Abstract
Background
Starting combination antiretroviral therapy (cART) during primary human immunodeficiency virus type 1 (HIV-1) infection results in a smaller HIV-1 latent reservoir, reduced immune activation, and less viral diversity compared to starting cART during chronic infection. We report results of a 4-year study designed to determine whether these properties would allow sustained virological suppression after simplification of cART to dolutegravir (DTG) monotherapy.
Methods
EARLY-SIMPLIFIED is a randomized, open-label, noninferiority trial. People with HIV (PWH) who started cART <180 days after a documented primary HIV-1 infection with suppressed viral load were randomized (2:1) to DTG monotherapy with 50 mg daily or continuation of cART. The primary endpoints were the proportion of PWH with viral failure at 48, 96, 144, and 192 weeks; noninferiority margin was 10%. After 96 weeks, randomization was lifted and patients were permitted to switch treatment groups as desired.
Results
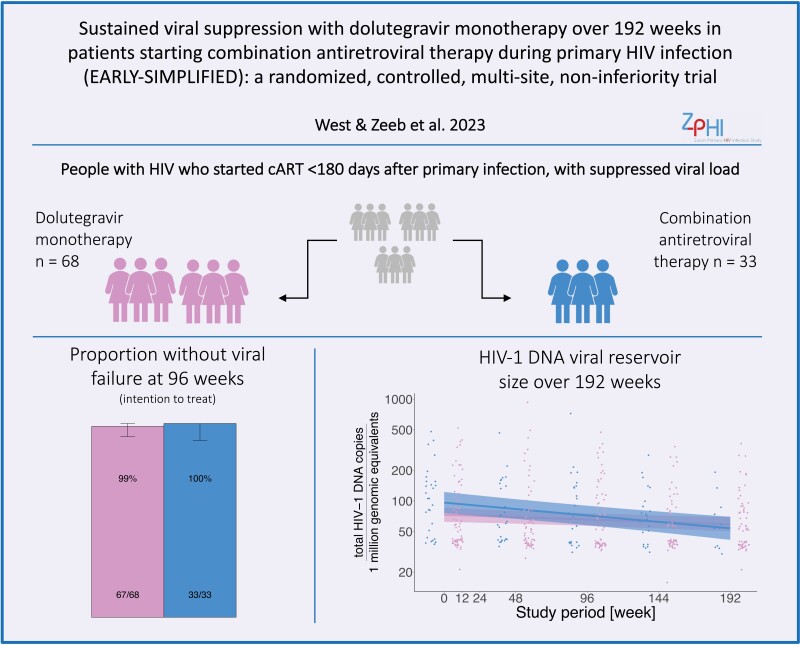
Of 101 PWH randomized, 68 were assigned to DTG monotherapy and 33 to cART. At week 96 in the per-protocol population, 64/64 (100%) showed virological response in the DTG monotherapy group versus 30/30 (100%) in the cART group (difference, 0.00%; upper bound of 95% confidence interval 6.22%). This demonstrated noninferiority of DTG monotherapy at the prespecified level. At week 192, the study end, no virological failure occurred in either group during 13 308 and 4897 person weeks of follow-up for the DTG monotherapy (n = 80) and cART groups, respectively.
Conclusions
This trial suggests that early cART initiation during primary HIV infection allows sustained virological suppression after switching to DTG monotherapy.
Keywords: primary HIV infection, dolutegravir, monotherapy, simplification, randomized controlled trial
EARLY-SIMPLIFIED is a 4-year open-label trial with randomized and observational phases showing noninferiority of dolutegravir monotherapy versus combination antiretroviral therapy in people with HIV-1 having initiated therapy during primary infection. At 192 weeks, no virological failure occurred in either group.
Graphical Abstract
Graphical Abstract.
(See the Editorial Commentary by Hocqueloux and Parienti on pages 1021–2.)
Long-term toxicity of combination antiretroviral therapy (cART), in particular due to nucleoside reverse transcriptase inhibitors (NRTIs), is a considerable cause of morbidity in people with human immunodeficiency virus type 1 (PWH) [1], as exemplified by the weight gain and emerging liver steatosis associated with tenofovir alafenamide treatment [2–4]. Hence, reducing the use of NRTIs is a potential benefit of approved newer dual antiretroviral therapy (ART) options including the combination of lamivudine and dolutegravir (DTG) as well as long-acting rilpivirine and cabotegravir [5], both of which have demonstrated noninferiority to cART in virologically suppressed patients [6, 7].
In contrast to DTG-based dual therapies, several randomized controlled trials that explored the efficacy of DTG monotherapy revealed inferiority compared to cART [8–10]. Importantly, all these DTG simplification studies concerned patients initiating cART during chronic HIV-1 infection. A recent meta-analysis pooling data from 4 randomized controlled trials investigating the efficacy of DTG monotherapy examined the factors associated with viral failure and showed strong associations for initiation of ART ≥90 days after acute HIV infection, CD4 T-cell nadir <350 cells/mm3, HIV RNA signal at baseline and reservoir size at baseline [11].
