Abstract
Cancer is considered the most important clinical, social and economic issue regarding cause-specific disability-adjusted life years among all human pathologies. Exogenous, endogenous and individual factors, including genetic predisposition, participate in cancer triggering. Telomeres are specific DNA structures positioned at the end of chromosomes and consist of repetitive nucleotide sequences, which, together with shelterin proteins, facilitate the maintenance of chromosome stability, while protecting them from genomic erosion. Even though the connection between telomere status and carcinogenesis has been identified, the absence of a universal or even a cancer-specific trend renders consent even more complex. It is indicative that both short and long telomere lengths have been associated with a high risk of cancer incidence. When evaluating risk associations between cancer and telomere length, a disparity appears to emerge. Even though shorter telomeres have been adopted as a marker of poorer health status and an older biological age, longer telomeres due to increased cell growth potential are associated with the acquirement of cancer-initiating somatic mutations. Therefore, the present review aimed to comprehensively present the multifaceted pattern of telomere length and cancer incidence association.
Keywords: telomere length, lung cancer, laryngeal cancer, bladder cancer, kidney cancer, non-Hodgkin's lymphoma, leukemia, melanoma of the skin, colorectal cancer, liver cancer
1. Introduction
Epidemiological studies have shown that cancer is the second cause of mortality worldwide following ischemic heart disease. However, cancer is considered the most important clinical, social and economic burden as regards cause-specific disability-adjusted life years among all human pathologies (1). Despite the fact that its existence was recognized >2,000 years ago by the ancient Greeks, the underlying causes leading to the uncontrolled growth of cells became a matter of research during the mid-20th century. Since then, tremendous advancements, not only in biology, but also in biochemistry and bioengineering, have made it possible to unveil some of the mechanisms of carcinogenesis (2). However, given the fact that cancer is not a single pathology, but rather a cluster of relative pathological entities, it is expected that certain mechanisms may have a different impact on different types of cancer. This is the case with human telomeres (and telomerase). Even though the connection between the telomere status and carcinogenesis has been identified, the absence of a universal or a cancer-specific trend complicates the thorough understanding to a great extent. It is indicative that both short and long telomere lengths have been associated with a high risk of cancer incidence. When evaluating risk associations between cancer and telomere length, a disparity appears to emerge. Even though shorter telomeres have been adopted as a marker of a poor health status and an older biological age, longer telomeres due to increased cell growth potential have been shown to be associated with cancer-initiating somatic mutations (3). Therefore, the aim of the present review was to comprehensively present the most recent information regarding the implication of telomeres in different types of cancer.
Telomeres are specific DNA structures positioned at the end of chromosomes and consist of repetitive nucleotide sequences (5′-TTAGGG-3′) (4). These functional non-coding sequences, with the contribution of shelterin proteins, facilitate the maintenance of chromosome stability and protect them from degradation and damage (4). Shelterin is a six-subunit protein complex that consists of a telomere repeat-binding factor (TRF)1 and TRF2, a nuclear protein 2 (TIN2), a repressor activator protein 1, a tripeptidyl-peptidase 1 (TPP1) and a protection of telomeres 1 (POT1) protein (5). Telomeres and shelterins form structures known as T-loops that prevent DNA repair mechanisms from processing telomeres and recognizing them as double-stranded DNA breaks (5). TRF2 depends on the DNA damage response (DDR) inhibition via T-loop structure formation. T-loops are created by the invasion of the long 3′overhang strand at the telomere end into the double-stranded telomeric DNA (3). Specifically, the 3′overhang is formed upon DNA replication and involves the exonucleolytic degradation of the telomeres' 5′ ends. The result of the respective processing and the concurrent inability of DNA polymerases to replicate the lagging ends of linear DNA molecules leads to the shortening of human telomeres by ~50 bp per cell division. This telomere is restrained by the action of telomerase reverse transcriptase (TERT), which places GGTTAG repeats to the chromosomal 3′DNA terminus at the end of the chromosome. The TERT gene is situated at chromosome 5p15.33 in humans, and is an integral and essential part of the telomerase holoenzyme. The TERT gene is 42 kb in length and consists of 15 introns and 16 exons, with a 260-bp promoter core (6). The reverse transcriptase domain is encoded by 5-9 exons. The TERT transcript can be spliced into 22 isoforms (7). While the transcriptional regulation of TERT has been studied in depth, recent research has evaluated the role of alternate splicing of mRNA transcripts. TERT can be translated from multiple differently spliced transcripts, with only the longest variant having reverse transcriptase enzymatic activity (8). Breast cancer cell lines with the overexpression of transcripts without catalytic function have been shown to exhibit a reduced apoptosis, conferring a survival advantage (9). This suggests novel functions of TERT beyond telomere extension TERT promoter (TERTp) region contains GC boxes that bind the zinc finger transcription factor Sp1, which increases TERT transcription, and E-boxes that bind both transcriptional enhancers and repressors. TERTp lacks a TATA box, but it contains binding sites for a variety of transcription factors (10). However, DNA polymerases cannot fully replicate the lagging strand of telomere DNA at the chromosome terminus during each mitotic cell division (4). As a result, there is an annual rate of telomere shortening of ~20-40 bp, causing cell proliferation arrest and cell senescence (4,11,12).
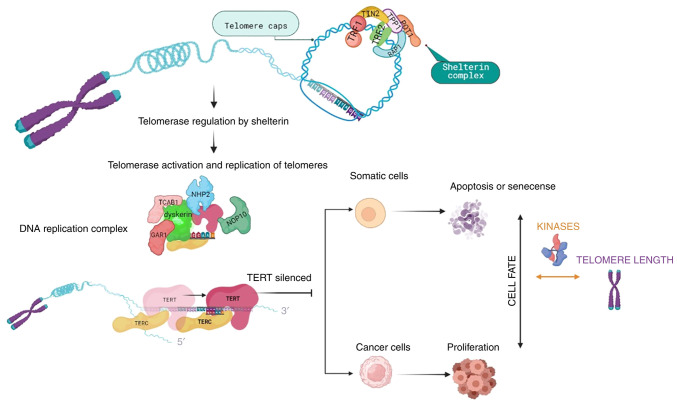
Telomerase can prevent telomere shortening. The activity of this reverse-transcriptase enzyme, using an RNA template, results in the telomeric DNA repeat synthesis (4,13). Telomerase consists of the reverse transcriptase (TERT), the telomerase RNA component, as well as proteins that are necessary for DNA synthesis, such as dyskerin, nucleolar protein 10, non-histone protein 2, GAR1 and telomerase Cajal body protein 1 (3) (Fig. 1). For cells not to replicate indefinitely, TERT is silenced and cells undergo apoptosis or cell senescence. However, cancer cells manage to overcome cell cycle arrest and activate telomerase, resulting in cells acquiring proliferative ability and developing mutations (Fig. 1). Therefore, telomere length can serve as a marker for biological aging (14).
Figure 1.
Overview of telomere length regulatory mechanisms and cell fate. Telomeres are protected by the shelterin complex and when telomeres need to be replicated, the activated telomerase consisting of TERT, the telomerase RNA component and proteins necessary for DNA synthesis (dyskerin, NOP10, NHP2, GAR1 and TCAB1), replicates the telomere sequences. As a prevention mechanism for continuous replication, TERT is silenced, allowing cells to undergo apoptosis or cell senescence. Cancer cells are able to overcome cell cycle arrest and activate telomerase, resulting in cells acquiring proliferation ability and mutations. Protein kinases, being part of the signaling regulating cell cycle checkpoints, can affect the telomere length dependent cell fate, by inhibiting DNA replication until damaged DNA is repaired, or by restoring cell-cycle progression into the S phase in senescent cells, when kinases are inhibited. TERT, telomerase reverse transcriptase; NOP10: nucleolar protein 10; NHP2, non-histone protein 2; TCAB1, telomerase Cajal body protein 1; TIN2, nuclear protein 2; Rap1, repressor activator protein 1; TRF, telomere repeat-binding factor; POT1, protection of telomeres 1.
A number of protein kinases participate in the signaling regulating DDR-activated cell cycle checkpoints, thus inhibiting DNA replication until damaged DNA is repaired (15). Therefore, protein kinases regulate the association between cell fate and telomere length. On the other hand, inhibiting protein kinases regulating specific damage checkpoints can restore cell cycle progression into the S phase in senescent cells. Thus, dysfunctional telomeres induce a DNA damage checkpoint response that initiates senescence.
Shorter telomeres and an attenuated telomerase activity contribute to the pathobiology of human disease (16). They have also been shown to be associated with a numbe rof age-related diseases, such as cancer, coronary heart (cardiovascular) disease, type 2 diabetes, stroke, arthritis, osteoporosis, hypertension, chronic obstructive pulmonary disease and dementia (17). Researchers have also presented a link between telomere length and stress, drug abuse, Alzheimer's disease and mental disorders, including depression and schizophrenia (13).
Telomere length is regulated by a myriad of factors, including genetic background, as short telomeres can be a hereditary trait passed by specific factors in parental gametes (4). In addition, there is evident sex dependence, as females have been shown to have longer telomeres than males, associated with a lower biological age (18). Moreover, environmental factors may also affect telomere lengths, such as physical activity, body mass index, hormone replacement therapy, smoking, chronic inflammation, oxidative stress, dietary antioxidants and vitamin intake (19). For instance, vitamin B12, C and E deficiency may result in genomic instability and telomere shortening (6). On the contrary, in vitro experiments have indicated that omega-3 polyunsaturated fatty acids, ascorbic acid and its derivatives, as well as α-tocopherol, can delay telomere shortening and protect telomeres against degradation. Thus, more studies must be conducted to better understand the correlation between supplement intake and telomere protection.
A less known mechanism that regulates telomere length is known as the alternative lengthening of telomeres (ALT). ALT is a telomerase-independent mechanism and is somewhat dependent on homologous recombination. The homologous recombination-mediated copying of one telomere by another is the simplest explanation for the spread of a DNA tag from one telomere to others. However, other types of elongation events may also occur, as it is observed in the telomerase null Type II survivors from the budding yeast species Saccharomyces cerevisiae and Kluyveromyces lactis (20,21). Even though the telomerase-dependent pathway appears to be the predominant mechanism of telomere elongation (85-90% of cases), there is a certain number of cancers, including some with particularly poor outcomes, that use the ALT pathway (roughly accounting for 10-15% of cases) (22). Notably, cells of mesenchymal origin appear to rely more on ALT for telomere elongation than on telomerase (23). In fact, in certain types of cancer, including osteosarcomas and cancers of the central nervous system, the rates of ALT positivity are approaching 90%, which escapes from possible mechanistic reasons for ALT development (24). The distribution is explained by the fact that cells of mesenchymal origin are more likely to have more a stringently regulated telomerase expression (25). Cancers with ALT difficult to treat, partially due to their distribution, the unique mechanism of maintenance and the early resection that is precluded, rendering them unaffected by therapies that are telomerase-targeted. ALT-positive cells have several uncommon features, such as extrachromosomal telomeric DNA which is separated from chromosome ends and it may be linear or circular (22). It appears that the optimal markers for ALT are partially single-stranded circles of telomeric DNA in which the C-rich (AATCCC)n strand is essentially intact and the G-rich (TTAGGG)n strand is gapped. This 'C-circle' DNA is associated with the amount of ALT activity. Promyelocytic leukemia (PML) bodies that have telomeric DNA are typical of ALT cells and are introduced as ALT-associated PML bodies (APBs). Large APBs have been shown to be associated with the senescence of ALT cells and the sequestration of extrachromosomal DNA, although it is considered that smaller APBs are sites where telomere lengthening can occur (22).
Of note, it is essential to state that telomeres can be measured in all nucleated cells. However, relative telomere length may vary from one cell population to another, even when only one disease is present (25). This is critical because, as it will become evident from the following description, there is no uniform trend in telomere length even in the same type of cancer. Therefore, where possible, adequate information regarding the cell population that was studied will be provided in the sections below.
