Abstract
Purpose: The optimal temporal resolution for free-breathing dynamic contrast-enhanced MRI (FBDCE-MRI) of the pancreas has not been determined. This study aimed to evaluate the appropriate temporal resolution to achieve good image quality and to perform pharmacokinetic analysis in FBDCE-MRI of the pancreas using golden-angle radial sparse parallel (GRASP).
Methods: Sixteen participants (53 ± 15 years, eight females) undergoing FBDCE-MRI were included in this prospective study. Images were retrospectively reconstructed at four temporal resolutions (1.8, 3.0, 4.8, and 7.8s). Two radiologists (5 years of experience) evaluated the image quality of each reconstructed image by assessing the visualization of the celiac artery (CEA), the common hepatic artery, the splenic artery, each area of the pancreas, and artifacts using a 5-point scale. Using Tissue-4D, pharmacokinetic parameters were calculated for each area in the reconstructed images at each temporal resolution for 16 examinations, excluding two with errors in the pharmacokinetic modeling analysis. Friedman and Bonferroni tests were used for analysis. A P value < 0.05 was considered statistically significant.
Results: During vascular assessment, only scores for the CEA at 7.8s were significantly lower than the other temporal resolutions. Scores of all pancreatic regions and artifacts were significantly lower at 1.8s than at 4.8s and 7.8s. In the pharmacokinetic analysis, all volume transfer coefficients (Ktrans), rate constants (Kep), and the initial area under the concentration curve (iAUC) in the pancreatic head and tail were significantly lower at 4.8s and 7.8s than at 1.8s. iAUC in the pancreatic body and extracellular extravascular volume fraction (Ve) in the pancreatic head were significantly lower at 7.8s than at 1.8s.
Conclusion: A temporal resolution of 3.0s is appropriate to achieve image quality and perform pharmacokinetic analysis in FBDCE-MRI of the pancreas using GRASP.
Keywords: dynamic contrast-enhanced magnetic resonance imaging, golden-angle radial sparse parallel, image quality, pharmacokinetic analysis, temporal resolution
Introduction
In recent years, dynamic contrast-enhanced MRI (DCE-MRI) has been used widely, not only for image evaluation but also for pharmacokinetic analysis.1–5 Ideally, image evaluation and pharmacokinetic analysis should be performed during a single imaging session; however, image quality and pharmacokinetic analysis are strongly interrelated. Although detailed pharmacokinetic analysis is possible at short temporal resolutions, the amount of data collected is small, and the image quality may be sub-optimal. Furthermore, even if long temporal resolution improves the image quality by increasing the amount of acquired data, it may not provide an accurate time-intensity curve, and thus it may impede an accurate description of the relevant pharmacokinetics.6,7
DCE-MRI using golden-angle radial sparse parallel (GRASP), which applies radial sampling8 and compressed sensing,9 allows images to be reconstructed at any temporal resolution after data acquisition. Therefore, by analyzing the DCE-MRI of different temporal resolutions during the examination of the same patient, it is possible to study the relationship between temporal resolution, image quality, and pharmacokinetic analysis more accurately.
Accordingly, we analyzed the effects of temporal resolution on image quality and pharmacokinetic analysis in pancreatic free-breathing DCE-MRI (FBDCE-MRI) using the aforementioned diagnostic method and investigated the optimal temporal resolution compatible with both image quality and pharmacokinetic analysis.
