Abstract
Objective:
Patients with medically treated type B aortic dissection (TBAD) remain at significant risk for late adverse events (LAEs). We hypothesize that not only initial morphological features, but also their change over time at follow-up are associated with LAEs.
Material and Methods:
Baseline and 188 follow-up CT scans with a median follow-up time of 4 years (range 10 days to 12.7 years) of 47 patients with acute uncomplicated TBAD were retrospectively reviewed. Morphologic features (n=8) were quantified at baseline and each follow-up. Medical records were reviewed for LAEs, which were defined according to current guidelines. To assess the effects of changes of morphological features over time, the linear mixed effects models were combined with Cox proportional hazards regression for the time- to-event outcome using a joint modeling approach.
Results:
LAE occurred in 21 of 47 patients after a median time of 6.6 (95% CI: 5.1 – 11.2) years. Among the 8 investigated morphologic features, the following 3 features showed strong association with LAE: Increase of partial false lumen thrombosis area (HR=1.39 [1.18–1.66] per cm2 increase; p<0.001), increase of major aortic diameter (HR=1.24 [1.13–1.37] per mm increase; p<0.001) and increase of circumferential extent of false lumen (HR=1.05 [1.01–1.10] per ° increase; p<0.001)
Conclusions:
In medically treated type B aortic dissection, increase in aortic diameter, new or increased partial false lumen thrombosis area and increase of circumferential extent of false lumen are strongly associated with LAEs.
Central Image
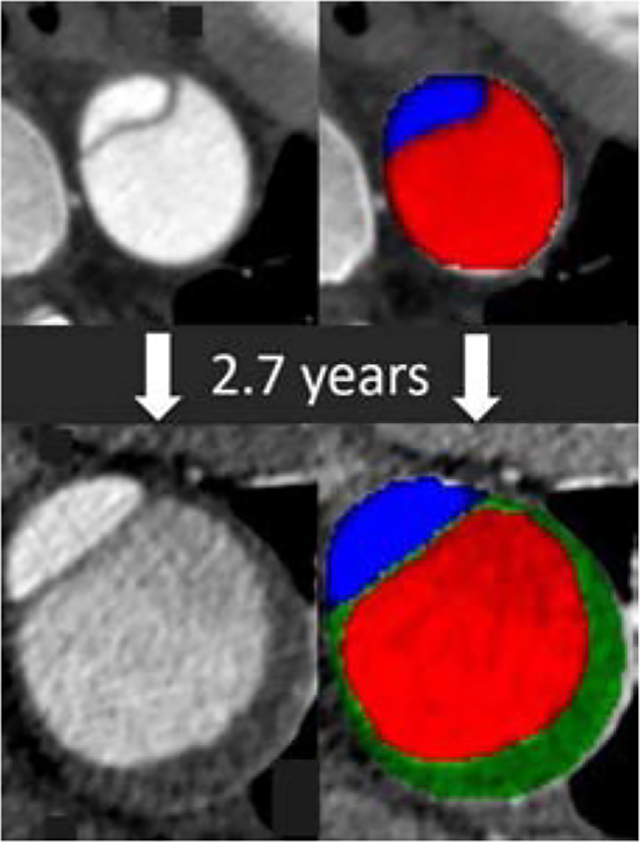
Native and segmented CT images show new thrombus (green) in a growing false lumen (red)
Central Message
Increase of maximum diameter, and increase or new partial false lumen thrombosis are associated with late adverse events in uncomplicated type B aortic dissections.
Introduction
Although thoracic endovascular aortic repair (TEVAR) has recently become a Food and Drug Administration (FDA) approved alternative to medical management in patients with acute uncomplicated type B aortic dissection, it is becoming increasingly clear that only a subset of high-risk patients may benefit from the favorable effects of early TEVAR on aortic remodeling (1). For patients with a low likelihood of developing late complications, the small but potentially devastating procedural risks of TEVAR – such as stroke, paraplegia or retrograde type A dissection – may not be warranted (2, 3). Risk stratification is thus highly desirable in this population and several groups have suggested the use of morphologic parameters extracted from imaging data to guide therapy (4–7).
Because morphologic parameters can change over time, an important prerequisite for any clinically useful risk prediction model for patients with uncomplicated type B aortic dissection is the ability to capture the evolution of these morphologic features over time and – if necessary – update an initial risk estimate with information obtained at each follow-up (8). The evolution of morphologic risk factors over time and the implication for future risk in patients with initially uncomplicated type B aortic dissection is currently unknown, however, with the exception of aortic diameter, which can also assume the role of an outcome variable in the form of a predefined size threshold for intervention (8).
The purpose of this study was to investigate if currently known or presumed morphologic risk factors change over time on serial imaging, and if such changes are independently associated with a risk for future events in patients with initially uncomplicated type B aortic dissection.
METHODS
This study was approved by our institutional review board. The requirement for written informed consent was waived due to the retrospective nature of this investigation.
Subjects
Patients were selected from an existing cohort of retrospectively identified patients presenting with acute, uncomplicated type B aortic dissection between January 2003 and 2012 (5, 8) (Figure 1). Patients with intramural hematoma were excluded. For the purposes of this investigation and due to the time consuming manual processing of serial, high-resolution imaging datasets, we restricted our analysis to the subjects of one of the participating aortic centers with follow-up until April 2017. Patients were included if they survived the index hospitalization under medical management without the development of acute complications within 30 days, including death, aortic rupture or signs of impending rupture, organ or limb ischemia, and uncontrollable pain or hypertension, and if at least one follow-up imaging study was available. Patient demographics, clinical characteristics (including history of hypertension, diabetes mellitus, or smoking), clinical course, and interventions were retrieved from patient electronic medical records (Table 1). Late adverse events (LAE) were defined as any of the following, occurring at least 30 days after the initial event: aneurysmal degeneration of the aorta (defined as maximum aortic diameter of more than 6 cm), rapid growth of more than 0.5 cm in a 6 month period, new dissection, rupture, malperfusion, or death. Patients were followed until the occurrence of a LAE, intervention or last available encounter on record.
