Abstract

Indolemethane derivatives are significant molecules in the study of N-heterocyclic chemistry. Herein, we designed and developed a highly efficient green synthesis of indolemethane compounds using a recyclable biodegradable glycerol-based carbon solid acid catalyst under solvent-free conditions at room temperature for 5 min with excellent yields. The synthesized compounds were subjected to cytotoxic activity against prostate (DU145), hepatocellular carcinoma (HepG2), and melanoma (B16) cell lines. The highest cytotoxicity effects were found with 1k (1.09 μM) and 1c (2.02 μM) against DU145, followed by 1a, 1d, 1f, 1n, and 1m between 5.10 and 8.18 μM concentrations. The anticancer activity is validated using molecular docking simulations, and comparing binding energies with the standard drug doxorubicin suggests that the title compounds are well fitted into the active site pocket of the target molecules..
Introduction
Indolemethane (IM) derivatives also known as bisindolylmethanes (BIMs) or diindolylmethanes (DIMs) are diverse molecules and the most active chemicals in the study of N-heterocyclic chemistry.1–5 BIMs exhibit a wide range of biological characteristics, such as antimicrobial, antifungal, antibacterial, antiviral, anti-inflammatory, antioxidant, analgesic, and radical scavenging activities.6–10 They have considerable medicinal applications (Figure 1), and they are prevalent in natural compounds and are essential in the treatment of many malignancies, including breast,11 colon,12 prostate,13 and pancreatic cancer.14 Over the past several decades, their use in drug development has increased, making them a vital part of different protocols.15 Numerous methods use conventional catalysts, which are poisonous, expensive, dangerous, difficult to handle, thermally unstable, and non-reusable.16–19 Hence, researchers are creating methods for green synthesis using ecologically friendly reagents to reduce costs, dangers, waste, and energy. Green synthesis uses solvent-free conditions or solvents such as water, glycerol, and ethylene glycol and also uses unconventional processes like grinding, microwave irradiation, and ultrasound methods.20–22
Figure 1.
Medicinally important indolemethane compounds.
Solid acid catalysts have been vital in the development of numerous techniques and catalysts used by researchers for organic conversions.23 In comparison to traditional homogeneous acid catalysts, heterogeneous solid acids have the benefit of being readily recovered and reused without losing effectiveness.24 Homogeneous liquid-phase mineral acids have been supplanted by carbon-based heterogeneous solid catalysts containing sulfonic acid due to their efficiency and reusability.25,26 These catalysts have faster reaction time, greater yield, and less pollution. We used the glycerol-based carbon sulfonic acid catalyst. It is a cost-efficient, eco-friendly, and potent catalyst in synthetic organic chemistry. It does not require column separation, catalyzes various reactions, and transforms organic functional groups. Prabhavathi et al.27,28 recommended using a sulfonic acid functionalized polycyclic fragrant carbon catalyst in order to recycle bioglycerol and glycerol pitch without losing much of its activity. This eco-friendly method has fast reaction rates, excellent yields, and little cleanup. Bio-glycerol-based carbon sulfonic acid is tested as an efficient catalyst for solvent-free synthesis of novel bisindolylmethane derivatives. Another potent and recyclable catalyst we used is bromodimethylsulfonyl bromide (BDMS), developed by Meerwein et al. in 1965. It catalyzes various brominating reactions and also transforms organic functional groups. Due to its inexpensive and safe handling, BDMS has gained extensive interest in organic synthesis.29–31
The significance of the indolemethane moiety attracted the attention of researchers, who used a variety of standard and unconventional techniques for the accomplishment. They employed different metal catalysts32–36 and nonconventional methods utilizing baker’s yeast,37 aromatic bromomethyl groups,38 meglumine-catalyzed,39 titanium(IV) salophen-catalyzed,40 sodium trifluoromethanesulfonate-mediated,41 and enzymatic approaches.42 Copper nanoparticles43 and the polyaniline sulfonic acid catalytic approach44 are the most recent notable reports. Although several methods are established, the majority of them suffer from any of the following disadvantages: long reaction times, unsatisfactory yields, use of organic solvents, and non-recyclability. A better catalytic approach is much needed to address these issues.
Results and Discussion
Chemistry
Despite the numerous approaches, we explored methods to address the aforementioned drawbacks in the synthesis of diindolylmethane derivatives under solvent-free, eco-friendly conditions utilizing simple, inexpensive, and easily available catalysts. Herein, we employed the bromodimethylsulfonyl bromide catalyst to produce BIM derivatives in a solvent-free environment with excellent yields. Initially, benzaldehyde was treated with indole to produce 3,3′-(phenylmethylene)bis(1H-indole) (1a) using a 5 mol % BDMS catalyst (Scheme 1). BDMS was optimized to give the best catalytic activity at 10 mol % (Table 1, entry 3); exceeding 10 mol %, the catalyst gave an insignificant increase in the yield of the product (Table 1, entries 4 and 5); however, the substrate conversion rate was reduced by employing a 5 mol % BDMS catalyst (Table 1, entry 1); when a solvent was employed, the conversion took substantially longer and produced lower yield (Table 1, entry 6–10). The synthesized product was isolated, characterized by 1H and 13C NMR and high-resolution mass spectrometry, then validated by crystallographic studies and matched to existing crystal data.
Scheme 1. BDMS-Catalyzed Synthesis of Turbomycin B.
Table 1. Optimization with the BDMS Catalyst.
| entry | BDMS (mol %) | solvent | time (minutes) | yield (%) |
|---|---|---|---|---|
| 1 | 5 | 5 | 55 | |
| 2 | 10 | 5 | 70 | |
| 3 | 10 | 10 | 95 | |
| 4 | 15 | 20 | 85 | |
| 5 | 20 | 60 | 90 | |
| 6 | 10 | CH3OH | 120 | 50 |
| 7 | 10 | CH2Cl2 | 120 | 40 |
| 8 | 10 | CHCl3 | 60 | 60 |
| 9 | 10 | C2H5CO2CH3 | 120 | 70 |
| 10 | 10 | CH3CN | 60 | 50 |
In spite of succeeding in synthesizing BIMs using the BDMS catalyst, in view of previous reports which focused primarily on KHSO4–SiO2- catalyzed conversion,45 and few other catalyzed conversions also reported so far,46 we concentrated our efforts precisely on a highly efficient and eco-friendly approach. Despite the BDMS catalyst being effective, the bio-glycerol-based carbon acid catalyst has reduced conversion time, and recyclability has been improved with lower catalytic loadings. The application of a solid acid catalyst is vital in the conversion of organic functional groups. Carbon solid acid catalysts have efficient catalytic activity toward these transformations. Consequently, we provide a safe and highly effective green procedure using a biodegradable glycerol-based carbon sulfonic acid catalyst to produce bisindoles in excellent yield under solvent-free conditions.
The optimization of the biodegradable glycerol-based carbon sulfonic acid catalyst was evaluated at ambient temperature under solvent-free conditions with excellent yields in 5 min (Scheme 2). The reactants were treated with various solvents; subsequently, the catalytic activity was evaluated (Table 2). The outcomes of the optimization studies reveal that the bio-glycerol-based carbon sulfonic acid catalyst efficiently synthesized the BIM derivatives with 5 mol % catalytic loading.
Scheme 2. Bioglycerol-Based C–SO3H-Catalyzed Synthesis of Turbomycin B.
Table 2. Optimization of the Bioglycerol-Based Carbon Sulfonic Acid Catalyst.
| entry | catalyst (5 mol %) | solvent | time (minutes) | yield (%)a |
|---|---|---|---|---|
| 1 | carbon–SO3H | H2O | 30 | 65 |
| 2 | carbon–SO3H | CH3OH | 30 | 50 |
| 3 | carbon–SO3H | C2H5OH | 10 | 95 |
| 4 | carbon–SO3H | 5 | 95 | |
| 5 | carbon–SO3H | C2H5OH | 5 | 90 |
| 6 | carbon–SO3H | C2H5OH: H2O | 15 | 93 |
| 7b | carbon–SO3H | 10 | 70 |
Isolated yield after the purification.
