Abstract
Herein, we demonstrated a silver/K2S2O8-mediated highly regio- and diastereoselective 6/5-exo trig radical cascade cyclization of alkyne-tethered cyclohexadienones with sulfonyl hydrazides or sodium sulfinates and subsequent selenation to access 6,6-dihydrochromenone and 6,5-fused tetrahydro benzofuranone derivatives. This reaction protocol features high functional group compatibility and has a wide substrate scope providing a variety of dihydrochromenones and tetrahydro benzofuranone derivatives in good to excellent yields. The reaction proceeds via the attack of a sulfonyl radical to alkyne over the activated Michael acceptor. The TEMPO quenching experiment implies the presence of a radical intermediate. Further synthetic versatility of 6,6- and 5,6-fused derivatives is also showcased.
Introduction
Organochalcogen compounds,1 particularly containing sulfonyl2 (R1SO2−) and seleno3 (R2Se−) groups, are highly versatile functional groups that are widely present in natural products and4 pharmaceuticals and play a substantial role in organic synthesis (nucleophiles, electrophiles, ligands, and catalysts), biochemistry,5 medicinal chemistry,6 and materials science.7 Additionally, molecules containing selenium and sulfone have been widely used in organic synthesis.8 The C–S and C–Se bonds are weak, making it simple to add, delete, and change them into different functional groups. The incorporation of chalcogen functionality into a single molecule might provide an opportunity to enhance the bioactivity of the drugs or original lead compounds.9 Therefore, the discovery of novel techniques for incorporating both seleno and sulfonyl groups into a single molecule is of enormous synthetic significance, enabling a rapid increase in molecular complexity.
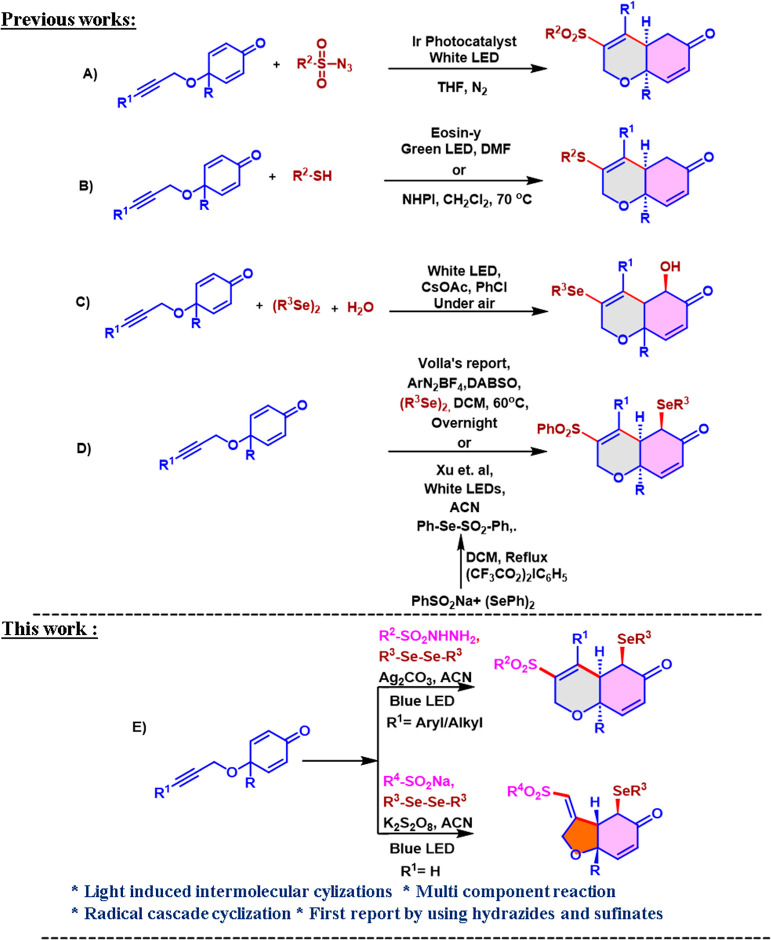
Visible-light photocatalysis10 has emerged as a key method for stimulating a variety of synthetic conversions for the creation of C–X and C–C bonds in the quest for energy-efficient and sustainable synthesis. For the production of radical ion intermediates under benign conditions, visible light-induced catalysis has proven to be one of the most reliable methods. On the other hand, the radical cascade cyclizations11 are privileged strategies that have been rapidly applied for the manufacture of complex molecular skeletons from commercially available acyclic substrates. In particular, the cascade cyclizations of 1,6-enynes12 have drawn a lot of attention for manufacturing of cyclic complex ring systems from acyclic moieties. Alkyne-tethered cyclohexadienones are used in manufacturing cyclic complex ring systems from acyclic moieties. Alkyne-tethered cyclohexadienones, which can be obtained by dearomatizing the appropriate phenols, are annulated in a variety of ways and are able to generate novel cyclic structural entities. In this context, the production of fused carbocycles from alkyne-tethered cyclohexadienones has received substantial research attention.13 As a result, great efforts have been made to synthesize these fused carbocycle motifs in a simple and effective synthetic approach. Although several strategies have been developed to construct decorated bicyclic frameworks via cyclizations of cyclohexadienones, recognized as powerful strategies, these structural entities are widely found in bioactive natural products. For instance, metal catalysts including Rh,14 Pd,15 Cu,16 and Ni17 have been widely studied in the synthesis of 5- and 6-fused carbocycles. There are only a few reports on the radical cascade cyclization of alkyne-tethered cyclohexadienones for the formation of 6,5- and 6,6-fused carbocycles. In a pioneering report, Lam and co-workers developed a radical cascade cyclization of alkyne-tethered cyclohexadienones with sulfonyl radicals produced from sulfonyl azides (Scheme 1,A)18 under visible light-induced iridium photocatalysis. Volla and co-workers demonstrated a Giese-type cyclization protocol for the highly diastereoselective synthesis of sulfenylated dihydrochromenones under visible-light irradiation (Scheme 1,B).19 Sahoo’s group also discovered a novel protocol for the construction of 3-thioaryl-bearing fused dihydrochromenones via regio- and chemoselective radical cyclization under N-hydroxyphthalimide (NHPI) catalysis (Scheme 1,B).20 Later, Xu developed a three-component tandem cyclization/substitution for the construction of highly substituted dihydrochromenones using cyclohexadienones, diselenides, and H2O (Scheme 1,C).21 Xu and colleagues reported the cascade cyclization of alkyne-tethered cyclohexadienones, although they employed a presynthesized starting material where the preparation required reflux temperature and a pricey hypervalent reagent (Scheme 1,D).22 Very recently, while this manuscript was under preparation, Volla and co-workers also demonstrated a multicomponent radical cascade reaction for the synthesis of highly functionalized dihydrochromenones using explosive aryldiazonium salts as aryl partners and expensive DABSO as a SO2 source and also as a redox mediator under reflux conditions (Scheme 1,D).22,23
Scheme 1. Comparative Approaches for Cascade Cyclizations of Alkyne-Tethered Cyclohexadienones between the Reported Work and This Work.
Inspired by these results and our previous experience on photoredox catalysis,24 we envisioned that sulfonyl radicals generated from sulfonyl hydrazides or sodium sulfinates might be added to alkynyl cyclohexadienones and undergo Giese-type cyclization, followed by selenation by diselenides to produce bis-functionalization (Scheme 1,E) (see Scheme 1).
Results and Discussion
To test the above hypothesis, we commenced our study to investigate the optimal reaction conditions (see Supporting Information Table S1). Under the optimized reaction conditions in hand, we turned our attention to probing the versatility of the developed protocol by independently screening with various cyclohexadienones, sulfonyl sources, and diaryl chalcogens (Scheme 2). First, the electronic effect of substituent R1 on the cyclohexadienones was investigated. Gratifyingly, both electron-withdrawing and -donating substituted arenes at the alkyne terminus of cyclohexadienones were well tolerated in this transformation and afforded the corresponding 6-exo trig cyclized products in good to excellent yields and with high diastereoselectivity, which indicated that electronic effects are not much effective in this protocol (Scheme 2,4a–e). We then examined the reaction efficiency with the replacement of arene at the alkyne terminus of cyclohexadienones with hetero aryl as well as alkyl substituent under the standard reaction conditions; the reaction proceeded well to afford the corresponding desired products in excellent yields (Scheme 2, 4f–g). Changing the substituent in the quaternary carbon of cyclohexadienones from methyl to iso-propyl or phenyl made the 6-exo trig transformation proceed smoothly and gave the desired products in good yields (Scheme 2, 4h–i). The radical cascade cyclization is also feasible for the cyclohexadienone having a methyl moiety at the α-position of carbonyl, affording the 6-exo trig cyclized product in an excellent yield (Scheme 2, 4j). Encouraged by these results, we were devoted to searching for the versatility of this present protocol to further extend the substrate scope with respect to diaryldiselenides.
Scheme 2. Substrate Scope of 6-Exo Trig Radical Cascade Cyclization of Cyclohexadienones.
Standard Condition for 6-Exo Trig Cyclization: Internal alkyne (1.0 equiv), hydrazide (1.0 equiv), diphenyl diselenide (0.5 equiv), and Ag2CO3 (1.0 equiv) were mixed in 2 mL ACN under an inert atmosphere with 5 W blue LED irradiation.
Delectably, electron-withdrawing and -donating groups were well tolerated at the ortho, para, and meta positions of the phenyl ring of diaryl diselenides, affording the desired cyclized products in good to excellent yields (Scheme 2, 4k–o). Notably, heteroaromatic-substituted diaryl diselenides underwent this transformation and afforded the corresponding 6-exo trig product in a good yield (Scheme 2, 4p). Furthermore, various sulfonyl hydrazides such as 4-methylphenyl and cyclopropyl sulfonyl hydrazides were also compatible with this catalytic system, leading to the expected 6-exo trig cyclized products 4r in excellent yields (Scheme 2, 4q–s). We also further examined the performance of reaction efficiency with ortho-substituted arene at the alkyne terminus of cyclohexadienone, and it was observed that the formation of the desired product in a good yield (Scheme 2, 4t), and it indicated there is no steric effect on this transformation. The addition of the aryl sulfonyl group occurred from the less sterically hindered exo side, and it is important to note that all of the products were produced as single diastereomers. Crystal X-ray diffraction analysis of 4a and 4g (Figure 1) indicates a clear relationship between all the stereocenters. However, the aliphatic selenides fail to give the desired product due to the low stability of the methyl selenide radical (Scheme 2, 4u). Next, we then investigated the scope of developed radical cascade cyclization with a variety of cyclohexadienones having terminal alkynes, sodium arylsulfinates, and diaryldiselenides in the presence of K2S2O8 under blue LED light irradiation (see Supporting Information Table S1). Our assumption was expected to be a 6-exo-trig cyclized product similar to internal alkynes, but the reaction surprisingly afforded the 5-exo-trig cyclized product as depicted in Scheme 3. The absolute structure of 6a was further confirmed with crystal X-ray diffraction analysis (Figure 1). Next, we explored the substrate scope with respect to various diaryl diselenides. Both electron donating as well as withdrawing groups bearing the phenyl ring of diaryl diselenides underwent this 5-exo-trig cyclization and we observed the desired products in good to excellent yields as well as excellent diastereoselective (Scheme 3, 6a–f). Notably, meta halo substituted diaryl diselenides gave the product 6d in good yield (78%) with a dr of 7:3.
