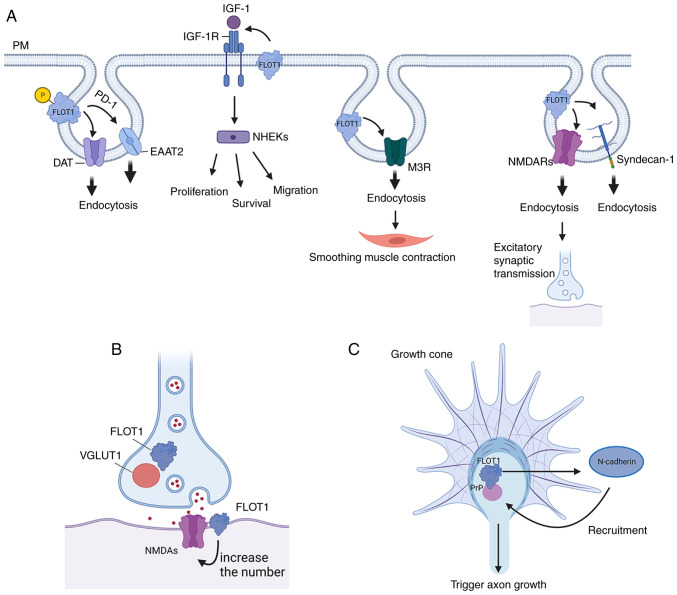
Firstly, FLOT1 is necessary for PKC-regulated endocytosis of DAT and EAAT2. Mechanistically, activated PKC can phosphorylate Ser315 of FLOT1 to promote endocytosis of DAT and EAAT2, rather than directly phosphorylating the transporter. Additionally, FLOT1 can maintain DAT in membrane rafts, and it is required for the reverse transport of DA, although it does not impact the DAT-mediated uptake of DA (15). In addition, the endocytosis of EAAT2 induced by FLOT1 is promoted by Parkinson's disease protein 7 (DJ-1), which is an early-onset autosomal recessive gene associated with PD (16). In addition, the IGF-1R signaling pathway can promote the proliferation, migration, and survival of keratinocytes (NHEKs). IGF-1 triggers endocytosis by activating IGF-1R, which is involved in regulating continuous signaling from the cell surface to the nucleus and cytoplasm. In human embryonic kidney cell lines, IGF-1R colocalizes with FLOT1. The endocytosis of IGF-1R is mediated by FLOT1 in lipid rafts and the AP2A1/2 complex located in clathrin vesicles of inclusion complexes. Notably, FLOT1-mediated endocytosis of IGF-1R is more sensitive when IGF-1R presence is low compared with the classical AP2A1/2 complex pathway, thus promoting rapid recovery of IGF-1R to regulate IGF-1R signaling and stimulate a more durable ligand response. Therefore, FLOT1-mediated endocytosis provides a novel avenue for targeted therapy in diseases where IGF-1R signaling is dysregulated (17). Similarly, as a G-protein-coupled receptor (GPCR) located in the PM, M3R is highly expressed in salivary glands and is related to physiological activities such as smoothing the contraction of muscle and salivary secretion. In addition, M3R can enter the cell by clathrin-mediated endocytosis (CME), while FLOT1 and FLOT2 are internalized by CIE. A study showed that FLOT1 and FLOT2 are partially related to the CME of M3R by promoting the internalization of M3R. However, the knockdown of FLOT1 or FLOT2 by siRNA reduces the CME of M3R. Therefore, FLOT1 and FLOT2 of salivary gland epithelial cells may play a role in the GPCR-mediated pathway (7). Additionally, PrPC forms a complex with FLOT1 under the stimulation of Cu2+ in the human neuroblastoma cells, and the PrPC-FLOT1 complex is transferred from the cell membrane to the cytoplasm under the treatment of Cu2+. However, the downregulation of FLOT1 in the human neuroblastoma cells notably eliminated the Cu2+-stimulated endocytosis process of PrPC. Therefore, the PrPC-FLOT1 complex may be involved in PrPC transport and endocytosis (6). In addition, FLOT1 can promote the degradation of anaplastic lymphoma kinase (ALK) in lysosomes through CIE. A related study also showed that the overexpression of FLOT1 promoted the endocytosis of ALK, while FLOT1 knockdown disrupted the lysosomal marker LAMP2 to inhibit the degradation of ALK, thus increasing the amount of ALK on the cell surface (28). Moreover, FLOT1 can promote the endocytosis of Syndecan-1, which is a receptor for C-TRLs (residual apolipoprotein B rich in cholesterol and triglycerides) (29). In addition, FLOT1 can trigger the endocytosis of α-synuclein (α-SYN), and the accumulation of α-SYN is a neuropathological hallmark of PD (30). Finally, FLOT1 may promote the internalization of NMDARs, which mediate excitatory synaptic transmission in the brain (22) (Fig. 2).
Figure 2.
The physiological effects of FLOT1: (A) FLOT1 can promote lipid raft protein-dependent or clathrin-independent endocytosis. (B) FLOT1 colocalizes with VGLUT1 and the number of glutamatergic synapses increases following overexpression of FLOT1. (C) FLOT1 can co-cluster with cellular PrP to transduce signals, resulting in the recruitment of N-cadherin to PrP-FLOT1 co-clusters in the growth cone, which can trigger axon growth. FLOT, flotillin protein; VGLUT1, vesicular glutamate transporter 1; PrP, Prion protein; DAT, dopamine transporter; EAAT2, glial glutamate transporter; IGF-1R, insulin-like growth factor-1 receptor; M3R, muscarinic type 3 receptor.
Formation of hippocampal synapses
In the nervous system, the development of hippocampal neurons plays an important role in learning and memory (31). Lipid rafts are a key factor affecting synaptic formation, while synaptic malformations are the basis of neurodevelopmental disease. Intact lipid rafts are necessary to maintain synaptic stability (18). The lipid raft-associated protein FLOT1 is directly related to synaptic plasticity (25). It plays an important role in promoting hippocampal neuronal differentiation and neurite growth in the early stages of neuronal development. FLOT1 colocalized with the glutamatergic presynaptic marker vesicular glutamate transporter 1 (VGLUT1) and synaptic NR1 (the obligatory subunit of NMDA receptors). Of note, overexpression of FLOT1 resulted in an increase in the presence of glutamatergic synapses, suggesting that FLOT1 is associated with the formation and induction of glutamatergic synapses. However, it should be noted that FLOT1 does not affect GABAergic synapses, suggesting that FLOT1 is a molecular target for regulating glutamatergic synaptogenesis (2). In addition, it has been shown that the frequency of miniature excitatory postsynaptic currents (mEPSCs) is increased by FLOT1 rather than miniature inhibitory postsynaptic currents (mIPSCs) (2). Another study identified that a series of synaptic adherence-like molecules (SALMs) promoted neurite growth in certain brain regions in rats. Both SALMs and FLOT1 can interact with NMDA receptors in glutamatergic synapses, and FLOT1 is found to be a molecular mediator of SALM4-induced neurite branching. Moreover, FlOT1 alone can induce neurite formation and branching, which is dependent on the presence of intact lipid rafts. In conclusion, SALM4 can regulate the FLOT1-associated pathway in hippocampal neurite branches (32). Another study showed that FLOT1 co-clustered with Prion protein (PrP) to transduce signals, causing N-cadherin to aggregate into the PrPC-FLOT1 complex in the growth cone, which triggered axonal growth (33). In addition, can induce filopodia formation in mammalian cell lines, thus promoting hippocampal neuronal differentiation and neurite outgrowth (2,33,34).
Other physiological effects
Gonadotropin-releasing hormone (GnRH) and a small amount of glucocorticoid receptor (GR) colocalize with the lipid raft protein FLOT1 at the PM, and colocalizes with FLOT1 independent of its ligands. GR and Gonadotropin-releasing hormone receptor (GnRHR) crosstalk in lipid rafts that mediate FLOT1-associated regulation of cell proliferation through the activation of PKC and the upregulation of SGK-1 (35). FLOT1 is expressed during formation of osteoclasts, where FLOT1-dominated rafts are converted to CAV1-rich rafts (36). In addition, FLOT1 plays a detectable role in the process of CD8+ T cell-mediated host monitoring under physiological conditions (37). FLOT1 can maintain the membrane integrity of B and T lymphocytes, as well as T-cell activation (38). Additionally, FLOT1 co-localizes with the inclusion membrane protein A (IncA) in the chlamydia pneumonia inclusion membranes and promotes bacterial intracellular growth by directly interacting with the chlamydia pathogen (39). In addition, exosomes are small extracellular membrane vesicles originating from late endosomes, that can mediate the intercellular transfer of RNA and protein (40). Exosomes are a subtype of EVs involved in breast cell-to-cell communication and immune processes and capable of transferring their materials to receptors (41). Meanwhile, exosome-mediated intercellular communication is the basis of cell senescence. Exercise, which promotes the release of exosomes, may be key to promoting intercellular communication and facilitating the adaptation of a system to exercise in aging or other diseases, such as type 2 diabetes mellitus, cardiovascular disease, and sarcopenia. As a significant marker of exosomes, FLOT1 may promote exosome function (19). In addition, FLOT1 is reported to participate in cell adhesion, and elevated FLOT1 enhances cell spreading (38).
5. The role of FLOT1 in tumors
Studies have shown that FLOT1 is upregulated in several types of cancer. For example, in the respiratory system, FLOT1 is overexpressed in lung adenocarcinoma (LUAD) (1), small cell lung cancer (SCLC), non-small cell lung cancer (NSCLC), and nasopharyngeal carcinoma. As for the digestive system, FLOT1 expression is increased in hepatocellular carcinoma (HCC), esophageal squamous cell carcinoma (ESCC), nasopharyngeal carcinoma (NPC), squamous cell carcinoma of the tongue, and colorectal cancer (CRC). In the urogenital system, it is elevated in clear cell renal cell carcinoma (ccRCC), bladder transitional cell carcinoma (BTCC), and PCa. In addition, FLOT1 expression is higher in gynecological cancers, such as CC, breast cancer (BC), and human epithelial ovarian neoplasms (4,28,42–53). However, FLOT1 is downregulated in neuroblastomas (28).
