Abstract
Objectives
The primary objective was to evaluate long-term treatment persistence and safety of natalizumab in Finnish multiple sclerosis patients. The secondary objectives were to assess patient characteristics, use of natalizumab-related safety protocol, and treatment persistence in patients with different anti-John Cunningham virus antibody statuses (John Cunningham virus status).
Materials & Methods
All adult multiple sclerosis patients in the Finnish multiple sclerosis register who started natalizumab between 1/2006 and 12/2018 were included in this study and followed retrospectively until treatment discontinuation or end of follow-up (12/2019).
Results
In total, 850 patients were included. Median duration of natalizumab treatment was 7.8 years in John Cunningham virus negative (n = 229) and 2.1 years in John Cunningham virus positive patients (n = 115; p < 0.001). The most common cause for treatment discontinuation was John Cunningham virus positivity. After natalizumab discontinuation, patients who had a washout duration of less than 6 weeks had fewer relapses during the first 6 months (p = 0.012) and 12 months (p = 0.005) compared with patients who had a washout duration of over 6 weeks. During the median follow-up of 3.6 years, 76% of patients remained stable or improved on their Expanded Disability Status Scale.
Conclusions
Treatment persistence was very high among John Cunningham virus negative patients. The study supports long-term effectiveness of natalizumab and a washout duration of less than 6 weeks after discontinuation.
Keywords: Multiple sclerosis, natalizumab, retrospective studies, duration of therapy, clinical decision-making, John Cunningham virus
Introduction
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disease of the central nervous system. 1 Natalizumab (NTZ) is a high-efficacy disease-modifying therapy (DMT) indicated for patients with highly active relapsing-remitting MS (RRMS) despite treatment with at least one DMT, and for patients with rapidly evolving severe RRMS. 2 NTZ has been demonstrated as highly efficacious in the treatment of RRMS both in clinical trials and real-world settings and has a well-established safety profile.3–8 However, real-world data on long-term treatment with NTZ is still limited, especially in patients with different anti-John Cunningham virus antibody statuses (JCV status).
The use of NTZ is limited by the increased risk of progressive multifocal leukoencephalopathy (PML), a rare but severe inflammatory disease caused by the JCV. The known risk factors for PML in NTZ-treated patients include positive JCV status, long NTZ treatment duration, and prior use of immunosuppressants. 9 Safety protocols including assessment of known risk factors, regular magnetic resonance imaging (MRI) scans, and careful screening of signs and symptoms of PML have been established to minimize the risk of PML.2,10 Also, a number of studies have reported an association between NTZ discontinuation and return of disease activity.11–13
The primary objective of this nationwide, observational, retrospective study was to evaluate the long-term treatment persistence and safety of NTZ in real-life clinical practice. The secondary objectives were to characterize Finnish MS patients treated with NTZ, assess the use of NTZ-related safety protocol, and examine the effect of JCV status on treatment persistence.
Materials and methods
Study population and data collection
This was a nationwide, observational, retrospective registry study designed to analyze the long-term treatment persistence and safety of NTZ in Finland. The data were collected retrospectively via the Finnish MS register and supplemented with data collected from electronic health records (EHRs) from the five Finnish university hospitals (Helsinki, Turku, Tampere, Kuopio, and Oulu University Hospitals). 14 All adult (≥18 years of age) patients with a diagnosis of MS disease (ICD-10: G35, the International Classification of Diseases, 10th edition) with at least one infusion of NTZ between 1/2006 and 12/2018 were included in the study cohort. The exclusion criterion was enrolment in a clinical trial (n = 2).
Two subgroups were formed: (a) The total cohort including all MS patients whose first NTZ infusion was between 1/2006 and 12/2018 and (2) a restricted cohort consisting of patients who had additional data collected from the EHRs of the university hospitals and whose first NTZ infusion was administered between 1/2014 and 12/2018. The restricted study cohort was formed to achieve a cohort with more comprehensive data on frequencies of MRI and anti-JCV antibody testing. The patients were followed up from the initiation of NTZ treatment until treatment discontinuation or the end of follow-up (12/2019). The possible follow-up period ranged from 12 months to 14 years.
Baseline and outcome measures
Age, gender, diagnosis (ICD-10: G35), date of MS onset, and date of diagnosis were collected at baseline. The JCV status in serum, information on NTZ infusions (date, dosage, and strength) and other DMTs, MS disease phenotype (RRMS, primary progressive MS, secondary progressive MS), frequency of MRI, and the Expanded Disability Status Scale (EDSS) score at baseline and during the follow-up were collected. The number of relapses during the year before the initiation of NTZ treatment and during the follow-up was collected. Also, reasons for NTZ discontinuation were collected. The disease duration was evaluated based on the time from MS diagnosis to the initiation of NTZ treatment. For the restricted cohort, additional data was collected directly from EHRs and consisted of NTZ infusion dates and dates of MRI and anti-JCV antibody testing.