As the above-mentioned risk factors are greatly reduced in patients who start cART during primary HIV infection, we hypothesized that this patient group would include the best candidates to maintain viral suppression after switching to DTG monotherapy. This hypothesis was supported by previously published interim results of the EARLY-SIMPLIFIED trial [12]. EARLY-SIMPLIFIED is a randomized, open-label, noninferiority trial comparing DTG monotherapy to cART among patients from the Zurich Primary HIV Infection (ZPHI) and Swiss HIV Cohort (SHCS) Studies [13, 14]. These patients started their first cART within 6 months of the estimated date of infection and had been successfully treated with cART for at least 48 weeks. Indeed, we were previously able to demonstrate noninferiority of DTG monotherapy compared to cART over 48 weeks in this proof-of-concept study. The aim of the current study was to assess the long-term efficacy of DTG monotherapy over 192 weeks of follow-up within the EARLY-SIMPLIFIED population.
METHODS
Study Population
We recruited the study population from the ZPHI, a multi-centric observational study (ClinicalTrials.gov, ID NCT00537966), and the Swiss Cohort Study (SHCS) (www.shcs.ch), a large prospective study [13, 14]. Participants enrolled in the ZPHI have a documented primary HIV infection and are followed up clinically every 3 months. Primary HIV-1 infection was defined as published elsewhere [15]. Within the studies, detailed clinical, laboratory, socioeconomic, and treatment data are recorded.
Inclusion/Exclusion Criteria
Our inclusion criteria for the study were: 18 years of age or older, no previous antiretroviral therapy (ART) failure, no prior treatment interruption, no major integrase inhibitor resistance, at least 48 weeks of HIV-1 plasma RNA <50 copies/mL, and negativity for hepatitis B virus surface antigen. We excluded patients who were pregnant, currently breastfeeding, using drugs contraindicated with DTG, or who described a prior DTG intolerance.
Participants were discontinued from the study if they developed virological failure, any HIV-related clinical condition [16], any serious adverse event related to the study drug, if they missed 2 or more consecutive study visits or withdrew consent.
Ethical Approval
The local ethics committee approved the clinical trial according to the 2008 Declaration of Helsinki principles with the identification number KEK-ZH-EK-1452. Study participants provided written informed consent before enrolment. This study is registered with ClinicalTrials.gov, number NCT02551523.
Study Endpoints
The primary endpoint was noninferiority between DTG monotherapy and standard of care cART, defined as the proportion of viral failures in patients within the DTG monotherapy group versus the cART group at 48, 96, 144, and 192 weeks. We defined viral failure as ≥2 consecutive viral load measurements above 50 HIV-1 plasma RNA copies/mL over at least 14 days. We set the threshold for noninferiority of DTG monotherapy at a 10% margin.
According to the original study protocol, we planned a follow-up period of 48 weeks after randomization. This was extended in Amendment 3 to 192 weeks: in response to data demonstrating noninferiority of the monotherapy arm at 48 weeks, we considered it ethical to lift randomization to allow all enrolled patients the opportunity to benefit from monotherapy during the second phase of the study. Thus, from 96 weeks onward, patients switched between DTG monotherapy and cART groups as desired. The main outcome of noninferiority of DTG monotherapy versus cART therefore pertains only to follow-up until week 96 in the randomized setting. For all secondary outcomes, we considered the entire follow-up period of 192 weeks.
We defined our secondary endpoints as: CD4+ T-cell changes over time within and between study groups, the differences of adverse events (AEs) between the study groups (particularly serious adverse events [SAEs] and [S]AEs causally related to the study drug), weight change on DTG monotherapy, the HIV DNA reservoir size, and occurrence of blips (defined as 1 viral load measurement above 50 and below 400 HIV-1 plasma RNA copies/mL, succeeded within 30 days by a value below/equal to 50 HIV-1 plasma RNA copies/mL).
Adherence
At every study visit, adherence to study drugs was assessed by checking ART packets for the number of remaining pills as well as actively asking patients about frequency of missed doses.
Study Protocol Amendments
Over the study course we implemented 4 amendments, which are described in the Supplementary Data.
Measurement of the Latent HIV Reservoir
The absolute HIV-1 DNA copy number per 1 million genomic equivalents was quantified using an in-house total HIV-1 DNA assay as previously published [17] on the QIAcuity digital polymerase chain reaction (PCR) system (Qiagen) and described in the Supplementary Data.
Statistical Analysis: Randomized Design Up to Week 96
We performed the randomization procedure of the study groups using SecuTrial, as detailed in the interim analysis [12]. We calculated the primary outcome, that is, the exact upper 95% inferiority confidence interval boundary, between DTG monotherapy and cART after 96 weeks, with the R package “ExactCIdiff” [18]. We refined the grid-parameters until convergence at a precision of 0.00001.
Statistical Analysis: Observational Follow-Up Beyond Week 96
For the secondary endpoints we used linear mixed models with study week, from week 0 to 192, as the predictor and further adjusted for study group to see differences between them. For the respective trajectories, within each study group, we ran the models stratified. We reset all patients who switched from cART to DTG monotherapy after study initiation to week 0 and combined them into the third group, “Switcher.” We performed all statistical analyses for the primary and secondary endpoints in R version 4.0.2. We did not correct for multiple testing.