Upstream regulation of FLOT1 promotes tumorigenesis
Emerging evidence has shown that the upstream regulation of FLOT1 plays a pivotal role in maintaining the homeostasis of normal physical processes; however, its expression is dysregulated in several types of cancer, contributing to the tumorigenesis (Table I).
lncRNAs and miRNAs in FLOT1-mediated tumorigenesis
MicroRNAs (miRNAs/miRs) are non-coding RNAs consisting of 17–24 nucleotides. miRNAs can interact with the 3′-untranslated regions (3′-UTRs) of target mRNAs, such as that of the FLOT1 mRNA, forming a silencing target mRNA transcription complex to inhibit and/or degrade the target mRNA (44), thus negatively regulating the expression of genes post-transcriptionally (54). Studies have shown that long intergenic non-coding RNAs (lncRNAs) and miRNAs are associated with the occurrence and development of tumors by targeting FLOT1 mRNA (44,54–56), suggesting that FLOT1 is a potential therapeutic target for cancer treatment (4,44,55). Homeobox (HOX) transcript antisense RNA (HOTAIR) is a lncRNA frequently reported to be involved in HCC tumorigenesis (57,58). The upregulation of HOTAIR induces the increased expression of FLOT1 by targeting miR-214-3p in hepatocytes. Therefore, the HOTAIR/miR-214-3p/FLOT1 axis is involved in the proliferation, invasion, and migration of HCC, and the downregulation of HOTAIR produces the opposite result (42). In BC, A1BG-AS1 (a lncRNA) is upregulated, resulting in the downregulation of miR-485-5p through sponging. FLOT1 is the direct target of miR-485-5p; thus, elevated A1BG-AS1 expression increases the expression of FLOT1, thus promoting the tumorigenesis of BC (44). ccRCC is a common urinary tract tumor in humans (56), with a high rate of metastasis and poor survival (55). Taurine upregulated gene 1 (TUG1) is a lncRNA that is significantly increased in the tissues and cells of ccRCC to participate in tumor progression. TUG1 positively modulated the expression of FLOT1 through sponging miR-31-5p. The overexpression of FLOT1 attenuates the inhibition of cell proliferation mediated by miR-31-5p and promotes apoptosis and autophagy, promoting the progression of ccRCC (45). In addition, FAM201A (a lncRNA) targets the Wnt/β-catenin pathway induced by the miR-1271-5p/FLOT1 axis. FAM201A is upregulated in CC, while miR-1271-5p is downregulated. The overexpression of FAM201A increases FLOT1 expression and CC tumorigenesis, cell viability, migration, and invasion in vivo, which may be reversed by the upregulation of miR-1271-5p (59). In addition, malignant glioma is the most common intracranial tumor in adults and is often fatal. FLOT1 expression is upregulated in glioma tissues and cells, where it serves as an oncogene. The upregulated nuclear-cap-binding subunit 3 (NCBP3) in gliomas binds to SNHG6 (a lncRNA) to stabilize the expression of SNHG6, inhibiting the transcription of gastrulation brain homeobox 2 (GBX2) via histone modification. GBX2 can reduce the promoter activity and downregulate the expression of the FLOT1 oncogene; thus the upregulation of NCBP3/SNHG6 and downregulation of GBX2 promotes the expression of FLOT1, promoting the proliferation, migration, invasion, and other malignant biological behaviors of glioma cells. Conversely, the downregulation of NCBP3 and SNHG6 and the upregulation of GBX2 can inhibit the malignant biological behaviors of tumor cells; highlighting a novel avenue for the targeted therapy of glioma (60).
A study showed that miR-6809-5p mediated HCC, induced by luteolin (a natural flavonoid), via targeting of FLOT1. miR-6809-5p is upregulated by luteolin, and miR-6809-5p directly targets FLOT1 in hepatocytes to inhibit the growth of HCC cells. However, knockdown of miR-6809-5p reversed the effect of luteolin to restrain the development of HCC (61). In addition, FLOT1 is a direct target of miR-124, and the ectopic expression of miR-124 can significantly inhibit FLOT1, suppressing the growth and migration of BC cells. Luciferase assays showed that miR-124 could directly bind to the 3′-UTRs of FLOT1 and inhibit its translation. In BC, the expression of miR-124 is downregulated, while FLOT1 is extensively upregulated. miR-124 is also involved in tumor lymph node metastasis (TNM) staging and the metastasis of lymph nodes (62). In CC, the expression of miR-1294 is decreased, and the overexpression of miR-1294 can block EMT and inhibit the expression of β-catenin to inhibit the viability and metastasis of CC cells. Notably, miR-1294 has been shown to target FLOT1 directly, which inhibits cell viability, migration, and invasion by inhibiting the expression of FLOT1. Therefore, miR-1294 acts as a tumor inhibitor of CC by regulating the expression of FLOT1 and blocking EMT (43). In ccRCC, FLOT1 is a potential target gene of miR-506 and the target gene of miR-124, which are associated with the genesis and development of ccRCC (55,56). FLOT1 is negatively correlated with the expression of miR-506 and is upregulated in ccRCC, and this upregulation of FLOT1 promotes the growth and metastasis of ccRCC cells. miR-506 is an independent prognostic marker of ccRCC patients, and its expression is positively associated with advanced clinical stages (56). In addition, the miRNA target network reveals that miR-124 is a key miRNA, and it leads to the acquisition of aggressive behaviors in ccRCC by targeting CAV1 and FLOT1. Patients with higher expression of FLOT1 and CAV1 exhibit lower miR-124-3p levels and shorter overall survival. miR-124-3p, miR-30a-5p, and miR-200c-3p are the most influential miRNAs in the pathogenesis of ccRCC, and the recovery of these miRNAs reduces the invasion, migration, and spread of ccRCC, which can be regarded as a potential therapeutic strategy for ccRCC (55). ESCC is one of the most aggressive tumors of the gastrointestinal tract. A related study showed that the knockdown of miR-138 upregulated various lipid rafts components, including FLOT1, FLOT2, and CAV1 to induce lipid raft formation, thus promoting the invasion of ESCC via increased expression of FLOT1. Increased miR-138 expression has the opposite effect, which suggests that miR-138 plays a tumor-suppressive role in ESCC by targeting FLOT1 (63).
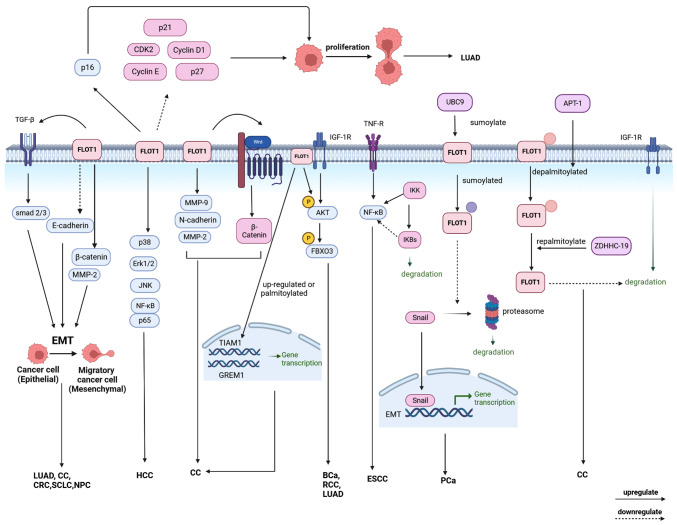
Upregulation, sumoylation, and palmitoylation of FLOT1 promote EMT
Epithelial-mesenchymal transition (EMT) is the process by which epithelial cells acquire the characteristics of mesenchymal cells (64). Numerous studies have shown that EMT is involved in the occurrence, invasion, and metastasis of tumors, as well as in the resistance to therapy in several types of cancer (64,65). Upregulation of FLOT1 promotes EMT by promoting TGF-β/Smad and AKT signaling pathways (1,52). Sumoylation of FLOT1 can also promote EMT and cancer metastasis by inhibiting Snail degradation, which is a transcription factor regulating the expression of EMT-related genes (5).
The EMT-related markers include N-cadherin, matrix metalloproteinase (MMP)-2, MMP-9 (66), and E-cadherin (67). EMT is primarily dependent on the TGF-β/Smad and AKT/mTOR pathways (68,69). FLOT1 can regulate EMT to promote the proliferation and metastasis of SCLC (50). In LUAD, FLOT1 can downregulate the epithelial marker E-cadherin and upregulate the mesenchymal markers β-catenin and MMP-2 to promote EMT. EMT promotes the growth, migration, and invasion of cancer cells and inhibits the apoptosis of cells. Therefore, targeting FLOT1 may be a potential therapeutic strategy for the management of LUAD (1). In addition, both the protein and mRNA levels of MMP-2, MMP-9, N-cadherin, and the Wnt/β-catenin signaling pathway are elevated following overexpression of FLOT1 in CC cells (59).
Snail is a major transcription factor involved in EMT, which mediates EMT gene expression and/or inhibits E-cadherin expression (5,70). In metastatic PCa, upregulated E2 conjugating enzyme UBC9 sumolylates FLOT1 at Lys51 and Lys195 following SUMO2/3 modification. The sumoylation of FLOT1 promotes EMT and cancer metastasis by interacting with Snail and inhibiting the degradation of Snail through the proteasome pathway in a sumoylation-dependent manner. Therefore, upregulation of UBC9 can promote the above process, thus targeting UBC9 can be used to regulate EMT in metastatic PCa, providing a novel therapeutic direction (5). CRC is one of the most common malignancies, the metastasis of which remains the primary cause of death clinically amongst patients with CRC, and it has been shown that FLOT1 can induce classical EMT, which is mediated by the TGF-β/Smad pathway and increase the migratory ability of CRC. FLOT1 is regulated by S100 calcium-binding protein A11 (S100A11) as its downstream factors at the post-transcriptional level instead of a transcriptional level, S100A11 can bind with LIM and SH3 protein 1 (LASP1) to regulate EMT mediated by TGF-β/Smad and the acquisition of a cell invasive phenotype (11). In CC, the upregulation and palmitoylation of FLOT1 are positively correlated with the induction of EMT genes, such as TIAM1 and GREM1, thus promoting the progression and metastasis of CC (13).
Upregulation of FLOT1 promotes the proliferation of cancer cells via regulation of the cell cycle
In LUAD, the overexpression of FLOT1 inhibits the expression of cyclin-dependent kinase 2 (CDK2), Cyclin E, and Cyclin D1 and elevates the expression of p16 to modulate the cell cycle. In addition, FLOT1 regulates the cell cycle by activating Erk/Akt signaling (1). A study showed that the knockdown of FLOT1 increased the proportion of cells in the G1 phase, suggesting that the suppression of FLOT1 could arrest SCLC cells at the G1 phase (50). In BC, the knockdown of FLOT1 could upregulate the cyclin-dependent kinase inhibitors p21 and p27, and reduce Cyclin D1 expression to inhibit the proliferation and tumorigenicity of BC cells (9). Another study showed that the knockdown of FLOT1 suppressed the proliferation and induced G1-phase arrest in BCa cells, which is related to AKT/forkhead box class O3a (FOXO3a) signaling (65).
FLOT1 affects signaling pathways
Gene expression profiling has shown that FLOT1 regulates the genes of AKT/FOXO3a, TGF-β-Smad2/3 (50), TNFR/NF-κB (63), and other signaling pathways. The upregulation of FLOT1 promotes the AKT/FOXO3a signaling pathway to promote the development of BC (9), BCa (71), LUAD (1), and RCC (72), and promoted TGF-β-Smad2/3 signaling to promote the development of SCLC (50) and NPC (52), and promoted TNFR/NF-κB signaling to promote the development of ESCC (63) and HCC (61) (Fig. 3).
Figure 3.
The mechanisms of FLOT1 leading to tumors: Upregulation, sumoylation, or palmitoylation of FLOT1 promotes EMT to promote the development of tumors. Upregulation of FLOT1 promotes the proliferation of cancer cells by regulating the cell cycle. In addition, FLOT1 is involved in the AKT/FOXO3a, TGF-βsmad2/3, TNFR/NF-κB, Wnt, and IGF-1R signaling pathways to mediate tumor proliferation, invasion, and metastasis in several types of cancer. EMT, epithelial-mesenchymal transition. FLOT, flotillin protein; FOXO3a, forkhead box class O3a; TGF-β, transforming growth factor β; TNF, tumor necrosis factor; TNFR, tumor necrosis factor-α receptor; IGF-1R, insulin-like growth factor-1 receptor.
Upregulation of FLOT1 promotes AKT/FOXO3a signaling pathway
In BC, a study showed that the knockdown of FLOT1 was related to the inhibition of Akt activity and the enhanced transcriptional activity of FOXO3a, which inhibits the proliferation of BC cells (9). In addition, miRNA-608 inhibits the proliferation and development of BCa cells by significantly downregulating the levels of p-AKT and p-FOXO3a to activate the AKT/FOXO3a signaling pathway, which is opposed to FLOT1. However, the upregulation of FLOT1 can reverse the inhibition of cell proliferation caused by miR-608 (71). In LUAD, the overexpression of FLOT1 upregulates the phosphorylation of Akt and downregulates the expression of FOXO3a to induce EMT and modulate the cell cycle, which promotes the growth, migration, and invasion of cancers cells and inhibits cells apoptosis (1). In RCC, the knockdown of FLOT1 decreased the phosphorylation of both FOXO3a and AKT, resulting in the inhibition of AKT/FOXO3a signaling (72).