2. Cancer burden
Based on the International Agency for Research on Cancer (IARC), in 2020, the cancer burden was increased to 19.3 million cases, while deaths related to cancer are estimated at 10 million. However, incidence rates differ depending on sex, cancer site and human development index (HDI). HDI is a statistical index that has been developed by the United Nations for the measurement of social and economic development levels in various countries. It consists of four parameters: The mean years of schooling, expected years of education, life expectancy at birth and gross national income per capita. HDI is used to follow changes in developmental levels over time and to make comparisons among different countries. The IARC provides statistics for the most common types of cancer according to sex and HDI, that are presented in the tables below. For example, HDI is inversely associated with the risk of prostate cancer, suggesting that socioeconomic parameters related to telomere status significantly affect cancer risk (26).
3. Modulation of human TERT in cancer
Over the past decades, studies have focused on the regulation of TERT in humans (hTERT) in cancer. As a result, several mechanisms of action for altering hTERT gene expression have been described. Of note, a previous study demonstrated how the hTERT promoter crucially regulates its transcription (27). The expression of hTERT has been shown to be induced by multiple genetic and epigenetic mechanisms, in tumors. More specifically, the mechanisms described include hTERT amplifications, structural variants, promoter mutations and promoter methylation (epigenetic modification) (28).
Amplification of hTERT
In cancer cells, the overexpression of amplified genes leads to the gain or loss of genetic material. Telomere dysfunctions, DNA copying errors and the presence of chromosomal fragile sites have been described as mechanisms that initiate gene amplification (29). In the case of hTERT, the proposed modes are telomere dysfunction, in addition to breakage at fragile sites and formation of chromosomal fusions (30).
Genomic rearrangements of hTERT
The overexpression of hTERT in cancer can also result by genomic rearrangements modulating the gene locus of hTERT (5p15.33). Genomic rearrangements lead to the increased proximity of active enhancers and the hTERT gene. The latter results in the interaction between promoter and the newly introduced enhancers, enhancing hTERT expression (31).
hTERT promoter mutation
hTERT promoter mutations are a common, yet distinct genetic modification that regulates hTERT telomerase activation and expression. The hTERT core promoter contains 260 base pairs and different transcription factor binding sites that modulate gene transcription and telomerase initiation (32). Different mutation loci in the promoter generate added E-twenty-six transcription factor family binding sites, therefore generating new possible sites of genetic regulation in cancer (33). hTERT promoter mutations mostly exist in low rate self-renewal cancers, such as brain tumors, liver tumors, melanocytes and also low-grade cancers, such as bladder cancers, proposing a triggering telomerase activation role (34).
hTERT epigenetic modifications: hTERT promoter methylations
DNA methylations exist genome-wide at CpG positions, located in non-coding gene sections. Approximately 70% of the human gene promoters enclose CpG sites; thus, DNA methylation is considered a crucial player in gene expression and regulation (35). Gene silencing and activation are both associated with the methylation of specific hTERT promoter sites, particularly upstream of the hTERT core promoter (36). Different mechanisms of hTERT promoter methylation have been described for hTERT stimulation. Castelo-Branco et al (37) indicated that DNA methylation prevents the binding of repressive elements. In addition, a more complex mechanism links DNA methylation and chromosome structural modifications (38). Finally, DNA methylation contributes to alterations in chromatin conformation, altering gene expression through differential transcription factor binding (39).
Effects of microRNAs (miRs/miRNAs) on hTERT modulation
In various types of cancer, several miRNAs have been identified as key modulators of hTERT. Such miRNAs have been found to negatively regulate hTERT expression, preventing carcinogenesis (40). miRNAs can act towards hTERT directly or indirectly. Direct binding is presented to the hTERT 3′untranslated region (3′UTR), that interferes with hTERT protein expression in cancer cells (41). In thyroid carcinoma, the inhibition of miR-138 has been found to be associated with an increased expression of hTERT and the imposed overexpression of miR-138 was found to attenuate hTERT expression through the association with the hTERT 3′UTR (41). Indirectly, miRNAs may modulate transcription factors known to regulate hTERT (33). Examples include miR-494 and miR-1294, that were found to downregulate c-Myc, a well-known transcriptional activator of hTERT, in pancreatic and esophageal squamous cell carcinomas (33)
4. Respiratory system
An altered telomere length has been well-identified to participate in lung cancer formation. Although several studies have reported this abnormality, a consensus has yet to be reached (42-46). Indeed, both short and long telomere lengths have been shown to be associated with a high risk of lung cancer formation (47).
Various epidemiological factors have been shown to affect the association between telomere length and lung cancer pathogenesis. A study on patients with small cell lung cancer (SCLC) with a history of heavy smoking demonstrated an association between a shorter telomere length and higher risk of mortality, particularly for those classified as having stage III/IV SCLC (42). Moreover, a stronger association for women >65 was indicated. Furthermore, Kachuri et al (42) determined an association between mortality and shorter telomere length in no-smoker cohorts of patients with non-small cell lung cancer (NSCLC) and adenocarcinoma. In a Chinese region characterized by high indoor pollution, a shorter telomere length was detected in the peripheral blood leukocytes of patients with lung cancer and chronic obstructive pulmonary disease (43). The association was attributed to the high levels of oxidative stress and inflammation in the airways and blood of patients (43). A recent study by Steiner et al (44) revealed that coffee was not associated with telomere length of cancers related to coffee intake, such as lung cancer. Age is also a putative factor that could affect such associations. Sun et al (45) concluded that age may influence the association of telomere length with cancer incidence, since younger patients with a shorter telomere length and an increased telomere length variation across all chromosome ends exhibited a higher risk of lung cancer presentation.
Jang et al (46), using peripheral blood lymphocytes, found that shorter telomeres indicated a higher risk of developing small cell carcinoma than squamous cell carcinoma and lung adenocarcinoma. On the contrary, Sanchez-Espiridion et al (47), also using peripheral blood leukocytes, presented a higher risk of lung squamous cell carcinoma for patients with shorter telomeres. In fact, they suggested that longer telomeres attenuated the development of squamous cell carcinoma, particularly in males. Of note, the same debate applies to telomerase activity as well. Jeon et al (48), using peripheral blood mononuclear cells, found that low telomerase activity was significantly linked to increased risk of lung cancer (adjusted odds ratio, 3.05; 95% confidence interval, 1.60-5.82; P=7×10−4). Dobija-Kubica et al (49) evaluated telomerase activity in 47 tissue specimens obtained from patients with NSCLC. According to their findings, 66.7% of healthy pulmonary parenchyma samples had a high telomerase activity, while a variable level of telomerase activity was reported in cancer cells. In detail, even though no association was found between the level of telomerase activity in NSCLC specimens and the 2-year survival rate of patients, there were significantly higher levels of telomerase activity in poorly differentiated (high grade) NSCLC tumors (grade 3), as compared to moderately differentiated (intermediate grade) tumors (grade 2) (49).
Genetic factors are implicated in telomere biology participation, as shown in a study in which patients with lung adenocarcinoma were treated with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (gefitinib) (50). Shorter telomeres were associated with a poor prognosis following such a treatment and with a shorter overall survival of lung cancer patients. Moreover, a short telomere length indicated an elevated risk of resistance regarding EGFR mutations (48). Therefore, short telomeres could act as a marker for this therapeutic response and the development of chromosomal instability (46). Furthermore, shorter telomeres may cause damage to immune cell function and promote immune senescence.
A longer telomere length has also been shown to be associated with a high risk of developing lung cancer, as indicated by a systematic review concluding that longer telomere length was associated with a higher risk of developing lung cancer (51). A previous study conducted in an East Asian region also demonstrated that longer telomere length was positively associated with the risk of developing lung cancer (52). Machiela et al (53) indicated that non-smoking women in Asia with a longer telomere length had an increased risk of developing lung cancer. Sanchez-Espiridion et al (47) suggested that patients with a longer telomere length had a higher risk of developing lung adenocarcinoma, particularly for women, individuals <60 years of age and light smokers. The findings in the study by Yuan et al (54) are in agrement with those of the study by results of Sanchez-Espiridion et al (47), where longer telomeres were associsated with an elevated risk of developing lung adenocarcinoma, but not squamous cell carcinoma. The aforementioned association may could be due to different mechanisms of tumorigenesis and may be associated with a specific type of cancer (47).
Indeed, since longer telomeres bestow an increased rate of proliferation to cells (54), the accumulation of somatic mutations in carcinogenesis is possible, leading to malignant transformations (52). Specifically, cells with longer telomeres have an increased telomerase activity and this may result in uncontrollable cellular and tumor development (53).
Notably, de-Torres et al (55) also suggested that long telomeres exhibited a high risk of lung cancer development, regardless of the presence of chronic obstructive pulmonary disease and/or emphysema. These authors suggested the existence of a potential mechanism termed the 'long telomere syndrome' that is associated with mutations in telomerase and shelterin genes (55). Consequently, both short and long telomere lengths may indicate telomere dysfunction (26). Indeed, telomere length may be used as a prognostic and therapeutic tool for specific cohorts of patients with lung cancer, bestowing sensitivity regarding therapeutic approaches and disease monitoring (56). Furthermore, the identification of more drivers could increase the specificity of these markers (57). However, a standing limitation concerning these studies is that different methods of measuring telomere length are used, which decreases the sensitivity of comparison (58) (Table I).
Table I.
Telomere length and cancer risk.
| Cancer type | Sample type | Origin of study population | TL (mean ± SD) | TL and cancer risk | Clinical significance | Authors/(Refs.) |
|---|---|---|---|---|---|---|
| Lung adenocarcinoma | European | Longer telomere length/higher risk | Risk prediction/intervention target for disease progression | Haycock et al (56) | ||
| East Asian | Longer telomere length/higher risk | Longer telomere length may play a role in carcinogenesis | Cao et al (52) | |||
| Peripheral blood leukocytes | Caucasian | 1.23±0.38 | Longer telomere length/higher risk | According to the histology of the tumor, cancer risk differs | Sanchez-Espiridion et al (47) | |
| Peripheral blood leukocytes | Asian (Chinese) | Longer telomere length/higher risk | Dose-dependent association for telomere length in peripheral blood leukocytes at baseline with an increased risk of lung adenocarcinoma | Yuan et al (54) | ||
| Interstitial lung disease | European | longer telomere length/lower risk | risk prediction/ intervention target for disease progression | Haycock et al (56) | ||
| Non-small cell lung cancer | Blood lymphocytes | In the younger age group, short telomere length and high TLV in blood lymphocytes jointly increased the risk of lung cancer by 8-fold compared with individuals who had long telomere length and low TLV | Age may be critical in establishing cancer risk | Sun et al (45) | ||
| Squamous cell carcinoma | East Asian | Marginal nonlinear association | Cao et al (52) | |||
| Peripheral blood leukocytes | Caucasian | 1.10±0.44 | Shorter telomere length/lower risk | Sanchez-Espiridion et al (47) | ||
| Lung cancer (all types) | Peripheral blood lymphocytes | Asian (Korean) | 1.59±0.75 | Shorter telomere length/higher risk (more pronounced in patients with small cell carcinoma than in those with squamous cell carcinoma and adenocarcinoma) | Short telomere-associated with risk of cancer development | Jang et al (46) |
| Peripheral white blood cell | Asian | Longer telomere length/higher risk | Machiela et al (53) | |||
| Peripheral blood leukocytes | Asian (Chinese) | 0.76±0.35 | Short telomere/ higher risk | 3.90- and 4.54-fold increased risk | Xue et al (43) |
TL, telomere length; TLV, telomere length variation.