RESULTS
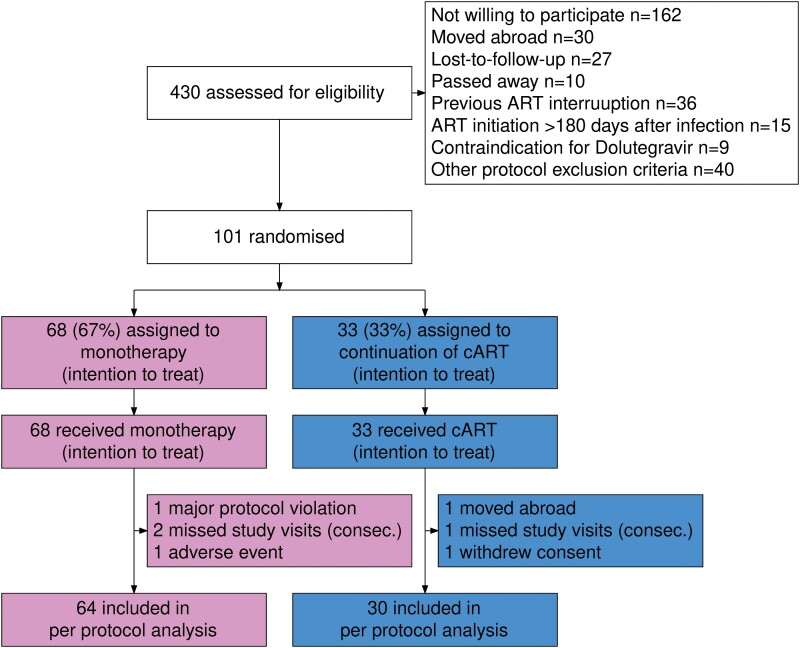
As described in our interim analysis [12], between November 2015 and March 2017, 430 PWH were assessed, and 101 were selected for participation in the study (Figure 1). Patients were randomized in a 2:1 ratio to DTG monotherapy (n = 68, 67%) or cART (n = 33, 33%), forming the basis for the intention to treat (ITT) analysis. As previously reported, participant baseline characteristics were well balanced between treatment groups (Table 1). Between study initiation and week 96, we excluded 4 patients in the DTG monotherapy group and 3 in the cART group due to adverse events, study protocol violations, missing visits, relocation, or withdrawal of consent, leaving 64 (68%) patients in the DTG monotherapy group and 30 (32%) in the cART group contributing to the per protocol analysis (PPI) (Figure 1). Overall, the total observed follow-up time was 18 205 weeks, of which 13 308 were on DTG monotherapy and 4897 on cART. Patients who switched from cART to DTG monotherapy after week 96 had a total follow-up time of 913 weeks.
Figure 1.
Trial profile up to week 96a. aData up to week 48 previously reported as part of the EARLY-SIMPLFIED interim analysis [12]. Abbreviations: ART, antiretroviral therapy; cART, combination antiretroviral therapy.
Table 1.
Baseline Characteristics of Study Participants Stratified by Study Arma
| Overall | cART | Monotherapy | Switchers | |
|---|---|---|---|---|
| n | 101 | 33 | 68 | 18 |
| Age (median [IQR]) | 42.00 [33.00–47.00] | 43.00 [35.00–46.00] | 42.00 [32.75–47.00] | 42.50 [35.25–46.00] |
| Gender male, n (%) | 97 (96.0) | 32 (97.0) | 65 (95.6) | 18 (100.0) |
| Ethnicity, n (%) | … | |||
| White | 93 (92.1) | 31 (93.9) | 62 (91.2) | 18 (100.0) |
| Black | 5 (5.0) | 1 (3.0) | 4 (5.9) | 0 (0.0) |
| Asian | 2 (2.0) | 0 (0.0) | 2 (2.9) | 0 (0.0) |
| Hispanic | 1 (1.0) | 1 (3.0) | 0 (0.0) | 0 (0.0) |
| HIV-1 transmission risk, n (%) | … | … | … | … |
| MSM | 84 (83.2) | 28 (84.8) | 56 (82.4) | 17 (94.4) |
| HET | 15 (14.9) | 5 (15.2) | 10 (14.7) | 1 (5.6) |
| Other | 2 (2.0) | 0 (0.0) | 2 (2.9) | 0 (0.0) |
| HIV-1 subtype Bb, n (%) | 63 (67.7) | 19 (59.4) | 44 (72.1) | 10 (58.8) |
| BMI (kg/m2) (median [IQR]) | 23.81 [22.39–26.56] | 24.16 [22.50–27.36] | 23.74 [22.10–26.23] | 23.68 [22.50–27.09] |
| Fiebig stage, n (%) | ||||
| I–II | 23 (22.8) | 6 (18.2) | 17 (25.0) | 3 (16.7) |
| III–IV | 11 (10.9) | 5 (15.2) | 6 (8.8) | 3 (16.7) |
| V–VI | 47 (46.5) | 17 (51.5) | 30 (44.1) | 10 (55.6) |
| Not determined | 20 (19.8) | 5 (15.2) | 15 (22.1) | 2 (11.1) |
| D from infection until ART start (median [IQR]) | 38.00 [28.00–77.50] | 36.00 [29.00–113.00] | 38.00 [27.50–73.00] | 35.50 [25.25–76.50] |
| Y on ART before study entry (median [IQR]) | 3.60 [1.96–5.98] | 3.27 [2.02–5.49 | 3.81 [1.93–6.08] | 3.56 [2.27–5.48] |
| CD4 cell count baseline (cells/µL) (median [IQR]) | 716 [584–918] | 669 [545–881] | 730 [610–920] | 722 [611–853] |
| CD4 cell count nadir (cells/µL) (median [IQR]) | 358 [265–486] | 329 [269–442] | 377 [263–496] | 302 [255–419] |
| DTG-based regimen at baseline, n (%) | 46 (45.5) | 13 (39.4) | 33 (48.5) | 7 (38.9) |
Data are median (interquartile range [IQR]) or n (%) and assessed at baseline (day of randomization).