Upregulation of FLOT1 promotes TGF-βsmad2/3 signaling
FLOT1 is upregulated in SCLC, and its expression is closely associated with the clinical stage, distant metastasis, and a poor survival rate. FLOT1 promotes EMT in SCLC by increasing the activities of TGF-β-smad2/3 and AKT signaling pathways. Therefore, the knockdown of FLOT1 reduces the growth, migration, and invasion of SCLC cells and reverses an EMT phenotype (50). NPC exhibits potent local invasion and a high frequency of regional lymph node metastasis, and patients with NPC often have a poor prognosis. A study showed that upregulation of FLOT1 induced the expression of transforming growth factor β1 (TGF-β1) and promoted EMT through activation of the TGF-β/Smad3 signaling pathway, which accelerates the invasion and metastasis of NPC. Therefore, FLOT1 is potentially important in the prognosis of NPC (52).
Upregulation of FLOT1 promotes the TNFR/NF-κB signaling pathway
The NF-κB pathway has been identified as a carcinogenic signaling pathway that plays an important role in inflammation and cancer (46). The activation of the NF-κB signaling pathway plays a crucial role in the occurrence and development of ESCC, and blocking the NF-κB signaling pathway can inhibit the proliferation of ESCC. A study showed that overexpression of FLOT1 could activate the NF-κB signaling pathway and promote the invasion of ESCC, which is inhibited by miR-138 (63). In addition, FLOT1 promotes tumor necrosis factor-α receptor (TNFR) signaling and NF-κB activation in ESCC. It was shown that FLOT1 promoted the recruitment of TNFR and IKK (NF-κB kinase) signalosomes to lipid rafts and promoted K63-linked polyubiquitin signaling. FLOT1 also promoted ubiquitin-coupled NF-κB signaling and maintained NF-κB activation. The recruitment of TNF receptor-associated factors (TRAFs) and receptor-interacting proteins (RIPs) to the receptor are ubiquitinated by a K63-linked polyubiquitin chain, facilitating the recruitment and activation of inhibitors of TGF-β-activated kinase-1 (TAK1) and IKK complexes. Activated IKK promotes the phosphorylation/proteasome degradation of NF-κB suppressor proteins (IKBs), leading to the activation of NF-κB (46). In HCC, downregulation of FLOT1 inactivates Erk1/2, p38, JNK, and NF-κB/p65 signaling pathways, thus inhibiting the growth of HCC cells; however, this effect can be reversed by upregulation of miR-6809-5p (61).
Other FLOT1-regulated signaling pathways in cancer
In CC, acyl protein thioesterases-1 (APT-1) promotes the depalmitoylation of FLOT1, and zinc finger DHHC domain-containing protein palmitoyltransferase-19 (ZDHHC-19) repalmitoylated FLOT1, which is frequently depalmitoylated in CC cells. The turnover of FLOT1 can prevent the desensitization of IGF-1R through endocytosis and lysosomal degradation, thus promoting the tumorigenesis of CC. Meanwhile, IGF-1 can promote palmitoylation of FLOT1 following IGF-1R activation (13). FLOT1 is involved in the acquisition of drug resistance in cancer. Multidrug resistance (MDR) of tumor cells is the leading cause of failure of chemotherapy and other anticancer drugs. A study showed that the knockdown of FLOT1 reduced drug resistance in CRC by downregulating the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway. After disruption of lipid rafts, increased cell membrane permeability may lead to increased drug accumulation in the cytoplasm, thus reversing resistance. Therefore, FLOT1 can be used as a potential therapeutic target in CC (73).
Other effects of FLOT1 in cancer
FLOT1 can induce the differentiation of certain cell types. It has been reported that lovastatin (lova) has dual effects on cancer cells. High levels of lova can induce apoptosis of thyroid cancer ARO cells, while low concentrations can induce differentiation of this cancer cell line; thus, lova may have potential as an adjuvant for cancer treatment. FLOT1 levels were increased in ARO cells following treatment with lova, and overexpression of FLOT1 increased the expression of thyroid differentiation markers, such as TG, TPO, TSHR, and SIS, suggesting that FLOT1 transformed ARO cells from an undifferentiated state to a differentiated state. These results suggest that FLOT may mediate lova-induced differentiation at least to a certain extent (74). In addition, FLOT1 plays an important role in cell proliferation, and its overexpression is associated with adverse outcomes in BC patients with LUAD (75). It was found that vacuolar protein sorting protein 33b (VPS33B) modulated exosome maturation and the secretion of proteins, and the lack of VPS33B may lead to a delay in leukemogenesis. Therefore, the study of FLOT1 and other exosome markers is conducive to the development of improved cancer treatment strategies (76).
In addition, FLOT1 is an independent prognostic indicator of several types of cancer. For example, a study showed that laryngeal cancer is a common type of cancer in men. Researchers established gene models and found that ACE2, FLOT1, and especially PRKD1 may have prognostic and biological significance. Therefore, these genes can be used as independent prognostic markers for postoperative recurrence of laryngeal squamous cell carcinoma (77). The prognosis of CRC patients after immunotherapy remains mixed; six immune-related gene markers (CCL22, LIMK1, MAPKAPK3, FLOT1, GPRC5B, and IL20RB) were found to be reliable prognostic indicators in CRC patients, providing insights into personalized cancer therapy and improving prognostic prediction in CRC patients (78).
The expression of FLOT1 in human ccRCC is involved in the progression of ccRCC and is associated with poor survival. Upregulated FLOT1 in ccRCC is involved in the tumorigenesis and progression of ccRCC. Therefore, FLOT1 can be used as a therapeutic target and an independent prognostic marker in patients with ccRCC (79). The prevalence of cutaneous squamous cell carcinoma (cSCC) was higher in patients who had undergone immunosuppressive organ transplantation than in the general population, and 16 T cell methylation domains (DMR) were found to be different between patients with and without cSCC following transplantation. An example of a gene annotated to DMR is FLOT1, which encodes a protein related to the migration of T-cells (80).
FLOT1-meidated inhibition of tumorigenesis
Neuroblastoma is one of the most common solid tumors in children, accounting for ~15% of childhood cancer-related deaths (81). FLOT1 is downregulated in neuroblastoma, and FLOT1 expression is inversely correlated with clinical malignancy. Decreased expression of FLOT1 in neuroblasts leads to dissociation of ALK from endosomes and membrane accumulation of ALK, promoting the expression or phosphorylation of ALK and the phosphorylation of the downstream mediators of ALK, such as ERK1/2, AKT and STAT3 signaling, which enhances the malignant features of neuroblastoma cells. Therefore, weakened FLOT1-ALK binding activates ALK signaling, promoting the malignant phenotype of neuroblastoma (28).
6. The role of FLOT1 in neurological diseases
Raft disruption is an important cause of several degenerative diseases including Alzheimer's disease (AD), PD, and prion disease. FLOT1 may contribute to the total degenerative process. Understanding the pathogenesis of lipid raft-related structures in neurological diseases may be helpful for the treatment of neurodegenerative diseases (8) (Fig. 4).
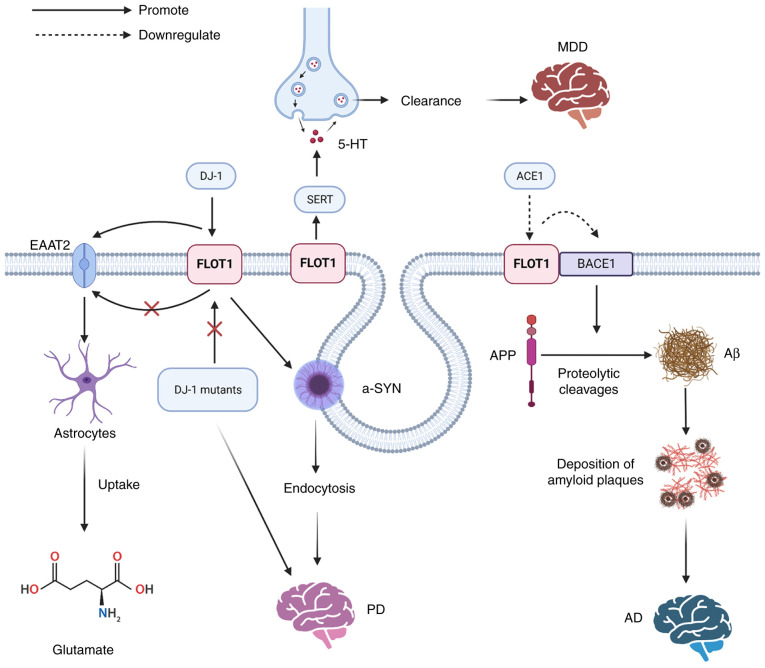
Figure 4.
The mechanisms of FLOT1 leading to neurological diseases: Aβ is produced by proteolytic cleavages of APP under the influence of BACE1, the accumulation of which can lead to the development of AD, and the overexpression of FLOT1 suppresses the activity of BACE1. DJ-1 can regulate the protein stability of FLOT1, but the PD-associated DJ-1 mutants fail to regulate the FLOT1. DJ-1 promotes the expression of EAAT2 by upregulating FLOT1, promoting the uptake of glutamate by astrocytes. FLOT1 contributes to the microdomain localization and regulation of SERT, and SERT can control the reuptake of released 5-HT from the synaptic cleft into the presynaptic terminal to regulate 5-HT clearance, leading to the development of MDD. Aβ, amyloid-β peptide; APP, amyloid precursor protein; BACE1, β-site APP cleaving enzyme 1; AD, Alzheimer's disease; PD, Parkinson's disease; FLOT, flotillin protein; DJ-1, PD protein 7; EAAT2, glial glutamate transporter; SERT, presynaptic high-affinity 5-HT transporter; MDD, major depressive disorder; EAAT2, glial glutamate transporter; ACE1, angiotensin-converting enzyme 1; -SYN, α-synuclein.
FLOT1 in AD
AD is the most common neurodegenerative disorder, accounting for ~80% of all dementia cases (82). The presence of amyloid plaques and the deposition of phosphorylated Tau in the brain are the neuropathological hallmarks of AD (82,83). Plaques are primarily composed of amyloid-β peptide (Aβ), which is produced by proteolytic cleavage of amyloid precursor protein (APP) under the influence of β-and γ-secretase. β-site APP cleaving enzyme 1 (BACE1) has been identified as a β-secretase. A study showed that BACE1 can interact with FLOT1, and part of BACE1 is recruited to lipid rafts in FLOT1-overexpressing cells. However, overexpression of FLOT1 suppresses β-secretase activity. It was speculated that the activity of β-secretase was inhibited as the binding of BACE1 to FLOT1 may conceal its active site (83). In addition, the rennin-angiotensin system (RAS) can enhance the expression of BACE1, which increases the accumulation of Aβ, which in turn stimulates the development of AD. Cleavage of APP into Aβ1-42 by BACE1 occurs in the lipid raft, and the lipid raft colocalizes with several receptors and enzymes involved in AD pathogenesis, such as estrogen receptor (Erα) and BACE1. However, the brain-penetrating angiotensin-converting enzyme 1 (ACE1) inhibitor perindopril can inhibit the expression of Aβ1-42 and decrease the expression of FLOT1. A study showed that the levels of FLOT1 in a hyperlipidemia AD model were significantly increased. FLOT1 was closely related to AD, but the molecular mechanism remains to be elucidated (82). In addition, a related study found differences in the expression of ELA protein levels in cerebrospinal fluid between AD and healthy controls, especially in proteins involved in endocytosis (84).