Figure 1:
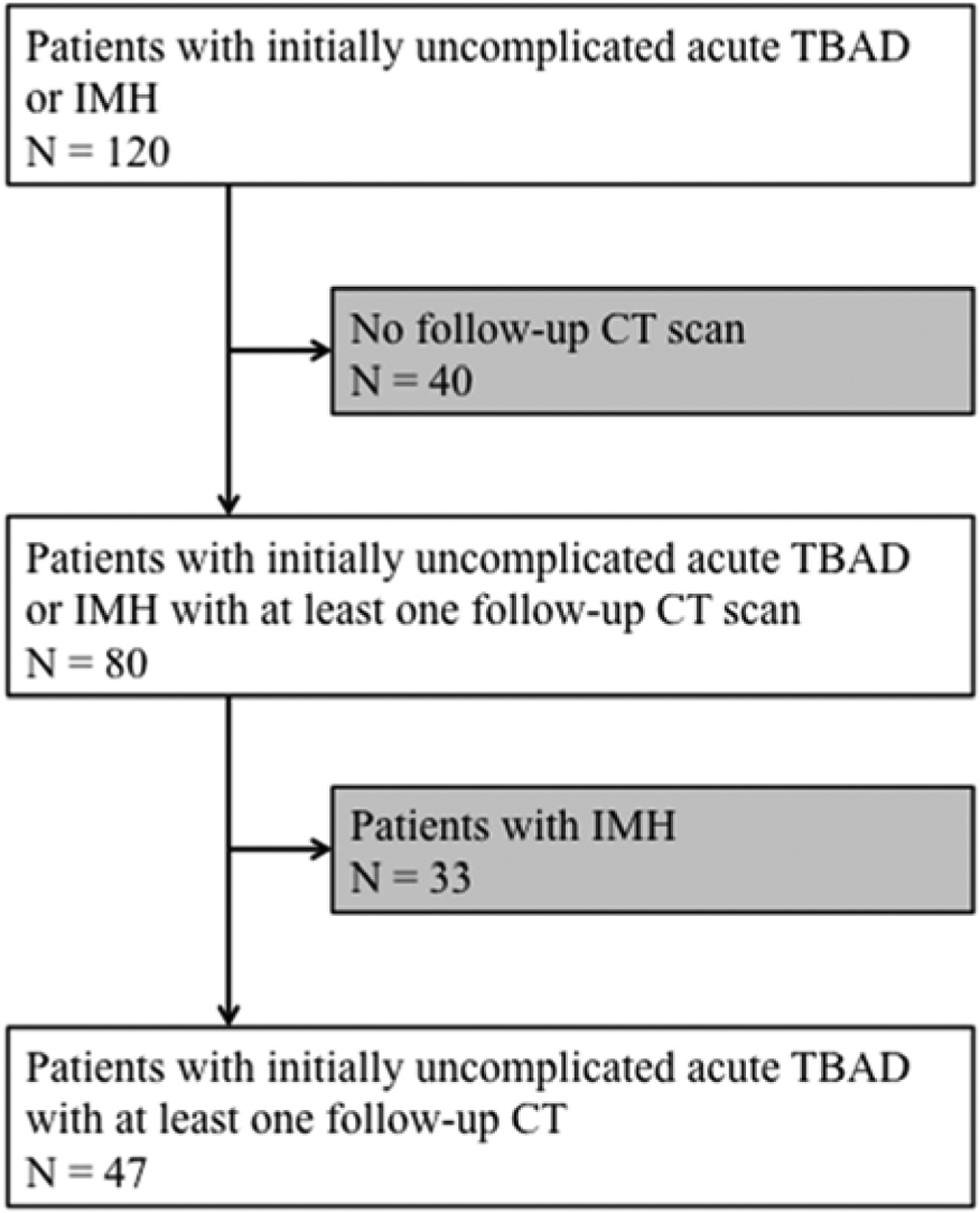
A consort diagram of the study population. TBAD: type B aortic dissection; IMH: intramural hematoma; CT: computed tomography.
Table 1.
Patient characteristics
| Patients characteristics | Total cohort |
|---|---|
| Number | N = 47 |
| Age in years (Median, IQR) | 50 (37– 59.5) |
| Sex (male) | 31 (66 %) |
| Hypertension | 43 (91%) |
| Hyperlipidemia | 14 (30%) |
| Diabetes Mellitus | 5 (11%) |
| Smoking* | 21 (45%) |
| Connective tissue disease | 12 (26%) |
| Number of events | 21 (45%) |
| Median time to event | NA |
| Median Number of follow-up | 4 (2– 5) |
| Median follow-up time (days) | 1449 (438– 2158) |
| (years) | 4.0 (1.3– 5.9) |
Note – IQR, interquartile range;
active or former smoker; NA: not applicable.
Imaging Data
All imaging datasets were obtained with state of the art multiple-detector row CT equipment (8 or more detector rows), with 0.75 to 1.25 mm thick sections reconstructed at 1 mm or smaller intervals. All baseline and follow-up CT scans were analyzed using dedicated 3D image post-processing software (Aquarius iNtuition, Version 4.4.12, TeraRecon, Inc, Foster City, CA, USA), which allows simultaneous review of spatially matched serial datasets. Morphologic features were reviewed and measured by two cardiovascular radiologists at baseline and at every time of follow-up imaging, which typically occurred at 3, 6, and 12 months, and yearly thereafter, but time intervals could vary between patients.
