Abstract
The antibiotic micrococcin is a potent growth inhibitor of the human malaria parasite Plasmodium falciparum, with a 50% inhibitory concentration of 35 nM. This is comparable to or less than the corresponding levels of commonly used antimalarial drugs. Micrococcin, like thiostrepton, putatively targets protein synthesis in the plastid-like organelle of the parasite.
Antibiotics of the thiocillin-thiazolyl class, which are collectively known as thiopeptides (16), are highly modified peptides whose site of action lies within eubacterial large subunit (LSU) rRNA (1). The most familiar member of this class of antibiotics is thiostrepton, which is produced by Streptomyces azureus. It binds tightly to a small region of eubacterial LSU rRNA (18) and inhibits the GTPase reaction catalyzed by ribosomes in the presence of EF-G (10). Although eukaryotic ribosomes are insensitive to thiostrepton (19), we found that thiostrepton inhibits growth of blood-stage cultures of the malaria parasite Plasmodium falciparum, probably by inhibiting protein synthesis in the unusual plastid-like organelle of the parasite (7). Thiostrepton also interacts directly with an RNA fragment derived from the plastid-encoded rRNA, and not with nucleus-encoded rRNA fragments (13). Since in Escherichia coli thiostrepton binding is correlated with inhibition of protein synthesis (18), the target for thiostrepton in P. falciparum is presumably plastid-encoded protein synthesis. The plastid genome was recently characterized in detail (20) and is, perhaps, a general feature of the phylum Apicomplexa (6, 8). Antibiotics that target the plastid may, therefore, be of considerable interest as novel chemotherapeutic agents against these parasites which are responsible for important human and animal diseases. In particular, the spread of chloroquine and antifolate resistance in P. falciparum suggests that modification of existing drugs may not circumvent the problem of multidrug-resistant parasites (12).
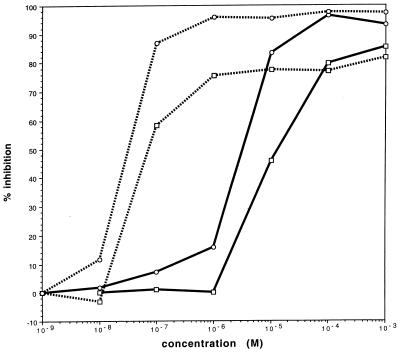
Like thiostrepton, micrococcin is a thiopeptide, although it is produced by Bacillus and Micrococcus spp. (2). Both compounds inhibit binding of aminoacyl-tRNA to the ribosomal A site as well as other functions linked to eubacterial ribosomal GTP hydrolysis (summarized in reference 1). However, subtle differences in the modes of action of the two drugs, as described below, prompted us to test the effects of micrococcin on parasite growth and protein synthesis, which were correlated with the uptake of [3H]hypoxanthine and [3H]leucine, respectively, in blood stage cultures (5). The results were calculated as the means of triplicate determinations (Fig. 1) and revealed effects of micrococcin on both growth (incorporation of [3H]hypoxanthine) and protein synthesis (incorporation of [3H]leucine) that are statistically significant. While the effects of thiostrepton were similar to those seen previously (7), comparable inhibition with micrococcin occurred at about 100-fold lower drug concentrations. For micrococcin, the estimated 50% inhibitory concentration (IC50) for growth was 35 ± 7.9 nM, while that of thiostrepton was 3.2 ± 0.9 M. Inhibition of protein synthesis also required higher drug concentrations; the IC50 for micrococcin was 90 ± 22 nM and that for thiostrepton 15 ± 4 M. The greater sensitivity of growth as opposed to protein synthesis was consistent with selective inhibition of a minor component of total protein synthesis, as expected if these drugs targeted plastid-encoded rRNA.
FIG. 1.
