Abstract
From 125 separate cloacal cultures from three turkey flocks fed virginiamycin, 104 Enterococcus faecium and 186 Enterococcus faecalis isolates were obtained. As the turkeys aged, there was a higher percentage of quinupristin-dalfopristin-resistant E. faecium isolates, with isolates from the oldest flock being 100% resistant. There were no vancomycin-resistant enterococci. Results of pulsed-field gel electrophoresis (PFGE) indicated there were 11 PFGE types of E. faecalis and 7 PFGE types of E. faecium that were in more than one group of flock cultures.
There has been a great deal learned about the epidemiology of nosocomial multiply resistant enterococci in the last 2 to 3 decades. Most research on antibiotic-resistant enterococci has been on isolates from patients in hospitals and extended-care facilities. Little is known, however, about the epidemiology of antibiotic-resistant enterococci in the community.
One source of resistant enterococci may lie in the feces of meat animals, including poultry, cattle, swine, and sheep. At least 80% of poultry are now given antibiotics in their feed (4, 12, 17, 18). Debate occurs over the possible hazards of this practice. In this study, we evaluated the antimicrobial resistance of enterococci cultured from three separate turkey flocks on two farms of a large poultry production company.
(This work was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 17 September 1996.)
Between October 1995 and April 1996, we studied three flocks of turkeys from two different farms. The farms were located near each other and represented the same large turkey-producing company. Each flock of turkeys contained approximately 30,000 birds per barn. The feeding and husbandry practices were the same on each farm. We collected 20 to 25 cloacal samples per flock of turkeys. Cloacal samples were obtained from individual turkeys, with routine aerobic culturettes, by catching, obtaining cultures from, and releasing the turkeys at random. The ages of the groups sampled were as follows: group 1, 24 days; group 2, 28 days; group 3, 59 days; group 4, 80 days; and group 5, 130 days. Groups 1 and 5 and groups 2 and 3 were the same flock. Group 5 was sampled alive at the farm 2 days before slaughter. The samples called group 6, or plant, were obtained during various stages of slaughter and processing of the turkey carcasses and were from animals in the three flocks from which cultures had previously been obtained.
All of the turkeys were given subtherapeutic levels of antimicrobials in their feed as growth promoters. Virginiamycin was given to the birds from about age 9 1/2 weeks to slaughter (18 weeks). Bacitracin in addition to virginiamycin was also given for 14 days to live animals in group 5.
All culturette swabs were plated within 48 h and cultured on SF media (Difco Laboratories, Detroit, Mich.) as described previously (8) and SF media containing gentamicin (Sigma, St. Louis, Mo. [50 μg/ml]), ampicillin (Sigma [16 μg/ml]) or vancomycin (Eli Lilly Co., Indianapolis, Ind. [8 μg/ml]). Three distinct colony types per plate were evaluated further. All of these isolates were identified by standard biochemical tests (14), and the MICs of all three antibiotics for them, as well as that of quinupristin-dalfopristin (Rhône-Poulenc Rorer, Collegeville, Pa.), were determined by published microdilution methods (25). Quinupristin-dalfopristin resistance was defined as a MIC of ≥4.0 μg/ml, ampicillin resistance was defined as a MIC of >16 μg/ml, high-level gentamicin resistance was defined as a MIC of >2,000 μg/ml, and vancomycin resistance was defined as a MIC of >16 μg/ml. Genomic DNA for contour-clamped homogeneous pulsed-field gel electrophoresis (PFGE) was prepared, and the results were interpreted by methods previously described (11, 30). All enterococci were evaluated by PFGE, and the results were used to exclude duplicate isolates from the same animal. Linear regression and chi-square analysis were used to determine the significance of differences between the culture groups.