The use of the NTZ-related safety protocol, as described in the summary of product characteristics (SPC) for Tysabri, was assessed based on the frequency of MRI and anti-JCV antibody testing. 2 According to the NTZ-related safety protocol, MRI should be performed before the initiation of treatment (usually within 3 months) and repeated at least every 12 months for patients without increased risk for PML and every 6 months for patients with increased risk for PML. A patient is considered at increased risk for PML if he/she (a) has tested positive for anti-JCV antibodies, NTZ treatment has been ongoing for at least 2 years, and has used immunosuppressive treatment prior NTZ or (b) NTZ treatment has been ongoing for at least 2 years, has no prior immunosuppressive treatment, but the anti-JCV antibody index is high (1.5 or greater). Anti-JCV antibody testing is recommended prior to initiating NTZ and every 6 months for JCV-negative patients to detect possible seroconversion. The adherence to safety protocol was evaluated in the restricted study cohort.
EDSS changes were assessed as the percentage of participants who had EDSS improvement (decrease of ≥0.5 points), EDSS stabilization (0.0 point change), or EDSS progression (increase of ≥0.5 points) compared with baseline. In addition, we examined the proportion of patients who showed accumulated disability which was defined as an increase of ≥1.5 points from the baseline EDSS score of 0.0, ≥ 1.0 points from the baseline EDSS score of ≥1.0 to <6.0, or ≥0.5 points from baseline EDSS score of ≥6.0.
Statistical analyses
Baseline and outcome variables were assessed descriptively by the number and proportion for categorical variables and by mean and standard deviation (SD) or median and first and third quartiles for continuous variables. In addition, the number and proportion of missing data was reported.
NTZ treatment persistence was analyzed with the Kaplan-Meier method among the total cohort and compared across patients with varying JCV status (positive and negative) using the log-rank test. JCV status was considered a time-dependent variable.
The descriptive statistics of EDSS changes and annualized relapse rate (ARR) were calculated for all patients with available information and with a treatment duration of at least 12 months. EDSS changes were assessed by comparing the baseline values to the last available value at the end of the follow-up, among patients who had both values available. All patients who had a baseline evaluation of EDSS within a year before the initiation of NTZ treatment and a follow-up evaluation within three months of NTZ discontinuation were included in the assessment.
EDSS changes were compared between treatment-naive patients and patients who used another DMT before NTZ initiation. The comparison was made using Fisher's exact test.
The effect of the washout period (less than 6 weeks vs. 6 weeks or more) on the frequency of relapses was investigated in two groups: Patients who had at least 6 months of follow-up available after the discontinuation of NTZ treatment, and those who had at least 12 months. These frequencies were compared using Fisher's exact test.
Two-tailed p values <.05 were considered statistically significant. Statistical analyses were conducted using RStudio (version 1.3.1073; http://www.rstudio.com).
Ethical considerations
According to Finnish legislation, the approval of an ethical committee was not required as the study was a non-interventional registry study in which patients were not contacted. The study was approved by the Finnish Institute of Health and Welfare (THL/2274/5.05.00/2019). The study was conducted in accordance with the Helsinki Declaration, Good Pharmacoepidemiology Practices (GPP), and Data Protection Directive (DPD).
Results
Patient characteristics
The total study cohort consisted of 850 MS patients (232 male; 618 female) who initiated NTZ treatment between 1/2006 and 12/2018 (Table 1). Most patients (90%) had RRMS phenotype at baseline with a mean disease duration of 5.4 years. The restricted study cohort consisted of 215 patients (57 male; 158 female) who had additional EHR information and started NTZ treatment between 1/2014 and 12/2018 (Table 1). NTZ dose was 300 mg for all patients. Information on dosing intervals was available for 441/850 patients. The median dosing interval per patient was 4.4 (IQR 4.2–4.6) weeks. Most patients (84%; 371/441) had a dosing interval of 4 weeks and 16% (70/441) of patients had a dosing interval of 5 weeks or more.
Table 1.
