Abstract
Monotherapy with isoniazid or amikacin or clarithromycin or combinations of two of these drugs showed nil to modest therapeutic activity in beige mice infected with Mycobacterium avium. However, the combination of all three, isoniazid-amikacin-clarithromycin, markedly reduced CFUs in both spleens and lungs after 91 days of infection.
Organisms belonging to the Mycobacterium avium-Mycobacterium intracellulare complex (MAC) may cause disseminated infections in patients with AIDS (8, 15). The Public Health Service Task Force on Prophylaxis and Therapy for MAC recommended clarithromycin (CLA)-containing regimens for therapy of these infections (11, 12). Isoniazid (INH), a powerful bactericidal drug largely used for the treatment of tuberculosis (14), was considered not to be effective for the therapy of MAC infections (6, 11, 12). Indeed, INH is inactive in vitro against the majority of MAC isolates (5, 7) and in general shows no demonstrable synergy with other drugs in its anti-MAC activity (3, 6, 13). However, in a previous study by our group, some activity of INH alone or in combination was observed in MAC-infected human macrophages (3). The purpose of the present investigation was to determine the in vivo activity of INH, used either alone or in combination with other drugs, against MAC infection in beige mice (4).
Two clinical isolates of MAC with different drug susceptibilities recovered from AIDS patients, namely, MAC strains 900 and 905 (3) (transparent colonies), were chosen for this study. MICs, as determined by the twofold agar dilution technique on Middlebrook 7H10 agar (Difco Laboratories, Detroit, Mich.), (2, 5) were, respectively: CLA, 1 and 8 μg/ml; amikacin (AMI), 1 and 8 μg/ml; ethambutol (EMB), 4 and 4 μg/ml; and INH, 2 and 4 μg/ml. Male beige mice (Charles River, Calco, Lecco, Italy) were infected intraperitoneally with 0.2-ml portions of bacterial suspensions containing approximately 107 CFUs. On day 1 after infection, six mice were sacrificed and the numbers of CFUs in the spleens, lungs, and livers were determined. Organs were aseptically removed, homogenized in Middlebrook 7H9 broth (Difco), and ultrasonicated for 10 s. To enumerate CFUs, appropriate dilutions of the homogenates were plated onto Middlebrook 7H10 agar and colonies were counted after 2 weeks of incubation at 37°C in a humidified 5% CO2 atmosphere. The remaining mice were allocated to untreated (control) groups and various drug-treated groups. The following drugs, either alone or in combinations, were given subcutaneously five times weekly: CLA, 50 mg/kg of body weight (9); AMI, 100 mg/kg (9); EMB, 100 mg/kg (1); and INH, 50 mg/kg (10). Untreated mice were injected with saline. At various times, some mice were sacrificed for CFU determination; to reduce the carryover effect of drugs in the organs, mice were sacrificed 72 h after the last dose.
CFU counts of MAC strain 900 in the spleens, lungs, and livers of mice receiving monotherapy with CLA, EMB, AMI, or INH for 56 days are shown in Table 1. The organism efficiently multiplied in control mice, as shown by a CFU increase of approximately 3 log10 CFUs in spleens and livers and 2 log10 CFUs in lungs. No mortality was observed both in untreated and treated mice (six mice/group). Compared to day 1 counts, bactericidal effects of CLA, EMB, AMI, and INH in spleens and livers (P < 0.01) (Student’s t test) were observed. In lungs, while CLA, AMI, and EMB were bacteriostatic, INH failed to limit mycobacterial proliferation. These results are in keeping with current knowledge on relevant anti-MAC activities of CLA, AMI, and EMB (9) alone but also showed some in vivo activity of INH. To investigate further this novel finding, in particular whether it could be dependent on the strain and the length of the treatment, and also to evaluate the potential use of INH in combined regimens, a MAC strain (strain 905) which is more resistant to those drugs than MAC 900 was tested.
TABLE 1.