Catalyst (10 mol %).
We extended our study using an optimized protocol to assess the recyclability of the catalyst. It was discovered that the bio-glycerol-based carbon sulfonic acid catalyst could be used for four cycles without further activation with much less yield loss (Table 3) and that it could be recovered by simple filtration, washed with ethyl acetate, dried, and reused.
Table 3. Recyclability of the Bioglycerol-Based Carbon Sulfonic Acid Catalyst.
| cycles | yield (product)% | recovery (catalyst)% |
|---|---|---|
| native | 95 | 96 |
| 1 | 93 | 93 |
| 2 | 90 | 88 |
| 3 | 87 | 85 |
| 4 | 82 | 80 |
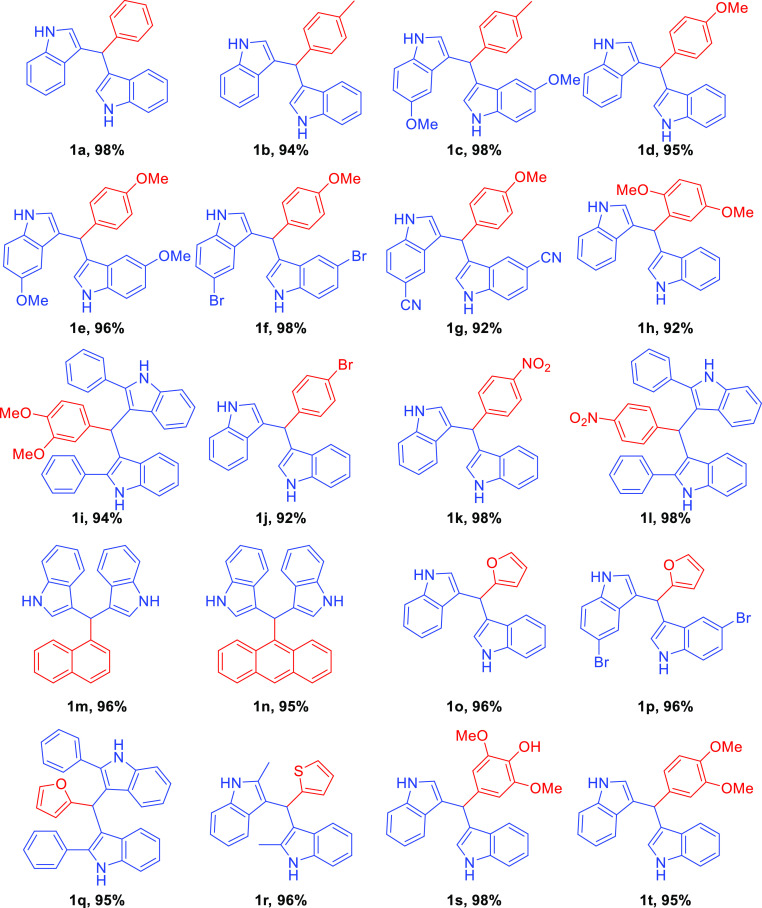
The substrate scope was evaluated by employing various aldehydes and indoles (Scheme 3). Initially, we used substituted benzaldehydes and indoles to generate BIM derivatives in the presence of the biodegradable glycerol-based carbon sulfonic acid catalyst at room temperature under solvent-free conditions for 5 min to obtain quantitative yields.
Scheme 3. Synthesis of Bisindolylmethane Derivatives.
To evaluate the use of indole and benzaldehyde substituents, the procedure is as follows. Initially, benzaldehyde was treated with indole under the above-mentioned conditions to afford 3,3′-(phenylmethylene)bis(1H-indole) (1a). Later, p-tolualdehyde was treated with indole and 5-methoxy indole to generate 3,3′-(p-tolylmethylene)bis(1H-indole) (1b) and 3,3′-(p-tolylmethylene)bis(5-methoxy-1H-indole) (1c), respectively. Further, 4-methoxy benzaldehyde was treated with indole, 5-methoxy indole, 5-bromo indole, and 5-cyano indole to give 3,3′-((4-methoxyphenyl)methylene)bis(1H-indole) (1d), 3, 3′-((4-methoxyphenyl)methylene)bis(5-methoxy-1H-indole) (1e), 3,3′-((4-methoxyphenyl)methylene)bis(5-bromo-1H-indole) (1f), and 3,3′-((4-methoxyphenyl)methylene)bis(1H-indole-5-carbonitrile) (1g), respectively. Furthermore, 2,5-dimethoxy benzaldehyde, veratraldehyde, syringaldehyde, 4-bromo benzaldehyde, 4-nitro benzaldehyde, 1-naphthaldehyde, 9-anthraldehyde were treated with indole, veratraldehyde, 4-nitro benzaldehyde were treated with 2-phenyl indole to produce 3,3′-((2,5-dimethoxyphenyl)methylene)bis(H-indole) (1h), 3, 3′-((4-bromophenyl)methylene)bis(1H-indole) (1j), 3,3′-((3,4-dimethoxyphenyl)methylene)bis(1H-indole) (1t), 4-(di(1H-indol-3-yl)methyl)-2,6-dimethoxyphenol (1s), 3,3′-((4-nitrophenyl)methylene)bis(1H-indole) (1k), 3,3′-((naphthalen-1-yl)methylene)bis(1H-indole) (1m), 3,3′-((anthracen-9-yl)methylene)bis(1H-indole) (1n), 3,3′-((4-nitrophenyl)methylene)bis(2-phenyl-1H-indole) (1i), 3,3′-((4-nitrophenyl)methylene)bis(2-phenyl-1H-indole) (1l), respectively. It is observed that there are no remarkable changes in the yields of the products in the presence of the electron-activating groups and the electron-withdrawing groups.
The scope was extended by using hetero aromatic aldehydes, such as 2-furaldehyde treated with indole, 5-bromo indole, and 2-phenyl indole, and thiophene-2-carboxaldehyde with 2-methylindole to generate 3,3′-((furan-2-yl)methylene)bis(1H-indole) (1o), 3,3′-((furan-2-yl)methylene)bis(5-bromo-1H-indole) (1p), 3,3′-((furan-2-yl)methylene)bis(2-phenyl-1H-indole) (1q), 3,3′-((thiophene-2-yl)methylene)bis(2-methyl-1H-indole) (1r), respectively (Scheme 4). All the synthesized indolemethane derivatives (1a–t) are represented in Figure 2 with the yields of the compounds.
Scheme 4. Bisindole Synthesis Using Heteroaromatic Aldehydes.
Figure 2.
Substrate scope.
Plausible Mechanism
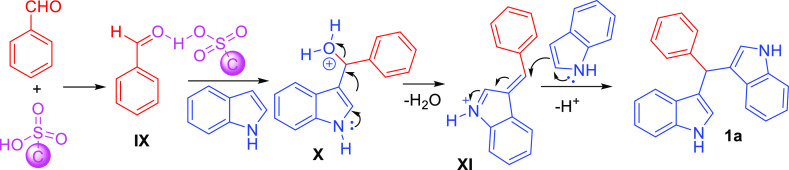
Figure 3 depicts a possible pathway for the synthesis of diindolylmethanes. Initially, a carbon solid acid catalyst reacts with benzaldehyde to form intermediate IX, then the first mole of indole reacts with IX to form intermediate X, and finally, it eliminates the water molecule to produce benzylidene intermediate XI. Later, it combines with the second mole of indole to form the diindolylmethane molecule (1a).
Figure 3.
Plausible mechanism.