Figure 1.
Crystal X-ray diffraction analysis of 4a, 4g, and 6a.
Scheme 3. Substrate Scope of 5-Exo Trig Radical Cascade Cyclization of Cyclohexadienones.
Standard Condition for 5-Exo Trig Cyclization: Terminal alkyne (1.0 equiv), sodium phenyl sulfinate (3.0 equiv), diphenyldiselenide (0.5 equiv), and K2S2O8 (2.0 equiv) were mixed in 2 mL of ACN under an inert atmosphere with 5 W blue LED irradiation.
Later, sodium arylsulfinates having functionalities such as 4-chlorophenyl and cyclopropyl were also tested to check the reaction efficiency, and the reaction proceeded well to afford the desired 5-exo-trig products in excellent yields (Schemes 3, 6g–h). The reaction also proceeded with cyclohexadienone having a methyl moiety at the α-position of carbonyl, affording the 5-exo-trig-cyclized product in an excellent yield (Scheme 3, 6j). Notably, 1,2-bis(2-fluorophenyl)diselane underwent 5-exo trig transformation smoothly and afforded the desired product in a good yield (Schemes 3, 6k). Aliphatic selenide failed to give the desired product (Schemes 3, 6l).
To demonstrate the synthetic utility further, a gram-scale reaction for the synthesis of 4a and 6i is carried out, as illustrated in Scheme 4. Internal alkyne (0.500 g, 1.0 equiv) and terminal alkyne (0.500 g, 1.0 equiv) were both subjected to this transformation and afforded the required products 4a and 6i in 85 and 91% yields, respectively. When chalcogenated dihydrochromenones 4a and tetrahydrobenzofuranones 6i were individually treated to react with m-CPBA, they generated deselenated products 7 and 8 in 75 and 80% yields, respectively (please see the Supporting Information).
Scheme 4. Gram-Scale Reaction and Synthetic Utility of 6/5-Exo Trig Radical Cascade Cyclization of Cyclohexadienones.
The reaction was carried out in the presence of the radical scavenger TEMPO (1.0 equiv) under optimal reaction circumstances to ensure the involvement of the radical pathway for both 5/6-exo trig cyclizations (Scheme 5). A trace amount of the desired product was observed. This result indicates that this reaction possibly proceeds through a radical pathway.
Scheme 5. Control Experiments.
Based on the control experiments and previous literature,25 a plausible mechanism of the present method for 6/5-exo-trig cyclization is shown in Scheme 6.
Scheme 6. Plausible Mechanism.
Initially, the interaction of sulfonyl hydrazide with Ag2CO3 produced the highly active species Ts•. The sulfonyl radical species was added to the internal alkyne of cyclohexadienones to give alkenyl radical II, which underwent an intramolecular Giese-type 6-exo trig cyclization, furnishing the α-carbonyl radical intermediate IV. Furthermore, the stereoselective attack of the aryl selenyl radical onto the intermediate IV generated the final product 4 (The stereoselective attack of aryl selenium is explained in the Supporting Information). Similarly, in the case of terminal alkyne-tethered cyclohexadienones, sulfonyl radicals were generated from sodium sulfonates, and further, this sulfonyl radical species was added to the terminal alkyne of cyclohexadienones to give alkenyl radical I, which underwent an intramolecular Giese-type 5-exo trig cyclization, furnishing the α-carbonyl radical intermediate III. Further, the radical cross-coupling of intermediate III and the aryl selenyl radical that was formed by homolytic cleavage of diaryl diselenide in the presence of light generated the final product 6.
In conclusion, highly functionalized fused chalcogenated dihydrochromenones and tetrahydrobenzofuranones have been developed via silver/K2S2O8-mediated highly regio- and diastereoselective 6/5 exo-trig radical cascade cyclization of alkyne-tethered cyclohexadienones sulfonylhydrazides/arylsulfinates and diselenides under visible-light irradiation. The reaction displayed good functional group tolerance for a wide variety of functional groups and proceeded under the radical mechanism and mild conditions, employing cheap and easily available Ag2CO3 and K2S2O8 as an additive and an oxidant, respectively. Initial mechanistic research supported a radical route. In addition, we also demonstrated the deselenation of dihydrochromenones and tetrahydrobenzofuranones using m-CPBA. Further studies on the enantiomeric radical cascade cyclization under visible-light catalysis are underway in our laboratory and will be disclosed in due course.
Experimental Section
General Information
Unless otherwise noted, all the commercial materials were used without further purification. All reactions were performed under an inert atmosphere and in oven-dried glassware with magnetic stirring. All solvents were dried before use following the standard procedures. Reactions were monitored by TLC on precoated silica gel 60 F-254. TLC plates were visualized with UV light (254 nm), iodine treatment, or p-anisaldehyde stain. Column chromatography was carried out using silica gel (60–120 and 100–200 mesh) packed in glass columns. NMR spectra were recorded at 300, 400, and 500 MHz (H) and at 75, 100, and 125 MHz (C), respectively. Chemical shifts (δ) are reported in ppm, using the residual solvent peak in CDCl3 (H: δ = 7.26 and C: δ = 77.00 ppm) as the internal standard, and coupling constants (J) are given in Hz. HRMS were recorded using ESI-TOF techniques.
General Procedure for the Cascade 6-Exo Trig Cyclization of Alkyne-Tethered Cyclohexadienones (i)
In an oven-dried glass vial, internal alkyne (0.05 g, 1.0 equiv), hydrazide (0.039 g, 1.0 equiv), diphenyldiselenide (0.032 g, 0.5 equiv), and Ag2CO3 (0.057 g, 1.0 equiv) were dissolved in 2 mL ACN; the vial was then sealed with a PTFE septum and purged with argon. The reaction mixture was stirred continuously for 6 h while being exposed to 5 W blue LED light at room temperature. Then, the reaction mixture was diluted with water. It was extracted with ethyl acetate (3 × 10 mL). Then, to the organic layer was added Na2SO4, and the mixture was concentrated under reduced pressure. The residue was directly subjected to flash chromatography on silica gel (hexane/ethyl acetate) to afford the desired product.
General Procedure for the Cascade 5-Exo Trig Cyclization of Alkyne-Tethered Cyclohexadienones (ii)
In an oven-dried glass vial, terminal alkyne (0.050 g, 1.0 equiv), sodium arylsulfinates (0.151 g, 3.0 equiv), diphenyl diselenide (0.048 g, 0.5 equiv), and K2S2O8 (0.166 g, 2.0 equiv) were dissolved in 2 mL ACN; the vial was then sealed with a PTFE septum and purged with argon. The reaction mixture was stirred continuously for 6 h while being exposed to 5 W blue LED light at room temperature. Then, the reaction mixture was diluted with water. It was extracted with ethyl acetate (3 × 10 mL). Then, to the organic layer was added Na2SO4, and the mixture was concentrated under reduced pressure. The residue was directly subjected to flash chromatography on silica gel (hexane/ethyl acetate) to afford the desired product.
Characterization Data
(4aR,5R,8aR)-8a-Methyl-4-phenyl-5-(phenylselanyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4a)
The title compound was prepared according to the general procedure as described above (i) in an 89% (102 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4a as a white solid (mp: 195–200 °C). 1H NMR (400 MHz, CDCl3): δ 7.41–7.39 (m, 2H), 7.36 (d, J = 8.3 Hz, 2H), 7.29 (dd, J = 12.7, 5.3 Hz, 2H), 7.26–7.23 (m, 2H), 7.21 (dd, J = 7.4, 4.0 Hz, 3H), 7.18–7.14 (m, 3H), 6.54 (d, J = 10.2 Hz, 1H), 6.09 (dd, J = 10.2, 1.3 Hz, 1H), 4.94 (d, J = 17.6 Hz, 1H), 4.66 (dd, J = 17.6, 2.5 Hz, 1H), 3.40 (dd, J = 4.2, 1.2 Hz, 1H), 2.85 (dd, J = 3.9, 2.2 Hz, 1H), 2.40 (s, 3H), 1.46 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.6, 146.3, 145.4, 144.2, 138.3, 138.1, 135.5, 134.0, 129.4, 129.1, 128.7, 128.3, 128.0, 127.64, 127.5, 68.4, 61.3, 48.4, 47.4, 22.6, 21.6. IR (neat)νmax: 3608, 1677, 1312, 1146, 751. HRMS (ESI-TOF): m/z calcd for C29H27O4SSe [M + H]+, 551.07726; found, 551.07898.
(4aR,5R,8aR)-8a-Methyl-5-(phenylselanyl)-4-(p-tolyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4b)
The title compound was prepared according to the general procedure as described above (i) in a 90% (100 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4b as a white solid (mp: 177–182 °C). 1H NMR (400 MHz, CDCl3): δ 7.38 (d, J = 8.0 Hz, 4H), 7.26–7.18 (m, 3H), 7.15 (d, J = 8.1 Hz, 2H), 7.00 (d, J = 7.9 Hz, 2H), 6.90 (s, 2H), 6.51 (d, J = 10.2 Hz, 1H), 6.07 (dd, J = 10.2, 0.8 Hz, 1H), 4.90 (d, J = 17.5 Hz, 1H), 4.62 (dd, J = 17.6, 2.3 Hz, 1H), 3.39 (d, J = 4.0 Hz, 1H), 2.83–2.81 (m, 1H), 2.40 (s, 3H), 2.33 (s, 3H), 1.43 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 193.6, 146.3, 145.7, 144.2, 138.7, 137.9, 134.0, 132.5, 130.3, 129.3, 129.0, 128.6, 128.2, 127.6, 127.4, 68.4, 61.3, 48.5, 47.5, 22.6, 21.6, 21.3. IR (neat)νmax: 3606, 3033, 2975, 1682, 1316, 1152, 1082, 755. HRMS (ESI-TOF): m/z calcd for C30H29O4SSe [M + H]+, 565.0926; found, 565.0946.
(4aR,5R,8aR)-4-(4-Methoxyphenyl)-8a-methyl-5-(phenylselanyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4c)
The title compound was prepared according to the general procedure as described above (i) in a 78% (84 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4c as a white solid (mp: 188–193 °C). 1H NMR (400 MHz, CDCl3): δ 7.38 (t, J = 7.8 Hz, 4H), 7.25–7.20 (m, 3H), 7.16 (d, J = 8.1 Hz, 2H), 6.95 (s, 2H), 6.73 (d, 2H), 6.53 (d, J = 10.2 Hz, 1H), 6.08 (dd, J = 10.2, 0.8 Hz, 1H), 4.94 (d, J = 17.6 Hz, 1H), 4.63 (dd, J = 17.6, 2.3 Hz, 1H), 3.81 (s, 3H), 3.37 (d, J = 3.3 Hz, 1H), 2.83 (d, J = 2.1 Hz, 1H), 2.40 (s, 3H), 1.44 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.64, 160.0, 146.3, 145.5, 144.1, 138.2, 138.2, 134.0, 130.3, 129.3, 129.1, 128.2, 127.7, 127.4, 113.4, 68.5, 61.4, 55.4, 48.5, 47.6, 22.6, 21.6. IR (neat)νmax: 3021, 2921, 1681, 1248, 753. HRMS (ESI-TOF): m/z calcd for C30H29O5SSe [M + H]+, 581.0879; found, 581.0895.