FLOT1 in PD
PD is the second most common neurodegenerative disorder (85), second to AD. FLOT1 expression is significantly elevated in brains with PD at the transcriptional and translational levels. The presence of α-SYN-positive Lewy bodies (LBs) and loss of catecholaminergic neurons are neuropathological features of PD; FLOT1 and DAT are the components of α-LB. A study showed that extracellular α-SYN promotes the binding of FLOT1-DAT and their accumulation at the cell surface prior to endocytosis, facilitating the endocytosis of DAT into dopaminergic neuron-like cells. Meanwhile, FLOT1 can trigger the endocytosis of α-SYN (30). In addition, a study found that DJ-1 can regulate the stability of the FLOT1 protein; however, PD-associated DJ-1 mutants fail to regulate FLOT1. DJ-1 promotes the expression of EAAT2 by upregulating FLOT1, promoting the uptake of glutamate by astrocytes. The overexpression of FLOT1 rescued the decreased glutamate uptake and decreased expression of EAAT2 caused by DJ-1 deficiency. These results suggest that the abnormal low expression of FLOT1 may lead to neurodegeneration (16).
FLOT1 in cerebrovascular diseases
Cerebral small vessel diseases (CSVD) are the primary leading cause of dementia and vascular cognitive impairment. Hypertension (HTN) is common in the elderly population and can lead to cerebral hemorrhage and other injuries. In the spontaneous hypertensive stroke predisposition (SHR-SP) model of HTN, the researchers found that FLOT1 is a key protein (86). In addition, FLOT1 is increased in the spontaneously hypertensive rat (SHR) model. Therefore, understanding the function of FLOT1 provides novel mechanistic insights into the development of these different forms of CSVD (87). Rupture of intracranial aneurysms is the primary cause of subarachnoid hemorrhage (SAH), it is important to study the specific gene expression profiles associated with intracranial aneurysms. It was found that FLOT1 may be a potential biomarker of SAH, which is more conducive to the differential diagnosis of aneurysmal SAH so as to avoid misdiagnosis and miss the optimal treatment opportunity (88).
FLOT1 in Major Depressive Disorder (MDD)
Major depressive disorder (MDD) is the most common psychiatric disorder (82,89). A study showed FLOT1 was significantly upregulated in the brain tissues of MDD patients. Several studies have identified FLOT1 as a novel MDD risk gene and MDD-associated genetic variants may confer a risk of MDD by affecting the expression of FLOT1 (90,91). Serotonin (5-hydroxytryptamine, 5-HT) is a neurotransmitter (92), the disruption of which is associated with a variety of brain disorders, such as MDD. The presynaptic high-affinity 5-HT transporter (SERT) can modulate the reuptake of released 5-HT from the synaptic cleft into the presynaptic terminal to regulate 5-HT clearance (92). SERT activity may be regulated by SERT-interacting proteins (SIPs), FLOT1 is a SIP, which is hypothesized to contribute to SERT microdomain localization and regulation. Therefore, FLOT1 may promote the clearance of 5-HT. Studying FLOT1 may help identify novel drug targets for the treatment of 5-HT-related diseases such as depression (89). In chronic corticosterone response (CORT), aberrant neurotransmission of 5-HT in the brain is hypothesized to be the central mechanism in neuropsychiatric disorders. As a SIP, FLOT1 is involved in the response to CORT therapy, and gene deletion of FLOT1 promotes chronic CORT-induced behavioral despair (92).
Other effects on the nervous system
NMDAR dysfunction can lead to several neurological disorders such as stroke or excitotoxic conditions. One potential role of FLOT1 may be to recruit NMDARs to lipid rafts to initiate a second messenger signaling system. The depletion of lipid rafts protects neurons from NMDAR-induced excitotoxicity (22). Prions are a type of infectious agent, which can cause a series of fatal neurodegenerative diseases, also known as TSEs. The primary cause of prion-related diseases is the conversion of the PrPC conformation encoded by the normal host prion gene PRNP to the abnormal conformation, PrPSc. However, the conversion of PrPC to PrPSc may occur in lipid rafts or via their associated intracellular processes. Moreover, in a prion-infected cell, PrPSc has been reported to highly colocalize with FLOT1 in the FLOT1-positive vesicles. However, the specific mechanism by which FLOT1 affects the conversion of PrPC into PrPSc has not been elaborated (6).
7. The role of FLOT1 in DCM/idiopathic (I)DCM
DCM is the primary cause of heart failure (HF), which is attributed to systolic dysfunction and ventricular dilatation. Currently, DCM is primarily treated by immunotherapy; however, there are significant individual differences, and the therapeutic effect requires further improvement. FLOT1 is overexpressed in DCM with HF, and FLOT1 may affect the development of DCM by activating T cells and accelerating cell dispersal, highlighting potential therapeutic targets of DCM (38). IDCM can affect the vascularization of myocardial tissues. Stromal cell-derived factor (SDF-1α)-mediated migration may affect endothelial recovery in patients. Significant colocalization of SDF-1α and FLOT1-specific markers have been observed in IDCM, and SDF-1α is also highly expressed in IDCM lipid rafts. A study provided novel insights into the function of lipid rafts in IDCM and hypothesized more effective treatments, although the mechanisms by which FLOT1 interacted with SDF-1α were not elucidated (93).
8. The role of FLOT1 in pathogenic microbial infections
A study showed that the FLOT1 gene was differentially expressed following parvovirus B19 infection and it was associated with integrin signaling, cytoskeleton, and tumor inhibition (94). Infection with Anaplasma phagocytophilum pathogens, also known as human granulocyte anaplasmosis (HGA) requires phosphatide protein recruitment of LDL cholesterol, and FLOT1 and FLOT2 as membrane proteins in heavy phagocytes bacillus infection and cholesterol played vital roles. FLOTs may contribute to anaplasma replication in host cells by aiding the blister transport of LDL-derived free cholesterol to anaplasma inclusions (3). A study showed that atherosclerosis was associated with chlamydia pneumoniae, an intracellular pathogen of the respiratory tract that can infect bronchoalveolar macrophages and can be transported to sites of vascular injury. Gene expression profiling of U937 human macrophages exposed to chlamydia pneumoniae and/or LDL revealed several interesting transcripts involved in structural integrity with respect to atherosclerosis, including FLOT1. The transcriptional alteration of FLOT1 was involved in atherosclerosis caused by chlamydia pneumoniae infection (95). Crohn's disease (CD) is a chronic and progressive disorder, and the etiology of CD may result from an abnormal interaction between microbiota and the enteric immune system in patients. Among them, invasive Escherichia coli (AIEC) is of great significance. Anti-TNF agents can limit AIEC survival within macrophages, and anti-TNF agents can induce the increase of FLOT1 and decrease mRNA levels of CHI3L1, which can promote the clearance of AIEC. Moreover, the levels of FLOT1 are negatively correlated with AIEC survival in CD patients treated with anti-TNF agents (96). FLOT1 in lipid rafts is an important part of the phagocytic lysosomal membrane of macrophages, thus FLOT1 plays an important role in anti-fungal immunity (97).
9. The role of FLOT1 in diabetes-related diseases
Diabetes mellitus (DM) is characterized by hyperinsulinemia and hyperglycemia (98,99). ANGPTL8, which has a unique characteristic in regulating lipid and glucose metabolism, is upregulated in diabetes and is becoming increasingly recognized as a potential drug target for the treatment of diabetes and related metabolic disorders (100,101). A study showed that the FLOT1 gene was co-expressed with ANGPTL8. It may be involved in the pathogenesis of diabetes and insulin resistance (102). Low FLOT1 expression in the livers of patients with Type 2 diabetes mellitus (T2DM) may lead to metabolic lipoproteinemia, which is due to impaired liver processing of C-TRLs. Syndecan-1 is a receptor for C-TRLs that mediates endocytosis through rafts. The interaction of C-TRLs and syndecan-1 enhances the association of syndecan-1/FLOT1 on liver cells. FLOT1 mRNA and protein levels are reduced in a rat model of T2DM. Knockdown of FLOT1 in cultured liver cells substantially inhibited endocytosis of syndecan-1, resulting in the accumulation of C-TRLs. FLOT1 is a relatively newer player in the treatment of harmful C-TRLs via syndecan-1 (29). Glucose and amino acid metabolism are altered during exercise and rehabilitation in patients with T2DM. The therapeutic benefits of physical activity for the prevention and treatment of T2DM are generally accepted. Pathway analysis of differentially regulated genes during exercise showed that FLOT1 and other genes were elevated in T2DM patients who exercised, highlighting novel insights into the underlying mechanisms that ameliorate the disturbances in glucose and amino acid metabolism associated with T2DM (103).
10. The role of FLOT1 in other diseases
Fabry disease (FD) is a rare and serious disorder caused by α-galactosidase a (GLA) enzyme deficiencies with often painful clinical features. Pain-related ion channels are related to the pain-like properties of FD, and their expression may also be influenced by the recruitment of lipid raft components such as several FLOT1-mediated receptors and channels from the nucleus to the cell membrane (104). A study showed that 15 miRNAs and 4 lncRNAs had potential functions as diagnostic markers of pediatric sepsis. FLOT1 has been shown to play a critical role in sepsis at the mRNA level (105). Moreover, the FLOT1 gene may be involved in the formation of rheumatoid arthritis (RA) (106), and a study identified novel genes in RA, in which FLOT1 expression differed significantly between RA patients and healthy controls (107). End-stage renal disease (ESRD) is the final stage of chronic kidney disease, and nocturnal hemodialysis (NHD) is a more favorable treatment approach in patients with ESRD. It is important to understand the expression of genes related to immune function in NHD in ESRD patients and to improve the immune response. Notably, it was found that the FLOT1 gene may be a potential target regulated by core transcription factors, which is related to the immunoreaction in NHD in ESRD patients (108).
FLOT1 is also related to certain gynecological diseases. In placental transcytosis, the abundant co-expression of FLOT1 and FLOT2 in cytotrophoblasts (CTs) and endothelial cells of full-term villous placenta, and the flotillin-dependent endocytosis may be important in the CT and endothelium. FLOT1 has potential implications for placental transcytosis (26). Abnormal adhesion of embryos to the endometrium leads to embryo implantation failure and infertility. Therefore, proteins involved in regulating adhesion in endometrial epithelial cells (HEECs) may be potential biomarkers or targets for infertility treatment. IL-11 is involved in HEEC adhesion, and its expression is dysregulated in infertile women. IL-11 can increase the expression of FLOT1 in HEEC membranes to regulate endometrial epithelial cell adhesion. Therefore, FLOT1 may serve as a marker of infertility or a pharmacological target for regulating fertility (14).