5. Laryngeal cancer
A relatively limited number of studies have examined the putative association between laryngeal cancer and telomere length. It has been suggested that telomeres are shorter in patients with laryngeal squamous cell carcinoma in the tumor differentiation grade 3 group than in the grade 1 and grade 2 groups. The grade 3 subgroup had the worst prognosis, with the highest mortality rate (59).
Genetic factors that affect telomere biology in laryngeal cancer pathogenesis have been identified. It has been well-documented that mutations within the OBFC1 gene result in a shorter telomere length in the cancer cells of patients with these mutations (60). The OBFC1 gene is associated with the replication and capping of telomeres. Therefore, it can be concluded that silencing such genes may reduce the risk of cancer and may exert a protective effect against tumorigenesis (60). Furthermore, it has been indicated that the hPOT1 gene is associated with telomere length and that mutations in this gene result in telomere dysfunction, telomere shortening, apoptosis and laryngeal cancer cell senescence (61). Lastly, it has been shown that the anti-telomerase treatment of laryngeal cancer cells is likely to activate the mechanisms of the alternative lengthening of telomeres monitored with the detection of ALT-specific promyelocytic leukemia bodies. Moreover, an enhanced exchange between telomeric sister-chromatids is evident, as well as the differential expression of telomere biology-related genes (62). Specifically, such cells exhibit a longer telomere length, an attenuated proliferation, and the development of a less invasive and tumorigenic phenotype (62). These data demonstrate the existence of two mechanisms maintaining telomere homeostasis, whose clarification might provide therapeutic targets for cancer.
6. Urinary/renal system
Bladder cancer
Epidemiological factors, such as age, sex, physiological status, genetic predisposition, or smoking have been associated with the development of bladder cancer. Notably, associations between epidemiological parameters and telomere length have been identified. Thus, smokers with shorter telomeres have been shown to have an increased risk of developing bladder cancer (63). Furthermore, it has been shown that patients who smoked present shorter telomeres than non-smokers (64). In addition, older patients with shorter telomeres exhibit a poorer prognosis (65). Notably, female patients have been found to have longer telomere lengths than males (66). Lin et al (67) demonstrated that depression could increase the risk of mortality in patients with a shorter telomere length compared to those with a longer telomere length, no signs of depression, and shorter cancer-free survival time. Specifically, they concluded that shorter telomeres could elevate the risk of mortality in depressed patients since the same neuroendocrine and immunological pathways are linked with depression and telomere length, and thus result in tumor progression and growth (67).
Specifically, genetic factors appear to be closely associated with telomere length during the process of tumorigenesis. Thus, patients with both short telomeres and GSTM1 homozygous deletions exhibit an increased risk of developing bladder cancer (68). Hosen et al (69) studied tumors with TERT promoter and fibroblast growth factor receptor 3 (FGFR3) mutations. More specifically, tumors solely with FGFR3 mutations (mainly in papillary carcinomas) had the shortest telomere length, followed by tumors with both mutations, then with TERT promoter mutations (found in both muscle-invasive and invasive tumors), and lastly by tumors not harboring the specific mutations (69).
The majority of studies concur that this type of cancer is associated with shorter telomeres. Indeed, short telomeres lead to chromosome instability in bladder cancer tissue (68). Moreover, telomere length appears to be associated with disease progression. Patients with muscle-invasive bladder cancer have been shown to have a shorter telomere length than those with non-muscle invasive bladder cancer, suggesting that telomere length is associated with cancer stage (64,68). In addition, shorter telomeres have been shown to be associated with a reduced survival rate, possibly due to poorer tolerance and higher chemotoxicity of therapy. Therefore, telomere length may be used as a marker of an optimal therapeutic strategy in bladder cancer (68).
However, some studies have found an association between bladder cancer and a longer telomere length. Fernandez-Gomez et al (70), using flow cytometry-based fluorescence in situ hybridization (FISH), observed a longer telomere length in more aggressive and aneuploid tumors compared to diploid ones. A separate study by Wang et al (71) indicated that a longer telomere and a higher telomere length variation could increase the risk of developing bladder cancer by 14-fold. Moreover, telomere length variation was increased in patients with bladder cancer, indicating severe telomere dysfunction (71). Furthermore, in another study, a specific genetic locus (rs398652on 14q21) was found to be associated with a longer telomere length, as well as a reduced risk of bladder cancer (72). This single nucleotide polymorphism (SNP) is associated with the PELI2 protein, which participates in the inflammatory response and cytokine production, protecting cells against chronic inflammation, closely associated with the process of cancerogenesis (72).
Even though short telomeres appear to be directly associated with the risk of developing bladder cancer, extreme telomere variation, including longer telomeres, has been associated with aggressive tumors.
Renal cancer
Even early reports identified an association between telomere length and kidney cancer development. Thus, in 1993, Holzmann et al (73) indicated that renal tumors were characterized by telomeric shortening, a process that could participate in tumor pathogenesis. The most common type of kidney cancer is renal cell carcinoma (RCC). Patients with RCC exhibit a shorter telomere length (74-85). However, Dahse et al (75) observed that telomere shortening occurred in distinct tumor cell populations, thus suggesting the heterogeneity of RCC. High-grade tumors exhibit shorter telomeres than low-grade tumors, associated with a high proliferation rate (76). In addition, shorter telomeres indicate a poorer disease-specific survival, since telomere shortening may facilitate tumor development and acceleration of immune cell senescence (77).
The examination of telomere length in cells in the blood of patients with RCC, however, has yielded somewhat contradictory results. Hoffman et al (78) did not find an association between pre-diagnostic leukocyte telomere length and the risk of developing RCC. Moreover, another study by Hofmann et al (79) did not find any association between blood cell telomere length and the risk of developing RCC. However, the study by Svenson et al (80) indicated that patients with a longer blood cell telomere length had a poorer prognosis than patients with a shorter one. In addition, patients with longer leukocyte telomeres and without any distant metastasis or capsule involvement, and patients with nuclear tumors of grade 1 to 3 had more unsatisfactory outcome (80).
However, Morais et al (81) hypothesized that telomeres may play a dual role: During early stages, shorter telomeres increase the risk of developing RCC due to the genetic instability that occurs during late carcinogenesis, while longer telomeres induce tumor progression. Genetically inferred telomere length, predictive of leukocyte telomere length, was established from the genotypes of nine telomere length-associated variants performed in six genome-wide association studies of RC (81). This approach suggested that individuals with an inherited predisposition exhibit more extended telomere length and harbor a higher risk of developing RCC (82). Notably, histologically different renal cancers exhibit a similar positive association with longer genetically inferred telomere length (82). On the other hand, it was demonstrated that the hTERT gene variantRs2736098 increased telomere length with each G allele added. Specifically, this G allele may enhance hTERT expression, thus increasing telomerase activity, elongating telomere length and reducing the risk of developing RCC (83).
As with other cancer types, an association between telomere length and cancer immune response was identified in renal cancer. Whole blood cell relative telomere length was positively associated with regulatory T-cells (Tregs), since they contribute to tumor angiogenesis and may promote tumor progression (84). Moreover, Svenson et al (84) indicated an association between cancer cell telomere length and serum levels of interleukin (IL)-7, -8 and -10 in RCC. These cytokines are critical immunological parameters. Specifically, IL-7 is associated with a poor survival, since it is imperative for the regulation of T- and B-cell development and T-cell homeostasis; IL-8 is a chemokine involved in tumor growth and development, and IL-10 induces immune suppression (84). Notably, patients with higher Treg levels exhibit longer T-cell telomeres. This association may indicate a suppressed immune system with attenuated cell division and subsequent lower telomere shortening (84).
It is noteworthy that, as previously demonstrated, after kidney transplants, pediatric cancer patients exhibited a shorter blood cell telomere length compared to the controls, but presented with elevated gene expression levels of telomere length-preserving proteins (85). Therefore, also in renal cancer, a significant association between the variation of telomere length and cancer risk has been established.
7. Hematogenous malignancies
Non-Hodgkin's lymphoma
Telomere length variations are strongly implicated in the pathogenesis of hematogenous malignancies. Thus, patients with non-Hodgkin's lymphoma were initially shown to have shorter telomeres length than the controls (86,87). Notably, patients with secondary diffuse large B-cell lymphoma were shown to have shorter telomeres than those with follicular lymphoma, indicating that telomere reduction induces disease progression (86). Furthermore, Widmann et al (87) demonstrated that patients had shorter telomeres in the myeloid subpopulations than the lymphoid ones.
On the other hand, Lan et al (88) were the first to associate longer telomere length with an elevated risk of developing non-Hodgkin's lymphoma. Specifically, it is suggested that longer telomeres create delayed senescence; thus, the cell can accumulate more mutations and increase the risk of transformation (88). Machiela et al (89) concurred with the aforementioned statement, indicating that longer telomeres bestow more significant replicative potential to hematogenous cancer cells.
It is essential to mention that patients undergoing chemotherapy have been shown to exhibit shorter telomeres, perhaps due to the proliferative stress of the high dose therapy in hematopoietic reconstruction (90). Notably, patients that relapsed exhibit shorter, longer as well as unaltered telomere lengths (91). The variations mentioned above may result from the selective loss of cells due to the therapy received or the surviving subclones having a specific telomere length constitution present in the tumor cell population (91).
Acute lymphocytic leukemia
The majority of studies focusing on leukemia progression and telomere biology have revealed an association with a shorter telomere length. Thus, patients with acute lymphocytic leukemia (ALL) are characterized by telomere shortening in their blood cells, a process that affects the pathogenesis of the disease (92). In a separate study, telomere lengths estimated from bone marrow samples were shorter than ones from peripheral blood of patients with ALL (93). However, upon chemotherapy, the mean telomere length increased, although it was later reduced due to the consolidation and maintenance of chemotherapy (93). The study by Borssén et al (94) concurred that the telomere length in blood cells of patients with ALL at the time of the diagnosis of lymphocytic leukemia was shorter than the telomere length measured at the end of therapy (94). Notably, a separate study demonstrated that the shortest telomeres were determined in the blood cells of relapsed patients, followed by newly diagnosed patients, and then by the complete remission group (95). Another study demonstrated that patients with late-stage ALL had a shorter telomere length and higher telomerase activity, associated with disease progression and more unsatisfactory outcomes; a short telomere length increased the risk of developing ALL, but was not associated with the TERT gene polymorphism (96). However, a separate study indicated that the rs16847897 CG genotype increased the risk of developing ALL by 29% compared to the CC genotype (97). Longer telomeres in low-risk B-cell precursor ALL indicated inferior outcomes compared with short telomeres (94). Considering these data, one can conclude that the effect of telomere variation in leukemia is subtype-dependent.
Acute myelogenous leukemia
An early study by Takauchi et al (98) indicated that patients with acute myelogenous leukemia (AML) had shorter telomere lengths. Furthermore, shorter telomere lengths were shown to be indicative of conversion from myelodysplastic syndrome to AML. The conversion was attributed either to heterogeneity or telomere shortening (99). However, telomere shortening is not an indication of cells undergoing a 'telomere crisis' (100). This may be due to the upregulation of telomerase activity in AML stem cells or the extensive replicative potential of normal blood-forming stem cells (100). Moreover, an inverse association between age and telomere length in AML has been shown (101).
Chromosomal aberrations are strongly associated with AML pathogenesis. Indeed, patients with AML with the loss or gain of chromosome fractions carry critically short telomeres, resulting in telomere dysfunction (102). Furthermore, patients with shorter telomeres are prone to jumping translocations (103), while FMS-like tyrosine kinase 3 (FLT3) and internal tandem duplication (ITD) mutations have also been shown to be associated with shorter telomeres (104). On the other hand, isocitrate dehydrogenase (IDH)1 and IDH2 mutations have been shown to be associated with longer telomeres and improved outcomes in patients with AML, possibly due to higher sensitivity to chemotherapy, the duration of aplasia, or other diseases/host factors (104).