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; cART, combination antiretroviral therapy; HET, heterosexual; HIV-1, human immunodeficiency virus type 1; MSM, men who have sex with men.
Data for cART and monotherapy groups previously reported as part of the EARLY-SIMPLFIED interim analysis [12].
Non-B subtypes: CRF01_AE, CRF02_AG, C, A, F, CRF12_BF, CRF20_BG-Recombinant.
Randomized Controlled Design Until Week 96
As the primary outcome, DTG monotherapy showed noninferiority compared to cART in the per-protocol analysis at 96 weeks (64/64 participants virally suppressed on DTG monotherapy vs 30/30 cART, 0%, 95% confidence interval [CI] [−100%, 6.22%]). Likewise, we confirmed noninferiority in the intention-to-treat analysis (67/68 DTG monotherapy vs 33/33 cART, 1.47%, 95% CI [−100%, 7.59%]). As already described in our interim publication [12], 1 viral failure occurred in a patient on monotherapy who was later excluded from the study as it was retrospectively noted the inclusion criteria had not been met. This participant was included in the intention-to-treat analysis. In addition, 3 patients in each group prematurely discontinued the study before reaching week 96: in the DTG monotherapy group 1 was due to weight gain and 2 were due to consecutive missed study visits and in the cART group 1 was due to withdrawal of consent, 1 was due to consecutive missed study visits, and 1 moved abroad. For a conservative estimate, we included these six patients without viral failure at the time when they left the study as not failed in the intention-to-treat analysis. We additionally performed an alternative analysis assuming viral failure in these 6 patients, which confirmed noninferiority at the prespecified level (64/68 DTG monotherapy vs 30/33 cART, −3.21%, 95% CI [−100%, 6.13%]) (Supplementary Figure 19).
Observational Follow-up Beyond Week 96
After lifting randomization at 96 weeks, 18 patients in the cART group chose to switch to DTG monotherapy (Supplementary Figure 1). No further episodes of viral failure were documented in either group. Two patients discontinued the study due to moving abroad, 1 in the cART group in week 109 and 1 in the DTG monotherapy group in week 145. One patient dropped out of the DTG monotherapy group in week 110 as his treatment was transferred to another center.
Safety
Of the 68 patients in the DTG monotherapy group, 17 (25%) experienced serious adverse events compared to 10 (30.3%) of the 33 on cART. No serious adverse event was classified as related to any ART regimen (Table 2). Study drug-related adverse events were seen in 15 out of 68 (22.1%) patients on DTG monotherapy and 10 out of 33 (30.3%) on cART. ART regimen change due to an adverse event was significantly more frequent in the cART group (5; 15.2%) compared to on DTG monotherapy (1; 1.5%) (for reasons see Supplementary Table 3).
Table 2.
Adverse Events Overall and Stratified by Study Arm at 192 Weeksa
| Overall (n (%)) | Current Therapy (n (%)) | Monotherapy (n (%)) | P value | Switchers (n (%)) | |
|---|---|---|---|---|---|
| n | 101 | 33 | 68 | 18 | |
| Any AE | 99 (98.0) | 32 (97.0) | 67 (98.5) | 1 | 17 (94.4) |
| Study drug-related AE | 24 (23.8) | 9 (27.3) | 15 (22.1) | .743 | 1 (5.6) |
| Any SAE | 27 (26.7) | 10 (30.3) | 17 (25.0) | .745 | 1 (5.6) |
| Antiretroviral therapy switch due to AE | 6 (5.9) | 5 (15.2) | 1 (1.5) | .023 | 0 (0.0) |
| Intensityb | |||||
| Mild | 98 (97.0) | 31 (93.9) | 67 (98.5) | .516 | 17 (94.4) |
| Moderate | 65 (64.4) | 22 (66.7) | 43 (63.2) | .907 | 4 (22.2) |
| Severe | 8 (7.9) | 3 (9.1) | 5 (7.4) | 1 | 0 (0.0) |
| Laboratory AE | 57 (56.4) | 16 (48.5) | 41 (60.3) | 0.364 | 7 (38.9) |
| Laboratory AE, intensityb | |||||
| Mild | 52 (51.5) | 14 (42.4) | 38 (55.9) | .29 | 6 (33.3) |
| Moderate | 5 (5.0) | 2 (6.1) | 3 (4.4) | 1 | 1 (5.6) |
| Arthralgia | 27 (26.7) | 8 (24.2) | 19 (27.9) | .877 | 0 (0.0) |
| Back pain | 28 (27.7) | 8 (24.2) | 20 (29.4) | .759 | 2 (11.1) |
| Depression | 13 (12.9) | 6 (18.2) | 7 (10.3) | .428 | 0 (0.0) |
| Diarrhea | 11 (10.9) | 5 (15.2) | 6 (8.8) | .537 | 0 (0.0) |
| Elective operation or intervention | 8 (7.9) | 4 (12.1) | 4 (5.9) | .486 | 0 (0.0) |
| Fatigue | 7 (6.9) | 2 (6.1) | 5 (7.4) | 1 | 0 (0.0) |
| Gastritis/GERD | 15 (14.9) | 6 (18.2) | 9 (13.2) | .721 | 1 (5.6) |
| Headache | 14 (13.9) | 5 (15.2) | 9 (13.2) | 1 | 2 (11.1) |
| Headache after lumbar puncture | 6 (5.9) | 3 (9.1) | 3 (4.4) | .628 | 0 (0.0) |
| Neoplasia | 4 (4.0) | 2 (6.1) | 2 (2.9) | .834 | 1 (5.6) |
| Psychosocial stress | 7 (6.9) | 2 (6.1) | 5 (7.4) | 1 | 0 (0.0) |
| Sexually transmitted infection | 46 (45.5) | 13 (39.4) | 33 (48.5) | .515 | 8 (44.4) |
| Skin rash | 24 (23.8) | 9 (27.3) | 15 (22.1) | .743 | 2 (11.1) |
| Sleeping disorder | 6 (5.9) | 1 (3.0) | 5 (7.4) | .679 | 0 (0.0) |
| Trauma | 22 (21.8) | 7 (21.2) | 15 (22.1) | 1 | 3 (16.7) |
| Viral URTI | 59 (58.4) | 17 (51.5) | 42 (61.8) | .444 | 8 (44.4) |
| Other infection (mild) | 35 (34.7) | 11 (33.3) | 24 (35.3) | 1 | 3 (16.7) |
| Other infection (moderate/severe) | 43 (42.6) | 12 (36.4) | 31 (45.6) | .506 | 4 (22.2) |
| Vitamin deficiency | 13 (12.9) | 4 (12.1) | 9 (13.2) | 1 | 3 (16.7) |
| Other | 72 (71.3) | 21 (63.6) | 51 (75.0) | .342 | 9 (50.0) |
Abbreviations: AE, adverse event; GERD, gastro-esophageal reflux disease; SAE, serious adverse event.