Serial Assessment of Morphologic Features
In addition to four baseline anatomic features (a-d) which have recently been associated with LAEs in a cohort which included the current study population (5), we also included several other plausible morphologic predictors of LAE (e-h) from the literature (4, 9, 10) to investigate if, and to what extent, any of these features changed over time, and if those changes were predictors of LAEs:
The maximum aortic diameter was defined as the greater of two orthogonal diameters (major and minor axis) obtained in a cross-sectional plane perpendicular to the aortic centerline, and measured using electronic calipers. In order to determine aortic growth, and because growth occurred consistently at the location of maximum diameter, the location of the maximum aortic diameter as determined in the last available CT scan was propagated to all prior scans of the same patient.
To capture the size of the false lumen, independent of the movement of the dissection flap over the cardiac cycle, we using an electronic protractor to measure the circumferential extent of the true and false lumen in angular degrees along the outer aortic wall as described previously (5). These measurements were performed at the level of maximum aortic diameter at each time-point.
The total number of visible intercostal arteries and their respective origins off the true vs. false lumen were counted and recorded based on transverse source images on each scan.
Aortic false lumen drainage pattern was estimated by visually assessing if the origins of the left subclavian, the visceral, the renal, and the iliac arteries arose off the true, the false, or both lumina, respectively, on each scan. Increased false lumen drainage has been shown to reduce the risk of LAEs.
The size of the primary intimal tear was measured using electronic calipers in two dimensions (transverse and craniocaudal) using transverse images with sagittal and coronal reformations of each scan.
The number of secondary/reentry tears were counted on transverse source images and noted on each scan.
The longitudinal extent (thoracic and/or abdominal), the thickness and mobility of the dissection flap was assessed on transverse images at the level of the celiac artery. The thickness of the dissection membrane was measured using electronic calipers. The dissection flap was defined as mobile if typical motion artifacts such as a double contour of the flap were visible; otherwise, the flap was considered stiff.
In an attempt to capture and quantify the presence and changes of partial false lumen thrombus – defined as the presence of a low-attenuation, non-enhancing filling defect within the contrast-opacified false lumen – we first used a 6-point visual grading system to semi-quantitatively describe the proportion of thrombosed vs. non-thrombosed false lumen: 1 = 0% (no thrombus), 2 = 1–24%, 3 = 25–50%, 4 = 51–75%, 5 = 76–99%, 6 = 100% (completely thrombosed). We also measured the cross-sectional area of false lumen thrombus, as well as corresponding true and false lumen areas, at the level of maximum false lumen thrombus area in each scan (Figure 2).
Figure 2:
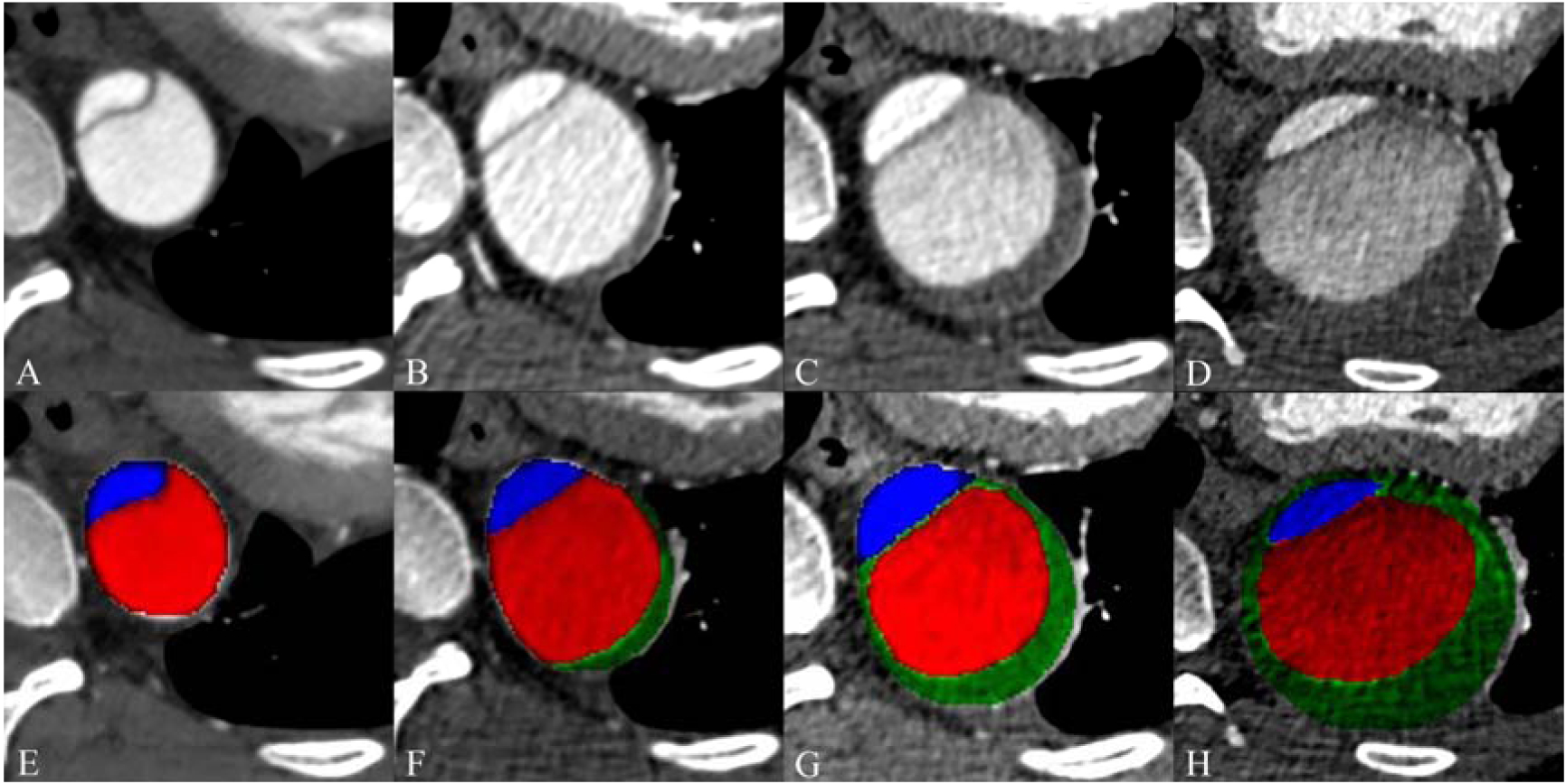
Imaging example of area measurement of true lumen, false lumen and false lumen thrombosis. Cross section image at baseline (A) and follow-up at 7 months (B), 2 years (C) and after 4 years (D). Corresponding baseline (E) and follow-up (F-H) images with color coding of measured areas for the true lumen (blue), false lumen (red), and thrombus within the false lumen (green, in F-H).