A previous study on long-term granulocyte-colony-stimulating factor treatment demonstrated an elevated risk of developing AML due to bone marrow stress from telomere shortening. Indeed, Li et al (105) suggested that this process may be associated with the early stages of leukemogenesis.
As regards pediatric AML, Aalbers et al indicated that these patients exhibited very short telomeres and an increased risk of FLT3/ITD molecular aberrations FLT3/ITD. However, no association was identified with the number of cytogenetic abnormalities, contrary to adult AML (106).
Chronic lymphocytic leukemia (CLL)
Short-length telomeres are a prominent characteristic of CLL. Notably, a shorter telomere length in CLL has been found to be associated with reduced hemoglobin levels and an adverse survival, particularly in patients with biallelic ATM defects (107). Moreover, ATM defects, as well as TP53 defects, have been shown to be associated with telomere shortening and the poor survival of patients with CLL (108). In addition, short telomeres and TP53 mutations increase chromosome instability since, with every cell cycle, the ability of telomeres to protect chromosome ends weakens, thus facilitating the creation of complex aberrations (109,110). Notably, an elevated risk of disease progression has also been found to be associated with TP53 abnormalities (111).
The association of specific mutations with telomere length was highlighted by Jebaraj et al (108), who demonstrated that individuals carrying 17p- and 11q-associated with TP53 and ATM loss had the shortest telomeres even when the abnormalities were minor. Furthermore, it was indicated that patients with two or more genetic abnormalities had shorter telomeres compared with individuals carrying a smaller number of congenital anomalies. Therefore, the authors suggested that telomere shortening was associated with genetic complexity (112).
Some exceptions are evident as patients with normal immunoglobulin variable heavy chain (IGHV) genes have shorter telomere lengths than those with mutated ones (113,114). On the other hand, Roos et al (115) observed an inverse correlation between telomere length and IGHV homology, further adding that shorter telomeres create genetic complexity by increasing the number and occurrence of unwanted chromosomal abnormalities.
Notably, the study by Lin et al (116) indicated that short telomeres were also prone to fusions. The prevalence mentioned above may lead to tumorigenic genomic rearrangements, particularly in patients with early-stage disease. Moreover, it was concluded that shorter telomeres were associated with more aggressive disease due to the high telomere attrition rate in highly proliferative tumors (117). Furthermore, patients with less advanced stages of CLL were shown to exhibit longer telomeres (118). However, both studies suggested that longer telomeres were associated with mutations in TERC, TERT and OBFC1, variants as well as with a higher risk of developing CLL (117,118).
Notably, Furtado et al (119) suggested that telomere shortening was an early event regarding leukemogenesis, since short telomeres are already present in small abnormal B-cell clones of high-count monoclonal B-cell lymphocytosis. This disease precedes CLL.
As regards methodology, both monochrome multiplex quantitative PCR and single telomere length analysis can provide clinically relevant information (111). However, Yang et al (120) suggested that telomere length should not be estimated from buccal samples, as telomere length in buccal and leukemic cells is not associated with patient survival or has any prognostic relevance.
In summary, it is suggested that telomere length can act as a potential prognostic factor, as it may improve risk stratification in patients with CLL for the early initiation of therapy (111,121).
Chronic myelogenous leukemia (CML)
Early studies on CML regarding telomere length demonstrated that patients with CML had shorter telomeres than healthy individuals (122,123). In continuation, it was indicated that more rapid telomere shortening occurs in leukemic rather than non-leukemic hematopoietic stem cells. This accelerated shortening has been shown to be positively associated with the leukemic clone size in the hematopoietic stem cell compartment (124). In addition, studies have indicated that patients with CML in the accelerated or blast phase have shorter telomeres than those in the chronic phase or cytogenic remission (123,125,126). Moreover, telomere shortening is more prominent in high-risk patients than in low-risk ones (126). Specifically, such shortening has been shown to be associated with disease progression/stage, indicating increased genetic instability and a high ability to accumulate secondary genetic events that may induce disease evolution (127).
Indeed, it was hypothesized that a high-risk subgroup of patients with CML who lack telomere maintenance mechanisms enter the accelerated phase of CML early (128). On the other hand, it was observed that patients with treatment-free remission (TFR) had shorter telomeres than those who relapsed (129). This may be attributed to the fact that the longer telomere-carrying CML cells can escape senescence and can divide following hte discontinuation of therapy (129).
Notably, Samassekou et al (130), examining telomere length at both ends of chromosomes, observed that p-ends carried longer telomeres than q-ends and that q-ends presented a higher shortening rate than p-ends). Furthermore, patients with CML in the chronic phase harbored specific telomere length changes of the longest individual telomeres on chromosomes 18p and Xp and the shortest individual telomeres on chromosomes 21p and 21q (130).
8. Integumentary system
Melanoma of the skin
Associations between telomere length and the presentation of cutaneous melanoma are heterogeneous, with the majority of studies concluding that shorter telomere lengths are associated with a decreased risk of developing skin melanoma. By contrast, longer telomeres exhibit a positive association (131-139). In the case of melanoma, shorter telomeres exhibit a protective function against the malignant transformation of melanocytes, since these cells have a limited proliferative ability and capability of undergoing apoptosis (132,136). Indeed, melanocytes carrying longer telomeres do not go through senescence or apoptosis; thus, there is increased melanocyte proliferation, as well as a propensity for nevi and melanoma development (138). Indeed, Viceconte et al (140) suggested that metastatic cutaneous melanoma cells carried longer telomeres, which provides these cells with sufficient replicative potential without activating a telomere maintenance mechanism, and finally contributing to tumor development. On the other hand, shorter telomeres have also been associated with an inferior survival, since critically short telomeres can trigger events that create genetic instability and tumorigenesis (139).
Notably, shorter telomeres have also been found to be associated with a lower number of skin moles (135), while longer telomeres are positively associated with a higher number of skin moles (133). Indeed, some authors have suggested that melanomas may develop from existing moles whose cells continue to proliferate because of delayed replicative senescence (133). Anic et al (133) also identified an association between longer telomeres and an elevated risk of developing melanoma in females, although no association was indicated for males.
However, the association between telomere length and the incidence of melanoma appears to differ between sporadic and familial melanoma. Thus, Menin et al (141) demonstrated that patients with sporadic melanoma exhibited a shorter telomere length than patients with familial melanoma. Indeed, even though shorter telomeres decreased the risk of developing familial melanoma, they tripled the risk of developing single sporadic melanoma (141). These data correlate well with the characterization of melanoma as a complex disease with a multifaceted etiology, and indicate that telomere length may affect each type of melanoma in a discrete manner (141). Undoubtedly, telomere-related genes are also related to the susceptibility of melanoma (134). However, further extensive studies need to be conducted to comprehend the role of telomeres in melanoma.
9. Endocrine system
Thyroid cancer
In 2000, Kammori et al (142) indicated that telomere length was reduced in thyroid cancer tissues and follicular adenomas, compared to normal tissues. However, it was shown that follicular adenomas and papillary carcinomas had elevated mean terminal restriction fragment values compared to the controls. Moreover, the mean terminal restriction fragment values were significantly shorter in telomerase-positive samples than in telomerase-negative ones in both follicular and papillary carcinomas (143).
Moreover, efforts were made to identify potential differences in telomere length among familial and sporadic thyroid cancer patients. This distinction may be critical as thyroid cancer exhibits the highest genetic predisposition among other cancer types (144), even though Jendrzejewski et al (145) did not detect any differences between telomere length in blood samples of familial papillary thyroid cancer (fPTC) and sporadic papillary thyroid cancer (sPTC) cases of papillary thyroid cancer. Capezzone et al (146) identified shorter telomeres in fPTC than in sPTC blood samples, as demonstrated using both quantitative PCR and FISH. Notably, a shorter telomere length was detected in all tissues of patients with fPTC in contrast to those with sPTC, indicating that the differences in telomere length were not restricted to tumor sites (147). These authors hypothesized that the shorter telomeres may have been inherited from parents (147). Indeed, it had been demonstrated that the relative telomere length in patients with second-generation fPTC was similar or even shorter to that of parents and unaffected siblings, suggesting that telomere length is partly transmitted to offspring (146).
On the other hand, patients with familial non-medullary thyroid cancer had shorter telomeres than the controls (148).
As regards cancer risk, no association between telomere length and the risk of thyroid subsequent malignant neoplasm was detected in childhood cancer survivors (149). Nonetheless, Li et al (150) demonstrated that telomere length was associated with the risk of papillary thyroid cancer. Specifically, a reverse U-shaped association between telomere length and the risk of cancer was identified, particularly in younger subjects, indicating that both short and long telomeres can be correlated with the risk of cancer development (150).
Therefore, a complex pattern between the risk of developing thyroid cancer and telomere length variation is emerging, and this warrants further analysis.
10. Reproductive system
Prostate cancer
Prostate cancer is characterized by significant telomere shortening, which results in genomic instability and even chromothripsis identified in >50% of prostate cancer precursor lesions (151). Indeed, short telomeres have been shown to be associated with an increased risk of developing prostate cancer, the risk of recurrence, and a worse prognosis due to the accelerated senescence of immune cells (152). Thus, more aggressive types of prostate cancer presented shorter telomeres (152). Tsai et al (153) also concurred with these results in a study conducted on African-American males. However, a separate study did not detect an association between telomere length and recurrence and prostate cancer-specific mortality. However, shorter telomeres detected in the stroma and epithelial cells were associated with metastasis (154). In another study telomere length was assessed in a cohort of 15 patients with prostate cancer who underwent radiotherapy utilizing telomere FISH (155). Length data were implemented in a machine learning model, XGBoost, trained on pre-irradiation (baseline) and in vitro exposed (4 Gy γ-rays) telomere length measurements, to predict post-irradiation telomeric outcomes. The authors of that study demonstrated that a machine learning model with individual telomere length data for the prediction of post-radiotherapy telomeric outcomes can provide an improved predictive power and novel insight into individual patient radiosensitivity and the risk of radiation-late toxicity. It could be used regardless of cancer type, radiation method, or genetic susceptibilities (155).
Genetic factors also appear to play a role. It was previously demonstrated that individuals carrying the RTEL1 rs2297441 variant AA had shorter telomeres and an increased risk of prostate cancer (156). Hurwitz et al (157) did not observe an association between leukocyte telomere length and prostate cancer in males from hereditary prostate cancer families. Still, they hypothesized that shorter telomeres may be associated with an elevated risk of developing prostate cancer in a subset of genetic diseases (157).
On the other hand, longer telomeres have also been shown to be associated with the risk of developing prostate cancer. The study by Julin et al (158) revealed a moderate association between longer telomeres and an increased risk of developing prostate cancer, particularly in males with a family history of the disease. In addition, longer telomeres increased overall mortality due to a suppressed immune system (158). In another study, longer telomeres were associated with a worse prostate cancer-specific and metastasis-free survival compared to shorter ones (160). Of note, Wulaningsih et al (161) first indicated that increased levels of total prostate-specific antigen were associated with longer telomeres.
In a separate study, the telomere lengths of prostatic small cell neuroendocrine carcinoma (SCNC) and prostatic adenocarcinoma (AdCa) were compared (162). Both cell types exhibited relatively similar telomere lengths, indicating their common origin, although longer telomeres were more common in SCNC (162). Furthermore, longer telomeres in AdCa were associated with more aggressive tumors of aggressive pathological and molecular characteristics (162).
Smoking has also been found to be associated with the development of prostate cancer. Notably, Mirabello et al (163) indicated that, particularly in the case of heavy smokers of the male sex without a family history of the disease, shorter telomeres were associated with a reduced risk of developing prostate cancer. However, another study did not detect any difference concerning telomere length, smoking and prostate cancer. Indeed, it was shown that recent smokers had an elevated variability in telomere length in prostate stromal and cancer cells than long-term smokers (164). Moreover, it was indicated that males of African origin with higher-grade disease had a higher variability in telomere length than Caucasian males with the same disease classification (165).