Descriptive statistics of the study cohort at baseline.
| Total cohort (N = 850) | Restricted cohorta (n = 215) | |
|---|---|---|
| Age at MS diagnosis, mean (SD), years | 30.5 (8.7) | 31.3 (9.0) |
| Age at MS onsetb, mean (SD), years | 28.5 (8.4) | 29.0 (8.4) |
| Disease durationc, mean (SD), years | 5.4 (5.6) | 5.0 (6.1) |
| Sex, n (%) | ||
| Male | 232 (27%) | 57 (26.5%) |
| Female | 618 (73%) | 158 (73.5%) |
| MS phenotype, n (%) | ||
| RRMS | 768 (90%) | 187 (87%) |
| PPMS | 7 (1%) | 2 (1%) |
| SPMS | 27 (3%) | 11 (5%) |
| Unspecified | 42 (5%) | 15 (7%) |
| Missing | 6 (1%) | 0 (0%) |
| EDSSd, median (Q1, Q3) | 3.0 (2.0, 4.5) | 2.5 (2.0, 4.4) |
| Number of previous DMTs, n (%) | ||
| 0 | 204 (24%) | 83 (39%) |
| 1 | 239 (28%) | 47 (22%) |
| ≥ 2 | 407 (48%) | 85 (40%) |
| JCV status, n (%) | ||
| Negative | 138 (16%) | 104 (48%) |
| Positive | 41 (5%) | 21 (10%) |
| Missing | 671 (79%) | 90 (42%) |
DMT: disease-modifying therapy; EDSS: Expanded Disability Status Scale; JCV: John Cunningham virus; MS: multiple sclerosis; PPMS: primary progressive multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SD: standard deviation; SPMS: secondary progressive multiple sclerosis.
The restricted study cohort included MS patients from the Finnish MS Register who had additional data from a Finnish university hospital and whose first natalizumab infusion was between 1/2014 and 12/2018.
127 patients in the total study cohort and 14 patients of restricted study cohort did not have information on the age at MS onset available.
The time from MS diagnosis to the initiation of natalizumab treatment.
484 patients in the total study cohort and 129 patients of restricted study cohort did not have EDSS information available.
Treatment persistence and reasons for discontinuation
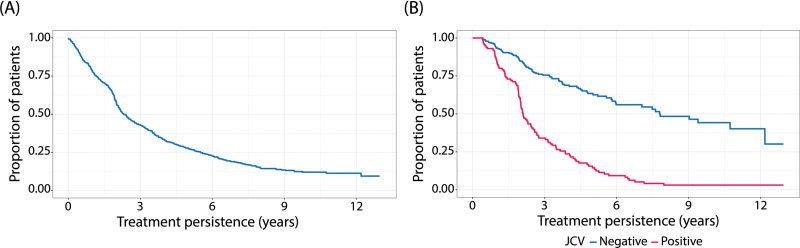
Altogether, 76% (642/850) of patients discontinued NTZ treatment during the 14-year observation period, and 208 patients had ongoing NTZ treatment at the end of the follow-up. The median treatment duration for all patients was 2.3 years (N = 850), and 4.4 years for patients who had JCV status reported. A total of 43% of patients persisted on NTZ treatment for three years or more (Figure 1A). A total of 76% of JCV-negative patients and 34% of JCV-positive patients persisted on NTZ treatment for at least three years (Figure 1B). The median duration of treatment was 7.8 years for JCV-negative patients (n = 229) and 2.1 years for JCV-positive patients (n = 115; p < 0.001).
Figure 1.
Kaplan–Meier estimate for natalizumab treatment persistence during follow-up for the (A) total population (N = 850) and (B) patients with a positive status for anti-john cunningham virus (JCV) antibodies (n = 115), and patients with a negative status for anti-JCV antibodies (n = 229).
The most common reason for NTZ treatment discontinuation was a positive JCV status, which accounted for 48% of treatment discontinuations in the total cohort, followed by disease activity (12%), adverse events (11%), patient's own decision (9%), and pregnancy (6%) (Table 2). The summary of adverse events leading to the discontinuation of NTZ treatment is shown in Table 3. Two cases of PML were reported during NTZ treatment.
Table 2.
Reasons for natalizumab treatment discontinuation.
| Reason for discontinuationa | Total cohort (n = 642) | |
|---|---|---|
| n | % | |
| Anti-John Cunningham virus (JCV) antibody positivity | 306 | 48 |
| Disease activity | 76 | 12 |
| Adverse events | 72 | 11 |
| Other | 70 | 11 |
| Patient's own decision | 57 | 9 |
| Pregnancy | 39 | 6 |
| Alteration of disease course | 36 | 6 |
| Drug antibodies | 32 | 5 |
| Missing – discontinued without specified reason | 32 | 5 |
a Individual patients may have more than one reason for discontinuation.