Statistical analysis
Statistical analyses were performed using R version 3.3.3. (R Foundation for Statistical Computing, Vienna, Austria). Patient characteristics are given as median and interquartile range, or count and percentage. Median LAE-free survival was quantified using the Kaplan Meier method.
We used a step-wise analytic approach to jointly model the longitudinal data (i.e. changes of morphologic features over time), with time-to-event outcomes (11). First, we used mixed-effects regression to assess changes in each predictor variable (i.e. the morphologic feature) over time. Each mixed effects model included a random intercept and random slope, to account for the clustering of multiple measurements per patient over time. We allowed linear and quadratic terms for time and used the Akaike Information Criterion (AIC) to determine the model that fitted best by choosing the model with the lowest AIC value. Next, we assessed the effects of longitudinal changes in predictor variables on time-to-event outcomes, by joining the mixed effects models with Cox proportional hazards regression. Initially the association for each morphological feature was assessed separately. For the multivariable models, each significant association was subsequently corrected by adding covariates to the Cox proportional hazards sub-model. As covariates, we considered baseline values of all other significant features as well as the presence of connective tissue disease.
Results
We identified a total of 47 patients. In 21 patients a late adverse event occurred over the course of follow-up. Patient demographics and clinical characteristics are given in Table 1. A total of 235 CT scans (47 baseline and 188 follow-up scans) were analyzed. The median (range) follow-up time was 4.0 years (10 days to 12.7 years). The median time to an LAE was 6.6 (95% CI: 5.1 – 11.2) years. A Kaplan-Meier curve showing probabilities of being LAE-free over the course of follow-up for the entire cohort is shown in Figure 3. The shaded area denotes the confidence bound around the Kaplan-Meier estimates. Events were predominantly driven by chronic aneurysm formation, occurring in 18 of 21 (86%) patients. One patient demonstrated rapid aortic growth, and one patient developed acute limb ischemia within the first year. One of 21 patients (5%) developed a new type A dissection 8 years after the initial event. LAEs were treated surgically in 14 patients, using endovascular repair in 5, and 2 patients were medically managed.
Figure 3:
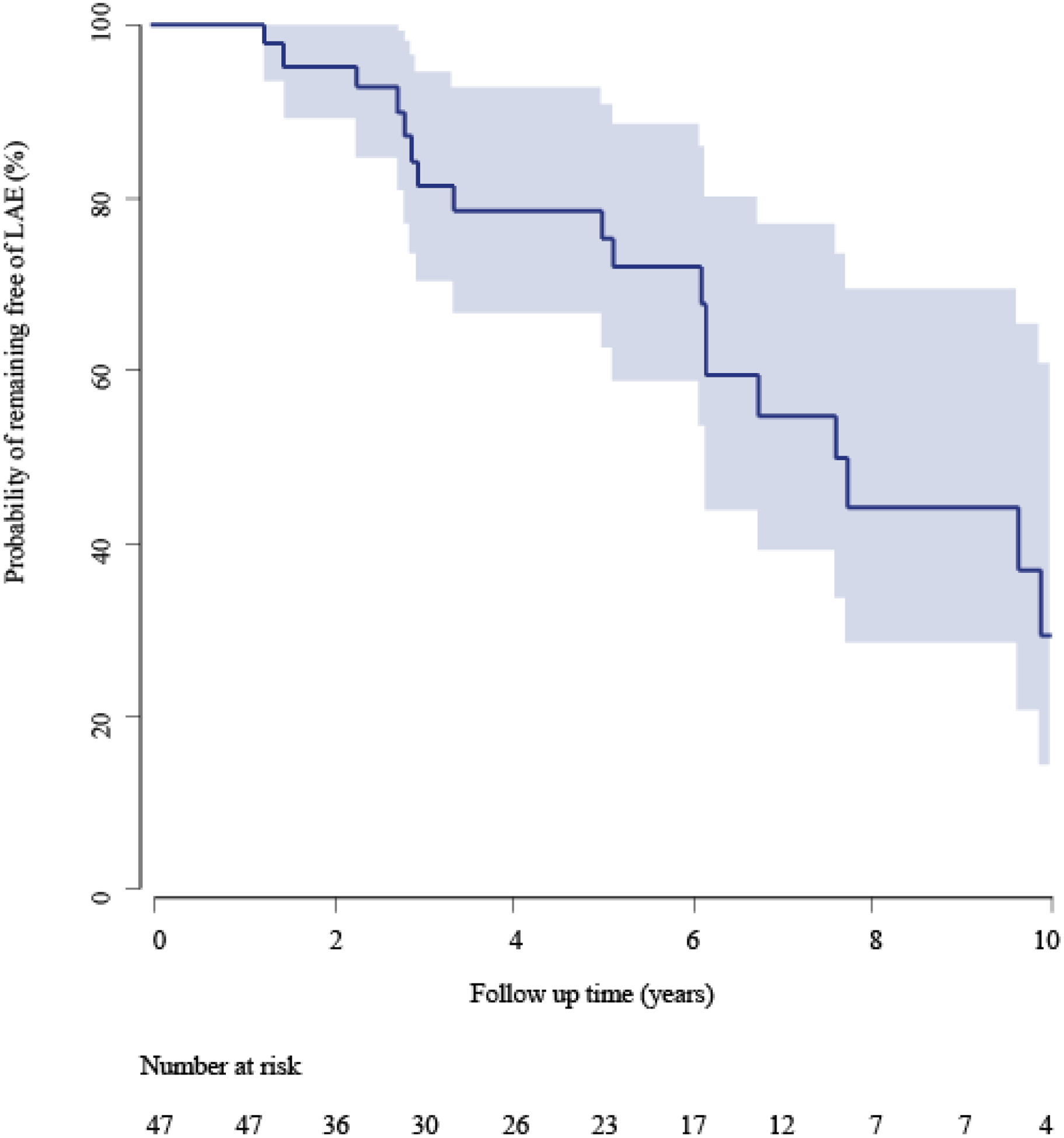
Kaplan Meier curve showing the probability of remaining free from late adverse event over the complete follow-up period. The blue area denotes the 95% confidence band around the Kaplan Meier estimates.