11. The bioinformatic analysis of FLOT1
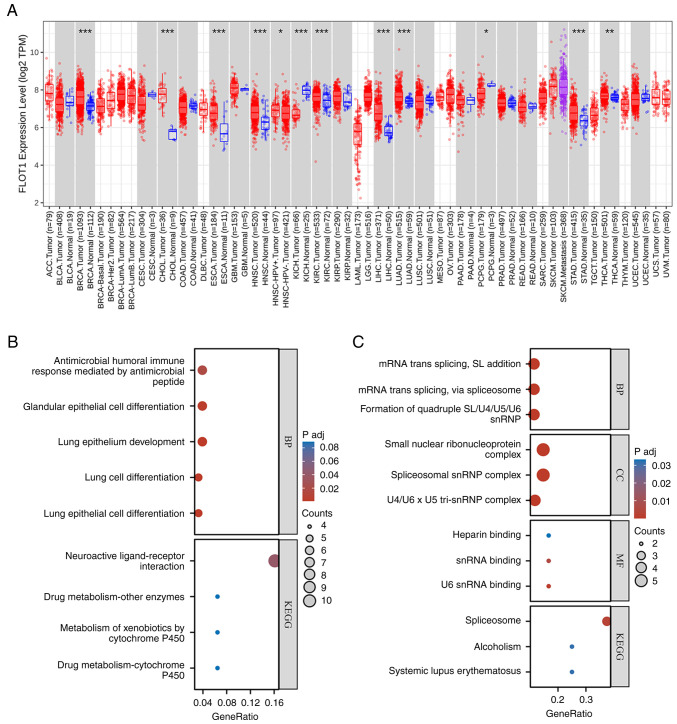
Following a pan-cancer analysis of FLOT1 in TIMER 2.0 (http://timer.cistrome.org/)at the mRNA level, the results indicated that the expression of FLOT1 in breast invasive carcinoma (BRCA), cholangiocarcinoma (CHOL), esophageal carcinoma (ESCA), head and neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), liver hepatocellular carcinoma (LIHC), LUAD, pheochromocytoma and paraganglioma (PCPG), stomach cancer (STAD), and thyroid cancer (THCA) differed from that in normal tissues (Fig. 5A), among which, the differences observed in LUAD and LIHC were significant. Therefore, Gene Ontology (GO; http://geneontology.org/) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) pathway enrichment analysis in LIHC (Fig. 5B) and LUAD (Fig. 5C) was performed. First, RNAseq data were obtained from The Cancer Genome Atlas-LIHC (HCC) project STAR process (https://portal.gdc.cancer.gov) in TPM format alongside the clinical data. The FLOT1 data was extracted from the data, and the DESeq2 package in R version 4.2.1 (http://www.R-project.org) (109) was used for differential analysis on the original Counts matrix of the selected public data according to a standard procedure (110). FLOT1 expression was stratified based on the median expression into low and high-expression groups. The high and low-expression groups consisted of 187 patients each, respectively. Following differential analysis, a total of 272 molecules were screened out based on a |log2FC|≥2 criterion, after which ID conversion was performed on the input molecule list, and enrichment analysis was performed using the clusterProfiler (111) package in R. A similar approach is used for LUAD. The results are shown as bubble diagrams (Fig. 5B and C). In LIHC, Gene Ontology-Biological Process (GO-BP) analyses showed that it was richer in ‘antimicrobial humoral immune response mediated by antimicrobial peptide’, ‘glandular epithelial cell differentiation’, ‘lung epithelium development’, ‘lung cell differentiation’, and ‘lung epithelial cell differentiation’. KEGG analyses showed that FLOT1 was associated with ‘neuroactive ligand-receptor interaction’, ‘drug metabolism-other enzymes’, ‘metabolism of xenobiotics by cytochrome P450’ and ‘drug metabolism-cytochrome P450’. In LUAD, GO-BP analyses showed that it was richer in ‘mRNA trans-splicing, SL addition’, and ‘mRNA trans-splicing via spliceosome’ and ‘formation of quadruple SL/U4/US/U6 snRNP’. Gene Ontology-Cellular Component (GO-CC) analyses showed that it was richer in ‘small nuclear ribonucleoprotein complex’, ‘spliceosomal snRNP complex’, and ‘U4/U6 × U5 tri-snRNP complex’. Gene Ontology-Molecular Function (GO-MF) analyses showed that it was closely related to ‘heparin binding’, ‘snRNA binding’, and ‘U6 snRNA binding’. KEGG analyses showed that it was enriched in the ‘spliceosome’, ‘alcoholism’, and ‘systemic lupus erythematosus’.
Figure 5.
Bioinformatic analysis of FLOT1: (A) Pan-cancer analyses of FLOT1 in TIMER 2.0 showed that at the mRNA level, the expression of FLOT1 in BRCA, CHOL, ESCA, HNSC, KICH, KIRC, LIHC, LUAD, PCPG, STAD, and THCA differed from that in normal tissues. *P<0.05, **P<0.01 and ***P<0.001. (B) In LIHC, GO-BP analyses showed that it was enriched in antimicrobial humoral immune response mediated by antimicrobial peptide, glandular epithelial cell differentiation, lung epithelium development, lung cell differentiation, and lung epithelial cell differentiation. KEGG analyses showed that it was enriched in neuroactive ligand-receptor interaction, drug metabolism - other enzymes, metabolism of xenobiotics by cytochrome P450 and drug metabolism - cytochrome P450. (C) In LUAD, GO-BP analyses showed that it was enriched in mRNA trans-splicing, SL addition, mRNA trans-splicing, via spliceosome, and formation of quadruple SL/U4/US/U6 snRNP. GO-CC analyses showed that it was enriched in small nuclear ribonucleoprotein complex, spliceosomal snRNP complex, and U4/U6 × U5 tri-snRNP complex. GO-MF analyses showed that it was closely associated with heparin binding, snRNA binding, and U6 snRNA binding. KEGG analyses showed that it was enriched in the spliceosome, alcoholism, and systemic lupus erythematosus. BRCA, breast invasive carcinoma; CHOL, cholangiocarcinoma; ESCA, esophageal carcinoma; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; STAD, stomach cancer; THCA, thyroid cancer; GO-BP, Gene Ontology Biological Process; KEGG, Kyoto Encyclopedia of Genes and Genomes.
12. Conclusions and future perspectives
The physiological effect and the role of FLOT1 in human diseases have received significant attention. FLOT1 can promote the internalization of DAT, α-SYN, and EAAT2. Abnormal regulation of these can lead to the occurrence of nervous system diseases, such as PD (16,30). In addition, FLOT1 can promote the formation of hippocampal synapses, increase the number of glutamatergic synapses, and trigger axonal growth through the recruitment of N-cadherin (2,25,33). Finally, FLOT1 plays a role in T-cell activation (38), regulating cell proliferation (35), and participating in cell adhesion (38), amongst other processes. FLOT1 is also involved in several pathological processes. The upregulation of FLOT1 promotes EMT to promote the development of LUAD, PCa, and CC (1,5,59), modulates the cell cycle in LUAD (1), and promotes the AKT/FOXO3a, TGF-βsmad2/3, and TNFR/NF-κB signaling pathways to promote the development of cancer (9,46,50), while it plays an inhibitory role in neuroblastoma recurrence (28). The reasons for this difference may be due to the tumor microenvironment or tumor heterogeneity.
However, the roles of FLOT1 in several tumors are relatively limited. For example, a study showed that FLOT1 may be an independent prognostic marker for laryngeal cancer patients (77); however, the mechanism of FLOT1 in laryngeal cancer requires further study. Additionally, FLOT1 plays a role in T-cell activation, although the specific process by which FLOT1 regulates T cells and whether it is related to immunity have not been reported in detail, nor has it been studied whether FLOT1 regulates tumor development by participating in immune escape. Therefore, the relationship between FLOT1 and immune responses requires further study.
FLOT1 can be upregulated by lncRNAs and downregulated by miRNAs, thus promoting the occurrence of certain types of tumors. Studying the mechanism of lncRNA/miRNA/FLOT1 axes in the tumor is beneficial to developing tumor treatments. In tumors in which FLOT1 acts as a tumor promoter, such as HCC and BC, the pathogenic pathway of FLOT1 may be blocked by targeting certain lncRNAs or upregulating associated miRNAs. For example, targeted inhibition of HOTAIR in HCC, A1BG-AS1 in BC, TUG1 in ccRCC, and FAM201A in CC may be considered, while in ccRCC, upregulation of miR-506 and miR-124-3p may be considered. However, in tumors in which FLOT1 acts as a tumor suppressor, such as neuroblastoma, there are no reports of the IncRNAs and miRNAs associated with FLOT1 in neuroblastoma. Therefore, additional studies into the mechanism of FLOT1 in neuroblastoma are required. Notably, although certain lncRNAs and miRNAs have been reported in tumors, there remain several corresponding downstream miRNAs and upstream lncRNAs that have not been mentioned. For example, SNHG6 a lncRNA can promote the development of malignant glioma by upregulating FLOT1; however, the downstream miRNA associated with SNHG6 has not been studied. In addition, the upstream lncRNAs of miR-6809-5p, miR-124, miR-1294, miR-138, miR-506, and miR-124-3p have not been studied as of yet. lncRNAs/miRNAs/FLOT1 axes in tumors require further study. Moreover, it was shown that FLOT1 played an important role in the prognosis of tumors and is a potential prognostic target for tumors, such as CRC (78), LUAD (112), and ccRCC (79), although it is mostly confined to mechanistic research, with clinical studies remaining limited, and therapeutic targeting of FLOT1 for tumor management requires further development and investigation.
FLOT1 also promotes the occurrence and development of other diseases, such as PD (30), AD (82), and MDD (89). However, whether FLOT1 can be used as a clinical drug target remains unknown, and further clinical trials are required to verify its value in targeted therapy. Additionally, although FLOT1 is associated with other diseases apart from cancer, such as cerebrovascular diseases, RA, TSEs (6,86,106), and DCM, its pathogenesis is not clear (38). As FLOT1 is closely associated with endocytosis, whether a disease involving dysregulated FLOT1 function is caused by aberrant endocytosis should be considered.
