Abstract
The anterior mediastinum is the most common location of mediastinal tumors, and thymic epithelial tumors are the most common mediastinal tumors. It is important to differentiate thymic epithelial tumors from malignant lymphomas and malignant germ cell tumors because of the different treatment strategies. Dynamic contrast-enhanced MRI and diffusion-weighted imaging can provide additional information on the differential diagnosis. Chemical shift imaging can detect tiny fat tissues in the lesion and is useful in differentiating thymic hyperplasia from other solid tumors such as thymomas. MRI findings reflect histopathological features of mediastinal tumors, and a comprehensive evaluation of MRI sequences is important for estimation of the histopathological features of the tumor. In this manuscript, we describe the MRI findings of anterior mediastinal solid tumors and the role of MRI in the differential diagnosis.
Keywords: anterior mediastinum, magnetic resonance imaging, thymic epithelial tumors, malignant lymphoma, germ cell tumor
Introduction
In the evaluation of mediastinal tumors, CT is the first choice imaging modality. MRI has higher soft-tissue contrast resolution compared with CT, and is useful in differentiating between solid and cystic lesions even without the intravenous administration of contrast agents.1 MRI performed in conjunction with dynamic contrast-enhanced MRI (DCE-MRI), chemical shift imaging (CSI), and/or diffusion-weighted imaging (DWI), can provide additional information about histopathological features of mediastinal tumors and is useful for the differential diagnosis.2–11
Anterior mediastinal solid tumors include various histopathological subtypes and show various MRI findings. Table 1 shows the differential diagnosis and MRI findings of anterior mediastinal solid tumors. The treatment strategy differs according to the type of tumor involved. In thymic epithelial tumors, surgery is the first choice of treatment. In contrast, biopsy and chemotherapy are usually performed for malignant lymphomas and malignant germ cell tumors (GCTs). Therefore, the differential diagnosis of anterior mediastinal solid tumors is important for the choice of treatment strategy.11
Table 1.
Differential diagnosis and MRI findings of anterior mediastinal solid tumors
| MRI findings | |
|---|---|
| Thymoma | Low-risk: round or oval shape, smooth contour, almost complete capsule, internal septaHigh-risk: irregular contour, partial capsule, internal septa, pleural dissemination |
| Thymic carcinoma | Invasive, heterogeneous, low-signal-intensity foci on T2WI, lymph node and hematogenous metastases |
| Thymic neuroendocrine tumors | Carcinoid: hypervascular, flow voidsLCNEC, small cell carcinoma: invasive, heterogeneous, lymph node and hematogenous metastasis |
| Germ cell tumors | Seminoma: limited necrosisNSGCTs: marked hemorrhagic necrosis, hematogenous metastasis |
| Malignant lymphomas | Invasive, heterogeneous, lymph node enlargement, low ADC values |
| Thymic hyperplasia | Flat triangular shape, decreasing signal intensity on opposed-phase image |
| Thymolipoma | Large and soft mass, intermingled signal intensity of the fat and thymic tissues |
| Intrathoracic goiter | Continuation to the thyroid gland or ectopic mediastinal lesion, cystic change, hypervascular, flow voids |
| Ectopic parathyroid adenoma | Hypervascular small nodule, cystic change |
| Schwannoma of the vagus and phrenic nerves | Target sign on T2WI, cystic change, thin capsule |
| Paraganglioma | Hypervascular, flow voids, hemorrhagic necrosis, salt and pepper appearance |
ADC, apparent diffusion coefficient, LCNEC, large cell neuroendocrine carcinoma, NSGCTs, non-seminomatous germ cell tumors, T2WI, T2-weighted image.
In this manuscript, we describe the MRI findings of anterior mediastinal solid tumors and role of MRI in the differential diagnosis.
MRI Sequences and MRI Findings of Mediastinal Tumors
Because MRI has higher soft-tissue contrast resolution compared with CT, MRI can provide additional information about the histopathological features of mediastinal tumors, such as cystic changes, hemorrhage, fat, and fibrous capsule and septa.12,13 Hemorrhage shows high signal intensity on T1-weighted images. Cystic change and myxoid matrix in the lesion show high signal intensity on T2-weighted images, while fibrous tissues show low signal intensity. T2-weighted images are reported to be superior to CT images for the detection of fibrous capsule and septa in thymic epithelial tumors.12 DCE-MRI can generate multiple phase images without radiation exposure, and the enhancement patterns reflect the vascularity and histopathological features of mediastinal tumors.9,11 Hypervascular tumors such as thymic carcinoid, intrathoracic goiter, ectopic parathyroid adenoma, paraganglioma, and hyaline vascular type Castleman’s disease show a rapid and intense enhancement on DCE-MRI.14,15 Myxoid and fibrous tissues show a gradual and persistent enhancement pattern. Fat saturation MR images are useful for the detection of fat tissue in mature cystic teratomas, thymolipomas, and myelolipomas. CSI is useful for the detection of intermingled and tiny fat tissues in normal thymus and thymic hyperplasia.3,16 DWI can provide additional information about the mobility of water molecules in tumors through the measurement of the apparent diffusion coefficient (ADC) in the tumor.5,8 On DWI, malignant tumors in various organs generally show lower ADC values due to increased cell density and resulting restriction of water molecules compared with benign tumors.5,8 Razek et al. compared the ADC values between malignant and benign mediastinal tumors using DWI with b values of 0, 300, and 600 sec/mm2.8 They reported that the ADC values of malignant tumors (1.09 ± 0.25 × 10−3 mm2/sec) were significantly lower than those of benign tumors (2.38 ± 0.25 × 10−3 mm2/sec).8 When an ADC value of 1.56 × 10−3 mm2/sec was used as a threshold value for differentiating malignancies from benign tumors, the best results were obtained with an accuracy of 95%, sensitivity of 96%, and specificity of 94%.8 Technical parameters and acquisition protocols of DWI and methods of ADC value measurement can affect ADC values. Priola et al. compared perfusion-free ADC value measurement with b values of 150 and 800 sec/mm2 to perfusion-sensitive ADC value measurement with b values of 0, 150, and 800 sec/mm2 in benign and malignant mediastinal lesions.7 They reported overestimation of the ADC values with the perfusion-sensitive measurement compared to those with the perfusion-free measurement in all lesions, benign lesions, and malignancies.7 They also reported that the perfusion-free ADC values (cut-point = 1.52 × 10−3 mm2/sec; sensitivity = 93.7%; specificity = 88.6%; accuracy = 90.8%) was superior to the perfusion-sensitive ADC values (cut-point = 1.75 × 10−3 mm2/sec; sensitivity = 75.0%; specificity = 79.5%; accuracy = 77.6%) in differentiating malignancies from benign lesions.7 Tumors with high cell density, such as malignant lymphomas, show high signal intensity on DWI, and the ADC values within the lesion are low.8,17 Myxoid tumors with low cell density, such as neurogenic tumors, usually show low signal intensity on DWI, and the ADC values are high. Even malignancy such as myxoid sarcomas with low cell density can show relative high ADC values. MRI findings reflect the histopathological features of mediastinal tumors, and a comprehensive evaluation of MRI sequences is important for estimation of the histopathological features of the tumor.
Thymic Epithelial Tumors
Thymomas
Thymoma is the most common anterior mediastinal neoplasm in adults and also the most common thymic epithelial tumor. Thymomas occur at almost all ages, with a peak incidence at 55–65 years.18 Myasthenia gravis (MG) is the most common paraneoplastic syndrome associated with thymoma; 30%–50% of patients with thymoma have symptoms of MG, whereas 10%–15% of patients with MG have thymoma.19 Hypogammaglobulinemia, pure red cell aplasia, and various other autoimmune diseases are also associated with thymomas. The 2021 World Health Organization (WHO) Classification of Tumors of the Thymus classifies thymomas into five major subtypes (types A, AB, B1, B2, and B3 thymoma) based on morphologic features of the epithelial cells and the lymphocyte-epithelial cell ratio. The histological classification also includes rare subtypes of thymoma such as micronodular thymoma with lymphoid stroma, metaplastic thymoma, and lipofibroadenoma (Table 2).18 Jeong et. al. simplified the WHO histological classification and classified type A, AB (Figs. 1 and 2) and B1 thymomas as low-risk and type B2 and B3 thymomas (Fig. 3) as high-risk according to the local invasion and prognosis of the tumors.20 Extracapsular invasion is more frequent and the prognosis is worse in high-risk thymomas than in low-risk thymomas.18,20 The Masaoka and modified Masaoka-Koga staging systems are based on microscopic invasion to the capsule and surrounding organs and correlate with the prognosis of thymomas. The correct prediction of the early stage (stages I and II) or advanced stage (stages III and IV) of thymomas before surgery based on imaging is important for decision of the treatment strategy.