Stable Morphologic Features over Time
Several key features of aortic dissection were remarkably stable over time without any noticeable or quantifiable changes when compared to baseline and therefore not included in the analyses: proximal and distal extent of the false lumen; location of the primary intimal tear; and large branch vessel blood supply from the true vs. the false lumen. This observation confirms that the longitudinal and circumferential boundaries of the false lumen in uncomplicated type B dissection are established at the time of the initial event. The thickness of the dissection membrane universally increased in all patients over time, with decreasing motion artifacts.
Univariable and multivariable analysis of changes of morphological features over time
Median and interquartile range for each of the evolving morphologic features at baseline and the regression coefficients of time and, if applicable, time-squared, are given in Table 2. The baseline values show the median values at time of inclusion accompanied by the interquartile range, and the coefficients show the average linear increase (or decrease in case of a negative coefficient) in the morphological feature per year following inclusion. In case a quadratic term was statistically significant, that coefficient was presented as well and would indicate a quadratic increase or decrease over time. The following morphological features showed significant change over time: max. major and max. minor aortic diameter, size of the primary intima tear (axial and sagittal), circumferential extent of FL, area of TL, area of FLT and total aortic cross-sectional area. Coefficients of time could not be modeled for the following features due to the distribution and were not further analyzed: Number of secondary tears and FLT grading.
Table 2:
Measurements of morphological features at baseline, and their change over time
| Baseline value | Coefficient of time (per year)† | P-value | |
|---|---|---|---|
| Max. major axis aortic diameter (mm) | 35.0 (33.0 – 38.5) | 3.19 (2.21 – 4.17) | < 0.001 |
| Max. minor axis aortic diameter (mm) | 32.0 (30.0 – 36.0) | 2.57 (1.67 – 3.47) | <0.001 |
| Size of intima tear axial (mm) | 7.0 (4.0 – 11.5) | 1.34 (0.89 – 1.87) | <0.001 |
| Size of intima tear sagittal (mm) | 5.0 (2.0 – 8.0) | 1.61 (1.11 – 2.11) | <0.001 |
| −0.08 (−0.13 – −0.04)‡ | <0.001 | ||
| Number of secondary tears (n)††† | 1.0 (0.0 – 1.0) | ||
| Circumferential extent of TL (°) | 103.0 (91.0 – 125.5) | −2.19 (−3.60 – −0.78) | 0.003 |
| Circumferential extent of FL (°) | 257.0 (234.5 – 269.0) | 2.19 (0.78 – 3.60) | 0.003 |
| Area TL (cm2) | 2.5 (2.0 – 3.3) | 0.32 (0.20 – 0.45) | <0.001 |
| −0.02 (−0.03 – −0.01)‡ | <0.001 | ||
| Area FL (cm2) | 5.0 (3.7 – 6.4) | 0.30 (−0.12 – 0.73) | 0.159 |
| Area FLT (cm2) | 2.1 (0.0 – 3.6) | 0.89 (0.41 – 1.38) | <0.001 |
| Total Area (cm2) | 8.0 (6.6 – 10.1) | 1.61 (0.98 – 2.25) | <0.001 |
| Grading FLT††† | 1.0 (0.0 – 1.0) | ||
| Number of ICA from TL (n) | 7.0 (3.5 – 9.0) | −0.08 (−0.21 – 0.05) | 0.222 |
| Number of ICA from FL (n) | 8.0 (5.0 – 11.5) | −0.19 (−0.36 – −0.02) | 0.033 |
| Total numbers of ICA (n) | 16.0 (13.0 – 18.0) | −0.57 (−0.81 – −0.32) | <0.001 |
| 0.04 (0.02 – 0.07)‡ | 0.002 |
Baseline values are given in median and interquartile range. Coefficients of time refer to the average linear (or quadratic) increase or decrease of each morphologic parameter per year. Max: Maximum; mm: millimeter; TL: true lumen, FL: false lumen; FLT: false lumen thrombosis; ICA: intercostal arteries
Computed using all follow-up measurements available, using mixed-effects regression. Estimates are accompanied by 95% confidence intervals.