Methyl 4-((4aR,5R,8aR)-8a-methyl-6-oxo-5-(phenylselanyl)-3-tosyl-4a,5,6,8a-tetrahydro-2H-chromen-4-yl)benzoate (4d)
The title compound was prepared according to the general procedure as described above (i) in a 93% (95 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4d as a white solid (mp:193–198 °C). 1H NMR (500 MHz, CDCl3): δ 7.88 (d, J = 8.0 Hz, 2H), 7.42 (d, J = 8.2 Hz, 2H), 7.38–7.37(m, 2H), 7.28 (d, J = 7.2 Hz, 2H), 7.24 (d, J = 6.9 Hz, 2H), 7.21 (d, J = 8.1 Hz, 3H), 6.54 (d, J = 10.2 Hz, 1H), 6.10 (dd, J = 10.2, 1.0 Hz, 1H), 4.91 (d, J = 17.8 Hz, 1H), 4.65 (dd, J = 17.8, 2.4 Hz, 1H), 3.94 (s, 3H), 3.36 (d, J = 3.3 Hz, 1H), 2.85 (dd, J = 3.5, 2.5 Hz, 1H), 2.43 (s, 3H), 1.47 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.4, 166.4, 146.3, 144.8, 144.3, 140.4, 138.9, 137.8, 133.8, 130.3, 130.1, 129.6, 129.2, 128.4, 127.7, 127.5, 68.3, 61.3, 52.3, 48.2, 47.2, 22.6, 21.6. IR (neat)νmax: 3605, 1723, 1684, 1286, 1152, 760. HRMS (ESI-TOF): m/z calcd for C31H29O6SSe [M + H]+, 609.0825; found, 609.0844.
(4aR,5R,8aR)-8a-Methyl-5-(phenylselanyl)-3-tosyl-4-(3-(trifluoromethyl)phenyl)-4a,8a-dihydro-2H-chromen-6(5H)-one (4e)
The title compound was prepared according to the general procedure(i) as described above (i) in an 86% (86 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4e as a white solid (mp: 203–208 °C). 1H NMR (400 MHz, CDCl3): δ 7.56 (d, J = 7.6 Hz, 2H), 7.40 (dd, J = 18.8, 6.8 Hz, 5H), 7.29 (dt, J = 5.7, 1.9 Hz, 1H), 7.24 (d, J = 7.4 Hz, 2H), 7.23–7.17 (m, 3H), 6.56 (d, J = 10.2 Hz, 1H), 6.11 (dd, J = 10.2, 1.2 Hz, 1H), 4.98 (d, J = 17.9 Hz, 1H), 4.72 (d, J = 17.9 Hz, 1H), 3.33 (d, J = 3.5 Hz, 1H), 2.83–2.81 (m, 1H), 2.40 (s, 3H), 1.51 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.4, 146.2, 144.9, 143.5, 140.0, 137.8, 136.3, 134.0, 129.6, 129.2, 128.5, 127.6, 127.3, 125.6, 125.5, 68.3, 61.3, 48.2, 47.2, 22.7, 21.5. IR (neat)νmax: 3604, 1683, 1329, 1158, 1083, 756. HRMS (ESI-TOF): m/z calcd for C30H26F3O4SSe [M + H]+, 619.0646; found, 619.0663.
(4aR,5R,8aR)-8a-Methyl-5-(phenylselanyl)-4-(thiophen-2-yl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4f)
The title compound was prepared according to the general procedure as described above (i) in an 83% (94 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4f as a white solid (mp: 198–203 °C). 1H NMR (400 MHz, CDCl3): δ 7.37–7.31 (m, 4H), 7.24 (dd, J = 5.1, 1.1 Hz, 1H), 7.21–7.16 (m, 2H), 7.15–7.08 (m, 4H), 6.88 (dd, J = 5.0, 3.6 Hz, 1H), 6.46 (d, J = 10.2 Hz, 1H), 6.03 (dd, J = 10.2, 1.2 Hz, 1H), 4.87 (dd, J = 25.8, 13.5 Hz, 1H), 4.64–4.59 (m, 1H), 3.38 (d, J = 5.5 Hz, 1H), 2.19–2.78 (m, 1H), 2.32 (s, 3H), 1.37 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.4, 146.2, 144.3, 141.0, 138.9, 137.6, 135.1, 134.5, 131.4, 130.2, 129.3, 129.1, 128.4, 128.1, 127.7, 127.4, 126.8, 68.6, 61.8, 49.2, 48.2, 22.7, 21.6. IR (neat)νmax: 3615, 1682, 1315, 1152, 1082, 755. HRMS (ESI-TOF): m/z calcd for C27H25O4S2Se [M + H]+, 557.0334; found, 557.0354.
(4aR,5R,8aR)-4,8a-Dimethyl-5-(phenylselanyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4g)
The title compound was prepared according to the general procedure as described above (i) in an 88% (118 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4g as a white solid (mp: 184–188 °C). 1H NMR (500 MHz, CDCl3): δ 7.89 (t, J = 6.5 Hz, 2H), 7.36 (d, J = 8.0 Hz, 2H), 7.30–7.26 (m, 1H), 7.22–7.18 (m, 4H), 6.50 (d, J = 10.2 Hz, 1H), 6.10 (dd, J = 10.2, 1.2 Hz, 1H), 4.64 (dd, J = 17.1, 1.8 Hz, 1H), 4.58–4.54 (m, 1H), 3.61 (dd, J = 4.3, 1.2 Hz, 1H), 2.64 (dd, J = 4.1, 1.7 Hz, 1H), 2.42 (s, 3H), 2.18 (t, J = 2.1 Hz, 3H), 1.38 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.77, 147.11, 144.69, 143.73, 138.37, 134.71, 129.89, 129.12, 128.58, 127.51, 127.40, 68.30, 61.50, 48.92, 47.43, 22.76, 21.66, 18.52. IR (neat)νmax: 3019, 2349, 1680, 1214, 1146, 743. HRMS (ESI-TOF): m/z calcd for C24H25O4SSe [M + H]+, 489.0617; found, 489.0633.
(4aR,5R,8aR)-8a-Isopropyl-4-phenyl-5-(phenylselanyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4h)
The title compound was prepared according to the general procedure as described above (i) in 91% (93 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4h as a white solid (mp: 188–192 °C). 1H NMR (400 MHz, CDCl3): δ 7.41–7.37 (m, 4H), 7.30–7.23 (m, 3H), 7.21–7.15 (m, 5H), 6.89 (s, 2H), 6.63 (d, J = 10.5 Hz, 1H), 6.23 (dd, J = 10.5, 0.9 Hz, 1H), 4.88 (d, J = 17.7 Hz, 1H), 4.65 (dd, J = 17.7, 2.2 Hz, 1H), 3.47 (dd, J = 4.2, 1.2 Hz, 1H), 3.13 (dd, J = 3.9, 1.9 Hz, 1H), 2.40 (s, 3H), 2.33 (dd, J = 13.7, 6.8 Hz, 1H), 1.17 (d, J = 6.7 Hz, 3H), 0.85 (d, J = 6.9 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ 193.6, 144.9, 144.2, 141.7, 138.2, 138.1, 135.8, 133.8,130.4, 129.3, 129.0, 128.5, 128.2, 128.0, 127.5, 73.1, 61.2, 48.4, 45.0, 29.3, 21.6, 18.5, 15.7. IR (neat)νmax: 3357, 2925, 1677, 1145, 1022, 748. HRMS (ESI-TOF): m/z calcd for C31H31O4SSe [M + H]+, 579.1082; found, 579.1102.
(4aR,5R,8aR)-4,8a-Diphenyl-5-(phenylselanyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4i)
The title compound was prepared according to the general procedure as described above (i) in an 85% (86 mg) yield; It was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4i as a white solid (mp: 228–233 °C). 1H NMR (500 MHz, CDCl3): δ 7.49–7.42(m, 8H), 7.33–7.21 (m, 7H), 6.93 (d, J = 8.2 Hz, 2H), 6.86 (d, J = 8.2 Hz, 2H), 6.63 (d, J = 10.2 Hz, 1H), 6.05 (d, J = 10.2 Hz, 1H), 4.94 (t, J = 15.1 Hz, 1H), 4.38–4.34 (m, 1H), 3.81 (s, 1H), 3.62 (d, J = 3.9 Hz, 1H), 2.32 (d, J = 24.0 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 193.6, 147.1, 145.7, 143.7, 139.0, 138.7, 137.8, 135.3, 134.3, 129.4, 129.1, 129.0, 128.9, 128.8, 128.4, 128.1, 127.1, 126.9, 126.2, 72.9, 62.1, 48.3, 45.0, 21.5. IR (neat)νmax: 3452, 2980, 1680, 1316, 1150, 761. HRMS (ESI-TOF): m/z calcd for C34H29O4SSe [M + H]+, 613.0929; found, 613.0946.
(4aR,5R,8aR)-7,8a-Dimethyl-4-phenyl-5-(phenylselanyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4j)
The title compound was prepared according to the general procedure as described above (i) in an 86% (96 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4j as a white solid (mp: 118–122 °C). 1H NMR (500 MHz, CDCl3): δ 7.30 (dd, J = 17.0, 7.4 Hz, 4H), 7.23–7.19 (m, 3H), 7.16 (d, J = 7.1 Hz, 2H), 7.15–7.10 (m, 3H), 7.07 (d, J = 7.7 Hz, 2H), 6.23 (s, 1H), 4.86 (d, J = 17.6 Hz, 1H), 4.56 (d, J = 17.5 Hz, 1H), 3.35 (d, J = 3.2 Hz, 1H), 2.79 (s, 1H), 2.33 (s, 3H), 1.80 (s, 3H), 1.37 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 194.1, 145.5, 144.2, 141.6, 138.3, 138.2, 135.7, 134.5, 134.0, 130.4, 129.3, 129.1, 128.7, 128.2,127.5, 68.9, 61.3, 48.6, 47.7, 22.9, 21.6, 16.3. IR (neat) νmax: 3340, 2950, 1676, 1315, 1151, 1097, 752. HRMS (ESI-TOF): m/z calcd for C30H29O4SSe [M + H]+, 565.0927; found, 565.0946.