Table 2.
Histological classification of thymomas18
| Thymoma, NOS | |
| Thymoma, type A | |
| Thymoma, type AB | |
| Thymoma, type B1 | |
| Thymoma, type B2 | |
| Thymoma, type B3 | |
| Micronodular thymoma with lymphoid stroma | |
| Metaplastic thymoma | |
| Lipofibroadenoma |
NOS, not otherwise specified.
Fig. 1.
Low-risk (type AB) thymoma (Masaoka-Koga stage I) in a 42-year-old woman. a) An axial half-Fourier acquisition single-shot turbo spin-echo (HASTE) T2-weighted image shows a well-circumscribed lobulated mass in the anterior mediastinum. A low-signal-intensity peripheral capsule (arrows) and septa (arrowhead) are seen in the mass. b and c) Axial dynamic contrast-enhanced images (b: 60 sec; c: 180 sec after the start of injection of the contrast material) show a rapid heterogeneous enhancement and a washout enhancement pattern of the mass. The internal fibrous septa (arrowhead) show a gradual enhancement.
Fig. 2.
Low-risk (type AB) thymoma (Masaoka-Koga stage I) in an 84-year-old woman. a) An axial turbo spin-echo (TSE) T2-weighted image shows a well-circumscribed round mass with a peripheral low-signal-intensity capsule (arrows) in the anterior mediastinum. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows high signal intensity of the mass (arrow). c) The mean ADC value within the mass (arrow) was relatively high (1.79 × 10−3 mm2/s) on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a rapid homogeneous enhancement and a washout enhancement pattern of the mass (arrow).
Fig. 3.
High-risk (type B3) thymoma (Masaoka-Koga stage III) in an 81-year-old woman. a) An axial TSE T2-weighted image shows an irregular mass (arrow) in the anterior mediastinum. A peripheral capsule and internal septa are not seen. The mass is widely adjacent to the ascending aorta and pulmonary artery. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows high signal intensity of the mass (arrow). c) The mean ADC value within the mass (arrow) was 1.14 × 10−3 mm2/s on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a heterogeneous and persistent enhancement pattern of the mass (arrow). Tumor invasion to the pericardium, left brachiocephalic vein, and adventitia of the aorta was confirmed by surgery (not shown).
Thymic carcinomas
Thymic carcinoma represents approximately 20% of thymic epithelial neoplasms and the mean age of patients at presentation is 50 years.18,21 Local invasion and lymph nodes or distant metastases are common in thymic carcinomas. The WHO classification includes various histological subtypes of thymic carcinomas (Table 3).18 Thymic squamous cell carcinoma (SCC) (Fig. 4) is the most common subtype of thymic carcinomas (approximately 70% of all cases). The 5-year overall survival of thymic SCCs is 57.6%–67.1%.18 Thymic basaloid carcinoma (Fig. 5) is an SCC characterized by solid and cystic papillary nests of medium- to small-sized cells with a high nuclear-to-cytoplasmic ratio and peripheral palisading.18 Thymic basaloid carcinomas account for less than 5% of all thymic carcinomas. Multilocular thymic cysts (MLTCs) were observed in 45% of reported cases of thymic basaloid carcinoma (Fig. 5).18 Lymphoepithelial carcinomas are undifferentiated or poorly differentiated SCCs accompanied by a prominent lymphoplasmacytic infiltrate, and morphologically similar to nasopharyngeal non-keratinizing SCC. They comprise 1.3%–6% of thymic carcinomas and are aggressive tumors with poor prognosis.18 Thymic adenocarcinomas not otherwise specified (NOS) show glandular differentiation and/or mucin production, and do not conform to low-grade papillary adenocarcinoma or enteric-type adenocarcinoma. They are aggressive tumors with poor prognosis.18 Thymic low-grade adenocarcinomas are composed of well-formed tubulopapillary structures, often associated with type A thymoma. They are reported to make up 3% of thymic carcinomas,22 and their prognosis is relatively good. Thymic carcinoma with adenoid cystic carcinoma-like features resembles salivary gland adenoid cystic carcinoma. Fewer than 10 cases have been reported, and their prognosis is relatively good.18 Thymic enteric-type adenocarcinomas have the same histological and immunohistochemical features as colorectal adenocarcinoma. They are reported to account for less than 5% of thymic carcinomas, and their prognosis is relatively good.18 Thymic adenosquamous carcinomas show both squamous and glandular differentiation, analogous to their pulmonary counterparts. They are very rare, and their epidemiology and prognosis are unclear.18 NUT carcinoma is a poorly differentiated carcinoma genetically defined by the presence of nuclear protein in testis (NUT) gene rearrangement and accounts for 2.7%–4% of thymic carcinomas.23,24 NUT carcinomas are extremely aggressive, and their prognosis is poor.25 Mucoepidermoid carcinomas are characterized by a combination of mucus-producing cells, intermediate cells, and squamoid cells. They account for 2.5% of thymic carcinomas, and some cases are associated with MLTCs.18 Survival is favorable in patients with low-grade thymic mucoepidermoid carcinomas, whereas patients with high-grade tumors have a poor prognosis. Clear cell carcinomas are rare thymic carcinomas, with only about 25 cases reported to date, and are composed predominantly or completely of clear cells. They are aggressive neoplasm, and their prognosis is poor.26 Sarcomatoid carcinomas (Fig. 6) are thymic carcinomas consisting partly or completely of spindle-shaped epithelial cells, and make up 2.5%–10% of thymic carcinomas.22,27 Tumors with heterologous sarcomatous elements are called carcinosarcomas. Thymic sarcomatoid carcinomas are highly aggressive, and their prognosis is poor. Undifferentiated carcinomas are rare thymic carcinomas showing no morphological or immunohistochemical differentiation, and are diagnosed by exclusion. They are high-grade carcinomas, and their prognosis is poor.18
Table 3.
Histological classification of thymic carcinomas18
| Squamous cell carcinomas |
| Squamous cell carcinoma, NOS |
| Basaloid carcinoma |
| Lymphoepithelial carcinoma |
| Adenocarcinomas |
| Adenocarcinoma, NOS |
| Low-grade papillary adenocarcinoma |
| Thymic carcinoma with adenoid cystic carcinoma-like features |
| Adenocarcinoma, enteric type |
| Adenosquamous carcinoma |
| NUT carcinoma |
| Salivary gland-like carcinomas |
| Mucoepidermoid carcinoma |
| Clear cell carcinoma |
| Sarcomatoid carcinoma |
| Carcinosarcoma |
| Carcinoma, undifferentiated, NOS |
| Thymic carcinoma, NOS |
NOS, not otherwise specified; NUT, nuclear protein of the testis.
Fig. 4.
Thymic squamous cell carcinoma in a 59-year-old man. a) An axial TSE T2-weighted image shows an irregular mass in the anterior mediastinum. A small low-signal-intensity focus which reflects fibrous tissues (small arrow) is seen in the mass. A capsule (large arrow) is seen in the right periphery of the mass, but the capsule is unclear in the left periphery, and invasion to the left lung and anterior chest wall are suspected (arrowheads). b) An axial diffusion-weighted image (b = 1000 s/mm2) shows high signal intensity of the mass (arrow). c) The mean ADC value within the mass (arrow) was 0.96 × 10−3 mm2/s on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a heterogeneous and persistent enhancement pattern of the mass (arrow). Multiple lymph node metastases and pleural dissemination were confirmed on PET-CT (not shown).