Materials and Methods
Patient characteristics
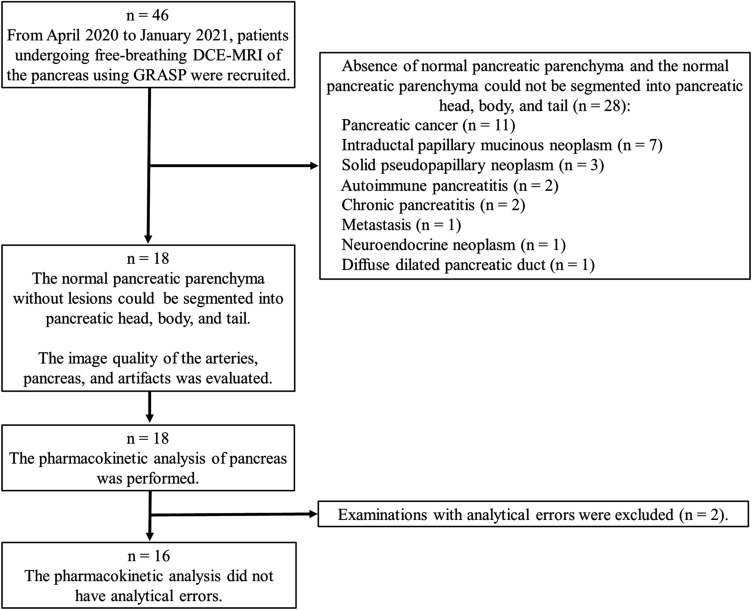
This prospective study was approved by our institutional review board. Written informed consent was obtained from all patients, and the procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We recruited 46 patients who underwent FBDCE-MRI of the pancreas using GRASP between April 2020 and January 2021. Subsequently, we excluded 28 examinations because we could not segment the normal pancreatic parenchyma of the pancreatic head, body, and tail from the lesioned area due to the presence of the tumor itself, as well as inflammation and a diffuse dilated pancreatic duct. The image quality evaluation and pharmacokinetic analysis were performed for 18 DCE-MRI examinations for 16 patients (eight men and eight women; mean age ± standard deviation, 53.2 ± 15.3 years). A flowchart of the patient selection is shown in Fig. 1. The characteristics of the study population, including indications for imaging and diagnosis, are shown in Table 1.
Fig. 1.
Summary flowchart of the patient selection process. DCE-MRI, dynamic contrast-enhanced MRI; GRASP, golden-angle radial sparse parallel.
Table 1.
Examination characteristics
| Image quality n = 18 | Pharmacokinetic analysis without analytical errors n = 16 | |
|---|---|---|
| Indications for imaging | ||
| Follow-up (n) | 9 | 7 |
| Suspected pancreas mass (n) | 4 | 4 |
| Preoperative assessment (n) | 3 | 3 |
| Tumor exclusion for genetic disorders (n) | 2 | 2 |
| Diagnosis | ||
| Neuroendocrine neoplasm (n) | 6 | 5 |
| No abnormalities (n) | 5 | 4 |
| Solid pseudopapillary neoplasm (n) | 2 | 2 |
| Intraductal papillary mucinous neoplasm (n) | 2 | 2 |
| Gastrointestinal stromal tumor (n) | 1 | 1 |
| Mucinous cystic neoplasm (n) | 1 | 1 |
| Pancreatic cancer (uncus) (n) | 1 | 1 |
MRI protocol
All MR images were obtained on a 3 T MR unit (MAGNETOM Vida; Siemens Healthcare, Erlangen, Germany) with a 30-channel body array coil and a spine matrix coil provided by the manufacturer. FBDCE-MR images were obtained using GRASP. The following sequence parameters were used: TR/TE, 2.61–2.87/1.24–1.29 ms; FOV, 360 × 360 or 380 × 380 mm; slice thickness, 2.0 mm; flip angle, 11˚; matrix, 288 × 288; and acquisition time, 180s. A standard dose of gadoterate meglumine (Magnescope; Fuji Pharma, Tokyo, Japan) (0.2 mL/kg) was administered to all patients. The contrast agent was injected into the cubital vein at a rate of 3 mL/s, followed by 50 mL of 0.9% saline at the same rate. A respiratory synchronization method called liver gate, which reconstructs images using a combination of 50% of the signals with the least variability within a certain time period, was used in combination with GRASP. DCE-MR images were retrospectively reconstructed at four temporal resolutions (1.8, 3.0, 4.8, and 7.8s) from raw data for each patient. In the GRASP sequence, the lowest number of sub-frame views was 10 and the number of sub-frame views was determined based on the Fibonacci sequence. In this study, 10, 17, 27, and 44 sub-frame views were used and the acquisition time of each view was 1.8, 3.0, 4.8, and 7.8s, respectively. The acceleration factor was 45.2 at a temporal resolution of 1.8s, 26.6 at 3.0s, 16.8 at 4.8s, and 10.2 at 7.8s.