Cytotoxicity
The cytotoxicity of the synthesized compounds (1a–t) was assessed using the MTT assay47 against human prostate cancer cell lines DU-145, hepatocellular carcinoma cell lines HepG2, and melanoma cancer cell lines B-16 by employing doxorubicin as a standard reference. The results showed that all of the test compounds were found to have good anticancer activity against all three cell lines (Table 4). Compound 1k was found to have potent activity against the DU-145 cell line with an IC50 value of 1.09 ± 0.92 μM. In addition, compounds 1a, 1c, 1d, 1f, and 1n showed good activity against DU-145 cells with IC50 values of 5.24 ± 0.67, 2.02 ± 0.74, 5.10 ± 0.26, 5.58 ± 0.17, and 6.87 ± 1.06 μM, respectively. The activity of compounds 1g, 1j, and 1m was noteworthy against DU-145 cells with IC50 values of 8.13 ± 0.77, 9.46 ± 1.23, and 8.18 ± 0.74 μM, respectively, whereas all other compounds displayed good to moderate activity against the prostate cancer cell line. In HepG2 cells, all the compounds (1a–t) showed potent cytotoxic effects with IC50 values ranging between 4.23 and 11.9 μM, among which compounds 1a, 1d, and 1f demonstrated highest activity with IC50 values of 4.62 ± 0.03, 4.93 ± 1.04, and 4.23 ± 1.09 μM, respectively. Similarly, in the B16 cell line, the activity of compounds 1a, 1d to 1h, 1j, 1m, 1s, and 1t was very good, with IC50 values ranging between 4.40 and 9.90 μM, whereas the rest of the compounds showed moderate activity. The activity of these compounds may be attributed to the presence of indole nuclei and different substituents present on them, and further, the structures varied with different substituents such as substituted phenyl rings, naphthalene, anthracene, furans, and thiophene attached to the chiral carbon. The toxicity of all of the compounds (1a–t) was tested against the normal cell line CHO–K1. Although all these compounds showed good cytotoxicity against all the tested cancer cells, they also showed moderate toxicity toward normal cells with IC50 ranging between 6.13 and 31.39 μM. Hence, it is inferred that all these compounds are more toxic to cancer cells compared to normal cells. It is known that chemotherapies are mostly toxic in nature. However, they are being used to increase the life expectancy of cancer patients.
Table 4. Cytotoxicity of 1a–ta,b.
| test compound | DU145 | HepG2 | B16 | CHO–K1 |
|---|---|---|---|---|
| 1a | 5.24 ± 0.67 | 4.62 ± 0.03 | 9.88 ± 1.19 | 29.32 ± 0.40 |
| 1b | 15.04 ± 1.02 | 7.18 ± 1.89 | 13.39 ± 1.01 | 31.39 ± 0.05 |
| 1c | 2.02 ± 0.74 | 5.47 ± 0.56 | 36.03 ± 1.09 | 16.24 ± 0.93 |
| 1d | 5.10 ± 0.26 | 4.93 ± 1.04 | 5.31 ± 0.07 | 18.63 ± 0.48 |
| 1e | 11.99 ± 0.46 | 8.27 ± 1.03 | 8.89 ± 0.26 | 16.82 ± 0.66 |
| 1f | 5.58 ± 0.17 | 4.23 ± 1.09 | 4.40 ± 0.35 | 6.13 ± 1.19 |
| 1g | 8.13 ± 0.77 | 6.33 ± 1.8 | 9.82 ± 0.52 | 46.55 ± 0.59 |
| 1h | 16.79 ± 0.65 | 6.23 ± 1.6 | 8.17 ± 0.37 | 11.60 ± 1.27 |
| 1i | 10.28 ± 0.87 | 7.43 ± 1.6 | 15.93 ± 0.25 | 13.53 ± 0.73 |
| 1j | 9.46 ± 1.23 | 5.24 ± 0.1 | 9.90 ± 1.61 | 6.38 ± 0.55 |
| 1k | 1.09 ± 0.92 | 5.28 ± 0.24 | 29.24 ± 2.38 | 26.8 ± 1.29 |
| 1l | 91.35 ± 0.81 | 9.73 ± 1.4 | 23.30 ± 0.65 | 24.23 ± 0.68 |
| 1m | 8.18 ± 0.74 | 7.54 ± 1.19 | 9.49 ± 1.97 | 16.5 ± 0.88 |
| 1n | 6.87 ± 1.06 | 11.9 ± 0.10 | 13.6 ± 0.92 | 12.36 ± 1.38 |
| 1o | 60.81 ± 1.24 | 5.87 ± 0.30 | 16.14 ± 0.79 | 10.12 ± 0.75 |
| 1p | 19.36 ± 0.88 | 11.39 ± 1.37 | 19.65 ± 0.87 | 30.49 ± 1.73 |
| 1q | 39.52 ± 1.02 | 5.00 ± 0.01 | 23.5 ± 7.0 | 9.18 ± 1.58 |
| 1r | 21.23 ± 2.01 | 5.82 ± 0.5 | 15.68 ± 2.10 | 30.46 ± 0.33 |
| 1s | 22.62 ± 0.98 | 7.01 ± 0.53 | 7.02 ± 0.32 | 15.84 ± 0.52 |
| 1t | 12.21 ± 0.96 | 8.12 ± 0.87 | 8.14 ± 1.45 | 15.21 ± 0.35 |
| standard (doxorubicin) | 0.7 ± 0.13 | 0.6 ± 0.12 | 0.8 ± 0.12 | |
| standard (Mitomycin C) | 13.3 ± 0.68 |
IC50 values (μM) (mean ± S.D.).
DU 145 -Prostate cancer cell lines; HepG2-Liver hepato cellular carcinoma cells; B16 -Melanoma cancer cell lines; CHO–K1-Chinese hamster ovary cells (normal).
Molecular Docking
To validate the anticancer activity of the title compounds, the crystal structures of the androgen receptor (AR) (PDB ID: 5T8E),48 proprotein convertase subtilisin/kexin type 9 (PCSK9) (PDB ID: 3GCW),49 and suppressor of cytokine signaling 6 (Socs6)50 were retrieved from the protein data bank (www.rcsb.org). Molecular docking simulations were performed in the active site pocket of receptors with all the ligand molecules 1a–t and compared binding energies with standard reference doxorubicin.
Molecular Docking against the AR
Modification of the AR causes prostate cancer, which is why targeting AR is a viable strategy to combat early-stage cancer.51–53 The docking scores of all molecules 1a–t are comparable to the doxorubicin score. The binding energies and binding interactions of all molecules with the AR (PDB ID: 5T8E) are presented in Table S1. The best active compound 1k scored a binding energy value of −8.8 kcal/mol, which was higher than that of doxorubicin, which scored −8.3 kcal/mol. It demonstrated two H-bond interactions with Arg752 and Asn756 with bond distances of 2.25 and 2.80 Å, respectively. And hydrophobic interactions were displayed with Glu681, Pro682, Val684, and Ala748 of the AR, whereas doxorubicin indicated H-bond interactions with Glu678, Trp751, Thr755, Pro682, Val684, Val685, Ala748, Arg752, Asn756, Pro801, Phe804, and Leu805 with the AR (Figure 4).
Figure 4.
Docking pose of (a) compound 1k and (b) doxorubicin, and binding interactions of (c) compound 1k and (d) doxorubicin in the cavity of the AR.
Molecular Docking against Proprotein Convertase Subtilisin/Kexin Type 9
PCSK2 is a serine protease enzyme mostly produced by the liver cell, and it can potentially be used as a drug target.54 All the novel synthesized compounds 1a–t were docked into the active site pocket of PCSK2 (PDB ID: 3GCW) along with doxorubicin. The docking scores of compounds range from −7.6 to −11.1 kcal/mol, whereas doxorubicin scored −8.8 kcal/mol against PCSK9, as presented in Table S2. The best active compound 1f scored a binding energy value of −9.6 kcal/mol and indicated key interactions with Ser329, Cys358, and Trp461 and hydrophobic interactions with Ala330, Pro331, Arg357, Cys358, Leu436, Pro438, Arg458, Thr459, Val460, Trp461, Arg476, and Ala478 of PCSK9. The standard compound doxorubicin showed H-bond interactions with Pro331, Arg357, Asp360, Arg412, Arg458, Ala463, and Arg476 and hydrophobic interactions with Pro331, Glu332, Arg357, Cys358, Asp360, Arg412, Ser462, Ala463, Ile474, and Arg476 of PCSK9 (Figure 5).