Breast cancer
Numerous studies have focused on the association between breast cancer risk and telomere length. Thus, longer, as well as shorter telomeres have been found to be associated with an increased risk of developing breast cancer. Indeed, it has been well-established that longer telomeres are associated with an enhanced telomerase activity and may facilitate the incidence of genetic mutations (166). In a previous study, longer telomeres were detected in patients with breast cancer compared with the controls (167). That study was performed on blood cells collected from 611 patients with breast cancer and 154 healthy women in Prague between 2002 and 2010 (167). A similar association on blood cell telomere length was determined in a Chinese female population (168), as well as in Indigenous American women (169).
However, shorter telomeres have also been shown to be associated with an increased risk of developing breast cancer, initially in older, premenopausal or postmenopausal women (170,171). Indeed, estrogen levels have been previously linked with telomere length; thus, the menopausal status could influence telomere length and its connection to insulin resistance and inflammation (171). However, no association between telomere length and the risk of hereditary breast cancer has been observed (172).
Varying results were also obtained when the putative association of telomere length with breast cancer progression was examined. For example, measuring peripheral leukocyte telomere length at baseline and 30 months post-diagnosis in a cohort of breast cancer survivors did not detect an association with either all-cause or breast cancer-specific mortality. However, participants whose telomeres exhibited shortening between baseline and 30 months exhibited a higher risk of breast cancer-specific and all-cause mortality (173). These authors hypothesized that longer telomeres may protect cells from entering into breakage-fusion-bridge cycles, especially those that induce cell senescence (173).
When telomere length and telomerase activity were examined in breast cancer cell lines with various levels of invasiveness, a paradoxical concurrence of enhanced telomerase activity and short telomeres was detected in the most aggressive cell lines. Furthermore, the intracellular localization of hTERT intracellular localization was associated with its activity levels (174). Indeed, it was suggest that telomere length and telomerase activity may be utilized as biomarkers for assessing the aggressiveness of breast cancer cells (174).
A clinical study examining a total of 44 breast cancer tissues, including 15 papillotubular, 17 scirrhous and 12 solid-tubular carcinomas, determined that telomeres measured using quantitative FISH were shorter in cancer cells compared to normal epithelial cells (175). In another clinical study, blood leukocyte telomere length was measured in 52 cancer patients and matching control subjects utilizing quantitative PCR. This approach demonstrated that the average telomere length of patients with advanced-stage disease was shorter compared to those with early-stage disease. Notably, patients with human epidermal growth factor receptor 2 (HER2)+ breast cancer had significantly longer telomeres than HER2− patients (176). HER2 is a biological marker for disease prognosis and disease aggressiveness, and its association with telomere length may provide insight into disease progression and malignancy (177). These data indicate the complexity of the roles of telomeres in breast cancer pathogenesis. Indeed, the association of telomeres with breast cancer progression appears to depend on disease stage, patient age and hormone receptor status.
A number of studies have confirmed the complex pattern of putative associations where genetic factors play a role. For example, it was previously demonstrated that patients homozygous for the variant allele (CC) of hTERC rs16847897 presented longer telomeres (167), while patients with the AA allele of rs2853677 had longer telomeres than those with AG (170). Other examples are BRCA1 and BRCA2 gene mutations concerning telomere length and breast cancer susceptibility in women with a high hereditary risk of developing breast cancer. Thus, Eyüboǧlu et al (177) indicated that patients with BRCA1 and/or BRCA2 mutations had a 12% telomere attrition compared with women with no BRCA1 and/or BRCA2 mutations. Notably, BRCA2 mutations have been shown to be associated with the maintenance of telomere length (178).
Thorvaldsdottir et al (179) also concurred with the latter result and indicated that patients with breast cancer had shorter telomeres compared with healthy women in the case of both BRCA2 mutation carriers and noncarriers. Moreover, BRCA2 mutation carriers with shorter telomeres exhibited an increased risk of developing increased breast cancer, which was not evidenced in non-carriers. Other factors, however, affect the connection to telomere biology. Shorter telomeres in patients with breast cancer have also been shown to be associated with low levels of physical activity (180,181). Indeed, physical activity may hinder cellular aging and protect individuals from age-related diseases (181).
Moreover, telomere length is associated with psychoneurological symptoms (PNS) in breast cancer survivors (182). Specifically, increased levels of pain and lower scores in the visual memory domain have been shown to be associated with shorter telomeres (182). Chemotherapy perhaps induces telomere breakage and chromosome instability, triggering immune surveillance pathways and causing inflammation (182). This may compromise tissue homeostasis and create genetic alteration, leading to the acquisition or persistence of PNS. To summarize, further studies are required in order to better understand the mechanistic aspects of telomere involvement in breast cancer development and progression and enhance telomere biology application in disease evaluation (Table II).
Table II.
Telomere length and breast cancer.
| Cancer type | Sample type | Origin of study population | Telomere length (mean ± SD) | Telomere length/cancer risk | Clinical significance | Authors/(Refs.) |
|---|---|---|---|---|---|---|
| Breast cancer | Blood sample | Turkish | Shorter telomere/higher risk | BRCA1/BRCA2 mutations are associated with shorter telomere length in women with a high hereditary risk of developing breast cancer | Eyüboğlu et al (177) | |
| Breast cancer | Peripheral blood leukocytes | Chinese | 1.07±0.22 | Longer telomere/higher risk | Longer telomeres may be a risk factor and act as a cancer risk predictor | Samavat et al (168) |
| Breast cancer | Leukocyte telomere length | Chinese | Shorter telomere/lower risk | Telomere length associated with breast cancer susceptibility | Luu et al (229) | |
| Breast cancer | Peripheral blood leukocytes | No correlation | Pavanello et al (172) | |||
| Breast cancer | Whole blood or mouthwash samples | US/Hispanic | Longer telomere/higher risk | Risk assessment appears to be modeled by genetic ancestry, specifically Indigenous American | Pellatt et al (169) | |
| Breast cancer | White blood cell | 0.70±0.33 | Shorter telomere/higher risk | More pronounced in pre-menopausal women | Shen et al (171) | |
| Breast cancer | Whole blood | Shorter telomere/higher risk | Such an association was observed in patients with BRCA2 mutations, not in non-carriers | Thorvaldsdottir et al (179) | ||
| Breast cancer | Peripheral blood | Chinese/Han | Shorter telomere/higher risk | This association applies to all subject groups (age >40 years, BMI ≤24 kg/m2 and post-menopausal women) | Wang et al (218) |
BMI, body mass index.
Ovarian cancer
Ovarian cancer is another hormone-responsive cancer whose pathogenesis is closely associated with fluctuations in sex hormones and discrete receptor expression. Initially, it was shown that the peripheral blood leukocytes of patients with ovarian cancer have shorter telomeres compared to those of age-matched healthy women (183). Moreover, it was determined that the strength of the association was inversely related to the telomere length of more aggressive types of tumors (183). That study was in agreement with the findings of the study by Kuhn et al (184), demonstrating that telomere length changes depending on the ovarian tumor histological type. Specifically, shorter telomeres were detected in high- and low-grade serous carcinomas and low-grade endometrioid carcinomas of the ovaries than clear cell ovarian carcinoma (184). However, these authors did not find an association between overall mortality and telomere length in these main ovarian cancer types (185). The exception was clear cell carcinoma of the ovaries, where the death hazard ratio among females with a telomere index >1 was higher when compared with those with a telomere index ≤1. The telomere index was defined as the mean telomere length of cancer cells relative that of to stromal cells (184).
Martinez-Delgado et al (186) demonstrated that sporadic, as well as familial cases of ovarian cancer had shorter telomeres than the controls when age-adjusted. Furthermore, these authors suggested that shorter telomeres were associated with an increased risk of developing ovarian cancer, particularly in younger females, with the risk progressively decreasing with age (186). In separate studies, shorter telomeres were associated with worse outcomes, as well as unplanned hospital admissions (187), while longer telomeres were associated with a reduced risk of non-severe and rapidly fatal cases (188). On the other hand, it was shown that the minor allele at the peak 2 SNP rs7705526 was associated with longer telomeres and an increased risk of developing low-malignant-potential ovarian cancer (the change in relative telomere length being 1.020-fold per allele) (189).
However, Terry et al (190) did not observe any difference between the telomere length of ovarian cancer cases and the controls, although they suggested that a genetic variation in the TERT gene could affect the risk for this malignancy. In addition, the study by Kotsopoulos et al (191) did not find any association between telomere length and ovarian cancer-specific mortality, suggesting that telomere length cannot predict outcome following diagnosis.
Several associations between telomere length and treatment strategies have been identified for patients with ovarian cancer. As regards therapies against ovarian carcinoma, some women are treated with glucose restriction combined with chemotherapy (192). Notably, telomerase is overexpressed in >80% of human cancers (193). It was previously shown that the administration of platinum-taxane chemotherapy, under fasting glucose conditions, significantly decreased telomerase expression, resulting in a 30% decrease in telomere length and in the attenuation of ovarian cancer cell immortalization (192). Notably, ovarian tissue cryopreservation is a process through which patients with ovarian cancer manage to preserve fertility (194). However, the mean telomere length is reduced following cryopreservation, inducing cellular senescence and DNA damage (194).
Therefore, these collective data indicate that the association between telomere length and ovarian cancer pathogenesis is influenced by the patients' age and the ovarian tumor histological type. These factors need to be taken into considerations before consensus can be reached.
Cervical cancer
The pattern of discrete associations between cancer incidence and telomere length is repeated in cervical cancer. Zhang et al (195) initially observed both the shortening and elongation of telomeres in patients with cervical cancer. However, in another study, telomere FISH assays revealed that early-stage cervical intraepithelial neoplasias (CINs), particularly CIN2, exhibited shorter telomeres compared to neighboring normal squamous epithelia. This was strongly associated with increased rates of chromosomal arm loss/gain (196). Moreover, cervical cancer tissue presented more significant heterogeneity as regards telomere length, suggesting that the progressive shortening of telomeres may facilitate the transformation of CIN to cervical cancer. On the other hand, no significant differences in the telomere length of the normal endometrium and endometrial hyperplasia and cancer were detected (196).
High-risk human papillomavirus (HR-HPV) can cause cervical cancer; however, a shortened telomere length in cervical exfoliated cells has been shown to be associated with a lower risk of developing cervical cancer among HR-HPV-positive women. Thus, it has been suggest that shorter telomeres may decrease the risk of developing cervical cancer in HR-HPV-positive patients (197). Indeed, in this case, the shorter telomeres may act as a suppressor and hinder proliferation (197).
It is noteworthy that telomeres are transcribed into heterogeneous long non-coding RNA, known as telomeric repeat-containing RNA (TERRA) (198). Of note, TERRA, which usually have a short half-life, tend to accumulate in rapidly-growing cancer cells, with the result that high TERRA levels are detected in various human cancer types (199). Thus, even though TERRA abundance was not found to be associated with telomere length in six cervical cancer cell lines, its abundance was found to be associated with RNA stability, and possibly, telomeres (200). Another example of genetic influence is the participation of homeobox containing 1 (HMBOX1) on telomere length. HMBOX1 was initially attributed to the properties of binding to double-stranded DNA (201). Moreover, this protein was identified as a positive regulator of telomere length (202). Furthermore, it was indicated that HMBOX1 knockdown induced radiosensitivity in cervical cancer cells and led to shorter telomeres, enhanced DNA damage response, and increased levels of apoptosis (203).
In summary, shorter telomeres appear to be associated with CIN transformation to cervical cancer, but not in HPV-positive patients, whereas the asscoiation with genetic factors may play a significant role (Table III).
Table III.