Concentration-response curves describing the in vitro inhibition of growth and protein synthesis in P. falciparum by micrococcin and thiostrepton. Data are the percent reduction values from no drug controls and are means of three determinations. Dashed lines, micrococcin; solid lines, thiostrepton. Incorporation of [3H]hypoxanthine (○) and [3H]leucine (□) was measured at the concentrations of drug shown. For the assays, an in vitro culture of P. falciparum (strains 3D7 and LF4) was diluted in microtiter plates as described previously (7). Micrococcin P (Mr, 1,120 [a kind gift from J. Walker]) and thiostrepton (Mr, 1,660 [Calbiochem]) were dissolved at 100 mM in sterile dimethyl sulfoxide (silylation grade [Pierce]); at the highest concentrations used, the drugs precipitated in the media. Parasites were grown for 48 h in the presence of serial dilutions of thiostrepton or micrococcin, together with drug-free controls. Labeling with [3H]hypoxanthine or [3H]leucine in medium lacking hypoxanthine or leucine, respectively, was as described elsewhere (7). Cells were harvested and lysed, and incorporated radioactivity was estimated. Data shown are for P. falciparum 3D7, but these were similar to data for LF4 (IC50 for growth, 39 nM).
It is intriguing why micrococcin is much more potent than thiostrepton in inhibition of the growth of P. falciparum, since both antibiotics interact in similar ways with eubacterial LSU rRNA (14). However, there are subtle differences in the ways in which the two drugs act. For example, micrococcin stimulates ribosome-dependent GTP hydrolysis in the presence of EF-G, whereas thiostrepton completely inhibits this process (2). Whether the mechanistic difference between the drugs is related to the 100-fold discrepancy in their potencies against P. falciparum is unclear, since the effectiveness of micrococcin might result from fortuitously concentrating in the plastid-like organelle. This is similar to accumulation of chloroquine in the food vacuole of the parasite (17), with resistance genetically linked to active transport of the drug (15). Since development of the erythrocytic stages of Plasmodium takes place in a cell with no organelles and little metabolic activity, the parasite forms Golgi-like intraerythrocytic tubovesicular structures (4), which are presumably contiguous with the parasite membrane, to traffic proteins and exchange nutrients. Either mechanistic differences or active transport would provide a framework for new drug development, targeting plastid protein synthesis.
The growth-inhibitory effects of micrococcin compare favorably with those of proven antimalarial drugs tested in vitro against sensitive strains of the parasite (Table 1). Antimalarials such as pyrimethamine, chloroquine, and mefloquine act rapidly and have in vitro IC50s that are in the nanomolar range for sensitive strains and can be as high as 100 nM for resistant strains. Antibiotics are generally poorly effective as antimalarial agents, since they are slow acting, although they are used in part because of their nontoxic effects (11). Since the IC50 of tetracycline derivatives for in vitro cultures of P. falciparum is 26 to 39 M (3), the 1,000-fold greater effectiveness of micrococcin prompts investigation of this perhaps forgotten antibiotic as a potential chemotherapeutic against Apicomplexa in general. Also, the potencies of these antibiotics against the mouse malaria parasite Plasmodium berghei, including chloroquine-resistant strains, are worth investigating.
TABLE 1.
Approximate in vitro IC50s for growth inhibition of P. falciparum
Acknowledgments
This work was supported by the NIH Intramural Research Program and by LamBed (E.C.).
We are grateful to Normal Heatley and James Walker (deceased) for supplies of micrococcin and micrococcin P, respectively.