Table 3.
Adverse events leading to natalizumab treatment discontinuation (total study cohort, n = 72).
| Adverse event | Number of patients | |
|---|---|---|
| n | % | |
| Airways, lungs, thoracic cavity, and mediastinum | ||
| Dyspnea | 1 | 1.4 |
| Upper airway infection | 2 | 2.8 |
| Alimentary tract | ||
| Abdominal pain | 2 | 2.8 |
| Blood and lymphatic tissue | ||
| Anemia | 1 | 1.4 |
| Leukocytosis | 1 | 1.4 |
| Neutropenia | 1 | 1.4 |
| Thrombocytopenia | 1 | 1.4 |
| Bone, skeletal muscle, and connective tissue | ||
| Backache | 2 | 2.8 |
| Flank pain | 1 | 1.4 |
| Limb pain | 3 | 4.2 |
| Muscle spasticity | 1 | 1.4 |
| Muscle weakness | 4 | 5.6 |
| Occipital pain | 1 | 1.4 |
| Cardiovascular | ||
| Deep venous thrombosis | 1 | 1.4 |
| Palpitation | 1 | 1.4 |
| Tachycardia | 1 | 1.4 |
| General | ||
| Chest pain | 1 | 1.4 |
| Fatigue | 3 | 4.2 |
| Fever | 2 | 2.8 |
| Headache | 1 | 1.4 |
| Shivering | 1 | 1.4 |
| Immune system | ||
| Anaphylactic reaction | 1 | 1.4 |
| Mild allergic reaction | 10 | 13.9 |
| Infections | ||
| Abscess (not at site of injection) | 1 | 1.4 |
| Progressive multifocal leukoencephalopathy (PML) | 2 | 2.8 |
| Unknown infection | 4 | 5.6 |
| Liver and biliary system | ||
| Abnormal liver function test | 6 | 8.3 |
| Hepatic insufficiency | 1 | 1.4 |
| Hyperbilirubinemia | 1 | 1.4 |
| Nervous system | ||
| Cognitive disorders | 1 | 1.4 |
| Tremor | 1 | 1.4 |
| Skin and subcutaneous tissue | ||
| Alopecia | 1 | 1.4 |
| Eczema | 4 | 5.6 |
| Urticaria | 4 | 5.6 |
| Unknown | 28 | 38.9 |
Safety protocol
In the restricted study cohort, baseline MRI was performed on 76% (163/215) of patients. During NTZ treatment, MRI was performed at least every 12 months in the 93% (155/167) of patients without increased PML risk, and at least every 6 months in the 75% (3/4) of patients at increased risk for PML. Anti-JCV antibody testing was performed at baseline for 58% (125/215) of patients and repeated at least every 6 months for 82% (85/104) of JCV-negative patients. The median interval of anti-JCV antibody testing was 6.0 (IQR 5.6–6.2) months.
Disability and relapses
EDSS information was available for 104 patients both at baseline and at the end of the follow-up. Mean follow-up time was 3.6 years in the patients with EDSS information and the mean interval between the EDSS evaluations was 3.8 (SD 2.5) years. Only patients with follow-up of at least 1 year were included in the analyses. EDSS improvement was observed in 32% (33/104), EDSS stabilization in 31% (32/104), and EDSS progression in 38% (39/104) of patients at the end of the follow-up. In addition, 24% (25/104) of the patients showed accumulated disability. There were no statistically significant differences in EDSS changes among treatment-naive patients (n = 22) and patients who had used another DMT before NTZ initiation (n = 82; p > 0.05, median [Q1, Q3] duration of previous treatment 1.1 [0.4, 2.5] years). The mean ARR was 1.3 (SD 1.1) during the year prior to starting NTZ and 0.1 (SD 0.4) during the follow-up (n = 663).
Effect of washout
The median washout time in those who switched from NTZ to another DMT was 13.1 weeks (IQR 8.4–21.9). There was a statistically significant difference in the frequency of relapses after NTZ discontinuation between patients who had a washout duration of less than 6 weeks and patients who had a washout duration of 6 weeks or more, for both the first 6 months (p = 0.012) and first 12 months (p = 0.005) periods. Patients with shorter washout duration were more often relapse-free than patients with longer washout duration (Table 4).
Table 4.