Figure 5.
Docking pose of (a) compound 1f and (b) doxorubicin, and binding interactions of (c) compound 1f and (d) doxorubicin in the cavity of PCSK2.
Molecular Docking against the Suppressor of Cytokine Signaling 6
The suppressor of cytokine signaling (Socs6) (PDB ID: 2VFI) is an attractive drug target for the inhibition of melanoma cancer cells.55,56 All the ligands 1a–t were docked into the active site pocket of Socs6, and the binding energies of compounds ranged from −6.9 to −10.1 kcal/mol, and the doxorubicin score was −8.9 kcal/mol, as presented in Table S3. The best active compound 1f scored a binding energy value of −8.2 kcal/mol, and it exhibited only hydrophobic interactions such as pi–pi stacking, pi-cation, and other alkyl interactions with Leu429, Thr431, Tyr442, Arg474, and Arg476 of Socs6, whereas doxorubicin scored −8.9 kcal/mol which showed H-bond interaction with Arg474, Arg476, Arg391, Phe422, His430, Arg432, Tyr443, Phe470, Cys471, and Arg474 of Socs6 (Figure 6).
Figure 6.
Docking pose of (a) compound 1f and (b) doxorubicin and binding interactions of (c) compound 1f and (d) doxorubicin in the cavity of Socs6.
Conclusions
In conclusion, we have developed highly efficient green protocols to synthesize indolemethane derivatives utilizing both bromodimethylsulfonyl bromide and bio-glycerol-based carbon sulfonic acid catalysts under solvent-free conditions with substituted benzaldehydes and indoles at room temperature in a short time. The biodegradable glycerol-based carbon sulfonic acid-catalyzed method was found to be more effective. The synthesized compounds exhibit excellent anticancer activity against DU145, HepG2, and B16 cell lines. The highest cytotoxicity effects were found with 1k (1.09 μM) against DU145 followed by 1c, 1f, 1n, and 1m between 2.02 and 8.18 μM concentrations. In HepG2 cells, all of the compounds (1a–t) showed potent cytotoxic effects with IC50 from 4.23 to 11.9 μM. Similarly, the compounds 1d–h, 1j, 1m, 1s, and 1t exhibited excellent to promising anticancer activity for the B16 cell line. These substances are more effective in preventing cancer cells than normal cells and are being used for improving the lifespan of cancer patients. The docking studies and binding interactions suggest that drugs are well fitted into the active site pocket of the target molecules, which supports the experimental evidence obtained from in vitro testing.
Experimental Section
Experimental Procedure for the Synthesis of Indolemethane Derivatives (1a–t)
Method 1
In a round-bottom flask, the mixture of benzaldehyde (1 mmol), indole (2 mmol), and bromodimethylsulfonyl bromide (10 mol %) was taken and stirred for 10 min. The completion of the reaction was monitored by TLC, and the reaction mixture was quenched with NaHCO3, extracted with ethyl acetate and water, and the ethyl acetate layer dried over Na2SO4 and concentrated to get corresponding indolemethane derivatives without further purification.
Method 2
In a round-bottom flask, the mixture of benzaldehyde (1 mmol), indole (2 mmol), and carbon sulfonic acid (5 mol %) was added and stirred for 5 min. The completion of the reaction was monitored by TLC, and the reaction mixture was filtered, and then the crude product was recrystallized with water then hexane to get the desired compounds without further purification.
3,3′-(Phenylmethylene)bis(1H-indole) (1a)
Red solid; (0.29 g, 98% yield); mp 124–125 °C; 1H NMR (500 MHz, CDCl3): δ 7.75 (d, J = 7.6 Hz, 2H), 7.29 (dt, J = 7.2, 1.9 Hz, 2H), 7.25 (m, 4H), 7.20–7.15 (m, 2H), 7.14–7.09 (m, 1H), 7.07 (m, 2H), 6.92 (m, 2H), 6.54 (dd, J = 7.3, 0.9 Hz, 2H), 5.79 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 144.1, 136.7, 128.8, 128.3, 127.1, 126.2, 123.7, 121.9, 120.0, 119.7, 119.2, 111.1, 40.2. Mass (ESI+): 321 [M – H]. HRMS (ESI+): m/z Calc. Mass for C23H17N2, [M – H]: 321.13838; found, 321.13863.
3,3-(p-Tolylmethylene)bis(1H-indole) (1b)
Orange–red solid; (0.26 g, 94% yield); mp 101–103 °C; 1H NMR (400 MHz, CDCl3): δ 7.81 (s, 2H), 7.31 (d, J = 7.8 Hz, 2H), 7.24 (s, 1H), 7.19–7.13 (m, 3H), 7.07 (t, J = 7.5 Hz, 2H), 6.99 (d, J = 7.7 Hz, 2H), 6.91 (t, J = 7.5 Hz,2H), 6.56 (d, J = 7.3 Hz, 2H), 5.76 (s, 1H), 2.24 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 141.0, 136.6, 128.9, 128.5, 127.0, 123.5, 121.8, 119.9, 119.1, 111.0, 39.8, 21.1. HRMS (ESI+): m/z calc. mass for C24H19N2, [M-H]: 335.15354; found, 335.15428.
3,3′-(p-Tolylmethylene)bis(5-methoxy-1H-indole) (1c)
Orange solid; (0.32 g, 98% yield); mp 195–198 °C; 1H NMR (400 MHz, CDCl3): δ 7.80 (s, 2H), 7.25–7.21 (m, 4H), 7.08 (d, J = 7.8 Hz, 2H), 6.83–6.78 (m, 4H), 6.65 (d, J = 7.0 Hz, 2H), 5.73 (s, 1H) 3.68(s, 6H), 2.31 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 153.7, 140.9, 135.5, 131.9, 128.9, 128.6, 128.0, 127.6, 124.4, 119.6, 111.8, 111.6, 102.1, 55.9, 39.9, 21.1. HRMS (ESI+): m/z calc. mass for C26H23O2N2, [M – H]: 395.17506; found, 395.17540.
3,3-((4-Methoxyphenyl)methylene)bis(1H-indole) (1d)
Orange–red solid; (0.24 g, 95% yield); mp 193–195 °C; 1H NMR (400 MHz, CDCl3): δ 7.90 (s, 1H), 7.38 (d, J = 7.9 Hz, 2H), 7.31 (d, J = 7.1 Hz, 2H), 7.25–7.21 (m, 2H), 7.13 (dd, J = 7.7, 1.6 Hz, 2H), 7.10–7.06 (m, 1H), 7.00–6.95 (m, 2H), 6.82–6.77 (m, 2H), 6.61 (s, 2H), 5.82 (d, J = 6.4 Hz, 1H), 3.76 (d, J = 7.7 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 157.8, 136.6, 136.2, 129.5, 127.0, 123.5, 121.8, 119.9, 119.1, 113.9, 113.5, 110.9, 55.2, 39.4. Mass (ESI+): 351 [M – H]. HRMS (ESI+): m/z calc. mass for C24H19ON2, [M – H]: 351.14839; found, 351.14919.
3,3-((4-Methoxyphenyl)methylene)bis(5-methoxy-1H-indole) (1e)
Reddish-brown solid; (0.29 g, 96% yield); mp 211–214 °C; 1H NMR (400 MHz, CDCl3): δ 7.88 (s, 2H), 7.37–7.34 (m, 1H), 7.23 (s, 1H), 6.91–6.82 (m, 8H), 6.31 (dd, J = 7.1, 1.9 Hz, 1H), 6.07 (s, 1H), 5.83 (s, 1H), 3.72 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 156.9, 153.8, 141.2, 131.6, 127.1, 123.7, 116.8, 112.0, 111.7, 110.1, 107.9, 106.6, 101.5, 55.9, 34.2. Mass (ESI+): 411 [M – H].