(4aR,5R,8aR)-8a-Methyl-4-phenyl-5-(p-tolylselanyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4k)
The title compound was prepared according to the general procedure as described above (i) in an 88% (98 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4k as a white solid (mp:175–178 °C). 1H NMR (400 MHz, CDCl3): δ 7.29 (d, J = 8.3 Hz, 2H), 7.21 (dt, J = 6.6, 5.2 Hz, 4H), 7.13 (d, J = 7.5 Hz, 2H), 7.07 (d, J = 8.0 Hz, 3H), 6.96 (d, J = 7.8 Hz, 2H), 6.45 (d, J = 10.2 Hz, 1H), 6.00 (dd, J = 10.2, 1.3 Hz, 1H), 4.86 (d, J = 17.6 Hz, 1H), 4.57 (dd, J = 17.6, 2.5 Hz, 1H), 3.26 (dd, J = 4.2, 1.2 Hz, 1H), 2.76 (dd, J = 3.9, 2.3 Hz, 1H), 2.32 (s, 3H), 2.23 (s, 3H), 1.38 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 210.8, 197.8, 193.3, 146.1, 145.4, 144.2, 138.5, 138.2, 138.0, 135.5, 134.5, 129.9, 129.3, 128.7, 127.9, 127.6, 127.4, 68.4, 61.2, 48.3, 47.3, 22.6, 21.6, 21.2. IR (neat)νmax: 3352, 2944, 2831, 1024, 750. HRMS (ESI-TOF): m/z calcd for C30H29O4SSe [M + H]+, 565.0926; found, 565.0946.
(4aR,5R,8aR)-5-((4-Methoxyphenyl)selanyl)-8a-methyl-4-phenyl-3-(phenylsulfonyl)-4a,8a-dihydro-2H-chromen-6(5H)-one (4l)
The title compound was prepared according to the general procedure as described above (i) in an 85% (93 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4l as a white solid (mp: 180–185 °C). 1H NMR (500 MHz, CDCl3): δ 7.51 (t, J = 7.4 Hz, 1H), 7.47 (d, J = 7.5 Hz, 2H), 7.36–7.28 (m, 6H), 7.22 (t, J = 7.1 Hz, 3H), 6.76 (d, J = 8.6 Hz, 2H), 6.53 (d, J = 10.2 Hz, 1H), 6.08 (d, J = 10.1 Hz, 1H), 4.97 (d, J = 17.7 Hz, 1H), 4.67 (dd, J = 17.7, 2.1 Hz, 1H), 3.76 (s, 3H), 3.26 (d, J = 3.4 Hz, 1H), 2.83 (s, 1H), 1.45 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.2, 160.1, 146.0, 145.9, 141.0, 138.1, 136.7, 135.3, 133.2, 128.7, 128.0, 127.6, 127.3, 120.2, 114.8, 68.4, 61.2, 55.2, 48.3, 48.1, 22.6. IR (neat)νmax: 3458, 2962, 1674, 1584, 1244, 1148, 692. HRMS (ESI-TOF): m/z calcd for C29H27O5SSe [M + H]+, 567.0723; found, 567.0738.
(4aR,5R,8aR)-5-((3-Fluorophenyl)selanyl)-8a-methyl-4-phenyl-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4m)
The title compound was prepared according to the general procedure as described above (i) in a 90% (99 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4m as a white solid (mp: 222–228 °C). 1H NMR (400 MHz, CDCl3): δ 7.35 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 7.5 Hz, 1H), 7.21–7.14 (m, 7H), 7.12–7.09 (m, 1H), 6.98–6.93 (m, 2H), 6.55 (d, J = 10.2 Hz, 1H), 6.09 (dd, J = 10.2, 1.2 Hz, 1H), 4.93 (d, J = 17.7 Hz, 1H), 4.66 (dd, J = 17.7, 2.4 Hz, 1H), 3.41 (dd, J = 4.2, 1.1 Hz, 1H), 2.86 (dd, J = 3.9, 2.3 Hz, 1H), 2.40 (s, 3H), 1.47 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.6, 163.5, 161.0, 146.6, 145.2, 144.4, 129.4, 129.1, 129.1, 128.8, 128.0, 127.6, 127.5, 120.4, 120.1, 115.3, 115.1, 68.4, 61.3, 48.3, 47.4, 22.5, 21.6. IR (neat)νmax: 3462, 2968, 1670, 1582, 1240, 1142, 720. HRMS (ESI-TOF): m/z calcd for C29H26FO4SSe [M + H]+, 569.0681; found, 569.0695.
(4aR,5R,8aR)-5-((2-Fluorophenyl)selanyl)-8a-methyl-4-phenyl-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4n)
The title compound was prepared according to the general procedure as described above (i) in an 80% (88 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4n as a white solid (mp: 178–183 °C). 1H NMR (500 MHz, CDCl3): δ 7.43–7.40 (m, 1H), 7.37 (d, J = 8.2 Hz, 2H), 7.32–7.27 (m, 2H), 7.23 (t, J = 7.7 Hz, 3H), 7.14 (d, J = 8.1 Hz, 2H), 7.05–7.00 (m, 3H), 6.55 (d, J = 10.2 Hz, 1H), 6.06 (dd, J = 10.2, 1.0 Hz, 1H), 4.92 (d, J = 17.7 Hz, 1H), 4.67 (dd, J = 17.7, 2.4 Hz, 1H), 3.55 (dd, J = 4.0, 0.9 Hz, 1H), 2.86 (dd, J = 3.5, 2.5 Hz, 1H), 2.38 (s, 3H), 1.48 (s, 3H). 13C NMR (100 MHz, CDCl3): δ. 193.0, 163.3, 160.9, 146.2, 145.2, 144.2, 138.2, 137.8, 136.4, 135.3, 130.7, 130.7, 129.4, 128.6, 127.9, 127.4, 127.3, 124.8, 124.8, 116.5, 116.2, 115.5, 115.3, 68.5, 61.3, 48.4, 45.6, 22.5, 21.5. IR (neat) νmax: 3022, 2976, 1674, 1443, 1146, 745. HRMS (ESI-TOF): m/z calcd for C29H26FO4SSe [M + H]+: 569.0681; found, 569.0695.
(4aR,5R,8aR)-5-((4-Methoxyphenyl)selanyl)-8a-methyl-4-phenyl-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4o)
The title compound was prepared according to the general procedure as described above (i) in a 95% (107 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4o as a white solid (mp: 205–209 °C). 1H NMR (400 MHz, CDCl3): δ 7.37–7.29 (m, J = 15.1, 7.4 Hz, 5H), 7.26–7.23 (m, 3H), 7.14 (d, J = 8.0 Hz, 3H), 6.76 (d, J = 8.6 Hz, 2H), 6.52 (d, J = 10.2 Hz, 1H), 6.07 (d, J = 10.2 Hz, 1H), 4.94 (d, J = 17.6 Hz, 1H), 4.64 (dd, J = 17.6, 2.1 Hz, 1H), 3.76 (s, 3H), 3.27 (d, J = 3.5 Hz, 1H), 2.83 (s, 1H), 2.39 (s, 3H), 1.44 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.3, 160.1, 146.1, 145.4, 144.2, 138.2, 138.0, 136.7, 135.5, 129.3, 128.7, 127.9, 127.6, 127.4, 120.3, 114.7, 68.4, 61.2, 55.2, 48.3, 48.2, 22.6, 21.6. IR (neat)νmax: 3420, 2948, 1680, 1495, 1248, 1151, 763. HRMS (ESI-TOF): m/z calcd for C30H29O5SSe [M + H]+, 581.0885; found, 581.0895.
(4aR,5R,8aR)-8a-Methyl-4-phenyl-5-(thiophen-2-ylselanyl)-3-tosyl-4a,8a-dihydro-2H-chromen-6(5H)-one (4p)
The title compound was prepared according to the general procedure as described above (i) in an 85% (92 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4p as a white solid (mp: 180–185 °C). 1H NMR (400 MHz, CDCl3): δ 7.37 (d, J = 7.5 Hz, 3H), 7.33–7.26 (m, 4H), 7.15 (d, J = 8.0 Hz, 3H), 7.08 (d, J = 2.8 Hz, 1H), 6.94 (dd, J = 5.1, 3.6 Hz, 1H), 6.53 (d, J = 10.2 Hz, 1H), 6.09 (d, J = 10.2 Hz, 1H), 4.92 (d, J = 17.6 Hz, 1H), 4.65 (dd, J = 17.7, 2.2 Hz, 1H), 3.29 (d, J = 3.2 Hz, 1H), 2.80 (s, 1H), 2.39 (s, 3H), 1.46 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 192.9, 146.1, 145.3, 144.3, 142.4, 138.2, 137.9, 137.12, 135.4, 132.1, 129.4, 128.8, 128.1, 128.0, 127.5, 127.4, 124.1, 68.5, 61.3, 50.4, 48.4, 40.2, 22.3, 21.6. IR (neat)νmax: 3428, 2938, 1677, 1312, 1146, 703. HRMS (ESI-TOF): m/z calcd for C27H25O4S2Se [M + H]+, 557.0339; found, 557.0354.
(4aR,5R,8aR)-8a-Methyl-4-phenyl-3-(phenylsulfonyl)-5-(p-tolylselanyl)-4a,8a-dihydro-2H-chromen-6(5H)-one (4q)
The title compound was prepared according to the general procedure as described above (i) in an 80% (85 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4q as a white solid (mp:140–145 °C). 1H NMR (400 MHz, CDCl3): δ 7.46–7.39 (m, 3H), 7.27 (dd, J = 14.6, 6.5 Hz, 3H), 7.24–7.19 (m, 3H), 7.13 (t, J = 7.6 Hz, 2H), 6.97 (d, J = 7.9 Hz, 3H), 6.46 (d, J = 10.2 Hz, 1H), 6.01 (dd, J = 10.2, 1.1 Hz, 1H), 4.89 (d, J = 17.7 Hz, 1H), 4.61 (dd, J = 17.7, 2.4 Hz, 1H), 3.26 (dd, J = 4.1, 1.0 Hz, 1H), 2.78–2.76 (m, 1H), 2.23 (s, 3H), 1.39 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 193.3, 146.1, 145.9, 141.0, 138.6, 138.1, 135.3, 134.6, 133.2, 130.0, 128.8, 128.7, 128.0, 127.6, 127.3, 126.3, 68.4, 61.3, 48.3, 47.6, 22.6, 21.2. IR (neat)νmax: 3450, 2980, 1678, 1313, 1151, 1082, 728. HRMS (ESI-TOF): m/z calcd for C29H27O4SSe [M + H]+, 551.07726; found, 551.07898.
(4aR,5R,8aR)-3-((4-Chlorophenyl)sulfonyl)-8a-methyl-4-phenyl-5-(phenylselanyl)-4a,8a-dihydro-2H-chromen-6(5H)-one (4r)
The title compound was prepared according to the general procedure as described above (i) in an 86% (95 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4r as a white solid (mp:155–160 °C). 1H NMR (500 MHz, CDCl3): δ 7.41–7.39 (m, 2H), 7.37–7.34 (m, 2H), 7.32–7.29 (m, 3H), 7.28–7.21 (m, 4H), 7.20 (t, J = 7.9 Hz, 2H), 6.98 (s, 1H), 6.54 (d, J = 10.2 Hz, 1H), 6.10 (dd, J = 10.2, 1.2 Hz, 1H), 4.96 (d, J = 17.7 Hz, 1H), 4.68 (dd, J = 17.7, 2.5 Hz, 1H), 3.39 (dd, J = 4.2, 1.2 Hz, 1H), 2.86 (dd, J = 3.8, 2.4 Hz, 1H), 1.47 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.4, 146.2, 139.9, 139.4, 138.1, 135.1, 133.9, 130.1, 129.1, 129.0, 128.8, 128.3, 128.1, 127.7, 68.4, 61.1, 48.4, 47.3, 22.6. IR (neat)νmax: 3020, 2852, 1673, 1313, 1148, 752. HRMS (ESI-TOF): m/z calcd for C28H24O4ClSSe [M + H]+, 571.0222; found, 571.0246.