The duration of washout and number of relapses during the first 6 months and 12 months after discontinuation of natalizumab. The results are presented for the total study cohort, n (%).
| Number of relapses | 6 months follow-upa (n = 538) |
12 months follow-upb (n = 519) |
||
|---|---|---|---|---|
| < 6 weeks washout (n = 107) | ≥ 6 weeks washout (n = 432) | < 6 weeks washout (n = 98) | ≥ 6 weeks washout (n = 422) | |
| 0 | 93 (87%) | 317 (73%) | 79 (81%) | 271 (64%) |
| 1 | 12 (11%) | 88 (20%) | 13 (13%) | 88 (21%) |
| 2+ | 2 (2%) | 27 (6%) | 6 (6%) | 63 (15%) |
A statistically significant difference (p = 0.012) was detected in the frequency of relapses between patients with a washout duration of less than 6 weeks and patients with a washout duration of 6 weeks or more, using Fisher's exact test.
A statistically significant difference (p = 0.005) was detected in the frequency of relapses between patients who had a washout duration of less than 6 weeks and patients who had a washout duration of 6 weeks or more, using Fisher's exact test.
Discussion
The results of this long-term registry study up to 14 years indicate that there is very high NTZ treatment persistence among JCV-negative patients and that JCV positivity is the most common reason for the discontinuation of NTZ treatment. In addition, our results support the association between prolonged washout duration and a higher risk of disease reactivation following NTZ discontinuation.
JCV negative patients (n = 229) had considerably longer treatment duration with a median of 7.8 years compared to JCV positive patients (n = 115) who had a median duration of 2.1 years. In an epoch-analysis of the Tysabri Observational Programme (TOP), the disability progression rate decreased further beyond 2 years of NTZ treatment. 15 Patients who responded well and remained on continuous NTZ therapy for over 4 years had sustained and potentially enhanced reductions in EDSS progression over time. Therefore, high persistence in NTZ treatment is clinically highly relevant for the long-term prognosis of patients.
The discontinuation of NTZ treatment in JCV-positive patients after 2 years of treatment is expected because of the increased risk of PML in these patients. 9 Although positive JCV status is known to be a critical factor limiting the duration of NTZ treatment, the treatment duration has not been previously reported separately for JCV negative and positive patients, to our knowledge.7,16 The result of the significant difference in long-term treatment duration between JCV negative and positive patients is also very relevant in light of the high prevalence estimates of JCV positivity among Finnish MS patients (57%) and multinational MS patient population (58%).17,18 Patients with negative JCV status are generally expected to have a long NTZ treatment duration even when considering the occurrence of seroconversion. Based on a recent meta-analysis, the pooled estimate of seroconversion incidence driven by JCV infection in NTZ-treated MS patients was 21% in European/American countries. 19
The median duration of NTZ treatment was 2.3 years for all patients (N = 850) and 43% of all patients persisted on NTZ treatment for at least 3 years. The discontinuation rate in our study was slightly higher than in some earlier reports. In the 10-year interim analysis of TOP, 52% had discontinued NTZ and the median duration of NTZ treatment was 3.3 years. 7 In a large Italian cohort of more than 5000 NTZ-treated RRMS patients, the median treatment duration was also 3.3 years, and 75% of patients persisted at 3.3 years of NTZ treatment. 16 Higher JCV index and EDSS at baseline predicted early NTZ discontinuation in that cohort. The discontinuers had higher ARR and EDSS evaluations at the last follow-up during NTZ treatment compared to continuers. 16 French TYSEDMUS cohort included 4055 patients treated with at least one infusion of NTZ in 2007–2012, and 31% of the patients discontinued NTZ treatment. 12
The risk of PML was the most critical factor affecting NTZ treatment persistence as the most common reason for discontinuation (48%) was positive JCV status. Altogether, 46% of discontinuations were due to the risk of PML in the TOP study, 7 83% in the previously mentioned Italian study, 16 and 35% in the TYSEDMUS study. 12 In this cohort, the second most common reason for discontinuation was disease activation but the proportion was low (12%) and similar to what has been reported in previous long-term studies (11%–13%).7,16
The long-term clinical effectiveness of NTZ was further demonstrated by the majority of patients showing EDSS improvement (32%) or stabilization (31%) from baseline to the end of follow-up. Less than one in four patients (24%) showed accumulated disability during follow-up. These results on disability and relapse rate are in line with previous long-term observational studies.6–8,20 In the TOP study, the reported cumulative probabilities of confirmed disability worsening and disability improvement were 28% and 33% at 10 years, respectively.7,20 The mean ARR was 0.1 during the follow-up of this study which is similar to previous long-term studies that have reported mean ARRs between 0.1 and 0.2 during NTZ treatment.6–8 Also, recent studies and meta-analysis have demonstrated that NTZ is still highly effective and viable immunotherapy for RRMS in comparison to other high-efficacy DMTs.21–23
Previous registry-based studies, including one Finnish study, have indicated that initiating treatment with a high-efficacy DMT in RRMS may reduce long-term disability progression and relapses compared to moderate-efficacy DMTs.24–27 In the restricted cohort of our study, we did not identify a statistically significant difference in disability progression as measured with EDSS change between 22 treatment-naive patients and 82 patients who had previously used another DMT. However, EDSS change was not comprehensively available for all patients limiting the statistical power of this analysis.