3,3-((4-Methoxyphenyl)methylene)bis(5-bromo-1H-indole) (1f)
Dark-red solid; (0.36 g, 98% yield); mp 219–221 °C; 1H NMR (400 MHz, CDCl3): δ 8.00 (s, 2H), 7.48 (d, J = 7.9 Hz, 2H), 7.19 (dd, J = 7.3, 2.0 Hz, 6H), 6.83 (d, J = 7.6 Hz, 2H), 6.62 (s, 2H), 5.69 (s, 1H), 3.79 (s,3H). 13C NMR (100 MHz, CDCl3): δ 158.2, 135.3, 129.5, 128.7, 125.0, 124.7, 122.3, 119.4, 113.8, 112.6, 55.3, 39.1. HRMS (ESI+): m/z calc. mass for C24H17ON2Br2, [M – H]: 506.96994; found, 506.97021.
3,3′-((4-Methoxyphenyl)methylene)bis(1H-indole-5-carbonitrile) (1g)
Brownish-orange solid; (0.27 g, 92% yield); mp 109–111 °C; 1H NMR (500 MHz, CDCl3): δ 8.00 (s, 2H), 7.47 (s, 2H), 7.26–7.16 (m, 6H), 6.82 (dd, J = 6.7, 1.9 Hz, 2H), 6.61 (d, J = 7.8 Hz, 2H), 5.68 (s, 1H), 3.78 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 157.9, 136.7, 136.3, 131.3, 129.6, 127.1, 123.6, 122.5, 121.9, 121.1, 120.0, 119.2, 114.9, 113.6, 111.1, 55.2, 39.4. HRMS (ESI+): m/z calc. mass for C26H17ON4, [M – H]: 401.13922; found, 401.13969.
3,3-((2,5-Dimethoxyphenyl)methylene)bis(1H-indole) (1h)
Dark-red solid; (0.21 g, 92% yield); mp 195–197 °C; 1H NMR (400 MHz, CDCl3): δ 7.90–7.81 (m, 2H), 7.27–7.19 (m, 3H), 7.08–6.97 (m, 6H), 6.82–6.73 (m, 4H), 6.38–6.35 (m, 1H) 3.62–3.57 (S, 3H), 3.47–3.43 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 164.5, 161.7, 149.5, 137.6, 130.0, 129.7, 129.0, 128.8, 128.3, 123.9, 123.5, 123.0, 117.2, 114.8, 67.6, 40.0. HRMS (ESI+): m/z calc. mass for C25H22O2N2, [M – H]: 381.15979; found, 381.15975.
3,3′-((3,4-Dimethoxyphenyl)methylene)bis(2-phenyl-1H-indole) (1i)
Red solid; (0.30 g, 94% yield); mp 236–239 °C; 1H NMR (400 MHz, CDCl3): δ 8.03 (s, 2H), 7.31 (d, J = 8.0 Hz, 2H), 7.18 (dt, J = 7.0, 1.2 Hz, 10H), 7.08 (s, 2H), 7.01(d, J = 7.8 Hz, 2H), 6.89–6.79 (m, 4H), 6.75 (d, J = 7.6 Hz, 1H), 6.04(s, 1H), 3.87 (s, 3H), 3.61 (s, 3H). 13C NMR(100 MHz, CDCl3): δ 135.9, 135.3, 133.1, 128.9, 128.3, 127.4, 121.8, 121.5, 119.6, 115.9, 112.8, 110.6, 77.4, 77.0, 76.7, 55.8, 39.8. Mass (ESI+): 533 [M – H]. HRMS (ESI+): m/z calc. mass for C37H29O2N2, [M – H]: 533.22208; found, 533.22235.
3,3-((4-Bromophenyl)methylene)bis(1H-indole) (1j)
Pink solid; (0.2 g, 92% yield); mp 102–104 °C; 1H NMR (400 MHz, CDCl3): δ 8.04 (s, 2H), 7.43 (d, J = 7.7 Hz, 2H), 7.29–7.21 (m, 7H), 7.12 (d, J = 7.3 Hz,2H), 6.61 (d, J = 7.3 Hz, 2H), 5.75 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 147.9, 141.8, 135.4, 129.8, 128.5, 125.2, 124.8, 122.1, 121.8, 120.9, 120.6, 118.6, 112.8, 39.3. HRMS (ESI+): m/z calc. mass for C23H16N2Br, [M – H]: 399.04841; found, 399.04914.
3,3′((4-Nitrophenyl)methylene)bis(1H-indole) (1k)
Yellow–orange solid; (0.23 g, 98% yield); mp 222–223 °C; 1H NMR (400 MHz, CDCl3): δ 8.13 (d, J = 7.5 Hz, 2H), 8.01 (s, 2H), 7.50 (d, J = 7.4 Hz, 2H), 7.38 (d, J = 7.1 Hz, 2H), 7.33 (d, J = 7.6 Hz, 2H), 7.19 (t, J = 7.6 Hz, 2H) 7.03 (t, J = 7.4 Hz, 2H), 6.68 (d, J = 7.2 Hz,2H), 5.98 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 151.9, 136.7, 129.6, 123.7, 122.4, 119.6, 118.2, 111.3, 40.2. HRMS (ESI+): m/z calc. mass for C23H16O2N3, [M – H]: 366.12318; found, 366.12369.
3,3-((4-Nitrophenyl)methylene)bis(2-phenyl-1H-indole) (1l)
Yellow–orange solid; (0.33 g, 98% yield); mp 256–259 °C; 1H NMR (400 MHz, CDCl3): δ 7.97 (s, 2H), 7.73 (d, J = 7.4 Hz, 1H), 7.64(d, J = 7.4 Hz, 1H), 7.43–7.33 (m, 9H), 7.25–7.15 (m, 6H), 7.06–7.00 (m, 3H), 6.68 (s, 2H), 5.87 (s, 1H). 13C NMR (125 MHz, CDCl3): δ 143.1, 139.9, 133.7, 132.9, 131.5, 131.3, 131.0, 130.5, 126.9, 123.6, 122.1, 119.8, 119.4, 119.1, 115.2, 111.1, 39.7. HRMS (ESI+): m/z calc. mass for C35H24O2N3, [M – H]: 518.18577; found, 518.18630.
3,3′-(Naphthalen-1-ylmethylene)bis(1H-indole) (1m)
Yellow–orange solid; (0.22 g, 96% yield); mp 165–166 °C; 1H NMR (400 MHz, CDCl3): δ 10.47 (s, 2H), 8.13 (d, J = 7.3 Hz, 1H), 7.86 (s, 1H), 7.79 (d, J = 7.9 Hz, 1H), 7.64 (d, J = 7.9 Hz, 1H), 7.39–7.27 (m, 4H), 7.20 (d, J = 6.9 Hz, 3H), 6.97 (t, J = 7.4 Hz, 2H), 6.78 (t, J = 7.4 Hz, 2H), 6.57 (s, 2H), 6.54 (s, 1H). 13C NMR (125 MHz, CDCl3): δ 145.3, 141.8, 138.7, 136.5, 133.5, 131.7, 131.0, 130.8, 130.8, 129.9, 129.4, 128.9, 126.0, 123.9, 123.4, 122.9, 116.6, 40.5. Mass (ESI+): 371 [M – H]. HRMS (ESI+): m/z calc. mass for C27H19N2, [M – H]: 371.15403; found, 371.15428.
3,3′-(Anthracen-9-ylmethylene)bis(1H-indole) (1n)
Dark-pink solid; (0.19 g, 95% yield); mp 188–190 °C; 1H NMR (400 MHz, CDCl3): δ 8.64 (d, J = 7.4 Hz, 2H), 8.45 (s, 1H), 8.00 (d, J = 7.2 Hz, 2H), 7.91 (s, 2H), 7.35 (dd, J = 7.2, 1.6 Hz, 6H), 7.11 (dd, J = 7.0, 1.2 Hz, 5H), 6.87 (s, 2H), 6.80 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 136.6, 135.1, 131.9, 129.1, 127.2, 124.7, 123.9, 121.8, 120.0, 119.2, 119.0, 111.0, 35.1. HRMS (ESI+): m/z calc. mass for C31H21N2, [M – H]: 421.16875; found, 421.16993.