REFERENCES
- 1.Cundliffe E. Recognition sites for antibiotics within rRNA. In: Hill W E, Moore P B, Dahlberg A, Schlessinger D, Garrett R A, Warner J R, editors. The ribosome: structure, function and evolution. Washington, D.C: American Society for Microbiology; 1990. pp. 479–490. [Google Scholar]
- 2.Cundliffe E, Thompson J. Concerning the mode of action of micrococcin upon bacterial protein synthesis. Eur J Biochem. 1981;118:47–52. doi: 10.1111/j.1432-1033.1981.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 3.Divo A A, Geary T G, Jensen J B. Oxygen- and time-dependent effects of antibiotics and selected mitochondrial inhibitors on Plasmodium falciparum in culture. Antimicrob Agents Chemother. 1985;27:21–27. doi: 10.1128/aac.27.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmendorf H G, Haldar K. Secretory transport in Plasmodium. Parasitol Today. 1993;9:98–102. doi: 10.1016/0169-4758(93)90216-3. [DOI] [PubMed] [Google Scholar]
- 5.Geary T G, Jensen J B. Effects of antibiotics on Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1983;32:221–225. doi: 10.4269/ajtmh.1983.32.221. [DOI] [PubMed] [Google Scholar]
- 6.Kohler S, Delwiche C F, Denny P W, Tilney L G, Webster P, Wilson R J M, Palmer J D, Roos D S. A plastid of probable green algal origin in Apicomplexan parasites. Science. 1997;275:1485–1489. doi: 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- 7.McConkey G A, Rogers M J, McCutchan T F. Inhibition of Plasmodium falciparum protein synthesis: targeting the plastid-like organelle with thiostrepton. J Biol Chem. 1997;272:2046–2049. doi: 10.1074/jbc.272.4.2046. [DOI] [PubMed] [Google Scholar]
- 8.McFadden G I, Reith M E, Munholland J, Lang-Unnasch N. Plastid in human parasites. Nature. 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 9.Milhous W K, Weatherly N F, Bowdre J H, Desjardins R E. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob Agents Chemother. 1985;27:525–530. doi: 10.1128/aac.27.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pestka S. Thiostrepton: a ribosomal inhibitor of translocation. Biochem Biophys Res Commun. 1970;40:667–674. doi: 10.1016/0006-291x(70)90956-3. [DOI] [PubMed] [Google Scholar]
- 11.Puri S K, Dutta G P. Antibiotics in the chemotherapy of malaria. Progr Drug Res. 1982;26:167–205. doi: 10.1007/978-3-0348-7111-2_5. [DOI] [PubMed] [Google Scholar]
- 12.Rieckmann K H. Falciparum malaria: the urgent need for safe and effective drugs. Annu Rev Med. 1983;34:321–335. doi: 10.1146/annurev.me.34.020183.001541. [DOI] [PubMed] [Google Scholar]
- 13.Rogers M J, Bukhman Y V, McCutchan T F, Draper D E. Interaction of thiostrepton with an RNA fragment derived from the plastid-encoded ribosomal RNA of the malaria parasite. RNA. 1997;3:815–820. [PMC free article] [PubMed] [Google Scholar]
- 14.Rosendahl G, Douthwaite S. The antibiotics micrococcin and thiostrepton interact directly with 23S rRNA nucleotides 1067A and 1095A. Nucleic Acids Res. 1994;22:357–363. doi: 10.1093/nar/22.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez C P, Wunsch S, Lanzer M. Identification of a chloroquine importer in Plasmodium falciparum: differences in import kinetics are genetically linked with the chloroquine-resistant phenotype. J Biol Chem. 1997;5:2652–2658. doi: 10.1074/jbc.272.5.2652. [DOI] [PubMed] [Google Scholar]
- 16.Strohl W R, Floss H G. Thiopeptides. Biotechnology. 1995;28:223–238. doi: 10.1016/b978-0-7506-9095-9.50015-0. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan D J J, Gluzman I Y, Russell D G, Goldberg D E. On the molecular mechanism of chloroquine’s antimalarial action. Proc Natl Acad Sci USA. 1996;93:11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson J, Cundliffe E. The binding of thiostrepton to 23S ribosomal RNA. Biochimie. 1991;73:1131–1135. doi: 10.1016/0300-9084(91)90156-u. [DOI] [PubMed] [Google Scholar]
- 19.Uchiumi T, Wada A, Kominami R. A base substitution within the GTPase-associated domain of mammalian 28 S ribosomal RNA causes high thiostrepton accessibility. J Biol Chem. 1995;270:29889–29893. doi: 10.1074/jbc.270.50.29889. [DOI] [PubMed] [Google Scholar]
- 20.Wilson R J M, Denny P W, Preiser P R, Rangachari K, Roberts K, Roy A, Whyte A, Strath M, Moore D J, Moore P W, Williamson D H. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J Mol Biol. 1996;261:155–172. doi: 10.1006/jmbi.1996.0449. [DOI] [PubMed] [Google Scholar]