The results in terms of species identified are shown in Table 1. In the six turkey culture groups studied, Enterococcus faecalis was isolated from 45 to 63% of the cultures and Enterococcus faecium was isolated from 22 to 38% of the cultures. Enterococcus casseliflavus isolates were cultured from the young birds (3 to 4 weeks) and were not found in the older turkeys. The in vitro susceptibilities of E. faecium isolates to quinupristin-dalfopristin are shown in Table 2. For E. faecium, ampicillin resistance occurred as follows: group 1, 4 of 17 (23%) isolates from four animals; group 2, 4 of 28 (14%) isolates from three animals; group 3, 10 of 16 (63%) isolates from nine animals; group 4, 11 of 16 (69%) isolates from nine animals; group 5, 7 of 9 (79%) isolates from five animals; and group 6, 0 (P = 0.035 for groups 1 to 5) isolates. High-level gentamicin resistance occurred as follows: group 1, 3 of 17 isolates (18%) from three animals; group 2, 1 of 28 (4%) isolates from one animal; group 3, 0 isolates; group 4, 1 of 16 (6%) isolates from one animal; groups 5 and 6, 0 isolates (P = not significant). In E. faecalis, high-level gentamicin resistance occurred as follows: group 1, 0 of 28 (0%) isolates; group 2, 22 of 47 (47%) isolates from 22 animals; group 3, 6 of 21 (28%) isolates from 6 animals; group 4, 7 of 27 (26%) isolates from 7 animals; group 5, 0 of 21 (0%) isolates; and group 6, 12 of 42 (29%) isolates from 12 animals. There were no ampicillin-resistant E. faecalis isolates. No vancomycin-resistant enterococci were found in any cultures from either the live or slaughtered birds or from selective media containing vancomycin.
TABLE 1.
Enterococcal species isolated from turkey flocks
| Enterococcus species (no. tested) | No. (%) positive in groupa:
|
|||||
|---|---|---|---|---|---|---|
| 1 (24 days)b | 2 (28 days) | 3 (59 days) | 4 (80 days) | 5 (130 days) | 6 (plant) | |
| E. faecium (104) | 17 (27) | 28 (31) | 16 (38) | 16 (37) | 9 (22) | 18 (24) |
| E. faecalis (186) | 28 (45) | 47 (53) | 21 (50) | 27 (63) | 21 (51) | 42 (57) |
| E. casseliflavus (17) | 6 (10) | 11 (12) | 0 | 0 | 0 | 0 |
| E. durans (13) | 0 | 2 (2) | 0 | 0 | 0 | 11 (15) |
| E. mundai (2) | 0 | 1 (1) | 0 | 0 | 0 | 1 (1) |
| E. hirae (7) | 0 | 0 | 4 (9) | 0 | 3 (77) | 0 |
| E. gallinarum (22) | 11 (18) | 0 | 1 (2) | 0 | 8 (19) | 2 (3) |
| Total | 62 | 89 | 42 | 43 | 41 | 74 |
Note that groups 1 and 5 are the same flock and groups 2 and 3 are the same flock.
Age of flock.
TABLE 2.
Summary of in vitro susceptibilities of E. faecium isolates to quinupristin-dalfopristin
| Group (age [days]) | No. (%) of resistanta isolates/total | No. (%) of animals with resistant strains/no. with E. faecium | Total no. of animals with culture (% of animals with resistant E. faecium) |
|---|---|---|---|
| 1 (24)b | 4/17 (23) | 4/13 (31) | 20 (20) |
| 2 (28)c | 0/0 (0) | 0/21 (0) | 25 (0) |
| 3 (59) | 3/16 (19) | 3/15 (20) | 20 (15) |
| 4 (80) | 9/16 (56) | 7/13 (54) | 20 (35) |
| 5 (130) | 9/9 (100)d | 5/5 (100)e | 20 (25) |
MIC of ≥4.0 defines resistance.
Groups 1 and 5 are the same flock (P = 0.0002 for isolates; P = 0.01 for group 1 versus group 5 animals).
Groups 2 and 3 are the same flock (P = 0.9 for isolates; P = 0.05 for group 2 versus group 3 animals).
P = 0.07 for comparison of isolates in groups 1 through 5.
P = 0.14 for comparison of animals in groups 1 through 5.
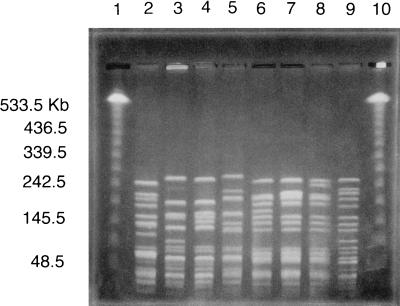
There were 34 E. faecalis strain types. Five clones had more than 10 isolates in each group. Eleven clones were found in more than one turkey culture group. For E. faecium, there were 45 strain types. Seven clones were found in more than one turkey culture group. The largest clone contained 13 isolates. The PFGE results in Fig. 1 show some of the related quinupristin-dalfopristin- and ampicillin-resistant strains of E. faecium cultured from different turkeys. Fourteen quinupristin-dalfopristin-resistant E. faecium clones (31 isolates) were identified in the turkeys from five of six culture groups (8 of these PFGE types were also ampicillin resistant). Four gentamicin-resistant E. faecium strain types (five isolates) were found in turkeys from three of six culture groups. One clone of quinupristin-dalfopristin-resistant E. faecium was found in two different flocks.