Fig. 5.
Thymic basaloid carcinoma associated with a multilocular thymic cyst in a 37-year-old woman. a) An axial TSE T2-weighted image shows a multilocular cystic mass (arrowhead) with a solid component (arrow) in the anterior mediastinum. b) axial diffusion-weighted image (b = 1000 s/mm2) shows high signal intensity of the solid component (arrow). c) The mean ADC value within the solid component (arrow) was relatively high (1.56 × 10−3 mm2/s) on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a plateau enhancement pattern of the solid component (arrow). The solid component was confirmed as basaloid carcinoma arising from a multilocular thymic cyst on the pathological findings (not shown).
Fig. 6.
Thymic sarcomatoid carcinoma in a 40-year-old woman. a) An axial TSE T2-weighted image shows a heterogeneous high-signal-intensity mass (arrows) in the anterior mediastinum. A peripheral capsule is not seen. The mass compresses the ascending aorta and pulmonary artery (arrowheads), which suggesting tumor invasion. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows heterogeneous high signal intensity of the mass. c) mean ADC value within the mass was 1.02 × 10−3 mm2/s on the ADC map. d, e, f) Coronal dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a heterogeneous and persistent enhancement pattern of the mass. Necrosis (arrows) is seen in the mass.
Thymic neuroendocrine tumors
Thymic neuroendocrine tumors (NETs) represent 2%–5% of all thymic epithelial tumors,28 and thymic carcinoids are approximately 0.4% of all carcinoids.29 The median age of the patients with thymic NETs is 57 years, and the male-to-female ratio is 3:1.29 In the WHO histological classification, thymic NETs are classified into typical carcinoid (low-grade), atypical carcinoid (Fig. 7) (intermediate-grade), and large cell neuroendocrine carcinoma (LCNEC) and small cell carcinoma (Fig. 8) (high-grade) according to tumor necrosis, mitosis, and cytology (Table 4).18 Approximately 25% of thymic NETs are associated with multiple endocrine neoplasia (MEN) type 1.30 The most common paraneoplastic syndrome associated with thymic NETs is Cushing’s syndrome due to ectopic production of adrenocorticotropic hormone (ACTH), whereas carcinoid syndrome is rare.18 The prognosis of typical carcinoid is slightly better than that of atypical carcinoid.18 Thymic LCNECs and small cell carcinomas are more aggressive. The reported 5-year survival rates of LCNECs range from 0% to 66%.18 Prognosis of thymic small cell carcinomas is the worst, with a median survival of 14 months.18
Fig. 7.
Recurrent thymic atypical carcinoid in an 83-year-old man. a) An axial TSE T2-weighted image with fat saturation shows a heterogeneous high-signal-intensity mass in the anterior mediastinum. A peripheral capsule is not seen. Necrosis (large arrow) in the mass and multiple flow voids (small arrows) in the mass and the periphery are seen. The mass compresses the ascending aorta and pulmonary artery, suggesting tumor invasion. A metastatic nodule (arrowhead) is also seen in the left lung. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows heterogeneous high signal intensity of the mass. c) The mean ADC value within the mass except necrotic areas was 1.15 × 10−3 mm2/s on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a heterogeneous and washout enhancement pattern of the mass. No enhancement is seen in the necrosis (arrows) in the mass.
Fig. 8.
Thymic small cell carcinoma in a 68-year-old woman. a) An axial TSE T2-weighted image shows a heterogeneous lobulated mass in the anterior mediastinum. A peripheral capsule is not seen. High-signal-intensity areas which reflect necrosis (arrows) are seen in the center of the mass. b) An axial contrast-enhanced T1-weighted image with fat saturation shows heterogeneous enhancement of the mass with central necrosis (arrows). c) An axial diffusion-weighted image (b = 1000 s/mm2) shows heterogeneous high signal intensity of the periphery of the mass (arrows). d) The mean ADC value of the periphery of the mass (arrows) was low (0.81 × 10−3 mm2/s) on the ADC map. At two months after surgery, local recurrence and left hilar lymph node metastasis appeared (not shown).
Table 4.
Histological classification of thymic neuroendocrine neoplasms18
| Neuroendocrine tumors |
| Carcinoid tumor, NOS/neuroendocrine tumor, NOS |
| Typical carcinoid/neuroendocrine tumor, grade 1 |
| Atypical carcinoid/neuroendocrine tumor, grade 2 |
| Neuroendocrine carcinomas |
| Small cell carcinoma |
| Combined small cell carcinoma |
| Large cell neuroendocrine carcinoma |
MRI Findings of Thymic Epithelial Tumors
Sadohara et al. compared CT and MRI findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas.12 They reported that thymic carcinomas (Figs. 4 and 6) showed an irregular contour more frequently (75%) than low-risk thymomas (3%) and high-risk thymomas (22%) on MR images.12 A complete or almost complete capsule (with a capsule perimeter visible for more than two-thirds of the perimeter of tumor) on T2-weighted images was seen in low-risk thymomas (27%) (Figs. 1a and 2a) and high-risk thymomas (17%) but not in thymic carcinomas.12 Internal septa were more common in low-risk (57%) (Fig. 1a) and high-risk thymomas (44%) than thymic carcinomas (8%).12 A peripheral capsule and internal septa on T2-weighted images are likely to be ill-defined in high-risk thymomas (Fig. 3a) compared to low-risk thymomas (Figs. 1a and 2a). Necrotic or cystic areas were more common in thymic carcinomas (67%) (Fig. 6) than low-risk thymomas (20%) and high-risk thymomas (28%).12 Thymic carcinomas were more heterogeneous on post-contrast MR images (100%) than low-risk thymomas (33%) and high-risk thymomas (56%).12 Mediastinal lymphadenopathy was more common in thymic carcinomas (58%) than low-risk thymomas (3%) and high-risk thymomas (6%).12 MRI was superior to CT for the detection of capsule (Figs. 1a and 2a), septum (Fig. 1a), and hemorrhage in the lesion. Thymic carcinomas more frequently invaded to the great vessels (42%) (Fig. 6) than low-risk thymomas (0%) and high-risk thymomas (6%) (Fig. 3).12 Because there was no significant difference in the diagnosis of tumor invasion to the great vessels between CT and MRI, MRI is considered to be useful in patients with contraindications to administration of contrast material, such as patients with asthma or renal failure. Inoue et al. evaluated MRI findings of the WHO histological subtypes of thymomas and thymic carcinomas and reported that type A thymomas were more likely to show a smooth contour, round shape, distinct capsule, and smaller size compared to any other type of thymic epithelial tumor.31 The frequency of heterogeneous intensity on T2-weighted images increased from type A thymomas to thymic carcinomas.31 Low-signal-intensity areas in the lesion on T2-weighted images, which may reflect abundant fibrous tissues, were more frequently seen in thymic carcinomas (56%) (Fig. 4a) than in subtypes of thymoma (from 0% to 27%).31 Mediastinal lymphadenopathy was common in thymic carcinomas (25%), but was not seen in thymomas.31
Fig. 12.
Nodular sclerosis Hodgkin lymphoma in a 32-year-old man. a) An axial HASTE T2-weighted image shows a lobulated and multinodular mass (arrows) in the anterior mediastinum. No peripheral capsule is seen. Right pleural effusion is seen. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows high-signal-intensity nodular areas (arrows) in the mass. c) The mean ADC value within the nodular areas (arrows) which show high signal intensity on the diffusion-weighted image was low (0.53 × 10−3 mm2/s) on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a heterogeneous and persistent enhancement pattern of the mass.