In general, the existing research provides support for the physiological and pathological effects of FLOT1, participating in the development of human diseases. As an important regulatory factor in human diseases such as cancer, FLOT1 provides a novel avenue for targeted therapy of diseases.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ALK
anaplastic lymphoma kinase
- APT-1
acyl protein thioesterases-1
- AD
Alzheimer's disease
- Aβ
amyloid-β peptide
- APP
amyloid precursor protein
- ACE1
angiotensin converting enzyme 1
- AIEC
invasive Escherichia coli
- ANGPTL8
angiopoietin-like protein 8
- BTCC
bladder transitional cell carcinoma
- BC
breast cancer
- BACE1
β-site APP cleaving enzyme 1
- B19
parvovirus B19
- CIE
clathrin-independent endocytosis
- CC
cervical cancer
- CRISPR
clustered regularly interspaced short palindromic repeats
- CRISPR/Cas9
clustered regularly interspaced short palindromic repeats-associated sequence 9
- CME
clathrin-mediated endocytosis
- C-TRLs
residual apolipoprotein B rich in cholesterol and triglycerides
- CRC
colorectal cancer
- ccRCC
clear cell renal cell carcinoma
- CDK2
cyclin-dependent kinase 2
- cSCC
cutaneous squamous cell carcinoma
- CSVD
cerebral small vessel disease
- CORT
chronic corticosterone response
- CD
Crohn's disease
- CT
cytotrophoblast
- DCM
dilated cardiomyopathy
- DAT
dopamine transporter
- Dsg2
Desmoglein 2
- Dsg2cacs
palmitoylated Dsg2
- DA
transport of dopamine
- DJ-1
Parkinson' disease protein 7
- DMR
differential methylation regions
- DM
Diabetes mellitus
- DRG
dorsal root ganglion
- EAAT2
glial glutamate transporter
- EV
extracellular vesicle
- EMT
epithelial-mesenchymal transition
- ER
endoplasmic reticulum
- EGF
epidermal growth factor
- ESCC
esophageal squamous cell carcinoma
- Erα
estrogen receptor α
- ESRD
end-stage renal disease
- FLOT
Flotillin protein
- FLOT1
FLOT-1/Reggie-2
- FLOT2
FLOT-2/Reggie-1
- FOXO3a
forkhead box class O3a
- FD
Fabry's disease
- GPI
glycosylphosphatidylinositol
- GPCR
G-protein-coupled receptor
- GnRH
gonadotropin-releasing hormone
- GnRHR
GnRH receptor
- GR
glucocorticoid receptor
- GBX2
gastrulation brain homeobox 2
- GLA
α-galactosidase A
- Gb3
globulinyl ceramide
- HCC
hepatocellular carcinoma
- HOX
homeobox
- HOTAIR
HOX transcript antisense RNA
- HSC
hematopoietic stem cells
- HTN
hypertension
- HF
heart failure
- HGA
human granulocyte form disease
- HEECs
human endometrial epithelial cells
- IGF-1
insulin-like growth factor-1
- IGF-1R
insulin-like growth factor-1 receptor
- IncA
inclusion membrane protein A
- IKK
NF-κB kinase
- IKBs
NF-κB inhibitory protein
- IDCM
idiopathic dilated cardiomyopathy
- IL-11
Interleukin-11
- lncRNAs
long intergenic non-coding RNAs
- LUAD
lung adenocarcinoma
- LASP1
LIM and SH3 protein 1
- lova
lovastatin
- LIC
leukemia initiating cell
- LBs
α-SYN-positive Lewy bodies
- M3R
muscarinic type 3 receptor, miRNA/miR microRNA
- mEPSC
miniature excitatory postsynaptic current
- mIPSC
miniature inhibitory postsynaptic current
- MMP-2
matrix metalloproteinase 2
- MMP-9
metalloproteinase 9
- MDR
multidrug resistance
- MDD
major depressive disorder
- NMDAR
N-methyl-d-aspartate receptor
- NSCLC
non-small cell lung cancer
- NPC
nasopharyngeal carcinoma
- NCBP3
nuclear cap-binding subunit 3
- NAFLD
non-alcoholic fatty liver disease
- NHD
nocturnal hemodialysis
- PHB
prohibition homology
- PM
plasma membrane
- PD
Parkinson's disease
- PTM
post-translational modification
- PKC
protein kinase C
- PCa
prostate cancer
- PrP
Prion protein
- PBMC
peripheral blood mononuclear cell
- RIP
receptor interacting protein
- RAS
renin-angiotensin system
- RA
rheumatoid arthritis
- SUMO
small ubiquitin-related modifier
- SALM
synaptic adherence-like molecule
- SCLC
small cell lung cancer
- S100A11
S100 calcium-binding protein A11
- SHR-SP
spontaneous hypertensive stroke predisposition
- SHR
spontaneously hypertensive rats
- SAH
subarachnoid hemorrhage
- SERT
presynaptic high-affinity 5-HT transporter
- SIP
SERT-interacting protein
- SDF-1α
stromal cell-derived factor
- TSEs
transmissible spongiform encephalopathy
- TUG1
taurine upregulated gene 1
- TNM
tumor lymph node metastasis
- TGF-β1
transforming growth factor β1
- TNF
tumor necrosis factor
- TNFR
TNF-α receptor
- TRAFs
TNF receptor-associated factors
- TAK1
TGF-β-activated kinase-1
- T2DM
type 2 diabetes mellitus
- VGLUT1
vesicular glutamate transporter 1
- VPS33B
vacuolar protein sorting protein 33b
- ZDHHC-19
zinc finger DHHC domain-containing protein palmitoyltransferase-19
- 3′-UTR
3′-untranslated region
- αc5-HT
5-hydroxytryptamine
Funding Statement
This review was funded by The National Natural Science Foundation of China (grant no. 32270821), The Natural Science Foundation of Ningbo (grant no. 2021J065), The Fundamental Research Funds for the Provincial Universities of Zhejiang (grant no. SJLZ2022004), and The K.C. Wong Magna Fund from Ningbo University (Ningbo, China).
Availability of data and materials
Not applicable.
Authors' contributions
ZZ conceived the subject of review, performed the investigation, and wrote and edited the original draft. XJ and MY wrote, reviewed, and edited the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhang L, Mao Y, Mao Q, Fan W, Xu L, Chen Y, Xu L, Wang J. FLOT1 promotes tumor development, induces epithelial-mesenchymal transition, and modulates the cell cycle by regulating the Erk/Akt signaling pathway in lung adenocarcinoma. Thorac Cancer. 2019;10:909–917. doi: 10.1111/1759-7714.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanwick CC, Shapiro ME, Vicini S, Wenthold RJ. Flotillin-1 promotes formation of glutamatergic synapses in hippocampal neurons. Dev Neurobiol. 2010;70:875–883. doi: 10.1002/dneu.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong Q, Lin M, Huang W, Rikihisa Y. Infection by anaplasma phagocytophilum requires recruitment of low-density lipoprotein cholesterol by flotillins. mBio. 2019;10:e02783–e02718. doi: 10.1128/mBio.02783-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Zhang Y, Chen SW, Li FJ, Zhuang SM, Wang LP, Zhang J, Song M. Prognostic significance of Flotillin1 expression in clinically N0 tongue squamous cell cancer. Int J Clin Exp Pathol. 2014;7:996–1003. [PMC free article] [PubMed] [Google Scholar]
- 5.Jang D, Kwon H, Choi M, Lee J, Pak Y. Sumoylation of flotillin-1 promotes EMT in metastatic prostate cancer by suppressing snail degradation. Oncogene. 2019;38:3248–3260. doi: 10.1038/s41388-018-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren K, Gao C, Zhang J, Wang K, Xu Y, Wang SB, Wang H, Tian C, Shi Q, Dong XP. Flotillin-1 mediates PrPc endocytosis in the cultured cells during Cu(2)(+) stimulation through molecular interaction. Mol Neurobiol. 2013;48:631–646. doi: 10.1007/s12035-013-8452-4. [DOI] [PubMed] [Google Scholar]
- 7.Park MY, Kim N, Wu LL, Yu GY, Park K. Role of flotillins in the endocytosis of GPCR in salivary gland epithelial cells. Biochem Biophys Res Commun. 2016;476:237–244. doi: 10.1016/j.bbrc.2016.05.103. [DOI] [PubMed] [Google Scholar]
- 8.Persaud-Sawin DA, Banach L, Harry GJ. Raft aggregation with specific receptor recruitment is required for microglial phagocytosis of Abeta42. Glia. 2009;57:320–335. doi: 10.1002/glia.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin C, Wu Z, Lin X, Yu C, Shi T, Zeng Y, Wang X, Li J, Song L. Knockdown of FLOT1 impairs cell proliferation and tumorigenicity in breast cancer through upregulation of FOXO3a. Clin Cancer Res. 2011;17:3089–3099. doi: 10.1158/1078-0432.CCR-10-3068. [DOI] [PubMed] [Google Scholar]
- 10.Flemming JP, Hill BL, Haque MW, Raad J, Bonder CS, Harshyne LA, Rodeck U, Luginbuhl A, Wahl JK, III, Tsai KY, et al. miRNA- and cytokine-associated extracellular vesicles mediate squamous cell carcinomas. J Extracell Vesicles. 2020;9:1790159. doi: 10.1080/20013078.2020.1790159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu Y, Shao Z, Wang H, Yang J, Zhang F, Luo Y, Xu L, Ding Y, Zhao L. LASP1-S100A11 axis promotes colorectal cancer aggressiveness by modulating TGFβ/smad signaling. Sci Rep. 2016;6:26112. doi: 10.1038/srep26112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riento K, Frick M, Schafer I, Nichols BJ. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J Cell Sci. 2009;122:912–918. doi: 10.1242/jcs.039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon H, Choi M, Ahn Y, Jang D, Pak Y. Flotillin-1 palmitoylation turnover by APT-1 and ZDHHC-19 promotes cervical cancer progression by suppressing IGF-1 receptor desensitization and proteostasis. Cancer Gene Ther. 2022;30:302–312. doi: 10.1038/s41417-022-00546-2. [DOI] [PubMed] [Google Scholar]
- 14.Yap J, Foo CF, Lee MY, Stanton PG, Dimitriadis E. Proteomic analysis identifies interleukin 11 regulated plasma membrane proteins in human endometrial epithelial cells in vitro. Reprod Biol Endocrinol. 2011;9:73. doi: 10.1186/1477-7827-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cremona ML, Matthies HJ, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469–477. doi: 10.1038/nn1211-1617a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JM, Cha SH, Choi YR, Jou I, Joe EH, Park SM. DJ-1 deficiency impairs glutamate uptake into astrocytes via the regulation of flotillin-1 and caveolin-1 expression. Sci Rep. 2016;6:28823. doi: 10.1038/srep28823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dam DHM, Jelsma SA, Yu JM, Liu H, Kong B, Paller AS. Flotillin and AP2A1/2 promote IGF-1 receptor association with clathrin and internalization in primary human keratinocytes. J Invest Dermatol. 2020;140:1743–1752. e1744. doi: 10.1016/j.jid.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM., Jr Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002;25:412–417. doi: 10.1016/S0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- 19.Estebanez B, Visavadiya NP, de Paz JA, Whitehurst M, Cuevas MJ, González-Gallego J, Huang CJ. Resistance training diminishes the expression of exosome CD63 protein without modification of plasma miR-146a-5p and cfDNA in the elderly. Nutrients. 2021;13:665. doi: 10.3390/nu13020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapahnke M, Banning A, Tikkanen R. Random splicing of several exons caused by a single base change in the target exon of CRISPR/Cas9 mediated gene knockout. Cells. 2016;5:45. doi: 10.3390/cells5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solis GP, Hoegg M, Munderloh C, Schrock Y, Malaga-Trillo E, Rivera-Milla E, Stuermer CAO. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403:313–322. doi: 10.1042/BJ20061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanwick CC, Shapiro ME, Yi Z, Chang K, Wenthold RJ. NMDA receptors interact with flotillin-1 and −2, lipid raft-associated proteins. FEBS Lett. 2009;583:1226–1230. doi: 10.1016/j.febslet.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Babuke T, Ruonala M, Meister M, Amaddii M, Genzler C, Esposito A, Tikkanen R. Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell Signal. 2009;21:1287–1297. doi: 10.1016/j.cellsig.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Yau TY, Sander W, Eidson C, Courey AJ. SUMO interacting motifs: Structure and function. Cells. 2021;10:2825. doi: 10.3390/cells10112825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohanraj N, Joshi NS, Poulose R, Patil RR, Santhoshkumar R, Kumar A, Waghmare GP, Saha AK, Haider SZ, Markandeya YS, et al. A proteomic study to unveil lead toxicity-induced memory impairments invoked by synaptic dysregulation. Toxicol Rep. 2022;9:1501–1513. doi: 10.1016/j.toxrep.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walton JR, Frey HA, Vandre DD, Kwiek JJ, Ishikawa T, Takizawa T, Robinson JM, Ackerman WE., IV Expression of flotillins in the human placenta: Potential implications for placental transcytosis. Histochem Cell Biol. 2013;139:487–500. doi: 10.1007/s00418-012-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polo S, Pece S, Di Fiore PP. Endocytosis and cancer. Curr Opin Cell Biol. 2004;16:156–161. doi: 10.1016/j.ceb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Tomiyama A, Uekita T, Kamata R, Sasaki K, Takita J, Ohira M, Nakagawara A, Kitanaka C, Mori K, Yamaguchi H, Sakai R. Flotillin-1 regulates oncogenic signaling in neuroblastoma cells by regulating ALK membrane association. Cancer Res. 2014;74:3790–3801. doi: 10.1158/0008-5472.CAN-14-0241. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Wu Q, Hu K, Yang C, Wu X, Cheung P, Williams KJ. Suppression of hepatic FLOT1 (Flotillin-1) by type 2 diabetes mellitus impairs the disposal of remnant lipoproteins via syndecan-1. Arterioscler Thromb Vasc Biol. 2018;38:102–113. doi: 10.1161/ATVBAHA.117.310358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi J, Hasegawa T, Sugeno N, Yoshida S, Akiyama T, Fujimori K, Hatakeyama H, Miki Y, Tomiyama A, Kawata Y, et al. Extracellular α-synuclein enters dopaminergic cells by modulating flotillin-1-assisted dopamine transporter endocytosis. FASEB J. 2019;33:10240–10256. doi: 10.1096/fj.201802051R. [DOI] [PubMed] [Google Scholar]