Data up to week 48 previously reported as part of the EARLY-SIMPLFIED interim analysis [12].
Mild indicates causing minimal symptoms and self-limiting, moderate indicates greater than minimal symptoms or requiring physician intervention but not meeting the criteria for SAE, severe indicates meeting the standard criteria for SAE.
HIV-1 DNA Reservoir
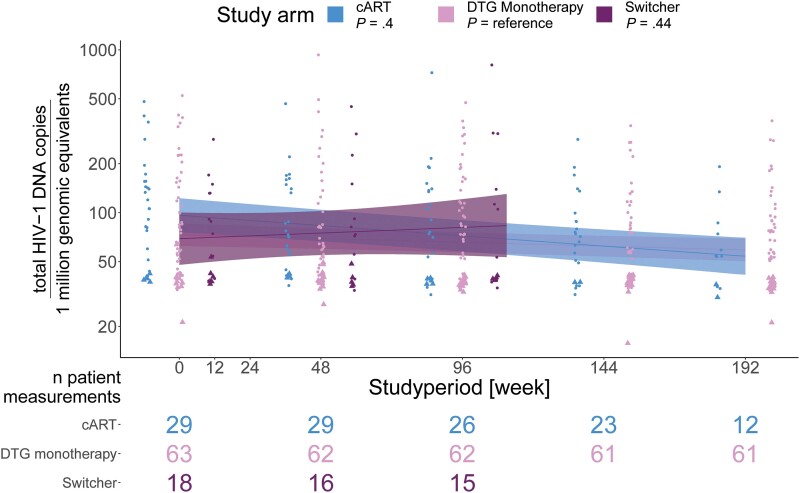
Patients in the cART group did not show a significantly greater decrease in HIV-1 DNA reservoir size over 192 weeks, compared to patients under DTG monotherapy (linear mixed model, P value .4) (Figure 2). At least 1 sample with a successful reservoir size measured was available from 63 patients in the DTG monotherapy group, 29 patients in the cART group, and 18 in the switcher group. The mean viral reservoir (log10 total HIV-1 DNA per 1 million genomic equivalents) decreased from week 0 to week 192 from 2 to 1.78 (95% CI difference .03, .4, P value .03) in the cART group, from 1.87 to 1.79 (95% CI difference −.02, .2, P value .1) in the DTG monotherapy group, and remained stable in the switcher group 1.84 to 1.9 (95% CI difference −.33, .2, P value .6).
Figure 2.
HIV-1 DNA viral reservoir size over 192 weeks in HIV-1 patients receiving DTG monotherapy (n = 63) or cART (n = 29). P values are calculated with a linear mixed model, using a random intercept model with unique patients, to assess the overall time trend with DTG monotherapy as the reference. Triangles indicate values below limit of detection. Data up to week 48 previously reported as part of the EARLY-SIMPLFIED interim analysis [12]. Abbreviations: cART, combination antiretroviral therapy; DTG, dolutegravir; HIV-1, human immunodeficiency virus type 1.
Blips
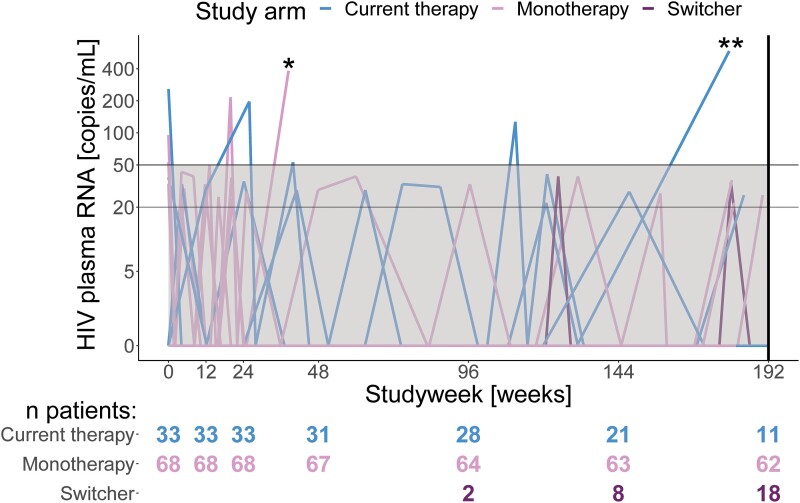
Three out of 68 patients on DTG monotherapy and 3 out of 33 in the cART group experienced blips, including 1 patient on cART twice (Figure 3). The proportional difference was not significant (prop. diff. 4.7%, 95% CI −8.5%, 17.9%, P value .63). In addition, 1 patient on cART had a single value of 586 HIV-1 plasma RNA copies/mL, which did not fit the formal definition of a blip or viral failure and returned to an unmeasurable value at the next measurement.