Sakai et al. evaluated the time intensity curves (TICs) on DCE-MRI of thymomas and non-thymomas, including thymic carcinomas, malignant lymphomas, malignant GCTs, and thymic carcinoids.9 They reported that the mean peak time of the TIC in thymomas (1.5 min) was significantly shorter than that in non-thymoma cases (3.2 min), and the mean peak time of the TIC in Masaoka stage I and II thymomas (1.3 min) was significantly shorter than that in stage III thymomas (2.5 min).9 Yabuuchi et al. evaluated TIC patterns of thymic epithelial tumors, malignant lymphomas, and malignant GCTs on DCE-MRI and categorized them as follows: persistent pattern, a time to peak of more than 120 sec; plateau pattern, a time to peak of 120 sec or less with a low washout ratio (30%); washout pattern, a time to peak of 120 sec or less with a high washout ratio (30%).11 They reported that the washout pattern was seen only in thymic epithelial tumors and was especially common in low-risk thymomas (Figs. 1 and 2), while all malignant lymphomas and malignant GCTs showed the persistent or plateau pattern.11
A few studies have discussed the DWI and ADC values of thymic epithelial tumors.2,4 Abdel Razek et al. measured the ADC values of low-risk thymomas, high-risk thymomas, and thymic carcinomas using DWI with b values of 0, 400, and 800 sec/mm2.2 They reported that the mean ADC values of high-risk thymomas (first reading: 1.16 × 10−3 mm2/sec; second reading: 1.14 × 10−3 mm2/sec) (Fig. 3c) and thymic carcinomas (1.18 × 10−3 mm2/sec and 1.06 × 10−3 mm2/sec) (Figs. 4c and 6c) were significantly lower than those of low-risk thymomas (1.30 × 10−3 mm2/sec and 1.29 × 10−3 mm2/sec) (Fig. 2c).2 And the mean ADC values of advanced-stage (Masaoka-koga stages III and IV) thymic epithelial tumors (1.18 × 10−3 mm2/sec and 1.17 × 10−3 mm2/sec) were significantly lower than those of early-stage (stages I and II) thymic epithelial tumors (1.31 × 10−3 mm2/sec and 1.29 × 10−3 mm2/sec)2. Priola et al. measured ADC values of only thymomas using DWI with b values of 150, 500, and 800 sec/mm2.4 They reported that the mean ADC values of high-risk thymomas (1.21 × 10−3 mm2/sec) (Fig. 3c) were significantly lower than those of low-risk thymomas (1.58 × 10−3 mm2/sec) (Fig. 2c). And the mean ADC values of advanced-stage thymomas (1.31 × 10−3 mm2/sec) (Fig. 3c) were significantly lower than those of early-stage thymomas (1.43 × 10−3 mm2/sec) (Fig. 2c).4 The mean ADC values of type B3 thymomas were significantly lower than those of other types of thymomas.4 Although there are overlaps of the ADC values between subtypes of thymic epithelial tumors, high grade and advanced-stage tumors can show lower ADC values than low grade and early-stage tumors. Seki et al. measured the ADC values of thymomas, thymic carcinomas, malignant lymphomas, malignant GCTs, and invasive lung carcinomas.10 They reported that the mean ADC values of thymomas (1.59 ± 0.24 × 10−3 mm2/s) were significantly higher than those of other malignant tumors (1.11 ± 0.40 × 10−3 mm2/s).10
Some thymomas and thymic carcinomas, such as basaloid carcinoma (Fig. 5) and mucoepidermoid carcinoma, can be associated with MLTC, and the reported cases show solid components in the MLTC (Fig. 5).
Limited studies about imaging findings of thymic NETs have been reported. Shimamoto et al. evaluated CT and MRI findings of 11 cases of thymic carcinoids (three typical carcinoids and eight atypical carcinoids).32 They reported that most of the lesions had an irregular margin (67% of cases) without a peripheral capsule (82%) (Fig. 7). They were heterogeneous with necrosis or cystic change (64%) (Fig. 7) and hemorrhage (18%), and septum within the mass was rare (18%).32 The lesions revealed heterogeneous (82%) and stronger enhancement than that of the chest wall muscle (91%), and tumor vessels or flow voids (Fig. 7a) were seen (18%).32 These findings suggest that thymic carcinoids are hypervascuar. Invasion to the surrounding tissues was common (64%), and hematogenous (18%) and lymph node metastases (36%) were also seen.32 A few reported cases of thymic LCNEC showed an irregular mass without a peripheral capsule in the anterior mediastinum and invasion to the surrounding tissues on MRI,33,34 and DWI showed high signal intensity of the mass in those cases.33 In our case, thymic small cell carcinoma (Fig. 8) showed a heterogeneous mass with central necrosis in the anterior mediastinum. The periphery of the mass showed high signal intensity on DWI (Fig. 8c) and low ADC values (Fig. 8d).
Germ Cell Tumors
Mediastinal germ cell tumors (GCTs) commonly occur in the anterior mediastinum. The WHO histological classification includes seminoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, mature teratoma, immature teratoma, mixed GCTs, teratoma with somatic-type malignancies, and GCTs with associated hematological malignancy (Table 5).18 While prepubertal GCTs consist only of teratomas (58% of cases) and yolk sac tumors (42%), postpubertal GCTs can be almost any of the histological types of GCTs. Teratomas (35%) and seminomas (32%) are common in postpubertal male patients, and most GCTs are teratoma (93%) in female postpubertal patients.18 Most malignant GCTs occur in adolescents and young adults. For therapeutic purposes, GCTs are grouped into seminomas and non-seminomatous GCTs (NSGCTs). Hematogenous metastasis is more common in NSGCTs than in seminomas, and the prognosis is worse in NSGCTs.
Table 5.
Histological classification of mediastinal germ cell tumors18
| Seminoma |
| Embryonal carcinoma |
| Yolk sac tumor |
| Choriocarcinoma |
| Mature teratoma |
| Immature teratoma of the thymus |
| Mixed germ cell tumor |
| Teratoma with somatic-type malignancies |
| Gem cell tumor associated with hematological malignancy |
Seminoma (Fig. 9) is composed of cells resembling primordial germ cells. Pure seminomas show a favorable response to radiotherapy and chemotherapy, and have an excellent prognosis, with a 5-year survival rate of 90%.18 Embryonal carcinoma is composed of large primitive cells of epithelial appearance, resembling cells of the embryonic gem disc. Pure embryonal carcinomas account for 2%–8% of all mediastinum GCTs.35,36 Virtually all patients have increased serum α-fetoprotein (AFP) levels. Yolk sac tumor (Fig. 10) is characterized by numerous patterns that recapitulate the yolk sac, allantois, and extra-embryonic mesenchyme. Pure yolk sac tumors in adolescents and adults account for 2%–12% of mediastinal GCTs,36–38 and AFP levels are elevated in most patients. The 5-year overall survival rate of children aged less than 15 years with NSGCT is 87%,39 whereas the rate is 45%–54% in patients after the age of 15-years.38,40–42 Choriocarcinoma is composed of syncytiotrophoblast, cytotrophoblast, and variably intermediate trophoblast. Only 3% of mediastinal GCTs are pure choriocarcinomas.18 The β-subunit of human chorionic gonadotropin (β-hCG) level is elevated in most patients. Choriocarcinomas are highly aggressive neoplasms, and most patients die of disseminated disease within months of the diagnosis. Teratoma is composed of somatic tissues derived from two or three of the germ layers (ectoderm, endoderm, and mesoderm). Mature teratomas are composed exclusively of mature, adult-type tissues. Immature teratomas contain immature, embryonic, or fetal tissues either exclusively or in addition to mature tissues.18 Mixed GCTs contain two or more histological types of GCTs. The most common components of mixed GCTs are yolk sac tumor and teratoma in children, and teratoma and embryonal carcinoma in adults.18 There is no significant difference in prognosis between mixed and pure NSGCTs. Teratomas with somatic-type malignancies are accompanied by a non-germ cell malignant component of sarcoma and carcinoma. The somatic-type malignant tumors are various sarcomas (63%), carcinomas (37%), or combinations of both, and rhabdomyosarcoma and colonic type adenocarcinoma are the most common subtypes.18 GCTs with associated hematological malignancy are GCTs accompanied by hematological malignancies that are clonally related to the underlying GCTs. Hematological malignancies develop in 2%–6% of mediastinal malignant NSGCTs.43,44
Fig. 9.