Adverse events were reported as a reason for discontinuation by 11% of the patients. Two cases of PML were diagnosed during the observation period. Both cases occurred in November 2011. 28 There have been no new PML cases after 2011 when the Tysabri SPC was updated with information on risk factors for PML and recommendations for anti-JCV antibody testing. The reported adverse events were consistent with the current safety profile of NTZ and no new safety concerns were identified.2,3
Besides the risk of PML, the risk of disease reactivation after NTZ discontinuation is a critical factor in clinical decision-making related to the initiation or discontinuation of NTZ treatment. Previous studies have demonstrated that NTZ cessation is often associated with disease reactivation.11–13 In the TYSEDMUS study, 37.1% of the patients who discontinued NTZ, experienced a relapse within 12 months after discontinuation. Relapse risk was associated with younger age and disease activity before NTZ initiation. Also, an increase of one point in EDSS was observed in 23% of patients at the end of follow-up period compared to the last NTZ infusion. 12 In our study, the median duration of washout after NTZ discontinuation was 13 weeks. In the past, longer washout durations were used in accordance with the Finnish treatment guidelines. Based on the fact that NTZ has pharmacodynamic effects for approximately 12 weeks after its last dose, starting other therapies during this time will result in a concomitant exposure to NTZ. 2 Previous real-world studies have reported that short washout duration (less than 2–3 months) and initiation of subsequent DMT may decrease the risk of disease reactivation.13,29–34 Accordingly, the updated Finnish Current Care Guidelines for MS now caution about the risk of disease reactivation after 2–3 months of NTZ discontinuation if the initiation of new therapy is delayed. 35 Our study supports the finding that a shorter washout (less than 6 weeks) is associated with a lower risk of disease reactivation during the first 6 and 12 months after NTZ cessation.
The main strength of this study is the nationwide coverage of the cohort and the long follow-up period. The Finnish MS register has a very high nationwide coverage (>90%). The treatment duration, reasons for discontinuation, and prior and subsequent DMTs were comprehensively reported in the total cohort. Other strengths include the high standard of healthcare in Finland and relatively uniform treatment practices across the country. This was a real-life, retrospective, non-interventional study in which data were collected from the Finnish MS register and supplemented with EHR data providing extensive clinical data for the patients included in the study cohort. The accuracy of available data collected from the Finnish MS register and EHRs can be considered high. 14 However, the key limitation is that not all variables were comprehensively available for all patients in the study cohort. JCV status was not systematically reported in the registry and, as a result, 40% (344/850) of patients did not have information available on their JCV status at baseline or during the follow-up. The results on disability and relapses can be considered as descriptive because only a subset of patients had enough available data. In addition, the data did not allow for confirming that the EDSS change in patients was sustained over time, therefore, it is possible that in some cases the EDSS change was due to short-term variation during the course of RRMS. Our study indicates that the treatment persistence was very high among JCV-negative patients who typically stayed on NTZ treatment for over 7 years. The most important reason for NTZ treatment discontinuation was positive JCV status and only a low proportion of patients discontinued due to disease activity. Monitoring PML risk in NTZ-treated MS patients is extensively adopted in clinical practice in Finland as compliance with the recommended frequency of MRI and anti-JCV antibody testing is good. Our study confirmed previous results on long-term clinical effectiveness of NTZ as the majority of the patients showed EDSS improvement or stabilization from baseline to the end of follow-up, and the mean ARR was low (0.1) during the follow-up. Our study also supports the earlier reports indicating that prolonged washout duration after NTZ discontinuation should be avoided. Taken together, the results of this study indicate that NTZ is a valuable long-term treatment option, especially, in JCV-negative MS patients.