Figure 3.
HIV-1 RNA viral load over 192 weeks within HIV-1 patients receiving DTG monotherapy (n = 68) or cART (n = 33)a. Patients had the option to switch from current therapy (n = 18) to dolutegravir monotherapy, irrespective of the primary outcome (viral failure), which some patients did after week 96 or later. *One patient in the dolutegravir monotherapy group showed viral failure on dolutegravir monotherapy but was excluded from the study due to a major protocol violation. **This patient on combination anti-retroviral therapy showed a single HIV-1 plasma RNA of 586 copies/mL, which, although above the defined level of a blip, did not constitute viral failure. During the next study visit, which occurred after week 192, an undetectable viral load on the same therapy was measured. aData up to week 48 previously reported as part of the EARLY-SIMPLFIED interim analysis [12]. Abbreviations: DTG, dolutegravir; HIV-1, human immunodeficiency virus type 1.
Measurable HIV-1 plasma RNA below the definition of a blip (above 20 and below 50 copies/mL) occurred in 5 out of 33 patients on cART and 13 out of 68 on DTG monotherapy, which did not represent a significant difference (prop. diff. −4%, 95% CI −21.6%, 13.7%, P value .83).
Adherence
The levels of adherence during the study for the monotherapy, current therapy, and switcher groups were 99.79% (interquartile range [IQR] 99.79%–100.00%), 99.62% (IQR 99.51%–100.00%), and 99.83% (IQR 99.74%–100.00%), respectively (t test P value .21). In the monotherapy, current therapy and switcher groups 97% (95% CI 88.8%–99.5%), 90.9% (95% CI 78.3%–98.9%), and 99.83% (95% CI 78.1%–100%) of individuals reported adherence levels of 100% (Supplementary Figure 20).
Adherence below 100% was not associated with risk of virological failure.
Weight Gain
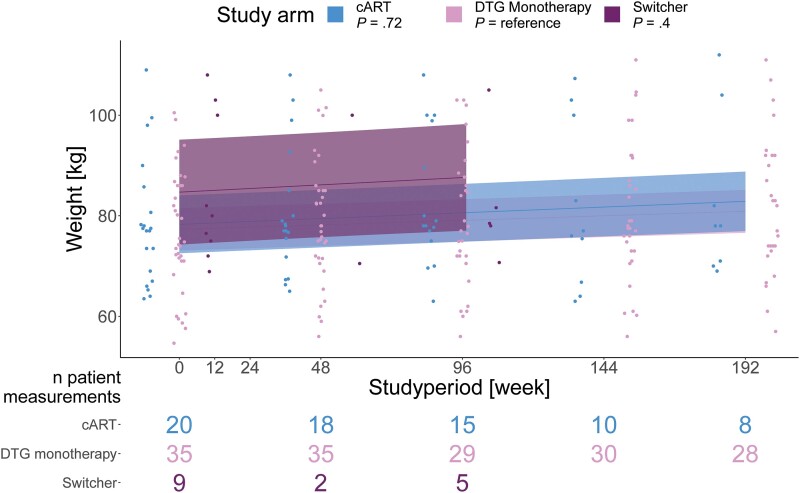
In an analysis restricted to patients without DTG intake prior to randomization, including 35 patients in the DTG monotherapy group and 20 in the cART group, a general weight increase was visible. However, over 192 weeks no significant difference in weight increase was visible between the study groups (Figure 4).
Figure 4.
Weight over 192 weeks for 55 randomized HIV-1 patients receiving DTG monotherapy (n = 35) or cART (n = 20). Patients switching from cART to DTG monotherapy after week 96 were included in the switcher group (n = 9) and had their timepoint reset to 0. Patients receiving DTG prior to randomization were excluded from this analysis. Shown are trajectories/P values stratified by study group and P values for differences in the trajectories between study groups, with DTG as the reference. P values are calculated with a linear mixed model, using a random intercept model with unique patients, to assess the overall time trend. The “n patient measurements” represent patients with useable measurements at the respective time. Abbreviations: cART, combination antiretroviral therapy; DTG, dolutegravir; HIV-1, human immunodeficiency virus type 1.
CD4+ T-Cell Level
Patients in both study groups showed an increase in their CD4+ T-cell count over the study period. However, there was no significant difference between the groups at 192 weeks (Supplementary Figure 18).
Changes In Metabolic Laboratory Parameters
The DTG monotherapy group showed a greater decrease in urine urea nitrogen (P value = .02) compared to the cART group after 192 weeks (Supplementary Tables 1 and 2, Supplementary Figure 2). In a range of further metabolic parameters there were no significant differences between DTG monotherapy and cART groups over the course of our follow-up (Supplementary Tables 1 and 2, Supplementary Figures 3–17).