Seminoma in a 38-year-old man. a) An axial TSE T2-weighted image shows a heterogeneous high-signal-intensity mass (large arrows) with an irregular margin in the anterior mediastinum. A peripheral capsule is not seen. Thin septa (small arrows) and low-signal-intensity areas which reflect hemorrhagic necrosis (arrowheads) are seen in the mass. The superior vena cava is involved by the mass. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows high signal intensity of the periphery of the mass. c) The mean ADC value of the periphery of the mass was low (0.85 × 10−3 mm2/s) on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a heterogeneous and persistent enhancement pattern of the mass. Hemorrhagic necrosis (arrowheads) is seen in the mass but is limited.
Fig. 10.
Yolk sac tumor in a 21-year-old man. a) An axial TSE T2-weighted image shows a heterogeneous lobulated mass (large arrows) in the anterior mediastinum. A peripheral capsule is not seen. High-signal-intensity areas which reflect marked hemorrhagic necrosis (arrowheads) can be seen in the center of the mass. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows high signal intensity of the central hemorrhagic necrosis in the mass (arrowheads). c) The mean ADC values of the hemorrhagic necrotic areas in the central and solid components of the periphery of the mass were 0.68 × 10−3 mm2/s and 0.78 × 10−3 mm2/s, respectively on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a gradual enhancement of the periphery of the mass and marked hemorrhagic necrosis (arrowheads) in the mass.
Clinically, patients with malignant GCTs tend to be younger than those with thymic epithelial tumors, and they are almost all males. Elevation of AFP and β-hCG is important for the diagnosis of GCTs. At the diagnosis, GCTs are usually larger than thymic epithelial tumors.11 And the maximum standard uptake (SUVmax) values of mediastinal GCTs on 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) are reported to be higher than those of thymomas.11
MRI findings of germ cell tumors
Seminomas show a well-defined, lobulated, or multinodular mass with relatively homogeneous signal intensity on MR images compared to NSGCTs. Cystic change, necrosis, and hemorrhage may be seen in seminomas but are usually limited (Fig. 9).13,45 Thin septa may be seen in the mass (Fig. 9a). On contrast-enhanced MRI, seminomas typically show relatively homogeneous enhancement of the mass.13,45 Malignant GCTs are reported to show a persistent or plateau enhancement pattern on DCE-MRI (Fig. 9d–9f).11 NSGCTs usually show a lobulated or irregular mass with heterogeneous signal intensity due to marked hemorrhagic necrosis in the tumor (Fig. 10).13,45 Invasion to the surrounding tissues and hematogenous and lymph node metastases are more common in NSGCTs than in pure seminomas. On contrast-enhanced MRI, NSGCTs typically show a heterogenous enhancement in the periphery of the mass (Fig. 10d–10f).13,45 In our cases, DWI showed high signal intensity of solid components of malignant GCTs (Fig. 9b), and the ADC values were low (Fig. 9c). Reported cases of teratoma with somatic-type solid malignancy usually show heterogeneous and invasive solid components in cystic teratoma.45–47
Malignant Lymphomas
The WHO histological classification of mediastinal hematolymphoid tumors includes primary mediastinal large B-cell lymphoma (PMBL), extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma), T-lymphoblastic leukemia/lymphoma, classic Hodgkin lymphoma, grey zone lymphoma, follicular dendritic cell sarcoma, and myeloid sarcoma (Table 6).18 The major subtypes of mediastinal malignant lymphomas are PMBL, T-lymphoblastic lymphoma (T-LBL), and Hodgkin lymphoma. PMBL (Fig. 11) is a mature aggressive large B-cell lymphoma of putative thymic B-cell origin, arising in the mediastinum. PMBL accounts for 2%–3% of all non-Hodgkin lymphomas, and commonly occurs in young adults (adults in their 30s and 40s).18 Thymic MALT lymphoma is rare low-grade primary extranodal B-cell lymphoma. Most patients are in their sixth or seventh decade with a female predominance and higher incidence in the Asian population.18,48 Thymic MALT lymphoma is strongly associated with autoimmune disease, especially Sjögren syndrome, and patients have an excellent outcome.18 T-lymphoblastic leukemia/lymphoma is a neoplasm of precursor lymphoid cells committed to T lineage, occurring either as a mass lesion with no or minimal blood or marrow involvement (T-lymphoblastic lymphoma) or with extensive blood and/or marrow involvement (T-lymphoblastic leukemia).18 Most T-LBLs occur in late childhood, adolescence, and young adulthood.18 Many of the patients present acute symptoms related to rapid growth of the mediastinal mass. Classic Hodgkin lymphoma is a clonal, malignant B-cell lymphoid proliferation in which a minority of malignant cells exhibit a characteristic immunophenotype, termed Hodgkin/Reed-Sternberg cells. Nodular sclerosis classical Hodgkin lymphoma (NSCHL) (Fig. 12) is the most common subtype (accounting for 50%–70% of primary mediastinal lymphomas), and is most prevalent between the ages of 15 and 34 years.18
Table 6.
Histological classification of mediastinal hematolymphoid tumors18
| Mediastinal large B-cell lymphoma |
| MALT lymphoma |
| T-lymphoblastic leukemia/lymphoma |
| Classic Hodgkin lymphoma, NOS |
| Classic Hodgkin lymphoma, nodular sclerosis |
| Classic Hodgkin lymphoma, mixed cellularity |
| Classic Hodgkin lymphoma, lymphocyte-rich |
| Classic Hodgkin lymphoma, lymphocyte depletion |
| Grey zone lymphoma |
| Follicular dendritic cell sarcoma |
| Myeloid sarcoma |
NOS: not otherwise specified.
Fig. 11.
Primary mediastinal large B-cell lymphoma in a 21-year-old man. a) An axial TSE T2-weighted image shows a heterogeneous lobulated mass (large arrows) in the anterior mediastinum. Thin septa (small arrows) and necrosis (arrowhead) are seen in the mass, and no peripheral capsule is evident. The mass compresses the superior vena cava, suggesting tumor invasion. Right pleural effusion is also seen. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows heterogeneous high signal intensity of the mass. c) The mean ADC value within the mass except necrotic areas of the mass was 0.79 × 10−3 mm2/s on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a heterogeneous and persistent enhancement pattern of the mass. No enhancement is seen in the necrosis (arrowheads) in the mass.
At diagnosis, the patient ages are younger and tumor sizes are larger in malignant lymphomas than in thymic epithelial tumors.11 And SUVmax values on FDG-PET are reported to be higher in malignant lymphomas than in thymomas.11 Lactate dehydrogenase (LDH) and soluble interleukin-2 receptor (sIL-2R) are frequently elevated in the patients with malignant lymphoma.
MRI findings of malignant lymphomas
PMBLs typically show a large lobulated or irregular mass without a peripheral capsule in the anterior mediastinum (Fig. 11). The lesion may be multinodular and show internal septa (Fig. 11a). Tumor invasion to the adjacent mediastinal structures such as the superior vena cava (Fig. 11), chest wall, and lung is common. Necrosis (Fig. 11), cystic change and hemorrhage are frequently seen in the lesion. Mediastinal lymph node involvements are common.13 About half of cases have pleural and/or pericardial effusion.49 Many of the reported cases with thymic MALT lymphomas show a multilocular cystic mass mimicking MLTC. Cases with a solid mass or a mass with a mixture of solid and cystic components have also been reported.48 Signal intensity in the cyst on T1-weighted image may vary according to the presence of serous, viscous, or hemorrhagic fluid in the thymic MALT lymphoma.48 T-LBLs are usually a large lobulated or irregular mass without a peripheral capsule and are heterogeneous due to necrosis, cystic change, and hemorrhage in the lesion. Tumor invasion to the adjacent mediastinal structures, pleural effusion, and pericardial effusion are common.49 Tumor spread to the extrathoracic lymph nodes, bone marrow, and/or central nervous system is commonly seen in extensive cases with T-LBL.49 NSCHLs are a lobulated or multinodular mass (Fig. 12), and thin septa may be seen in the mass. Necrotic and cystic changes are limited in NSCHLs, and NSCHLs are relatively homogeneous compared to PMBLs and T-LBLs. Mediastinal lymph node enlargements adjacent to the primary lesion may be seen.13 Pleural and pericardial effusion are rare compared to PMBLs and T-LBLs.49
Calcification is very rare in any subtypes of malignant lymphomas prior to chemotherapy. Penetration of vessels in the lesion is one of the characteristic findings of malignant lymphomas. On DCE-MRI, malignant lymphomas are reported to show a persistent or plateau enhancement pattern (Figs. 11 and 12).11 Some studies reported that the ADC values of mediastinal malignant lymphoma were low (Figs. 11c and 12c).8,17 Zhang et al. performed a comparative ADC histogram analysis of thymic carcinomas versus malignant lymphomas.17 They reported that the mean, median, 10th percentile, and 90th percentile ADC values of malignant lymphomas were significantly lower than those of thymic carcinomas, and the results suggested that malignant lymphomas have high tumor cell density.17
Thymic Hyperplasia
Thymic hyperplasia is histologically divided into true thymic hyperplasia and thymic lymphoid hyperplasia. True thymic hyperplasia is an enlargement of the thymus with normal histological features of the thymic tissues. Rebound thymic hyperplasia is common type of true hyperplasia and is secondary to atrophy caused by such as chemotherapy for malignancy.13 Differential diagnosis between thymic rebound hyperplasia and recurrence is clinically important in patients after chemotherapy for malignancy. Thymic lymphoid hyperplasia is pathologically characterized by hyperplastic lymphoid follicles with germinal centers in the medulla of the thymus, and is associated with various autoimmune diseases such as MG and collagen vascular diseases (Fig. 13).13
Fig. 13.