(4aR,5R,8aR)-3-(Cyclopropylsulfonyl)-8a-methyl-4-phenyl-5-(phenylselanyl)-4a,8a-dihydro-2H-chromen-6(5H)-one (4s)
The title compound was prepared according to the general procedure as described above (i) in an 82% (79 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4s as a white solid (mp: 144–148 °C). 1H NMR (500 MHz, CDCl3): δ 7.50–7.47 (m, 2H), 7.38 (s, 4H), 7.31–7.24 (m, 4H), 6.58 (d, J = 10.2 Hz, 1H), 6.12 (dd, J = 10.2, 1.3 Hz, 1H), 4.83 (d, J = 17.6 Hz, 1H), 4.58 (dd, J = 17.6, 2.5 Hz, 1H), 3.51 (dd, J = 4.3, 1.2 Hz, 1H), 3.03 (dd, J = 3.9, 2.4 Hz, 1H), 2.04 (dq, J = 8.0, 4.9 Hz, 1H), 1.57 (s, 3H), 1.21 (ddt, J = 10.0, 6.8, 4.9 Hz, 1H), 1.12–1.06 (m, 1H), 0.97–0.92 (m, 1H), 0.89–0.84 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 193.5, 146.3, 144.3, 137.8, 136.1, 134.0, 130.1, 129.2, 129.2, 128.4, 128.3, 127.6, 68.4, 61.3, 48.2, 47.1, 32.5, 22.6, 5.8, 5.3. IR (neat)νmax: 3051, 2976, 1672, 1300, 1144, 701. HRMS (ESI-TOF): m/z calcd for C25H25O4SSe [M + H]+, 501.0621; found, 501.0633.
(4aR,5R,8aR)-4-(2-Fluorophenyl)-8a-methyl-5-(phenylselanyl)-3-(phenylsulfonyl)-4a,8a-dihydro-2H-chromen-6(5H)-one (4t)
The title compound was prepared according to the general procedure as described above (i) in a 75% (85 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 4t as a white solid (mp: 154–158 οC) 1H NMR (400 MHz, CDCl3): δ 7.50–7.42 (m, 4H), 7.38–7.29 (m, 3H), 7.27–7.20 (m, 4H), 7.05–6.96 (m, 3H), 6.79–6.75 (m, 1H), 6.49 (d, J = 10.2 Hz, 1H), 6.00 (dd, J = 10.2, 1.3 Hz, 1H), 4.89 (d, J = 17.9 Hz, 1H), 4.65 (dd, J = 17.9, 2.4 Hz, 1H), 3.52 (dd, J = 4.2, 1.1 Hz, 1H), 2.88 (dd, J = 3.9, 2.3 Hz, 1H), 1.42 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 192.9, 146.3, 140.8, 140.3, 140.0, 136.8, 133.4, 132.1, 131.0, 131.0, 128.9, 127.2, 124.9, 124.8, 123.6, 123.6, 115.6, 115.4, 115.3, 68.7, 61.5, 46.3, 45.8, 22.2.IR (neat)νmax: 3022, 2976, 1674, 1443, 1146, 745. HRMS (ESI-TOF): m/z calcd for C28H24FO4SSe [M + H]+, 554.0466; found, 554.0476.
(3aS,4R,7aS,Z)-7a-Methyl-4-(phenylselanyl)-3-(tosylmethylene)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6a)
The title compound was prepared according to the general procedure as described above (ii) in an 88% (140 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6a as a white solid(155–160 °C). 1H NMR (400 MHz, CDCl3): δ 7.65 (d, J = 8.0 Hz, 2H), 7.57 (d, J = 7.0 Hz, 2H), 7.39–7.29 (m, 5H), 6.44 (d, J = 10.2 Hz, 1H), 6.07 (d, J = 2.0 Hz, 1H), 5.98 (d, J = 10.4 Hz, 1H), 5.12 (dd, J = 17.4, 1.3 Hz, 1H), 4.56 (d, J = 17.4 Hz, 1H), 3.85 (s, 1H), 3.29 (s, 1H), 2.41 (s, 3H), 1.72 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 191.2, 158.5, 148.5, 144.9, 137.6, 135.3, 130.0, 129.5, 129.3, 128.4, 127.2, 123.7, 78.7, 68.2, 53.8, 45.4, 25.0, 21.6. IR (neat)νmax: 3405, 2940, 1683, 1444, 1303, 1145, 742. HRMS (ESI-TOF): m/z calcd for C23H23O4SSe [M + H]+, 475.0466, Found 475.0476.
(3aS,4R,7aS,Z)-7a-Methyl-4-(p-tolylselanyl)-3-(tosylmethylene)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6b)
The title compound was prepared according to the general procedure as described above (iii) in an 87% (143 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6b as a white solid (mp: 175–180 °C). 1H NMR (500 MHz, CDCl3): δ 7.64 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.0 Hz, 2H), 7.29 (d, J = 8.0 Hz, 2H), 7.13 (d, J = 7.8 Hz, 2H), 6.43 (dd, J = 10.4, 1.7 Hz, 1H), 6.04 (d, J = 2.4 Hz, 1H), 5.97 (dd, J = 10.4, 0.9 Hz, 1H), 5.12 (dd, J = 17.4, 2.2 Hz, 1H), 4.58–4.53 (m, 1H), 3.80 (s, 1H), 3.28 (s, 1H), 2.41 (s, 3H), 2.35 (s, 3H), 1.72 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 191.1, 158.6, 148.4, 144.8, 139.7, 137.6, 135.7, 130.4, 130.0, 128.4, 127.2, 124.4, 123.6, 78.7, 68.2, 53.7, 45.6, 25.0, 21.6, 21.3. IR (neat)νmax:3482, 2926, 1675, 1306, 1148, 809. HRMS (ESI-TOF): m/z calcd for C24H25O4SSe [M + H]+, 489.0621; found, 489. 0633
(3aS,4R,7aS,Z)-4-((4-Methoxyphenyl)selanyl)-7a-methyl-3-(tosylmethylene)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6c)
The title compound was prepared according to the general procedure as described above (ii) in a 92% (156 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6c as a white solid (mp:153–156 °C). 1H NMR (500 MHz, CDCl3): δ 7.64 (d, J = 7.9 Hz, 2H), 7.47 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 7.8 Hz, 2H), 6.84 (d, J = 8.3 Hz, 2H), 6.43 (d, J = 10.3 Hz, 1H), 6.03 (s, 1H), 5.97 (d, J = 10.4 Hz, 1H), 5.12 (d, J = 17.4 Hz, 1H), 4.56 (d, J = 17.4 Hz, 1H), 3.80 (s, 3H), 3.74 (s, 1H), 3.27 (s, 1H), 2.41 (s, 3H), 1.72 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 191.1, 160.8, 158.6, 148.4, 144.9, 137.8, 137.6, 130.0, 128.4, 127.2, 123.5, 118.3, 115.2, 78.7, 68.2, 55.3, 53.6, 46.0, 25.0, 21.6. IR (neat)νmax: 3498, 2979, 1676, 1491, 1246, 1146, 819. HRMS (ESI-TOF): m/z calcd for C24H25O5SSe [M + H]+, 505.0569; found, 505.0582.
(3aS,4R,7aS,Z)-4-((3-Fluorophenyl)selanyl)-7a-methyl-3-(tosylmethylene)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6d)
The title compound was prepared according to the general procedure as described above (ii) in a 78% (118 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6d as a white solid (mp:133–146 °C). 1H NMR (500 MHz, CDCl3): δ 7.76 (d, J = 8.2 Hz, 0.7H), 7.66 (d, J = 8.3 Hz, 2H), 7.35–7.26 (m, 6H), 7.22–6.98 (m, 2.2.H), 6.92 (d, J = 1.9 Hz, 0.35H), 6.54 (d, J = 10.2 Hz, 0.35H), 6.45 (dd, J = 10.4, 1.7 Hz, 1H), 6.09 (dd, J = 5.0, 2.4 Hz, 1H), 6.04 (d, J = 10.2 Hz, 0.35H), 5.99 (dd, J = 10.4, 1.1 Hz, 1H), 5.12 (dd, J = 17.4, 2.2 Hz, 1H), 5.00 (dd, J = 17.4, 2.3 Hz, 0.35H), 4.93–4.89 (m, 0.35H), 4.57 (dt, J = 17.4, 2.4 Hz, 1H), 4.03 (d, J = 4.9 Hz, 0.35H), 3.87–3.87 (m, 1H), 3.30–3.30 (m, 1H), 3.14–3.17 (m, 0.35H), 2.43 (s, 1H), 2.42 (s, 3H), 1.70 (s, 3H), 1.39 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 192.3, 191.0, 163.6, 161.1, 158.2, 156.9, 148.7, 148.0, 144.9, 137.9, 137.5, 130.8, 130.7, 130.6, 130.6, 130.4, 130.3, 130.0, 129.5, 129.4, 128.5, 128.3, 127.2, 127.1, 123.8, 123.7, 122.0, 121.8, 121.5, 121.3, 116.5, 116.3, 116.0, 115.8, 79.5, 78.6, 69.1, 68.2, 53.8, 52.3, 49.7, 45.3, 25.0, 22.7, 21.6 IR (neat)νmax: 3049, 2929, 1672, 1475, 1280, 1148, 811. HRMS (ESI-TOF): m/z calcd for C23H22O4FSSe [M + H]+, 493.0367; found, 493.0382.
(3aS,4R,7aS,Z)-7a-Methyl-3-((phenylsulfonyl)methylene)-4-(p-tolylselanyl)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6e)
The title compound was prepared according to the general procedure as described above (ii) in a 91% (145 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6e as a white solid (mp: 158–163 °C). 1H NMR (400 MHz, CDCl3): δ 7.78–7.76(m, 2H), 7.61–7.53 (m, 1H), 7.51–7.44 (m, 2H), 7.26–7.72 (m, 2H), 7.13 (d, J = 7.8 Hz, 2H), 6.43 (dd, J = 10.4, 1.8 Hz, 1H), 6.43 (dd, J = 10.4, 1.8 Hz, 1H), 6.07 (q, J = 2.5 Hz, 1H), 5.97 (dd, J = 10.4, 1.2 Hz, 1H), 5.13 (dd, J = 17.5, 2.3 Hz, 1H), 4.56 (dt, J = 17.5, 2.5 Hz, 1H), 3.80 (dd, J = 2.3, 1.3 Hz, 1H), 3.29–3.28 (m, 1H), 2.35 (s, 3H), 1.73 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 191.10, 159.29, 148.38, 140.51, 139.71, 135.68, 133.77, 130.38, 129.35, 128.42, 127.13, 124.39, 123.30, 78.72, 77.00, 68.17, 53.75, 45.60, 24.96, 21.27. IR (neat) νmax: 3492, 2974, 1677, 1305, 1147, 1082, 802. HRMS (ESI-TOF): m/z calcd for C23H23O4SSe [M + H]+, 475.0466; found, 475.0476.