CFU counts in different organs in MAC 900-infected mice treated with single drugs for 56 days
| Treatment group (dose [mg/kg]) | Log10 CFUs ± SD
|
||
|---|---|---|---|
| Spleen | Liver | Lung | |
| Control | |||
| Day 1 | 5.48 ± 0.08 | 5.49 ± 0.13 | 4.27 ± 0.19 |
| Day 28 | 7.94 ± 0.09 | 7.26 ± 0.06 | 5.55 ± 0.21 |
| Day 56 | 8.48 ± 0.09 | 8.51 ± 0.05 | 6.45 ± 0.14 |
| CLA (50) | 4.60 ± 0.27 | 4.56 ± 0.16 | 4.25 ± 0.24 |
| EMB (100) | 4.90 ± 0.24 | 4.69 ± 0.20 | 4.41 ± 0.38 |
| AMI (100) | 5.01 ± 0.24 | 5.14 ± 0.10 | 4.35 ± 0.14 |
| INH (50) | 5.09 ± 0.31 | 4.76 ± 0.08 | 4.92 ± 0.26 |
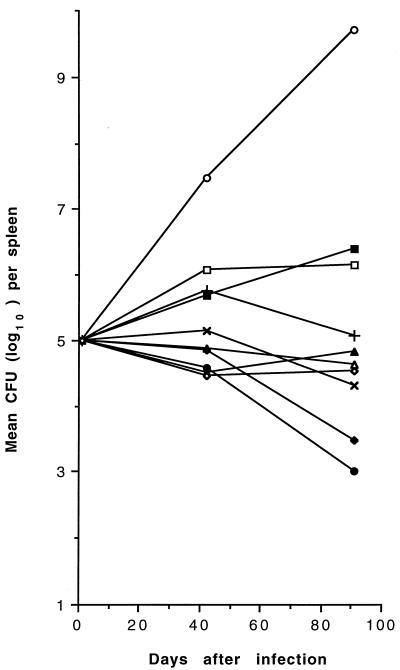
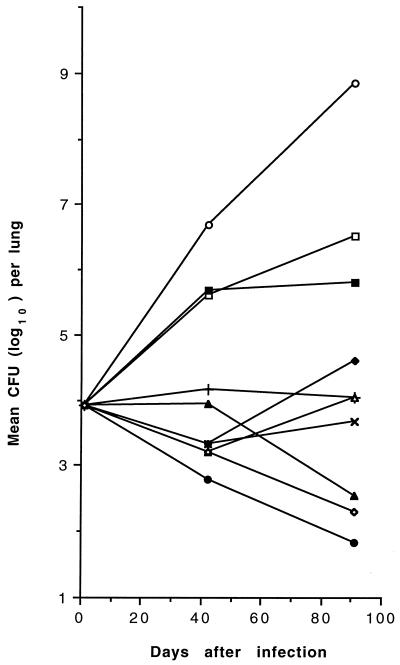
CFU counts in mice infected with MAC 905 receiving therapy with CLA, AMI, EMB, INH, INH-CLA, INH-AMI, CLA-AMI, INH-AMI-CLA, and INH-EMB-CLA for 91 days are shown in Fig. 1 (spleens) and Fig. 2 (lungs). MAC efficiently multiplied in control mice, with a mean increase of 4.71 and 4.93 log10 CFUs in spleens and lungs, respectively. During the period of observation, 3 (17.6%) of the 17 untreated mice died, while in the drug-treated groups (10 to 16 mice per group), mortality ranged from 0 to 42.9%; mortality of mice treated with all three drugs, INH-AMI-CLA, was 18.7%. Compared with day 1 counts, monotherapy with EMB or INH partially inhibited MAC growth while CLA was bacteriostatic in spleens and lungs and AMI displayed modest but significant degrees of bactericidal activity (P < 0.05) in spleens. While the two-drug combination INH-CLA was bacteriostatic in both organs, INH-AMI and CLA-AMI were bactericidal (P < 0.01) in lungs and spleens, respectively, and regrowth was consistently observed after day 42 in spleens and lungs treated with INH-AMI and CLA-AMI, respectively. Unlike the two-drug combinations, the three-drug combination INH-AMI-CLA was significantly (P < 0.01) bactericidal in both organs and caused ≥2 and ≥6.7 log10 CFU reductions in comparison with day 1 and day 91 control CFUs, respectively. The three-drug combination INH-EMB-CLA was less effective than INH-AMI-CLA in both organs. No MAC 905 drug-resistant mutant was selected in spleens after 91 days of therapy with combined regimens, as observed by plating dilutions of homogenates onto Middlebrook 7H10 medium containing drug concentrations corresponding to eight times the MICs (results not shown).
FIG. 1.
Mean CFU counts in the spleens of untreated and drug-treated MAC 905-infected beige mice. Standard deviations are not shown for clarity. Mice (10 to 16 per group) were inoculated intraperitoneally with approximately 107 organisms, and treatments were begun 1 day later. Symbols: ○, control; +, CLA; ×, AMI; □, INH; ▪, EMB; ▵, INH-CLA; ▴, INH-AMI; ⧫, CLA-AMI; •, INH-AMI-CLA; ◊, INH-EMB-CLA.
FIG. 2.
Mean CFU counts in the lungs of untreated and drug-treated MAC 905-infected beige mice. Standard deviations are not shown for clarity. Mice (10 to 16 per group) were inoculated intraperitoneally with approximately 107 organisms, and treatments were begun 1 day later. Symbols: ○, control; +, CLA; ×, AMI; □, INH; ▪, EMB; ▵, INH-CLA; ▴, INH-AMI; ⧫, CLA-AMI; •, INH-AMI-CLA; ◊, INH-EMB-CLA.