Results of time-squared to accommodate non-linear change over time.
Coefficient of time could not be modeled due to the distribution.
The results of our univariable and multivariable analyses of the longitudinal predictors on LAEs are given in Table 3. Among the investigated morphologic features, multivariable analysis revealed strong association of the following morphological features with LAE: increase of major aortic diameter (HR=1.24 [1.13–1.37] per mm increase; p<0.001), increase of minor aortic diameter (HR=1.16 [1.07–1.25] per mm increase; p<0.001), increase of circumferential extent of false lumen (HR=1.05 [1.01–1.10] per ° increase; p<0.001), increase of partial false lumen thrombosis area (HR=1.39 [1.18–1.66] per cm2 increase; p<0.001) and increase of total area (HR=1.18 [1.23–1.35] per cm2 increase; p<0.001). Supplementary tables 1 through 7 show the coefficients of the baseline values of morphological features in addition to the multivariable adjusted longitudinal predictors.
Table 3:
Associations between changes in morphological features over time and the occurrence of late adverse events
| Univariable HR (95% CI) | P-value | Multivariable‡ HR (95% CI) | P-value | |
|---|---|---|---|---|
| Max. major aortic diameter (mm) | 1.21 (1.13 – 1.30) | < 0.001 | 1.25 (1.13 – 1.37) | <0.001 |
| Max. minor aortic diameter (mm) | 1.16 (1.10 – 1.23) | < 0.001 | 1.16 (1.08 – 1.24) | <0.001 |
| Size of intima tear axial (mm) | 1.03 (0.99 – 1.03) | 0.145 | NA | |
| Size of intima tear sagittal (mm) | 1.04 (0.98 – 1.10) | 0.171 | NA | |
| Circumferential extent of TL (°) | 0.96 (0.94 – 0.98) | < 0.001 | 0.96 (0.94 – 0.98) | < 0.001 |
| Circumferential extent of FL (°) | 1.03 (1.01 – 1.05) | 0.004 | 1.04 (1.01 – 1.06) | 0.004 |
| Area TL (cm2) | 0.97 (0.68 – 1.38) | 0.874 | NA | |
| Area FL (cm2) | 1.13 (1.02 – 1.25) | 0.017 | 1.06 (0.94 – 1.20) | 0.326 |
| Area FLT (cm2) | 1.30 (1.16 – 1.46) | < 0.001 | 1.53 (1.25 – 1.88) | <0.001 |
| Total Area (cm2) | 1.18 (1.10 – 1.27) | <0.001 | 1.26 (1.14 – 1.39) | <0.001 |
| Number of ICA from TL (n) | 0.93 (0.80 – 1.08) | 0.330 | NA | |
| Number of ICA from FL (n) | 1.09 (0.95 – 1.25) | 0.235 | NA | |
| Total numbers of ICA (n) | 1.06 (0.92 – 1.23) | 0.407 | NA |
Association between morphological feature change over time and time-to-occurrence of a LAE corrected for the baseline values of all other features significant in the univariable analysis, and corrected for the presence of connective tissue disease. HR: hazard ratio; CI: confidence interval; Max: maximum; mm: millimeter; TL: true lumen; FL: false lumen; FLT: false lumen thrombosis; ICA: intercostal arteries; NA: not applicable (not significant in univariable analysis).
Discussion
The evolution of morphologic features associated with late adverse events in patients with initially uncomplicated acute type B aortic dissection is largely unknown. This is a crucial gap in our understanding of the subacute and chronic phase of the disease, because survivors of the initial event require life-long clinical follow-up and imaging surveillance, and it is plausible that ongoing changes to these morphologic features would influence an individual patient’s long-term risk profile. Our primary conclusions from serial imaging data in patients with uncomplicated type B aortic dissection are that 1) some, but not all, baseline morphologic predictors of LAE change over time, 2) new prognostic features, such as partial false lumen thrombosis, may arise after the initiating event, and 3) quantitative changes of morphologic predictors over time convey potentially important prognostic information. While many details remain to be elucidated, the fundamental implication of these observations is that imaging-based risk-prediction models aimed at improved patient selection in the acute phase of an uncomplicated type B aortic dissection cannot necessarily be applied to the much larger number of expected follow-up imaging studies in the subacute and chronic phase of the disease. Examples of five of the eight morphological features, and how they change over time are provided in Figure 4 (Graphical Abstract).
Figure 4 (Graphical Abstract).