Telomere length and HPV infection.
| Sample type | Measurement method | Association/significance | Authors/(Refs.) |
|---|---|---|---|
| Tissue sample | PCR-based TRAP assay | No correlation between HPV infection/telomere length | Zhang et al (233) |
| Cervical exfoliated cells | PCR | Shorter telomere length/lower risk of cancer in HPV-positive women; telomere length may function as a biomarker to detect high-risk individuals during screening | Chen et al (197) |
TRAP, telomerase repeat amplification protocol; HPV, human papillomavirus.
11. Digestive system
Esophageal cancer
A complex pattern emerges when analyzing data on the association between telomere length and esophageal cancer. Both short and long telomeres are implicated with a U-shaped association. As with the other types of cancer, the findings of research studies vary considerably, depending on the clinical outcomes and the parameters under investigation. To begin with, multiple studies using esophageal squamous cell carcinoma (ESCC) cells have proven that these cells possess shorter telomeres than the controls (204-206). Moreover, it has also been shown that telomere alterations not only affect the esophageal epithelium, but also stromal cells. This is crucial, as, in this case, stromal cells of cancer lesions have been identified to have longer telomeres resulting in chromosome 4q, 13q and 15q instability (207). Notably, Xing et al (205) indicated that shorter telomeres were detected, particularly on chromosomes 17p and 12q, but not 11q and 2p of ESCC cells. This may occur since p53 and other tumor suppressor genes are located on 17p (205). On the other hand, Du et al (204) observed a U-shaped association between telomere length and ESCC risk, indicating that both extremely short and long telomeres may affect tumor progression. However, Lin et al (207) did not find any association between telomere length and ESCC precursor lesions. Furthermore, genotyping studies have identified several SNPs related to telomere length that are associated with the susceptibility to ESCC (208-211). Specifically, CXCR4 rs6430612, TERT rs13172201 and OBFC1 rs4387287 in short telomeres were found to increase the risk of developing ESCC (208). At the same time, the A allele of telomere-related SNP rs2736108 was associated with longer telomeres, as well as a more prolonged survival (209) suggesting an underlying protective mechanism against ESCC (210). It was shown that the rs621559 AA genotype decreased the risk of developing ESCC, compared to the GG genotype, while the 14q21 rs398652 G allele exhibited an increased cancer risk (210). These associations were sex-dependent, with stronger associations detected in males (210). Lastly, Hao et al (211) indicated that patients with p53 somatic mutations had shorter telomeres, inducing increased proliferation and susceptibility to tumor development. Moreover, the rs12951053 CC genotype and the rs1042522 GG genotype were shown to be associated with shorter telomeres (211). Yu et al (212) demonstrated that short telomeres in combination with Arg/Pro or Arg/Arg genotypes and HPV-16 seropositivity increased the risk of ESCC 12.08-fold. On the other hand, in another study, no association between leukocyte telomere length and disease was detected in esophageal adenocarcinoma (213). Of note, Pan et al (214) demonstrated that when short telomeres were combined with epidemiological factors, such as smoking and excessive alcohol intake, there was a 16.82-fold increase in the risk of developing ESCC.
Gastric cancer
Even though gastric cancer is a rather complex entity where genetic, environmental and microbial parameters appear to be involved (215). Tahara et al (215) demonstrated that patients with gastric cancer exhibited shorter leukocyte telomeres than the healthy controls, this observation is in agreement with the results from previous and later studies (216,217). When an analysis of telomere length in patients with gastric cancer and age- and sex-matched controls was performed, an association with aging, a history of smoking, a decreased fruit intake and Helicobacter pylori positivity with short telomeres was established (216). Shorter leukocyte telomeres were also shown to be associated with a worse overall survival and progression-free survival in patients with advanced-stage disease (215). An association between peripheral blood leukocyte telomere length and the risk of gastric cancer was prospectively assessed in a cohort of 26,540 middle-aged or older Chinese patients. This strategy identified a significantly higher risk of developing gastric cancer with the lowest or the highest quintile of telomere length, most apparent in males and younger individuals (218). On the other hand, a prospective study encompassing a cohort of 40,000 European participants did not identify an association between the risk of gastric cancer and telomere length (219). These data collectively suggest that the effect of telomere length may differ depending on the disease stage, sex, or even racial origin.
Furthermore, a reduced immune response and a higher percentage of CD4+ T-cells and CD19+ IL-10+ Breg percentage in B-cells and plasma IL-10 concentration were shown to be associated with shorter telomeres in cancer patients (217). Shorter telomeres were also linked associated with Helicobacter pylori-positive patients, smokers with a low fruit/vegetable intake, moderate or severe gastritis and intestinal metaplasia (215,216). In separate studies, smoking was also found to be associated with the risk of developing gastric cancer and a shorter telomere length (220). Moreover, leukocyte telomere length was found to strongly contribute to the predisposition to gastric cardia carcinoma in a cohort of the Chinese Han population. Notably, the combination of shorter telomere length and smoking enhanced the development of this cancer type (221). Thus, Hou et al (216) concluded that shorter telomeres increased the risk of developing gastric cancer, perhaps through the impairment of cellular functions, creating chromosome instability.
The known association between Helicobacter pylori and gastric cancer has also been linked to telomere length. Thus, patients infected with Helicobacter pylori infection have shorter telomeres, perhaps due to related inflammatory cytokine release (222). Furthermore, the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) amplification could possibly be an integral part of carcinogenesis (222,223). Therefore, Tahara et al (222) suggested that Helicobacter pylori eradication could significantly decrease the risk and mortality from gastric cancer.
Genotypic factors associated with telomere length, such as different TERT variants, can increase the risk of developing gastric cancer (224). For instance, TERT variants such as rs10069690, rs2242652 and rs2853676, and TN1P1 variants such as rs7708392 and rs10036748 have been shown to be associated with an increased risk of gastric cancer (224). In addition, Du et al (225) indicated that the G allele of rs2736100 in TERT at 5p15.33 was associated with longer telomeres; Choi et al (226) indicated that in the same variant, the CC genotype had longer telomeres than the AA. Notably, Du et al (225) also identified a U-shaped association between telomere length and the risk of developing gastric cancer.
Pancreatic cancer
Multiple studies have suggested that short telomeres increase the risk of developing pancreatic cancer. Telomere shortening is one of the earliest events in tumorigenesis; thus, shorter telomeres may increase the risk of developing pancreatic cancer and also with its progression (227,228). On the other hand, some studies have demonstrated that longer telomeres increase the risk of developing pancreatic cancer, as they bestow an elevated ability to proliferate to transformed cells (229,230). According to Mormile (231), long telomeres result from high telomerase activity, set off by surviving overexpression through transcriptional activation of hTERT. However, the results of extensive epidemiological research are inconclusive regarding the association between the risk of pancreatic ductal adenocarcinoma (PDAC) and telomere length. This is attributed to various reasons, including the study design and method of telomere measurement, as discussed by Duell (228).
Other studies, however, have indicated that both extremely long and short telomeres increase the risk of developing this type of cancer. Skinner et al (232) demonstrated a skewed U-shape association between telomere length and pancreatic cancer risk, whereas Zhang et al (233) concurred with these results for an Asian population cohort.
In a previous study, polymorphisms previously associated with variations in telomere length were genotyped to assess the association of genetically predicted short telomere length with the risk of developing PDAC in light of the conflicting findings. This approach revealed that genetically predicted short telomere length was not associated with the risk of developing PDAC (234). Indeed, since genetically predicted short telomere length is not associated with the risk of developing PDAC, it is suggested that telomere length may be a marker of long-term exposure to various risk factors, such as obesity, smoking and diabetes (233).
In continuation, it was determined that treatment-naïve short leukocyte short telomere length was associated with a higher risk of developing PDAC. The association was not affected by the germline variation of the genotyped SNPs (235). Furthermore, treatment-naïve short leukocyte short telomere length was associated with the poorer overall survival of patients with PDAC (235). The association may partially be attributed to the fact that shorter telomeres create chromosome instability and can enhance the progression rapidly from precursor lesions to invasive ductal carcinoma (236). Notably, the minor TERT allele rs401681 was also found to be associated with short telomeres, resulting in an increased risk of developing pancreatic cancer (237).
The study by Posch et al (238) indicated that patients with sporadic pancreatic neuroendocrine neoplasms with promoter mutations had longer telomeres than those with wild-type ones. By contrast, another study demonstrated that IL-6 cytokine production was reduced in individuals with longer telomeres (239). It was hypothesized that shorter telomeres were a consequence and not a result, since they may indicate a model of pancreatic tumorigenesis with increased levels of IL-6 accounting for the strong STAT3 (major pro-tumorigenic IL-6 effector and can influence KRAS-induced pancreatic tumorigenesis) activation implicated in KRAS-driven pancreatic cancer (239). Both epidemiological and environmental factors appear to affect the association of telomere length with the risk of developing pancreatic cancer.
Colorectal cancer
The latest advances regarding colorectal cancer suggest that telomere length in tumor tissues is shorter than in the adjacent mucosa (240,241). Furthermore, tumors with a higher number of somatic mutations present shorter telomeres (241). Moreover, an association between tumor stage and telomere length was previously identified, as lower-stage tumor tissues exhibited shorter telomeres than advanced-stage and metastatic tumors (240). These studies characterize colorectal cancer tissue with both chromosomal or microsatellite instability (240,241). Moreover, Piñol-Felis et al (242) concluded that telomere length could be used as a reliable prognostic factor, since telomere shortening was, in their study, associated with an early stage of tumorigenesis.
However, some studies have identified an association between the risk of developing colorectal cancer and longer telomeres (240,243). The two different associations detected in patients with colon cancer are suggested to reflect alternative mechanisms of tumorigenesis and specific disease stages (240,243). Likewise, the studies by Luu et al (244) and Peacock et al (245) demonstrated an association between longer telomeres and an increased risk of colorectal cancer. Specifically, longer telomeres exhibited an elevated risk of accumulating mutations that could lead to transformation and cancer progression (244,245). However, a meta-analysis study indicated no association between telomere length and the risk of colorectal cancer (246).
Furthermore, in another study, the analysis of telomere-related protein expression in colorectal cancer tissues revealed differences relative to the adjacent mucosa. A positive association between hTERT expression and patient age in a Saudi Arabian cohort was identified and was associated with the patients' clinicopathological characteristics (247).
Genetic factors affect the association between the risk of developing colorectal cancer and telomere length. Park et al (248) identified that telomere shortening in cases of tubular adenomas was mainly caused by the PIK3CA amplification. Bu contrast, telomere shortening in serrated polyps was attributed to BRAF mutations (248). Furthermore, these authors suggested that tumor genotyping may be a helpful tool to monitor tumor progression (248). Moreover, a rare P507L variant in TPP1 may increase the risk of developing colorectal cancer by interrupting the TPP1-TIN2 interaction, thus impairing telomerase activity and decreasing telomeres (249).
Epidemiological factors appear to be involved, as a study detected shorter telomeres in depressed individuals and identified education and social support as factors towards alterations in telomere length (250). However, a separate study failed to detect an association between religiosity and telomere length (251).
A complex pattern on the association between colon cancer risk and telomere length is emerging, which warrants further validation through studies performed with a larger number of patients (Table IV).
Table IV.
Telomere length and colorectal cancer.
| Cancer type | Sample type | Origin of study population | Telomere length/cancer risk | Clinical significance | Authors/(Refs.) |
|---|---|---|---|---|---|
| Colorectal adenoma | Colonic tissue samples | Longer telomere/higher risk | Peacock et al (245) | ||
| Colorectal cancer | Chinese | Shorter telomere/higher risk | A rare variant, P507L in TPP1 increases the risk of colorectal cancer through telomere shortening | Li et al (150) | |
| Colorectal cancer | White blood cells | Chinese | Longer telomere/higher risk | Particularly for rectal cancer, longer telomeres could play a role in cancer pathogenesis, thus acting as a biomarker | Luu et al (244) |
| Colorectal cancer | Peripheral blood leucocytes | Inconclusive results (metanalysis of seven studies) | The complex relationship between telomere length and cancer risk | Naing et al (246) |
TPP1, tripeptidyl-peptidase 1.