Image analysis
In the imaging evaluation, two radiologists with 5 years of experience independently evaluated the visualization of the celiac artery (CEA), the splenic artery (SA), the common hepatic artery (CHA), the pancreatic head, pancreatic body, and pancreatic tail and the degree of artifacts on a 5-point scale during the arterial predominant phase. All temporal resolution images from 18 examinations were classified as follows: for visualization, score 1–5 meant not visible, undiagnostic and poor, undiagnostic but visible, diagnostic and good, and diagnostic and excellent, respectively; for the degree of artifacts: score 1–5 meant distinct artifact, intermediate artifact, moderate artifact, faint artifact, and no artifact, respectively. When readers gave difference scores, final values were determined by discussion and consensus.
For the pharmacokinetic analysis, the axial sections of the head, body, and tail of the pancreas to be measured in each examination were defined by the readers’ agreement. At defined axial sections, each pancreatic region was manually surrounded by ROIs for the arterial predominant phase of all temporal resolutions to obtain a concentration curve within the ROI. The axial sections in which the readers were able to enclose > 3 cm2 of the normal pancreatic parenchyma without lesioned areas were selected; however, in difficult examinations, the section with the largest area of the normal pancreatic parenchyma without lesioned areas was chosen instead. Pharmacokinetic parameters (volume transfer coefficient [Ktrans], rate constant [Kep], extracellular extravascular volume fraction [Ve], and initial area under the concentration curve [iAUC]) in the ROI were calculated according to the Tofts model (Tissue-4D software program; Siemens Healthcare). For pharmacokinetic analysis, the rapid function was chosen as the arterial input function, which has the lowest Chi-square (χ2), an error index representing the fit of the model.
Statistical analysis
When the readers provided different scores for image quality evaluation, the final score was decided by consensus. Inter-rater agreement of the scores was analyzed using Cohen’s kappa statistics with the kappa values defined as follows: ≤ 0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, excellent agreement.
In the pharmacokinetic analysis, examinations with χ2 > 0.5 were excluded as analytical errors, and the mean of the parameters measured by the two radiologists was used.
Statistical analyses were performed using SPSS version 27 (IBM, Armonk, NY, USA) and EXCEL Toukei Version. 7.1 (ESUMI, Tokyo, Japan). Friedman tests were performed to assess the differences in the pharmacokinetic parameters and image quality between all temporal resolutions. Multiple comparisons were accounted for using the Bonferroni correction. A P value < 0.05 was considered to indicate statistical significance.