DISCUSSION
We previously reported the first 48 weeks of our proof-of-concept EARLY-SIMPLIFIED randomized, open-label trial demonstrating noninferiority of DTG monotherapy to cART [12]. Our final results, including 96 weeks of randomized follow-up and a further 96 weeks of observation, demonstrate continued successful viral suppression in patients who initiated cART during primary HIV-1 infection, with no additional episodes of viral failure. We chose to extend EARLY-SIMPLIFIED and include this observational phase due to high demand from patients and the ethical arguments for monotherapy in response to the encouraging evidence for its noninferiority in the 48 week analysis.
Recent guidelines underscore a paradigm shift in HIV therapeutics towards dual ART in many patients, with the goal of limiting antiretroviral toxicity and costs [19–21]. By contrast, DTG monotherapy has been associated with unacceptably high levels of virological failure and the development of integrase inhibitor resistance in patients who started cART during chronic HIV-1 infection in several randomized studies [8–10], with risk increasing over time and reaching up to 8.9% at 48 weeks [22]. Relevant risk factors for this viral failure were elucidated in a recent meta-analysis and include low CD4-nadir, longer timespan between HIV-1 diagnosis and ART initiation and larger HIV-1 reservoir [11]. Our study addressed these factors by restricting participation to those patients having commenced cART during primary HIV-1 infection and showed no cases of viral failure in either per protocol treatment group during a total of 192 weeks of follow-up. Importantly, our study showed no significant difference in the trajectory of viral reservoir change over time between DTG monotherapy and cART groups, suggesting robust suppression of viral replication on monotherapy below the limit of detection.
The overall rates of adverse events, including data from randomized and extended follow-up phases, were similar across treatment groups, indicating comparable safety of dolutegravir monotherapy to cART over a protracted follow-up period of up to four years per patient. However, significantly fewer patients in the monotherapy group experienced adverse events leading to discontinuation of their antiretroviral regimen, suggesting better tolerability of monotherapy than cART. Both groups showed weight increase, which is expected among patients on effective ART over a follow-up of 4 years, as has been documented in large observational studies [23, 24]. A contribution of DTG to this increase, as previously described [2], is likely.
The major strengths of EARLY-SIMPLIFIED are that it provides the longest follow-up of any DTG monotherapy study, the detail in which study participants could be described as well as the longitudinal characterization of the latent reservoir size on DTG monotherapy. The study is weakened by our decision to limit the randomized phase to 96 weeks, although we believe the switch to an observational design was justified in the interest of participants. In addition, our findings have limited generalizability due to our highly selected patient population.
We are aware of differences in reservoir size compared to our previous report [12]. For the current analysis we used digital PCR instead of droplet digital PCR as previously. We assume the difference is due to the lower sensitivity of digital PCR especially at very low reservoir sizes. However, while the baseline differs between study groups, the overall trend is ultimately the same. Taken together with the meta-analysis by Fournier et al [11], our study strongly suggests that the size of the reservoir may matter for treatment outcome in PWH. To date, this concept has never influenced the design of clinical trials or therapy as a predictor for failure because for reasons of simplicity all PWH tend to be treated alike. However, given the shift toward treating patients rapidly after diagnosis, the fraction of PWH harboring a limited reservoir size will increase and these individuals potentially could be treated with a single drug. The impact of reservoir size may also be highly relevant for patients on long-acting drugs; thus, in an optimal setting, this should be assessed, for example, by total or intact proviral PCR-based DNA assays, which are feasible to conduct in larger patient populations [17]. Due to the lack of viral failure in our study, our data alone cannot define appropriate reservoir cut-off values for clinical use. However, in the meta-analysis [11] also including our patients, a proviral DNA load under 2.7 log10 copies/million PBMC in conjunction with a CD4 nadir above 350 cells/µL showed good prediction for treatment success in patients on monotherapy.
In conclusion, this proof-of-concept study underscores the differing ART requirements between PWH and the need for patient stratification according to predictors of viral failure with the goal of minimizing ART toxicity. In light of robust evidence for the efficacy and low side-effect profile of dual ART with DTG and lamivudine, we see no widespread indication for DTG monotherapy. However, we believe our study contributes to existing evidence that triple-ART represents over-treatment of HIV infection in a significant proportion of patients. We hope to pave the way for additional work to reduce ART burden by patient stratification according to latent reservoir size or duration of active infection before starting therapy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Emily West, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Marius Zeeb, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Christina Grube, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Herbert Kuster, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Katrin Wanner, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Thomas Scheier, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Kathrin Neumann, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Lisa Jörimann, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Benjamin Hampel, Checkpoint Zurich, Zurich, Switzerland; Department of Public and Global Health, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Karin J Metzner, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Roger D Kouyos, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Dominique L Braun, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Huldrych F Günthard, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, University of Zurich, Zurich, Switzerland; Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Notes
Author contributions. The study was designed by D. L. B. and H. F. G. Data acquisition was done by D. L. B., E. W., C. G., H. K., K. W., T. S., K. N., L. J., B. H., K. M., and H. F. G. Statistical analysis was performed by M. Z. and R. D. K. H. F. G. supervised the study. E. W. wrote the first draft. All investigators contributed to data collection and interpretation of the data, reviewed drafts of the manuscript, and approved the final manuscript.