Thymic hyperplasia in a 50-year-old woman with rheumatoid arthritis. a) An axial in-phase image shows a lobulated and flat lesion (arrows) in the anterior mediastinum. b) An axial opposed-phase image shows decreasing-signal-intensity areas (arrowheads) which reflect intermingled fat tissues in the lesion compared with the in-phase image. c) An axial diffusion-weighted image shows slightly high signal intensity of the lesion (arrows). d) The mean ADC value within the lesion (arrows) was high (2.49 × 10−3 mm2/s) on the ADC map.
MRI findings of thymic hyperplasia
Thymic hyperplasia typically manifests as a round or box-shaped mass in children and a flat or triangular mass (Fig. 13) in adults. In patients with thymic hyperplasia older than 15 years of age, CSI can detect fatty infiltration within the thymic hyperplasia (Fig. 13a, 13b) and is useful in its differentiation from thymic tumors such as thymoma. CSI has been reported to detect fatty infiltration of the normal thymus in 50% of persons aged 11–15 years and none of those under 10 years.16 Inaoka et al. evaluated CSI in patients with thymic hyperplasia and thymic tumors, and reported that all cases with hyperplasia but none of the cases with thymic tumors showed a signal decrease on opposed-phase images compared with in-phase images, with no overlap in the range of chemical shift ratio between the two groups.3 CSI may be useful in differentiating between rebound thymic hyperplasia and recurrence of malignancy after chemotherapy, especially if thymic hyperplasia presents with normal physiological uptake at FDG-PET. Although the SUVmax is reported to be useful in differentiating rebound thymic hyperplasia from thymic tumors, there is a considerable overlap of the SUVmax values between them.50,51
DWI demonstrates unrestricted diffusion with high ADC values in thymic hyperplasia (Fig. 13d) and is useful for differentiation of thymic hyperplasia from thymic tumors such as malignant lymphoma, especially in subjects younger than 15 years, who may present uptake on FDG-PET and no signal intensity loss on CSI within the normal thymic tissue. DWI may differentiate rebound thymic hyperplasia from recurrent malignant lymphoma in children and adolescents without invasive procedures such as biopsy and radiation exposure by CT or FDG-PET. Priola et al. measured the ADC values between a normal thymus group showing no signal suppression on opposed-phase CSI and a malignant lymphoma group in young subjects.6 They reported that the mean ADC values of the normal thymus group (2.48 ± 0.38 × 10−3 mm2/s) were significantly higher than those of the malignant lymphoma group (1.24 ± 0.23 × 10−3 mm2/s).6 In another study, Priola et al. reported that the mean ADC values differed significantly between benign conditions (normal thymus and lymphoid thymic hyperplasia, mean ADC of 1.92 ± 0.21 × 10−3 mm2/s) and thymomas (mean ADC of 1.36 ± 0.33 × 10−3 mm2/s), with an optimal ADC cut-point of 1.625 × 10−3mm2/s (sensitivity and specificity of 96.8% and 79.2%, respectively).5
Other Anterior Mediastinal Solid Tumors and MRI Findings
Thymolipomas are a rare benign anterior mediastinum tumor which contains fat and thymic tissues and are common in young adults (the average age is 22–26 years) with no gender predominance.52 On MRI, thymolipomas show a large, soft, and well-defined mass comprised of high-signal-intensity areas which reflect fat tissues and intermediate-signal-intensity areas which reflect thymic and fibrous tissues.52
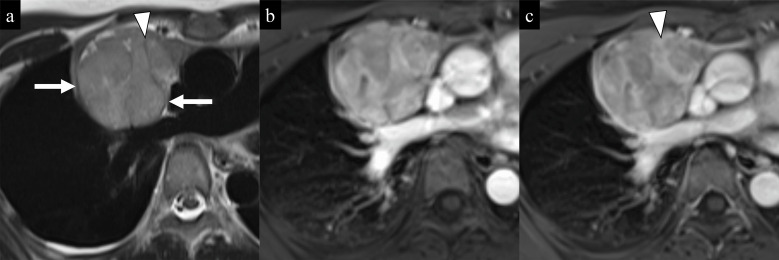
Intrathoracic goiters are common in the superior portion of the mediastinum and may extend to the anterior and middle mediastinum.53 Although the majority of cases are extensions of cervical goiters, approximately 2% of cases arise from a mediastinal ectopic thyroid gland and show no connection to the cervical thyroid gland (Fig. 14).54 Ectopic mediastinal goiters may mimic thymic epithelial tumors. Most intrathoracic goiters are adenomatous goiters histologically, and thyroid carcinomas are rare. Thyroid tissues containing iodine may show high attenuation on plain CT. Adenomatous goiters show multiple nodules, and calcifications, cystic change (Fig. 14), and hemorrhage are common in the lesion.53 Because intrathoracic goiters are hypervascular, flow voids on T2-weighted images (Fig. 14a) and a rapid intense enhancement on DCE-MRI (Fig. 14d–14f) may be seen in the lesion.14,53 123I-scintigraphy shows uptake in the thyroid tissues and is useful for the diagnosis of ectopic mediastinal goiters.53
Fig. 14.
Ectopic mediastinal goiter in a 73-year-old woman. a) An axial HASTE T2-weighted image shows a well-circumscribed and heterogeneous mass (large arrows) with multiple cystic changes in the anterior mediastinum. Flow voids (arrowheads) are seen in the periphery of the mass. b) An axial diffusion-weighted image (b = 1000 s/mm2) shows slightly high signal intensity of the mass (arrow). c) The mean ADC value within the mass (arrow) was high (1.62 × 10−3 mm2/s) on the ADC map. d, e, f) Axial dynamic contrast-enhanced images (a: precontrast; b: 30 sec; c: 180 sec after the start of injection of the contrast material) show a rapid intense enhancement and a washout enhancement pattern of the mass (arrow). No enhancement is seen in cystic changes (arrowheads) in the mass.Diagnosis of ectopic mediastinal adenomatous goiter without connection to the cervical thyroid gland was confirmed by surgery (not shown).