Acknowledgments
The authors were assisted in preparing the manuscript by Noora Lindgrén from MedEngine Oy. Harlan Barker, MSc, from MedEngine Oy is acknowledged for the language review. The authors provided final approval of all content and were responsible for the decision to submit it for publication.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K.H. and S.L. are employees of Biogen Finland Oy. M.V. is an employee of StellarQ Oy. T.Y. is the owner of MedEngine Oy and J.M. is an employee of MedEngine Oy. M.S-H. has received fees for lectures from Biogen, Novartis, Merck, Roche, Sanofi Genzyme, Teva; congress expenses from Biogen, Merck, Roche, Sanofi Genzyme, Teva; advisory boards from Biogen, Novartis, Merck, Roche, Sanofi Genzyme, Teva. H.K. has received honorarium from Biogen, Merck, Sanofi, Jansen Cilag, Celgene, and Roche. A.V-A. and P.H. have no conflict of interest to report.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Biogen provided funding for the study and writing support.
ORCID iDs: Hanna Kuusisto https://orcid.org/0000-0002-3697-4961
Sanni Lahdenperä https://orcid.org/0000-0001-7306-3502
Merja Soilu-Hänninen https://orcid.org/0000-0001-6930-0229
Contributor Information
Auli Verkkoniemi-Ahola, Department of Neurology, Helsinki University Hospital and University of Helsinki, Helsinki, Finland.
Päivi Hartikainen, Neuro Center, Neurology Outpatient Clinic, Kuopio University Hospital, Kuopio, Finland.
Katja Hassi, Biogen Finland Oy, Espoo, Finland.
Hanna Kuusisto, Department of Neurology, Tampere University Hospital, Tampere, Finland; Kanta-Häme Central Hospital, Hämeenlinna, Finland; Department of Health and Social Management, University of Eastern Finland, Kuopio, Finland.
Sanni Lahdenperä, Biogen Finland Oy, Espoo, Finland.
Juha Mehtälä, MedEngine Oy, Helsinki, Finland.
Matias Viitala, StellarQ Ltd, Turku, Finland.
Tero Ylisaukko-oja, MedEngine Oy, Helsinki, Finland; Faculty of Medicine, Center for Life Course Health Research, University of Oulu, Oulu, Finland.
Merja Soilu-Hänninen, Department of Clinical Neurosciences, University of Turku, Turku, Finland; Neurocenter, Turku University Hospital, Turku, Finland.
References
- 1.Dobson R, Giovannoni G. Multiple sclerosis—a review. Eur J Neurol 2019; 26: 27–40. [DOI] [PubMed] [Google Scholar]
- 2. Tysabri EPAR Annex I . Available at: https://www.ema.europa.eu/en/documents/product-information/tysabri-epar-product-information_en.pdf.
- 3.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910. [DOI] [PubMed] [Google Scholar]
- 4.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354: 911–923. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor P, Goodman A, Kappos L, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS study. Neurology 2014; 83: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correia I, Batista S, Galego O, et al. Long-term effectiveness and safety of natalizumab in a Portuguese population. Int Immunopharmacol 2017; 46: 105–111. [DOI] [PubMed] [Google Scholar]
- 7.Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the tysabri observational program (TOP). J Neurol Neurosurg Psychiatry 2020; 91: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efthimios D, Georgios K, Antonia A, et al. Long-Term effectiveness of natalizumab in patients with relapsing-remitting multiple sclerosis treated in the routine care in Greece: Results from the multicenter, observational 5-year prospective study “TOPICS Greece.”. Clin Drug Investig 2021; 41: 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho PR, Koendgen H, Campbell N, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: A retrospective analysis of data from four clinical studies. Lancet Neurol 2017; 16: 925–933. [DOI] [PubMed] [Google Scholar]
- 10.Iaffaldano P, D’Onghia M, Trojano M. Safety profile of tysabri: international risk management plan. Neurol Sci 2009; 30: S159–S162. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 2011; 76: 1858–1865. [DOI] [PubMed] [Google Scholar]
- 12.Papeix C, Vukusic S, Casey R, et al. Risk of relapse after natalizumab withdrawal: Results from the French TYSEDMUS cohort. Neurol Neuroimmunol Neuroinflamm 2016; 3: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prosperini L, Kinkel RP, Miravalle AA, et al. Post-natalizumab disease reactivation in multiple sclerosis: Systematic review and meta-analysis. Ther Adv Neurol Disord 2019; 12: 1756286419837809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laakso SM, Viitala M, Kuusisto H, et al. Multiple sclerosis in Finland 2018-data from the national register. Acta Neurol Scand 2019; 140: 303–311. [DOI] [PubMed] [Google Scholar]