(3aS,4R,7aS,Z)-4-((4-Methoxyphenyl)selanyl)-7a-methyl-3-((phenylsulfonyl)methylene)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6f)
The title compound was prepared according to the general procedure as described above (ii) in an 86% (142 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6f as a white solid (mp: 160–165 °C). 1H NMR (400 MHz, CDCl3): δ 7.76 (dd, J = 5.2, 3.3 Hz, 2H), 7.62–7.58 (m, 1H), 7.52–7.45 (m, 4H), 6.86–6.82 (m, 2H), 6.43 (dd, J = 10.4, 1.8 Hz, 1H), 6.06 (q, J = 2.5 Hz, 1H), 5.97 (dd, J = 10.4, 1.2 Hz, 1H), 5.12 (dd, J = 17.5, 2.3 Hz, 1H), 4.56 (dt, J = 17.5, 2.5 Hz, 1H), 3.79 (s, 3H), 3.75 (dd, J = 2.2, 1.3 Hz, 1H), 3.33–3.23 (m, 1H), 1.72 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 191.1, 160.8, 159.3, 148.3, 140.5, 137.7, 133.8, 129.3, 128.4, 127.1, 123.3, 118.2, 115.2, 78.7, 68.2, 55.3, 53.7, 45.9, 25.0. IR (neat)νmax:3499, 2478, 1678, 1298, 1249, 1150, 755 HRMS (ESI-TOF): m/z calcd for C23H23O5SSe [M + H]+, 491.0415; found, 491.0421.
(3aS,4R,7aS,Z)-3-(((4-Chlorophenyl)sulfonyl)methylene)-7a-methyl-4-(phenylselanyl)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6g)
The title compound was prepared according to the general procedure as described above (ii) in an 80% (133 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6g as a white solid (mp: 194–198 °C). 1H NMR (500 MHz, CDCl3): δ 7.70 (d, J = 8.5 Hz, 2H), 7.57 (d, J = 7.0 Hz, 2H), 7.48 (d, J = 8.4 Hz, 2H), 7.35 (dt, J = 24.7, 7.2 Hz, 3H), 6.44 (dd, J = 10.4, 1.4 Hz, 1H), 6.07 (d, J = 0.9 Hz, 1H), 5.99 (d, J = 10.4 Hz, 1H), 5.11 (dd, J = 17.6, 1.8 Hz, 1H), 4.55 (dt, J = 17.6, 2.4 Hz, 1H), 3.86 (d, J = 0.7 Hz, 1H), 3.31 (s, 1H), 1.73 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 191.18, 159.90, 148.42, 140.56, 138.93, 135.32, 129.70, 129.55, 129.32, 128.61, 128.45, 128.03, 122.99, 78.74, 77.00, 68.13, 53.92, 45.34, 24.96. IR (neat)νmax;3049, 2929, 1672, 1313, 1148, 1086, 752. HRMS (ESI-TOF): m/z calcd for C22H20O4ClSSe [M + H]+, 494.9913; found, 494.9930.
(3aS,4R,7aS,Z)-3-((Cyclopropylsulfonyl)methylene)-7a-methyl-4-(phenylselanyl)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6h)
The title compound was prepared according to the general procedure as described above (ii) in a 79% (112 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6h as a white solid (mp: 172–177 °C). 1H NMR (500 MHz, CDCl3): δ 7.64–7.62 (m, 2H), 7.42–7.36 (m, 3H), 6.46 (dd, J = 10.4, 1.8 Hz, 1H), 6.09 (q, J = 2.6 Hz, 1H), 6.04 (dd, J = 10.4, 1.3 Hz, 1H), 5.02 (dd, J = 17.4, 2.3 Hz, 1H), 4.48 (dt, J = 17.4, 2.5 Hz, 1H), 3.95 (dd, J = 2.3, 1.3 Hz, 1H), 3.35 (dt, J = 4.2, 2.0 Hz, 1H), 2.30 (tt, J = 8.0, 4.8 Hz, 1H), 1.75 (s, 3H), 1.22–1.10 (m, 2H), 1.04–0.93 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 191.4, 159.3, 148.6, 135.3, 129.6, 129.3, 128.4, 128.2, 121.6, 78.6, 68.1, 53.8, 45.6, 31.6, 25.0, 5.2, 4.9. IR (neat)νmax: 3047, 2930, 1671, 1285, 1131, 1048, 746. HRMS (ESI-TOF): m/z calcd for C19H21O4SSe [M + H]+, 425.0305; found, 425.0320.
(3aS,4R,7aS,Z)-7a-Methyl-4-(phenylselanyl)-3-((phenylsulfonyl)methylene)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6i)
The title compound was prepared according to the general procedure as described above (ii) in a 93% (144 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6i as a white solid (mp: 162–168 °C). 1H NMR (400 MHz, CDCl3): δ 7.79–7.71 (m, 2H), 7.61–7.51 (m, 6H), 7.36–7.3 (m, 2H), 6.44 (dd, J = 10.4, 1.8 Hz, 1H), 6.09 (q, J = 2.5 Hz, 1H), 5.98 (dd, J = 10.4, 1.2 Hz, 1H), 5.13 (dd, J = 17.5, 2.3 Hz, 1H), 4.57 (dt, J = 17.5, 2.5 Hz, 1H), 3.86 (dd, J = 2.3, 1.3 Hz, 1H), 3.32–3.31 (m, 1H), 1.73 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 191.2, 159.2, 148.5, 135.3, 133.8, 129.6, 129.4, 129.3, 128.5, 127.2, 123.4, 78.7, 68.2, 53.9, 45.4, 25.0. IR (neat)νmax: 3492, 2974, 1677, 1305, 1147, 1082, 802. HRMS (ESI-TOF): m/z calcd for C22H21O4SSe [M + H]+, 461.0302; found, 461.0320.
(3aS,4R,7aS,Z)-6,7a-Dimethyl-4-(phenylselanyl)-3-(tosylmethylene)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6j)
The title compound was prepared according to the general procedure as described above (ii) in an 85% (127 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6j as a white solid (mp:143–146 °C). 1H NMR (400 MHz, CDCl3): δ 7.57 (d, J = 8.0 Hz, 2H), 7.48 (d, J = 6.9 Hz, 2H), 7.31–7.18 (m, 5H), 6.12 (s, = 1H), 5.99 (d, J = 1.9 Hz, 1H), 5.00 (d, J = 17.4 Hz, 1H), 4.44 (d, J = 17.4 Hz, 1H), 3.80 (d, J = 1.9 Hz, 1H), 3.20 (s, 1H), 2.34 (s, 3H), 1.69 (s, 3H), 1.62 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 191.8, 159.2, 144.8, 143.6, 137.7, 135.2, 129.9, 129.5, 129.1, 127.1, 123.5, 79.1, 68.0, 54.1, 45.8, 25.4, 21.6, 16.3. IR (neat)νmax: 3478, 2977, 1672, 1302, 1145, 1047, 741. HRMS (ESI-TOF): m/z calcd for C24H25O4SSe [M + H]+, 489.0621; found, 457.0633.
(3aS,4R,7aS,Z)-4-((2-Fluorophenyl)selanyl)-7a-methyl-3-((phenylsulfonyl)methylene)-2,3,3a,7a-tetrahydrobenzofuran-5(4H)-one (6k)
The title compound was prepared according to the general procedure as described above (ii) in a 78% (125 mg) yield; it was purified by column chromatography (20% EtOAc/hexane; Rf = 0.2) to afford 6k as a white solid (mp:133–136 οC). 1H NMR (400 MHz, CDCl3)δ 7.73–7.71 (m, 2H), 7.55–7.53 (m, 1H), 7.50–7.43 (m, 3H), 7.32 (tdd, J = 7.2, 5.3, 1.7 Hz, 1H), 7.08–7.02 (m, 2H), 6.39 (dd, J = 10.4, 1.8 Hz, 1H), 6.05 (q, J = 2.5 Hz, 1H), 5.90 (dd, J = 10.4, 1.3 Hz, 1H), 5.06 (dd, J = 17.5, 2.3 Hz, 1H), 4.50 (dt, J = 17.4, 2.5 Hz, 1H), 3.93 (dd, J = 2.2, 1.3 Hz, 1H), 3.24–3.22 (m, 1H), 1.67 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 190.62, 159.12, 148.57, 140.48, 137.26, 133.81, 131.93, 131.85, 129.38, 128.29, 127.13, 125.15, 123.48, 116.08, 115.85, 114.64, 114.42, 78.59, 68.21, 53.45, 43.70, 24.92. IR (neat)νmax: 3049, 2929, 1672, 1475, 1280, 1148, 811. HRMS (ESI-TOF):m/z calcd for C22H20FO4SSe [M + H]+, 478.0153; found, 478.0158.
8a-Methyl-4-phenyl-3-tosyl-2H-chromen-6(8aH)-one (7)
The title compound was prepared according to the general procedure as described above in a 75% (25 mg) yield; it was purified by column chromatography (30% EtOAc/hexane; Rf = 0.2) to afford 7 as a colorless sticky solid. 1H NMR (500 MHz, CDCl3): δ 7.24 (ddd, J = 7.6, 2.5, 1.2 Hz, 1H), 7.13 (t, J = 7.9 Hz, 4H), 7.03–7.01 (m, 2H), 6.99 (s, 1H), 6.88 (d, J = 7.2 Hz, 2H), 6.48 (s, 1H), 6.26 (d, J = 10.0 Hz, 1H), 4.93 (d, J = 18.9 Hz, 1H), 4.79 (d, J = 18.9 Hz, 1H), 2.31 (s, 3H), 1.50 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 181.2, 153.8, 144.5, 144.2, 142.3, 138.1, 134.7, 133.8, 129.4, 128.0, 127.4, 123.9, 70.6, 62.0, 23.8, 21.6. IR (neat)νmax: 1655,1485,1070,958,751. HRMS (ESI-TOF): m/z calcd for C23 H19 O 4 S = 391.10112[M + H]+, 391.0998; found, 391.0998.
7a-Methyl-3-((phenylsulfonyl)methylene)-2,3-dihydrobenzofuran-5(7aH)-one (8)
The title compound was prepared according to the general procedure as described above in an 80% (26 mg) yield; it was purified by column chromatography (EtOAc/hexane) to afford 8 as a colorless sticky solid. 1H NMR (400 MHz, CDCl3): δ 7.95–7.92 (m, 2H), 7.72–7.68 (m, 1H), 7.62–7.59 (m, 2H), 7.18 (d, J = 9.9 Hz, 1H), 6.72 (t, J = 2.7 Hz, 1H), 6.27 (d, J = 1.4 Hz, 1H), 6.19–6.16 (m, 1H), 5.26 (dd, J = 17.4, 2.6 Hz, 1H), 5.11 (dd, J = 17.4, 2.7 Hz, 1H), 1.40 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 185., 159.4, 148.1, 147.9, 140.1, 134.3, 129.7, 128.0, 127.6, 123.6, 120.1, 78.7, 68.7, 25.6. IR (neat)νmax: 1669,1303,1076,672 HRMS (ESI-TOF):m/z calcd for C16H15O4S [M – H]+, 303.0696; found, 303.06856.