Results
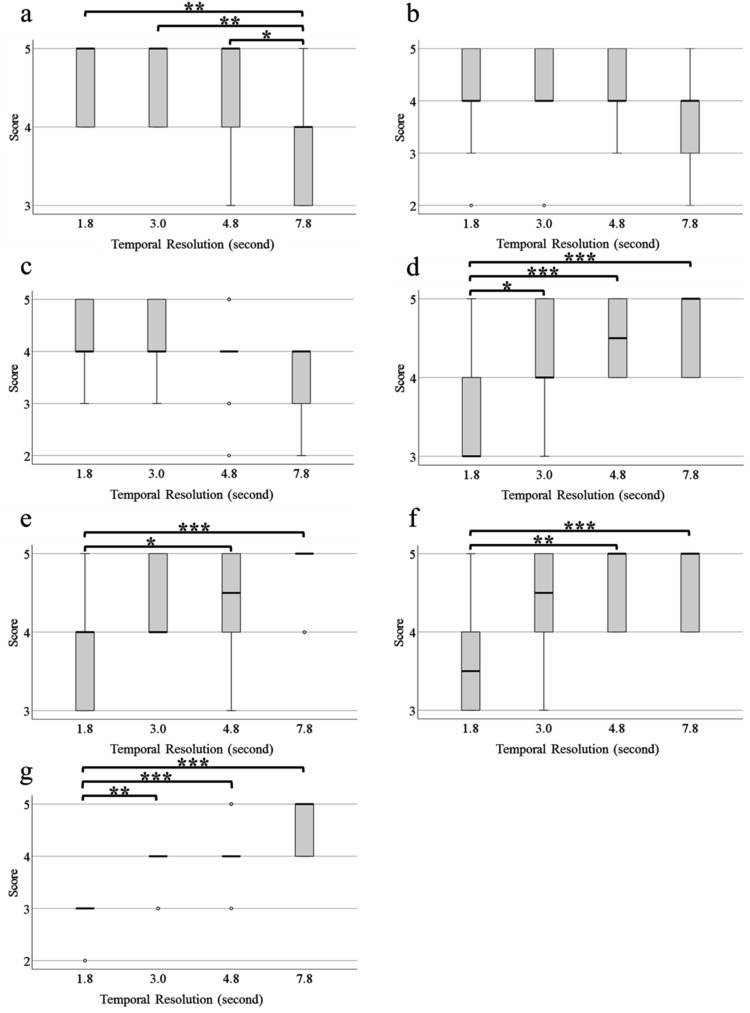
When scores ≤ 3 were considered non-diagnostic and scores ≥ 4 were considered diagnostic, the kappa values before the final consultation for the two readers in the imaging evaluation were 0.578 (95% confidence interval [CI] 0.275 to 0.882) for CEA, 0.579 (95% CI 0.347 to 0.812) for SA, 0.601 (95% CI 0.351 to 0.850) for CHA, 0.702 (95% CI 0.480 to 0.923) for the pancreatic head, 0.684 (95% CI 0.395 to 0.973) for the pancreatic body, 0.464 (95% CI 0.183 to 0.746) for the pancreatic tail, and 0.646 (95% CI 0.449 to 0.843) for the artifacts; the kappa values indicated moderate or good agreement. The visualization scores for the CEA, SA, CHA, pancreatic head, pancreatic body, and pancreatic tail and degree of artifacts at all temporal resolutions are shown in Fig. 2. The scores for CEA and CHA at 7.8s were lower than at other temporal resolutions, with significant differences only for CEA (1.8s, P < 0.01; 3.0s, P < 0.01; 4.8s, P < 0.05). The SA score decreased with an increase in temporal resolution, but there was no significant difference between the temporal resolutions. Scores for the pancreatic head, pancreatic body, pancreatic tail, and artifacts increased as the temporal resolution increased. These scores were significantly lower at 1.8s than at 4.8s and 7.8s (pancreatic head: 4.8s, P < 0.001; and 7.8s, P < 0.001; pancreatic body: 4.8s, P < 0.05; and 7.8s, P < 0.001; pancreatic tail: 4.8s, P < 0.01; and 7.8s, P < 0.001; and artifacts: 4.8s, P < 0.001; and 7.8s, P < 0.001). The same was true for the pancreatic head (P < 0.05) and artifacts (P < 0.01) at 1.8s compared with the values at 3.0s. The streaking artifacts were more pronounced at 1.8s than at 7.8s (Fig. 3).
Fig. 2.
Quality of the visualization of blood vessels and pancreas, and degree of artifacts on all temporal resolution images using a 5-point scale. Two radiologists evaluate the images independently; differences in scores between readers are discussed, and final values are determined. (a) CEA; (b) CHA; (c) SA; (d) pancreatic head; (e) pancreatic body; (f) pancreatic tail; and (g) artifacts. *P < 0.05; **P < 0.01; and ***P < 0.001. CEA, celiac artery; CHA, common hepatic artery; SA, splenic artery.
Fig. 3.
Degree of streaking artifacts on each temporal resolution image: (a) 1.8s and (b) 7.8s. The streaking artifacts are more pronounced at 1.8s.