3,3′-(Furan-2-ylmethylene)bis(1H-indole) (1o)
Pinkish-red solid; (0.31 g, 96% yield); mp 311–314 °C; 1H NMR (400 MHz, CDCl3): δ 7.95 (s, 2H), 7.47 (d, J = 7.5 Hz, 2H), 7.33 (d, J = 7.6 Hz, 3H), 7.19–7.12 (m, 2H), 7.06–6.9 (m, 2H), 6.85 (s, 2H), 6.28 (dd, J = 7.0, 1.8 Hz, 1H), 6.04 (d, J = 7.1 Hz,1H), 5.93 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 157.0, 141.2, 136.5, 126.7, 123.0, 122.0, 119.6, 119.3, 117.1, 111.0, 110.1, 106.5, 34.0. HRMS (ESI+): m/z calc. mass for C21H15ON2, [M – H]: 311.11705; found, 311.1178.
3,3′-(Furan-2-ylmethylene)bis(5-bromo-1H-indole) (1p)
Blackish-brown solid; (0.46 g, 96%); mp 331–334 °C; 1H NMR (400 MHz, CDCl3): δ 8.06 (s, 2H), 7.58–7.52(m, 2H), 7.38 (dd, J = 7.8, 0.9 Hz, 1H), 7.24 (dd, J = 7.2, 1.6 Hz, 4H), 6.88 (dd, J = 7.5, 1.6 Hz, 2H), 6.33 (dd, J = 7.2, 1.9 Hz, 1H), 6.05–5.98 (m, 1H), 5.81 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 162.9, 140.2, 137.0, 128.9, 126.8, 124.6, 122.0, 116.8, 27.8. HRMS (ESI+): m/z calc. mass for C21H13ON2Br2, [M – H]: 466.93835; found, 466.93891.
3,3′-(Furan-2-ylmethylene)bis(2-phenyl-1H-indole) (1q)
Purple–pink solid; (0.45 g, 95% yield); mp 319–323 °C; 1H NMR (400 MHz, CDCl3): δ 7.92 (s, 2H), 7.30 (s, 1H), 7.22 (d, J = 7.0 Hz, 2H), 7.15–7.06 (m, 10H), 7.02(t, J = 7.1 Hz, 4H), 6.82 (t, J = 7.6 Hz, 2H), 6.30–6.24 (m, 1H), 6.03–5.94 (m, 2H). 13C NMR (100 MHz, CDCl3 + DMSO-d6): δ 162.9, 140.2, 137.0, 128.9, 126.8, 124.6, 122.0, 116.8, 27.8. Mass (ESI+): 487 [M + Na]. HRMS (ESI+): m/z calc. mass for C33H24ON2, [M] 464.18778; found, 464.18831.
3,3′-(Thiophen-2-ylmethylene)bis(2-methyl-1H-indole) (1r)
Green solid; (0.30 g, 96% yield); mp 148–151 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.77 (s, 4H), 7.32 (d, J = 7.2 Hz, 4H), 7.20 (d, J = 7.9 Hz, 5H), 6.91 (dd, J = 7.2, 1.4 Hz, 11H), 6.76–6.66 (m, 7H), 6.10 (s, 2H), 2.14 (s, 14H). 13C NMR (100 MHz, CDCl3): δ 135.1, 132.1, 129.8, 128.4, 123.4, 121.0, 119.4, 119.0, 111.9, 110.4, 110.2, 39.4, 12.5. Mass (ESI+): 355 [M – H]. HRMS (ESI+): m/z calc. mass for C23H19N2S, [M – H]: 355.12623; found, 355.12635.
4-(Di(1H-indol-3-yl)methyl)-2,6-dimethoxyphenol (1s)
Dark red; (0.25 g, 98% yield); mp 115–116 °C; 1H NMR (500 MHz, CDCl3): δ 7.92 (s, 2H), 7.33 (d, J = 7.9 Hz, 2H), 7.29 (d, J = 7.6 Hz, 2H), 7.19 (s, 2H), 6.96–6.90 (m, 2H), 6.60 (s, 2H), 6.52 (s, 2H), 5.74 (s, 1H), 3.70–3.64 (m, 6H). 13C NMR (125 MHz, CDCl3): δ 152.05, 135.10, 132.17, 129.86, 123.45, 121.28, 121.04, 119.46, 119.05, 112.51, 111.87, 110.48, 110.29, 39.44, 25.11. HRMS (ESI+): m/z calc. mass for C25H22O3N2, [M – H]: 397.15423.
3,3′-((3,4-Dimethoxyphenyl)methylene)bis(1H-indole) (1t)
Orange–red solid; (0.21 g, 95% yield); mp 208–209 °C; 1H NMR (400 MHz, CDCl3): δ 8.10 (dd, J = 7.6, 1.7 Hz 2H), 7.85 (s,2H), 7.42 (d, J = 7.8 Hz, 2H), 7.28 (s, 1H), 7.07 (m, 3H), 6.88 (dt, J = 7.2, 1.5 Hz, 5H), 6.05 (s, 1H), 2.08 (s, 6H).
Acknowledgments
The authors express their gratitude to the Head, Dean, and Principal of the Department of Chemistry at the University College of Science, Osmania University, Hyderabad, as well as the Director of CSIR-IICT, Hyderabad, for providing laboratory facilities. P.M.R. acknowledges financial support from TSCOST under Project Relate Grants (File No. 03/TSCOST/DST-PRG/2021-22, Dt: 31.12.2021). P.M.R. also extends thanks to the UGC-UPE FAR & DST-PURSE PROGRAMME (2017-22) at Osmania University, Hyderabad, for their financial support. Anren Hu would like to express their gratitude to the Ministry of Science and Technology, Taiwan, for providing financial support for this research under contract numbers NSTC 112-2113-M-320-001 and MOST 111-2113-M-320-001. Additionally, they extend their thanks to Tzu-Chi University and Tzu Chi Hospital for their financial support of this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c05293.