FIG. 1.
PFGE of SmaI-digested genomic DNA from turkey isolates of ampicillin- and quinupristin-dalfopristin-resistant E. faecium. Lanes: 1 and 10; lambda ladder PFGE marker; 2 and 3; isolates from turkey culture group 3 (age, 59 days); 4 and 5, isolates from group 4 (age, 80 days); 6 and 7, isolates from group 1 (age, 24 days); 8 and 9, isolates from group 5 (age, 130 days). Lanes 3 and 4 are clonally related isolates from two different flocks. Lanes 6, 7, and 8 are clonally related isolates from the same flock at different ages.
Nonhuman sources for resistant enterococci have included the inanimate environment and farm, pet, and veterinary animal isolates (1–3, 5, 6, 10, 16, 19–21). Studies in 1985 indicated evidence for Tn917 genes in enterococci cultures obtained from humans and farm animals (22, 28). In Europe, where the glycopeptide avoparcin has been used in animal feed, there has been increasing evidence that the use of this agent is associated with vancomycin-resistant enterococci in animal isolates (1–3, 5, 6, 15, 16). Of great concern is that enterococci causing human disease can be acquired through these environmental sources (15, 16, 27, 32). In the United States, vancomycin-resistant enterococci have been found predominantly in hospitals (9, 24, 29) or extended-care facilities.
In this study, we evaluated the in vitro antibiotic susceptibility and molecular relatedness of enterococcal isolates from turkeys fed virginiamycin in animal feed. Virginiamycin is a mixture of virginiamycin M (streptogramin A type) and virginiamycin S (streptogramin B type) antibiotics. Quinupristin-dalfopristin is a combination of streptogramin B (quinupristin) and A (dalfopristin) class antibiotics. In vitro resistance to the combination of quinupristin-dalfopristin in E. faecium is rare in human isolates (7, 23). In this study, we found that in E. faecium, ampicillin resistance occurred in up to 78% of isolates (45% of animals from which cultures were obtained), and high-level gentamicin resistance occurred in up to 18% of isolates (15% of animals from which cultures were obtained). For E. faecalis, high-level gentamicin resistance occurred in up to 47% of the isolates studied (88% of animals from which cultures were obtained). There were no vancomycin-resistant enterococci. Importantly, in isolates of E. faecium, resistance to quinupristin-dalfopristin occurred in up to 100% of isolates (25% of animals from which cultures were obtained) at the time of processing. Results of molecular typing by PFGE indicated identical clones of ampicillin-, quinupristin-dalfopristin-, and gentamicin-resistant strains from turkeys in different culture groups, suggesting dissemination of strains between animals.
In older turkeys, we found a significantly greater incidence of resistance to ampicillin and quinupristin-dalfopristin, which was probably related to their longer exposure to antibiotics and exposure to animals with resistant strains. Samples from the processing plant showed lower levels of resistance, possibly reflecting that the specimens obtained were not from individual turkeys and that animals given bacitracin had a lower prevalence of gentamicin-resistant enterococci. Bacitracin has excellent in vitro activity versus enterococci (13, 26) and may have reduced some of the enteric colonization by resistant strains. In this study, we did not evaluate a link between resistant strains in animals and those in humans. Since there was no control group of animals that were not given virginiamycin, conclusions about virginiamycin causing resistance are limited. In a separate study of farm animal isolates in southeastern Michigan, however, we found that gentamicin- and ampicillin-resistant enterococci were rare in animals that did not receive antimicrobial agents (31); therefore, the amount of resistance we found was higher than expected. It is possible that animals given antimicrobials other than virginiamycin in feed may also be colonized by streptogramin-resistant enterococci. The significance of the findings of this study are that we found quinupristin-dalfopristin-resistant strains in animals before the drug combination had been utilized in humans. Quinupristin-dalfopristin has completed phase III clinical trials in the United States and Europe. Initial studies have shown that the quinupristin-dalfopristin combination has promise as an agent for serious vancomycin-resistant enterococcal infections (33). Since antibiotic-resistant bacteria that can cause human infection may be transferred via food from animals to humans, until further information is available, great caution should be exercised in the use of streptogramins in animals.