Acknowledgments
V.S. thanks DST-Inspire, V.R. thanks CSIR, and M.G. thanks UGC, for their fellowship. We thank CSIR for financial support (ref. no. 34/1/TD CLP/NCP FBR 2020 RPPBDD TMD–Se MI). The authors thank the Director of CSIR-IICT for the generous support (IICT/Pubs./2023/102).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c03362.
Copies of 1H and 13C NMR spectra for all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Yang X.; Elrod L. C.; Le T.; Vega V. S.; Naumann H.; Rezenom Y.; Reibenspies J. H.; Hall M. B.; Darensbourg M. Y. Controlling O2 Reactivity in Synthetic Analogues of [NiFeS]- and [NiFeSe]-Hydrogenase Active Sites. J. Am. Chem. Soc. 2019, 141, 15338–15347. 10.1021/jacs.9b07448. [DOI] [PubMed] [Google Scholar]; b Huang S.; Li H.; Xie T.; Wei F.; Tung H.; Xu Z. Scandium-catalyzed electrophilic alkene difunctionalization: regioselective synthesis of thiosulfone derivatives. Org. Chem. Front. 2019, 6, 1663–1666. 10.1039/C9QO00138G. [DOI] [Google Scholar]; c Wang H.; Li Y.; Lu Q.; Yu M.; Bai X.; Wang S.; Cong H.; Zhang H.; Lei A. Oxidation-Induced β-Selective C-H Bond Functionalization: Thiolation and Selenation of N-Heterocycles. ACS Catal. 2019, 9, 1888–1894. 10.1021/acscatal.8b05054. [DOI] [Google Scholar]; d Qin Y.; Han Y.; Tang Y.; Wei J.; Yang M. A general method for site-selective Csp3-S bond formation via cooperative catalysis. Chem. Sci. 2020, 11, 1276–1282. 10.1039/C9SC04169A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yamada M.; Ichikawa T.; Ii M.; Itoh K.; Tamura N.; Kitazaki T. Novel cyclohexene derivatives as anti-sepsis agents: Synthetic studies and inhibition of NO and cytokine production. Bioorg. Med. Chem. 2008, 16, 3941–3958. 10.1016/j.bmc.2008.01.030. [DOI] [PubMed] [Google Scholar]; b Li J.; Rao W.; Wang S.-Y.; Ji S.-J. Nickel-Catalyzed Defluorinative Reductive Cross-Coupling Reaction of gem-Difluoroalkenes with Thiosulfonate or Selenium Sulfonate. J. Org. Chem. 2019, 84, 11542–11552. 10.1021/acs.joc.9b01387. [DOI] [PubMed] [Google Scholar]; c Scott J. P.; Lieberman D. R.; Beureux O. M.; Brands K. M. J.; Davies A. J.; Gibson A. W.; Hammond D. C.; McWilliams C. J.; Stewart G. W.; Wilson R. D.; Dolling U.-H. A Practical Synthesis of a γ-Secretase Inhibitor. J. Org. Chem. 2007, 72, 4149–4155. 10.1021/jo070407n. [DOI] [PubMed] [Google Scholar]
- a Wei Q.; Wang Y.-Y.; Du Y.-L.; Gong L.-Z. Organocatalytic asymmetric selenofunctionalization of tryptamine for the synthesis of hexahydropyrrolo[2,3-b] indole derivatives. Beilstein J. Org. Chem. 2013, 9, 1559–1564. 10.3762/bjoc.9.177. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kodama S.; Saeki T.; Mihara K.; Higashimae S.; Kawaguchi S.-i.; Sonoda M.; Nomoto A.; Ogawa A. A Benzoyl Peroxide/Diphenyl Diselenide Binary System for Functionalization of Alkynes Leading to Alkenyl and Alkynyl Selenides. J. Org. Chem. 2017, 82, 12477–12484. 10.1021/acs.joc.7b02276. [DOI] [PubMed] [Google Scholar]; c Liao L.; Zhang H.; Zhao X. Selenium-π-Acid Catalyzed Oxidative Functionalization of Alkynes: Facile Access to Ynones and Multisubstituted Oxazoles. ACS Catal. 2018, 8, 6745–6750. 10.1021/acscatal.8b01595. [DOI] [Google Scholar]
- a Mugesh G.; du Mont W.-W.; Sies H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. 10.1021/cr000426w. [DOI] [PubMed] [Google Scholar]; b Morrill L. A.; Susick R. B.; Chari J. V.; Garg N. K. Total Synthesis as a Vehicle for Collaboration. J. Am. Chem. Soc. 2019, 141, 12423–12443. 10.1021/jacs.9b05588. [DOI] [PubMed] [Google Scholar]; c Li J.; Ye Y.; Zhang Y. Cycloaddition/annulation strategies for the construction of multisubstituted pyrrolidines and their applications in natural product synthesis. Org. Chem. Front. 2018, 5, 864–892. 10.1039/C7QO01077J. [DOI] [Google Scholar]
- a Wu Q.; Zhao B.; Weng Y.; Shan Y.; Li X.; Hu Y.; Liang Z.; Yuan H.; Zhang L.; Zhang Y. Site-Specific Quantification of Persulfidome by Combining an Isotope-Coded Affinity Tag with Strong Cation-Exchange-Based Fractionation. Anal. Chem. 2019, 91, 14860–14864. 10.1021/acs.analchem.9b04112. [DOI] [PubMed] [Google Scholar]; b Reich H. J.; Hondal R. J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. 10.1021/acschembio.6b00031. [DOI] [PubMed] [Google Scholar]; c Bhushan B.; Lin Y. A.; Bak M.; Phanumartwiwath A.; Yang N. M.; Bilyard K.; Tanaka T.; Hudson K. L.; Lercher L.; Stegmann M.; Mohammed S.; Davis B. G. Genetic Incorporation of Olefin Cross-Metathesis Reaction Tags for Protein Modification. J. Am. Chem. Soc. 2018, 140, 14599–14603. 10.1021/jacs.8b09433. [DOI] [PubMed] [Google Scholar]
- Cheignon C.; Cordeau E.; Prache N. S.; Cantel S.; Martinez J.; Subra G.; Arnaudguilhem C.; Bouyssiere B.; Enjalbal C. Receptor-Ligand Interaction Measured by Inductively Coupled Plasma Mass Spectrometry and Selenium Labeling. J. Med. Chem. 2018, 61, 10173–10184. 10.1021/acs.jmedchem.8b01320. [DOI] [PubMed] [Google Scholar]
- a Hoover G. C.; Seferos D. S. Photoactivity and optical applications of organic materials containing selenium and tellurium. Chem. Sci. 2019, 10, 9182–9188. 10.1039/C9SC04279B. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Buriak J. M.; Sikder M. D. H. From Molecules to Surfaces: Radical-Based Mechanisms of Si-S and Si-Se Bond Formation on Silicon. J. Am. Chem. Soc. 2015, 137, 9730–9738. 10.1021/jacs.5b05738. [DOI] [PubMed] [Google Scholar]; c Hu M.; Hauger T. C.; Olsen B. C.; Luber E. J.; Buriak J. M. UV-Initiated Si-S, Si-Se, and Si-Te Bond Formation on Si (111): Coverage, Mechanism, and Electronics. J. Phys. Chem. C 2018, 122, 13803–13814. 10.1021/acs.jpcc.8b00910. [DOI] [Google Scholar]
- a Cui F.; Chen J.; Mo Z.; Su S.; Chen Y.; Ma X.; Tang H.; Wang H.; Pan Y.; Xu Y. Copper-Catalyzed Decarboxylative/Click Cascade Reaction: Regioselective Assembly of 5-Selenotriazole Anticancer Agents. Org. Lett. 2018, 20, 925–929. 10.1021/acs.orglett.7b03734. [DOI] [PubMed] [Google Scholar]; b Liu H. Y.; Zhang J. R.; Huang G. B.; Zhou Y. H.; Chen Y. Y.; Xu Y. L. Visible Light-Promoted Selenylation/Cyclization of Enaminones toward the Formation of 3-Selanyl-4H-Chromen-4- Ones. Adv. Synth. Catal. 2021, 363, 1656–1661. 10.1002/adsc.202001474. [DOI] [Google Scholar]; c Zhang J.; Liu H.; Fan T.; Chen Y.; Xu Y. Synthesis of Indolo[2,1-a]isoquinolin-6(5H)-Ones Derivatives via Fe(OTf)3-Promoted Tandem Selenylation/Cyclization of 2-Arylindoles. Adv. Synth. Catal. 2021, 363, 497–504. 10.1002/adsc.202000983. [DOI] [Google Scholar]; d Sun S. S.; Mo Z. Y.; Chen Y. Y.; Xu Y. L. Synthesis of Selenyl-Substituted Quinoline Derivatives via Substrate-Controlled Three-Component Domino Reactions. J. Org. Chem. 2022, 87, 12447–12454. 10.1021/acs.joc.2c01260. [DOI] [PubMed] [Google Scholar]
- a Klyushova L. S.; Kandalintseva N. V.; Grishanova A. Y. Antioxidant Activity of New Sulphur- and SeleniumContaining Analogues of Potassium Phenosan against H2O2-Induced Cytotoxicity in Tumour Cells. Curr. Issues. Mol. Biol. 2022, 44, 3131–3145. 10.3390/cimb44070216. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Alfieri M. L.; Panzella L.; Amorati R.; Cariola A.; Valgimigli L.; Napolitano A. Role of Sulphur and Heavier Chalcogens on the Antioxidant Power and Bioactivity of Natural Phenolic Compounds. Biomolecules 2022, 12, 90. 10.3390/biom12010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Schultz D. M.; Yoon T. P. Solar Synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 985. 10.1126/science.1239176. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhou Q.-Q.; Zou Y.-Q.; Lu L.-Q.; Xiao W.-J. Visible-Light-Induced Organic Photochemical Reactions through Energy-Transfer Pathways. Angew. Chem., Int. Ed. 2019, 58, 1586–1604. 10.1002/anie.201803102. [DOI] [PubMed] [Google Scholar]; c Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Shaw M. H.; Twilton J.; MacMillan D. W. C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Huang M.-H.; Hao W.-J.; Li G.; Tu S.-J.; Jiang B. Recent advances in radical transformations of internal alkynes. Chem. Commun. 2018, 54, 10791–10811. 10.1039/C8CC04618B. [DOI] [PubMed] [Google Scholar]; b Li H.; Cheng Z.; Tung C.-H.; Xu Z. Atom Transfer Radical Addition to Alkynes and Enynes: A Versatile Gold/Photoredox Approach to Thio-Functionalized Vinylsulfones. ACS Catal. 2018, 8, 8237–8243. 10.1021/acscatal.8b02194. [DOI] [Google Scholar]; c Huang M.-H.; Hao W.-J.; Jiang B. Recent Advances in Radical-Enabled Bicyclization and Annulation/1, n-Bifunctionalization Reactions. Chem.—Asian J. 2018, 13, 2958–2977. 10.1002/asia.201801119. [DOI] [PubMed] [Google Scholar]