Ectopic mediastinal parathyroid adenomas are reported to be detected in 22% of patients with hyperparathyroidism.55 They usually arise from the inferior parathyroid glands and are common near or within the thymus in the superior portion of the mediastinum and anterior mediastinum.56 Ectopic parathyroid adenomas show a small solid nodule and a rapid intense enhancement on DCE-MRI because of hypervascularity of the lesion.14,53 Calcification, cystic change, and hemorrhage may be seen in the lesion. 99mTc-methoxy-isobutyl-isonitrile (MIBI) scintigraphy is useful for the detection of ectopic parathyroid adenomas.57
Most mediastinal neurogenic tumors occur in the posterior mediastinum, but vagus or phrenic nerve schwannomas can occur in the superior portion of the mediastinum and anterior mediastinum.15 On MRI, benign peripheral nerve tumors are a well-circumscribed solid mass with a thin fibrous capsule. Cystic change and hemorrhage are frequently seen in schwannomas. Schwannomas histologically contain the Antoni A areas composed of cellular spindle cells and Antoni B areas which are hypocellular with abundant myxomatous stroma. The Antoni A areas show relatively low signal intensity on T2-weighted images, relatively low ADC values, and relatively intense contrast enhancement on DCE-MRI. The Antoni B areas show very high signal intensity on T2-weighted images, relatively high ADC values, and gradual and weak contrast enhancement on DCE-MRI.58 Signal intensity of neurofibromas on T2-weighted images depends on the ratio of the myxomatous and fibrous tissues in the tumor, and the ADC values are usually relatively high. On DCE-MRI, neurofibromas usually show a gradual and weak contrast enhancement which reflects myxomatous and fibrous tissues in the lesion.58 Mediastinal paragangliomas mainly occur in two locations. The aortosympathetic paraganglia are located along the sympathetic chain, and aortosympathetic paragangliomas occur in the paravertebral region. The aortopulmonary paraganglia, also known as aortic bodies, are located along the great vessels,59 and aortopulmonary paragangliomas occur in the superior portion of the mediastinum, anterior mediastinum and middle mediastinum.15 On MRI, paragangliomas are a well-defined or invasive heterogeneous mass, and hemorrhage and necrosis are common in the mass.58 Flow voids within or around the mass on T2-weigted images and a rapid and intense enhancement on DCE-MRI can be seen, which reflect hypervascularity of the tumor.58 A “salt and pepper” appearance indicates adjacent high-signal-intensity regions which reflect slow flow within the tumor vessels or hemorrhage and low-signal-intensity regions which reflect high flow within tumor vessels on T2-weighted images and may be seen in paragangliomas.60
Conclusion
MRI has high soft-tissue contrast resolution and can provide additional information about the differential diagnosis of anterior mediastinal solid tumors. T2-weighted images are useful for evaluating a fibrous capsule and septa in thymic epithelial tumors. Enhancement patterns on DCE-MRI reflect the vascularity and histopathological features of the tumor, and anterior mediastinal solid tumors can show different enhancement patterns. ADC values reflect the tumor cell density and can provide additional information about the histopathological features of the tumor and histological grade of malignancy. CSI can detect tiny fat tissues and is useful in differentiating thymic hyperplasia from other tumors. A comprehensive evaluation of MRI sequences is useful for estimating the histopathological features of anterior mediastinal solid tumors and narrowing the differential diagnosis.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Tomiyama N, Honda O, Tsubamoto M, et al. Anterior mediastinal tumors: diagnostic accuracy of CT and MRI. Eur J Radiol 2009; 69:280–288. [DOI] [PubMed] [Google Scholar]
- 2.Abdel Razek AA, Khairy M, Nada N. Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology 2014; 273:268–275. [DOI] [PubMed] [Google Scholar]
- 3.Inaoka T, Takahashi K, Mineta M, et al. Thymic hyperplasia and thymus gland tumors: differentiation with chemical shift MR imaging. Radiology 2007; 243:869–876. [DOI] [PubMed] [Google Scholar]
- 4.Priola AM, Priola SM, Giraudo MT, et al. Diffusion-weighted magnetic resonance imaging of thymoma: ability of the apparent diffusion coefficient in predicting the World Health Organization (WHO) classification and the Masaoka-Koga staging system and its prognostic significance on disease-free survival. Eur Radiol 2016; 26:2126–2138. [DOI] [PubMed] [Google Scholar]
- 5.Priola AM, Priola SM, Giraudo MT, et al. Chemical-shift and diffusion-weighted magnetic resonance imaging of thymus in myasthenia gravis: usefulness of quantitative assessment. Invest Radiol 2015; 50:228–238. [DOI] [PubMed] [Google Scholar]
- 6.Priola AM, Priola SM, Gned D, Giraudo MT, Veltri A. Nonsuppressing normal thymus on chemical-shift MR imaging and anterior mediastinal lymphoma: differentiation with diffusion-weighted MR imaging by using the apparent diffusion coefficient. Eur Radiol 2018; 28:1427–1437. [DOI] [PubMed] [Google Scholar]
- 7.Priola AM, Priola SM, Gned D, et al. Diffusion-weighted quantitative MRI to diagnose benign conditions from malignancies of the anterior mediastinum: Improvement of diagnostic accuracy by comparing perfusion-free to perfusion-sensitive measurements of the apparent diffusion coefficient. J Magn Reson Imaging 2016; 44:758–769. [DOI] [PubMed] [Google Scholar]
- 8.Razek AA, Elmorsy A, Elshafey M, Elhadedy T, Hamza O. Assessment of mediastinal tumors with diffusion-weighted single-shot echo-planar MRI. J Magn Reson Imaging 2009; 30:535–540. [DOI] [PubMed] [Google Scholar]
- 9.Sakai S, Murayama S, Soeda H, Matsuo Y, Ono M, Masuda K. Differential diagnosis between thymoma and non-thymoma by dynamic MR imaging. Acta Radiol 2002; 43:262–268. [DOI] [PubMed] [Google Scholar]
- 10.Seki S, Koyama H, Ohno Y, et al. Diffusion-weighted MR imaging vs. multi-detector row CT: Direct comparison of capability for assessment of management needs for anterior mediastinal solitary tumors. Eur J Radiol 2014; 83:835–842. [DOI] [PubMed] [Google Scholar]
- 11.Yabuuchi H, Matsuo Y, Abe K, et al. Anterior mediastinal solid tumours in adults: characterisation using dynamic contrast-enhanced MRI, diffusion-weighted MRI, and FDG-PET/CT. Clin Radiol 2015; 70:1289–1298. [DOI] [PubMed] [Google Scholar]
- 12.Sadohara J, Fujimoto K, Müller NL, et al. Thymic epithelial tumors: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol 2006; 60:70–79. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Al-Janabi NJ. Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging 2010; 32:1325–1339. [DOI] [PubMed] [Google Scholar]
- 14.Nakazono T, Yamaguchi K, Egashira R, Mizuguchi M, Irie H. Anterior mediastinal lesions: CT and MRI features and differential diagnosis. Jpn J Radiol 2021; 39:101–117. [DOI] [PubMed] [Google Scholar]
- 15.Nakazono T, Yamaguchi K, Egashira R, et al. CT-based mediastinal compartment classifications and differential diagnosis of mediastinal tumors. Jpn J Radiol 2019; 37 117–134. [DOI] [PubMed] [Google Scholar]
- 16.Inaoka T, Takahashi K, Iwata K, et al. Evaluation of normal fatty replacement of the thymus with chemical-shift MR imaging for identification of the normal thymus. J Magn Reson Imaging 2005; 22:341–346. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Zhou Y, Xu XQ, et al. A whole-tumor histogram analysis of apparent diffusion coefficient maps for differentiating thymic carcinoma from lymphoma. Korean J Radiol 2018; 19:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The WHO classification of Tumours Editorial Board. WHO classification of tumours. Toracic tumours, 5th edition, In. Lyon:International Agency for Research on Cancer (IARC), 2021. [Google Scholar]