Acknowledgments
This work was supported by the William Beaumont Hospital Research Institute and by Public Health Service grant H50/CCH513220-01 from the Centers for Disease Control and Prevention.
We thank Stuart Levy for assistance with this work and Rosalind Smith for assistance in the preparation of the manuscript.
REFERENCES
- 1.Aarestrup F M. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from conventional and ecological poultry farms. Microb Drug Resist. 1995;1:255–257. doi: 10.1089/mdr.1995.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup F M, Ahrens P, Madsen M, Pallesen L V, Poulsen R L, Westh H. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the vanA cluster. Antimicrob Agents Chemother. 1996;40:1938–1940. doi: 10.1128/aac.40.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarestrup F M, Bager F, Madsen M, et al. 35th Interscience Conference on Antimicrobial Agents and Chemotherapy Program Addendum. Washington, D.C: American Society for Microbiology; 1995. The effect of avoparcin as a feed additive on the occurrence of vancomycin-resistant Enterococcus faecium in pig and poultry production, abstr. LB-27; p. 7. [Google Scholar]
- 4.Barnes E M, Mead G C, Impey C S, Adams B W. The effect of dietary bacitracin on the incidence of Streptococcus faecalis subspecies liquefaciens and related streptococci in the intestines of young chicks. Br Poultry Sci. 1978;19:713–723. [Google Scholar]
- 5.Bates J, Jordens J Z, Griffiths D T. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J Antimicrob Chemother. 1994;34:507–517. doi: 10.1093/jac/34.4.507. [DOI] [PubMed] [Google Scholar]
- 6.Bates J, Jordens J Z, Selkon J B. Evidence for an animal origin of vancomycin-resistant enterococci. Lancet. 1993;342:490–491. doi: 10.1016/0140-6736(93)91613-q. [DOI] [PubMed] [Google Scholar]
- 7.Bonilla H F, Perri M B, Kauffman C A, Zervos M J. Comparative in vitro activity of quinupristin/dalfopristin against multidrug resistant Enterococcus faecium. Diagn Microbiol Infect Dis. 1996;25:127–131. doi: 10.1016/s0732-8893(96)00123-x. [DOI] [PubMed] [Google Scholar]
- 8.Coque T M, Arduino R C, Murray B E. High-level resistance to aminoglycosides: comparison of community and nosocomial fecal isolates of enterococci. Clin Infect Dis. 1995;20:1048–1051. doi: 10.1093/clinids/20.4.1048. [DOI] [PubMed] [Google Scholar]
- 9.Coque T M, Tomayko J F, Ricke S C, Okhyusen P C, Murray B E. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob Agents Chemother. 1996;40:2605–2609. doi: 10.1128/aac.40.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devriese L A, Ieven M, Goossens H, Vandamme P, Pot B, Hommez J, Haesebrouck F. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob Agents Chemother. 1996;40:2285–2287. doi: 10.1128/aac.40.10.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donabedian S, Chow J W, Shlaes D M, Green M, Zervos M J. DNA probes and contour-clamped homogeneous electric field electrophoresis for identification of enterococci to the species level. J Clin Microbiol. 1995;33:141–145. doi: 10.1128/jcm.33.1.141-145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DuPont H L, Steele J H. Use of antimicrobial agents in animal feeds: implications for human health. Rev Infect Dis. 1987;9:447–460. doi: 10.1093/clinids/9.3.447. [DOI] [PubMed] [Google Scholar]
- 13.Dutta G N, Devriese L A. Susceptibility of fecal streptococci of poultry origin to nine growth-promoting agents. Appl Environ Microbiol. 1982;44:832–837. doi: 10.1128/aem.44.4.832-837.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facklam R R, Collins M D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordts B, Van Landuyt H, Ieven M, Vandamme P, Goossens H. Vancomycin-resistant enterococci colonizing the intestinal tracts of hospitalized patients. J Clin Microbiol. 1995;33:2842–2846. doi: 10.1128/jcm.33.11.2842-2846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordens J Z, Bates J, Griffiths D T. Faecal carriage and nosocomial spread of vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother. 1994;34:515–528. doi: 10.1093/jac/34.4.515. [DOI] [PubMed] [Google Scholar]