The concentration curves obtained from the ROI of each pancreatic region showed wider peaks and generally lower amplitude as the temporal resolution increased (Fig. 4).
Fig. 4.
Concentration curve for all temporal resolutions: (a) 1.8s; (b) 3.0s; (c) 4.8s; and (d) 7.8s. The concentration curves obtained from the ROI of each pancreatic region show wider peaks and generally lower amplitude as the temporal resolution increases.
In the pharmacokinetic analysis, two examinations with χ2 > 0.5 were excluded as analytical errors. Pharmacokinetic parameters at all temporal resolutions for 16 examinations with χ2 ≤ 0.5 are shown in Fig. 5. Ktrans and Kep decreased at all pancreatic regions as the temporal resolution increased. Ktrans and Kep were significantly lower at 4.8s and 7.8s than at 1.8s (Ktrans: pancreatic head, 4.8s, P < 0.001; 7.8s, P < 0.001; pancreatic body, 4.8s, P < 0.001; 7.8s, P < 0.001; pancreatic tail, 4.8s, P < 0.01; 7.8s, P < 0.001; Kep, pancreatic head, 4.8s, P < 0.001; 7.8s, P < 0.001; pancreatic body, 4.8s, P < 0.001; 7.8s, P < 0.001; pancreatic tail, 4.8s, P < 0.001; 7.8s, P < 0.001). Moreover, there were significant differences in Ktrans and Kep in all pancreatic regions between 3.0s and 7.8s (Ktrans pancreatic head, P < 0.001; Ktrans pancreatic body, P < 0.001; Ktrans pancreatic tail, P < 0.001; Kep pancreatic head, P < 0.001; Kep pancreatic body, P < 0.001; and Kep pancreatic tail, P < 0.001). In addition, there was a significant difference in Ktrans (P < 0.05) in the pancreatic tail between 4.8s and 7.8s and in Kep in the pancreatic head (P < 0.05) between 3.0s and 4.8s. The color map of Kep at the pancreatic tail clearly demonstrated that the color fades as the temporal resolution increases (Fig. 6). Ve at 7.8s was significantly lower than that at other temporal resolutions in the pancreatic head (1.8s, P < 0.01; 3.0s, P < 0.05; 4.8s, P < 0.01) and lower than Ve at 4.8s in the pancreatic body (P < 0.01). iAUC decreased as the temporal resolution increased at all pancreatic regions, and it was significantly lower at 7.8s than at 1.8s and 3.0s in all pancreatic regions (pancreatic head, 1.8s, P < 0.001; 3.0s, P < 0.001; pancreatic body, 1.8s, P < 0.001; 3.0s, P < 0.001; pancreatic tail, 1.8s, P < 0.001; 3.0s, P < 0.01). In the pancreatic head (P < 0.05) and body (P < 0.05), values at 7.8s were significantly lower than those at 4.8s, whereas in the pancreatic head (P < 0.05) and tail (P < 0.05), values at 4.8s were significantly lower than those at 1.8s.
Fig. 5.
Pharmacokinetic analysis of the pancreas. The mean of the parameters determined by two radiologists is used. (a) Ktrans pancreatic head; (b) Ktrans pancreatic body; (c) Ktrans pancreatic tail; (d) Kep pancreatic head; (e) Kep pancreatic body; (f) Kep pancreatic tail; (g) Ve pancreatic head; (h) Ve pancreatic body; (i) Ve pancreatic tail; (j) iAUC pancreatic head; (k) iAUC pancreatic body; and (l) iAUC pancreatic tail. *P < 0.05; **P < 0.01; and ***P < 0.001. Ktrans, volume transfer coefficient; Kep, rate constant; Ve, extracellular extravascular volume fraction; iAUC, initial area under the concentration curve.
Fig. 6.
Color map of Kep at the pancreatic tail for all temporal resolutions: (a) 1.8s; (b) 3.0s; (c) 4.8s; and (d) 7.8s. This visualization clearly demonstrates that the color fades as the temporal resolution increases. Kep, rate constant.