General information, experimental procedures, docking experimental tables, and characterization spectra of 1a–t (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Humphrey G. R.; Kuethe J. T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106 (7), 2875–2911. 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]
- Bandini M.; Eichholzer A. Catalytic functionalization of indoles in a new dimension. Angew. Chem., Int. Ed. Engl. 2009, 48 (51), 9608–9644. 10.1002/anie.200901843. [DOI] [PubMed] [Google Scholar]
- Pathak T. P.; Osiak J. G.; Vaden R. M.; Welm B. E.; Sigman M. S. Synthesis and preliminary biological study of bisindolylmethanes accessed by an acid-catalyzed hydroarylation of vinyl indoles. Tetrahedron 2012, 68 (26), 5203–5208. 10.1016/j.tet.2012.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernon M.; Wu M.; Buszta T.; Janney P. Environmental benefits of methanesulfonic acid. Green Chem. 1999, 1 (3), 127–140. 10.1039/a900157c. [DOI] [Google Scholar]
- Gönül Z.; Öztürk D. A.; Küçükbay F.; Tekin S.; Tekin Z.; Küçükbay H. Antioxidant and cytotoxic properties of some new dipeptide-indole conjugates. J. Heterocycl. Chem. 2023, 60 (1), 86–95. 10.1002/jhet.4564. [DOI] [Google Scholar]
- Bao B.; Sun Q.; Yao X.; Hong J.; Lee C. O.; Sim C. J.; Im K. S.; Jung J. H. Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp. J. Nat. Prod. 2005, 68 (5), 711–715. 10.1021/np049577a. [DOI] [PubMed] [Google Scholar]
- Garbe T. R.; Kobayashi M.; Shimizu N.; Takesue N.; Ozawa M.; Yukawa H. Indolyl carboxylic acids by condensation of indoles with alpha-keto acids. J. Nat. Prod. 2000, 63 (5), 596–598. 10.1021/np990517s. [DOI] [PubMed] [Google Scholar]
- Sakemi S.; Sun H. H. Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991, 56 (13), 4304–4307. 10.1021/jo00013a044. [DOI] [Google Scholar]
- Gunasekera S. P.; McCarthy P. J.; Kelly-Borges M. Hamacanthins A and B, new antifungal bis indole alkaloids from the deep-water marine sponge, Hamacantha sp. J. Nat. Prod. 1994, 57 (10), 1437–1441. 10.1021/np50112a014. [DOI] [PubMed] [Google Scholar]
- Malghe Y. S.; Thorat V. V.; Chowdhary A. S.; Bobade A. S. Synthesis, characterization and biological activities of new bis-1, 3, 4-oxadiazoles. J. Chem. Pharm. Res. 2015, 7 (6), 392–398. [Google Scholar]
- Kim Y. S.; Milner J. A. Targets for indole-3-carbinol in cancer prevention. J. Nutr. Biochem. 2005, 16 (2), 65–73. 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lerner A.; Grafi-Cohen M.; Napso T.; Azzam N.; Fares F. The indolic diet-derivative, 3, 3′-diindolylmethane, induced apoptosis in human colon cancer cells through upregulation of NDRG1. BioMed Res. Int. 2012, 2012, 1–5. 10.1155/2012/256178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelbaqi K.; Lack N.; Guns E. T.; Kotha L.; Safe S.; Sanderson J. T. Antiandrogenic and growth inhibitory effects of ring-substituted analogs of 3,3′-diindolylmethane (ring-DIMs) in hormone-responsive LNCaP human prostate cancer cells. Prostate 2011, 71 (13), 1401–1412. 10.1002/pros.21356. [DOI] [PubMed] [Google Scholar]
- Yoon K.; Lee S. O.; Cho S. D.; Kim K.; Khan S.; Safe S. Activation of nuclear TR3 (NR4A1) by a diindolylmethane analog induces apoptosis and proapoptotic genes in pancreatic cancer cells and tumors. Carcinogenesis 2011, 32 (6), 836–842. 10.1093/carcin/bgr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-J.New indole-containing medicinal compounds. Heterocyclic Scaffolds II: Reactions Applications of Indoles; Springer Berlin Heidelberg, Vol. 26, 2010; pp 1–29. [Google Scholar]
- Esmaielpour M.; Akhlaghinia B.; Jahanshahi R. Green and efficient synthesis of aryl/alkylbis (indolyl) methanes using Expanded Perlite-PPA as a heterogeneous solid acid catalyst in aqueous media. J. Chem. Sci. 2017, 129, 313–328. 10.1007/s12039-017-1246-x. [DOI] [Google Scholar]
- Ghosh R.; Maiti S. Advances in indium triflate catalyzed organic syntheses. J. Mol. Catal. A: Chem. 2007, 264 (1–2), 1–8. 10.1016/j.molcata.2006.08.086. [DOI] [Google Scholar]
- Kokare N. D.; Sangshetti J. N.; Shinde D. B. Oxalic acid as a catalyst for efficient synthesis of bis-(indolyl) methanes, and 14-aryl-14H-dibenzo [a, j] xanthenes in water. Chin. Chem. Lett. 2008, 19 (10), 1186–1189. 10.1016/j.cclet.2008.07.015. [DOI] [Google Scholar]
- Parameswaran P.; Majik M.; Praveen P. Bis (indolyl) methane alkaloids: isolation, bioactivity, and syntheses. Synthesis 2015, 47, 1827–1837. 10.1055/s-0034-1380415. [DOI] [Google Scholar]
- Yang Q.; Yin Z. L.; Ouyang B. L.; Peng Y. Y. Pyridinium tribromide catalyzed condensation of indoles and aldehydes to form bisindolylalkanes. Chin. Chem. Lett. 2011, 22 (5), 515–518. 10.1016/j.cclet.2010.11.026. [DOI] [Google Scholar]
- Azizi N.; Manocheri Z. Eutectic salts promote green synthesis of bis (indolyl) methanes. Res. Chem. Intermed. 2012, 38, 1495–1500. 10.1007/s11164-011-0479-4. [DOI] [Google Scholar]
- Bai G. Y.; Ma Z.; Shi L.; Li T.; Han J.; Chen G.; Li N.; Liu P. An effective lactic acid-modified Hβ zeolite for synthesis of bis (indolyl) methanes. Res. Chem. Intermed. 2012, 38, 2501–2510. 10.1007/s11164-012-0567-0. [DOI] [Google Scholar]
- Wang L.; Wei W.; Guo Y.; Xu J.; Shao S. Nitro-substituted 3, 3′-bis (indolyl) methane derivatives as anion receptors: Electron-withdrawing effect and tunability of anion binding properties. Spectrochim. Acta, Part A 2011, 78 (2), 726–731. 10.1016/j.saa.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Lee A. F.; Wilson K. Recent developments in heterogeneous catalysis for the sustainable production of biodiesel. Catal. Today 2015, 242, 3–18. 10.1016/j.cattod.2014.03.072. [DOI] [Google Scholar]
- Steffen W.; Hughes L.. Critical Decade 2013: Climate Change Science, Risks and Response 2013.
- Wilson K.; Clark J. H. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. Chem. 2000, 72 (7), 1313–1319. 10.1351/pac200072071313. [DOI] [Google Scholar]
- Prabhavathi Devi B.; Gangadhar K.; Siva Kumar K.; Shiva Shanker K.; Prasad R.; Sai Prasad P. Synthesis of sulfonic acid functionalized carbon catalyst from glycerol pitch and its application for tetrahydropyranyl protection/deprotection of alcohols and phenols. J. Mol. Catal. A: Chem. 2011, 345 (1–2), 96–100. 10.1016/j.molcata.2011.05.025. [DOI] [Google Scholar]
- Prabhavathi Devi B. L.; Gangadhar K. N.; Sai Prasad P. S.; Jagannadh B.; Prasad R. B. A glycerol-based carbon catalyst for the preparation of biodiesel. ChemSusChem 2009, 2 (7), 617–620. 10.1002/cssc.200900097. [DOI] [PubMed] [Google Scholar]
- Karthik M.; Tripathi A.; Gupta N.; Palanichamy M.; Murugesan V. Zeolite catalyzed electrophilic substitution reaction of indoles with aldehydes: synthesis of bis (indolyl) methanes. Catal. Commun. 2004, 5 (7), 371–375. 10.1016/j.catcom.2004.04.007. [DOI] [Google Scholar]
- Rezaei N.; Ranjbar P. R. The efficient synthesis of Hantzsch 1, 4-dihydropyridines via metal-free oxidative CC coupling by HBr and DMSO. Tetrahedron Lett. 2018, 59 (46), 4102–4106. 10.1016/j.tetlet.2018.10.010. [DOI] [Google Scholar]
- Choudhury L. H.; Parvin T.; Khan A. T. Recent advances in the application of bromodimethylsulfonium bromide (BDMS) in organic synthesis. Tetrahedron 2009, 65 (46), 9513–9526. 10.1016/j.tet.2009.07.052. [DOI] [Google Scholar]