- a Jimenez-Nunez E.; Echavarren A. M. Gold-Catalyzed Cycloisomerizations of Enynes: A Mechanistic Perspective. Chem. Rev. 2008, 108, 3326–3350. 10.1021/cr0684319. [DOI] [PubMed] [Google Scholar]; b Hu Y.; Bai M.; Yang Y.; Zhou Q. Metal-catalyzed enyne cycloisomerization in natural product total synthesis. Org. Chem. Front. 2017, 4, 2256–2275. 10.1039/c7qo00702g. [DOI] [Google Scholar]; c Li J.; Lee D. Enyne-Metathesis-Based Tandem Processes. Eur. J. Org Chem. 2011, 2011, 4269–4287. 10.1002/ejoc.201100438. [DOI] [Google Scholar]
- a Teng Q.; Thirupathi N.; Tung C.-H.; Xu Z. Hydroalkynylative Cyclization of 1,6-Enynes with Terminal Alkynes. Chem. Sci. 2019, 10, 6863–6867. 10.1039/C9SC02341K. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Takenaka K.; Mohanta S. C.; Sasai H. Palladium Enolate Umpolung: Cyclative Diacetoxylation of Alkynyl Cyclohexadienones Promoted by a Pd/SPRIX Catalyst. Angew. Chem., Int. Ed. 2014, 53, 4675–4679. 10.1002/anie.201311172. [DOI] [PubMed] [Google Scholar]
- a Qi Z.; Li X. Rhodium (III)-Catalyzed Coupling of Arenes with 7-Oxa/Azabenzonorbornadienes by C-H Activation. Angew. Chem., Int. Ed. 2013, 52, 8995–9000. 10.1002/anie.201303507. [DOI] [PubMed] [Google Scholar]; b Burns D. J.; Best D.; Wieczysty M. D.; Lam H. W. All-Carbon [3 + 3] Oxidative Annulations of 1,3-Enynes by Rhodium (III)-Catalyzed C-H Functionalization and 1,4-Migration. Angew. Chem., Int. Ed. 2015, 54, 9958–9962. 10.1002/anie.201503978. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang P.; Rao H.; Hua R.; Li C. Rhodium-Catalyzed Xanthone Formation from 2-Aryloxybenzaldehydes via Cross-Dehydrogenative Coupling (CDC). Org. Lett. 2012, 14, 902–905. 10.1021/ol203381q. [DOI] [PubMed] [Google Scholar]; d Huang J. R.; Dong L.; Han B.; Peng C.; Chen Y. C. Synthesis of Aza-Fused Polycyclic Quinolines via Double C-H Bond Activation. Chem.—Eur. J. 2012, 18, 8896–8900. 10.1002/chem.201201207. [DOI] [PubMed] [Google Scholar]
- a Heck R. F.; Nolley J. P. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972, 37, 2320–2322. 10.1021/jo00979a024. [DOI] [Google Scholar]; b Milstein D.; Stille J. K. Palladium-catalyzed coupling of tetraorganotin compounds with aryl and benzyl halides. Synthetic utility and mechanism. J. Am. Chem. Soc. 1979, 101, 4992–4998. 10.1021/ja00511a032. [DOI] [Google Scholar]; c Arcadi A.; Blesi F.; Cacchi S.; Fabrizi G.; Goggia-mani A.; Marinelli F. Palladium-Catalyzed Cascade Reactions of 1-(3-Arylprop-2-ynyloxy)-2-bromo Benzene Derivatives with Organoboron Compounds. J. Org. Chem. 2013, 78, 4490–4498. 10.1021/jo400503f. [DOI] [PubMed] [Google Scholar]
- a Li X.; He L.; Chen H.; Wu W.; Jiang H. Copper-Catalyzed Aerobic C(sp2)-H Functionalization for C-N Bond Formation: Synthesis of Pyrazoles and Indazoles. J. Org. Chem. 2013, 78, 3636–3646. 10.1021/jo400162d. [DOI] [PubMed] [Google Scholar]; b Sherman E. S.; Chemler S. R. Copper (II)-Catalyzed Aminooxygenation and Carboamination of N-Aryl-2-allylanilines. Adv. Synth. Catal. 2009, 351, 467–471. 10.1002/adsc.200800705. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cho S. H.; Yoon J.; Chang S. Intramolecular Oxidative C-N Bond Formation for the Synthesis of Carbazoles: Comparison of Reactivity between the Copper-Catalyzed and Metal-Free Conditions. J. Am. Chem. Soc. 2011, 133, 5996–6005. 10.1021/ja111652v. [DOI] [PubMed] [Google Scholar]; d Takamatsu K.; Hirano K.; Satoh T.; Miura M. Synthesis of Carbazoles by Copper-Catalyzed Intramolecular C-H/N-H Coupling. Org. Lett. 2014, 16, 2892–2895. 10.1021/ol501037j. [DOI] [PubMed] [Google Scholar]
- a Rosen B. M.; Quasdorf K. W.; Wilson D. A.; Zhang N.; Resmerita A.-M.; Garg N. K.; Percec V. Nickel-Catalyzed Cross-Couplings Involving Carbon-Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jana R.; Pathak T. P.; Sigman M. S. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pototschnig G.; Maulide N.; Schnürch M. Direct Functionalization of C-H Bonds by Iron, Nickel, and Cobalt Catalysis. Chem.—Eur. J. 2017, 23, 9206–9232. 10.1002/chem.201605657. [DOI] [PubMed] [Google Scholar]
- Zhu S. Q.; Pathigoolla A.; Lowe G.; Walsh D. A.; Cooper M.; Lewis W.; Lam H. W. Sulfonylative and Azidosulfonylative Cyclizations by Visible-Light-Photosensitization of Sulfonyl Azides in THF. Chem.—Eur. J. 2017, 23, 17598–17604. 10.1002/chem.201704380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nair A. M.; Kumar S.; Volla C. M. R. Visible Light Mediated Sulfenylation-Annulation Cascade of Alkyne Tethered Cyclohexadienones. Adv. Synth. Catal. 2019, 361, 4983–4988. 10.1002/adsc.201900874. [DOI] [Google Scholar]
- Mallick R. K.; Dutta S.; Vanjari R.; Voituriez A.; Sahoo A. K. Thioarylative Radical Cyclization of Yne-Dienone. J. Org. Chem. 2019, 84, 10509–10517. 10.1021/acs.joc.9b01445. [DOI] [PubMed] [Google Scholar]
- Ma X.-L.; Wang Q.; Feng X.-Y.; Mo Z.-Y.; Pan Y.-M.; Chen Y.-Y.; Xin M.; Xu Y.-L. Metal-free visible-light induced cyclization/substitution cascade reaction of alkyne-tethered cyclohexadienones and diselenides: access to 5-hydroxy-3-selenyl-4a,8a-dihydro-2H-chromen-6(5H)-ones. Green Chem. 2019, 21, 3547–3551. 10.1039/C9GC00570F. [DOI] [Google Scholar]
- Nair A. M.; Halder I.; Volla C. M. R. A metal-free four-component sulfonylation, Giese cyclization, selenylation cascade via insertion of sulfur dioxide. Chem. Commun. 2022, 58, 6950–6953. 10.1039/D2CC02315F. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Zhang N.; Li Y.; Mo Z.; Ma X.; Chen Y.; Xu Y. Metal-free synthesis of 3-sulfonyl-5-selanyl-4a,8adihydro-2H-chromen-6(5H)-ones via visible light driven intermolecular cascade cyclization of alkyne-tethered cyclohexadienones and selenosulfonates. Green Synth. Catal. 2021, 2, 397–400. 10.1016/j.gresc.2021.07.004. [DOI] [Google Scholar]
- a Ramesh V.; Gangadhar M.; Nanubolu J. B.; Adiyala P. R. Visible-Light-Induced Deaminative Alkylation/Cyclization of Alkyl Amines with N-Methacryloyl-2-phenylbenzoimidazoles in Continuous-Flow Organo-Photocatalysis. J. Org. Chem. 2021, 86, 12908–12921. 10.1021/acs.joc.1c01555. [DOI] [PubMed] [Google Scholar]; b Kishor G.; Ramesh V.; Rao V. R.; Pabbaraja S.; Adiyala P. R. Regioselective C-3-alkylation of quinoxalin-2(1H)-ones via C-N bond cleavage of amine derived Katritzky salts enabled by continuous-flow photoredox catalysis. RSC Adv. 2022, 12, 12235–12241. 10.1039/D2RA00753C. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Adiyala P. R.; Sastry K. N. V.; Kovvuri J.; Nagarajan A.; Reddy V. G.; Sayeed I. B.; Nayak V. L.; Maurya R. A.; Kamal A. Visible Light Driven Coupling of 2-aminopyridines and α-Keto Vinyl Azides for the Synthesis of Imidazo[1, 2-a]pyridines and Their Cytotoxicity. ChemistrySelect 2017, 2, 8158–8161. 10.1002/slct.201700952. [DOI] [Google Scholar]; d Adiyala P. R.; Jang S.; Vishwakarma N. K.; Hwang Y.-H.; Kim D.-P. Continuous-flow photo-induced decarboxylative annulative access to fused imidazole derivatives via a microreactor containing immobilized ruthenium. Green Chem. 2020, 22, 1565–1571. 10.1039/C9GC03496J. [DOI] [Google Scholar]
- a Yang S.; Wang L.; Wang L.; Li H. Visible-Light Photoredox-Catalyzed Regioselective Sulfonylation of Alkenes Assisted by Oximes via [1,5] H Migration. J. Org. Chem. 2020, 85, 564–573. 10.1021/acs.joc.9b02646. [DOI] [PubMed] [Google Scholar]; b Liang D.; Dong Q.; Xu P.; Dong Y.; Li W.; Ma Y. Synthesis of CF3CH2-Containing Indolines by Transition-Metal-Free Aryltrifluoromethylation of Unactivated Alkenes. J. Org. Chem. 2018, 83, 11978–11986. 10.1021/acs.joc.8b01861. [DOI] [PubMed] [Google Scholar]; c Liu C.; Wang B.; Guo Z.; Zhang J.; Xie M. Metal-free cascade rearrangement/radical addition/oxidative C-H annulation of propargyl alcohols with sodium sulfinates: access to 2-sulfenylindenones. Org. Chem. Front. 2019, 6, 2796–2800. 10.1039/C9QO00688E. [DOI] [Google Scholar]; d Wei W.; Wen J.; Yang D.; Liu X.; Guo M.; Dong R.; Wang H. Metal-Free Direct Trifluoromethylation of Activated Alkenes with Langlois’ Reagent Leading to CF3-Containing Oxindoles. J. Org. Chem. 2014, 79, 4225–4230. 10.1021/jo500515x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.