Discussion
Previous studies have reported that high temporal resolution images can accurately show the peak of the contrast effect in the arteries.10,11 In the image quality evaluation of this study, the CEA, CHA, and SA scores were lower with long temporal resolution than with short temporal resolution, and the pancreatic head, pancreatic body, pancreatic tail, and artifact scores were higher with long temporal resolution than with short temporal resolution. This could mean that longer temporal resolution made it more difficult to demonstrate the peak of the contrast effect in the arteries, thereby resulting in lower scores. The reason why only the CEA score (and not the CHA or SA score) at 7.8s was significantly different from the other temporal resolutions is that the CEA is thicker than other arteries. Therefore, the CEA was more clearly delineated and the CEA scores were generally higher than other arteries scores at short temporal resolutions that capture the peak contrast effect, resulting in a significant difference compared to long temporal resolution images, which do not capture the peak contrast effect. We attributed the deterioration in the quality of the pancreatic parenchyma images to a fewer number of spokes on higher temporal sub-frame images; that is, the number of acquired data in high temporal sub-frame images was lower than that of low temporal sub-frame images. Considering these results, the image quality of the artery and pancreatic parenchyma are closely interrelated. The CEA, CHA, and SA scores were ≥ 4 and not significantly different between 1.8s and 4.8s. The morphology of the pancreas and artifacts was ≥ 4 with no significant differences between 3.0s and 7.8s, which were diagnostic and well-delineated values. Thus, a temporal resolution at 3.0–4.8s is considered suitable for image quality evaluation.
In the pharmacokinetic analysis, as reported for breast and prostate cancer,12,13 the effect of temporal resolution on pharmacokinetic parameters depends on the type of tissue; however, we were unable to identify any previous studies on the pancreas. Regarding this study, there was a significant difference in parameters at 4.8s and 7.8s compared with those at 1.8s, except for Ve, in all pancreatic regions and iAUC in the pancreatic body, which was attributed to the overall lower concentration curve with the longer temporal resolution. The reason for the lack of significant differences in Ve was that Ve is defined as Ve = Ktrans/Kep; further, Ktrans and Kep showed a similar decrease with an increase in temporal resolution. All parameters except Ve were significantly lower at 4.8s and 7.8s than at 1.8s, but there were no significant differences in any parameters between 1.8s and 3.0s. Therefore, by assuming that pharmacokinetic analysis can be performed more accurately with a shorter temporal resolution, the results indicate that pharmacokinetic analysis can be performed more accurately with a temporal resolution of up to 3.0s. In addition, two examinations were excluded from the pharmacokinetic analysis owing to analytical errors (χ2 > 0.5) because streaking artifacts are noticeable at 1.8s, and the parameters vary with these artifacts, resulting in sub-optimal fitting of the model. This is probably because 1.8s is shorter than the respiratory cycle, and even data acquisition collection using Liver gate does not provide sufficient expiratory data, resulting in a mixture of inspiratory data and strong streaking artifacts. Due to these artifacts, an accurate analysis at 1.8s is difficult in some examinations, and a temporal resolution of 3.0s is considered suitable for pharmacokinetic analysis. The novelty of this study was its comparison of data acquisition time and pharmacokinetic parameters. Previous studies on other organs have compared acquisition timing and pharmacokinetic parameters; however, we could not find any studies that compared data acquisition time and pharmacokinetic parameters in the pancreas. For example, if the short temporal resolution is 5s, and the long temporal resolution is 30s, the long temporal resolution image will consist of 5s of acquired data for every 30s using the past research methods, but 3s of acquired data with the current research method.
Based on the findings of this research, a temporal resolution of 3.0–4.8s is suitable for image quality assessment of the pancreas, and a resolution of 3.0s is suitable for pharmacokinetic analysis; therefore, the optimal temporal resolution to perform pharmacokinetic analysis and obtain good image quality in FBDCE-MRI of the pancreas is 3.0s.