- 19.Osserman KE, Genkins G. Studies in myasthenia gravis: review of a twenty-year experience in over 1200 patients. Mt Sinai J Med 1971; 38:497–537. [PubMed] [Google Scholar]
- 20.Jeong YJ, Lee KS, Kim J, Shim YM, Han J, Kwon OJ. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis?. AJR Am J Roentgenol 2004; 183:283–289. [DOI] [PubMed] [Google Scholar]
- 21.Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics 2010; 30:413–428. [DOI] [PubMed] [Google Scholar]
- 22.Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol 2012; 138:103–114. [DOI] [PubMed] [Google Scholar]
- 23.Evans AG, French CA, Cameron MJ, et al. Pathologic characteristics of NUT midline carcinoma arising in the mediastinum. Am J Surg Pathol 2012; 36:1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrini P, French CA, Rajan A, et al. NUT rearrangement is uncommon in human thymic epithelial tumors. J Thorac Oncol 2012; 7:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012; 18:5773–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasserjian RP, Klimstra DS, Rosai J. Carcinoma of the thymus with clear-cell features. Report of eight cases and review of the literature. Am J Surg Pathol 1995; 19:835–841. [DOI] [PubMed] [Google Scholar]
- 27.Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991; 67:1025–1032. [DOI] [PubMed] [Google Scholar]
- 28.Chaer R, Massad MG, Evans A, Snow NJ, Geha AS. Primary neuroendocrine tumors of the thymus. Ann Thorac Surg 2002; 74:1733–1740. [DOI] [PubMed] [Google Scholar]
- 29.Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010; 251:1117–1121. [DOI] [PubMed] [Google Scholar]
- 30.Gibril F, Chen YJ, Schrump DS, et al. Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab 2003; 88:1066–1081. [DOI] [PubMed] [Google Scholar]
- 31.Inoue A, Tomiyama N, Fujimoto K, et al. MR imaging of thymic epithelial tumors: correlation with World Health Organization classification. Radiat Med 2006; 24:171–181. [DOI] [PubMed] [Google Scholar]
- 32.Shimamoto A, Ashizawa K, Kido Y, et al. CT and MRI findings of thymic carcinoid. Br J Radiol 2017; 90:20150341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domen H, Hida Y, Sato M, et al. Resected thymic large cell neuroendocrine carcinoma: report of a case. Surg Case Rep 2018; 4:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa F, Iyoda A, Amano H, et al. Thymic large cell neuroendocrine carcinoma: report of a resected case - a case report. J Cardiothorac Surg 2010; 5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knapp RH, Hurt RD, Payne WS, et al. Malignant germ cell tumors of the mediastinum. J Thorac Cardiovasc Surg 1985; 89:82–89. [PubMed] [Google Scholar]
- 36.Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer 1997; 80:681–690. [PubMed] [Google Scholar]
- 37.Kesler KA, Rieger KM, Hammoud ZT, et al. A 25-year single institution experience with surgery for primary mediastinal nonseminomatous germ cell tumors. Ann Thorac Surg 2008; 85:371–378. [DOI] [PubMed] [Google Scholar]
- 38.Sarkaria IS, Bains MS, Sood S, et al. Resection of primary mediastinal non-seminomatous germ cell tumors: a 28-year experience at memorial sloan-kettering cancer center. J Thorac Oncol 2011; 6:1236–1241. [DOI] [PubMed] [Google Scholar]
- 39.Schneider DT, Calaminus G, Reinhard H, et al. Primary mediastinal germ cell tumors in children and adolescents: results of the German cooperative protocols MAKEI 83/86, 89, and 96. J Clin Oncol 2000; 18:832–839. [DOI] [PubMed] [Google Scholar]
- 40.Fedyanin M, Tryakin A, Mosyakova Y, et al. Prognostic factors and efficacy of different chemotherapeutic regimens in patients with mediastinal nonseminomatous germ cell tumors. J Cancer Res Clin Oncol 2014; 140:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann JT, Nichols CR, Droz JP, et al. Prognostic variables for response and outcome in patients with extragonadal germ-cell tumors. Ann Oncol 2002; 13:1017–1028. [DOI] [PubMed] [Google Scholar]
- 42.Rodney AJ, Tannir NM, Siefker-Radtke AO, et al. Survival outcomes for men with mediastinal germ-cell tumors: the University of Texas M. D. Anderson Cancer Center experience. Urol Oncol 2012; 30:879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartmann JT, Fossa SD, Nichols CR, et al. Incidence of metachronous testicular cancer in patients with extragonadal germ cell tumors. J Natl Cancer Inst 2001; 93:1733–1738. [DOI] [PubMed] [Google Scholar]
- 44.Neiman RS, Orazi A. Mediastinal non-seminomatous germ cell tumours: their association with non-germ cell malignancies. Pathol Res Pract 1999; 195:589–594. [DOI] [PubMed] [Google Scholar]
- 45.Drevelegas A, Palladas P, Scordalaki A. Mediastinal germ cell tumors: a radiologic-pathologic review. Eur Radiol 2001; 11:1925–1932. [DOI] [PubMed] [Google Scholar]
- 46.Athanasiou A, Vanel D, El Mesbahi O, Theodore C, Fizazi K. Non-germ cell tumours arising in germ cell tumours (teratoma with malignant transformation) in men: CT and MR findings. Eur J Radiol 2009; 69:230–235. [DOI] [PubMed] [Google Scholar]
- 47.Sumi A, Nagata S, Zaizen M, et al. Mature cystic teratoma with an element of hepatocellular carcinoma in anterior mediastinum: Magnetic resonance-pathologic correlation. J Thorac Imaging 2017; 32:W84-86. [DOI] [PubMed] [Google Scholar]
- 48.Kuroki S, Nasu K, Murakami K, et al. Thymic MALT lymphoma: MR imaging findings and their correlation with histopathological findings on four cases. Clin Imaging 2004; 28:274–277. [DOI] [PubMed] [Google Scholar]
- 49.Tateishi U, Müller NL, Johkoh T, et al. Primary mediastinal lymphoma: characteristic features of the various histological subtypes on CT. J Comput Assist Tomogr 2004; 28:782–789. [DOI] [PubMed] [Google Scholar]
- 50.Gawande RS, Khurana A, Messing S, et al. Differentiation of normal thymus from anterior mediastinal lymphoma and lymphoma recurrence at pediatric PET/CT. Radiology 2012; 262:613–622. [DOI] [PubMed] [Google Scholar]
- 51.Jerushalmi J, Frenkel A, Bar-Shalom R, Khoury J, Israel O. Physiologic thymic uptake of 18F-FDG in children and young adults: a PET/CT evaluation of incidence, patterns, and relationship to treatment. J Nucl Med 2009; 50:849–853. [DOI] [PubMed] [Google Scholar]
- 52.Rosado-de-Christenson ML, Pugatch RD, Moran CA, Galobardes J. Thymolipoma: analysis of 27 cases. Radiology 1994; 193:121–126. [DOI] [PubMed] [Google Scholar]
- 53.Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest 1997; 112:511–522. 10.1378/chest.112.2.511 [DOI] [PubMed] [Google Scholar]
- 54.Maeda A, Shimizu K, Yukawa T, Hirami Y, Nakata M. A case of aberrant mediastinal goiter. Jpn J Assoc Chest Surg. 2010; 24:195–199. (in Japanese) [Google Scholar]
- 55.Clark OH. Mediastinal parathyroid tumors. Arch Surg 1988; 123:1096–1100. [DOI] [PubMed] [Google Scholar]
- 56.Nathaniels EK, Nathaniels AM, Wang CA. Mediastinal parathyroid tumors: a clinical and pathological study of 84 cases. Ann Surg 1970; 171:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gotthardt M, Lohmann B, Behr TM, et al. Clinical value of parathyroid scintigraphy with technetium-99m methoxyisobutylisonitrile: discrepancies in clinical data and a systematic metaanalysis of the literature. World J Surg 2004; 28:100–107. [DOI] [PubMed] [Google Scholar]
- 58.Nakazono T, White CS, Yamasaki F, et al. MRI findings of mediastinal neurogenic tumors. AJR Am J Roentgenol 2011; 197:W643-W652. [DOI] [PubMed] [Google Scholar]
- 59.Balcombe J, Torigian DA, Kim W, Miller WT., Jr. Cross-sectional imaging of paragangliomas of the aortic body and other thoracic branchiomeric paraganglia. AJR Am J Roentgenol 2007; 188:1054–1058. [DOI] [PubMed] [Google Scholar]
- 60.Olsen WL, Dillon WP, Kelly WM, Norman D, Brant-Zawadzki M, Newton TH. MR imaging of paragangliomas. AJR Am J Roentgenol 1987; 148:201–204. [DOI] [PubMed] [Google Scholar]