The finding that the activity of INH alone was modest or nil is possibly related to the MIC for the strain. Indeed, MAC strain 101 (MIC of INH of 12.5 μg/ml) (13) was inhibited at a lower level than MAC strain 905 in beige mice, but the results of those researchers are hardly comparable to our own results due to differences in dosage and duration of treatment. These investigators demonstrated that the combination of INH with the aminoglycoside streptomycin consistently decreased MAC strain 101 CFUs (13). Our study shows that the combination of INH with the aminoglycoside AMI is significantly more active than AMI alone in lungs (P < 0.01), but we found that the addition of CLA to INH-AMI markedly decreased CFUs in both organs. The strong activity in vivo of the combination INH-AMI-CLA is in good accordance with our previous results in human macrophages (3). Coupled with the fact that this therapeutic activity is seen in mice with deficiencies of natural immunity, the data support the notion that this combination could be useful for therapy.
Acknowledgments
We thank Antonio Cassone, Istituto Superiore di Sanità, Rome, Italy, for help in reviewing the manuscript. We also thank Raffaela Teloni, Istituto Superiore di Sanità, for valuable technical assistance.
This work was supported in part by the Italian AIDS Project, Istituto Superiore di Sanità-Ministero della Sanità (grant 940/N).
REFERENCES
- 1.Bermudez L E, Kolonowski P, Young L S, Inderlied C B. Activity of KRM 1648 alone or in combination with ethambutol or clarithromycin against Mycobacterium avium in beige mouse model of disseminated infection. Antimicrob Agents Chemother. 1994;38:1844–1848. doi: 10.1128/aac.38.8.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fattorini L, Hu C Q, Jin S H, Santoro C, Tsang A Y, Mascellino M T, Mandler F, Orefici G. Activity of antimicrobial agents against Mycobacterium avium-intracellulare complex (MAC) strains isolated in Italy from AIDS patients. Zentralbl Bakteriol. 1992;276:512–520. [PubMed] [Google Scholar]
- 3.Fattorini L, Li B, Piersimoni C, Tortoli E, Xiao Y, Santoro C, Ricci M L, Orefici G. In vitro and ex vivo activities of antimicrobial agents used in combination with clarithromycin, with or without amikacin, against Mycobacterium avium. Antimicrob Agents Chemother. 1995;39:680–685. doi: 10.1128/AAC.39.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangadharam P R J. Beige mouse model for Mycobacterium avium complex disease. Antimicrob Agents Chemother. 1995;39:1647–1654. doi: 10.1128/aac.39.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heifets L B. Susceptibility testing of Mycobacterium avium complex isolates. Antimicrob Agents Chemother. 1996;40:1759–1767. doi: 10.1128/aac.40.8.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heifets L B, Iseman M D. Individualized therapy versus standard regimens in the treatment of Mycobacterium avium infections. Am Rev Respir Dis. 1991;144:1–2. doi: 10.1164/ajrccm/144.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Inderlied C B. Antimycobacterial agents: in vitro susceptibility testing, spectrums of activity, mechanisms of action and resistance, and assays for activity in biological fluids. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 134–197. [Google Scholar]
- 8.Inderlied C B, Kemper C A, Bermudez L E M. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji B, Lounis N, Truffot-Pernot C, Grosset J. Effectiveness of various antimicrobial agents against Mycobacterium avium complex in the beige mouse model. Antimicrob Agents Chemother. 1994;38:2521–2529. doi: 10.1128/aac.38.11.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klemens S P, DeStefano M S, Cynamon M H. Therapy of multidrug-resistant tuberculosis: lessons from studies with mice. Antimicrob Agents Chemother. 1993;37:2344–2347. doi: 10.1128/aac.37.11.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korvick J A, Benson C A. Advances in the treatment and prophylaxis of Mycobacterium avium complex in individuals infected with human immunodeficiency virus. In: Korvick J A, Benson C A, editors. Mycobacterium avium-complex infection. Progress in research and treatment. New York, N.Y: Marcel Dekker Inc.; 1996. pp. 241–262. [Google Scholar]
- 12.Masur H. Recommendations on prophylaxis and therapy for disseminated Mycobacterium avium complex disease in patients infected with the human immuno-deficiency virus. N Engl J Med. 1993;329:898–904. doi: 10.1056/NEJM199309163291228. [DOI] [PubMed] [Google Scholar]
- 13.Reddy M V, Srinivasan S, Gangadharam P R J. In vitro and in vivo synergistic effect of isoniazid with streptomycin and clofazimine against Mycobacterium avium complex (MAC) Tubercle Lung Dis. 1994;75:208–212. doi: 10.1016/0962-8479(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 14.Sepkowitz K A, Raffalli J, Riley L, Kiehn T E, Amstrong D. Tuberculosis in the AIDS era. Clin Microbiol Rev. 1995;8:180–199. doi: 10.1128/cmr.8.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torriani F J, Behling C A, McCutchan J A, Haubrich R H, Havlir D V. Disseminated Mycobacterium avium complex: correlation between blood and tissue burden. J Infect Dis. 1996;173:942–949. doi: 10.1093/infdis/173.4.942. [DOI] [PubMed] [Google Scholar]