Liver cancer
The association between the incidence of hepatocellular cancer (HCC) and telomere length is dependent on cancer stage. Patients with HCC have shorter telomeres compared to healthy controls, although longer telomere lengths have been detected in patients with advanced-stage disease (252-255). Furthermore, shorter telomeres have been shown to be associated with a decreased survival, increased recurrence and numerous TERT promoter mutations (253,255). On the other hand, longer telomeres have been found to be associated with more aggressive types of tumors and a poor prognosis (252,254). In addition, it is suggested that longer telomeres may prevent telomere attrition by suppressing reactive oxygen species or phosphorylated AKT levels (252).
Notably, longer telomeres, in combination with hepatitis B virus (HBV) or hepatitis C virus (HCV) infections, increased the risk of developing HCC (256,257). However, Cheng et al (257) detected a U-shaped association between telomere length and the risk of developing HCC. At the same time, Zeng et al (256) indicated that 5 years prior to diagnosis, shorter telomeres were associated with an increased risk of the disease. On the contrary, longer telomere length detected 10 years prior to diagnosis contributed to the risk of developing HCC (256). Finally, Feng et al (258) indicated that peripheral blood samples could be used to measure telomere length in HBV or HCV-infected patients, but not in non-infected ones. The viral genome may affect telomerase activity, thus affecting disease development and persistence (258).
12. Conclusions and future perspectives
Telomeres, chromosome-end DNA-protein structures, are known to progressively shorten over time in the majority of somatic cells. These genome-protecting structures are markers of aging. Notably, short telomeres have been associated with an older age and chronic diseases. An association between cancer and telomere length has been suggested with related uncertainties due to objective difficulties in designing studies of sufficient robustness. A disparity appears to emerge when evaluating risk associations between cancer and telomere length. Even though shorter telomeres have been adopted as a marker of a poorer health status and an older biological age, longer telomeres due to increased growth potential are associated with acquiring cancer-initiating somatic mutations (56). Indeed, the majority of retrospective studies report an increased risk of cancer in individuals carrying shorter telomeres (27,134,145,163,209) whereas prospective observational studies have detected a weak positive association between longer leukocyte telomeres with the risk of cancer (51,88,123,136,245,256). This association pattern may be partly accounted for by the varying ability of somatic cells to grow; thus, telomere length could exert discrete potential effects. Moreover, a U-shaped curve of telomere length effects has been detected in various types of cancer (150,156,216,237,256). This suggests that the association with cancer risk would vary among telomere length distribution and would not be linear. The pivotal clinical relevance of this knowledge stands on the fact that oncologists who treat these malignancies may seek for another tool in their effort to tackle cancer. Moreover, pathologists may use telomeres, telomerase, hTERT or any of the implicated proteins as potential biomarkers for cancer prognosis (259,260). Furthermore, the cancer-type-specific association is influenced by co-factors, e.g., virus load, inflammatory status, or SNP-disease association. Moreover, the effect of telomere length may differ, depending on the disease stage, sex, or even racial belonging. This is critical, particularly for treatment strategies targeting telomerase, since tumors that present long telomeres may be affected, but others that exhibit short telomeres may not have the same response (261). Therefore, telomere length data may provide valuable input on cancer development and progression for certain types of cancers; however, further studies are required for more generalized conclusions.
Acknowledgments
Not applicable.
Abbreviations
- HDI
human development index
- TRF
telomere repeat-binding factor
- TIN2
nuclear protein 2
- TPP1
tripeptidyl-peptidase 1
- POT1
protection of telomeres 1
- DDR
DNA damage response
- TERT
telomerase reverse transcriptase
- ALT
alternative lengthening of telomeres
- PML
promyelocytic leukemia
- APBs
ALT-associated PML bodies
- IARC
International Agency for Research on Cancer
- SCLC
small cell lung cancer
- NSCLC
non-small cell lung cancer
- RCC
renal cell carcinoma
- ALL
acute lymphocytic leukemia
- AML
acute myelogenous leukemia
- CLL
chronic lymphocytic leukemia
- CML
chronic myelogenous leukemia
- fPTC
familial papillary thyroid cancer
- sPTC
sporadic papillary thyroid cancer
- PDAC
pancreatic ductal adenocarcinoma
- SCNC
small cell neuroendocrine carcinoma
- AdCa
prostatic adenocarcinoma
- CINs
cervical intraepithelial neoplasias
- HR-HPV
high-risk human papillomavirus
- HMBOX1
homeobox containing 1
- ESCC
esophageal squamous cell carcinoma
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
Funding Statement
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (AT, TO, TKN, EV, MTz, MF, ER, PF, EI, MB, VK, IK, FK, EH, MTo, AAS, DAS, DN, JT and AB) contributed to the conception and design of the study. TO, TKN, EV, MF, ER, PF, EI, MB, VK, IK and FK searched the literature for studies to be included in the review; the selected literature was then examined and reviewed by EH, DAS, JT, DN, AAS, EV, AB and MTz drafted and wrote the manuscript. AT, AAS, AB and DAS provided advice and critically revised the manuscript. All authors have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9:217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzanakakis G, Giatagana EM, Kuskov A, Berdiaki A, Tsatsakis A, Neagu M, Nikitovic D. Proteoglycans in the pathogenesis of hormone-dependent cancers: Mediators and effectors. Cancers (Basel) 2020;12:2401. doi: 10.3390/cancers12092401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maciejowski J, de Lange T. Telomeres in cancer: Tumor suppression and genome instability. Nat Rev Mol Cell Biol. 2017;18:175–186. doi: 10.1038/nrm.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shay JW, Wright WE. Telomeres and telomerase: Three decades of progress. Nat Rev Genet. 2019;20:299–309. doi: 10.1038/s41576-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 5.Lim CJ, Cech TR. Shaping human telomeres: From shelterin and CST complexes to telomeric chromatin organization. Nat Rev Mol Cell Biol. 2021;22:283–298. doi: 10.1038/s41580-021-00328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dratwa M, Wysoczańska B, Łacina P, Kubik T, Bogunia-Kubik K. TERT-Regulation and roles in cancer formation. Front Immunol. 2020;11:2930. doi: 10.3389/fimmu.2020.589929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hrdličková R, Nehyba J, Bose HR. Alternatively spliced telomerase reverse transcriptase variants lacking telomerase activity stimulate cell proliferation. Mol Cell Biol. 2012;32:4283–4296. doi: 10.1128/MCB.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sæbøe-Larssen S, Fossberg E, Gaudernack G. Characterization of novel alternative splicing sites in human telomerase reverse transcriptase (hTERT): Analysis of expression and mutual correlation in mRNA isoforms from normal and tumour tissues. BMC Mol Biol. 2006;7:26. doi: 10.1186/1471-2199-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Listerman I, Sun J, Gazzaniga F, Lukas JL, Blackburn EH. The major reverse transcriptase-incompetent splice variant of the human telomerase protein inhibits telomerase activity but protects from apoptosis. Cancer Res. 2013;73:2817–2828. doi: 10.1158/0008-5472.CAN-12-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 11.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Vakonaki E, Salataj E, Kouvidi E, Nikitovic D, Kovatsi L, et al. Association of nutraceutical supplements with longer telomere length. Int J Mol Med. 2019;44:218–226. doi: 10.3892/ijmm.2019.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasilopoulos E, Fragkiadaki P, Kalliora C, Fragou D, Docea AO, Vakonaki E, Tsoukalas D, Calina D, Buga AM, Georgiadis G, et al. The association of female and male infertility with telomere length (Review) Int J Mol Med. 2019;44:375–389. doi: 10.3892/ijmm.2019.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vakonaki E, Tsiminikaki K, Plaitis S, Fragkiadaki P, Tsoukalas D, Katsikantami I, Vaki G, Tzatzarakis MN, Spandidos DA, Tsatsakis AM. Common mental disorders and association with telomere length. Biomed Rep. 2018;8:111–116. doi: 10.3892/br.2018.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razgonova MP, Zakharenko AM, Golokhvast KS, Thanasoula M, Sarandi E, Nikolouzakis K, Fragkiadaki P, Tsoukalas D, Spandidos DA, Tsatsakis A. Telomerase and telomeres in aging theory and chronographic aging theory (Review) Mol Med Rep. 2020;22:1679–1694. doi: 10.3892/mmr.2020.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336:593–597. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasco MA. Telomeres and human disease: Ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 17.Jäger K, Walter M. Therapeutic targeting of telomerase. Genes (Basel) 2016;7:39. doi: 10.3390/genes7070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsatsakis A, Tsoukalas D, Fragkiadaki P, Vakonaki E, Tzatzarakis M, Sarandi E, Nikitovic D, Tsilimidos G, Alegakis AK. Developing BIOTEL: A Semi-Automated spreadsheet for estimating telomere length and biological age. Front Genet. 2019;10:84. doi: 10.3389/fgene.2019.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26:447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]
- 20.Teng SC, Zakian VA. Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:8083–8093. doi: 10.1128/MCB.19.12.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cesare A, Reddel R. Alternative lengthening of telomeres: Models mechanisms and implications. Nat Rev Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 22.Henson J, Neumann AA, Yeager TR, Reddel RR. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21:598–610. doi: 10.1038/sj.onc.1205058. [DOI] [PubMed] [Google Scholar]
- 23.Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, Netto GJ, Epstein JI, Lotan TL, Westra WH, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179:1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demanelis K, Jasmine F, Chen LS, Chernoff M, Tong L, Delgado D, Zhang C, Shinkle J, Sabarinathan M, Lin H, et al. Determinants of telomere length across human tissues. Science. 2020;369:eaaz6876. doi: 10.1126/science.aaz6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peleteiro B, Padrão P, Castro C, Ferro A, Morais S, Lunet N. Worldwide burden of gastric cancer in 2012 that could have been prevented by increasing fruit and vegetable intake and predictions for 2025. Br J Nutr. 2016;115:851–859. doi: 10.1017/S000711451500522X. [DOI] [PubMed] [Google Scholar]
- 26.Doherty JA, Grieshober L, Houck JR, Barnett MJ, Tapsoba JD, Thornquist M, Wang CY, Goodman GE, Chen C. Telomere length and lung cancer mortality among heavy smokers. Cancer Epidemiol Biomarkers Prev. 2018;27:829–837. doi: 10.1158/1055-9965.EPI-17-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leão R, Apolónio JD, Lee D, Figueiredo A, Tabori U, Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: Clinical impacts in cancer. J Biomed Sci. 2018;251:22. doi: 10.1186/s12929-018-0422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barthel FP, Wei W, Tang M, Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang Q, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49:349–357. doi: 10.1038/ng.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albertson DG. Gene amplification in cancer. Trends Genet. 2006;22:447–455. doi: 10.1016/j.tig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 30.McClintock B. The fusion of broken ends of chromosomes following nuclear fusion. Proc Natl Acad Sci. 1942;28:458–463. doi: 10.1073/pnas.28.11.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentijn L, Koster J, Zwijnenburg D, Hasselt NE, van Sluis P, Volckmann R, van Noesel MM, George RE, Tytgat GA, Molenaar JJ, Versteeg R. NH-N and 2015 undefined: TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet. 2015;47:1411–1414. doi: 10.1038/ng.3438. [DOI] [PubMed] [Google Scholar]