- 17.Kaukas A, Hinton M, Linton A H. The effect of ampicillin and tylosin on the faecal enterococci of healthy young chickens. J Appl Bacteriol. 1987;62:441–447. doi: 10.1111/j.1365-2672.1987.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaukas A, Hinton M, Linton A H. The effect of growth-promoting antibiotics on the faecal enterococci of healthy young chickens. J Appl Bacteriol. 1988;64:57–64. doi: 10.1111/j.1365-2672.1988.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 19.Klare I, Heier H, Claus H, Böhme G, Marin S, Seltman G, Hakenbeck R, Antanassova V, Witte W. Enterococcus faecium strains with vanA-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community. Microb Drug Resist. 1995;1:265–272. doi: 10.1089/mdr.1995.1.265. [DOI] [PubMed] [Google Scholar]
- 20.Klare, I., H. Heier, H. Claus, R. Reissbrodt, and W. Witte. vanA mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol. Lett. 125:165–172. [DOI] [PubMed]
- 21.Klare I, Heier H, Claus H, Witte W. Environmental strains of Enterococcus faecium with inducible high-level resistance to glycopeptides. FEMS Microbiol Lett. 1993;106:23–30. doi: 10.1111/j.1574-6968.1993.tb05930.x. [DOI] [PubMed] [Google Scholar]
- 22.LeBlanc D J, Inamine J M, Lee L N. Broad geographical distribution of homologous erythromycin, kanamycin, and streptomycin resistance determinants among group D streptococci of human and animal origin. Antimicrob Agents Chemother. 1986;29:549–555. doi: 10.1128/aac.29.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low D E. Quinupristin/dalfopristin: spectrum of activity, pharmacokinetics, and initial clinical experience. Microb Drug Resist. 1995;1:223–234. doi: 10.1089/mdr.1995.1.223. [DOI] [PubMed] [Google Scholar]
- 24.Morena F, Grota P, Crisp C, Magnon K, Melcher G P, Jorgensen J H, Patterson J E. Clinical and molecular epidemiology of vancomycin-resistant Enterococcus faecium during its emergence in a city in southern Texas. Clin Infect Dis. 1995;21:1234–1237. doi: 10.1093/clinids/21.5.1234. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 2nd ed. Approved standard. NCCLS document M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 26.O’Donovan C A, Fan-Havard P, Tecson-Tumang F T, Smith S M, Eng R H K. Enteric eradication of vancomycin-resistant Enterococcus faecium with oral bacitracin. Diagn Microbiol Infect Dis. 1994;18:105–109. doi: 10.1016/0732-8893(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 27.Piddock L J. Does the use of antimicrobial agents in veterinary medicine and animal husbandry select antibiotic-resistant bacteria that infect man and compromise antimicrobial chemotherapy. J Antimicrob Chemother. 1996;38:1–3. doi: 10.1093/jac/38.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Rollins L D, Lee L N, LeBlanc D J. Evidence for a disseminated erythromycin resistance determinant mediated by Tn917-like sequences among group D streptococci isolated from pigs, chickens, and humans. Antimicrob Agents Chemother. 1985;27:439–444. doi: 10.1128/aac.27.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman J, Perri M B, Bostic G, Zervos M J. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Characterization of antimicrobial resistance in community fecal isolates of enterococci, abstr. C26; p. 86. [Google Scholar]
- 30.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thal L A, Chow J W, Mahayni R, Bonilla H, Perri M B, Donabedian S A, Silverman J, Taber S, Zervos M J. Characterization of antimicrobial resistance in enterococci of animal origin. Antimicrob Agents Chemother. 1995;39:2112–2115. doi: 10.1128/aac.39.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Auwera P, Pensart N, Korten V, Murray B E, Leclercq R. Influence of oral glycopeptides in the fecal flora of human volunteers: selection of highly glycopeptide-resistant enterococci. J Infect Dis. 1996;173:1129–1136. doi: 10.1093/infdis/173.5.1129. [DOI] [PubMed] [Google Scholar]
- 33.Zervos M J. Vancomycin-resistant Enterococcus faecium infections in the ICU. New Horizons. 1996;4:385–392. [PubMed] [Google Scholar]