- 15.Wiendl H, Butzkueven H, Kappos L, et al. Epoch analysis of on-treatment disability progression events over time in the tysabri observational program (TOP). PloS One 2016; 11: e0144834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chisari CG, Comi G, Filippi M, et al. PML Risk is the main factor driving the choice of discontinuing natalizumab in a large multiple sclerosis population: results from an Italian multicenter retrospective study. J Neurol 2022; 269: 933–944. [DOI] [PubMed] [Google Scholar]
- 17.Kolasa M, Hagman S, Verkkoniemi-Ahola A, et al. Anti-JC virus seroprevalence in a Finnish MS cohort. Acta Neurol Scand 2016; 133: 391–397. [DOI] [PubMed] [Google Scholar]
- 18.Olsson T, Achiron A, Alfredsson L, et al. Anti-JC virus antibody prevalence in a multinational multiple sclerosis cohort. Mult Scler 2013; 19: 1533–1538. [DOI] [PubMed] [Google Scholar]
- 19.Azimi A, Hanaei S, Sahraian MA, et al. Incidence of seroconversion and sero-reversion in patients with multiple sclerosis (MS) who had been treated with natalizumab: A systematic review and meta-analysis. J Clin Neurosci 2020; 71: 129–134. [DOI] [PubMed] [Google Scholar]
- 20.Wiendl H, Spelman T, Butzkueven H, et al. Real-world disability improvement in patients with relapsing-remitting multiple sclerosis treated with natalizumab in the tysabri observational program. Mult Scler 2021; 27: 719–728. [DOI] [PubMed] [Google Scholar]
- 21.Kalincik T, Brown W, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: A cohort study. Lancet Neurol 2017; 16: 271–281. [DOI] [PubMed] [Google Scholar]
- 22.Rauma I, Mustonen T, Seppä J, et al. Safety of alemtuzumab in a nationwide cohort of Finnish multiple sclerosis patients. Journal of Neurology 2022; 269: 824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sladowska K, Kawalek P, Holko P, et al. Comparative safety of high-efficacy disease-modifying therapies in relapsing-remitting multiple sclerosis: A systematic review and network meta-analysis. Neurol Sci 2022 Sep; 43: 5479–5500. [DOI] [PubMed] [Google Scholar]
- 24.Hänninen K, Viitala M, Atula S, et al. Initial treatment strategy and clinical outcomes in Finnish MS patients: A propensity-matched study. J Neurol 2022; 269: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol 2019; 76: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buron MD, Chalmer TA, Sellebjerg F, et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: A nationwide cohort study. Neurology 2020; 95: e1041–e1051. [DOI] [PubMed] [Google Scholar]
- 27.Filippi M, Amato MP, Centonze D, et al. Early use of high-efficacy disease modifying therapies makes the difference in people with multiple sclerosis: An expert opinion. J Neurol 2022 Oct; 269: 5382–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soilu-Hänninen M, Päivärinta M, Sonninen Pet al. et al. Progressiivinen multifokaalinen leukoenkefalopatia natalitsumabihoidon komplikaationa. Lääketieteellinen Aikakauskirja Duodecim 2013; 129: 765–770. [PubMed] [Google Scholar]
- 29.Butzkueven H, Trojano M, Kappos L, et al. Clinical outcomes in patients who discontinue natalizumab therapy after 2 years in the tysabri® observational program (TOP). Mult Scler 2021; 27: 410–419. [DOI] [PubMed] [Google Scholar]
- 30.Iaffaldano P, Lucisano G, Pozzilli C, et al. Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain J Neurol 2015; 138: 3275–3286. [DOI] [PubMed] [Google Scholar]
- 31.Kappos L, Radue EW, Comi G, et al. Switching from natalizumab to fingolimod: A randomized, placebo-controlled study in RRMS. Neurology 2015; 85: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Re M, Capobianco M, Ragonese P, et al. Natalizumab discontinuation and treatment strategies in patients with multiple sclerosis (MS): A retrospective study from two Italian MS centers. Neurol Ther 2015; 4: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salhofer-Polanyi S, Baumgartner A, Kraus J, et al. What to expect after natalizumab cessation in a real-life setting. Acta Neurol Scand 2014; 130: 97–102. [DOI] [PubMed] [Google Scholar]
- 34.Mustonen T, Rauma I, Hartikainen P, et al. Risk factors for reactivation of clinical disease activity in multiple sclerosis after natalizumab cessation. Mult Scler Relat Disord 2020; 38: 101498. [DOI] [PubMed] [Google Scholar]
- 35. MS disease . Current Care Guidelines. Working group set up by the Finnish Medical Society Duodecim and the Finnish Cardiac Society. Helsinki: The Finnish Medical Society Duodecim, 2020, Accessed April 21, 2022. Available online at: www.kaypahoito.fi. [Google Scholar]