- Armstrong E. L.; Grover H. K.; Kerr M. A. Scandium triflate-catalyzed nucleophilic additions to indolylmethyl meldrum’s acid derivatives via a gramine-type fragmentation: synthesis of substituted indolemethanes. J. Org. Chem. 2013, 78 (20), 10534–10540. 10.1021/jo4017524. [DOI] [PubMed] [Google Scholar]
- Xu X.-F.; Xiong Y.; Ling X.-G.; Xie X.-M.; Yuan J.; Zhang S.-T.; Song Z.-R. A practical synthesis of bis (indolyl) methanes catalyzed by BF3· Et2O. Chin. Chem. Lett. 2014, 25 (3), 406–410. 10.1016/j.cclet.2013.11.038. [DOI] [Google Scholar]
- Veisi H.; Maleki B.; Eshbala F. H.; Veisi H.; Masti R.; Ashrafi S. S.; Baghayeri M. In situ generation of Iron (III) dodecyl sulfate as Lewis acid-surfactant catalyst for synthesis of bis-indolyl, tris-indolyl, Di (bis-indolyl), Tri (bis-indolyl), tetra (bis-indolyl) methanes and 3-alkylated indole compounds in water. RSC adv. 2014, 4 (58), 30683–30688. 10.1039/C4RA03194F. [DOI] [Google Scholar]
- Nguyen N.-K.; Tran D. L.; Hung T. Q.; Le T. M.; Son N. T.; Trinh Q. T.; Dang T. T.; Langer P. Facile access to bis (indolyl) methanes by copper-catalysed alkylation of indoles using alcohols under air. Tetrahedron Lett. 2021, 68, 152936. 10.1016/j.tetlet.2021.152936. [DOI] [Google Scholar]
- Griffiths K.; Kumar P.; Akien G. R.; Chilton N. F.; Abdul-Sada A.; Tizzard G. J.; Coles S. J.; Kostakis G. E. Tetranuclear Zn/4f coordination clusters as highly efficient catalysts for Friedel-Crafts alkylation. Chem. Commun. 2016, 52 (50), 7866–7869. 10.1039/C6CC03608B. [DOI] [PubMed] [Google Scholar]
- Singh N. G.; Nongrum R.; Kathing C.; Rani J. W. S.; Nongkhlaw R. Bakers’ yeast: an environment benign catalyst for the one-pot synthesis of indolyl chromenes and bisindolyl alkanes. Green Chem. Lett. Rev. 2014, 7 (2), 137–144. 10.1080/17518253.2014.902506. [DOI] [Google Scholar]
- Kalla R. M. N.; Hong S. C.; Kim I. Synthesis of bis (indolyl) methanes using hyper-cross-linked polyaromatic spheres decorated with bromomethyl groups as efficient and recyclable catalysts. ACS Omega 2018, 3 (2), 2242–2253. 10.1021/acsomega.7b01925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemallapudi B. R.; Zyryanov G. V.; Avula B.; Guda M. R.; Gundala S. An effective green and ecofriendly catalyst for synthesis of bis (indolyl) methanes as promising antimicrobial agents. J. Heterocycl. Chem. 2019, 56 (12), 3324–3332. 10.1002/jhet.3729. [DOI] [Google Scholar]
- Qiao J.; Gao S.; Wang L.; Wei J.; Li N.; Xu X. Air-stable μ-oxo-bridged binuclear titanium (IV) salophen perfluorooctanesulfonate as a highly efficient and recyclable catalyst for the synthesis of bis (indolyl) methane derivatives. J. Organomet. Chem. 2020, 906, 121039. 10.1016/j.jorganchem.2019.121039. [DOI] [Google Scholar]
- Yang T.; Lu H.; Shu Y.; Ou Y.; Hong L.; Au C.-T.; Qiu R. CF3SO2Na-mediated, UV-light-induced Friedel-Crafts alkylation of indoles with ketones/aldehydes and bioactivities of products. Org. Lett. 2020, 22 (3), 827–831. 10.1021/acs.orglett.9b04272. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Lu Z.; Fang K.; He X.; Xu H.; Hu Y. Enzymatic approach to cascade synthesis of bis(indolyl)methanes in pure water. RSC Adv. 2020, 10 (18), 10848–10853. 10.1039/C9RA10014H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Mercado E.; Rivas-Loaiza J. A.; García-Merinos J. P.; López Y.; González-Campos J. B. Chitosan-supported copper salt and copper metal nanoparticles/copper (I) oxide Microcrystals: Efficient and Recyclable Heterogeneous Catalysts for the Synthesis of Bis (indolyl) methanes. Chem. Eng. Process.: Process Intensif. 2021, 159, 108201. 10.1016/j.cep.2020.108201. [DOI] [Google Scholar]
- Wu Z.; Wang G.; Yuan S.; Zhan H.; Liu W.; Bi S.; Li H.; Ma B.; Sun Y. Synthesis, characterization, and properties of highly hydrophilic polyaniline sulfonic acid. Russ. J. Gen. Chem. 2020, 90, 1055–1061. 10.1134/S1070363220060195. [DOI] [Google Scholar]
- Naidu K. R. M.; Khalivulla S. I.; Kumar P. C. R.; Lasekan O. KHSO4-SiO 2 catalyzed facile synthesis of bis (indolyl) methanes. Org. Commun. 2012, 5 (3), 150–159. [Google Scholar]
- Gong H. W.; Xie Z. F. Research Progress of Synthesis of Bis(indolyl)methanes. Chin. J. Org. Chem. 2012, 32 (07), 1195–1207. 10.6023/cjoc1110263. [DOI] [Google Scholar]
- Deshpande S. S.; Veeragoni D.; Rachamalla H. K.; Misra S. Anticancer properties of ZnO-Curcumin nanocomposite against melanoma cancer and its genotoxicity profiling. J. Drug Delivery Sci. Technol. 2022, 75, 103703. 10.1016/j.jddst.2022.103703. [DOI] [Google Scholar]
- Asano M.; Hitaka T.; Imada T.; Yamada M.; Morimoto M.; Shinohara H.; Hara T.; Yamaoka M.; Santou T.; Nakayama M.; Imai Y.; Habuka N.; Yano J.; Wilson K.; Fujita H.; Hasuoka A. Synthesis and biological evaluation of novel selective androgen receptor modulators (SARMs). Part II: Optimization of 4-(pyrrolidin-1-yl)benzonitrile derivatives. Bioorg. Med. Chem. Lett. 2017, 27 (9), 1897–1901. 10.1016/j.bmcl.2017.03.038. [DOI] [PubMed] [Google Scholar]
- McNutt M. C.; Kwon H. J.; Chen C.; Chen J. R.; Horton J. D.; Lagace T. A. Antagonism of secreted PCSK9 increases low density lipoprotein receptor expression in HepG2 cells. J. Biol. Chem. 2009, 284 (16), 10561–10570. 10.1074/jbc.M808802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadjali F.; Pike A. C.; Vesterlund M.; Sun J.; Wu C.; Li S. S.; Ronnstrand L.; Knapp S.; Bullock A. N.; Flores-Morales A. Structural basis for c-KIT inhibition by the suppressor of cytokine signaling 6 (SOCS6) ubiquitin ligase. J. Biol. Chem. 2011, 286 (1), 480–490. 10.1074/jbc.M110.173526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikwu F. A.; Shallangwa G. A.; Mamza P. A. QSAR, QSTR, and molecular docking studies of the anti-proliferative activity of phenylpiperazine derivatives against DU145 prostate cancer cell lines. J. Basic Appl. Sci. 2020, 9, 35. 10.1186/s43088-020-00054-y. [DOI] [Google Scholar]
- Pulukuri S. M.; Gondi C. S.; Lakka S. S.; Jutla A.; Estes N.; Gujrati M.; Rao J. S. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J. Biol. Chem. 2005, 280 (43), 36529–36540. 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Recouvreux M. V.; Wu J. B.; Gao A. C.; Zonis S.; Chesnokova V.; Bhowmick N.; Chung L. W.; Melmed S. Androgen Receptor Regulation of Local Growth Hormone in Prostate Cancer Cells. Endocrinology 2017, 158 (7), 2255–2268. 10.1210/en.2016-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbe N. B.; Lagos C. F.; Ortiz C. A. V.; Tambuwala M.; Palakurthi S. S.; Zacconi F. C. PCSK9 conjugated liposomes for targeted delivery of paclitaxel to the cancer cell: A proof-of-concept study. Biomed. Pharmacother. 2022, 153, 113428. 10.1016/j.biopha.2022.113428. [DOI] [PubMed] [Google Scholar]
- Tobelaim W. S.La double face de la protéine SOCS1 dans la carcinogenèse colorectale; Université de Sherbrooke, 2015.
- Tagami-Nagata N.; Serada S.; Fujimoto M.; Tanemura A.; Nakatsuka R.; Ohkawara T.; Murota H.; Kishimoto T.; Katayama I.; Naka T. Suppressor of cytokine signalling-1 induces significant preclinical antitumor effect in malignant melanoma cells. Exp. Dermatol. 2015, 24 (11), 864–871. 10.1111/exd.12802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.