Acknowledgments. The authors are grateful to all patients who participated in the ZPHI Study; the various human immunodefiency virus (HIV) physicians from the ZPHI study for their dedicated patient care; Christine Leemann and Dominique Klimpel for excellent laboratory assistance; the Institute for Medical Virology of the University of Zurich for the excellent laboratory work. They thank Roche Switzerland Ltd. for partially funding the study.
Data sharing agreement. The study protocol and individual participant data that underlie the results reported in this article will be available after de-identification following article publication to investigators whose proposed use of the data has been approved by an independent review committee to achieve aims in the approved proposal. Proposals should be directed to huldrych.guenthard@usz.ch; to gain access, data requestors will need to sign a data access agreement.
Financial support. This work was supported by the Swiss National Science Foundation (SNF) (grant number 179571 to H. F. G.); and the University of Zurich's Clinical Research Priority Program's Viral Infectious Diseases: Zurich Primary HIV Infection Study (ZPHI) to H. F. G. and D. L. B. R. D. K. was supported by the SNF (grant numbers PZ00P3-142411 and BSSGI0_155851). Roche Diagnostics (Switzerland) Ltd. provided free of charge 480 tests of the CAP/CTM HIV version 2. Roche had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Keiser O, Fellay J, Opravil M, et al. Adverse events to antiretrovirals in the Swiss HIV cohort study: effect on mortality and treatment modification. Antivir Ther 2007; 12:1157–64. [PubMed] [Google Scholar]
- 2. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bischoff J, Gu W, Schwarze-Zander C, et al. Stratifying the risk of NAFLD in patients with HIV under combination antiretroviral therapy (cART). EClinicalMedicine 2021; 40:101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riebensahm C, Berzigotti A, Surial B, et al. Factors associated with liver steatosis in people with human immunodeficiency virus on contemporary antiretroviral therapy. Open Forum Infect Dis 2022; 9:ofac538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European AIDS Clinical Society Guidelines Version 11.0, October 2021. Available at https://www.eacsociety.org/guidelines. Accessed 8 December 2022.
- 6. van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis 2020; 71:1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-Acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–23. [DOI] [PubMed] [Google Scholar]
- 8. Wijting I, Rokx C, Boucher C, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV 2017; 4:e547–54. [DOI] [PubMed] [Google Scholar]
- 9. Blanco JL, Rojas J, Paredes R, et al. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73:1965–71. [DOI] [PubMed] [Google Scholar]
- 10. Hocqueloux L, Raffi F, Prazuck T, et al. Dolutegravir monotherapy versus dolutegravir/Abacavir/lamivudine for virologically suppressed people living with chronic human immunodeficiency virus infection: the randomized noninferiority MONotherapy of TiviCAY trial. Clin Infect Dis 2019; 69:1498–505. [DOI] [PubMed] [Google Scholar]
- 11. Fournier AL, Hocqueloux L, Braun DL, et al. Dolutegravir monotherapy as maintenance strategy: a meta-analysis of individual participant data from randomized controlled trials. Open Forum Infect Dis 2022; 9:ofac107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braun DL, Turk T, Tschumi F, et al. Noninferiority of simplified dolutegravir monotherapy compared to continued combination antiretroviral therapy that was initiated during primary human immunodeficiency virus infection: a randomized, controlled, multisite, open-label, noninferiority trial. Clin Infect Dis 2019; 69:1489–97. [DOI] [PubMed] [Google Scholar]
- 13. Rieder P, Joos B, Scherrer AU, et al. Characterization of human immunodeficiency virus type 1 (HIV-1) diversity and tropism in 145 patients with primary HIV-1 infection. Clin Infect Dis 2011; 53:1271–9. [DOI] [PubMed] [Google Scholar]
- 14. Scherrer AU, Traytel A, Braun DL, et al. Cohort profile update: the Swiss HIV Cohort Study (SHCS). Int J Epidemiol 2022; 51:33–4j. [DOI] [PubMed] [Google Scholar]
- 15. Braun DL, Marzel A, Steffens D, et al. High rates of subsequent asymptomatic sexually transmitted infections and risky sexual behavior in patients initially presenting with primary human immunodeficiency virus-1 infection. Clin Infect Dis 2018; 66:735–42. [DOI] [PubMed] [Google Scholar]
- 16. From the Centers for Disease Control and Prevention . 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA 1993; 269:729–30. [PubMed] [Google Scholar]
- 17. Bachmann N, von Siebenthal C, Vongrad V, et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun 2019; 10:3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shan G, Wang W. ExactCIdiff: an R package for computing exact confidence intervals for the difference of two proportions. R Journal 2013;5:62–70. [Google Scholar]
- 19. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA panel. JAMA 2023;329:63–84. [DOI] [PubMed] [Google Scholar]
- 21. Ryom L, De Miguel R, Cotter AG, et al. Major revision version 11.0 of the European AIDS Clinical Society guidelines 2021. HIV Med 2022; 23:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wandeler G, Buzzi M, Anderegg N, et al. Virologic failure and HIV drug resistance on simplified, dolutegravir-based maintenance therapy: systematic review and meta-analysis. F1000Res 2018; 7:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bansi-Matharu L, Phillips A, Oprea C, et al. Contemporary antiretrovirals and body-mass index: a prospective study of the RESPOND cohort consortium. Lancet HIV 2021; 8:e711–22. [DOI] [PubMed] [Google Scholar]
- 24. Surial B, Mugglin C, Calmy A, et al. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med 2021; 174:758–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.