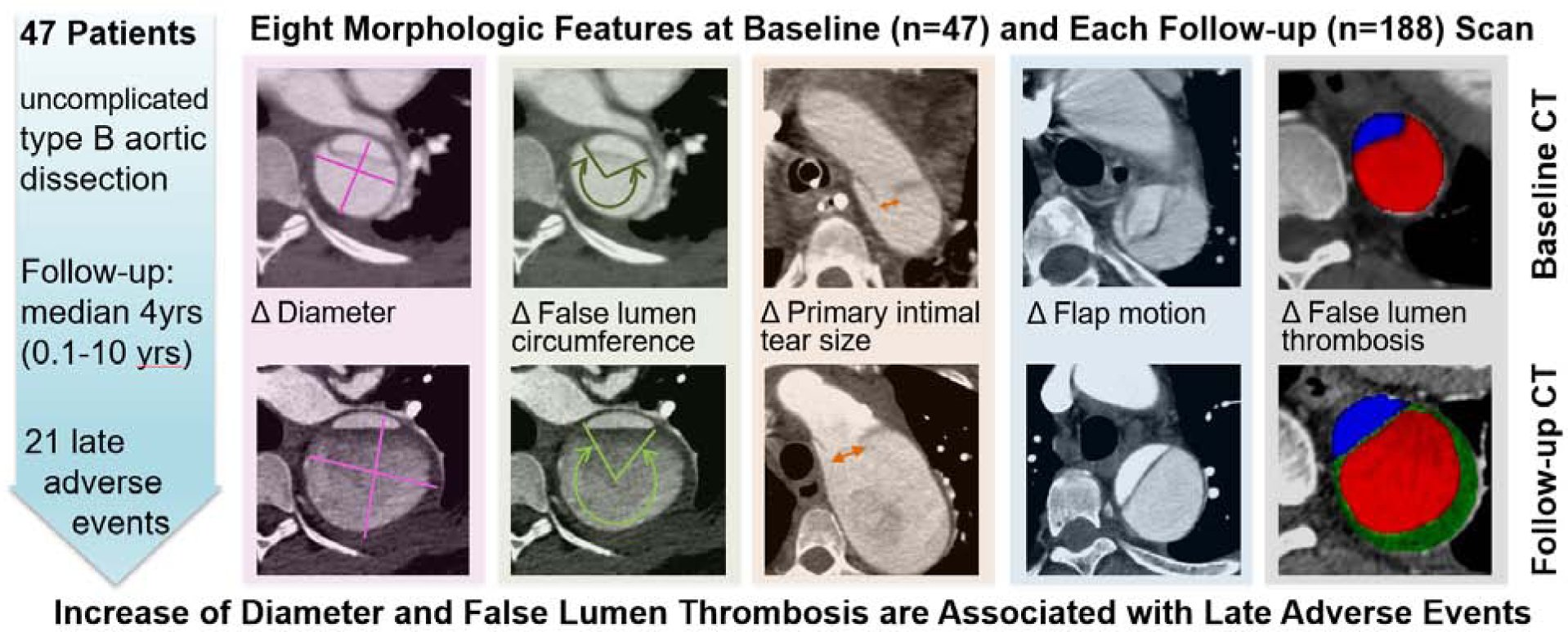
Graphical abstract exemplifies five out of eight of the morphological features, measured and recorded on baseline CT scans (top row). Follow-up CT scans (bottom row) show how these features changed over time. In a cohort of 47 patients with initially uncomplicated type B aortic dissection, an increase of aortic diameter and the development of new or increasing partial false lumen thrombosis were associated with late adverse events.
Our findings also suggest that morphologic risk factors observed at any given time after the initial event need to be interpreted in the context of the entire preceding morphologic evolution: e.g. a 4 cm maximum aortic diameter may convey a different risk at baseline vs at 2 year follow-up; a 4 cm diameter at one year follow-up may also convey a different risk if the prior measurement at 12 months was 3 cm versus 3.5 cm. Interpretation of imaging findings that accounts for a patient’s entire history of imaging findings is highly desired by clinicians, and our data support the need for analysis and interpretation of imaging data that includes changes over time (12, 13).
Our study once again corroborates the importance of both maximum aortic diameter and change in maximum aortic diameter over time as a strong predictor for LAEs. Others have demonstrated this as well (8, 14). It will be interesting to further investigate the interaction between size and growth in a larger cohort in the future.
The relative circumference – expressed in angular degrees – of the proportion of the outer aortic wall enclosing the false lumen, has recently been shown to be an independent baseline predictor for LAEs (5). It is not surprising that an increase of the false lumen circumference over time is also strongly associated with LAEs. Unfortunately, our analysis cannot determine if the increase of false lumen circumference – or total aortic area – is also an independent risk factor over time, of if it is simply associated with diameter growth, which is almost exclusively driven by false lumen expansion. We acknowledge at this point, that the circumferential extent of false lumen outer aortic wall measured in angular degrees is difficult to measure and interpret when the aortic cross-section becomes more elliptical rather than circular over time.
The primary intimal tear appears to get larger over time, but this did not translate to an increased risk in our small cohort. We have not found the size of the primary intimal tear to be a significant baseline risk factor in uncomplicated type B dissection previously (5), although others have shown a higher rate of complications if both complicated and uncomplicated type B dissections were included (4). The hemodynamic effects of increasing primary intimal tear size while the dissection membrane becomes thicker and less mobile over time are currently unclear. Computational fluid dynamics modeling may improve our understanding of this evolution in the future (15). Similarly, we observed an increase of secondary tears/communications between true and false lumen over time – again without a detectable effect on risk. It is possible that some of the subtle observations in our series reflect better detectability of preexisting small tears over time within an increasingly thicker and less mobile dissection membrane.
Partial false lumen thrombosis has been described as a risk factor for adverse events in patients with type B dissection (9), although this has not been a universal finding (16). In our own experience, partial false lumen thrombosis was uncommon at baseline and did not predict late adverse events (5). In this analysis of serial imaging, however, we found that new development or increase of thrombus in the perfused false lumen is a significant risk factor. In contradistinction to the proposed hypothesis that partial thrombosis of the FL causes diminished outflow and thus increased FL mean pressure (9), our findings are better compatible with the hypothesis that false lumen thrombus formation is the consequence of slow, stagnating blood flow (17), due to poor FL drainage/outflow, which is associated with increased diastolic pressure and in turn faster aneurysmal degeneration (8).
The main limitations of this study are the retrospective design and the relatively small population size. While we analyzed a large number of CT datasets (n=235), the lengthy processing-time required to manually analyze each individual dataset prevented us from including a larger number of patients for serial analysis, and the study may be underpowered to identify additional morphological features associated with LAE. Further investigation of the quantitative assessment of aortic remodeling over time, which would be facilitated by new software-based tools for automatic or semi-automatic extraction of a wide range of anatomic features over time, will be needed. Machine learning algorithms may also take advantage of the currently unexploited information contained in modern high-resolution imaging datasets and uncover novel morphologic prognostic factors (18). Such developments are not only a prerequisite for expanding similar research efforts to larger cohorts but also for using morphologic features and their changes over time in a clinical setting. Finally, we were unable to correct the association of each of the longitudinal features on LAEs, for all other features measured over time due to the fact that current joint models of longitudinal and time-to-event data have not been extended to allow inclusion of a large number of time-varying covariates. We did correct for baseline values of the important predictors, notably aortic diameter.