- 31.Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 32.Swanwick CC, Shapiro ME, Vicini S, Wenthold RJ. Flotillin-1 mediates neurite branching induced by synaptic adhesion-like molecule 4 in hippocampal neurons. Mol Cell Neurosci. 2010;45:213–225. doi: 10.1016/j.mcn.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Stuermer CA. How reggies regulate regeneration and axon growth. Cell Tissue Res. 2012;349:71–77. doi: 10.1007/s00441-012-1343-6. [DOI] [PubMed] [Google Scholar]
- 34.Stuermer CA. The reggie/flotillin connection to growth. Trends Cell Biol. 2010;20:6–13. doi: 10.1016/j.tcb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Wehmeyer L, Du Toit A, Lang DM, Hapgood JP. Lipid raft- and protein kinase C-mediated synergism between glucocorticoid- and gonadotropin-releasing hormone signaling results in decreased cell proliferation. J Biol Chem. 2014;289:10235–10251. doi: 10.1074/jbc.M113.544742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hada N, Okayasu M, Ito J, Nakayachi M, Hayashida C, Kaneda T, Uchida N, Muramatsu T, Koike C, Masuhara M, et al. Receptor activator of NF-kappaB ligand-dependent expression of caveolin-1 in osteoclast precursors, and high dependency of osteoclastogenesis on exogenous lipoprotein. Bone. 2012;50:226–236. doi: 10.1016/j.bone.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Ficht X, Ruef N, Stolp B, Samson GPB, Moalli F, Page N, Merkler D, Nichols BJ, Diz-Muñoz A, Legler DF, et al. In vivo function of the lipid raft protein flotillin-1 during CD8(+) T cell-mediated host surveillance. J Immunol. 2019;203:2377–2387. doi: 10.4049/jimmunol.1900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Guan H, Liu W, Li H, Ding J, Feng Y, Chen Z. Identification of immune markers in dilated cardiomyopathies with heart failure by integrated weighted gene coexpression network analysis. Genes (Basel) 2022;13:393. doi: 10.3390/genes13030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korhonen JT, Puolakkainen M, Häivälä R, Penttilä T, Haveri A, Markkula E, Lahesmaa R. Flotillin-1 (Reggie-2) contributes to Chlamydia pneumoniae growth and is associated with bacterial inclusion. Infect Immun. 2012;80:1072–1078. doi: 10.1128/IAI.05528-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W, et al. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13:1672–1686. doi: 10.1002/pmic.201200562. [DOI] [PubMed] [Google Scholar]
- 41.Reutgen H, Eckert H, Christ R. The Kveim test in the diagnosis of sarcoidosis. Z Erkr Atmungsorgane. 1988;170:306–313. (In German) [PubMed] [Google Scholar]
- 42.Liu C, Shang Z, Ma Y, Ma J, Song J. HOTAIR/miR-214-3p/FLOT1 axis plays an essential role in the proliferation, migration, and invasion of hepatocellular carcinoma. Int J Clin Exp Pathol. 2019;12:50–63. [PMC free article] [PubMed] [Google Scholar]
- 43.Kan XQ, Li YB, He B, Cheng S, Wei Y, Sun J. MiR-1294 acts as a tumor inhibitor in cervical cancer by regulating FLOT1 expression. J Biol Regul Homeost Agents. 2020;34:10.23812/20–10A. doi: 10.23812/20-10A. [DOI] [PubMed] [Google Scholar]
- 44.Cai S, Zhou Y, Pan Y, Liu P, Yu K, Chen S. Long non-coding RNA A1BG-AS1 promotes tumorigenesis in breast cancer by sponging microRNA-485-5p and consequently increasing expression of FLOT1 expression. Hum Cell. 2021;34:1517–1531. doi: 10.1007/s13577-021-00554-8. [DOI] [PubMed] [Google Scholar]
- 45.Lv D, Xiang Y, Yang Q, Yao J, Dong Q. Long non-coding RNA TUG1 promotes cell proliferation and inhibits cell apoptosis, autophagy in clear cell renal cell carcinoma via MiR-31-5p/FLOT1 axis. Onco Targets Ther. 2020;13:5857–5868. doi: 10.2147/OTT.S254634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song L, Gong H, Lin C, Wang C, Liu L, Wu J, Li M, Li J. Flotillin-1 promotes tumor necrosis factor-alpha receptor signaling and activation of NF-κB in esophageal squamous cell carcinoma cells. Gastroenterology. 2012;143:995–1005. e1012. doi: 10.1053/j.gastro.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Wang RM, Liu SG, Zhang JP, Luo JY, Zhang BJ, Zhang XG. Abnormal expression of FLOT1 correlates with tumor progression and poor survival in patients with non-small cell lung cancer. Tumour Biol. 2014;35:3311–3315. doi: 10.1007/s13277-013-1434-3. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Zuo X, Shi J, Zhang J, Duan X, Xu G. Flotillin 1 is differentially expressed in human epithelial ovarian tumors. Neoplasma. 2018;65:561–571. doi: 10.4149/neo_2018_170714N483. [DOI] [PubMed] [Google Scholar]
- 49.Guan Y, Song H, Zhang G, Ai X. Overexpression of flotillin-1 is involved in proliferation and recurrence of bladder transitional cell carcinoma. Oncol Rep. 2014;32:748–754. doi: 10.3892/or.2014.3221. [DOI] [PubMed] [Google Scholar]
- 50.Zhao L, Li J, Liu Y, Zhou W, Shan Y, Fan X, Zhou X, Shan B, Song Y, Zhan Q. Flotillin1 promotes EMT of human small cell lung cancer via TGF-β signaling pathway. Cancer Biol Med. 2018;15:400–414. doi: 10.20892/j.issn.2095-3941.2018.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baig N, Li Z, Lu J, Chen H, Yu S, Li T, Niu Z, Niu J. Clinical significance and comparison of flotillin 1 expression in left and right colon cancer. Oncol Lett. 2019;18:997–1004. doi: 10.3892/ol.2019.10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao S, Cui Y, Xiao H, Mai M, Wang C, Xie S, Yang J, Wu S, Li J, Song L, et al. Upregulation of flotillin-1 promotes invasion and metastasis by activating TGF-beta signaling in nasopharyngeal carcinoma. Oncotarget. 2016;7:4252–4264. doi: 10.18632/oncotarget.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang R, Chen Z, Zhang Y, Ziao S, Zhang W, Hu X, Xiao Q, Liu Q, Wang X. Flotillin-1 is a prognostic biomarker for glioblastoma and promotes cancer development through enhancing invasion and altering tumour microenvironment. J Cell Mol Med. 2023;27:392–402. doi: 10.1111/jcmm.17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butz H, Szabo PM, Khella HW, Nofech-Mozes R, Patocs A, Yousef GM. miRNA-target network reveals miR-124as a key miRNA contributing to clear cell renal cell carcinoma aggressive behaviour by targeting CAV1 and FLOT1. Oncotarget. 2015;6:12543–12557. doi: 10.18632/oncotarget.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang FQ, Zhang HM, Chen SJ, Yan Y, Zheng JH. MiR-506 is down-regulated in clear cell renal cell carcinoma and inhibits cell growth and metastasis via targeting FLOT1. PLoS One. 2015;10:e0120258. doi: 10.1371/journal.pone.0120258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang B, Zhang M, Yang Y, Li Q, Yu J, Zhu S, Niu Y, Shang Z. Targeting KDM4A-AS1 represses AR/AR-Vs deubiquitination and enhances enzalutamide response in CRPC. Oncogene. 2022;41:387–399. doi: 10.1038/s41388-021-02103-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Zhang Y, Cheng X, Liang H, Jin Z. Long non-coding RNA HOTAIR and STAT3 synergistically regulate the cervical cancer cell migration and invasion. Chem Biol Interact. 2018;286:106–110. doi: 10.1016/j.cbi.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Wang Z, Cheng K, Hao Q. FAM201A promotes cervical cancer progression and metastasis through miR-1271-5p/Flotillin-1 axis targeting-induced Wnt/β-catenin pathway. J Oncol. 2022;2022:1123839. doi: 10.1155/2022/1123839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Zhang F, Ma J, Ruan X, Liu X, Zheng J, Liu Y, Cao S, Shen S, Shao L, et al. NCBP3/SNHG6 inhibits GBX2 transcription in a histone modification manner to facilitate the malignant biological behaviour of glioma cells. RNA Biol. 2021;18:47–63. doi: 10.1080/15476286.2020.1790140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang PW, Lu ZY, Pan Q, Chen TT, Feng XJ, Wang SM, Pan YC, Zhu MH, Zhang SH. MicroRNA-6809-5p mediates luteolin-induced anticancer effects against hepatoma by targeting flotillin 1. Phytomedicine. 2019;57:18–29. doi: 10.1016/j.phymed.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 62.Li L, Luo J, Wang B, Wang D, Xie X, Yuan L, Guo J, Xi S, Gao J, Lin X, et al. Microrna-124 targets flotillin-1 to regulate proliferation and migration in breast cancer. Mol Cancer. 2013;12:163. doi: 10.1186/1476-4598-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gong H, Song L, Lin C, Liu A, Lin X, Wu J, Li M, Li J. Downregulation of miR-138 sustains NF-κB activation and promotes lipid raft formation in esophageal squamous cell carcinoma. Clin Cancer Res. 2013;19:1083–1093. doi: 10.1158/1078-0432.CCR-12-3169. [DOI] [PubMed] [Google Scholar]
- 64.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Nachiyappan A, Gupta N, Taneja R. EHMT1/EHMT2 in EMT, cancer stemness and drug resistance: Emerging evidence and mechanisms. FEBS J. 2022;289:1329–1351. doi: 10.1111/febs.16334. [DOI] [PubMed] [Google Scholar]
- 66.Peng L, Wen L, Shi QF, Gao F, Huang B, Meng J, Hu CP, Wang CM. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020;11:978. doi: 10.1038/s41419-020-03178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang W, Dong L, Zhao B, Lu J, Zhao Y. E-cadherin is downregulated by microenvironmental changes in pancreatic cancer and induces EMT. Oncol Rep. 2018;40:1641–1649. doi: 10.3892/or.2018.6528. [DOI] [PubMed] [Google Scholar]
- 68.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roshan MK, Soltani A, Soleimani A, Kahkhaie KR, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–234. doi: 10.1016/j.biochi.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13:963–972. doi: 10.2174/15680096113136660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang Z, Wang X, Xu X, Xie B, Ji A, Meng S, Li S, Zhu Y, Wu J, Hu Z, et al. MicroRNA-608 inhibits proliferation of bladder cancer via AKT/FOXO3a signaling pathway. Mol Cancer. 2017;16:96. doi: 10.1186/s12943-017-0664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu X, Wu J, Li S, Hu Z, Xu X, Zhu Y, Liang Z, Wang X, Lin Y, Mao Y, et al. Downregulation of microRNA-182-5p contributes to renal cell carcinoma proliferation via activating the AKT/FOXO3a signaling pathway. Mol Cancer. 2014;13:109. doi: 10.1186/1476-4598-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye DM, Ye SC, Yu SQ, Shu FF, Xu SS, Chen QQ, Wang YL, Tang ZT, Pan C. Drug-resistance reversal in colorectal cancer cells by destruction of flotillins, the key lipid rafts proteins. Neoplasma. 2019;66:576–583. doi: 10.4149/neo_2018_180820N633. [DOI] [PubMed] [Google Scholar]