This study had several limitations. First, it was performed at a single institution over a short period of time and the sample size was small, thereby causing potential selection bias. Second, patients with a χ2 (an error index representing the fit of the model) > 0.5 were excluded. Although a lower χ2 is desirable, there is no strict rule regarding the threshold for error detection, which may also have caused a selection bias. Lastly, regarding the segmentation of the pancreas for pharmacokinetic analysis, we performed a 2D evaluation of a single cross section and a 3D evaluation was not performed. In addition, segmentation was performed manually by each radiologist.
Conclusion
In conclusion, a temporal resolution of 3.0s is appropriate to achieve both pharmacokinetic analysis and high image quality in FBDCE-MRI of the pancreas using GRASP.
Acknowledgments
The authors thank the radiographers at our hospital for their cooperation, Editage (www.editage.com) for editing the draft of this manuscript.
Funding Statement
This work was supported by the Japan Society for the Promotion of Science for grant support (JSPS KAKENHI grant Number 20K07992) and Bayer Yakuhin for grant support (Bayer Academic Support application number BASJ20210406008).
Footnotes
Conflicts of Interest
Marcel D. Nickel is an employee of Siemens Healthcare GmbH; Katsuya Maruyama is an employee of Siemens Healthcare K.K.; the other authors have no conflicts of interest.
References
- 1.Harrington KA, Shukla-Dave A, Paudyal R, Do RKG. MRI of the pancreas. J Magn Reson Imaging 2021; 53:347–359. [DOI] [PubMed] [Google Scholar]
- 2.Bali MA, Metens T, Denolin V, et al. Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology 2011; 261:456–466. [DOI] [PubMed] [Google Scholar]
- 3.Akisik MF, Sandrasegaran K, Bu G, Lin C, Hutchins GD, Chiorean EG. Pancreatic cancer: utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology 2010; 256:441–449. [DOI] [PubMed] [Google Scholar]
- 4.Lecler A, Balvay D, Cuenod CA, et al. Quality-based pharmacokinetic model selection on DCE-MRI for characterizing orbital lesions. J Magn Reson Imaging 2019; 50:1514–1525. [DOI] [PubMed] [Google Scholar]
- 5.Guan Y, Peck KK, Lyo J, et al. T1-weighted dynamic contrast-enhanced MRI to differentiate nonneoplastic and malignant vertebral body lesions in the spine. Radiology 2020; 297:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Li D, Shen Z, Normolle D. Sensitivity of quantitative metrics derived from DCE MRI and a pharmacokinetic model to image quality and acquisition parameters. Acad Radiol 2010; 17:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging of the breast: trade-off between spatial and temporal resolution. Radiology 2005; 236:789–800. [DOI] [PubMed] [Google Scholar]
- 8.Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the golden ratio for time-resolved MRI. IEEE Trans Med Imaging 2007; 26:68–76. [DOI] [PubMed] [Google Scholar]
- 9.Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H. Compressed sensing for body MRI. J Magn Reson Imaging 2017; 45:966–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujinaga Y, Ohya A, Tokoro H, et al. Radial volumetric imaging breath-hold examination (VIBE) with k-space weighted image contrast (KWIC) for dynamic gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver: advantages over Cartesian VIBE in the arterial phase. Eur Radiol 2014; 24:1290–1299. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal MD, Spincemaille P, Mennitt KW, et al. Improved hepatic arterial phase MRI with 3-second temporal resolution. J Magn Reson Imaging 2013; 37:1129–1136. [DOI] [PubMed] [Google Scholar]
- 12.Matsukuma M, Furukawa M, Yamamoto S, et al. The kinetic analysis of breast cancer: an investigation of the optimal temporal resolution for dynamic contrast-enhanced MR imaging. Clin Imaging 2020; 61:4–10. [DOI] [PubMed] [Google Scholar]
- 13.Othman AE, Falkner F, Weiss J, et al. Effect of temporal resolution on diagnostic performance of dynamic contrast-enhanced magnetic resonance imaging of the prostate. Invest Radiol 2016; 51:290–296. [DOI] [PubMed] [Google Scholar]