In conclusion, increase in aortic diameter and increase or new development of false lumen thrombus are both associated with late adverse events in patients with initially uncomplicated type B aortic dissection. Future individual risk prediction models for patients with chronic dissection may be more accurate if they are based on both current and prior assessments, requiring recalibration of model parameters with each follow-up encounter.
Supplementary Material
Perspective Statement.
Morphologic features extracted from imaging data may improve risk stratification in patients with initially uncomplicated type B aortic dissection. We demonstrate that aortic growth and the increase or new development of false lumen thrombus are associated with late adverse events, suggesting that follow-up imaging data should be included in future risk prediction models.
Source of funding:
Kai Higashigaito is funded by an ‘Early.Postdoc Mobility Grant (P2ZHP3_168559) by the Swiss National Research Foundation; Martin J. Willemink is supported by the American Heart Association (18POST34030192); This work was supported by a National Institute of Health Clinical and Translational Science Award UL1 RR025744
Glossary of Abbreviations
- AIC
Akaike Information Criterion
- CT
Computed tomography
- FDA
Food and drug administration
- HR
Hazard ratio
- LAE
Late adverse events
- TBAD
Type B aortic dissection
- TEVAR
Thoracic endovascular aortic repair
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registry: not applicable
Conflict of interest: No conflict of interests
References
- 1.Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circulation Cardiovascular interventions. 2013;6(4):407–16. [DOI] [PubMed] [Google Scholar]
- 2.Buth J, Harris PL, Hobo R, van Eps R, Cuypers P, Duijm L, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. Journal of vascular surgery. 2007;46(6):1103–10; discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 3.Lee WA. Failure modes of thoracic endografts: Prevention and management. Journal of vascular surgery. 2009;49(3):792–9. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz SI, Durham C, Clouse WD, Patel VI, Lancaster RT, Cambria RP, et al. Predictors of late aortic intervention in patients with medically treated type B aortic dissection. Journal of vascular surgery. 2018;67(1):78–84. [DOI] [PubMed] [Google Scholar]
- 5.Sailer AM, van Kuijk SM, Nelemans PJ, Chin AS, Kino A, Huininga M, et al. Computed Tomography Imaging Features in Acute Uncomplicated Stanford Type-B Aortic Dissection Predict Late Adverse Events. Circulation Cardiovascular imaging. 2017;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afifi RO, Sandhu HK, Leake SS, Boutrous ML, Kumar V, Azizzadeh A, et al. Outcomes of Patients With Acute Type B (DeBakey III) Aortic Dissection. A 13-Year, Single-Center Experience. 2015;132(8):748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krol E, Panneton JM. Uncomplicated Acute Type B Aortic Dissection: Selection Guidelines for TEVAR. Ann Vasc Dis. 2017;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sailer AM, Nelemans PJ, Hastie TJ, Chin AS, Huininga M, Chiu P, et al. Prognostic significance of early aortic remodeling in acute uncomplicated type B aortic dissection and intramural hematoma. J Thorac Cardiovasc Surg. 2017;154(4):1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357(4):349–59. [DOI] [PubMed] [Google Scholar]
- 10.Spinelli D, Benedetto F, Donato R, Piffaretti G, Marrocco-Trischitta MM, Patel HJ, et al. Current evidence in predictors of aortic growth and events in acute type B aortic dissection. Journal of vascular surgery. 2018. [DOI] [PubMed] [Google Scholar]
- 11.Rizopoulos D Joint models for longitudinal and time to event data. With Applications in R. Jones B, Liu JP, Peace KE, Turnbull BW, editors. Boca Raton, FL: CRC Press Taylor & Francis Group; 2012. [Google Scholar]
- 12.Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Current problems in cardiology. 2008;33(5):203–77. [DOI] [PubMed] [Google Scholar]
- 13.Elefteriades JA, Rizzo JA, Coady MA. Thoracic aorta. Radiology. 1999;211(3):889. [DOI] [PubMed] [Google Scholar]
- 14.Hosn MA, Goffredo P, Zavala J, Sharp WJ, Katragunta N, Kresowik T, et al. Analysis of Aortic Growth Rates in Uncomplicated Type B Dissection. Annals of vascular surgery. 2017. [DOI] [PubMed] [Google Scholar]
- 15.Alimohammadi M, Sherwood JM, Karimpour M, Agu O, Balabani S, Diaz-Zuccarini V. Aortic dissection simulation models for clinical support: fluid-structure interaction vs. rigid wall models. Biomedical engineering online. 2015;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trimarchi S, Tolenaar JL, Jonker FHW, Murray B, Tsai TT, Eagle KA, et al. Importance of false lumen thrombosis in type B aortic dissection prognosis. The Journal of Thoracic and Cardiovascular Surgery. 2013;145(3, Supplement):S208–S12. [DOI] [PubMed] [Google Scholar]
- 17.Naim WNWA Ganesan PB, Sun Z, Liew YM, Qian Y, Lee C-J, et al. Prediction of thrombus formation using vortical structures presentation in Stanford type B aortic dissection: A preliminary study using CFD approach. Applied Mathematical Modelling. 2016;40(4):3115–27. [Google Scholar]
- 18.McBee MP, Awan OA, Colucci AT, Ghobadi CW, Kadom N, Kansagra AP, et al. Deep Learning in Radiology. Academic radiology. 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