- 74.Shui HA, Hsia CW, Chen HM, Chang TC, Wang CY. Proteomics and bioinformatics analysis of lovastatin-induced differentiation in ARO cells. J Proteomics. 2012;75:1170–1180. doi: 10.1016/j.jprot.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 75.Arkhipova KA, Sheyderman AN, Laktionov KK, Mochalnikova VV, Zborovskaya IB. Simultaneous expression of flotillin-1, flotillin-2, stomatin and caveolin-1 in non-small cell lung cancer and soft tissue sarcomas. BMC Cancer. 2014;14:100. doi: 10.1186/1471-2407-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu H, Chen C, Hao X, Wang C, Zhang X, Li Z, Shao H, Zeng H, Yu Z, Xie L, et al. Sorting protein VPS33B regulates exosomal autocrine signaling to mediate hematopoiesis and leukemogenesis. J Clin Invest. 2016;126:4537–4553. doi: 10.1172/JCI87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fountzilas E, Kotoula V, Angouridakis N, Karasmanis I, Wirtz RM, Eleftheraki AG, Veltrup E, Markou K, Nikolaou A, Pectasides D, Fountzilas G. Identification and validation of a multigene predictor of recurrence in primary laryngeal cancer. PLoS One. 2013;8:e70429. doi: 10.1371/journal.pone.0070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai S, Xu S, Ye Y, Ding K. Identification of an immune-related gene signature to improve prognosis prediction in colorectal cancer patients. Front Genet. 2020;11:607009. doi: 10.3389/fgene.2020.607009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Li J, Song Y, Chen F, Pei Y, Yao F. Flotillin-1 expression in human clear-cell renal cell carcinoma is associated with cancer progression and poor patient survival. Mol Med Rep. 2014;10:860–866. doi: 10.3892/mmr.2014.2310. [DOI] [PubMed] [Google Scholar]
- 80.Peters FS, Peeters AMA, Mandaviya PR, van Meurs JBJ, Hofland LJ, van de Wetering J, Betjes MGH, Baan CC, Boer K. Differentially methylated regions in T cells identify kidney transplant patients at risk for de novo skin cancer. Clin Epigenetics. 2018;10:81. doi: 10.1186/s13148-018-0519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zafar A, Wang W, Liu G, Wang X, Xian W, McKeon F, Foster J, Zhou J, Zhang R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med Res Rev. 2021;41:961–1021. doi: 10.1002/med.21750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Messiha BAS, Ali MRA, Khattab MM, Abo-Youssef AM. Perindopril ameliorates experimental Alzheimer's disease progression: Role of amyloid β degradation, central estrogen receptor and hyperlipidemic-lipid raft signaling. Inflammopharmacology. 2020;28:1343–1364. doi: 10.1007/s10787-020-00724-4. [DOI] [PubMed] [Google Scholar]
- 83.Hattori C, Asai M, Onishi H, Sasagawa N, Hashimoto Y, Saido TC, Maruyama K, Mizutani S, Ishiura S. BACE1 interacts with lipid raft proteins. J Neurosci Res. 2006;84:912–917. doi: 10.1002/jnr.20981. [DOI] [PubMed] [Google Scholar]
- 84.Krance SH, Wu CY, Chan ACY, Kwong S, Song BX, Xiong LY, Ouk M, Chen MH, Zhang J, Yung A, et al. Endosomal-lysosomal and autophagy pathway in alzheimer's disease: A systematic review and meta-analysis. J Alzheimers Dis. 2022;88:1279–1292. doi: 10.3233/JAD-220360. [DOI] [PubMed] [Google Scholar]
- 85.Cabreira V, Massano J. Parkinson's disease: Clinical review and update. Acta Med Port. 2019;32:661–670. doi: 10.20344/amp.11978. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 86.Schrader JM, Stanisavljevic A, Xu F, Van Nostrand WE. Distinct brain proteomic signatures in cerebral small vessel disease rat models of hypertension and cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2022;81:731–745. doi: 10.1093/jnen/nlac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng Y, Sun D, Zhu B, Zhou W, Lv C, Kou F, Wei H. Integrative metabolic and proteomic profiling of the brainstem in spontaneously hypertensive rats. J Proteome Res. 2020;19:4114–4124. doi: 10.1021/acs.jproteome.0c00585. [DOI] [PubMed] [Google Scholar]
- 88.Zhao H, Li ST, Zhu J, Hua XM, Wan L. Analysis of peripheral blood cells' transcriptome in patients with subarachnoid hemorrhage from ruptured aneurysm reveals potential biomarkers. World Neurosurg. 2019;129:e16–e22. doi: 10.1016/j.wneu.2019.04.125. [DOI] [PubMed] [Google Scholar]
- 89.Quinlan MA, Robson MJ, Ye R, Rose KL, Schey KL, Blakely RD. Ex vivo quantitative proteomic analysis of serotonin transporter interactome: Network impact of the SERT Ala56 coding variant. Front Mol Neurosci. 2020;13:89. doi: 10.3389/fnmol.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhong J, Li S, Zeng W, Li X, Gu C, Liu J, Luo XJ. Integration of GWAS and brain eQTL identifies FLOT1 as a risk gene for major depressive disorder. Neuropsychopharmacology. 2019;44:1542–1551. doi: 10.1038/s41386-019-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu W, Li W, Cai X, Yang Z, Li H, Su X, Song M, Zhou DS, Li X, Zhang C, et al. Identification of a functional human-unique 351-bp Alu insertion polymorphism associated with major depressive disorder in the 1p31.1 GWAS risk loci. Neuropsychopharmacology. 2020;45:1196–1206. doi: 10.1038/s41386-020-0659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reisinger SN, Kong E, Molz B, Humberg T, Sideromenos S, Cicvaric A, Steinkellner T, Yang JW, Cabatic M, Monje FJ, et al. Flotillin-1 interacts with the serotonin transporter and modulates chronic corticosterone response. Genes Brain Behav. 2019;18:e12482. doi: 10.1111/gbb.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roura S, Galvez-Monton C, Pujal JM, Casani L, Fernández MA, Astier L, Gastelurrutia P, Domingo M, Prat-Vidal C, Soler-Botija C, et al. New insights into lipid raft function regulating myocardial vascularization competency in human idiopathic dilated cardiomyopathy. Atherosclerosis. 2013;230:354–364. doi: 10.1016/j.atherosclerosis.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 94.Kerr JR, Kaushik N, Fear D, Baldwin DA, Nuwaysir EF, Adcock IM. Single-nucleotide polymorphisms associated with symptomatic infection and differential human gene expression in healthy seropositive persons each implicate the cytoskeleton, integrin signaling, and oncosuppression in the pathogenesis of human parvovirus B19 infection. J Infect Dis. 2005;192:276–286. doi: 10.1086/430950. [DOI] [PubMed] [Google Scholar]
- 95.Lim WC, Chow VT. Gene expression profiles of U937 human macrophages exposed to Chlamydophila pneumoniae and/or low density lipoprotein in five study models using differential display and real-time RT-PCR. Biochimie. 2006;88:367–377. doi: 10.1016/j.biochi.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 96.Douadi C, Vazeille E, Chambon C, Hébraud M, Fargeas M, Dodel M, Coban D, Pereira B, Birer A, Sauvanet P, et al. Anti-TNF agents restrict adherent-invasive escherichia coli replication within macrophages through modulation of chitinase 3-like 1 in patients with Crohn's Disease. J Crohns Colitis. 2022;16:1140–1150. doi: 10.1093/ecco-jcc/jjab236. [DOI] [PubMed] [Google Scholar]
- 97.Schmidt F, Thywissen A, Goldmann M, Cunha C, Cseresnyés Z, Schmidt H, Rafiq M, Galiani S, Gräler MH, Chamilos G, et al. Flotillin-dependent membrane microdomains are required for functional phagolysosomes against fungal infections. Cell Rep. 2020;32:108017. doi: 10.1016/j.celrep.2020.108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salazar J, Angarita L, Morillo V, Navarro C, Martínez MS, Chacín M, Torres W, Rajotia A, Rojas M, Cano C, et al. Microbiota and diabetes mellitus: Role of lipid mediators. Nutrients. 2020;12:3039. doi: 10.3390/nu12103039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rojas-Carranza CA, Bustos-Cruz RH, Pino-Pinzon CJ, Ariza-Marquez YV, Gomez-Bello RM, Canadas-Garre M. Diabetes-related neurological implications and pharmacogenomics. Curr Pharm Des. 2018;24:1695–1710. doi: 10.2174/1381612823666170317165350. [DOI] [PubMed] [Google Scholar]
- 100.Abu-Farha M, Abubaker J, Tuomilehto J. ANGPTL8 (betatrophin) role in diabetes and metabolic diseases. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2919. 10.1002/dmrr.29. [DOI] [PubMed] [Google Scholar]
- 101.Zhao Z, Deng X, Jia J, Zhao L, Wang C, Cai Z, Guo C, Yang L, Wang D, Ma S, et al. Angiopoietin-like protein 8 (betatrophin) inhibits hepatic gluconeogenesis through PI3K/Akt signaling pathway in diabetic mice. Metabolism. 2022;126:154921. doi: 10.1016/j.metabol.2021.154921. [DOI] [PubMed] [Google Scholar]
- 102.Siddiqa A, Cirillo E, Tareen SHK, Ali A, Kutmon M, Eijssen LMT, Ahmad J, Evelo CT, Coort SL. Biological pathways leading from ANGPTL8 to diabetes mellitus-a co-expression network based analysis. Front Physiol. 2018;9:1841. doi: 10.3389/fphys.2018.01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hansen JS, Zhao X, Irmler M, Liu X, Hoene M, Scheler M, Li Y, Beckers J, de Angelis MH, Häring HU, et al. Type 2 diabetes alters metabolic and transcriptional signatures of glucose and amino acid metabolism during exercise and recovery. Diabetologia. 2015;58:1845–1854. doi: 10.1007/s00125-015-3584-x. [DOI] [PubMed] [Google Scholar]
- 104.Spitzel M, Wagner E, Breyer M, Henniger D, Bayin M, Hofmann L, Mauceri D, Sommer C, Üçeyler N. Dysregulation of immune response mediators and pain-related ion channels is associated with pain-like behavior in the GLA KO mouse model of fabry disease. Cells. 2022;11:1730. doi: 10.3390/cells11111730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X, Cui Y, Ding X, Liu S, Han B, Duan X, Zhang H, Sun T. Analysis of mRNAlncRNA and mRNAlncRNA-pathway coexpression networks based on WGCNA in developing pediatric sepsis. Bioengineered. 2021;12:1457–1470. doi: 10.1080/21655979.2021.1908029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mo XB, Sun YH, Zhang YH, Lei SF. Integrative analysis highlighted susceptibility genes for rheumatoid arthritis. Int Immunopharmacol. 2020;86:106716. doi: 10.1016/j.intimp.2020.106716. [DOI] [PubMed] [Google Scholar]
- 107.Zhu H, Xia W, Mo XB, Lin X, Qiu YH, Yi NJ, Zhang YH, Deng FY, Lei SF. Gene-based genome-wide association analysis in european and asian populations identified novel genes for rheumatoid arthritis. PLoS One. 2016;11:e0167212. doi: 10.1371/journal.pone.0167212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dai H, Zhou J, Zhu B. Gene co-expression network analysis identifies the hub genes associated with immune functions for nocturnal hemodialysis in patients with end-stage renal disease. Medicine (Baltimore) 2018;97:e12018. doi: 10.1097/MD.0000000000012018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prates L, Lemes RB, Hunemeier T, Leonardi F. Population-based change-point detection for the identification of homozygosity islands. Bioinformatics. 2023;39:btad170. doi: 10.1093/bioinformatics/btad170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McDermaid A, Monier B, Zhao J, Liu B, Ma Q. Interpretation of differential gene expression results of RNA-seq data: Review and integration. Brief Bioinform. 2019;20:2044–2054. doi: 10.1093/bib/bby067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo AY, Liang XJ, Liu RJ, Li XX, Bi W, Zhou LY, Tang CE, Yan A, Chen ZC, Zhang PF. Flotilin-1 promotes the tumorigenicity and progression of malignant phenotype in human lung adenocarcinoma. Cancer Biol Ther. 2017;18:715–722. doi: 10.1080/15384047.2017.1360445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.