- 32.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Wiley Online Libr. 2008;99:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis KA, Tollefsbol TO. Regulation of the telomerase reverse transcriptase subunit through epigenetic mechanisms. Front Genet. 2016;7:83. doi: 10.3389/fgene.2016.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng L, Montironi R, Lopez-Beltran A. TERT promoter mutations occur frequently in urothelial papilloma and papillary urothelial neoplasm of low malignant potential. Eur Urol. 2017;71:497–498. doi: 10.1016/j.eururo.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castelo-Branco P, Choufani S, Mack S, Gallagher D, Zhang C, Lipman T, Zhukova N, Walker EJ, Martin D, Merino D, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: An integrative genomic and molecular study. Lancet Oncol. 2013;14:534–542. doi: 10.1016/S1470-2045(13)70110-4. [DOI] [PubMed] [Google Scholar]
- 37.Castelo-Branco P, Leão R, Lipman T, Campbell B, Lee D, Price A, Zhang C, Heidari A, Stephens D, Boerno S, et al. A cancer specific hypermethylation signature of the TERT promoter predicts biochemical relapse in prostate cancer: A retrospective cohort study. Oncotarget. 2016;7:57726–57736. doi: 10.18632/oncotarget.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sepehri Z, Beacon TH, Osman FDS, Jahan S, Davie JR. DNA methylation and chromatin modifications. Nutritional Epigenomics. 2019:13–36. doi: 10.1016/B978-0-12-816843-1.00002-3. [DOI] [Google Scholar]
- 39.Bert SA, Robinson MD, Strbenac D, Statham AL, Song JZ, Hulf T, Sutherland RL, Coolen MW, Stirzaker C, Clark SJ. Regional Activation of the cancer genome by long-range epigenetic remodeling. Cancer Cell. 2013;23:9–22. doi: 10.1016/j.ccr.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Hrdličková R, Nehyba J, Bargmann W, Bose HR. Multiple tumor suppressor microRNAs regulate telomerase and TCF7 an important transcriptional regulator of the Wnt pathway. PLoS One. 2014;9:e86990. doi: 10.1371/journal.pone.0086990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitomo S, Maesawa C, Ogasawara S, Iwaya T, Shibazaki M, Yashima-Abo A, Kotani K, Oikawa H, Sakurai E, Izutsu N, et al. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci. 2008;99:280–286. doi: 10.1111/j.1349-7006.2007.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kachuri L, Helby J, Bojesen SE, Christiani DC, Su L, Wu X, Tardón A, Fernández-Tardón G, Field JK, Davies MP, et al. Investigation of leukocyte telomere length and genetic variants in chromosome 5p15.33 as prognostic markers in lung cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:1228–1237. doi: 10.1158/1055-9965.EPI-18-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue Y, Guo X, Huang X, Zhu Z, Chen M, Chu J, Yang G, Wang Q, Kong X. Shortened telomere length in peripheral blood leukocytes of patients with lung cancer, chronic obstructive pulmonary disease in a high indoor air pollution region in China. Mutat Res. 2020;858-860:503250. doi: 10.1016/j.mrgentox.2020.503250. [DOI] [PubMed] [Google Scholar]
- 44.Steiner B, Ferrucci LM, Mirabello L, Lan Q, Hu W, Liao LM, Savage SA, De Vivo I, Hayes RB, Rajaraman P, et al. Association between coffee drinking and telomere length in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. PLoS One. 2020;15:e0226972. doi: 10.1371/journal.pone.0226972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun B, Wang Y, Kota K, Shi Y, Motlak S, Makambi K, Loffredo CA, Shields PG, Yang Q, Harris CC, Zheng YL. Telomere length variation: A potential new telomere biomarker for lung cancer risk. Lung Cancer. 2015;88:297–303. doi: 10.1016/j.lungcan.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang JS, Choi YY, Lee WK, Choi JE, Cha SI, Kim YJ, Kim CH, Kam S, Jung TH, Park JY. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99:385–1389. doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Espiridion B, Chen M, Chang JY, Lu C, Chang DW, Roth JA, Wu X, Gu J. Telomere length in peripheral blood leukocytes and lung cancer risk: A large case-control study in Caucasians. Cancer Res. 2014;74:2476–2486. doi: 10.1158/0008-5472.CAN-13-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon HS, Choi JE, Jung DK, Choi YY, Kang HG, Lee WK, Yoo SS, Lim JO, Park JY. Telomerase activity and the risk of lung cancer. J Korean Med Sci. 2012;27:141–145. doi: 10.3346/jkms.2012.27.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dobija-Kubica K, Zalewska-Ziob M, Bruliński K, Rogoziński P, Wiczkowski A, Gawrychowska A, Gawrychowski J. Telomerase activity in non-small cell lung cancer. Kardiochir Torakochirurgia Pol. 2016;13:15–20. doi: 10.5114/kitp.2016.58959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Zhang L, Zhu H, Pan W, Zhang N, Li Y, Yang M. Leukocyte telomere length and clinical outcomes of advanced lung adenocarcinoma patients with epidermal growth factor receptor tyrosine kinase inhibitors treatment. DNA Cell Biol. 2018;37:903–908. doi: 10.1089/dna.2018.4337. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Zhao Q, Zhu W, Liu T, Xie SH, Zhong LX, Cai YY, Li XN, Liang M, Chen W, et al. The association of telomere length in peripheral blood cells with cancer risk: A systematic review and meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2017;26:1381–1390. doi: 10.1158/1055-9965.EPI-16-0968. [DOI] [PubMed] [Google Scholar]
- 52.Cao X, Huang M, Zhu M, Fang R, Ma Z, Jiang T, Dai J, Ma H, Jin G, Shen H, et al. Mendelian randomization study of telomere length and lung cancer risk in East Asian population. Cancer Med. 2019;8:7469–7476. doi: 10.1002/cam4.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machiela MJ, Hsiung CA, Shu XO, Seow WJ, Wang Z, Matsuo K, Hong YC, Seow A, Wu C, Hosgood HD, et al. Genetic variants associated with longer telomere length are associated with increased lung cancer risk among never-smoking women in Asia: A report from the female lung cancer consortium in Asia. Int J Cancer. 2015;137:311–319. doi: 10.1002/ijc.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan JM, Beckman KB, Wang R, Bull C, Adams-Haduch J, Huang JY, Jin A, Opresko P, Newman AB, Zheng YL, et al. Leukocyte telomere length in relation to risk of lung adenocarcinoma incidence: Findings from the Singapore Chinese Health Study. Int J Cancer. 2018;142:2234–2243. doi: 10.1002/ijc.31251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de-Torres JP, Sanchez-Salcedo P, Bastarrika G, Alcaide AB, Pío R, Pajares MJ, Campo A, Berto J, Montuenga L, Del Mar Ocon M, et al. Telomere length, COPD and emphysema as risk factors for lung cancer. Eur Respir J. 2017;49:1601521. doi: 10.1183/13993003.01521-2016. [DOI] [PubMed] [Google Scholar]
- 56.Telomeres Mendelian Randomization Collaboration. Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, Bowden J, Wade KH, Timpson NJ, Evans DM, et al. Association between telomere length and risk of cancer and non-neoplastic diseases: A mendelian randomization study. JAMA Oncol. 2017;3:636–651. doi: 10.1001/jamaoncol.2017.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goh F, Yang IA, Bowman RV, Fong KM. Subtype variation and actionability of telomere length abnormality in lung cancer. Transl Lung Cancer Res. 2018;7(Suppl 3):S251–S253. doi: 10.21037/tlcr.2018.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kachuri L, Latifovic L, Liu G, Hung RJ. Systematic review of genetic variation in chromosome 5p15.33 and telomere length as predictive and prognostic biomarkers for lung cancer. Cancer Epidemiol Biomarkers Prev. 2016;25:1537–1549. doi: 10.1158/1055-9965.EPI-16-0200. [DOI] [PubMed] [Google Scholar]
- 59.Vaiciulis P, Liutkeviciene R, Liutkevicius V, Vilkeviciute A, Gedvilaite G, Uloza V. Association of relative leucocyte telomere length and gene single nucleotide polymorphisms (TERT, TRF1, TNKS2) in laryngeal squamous cell carcinoma. Cancer Genomics Proteomics. 2020;17:431–439. doi: 10.21873/cgp.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han P, Dang Z, Shen Z, Dai H, Bai Y, Li B, Shao Y. Association of SNPs in the OBFC1 gene and laryngeal carcinoma in Chinese Han male population. Int J Clin Oncol. 2019;24:1042–1048. doi: 10.1007/s10147-019-01442-w. [DOI] [PubMed] [Google Scholar]
- 61.Lei H, Feng D, Zhou F, Xu H, Tang T, Yu H, Xie C, Zhou Y. Expression of human protection of telomere 1 correlates with telomere length and radiosensitivity in the human laryngeal cancer Hep-2 cell line. Oncol Lett. 2015;10:1149–1154. doi: 10.3892/ol.2015.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen W, Xiao BK, Liu JP, Chen SM, Tao ZZ. Alternative lengthening of telomeres in hTERT-inhibited laryngeal cancer cells. Cancer Sci. 2010;101:1769–1776. doi: 10.1111/j.1349-7006.2010.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broberg K, Björk J, Paulsson K, Höglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26:1263–1271. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 64.Chen M, Xu Y, Xu J, Chancoco H, Gu J. Leukocyte telomere length and bladder cancer risk: A large case-control study and mendelian randomization analysis. Cancer Epidemiol Biomarkers Prev. 2021;30:203–209. doi: 10.1158/1055-9965.EPI-20-0351. [DOI] [PubMed] [Google Scholar]
- 65.Pavanello S, Carta A, Mastrangelo G, Campisi M, Arici C, Porru S. Relationship between telomere length, genetic traits and Environmental/Occupational exposures in bladder cancer risk by structural equation modelling. Int J Environ Res Public Health. 2017;15:5. doi: 10.3390/ijerph15010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 67.Lin J, Blalock JA, Chen M, Ye Y, Gu J, Cohen L, Cinciripini PM, Wu X. Depressive symptoms and short telomere length are associated with increased mortality in bladder cancer patients. Cancer Epidemiol Biomarkers Prev. 2015;24:336–343. doi: 10.1158/1055-9965.EPI-14-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu C, Hequn C, Longfei L, Long W, Zhi C, Feng Z, Jinbo C, Chao L, Xiongbing Z. GSTM1 and GSTT1 polymorphisms are associated with increased bladder cancer risk: Evidence from updated meta-analysis. Oncotarget. 2017;8:3246–3258. doi: 10.18632/oncotarget.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosen I, Rachakonda PS, Heidenreich B, de Verdier PJ, Ryk C, Steineck G, Hemminki K, Kumar R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. Int J Cancer. 2015;137:1621–1629. doi: 10.1002/ijc.29526. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez-Gomez J, Escaf Barmadah S, Gosalbez D, Rodriguez-Faba O, Jalon A, Gonzalez R, Garcia Miralles T, Calas A. Telomere length on bladder washing samples from patients with bladder cancer correlates with tumor characteristics flow cytometry method for quantitative fluorescence in situ hybridization (flow-FISH technique) Eur Urol. 2005;48:432–437. doi: 10.1016/j.eururo.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 71.Wang H, Wang Y, Kota KK, Kallakury B, Mikhail NN, Sayed D, Mokhtar A, Maximous D, Yassin EH, Gouda I, et al. Strong association between long and heterogeneous telomere length in blood lymphocytes and bladder cancer risk in Egyptian. Carcinogenesis. 2015;36:1284–1290. doi: 10.1093/carcin/bgv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu J, Chen M, Shete S, Amos CI, Kamat A, Ye Y, Lin J, Dinney CP, Wu X. A genome-wide association study identifies a locus on chromosome 14q21 as a predictor of leukocyte telomere length and as a marker of susceptibility for bladder cancer. Cancer Prev Res (Phila) 2011;4:514–521. doi: 10.1158/1940-6207.CAPR-11-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holzmann K, Blin N, Welter C, Zang KD, Seitz G, Henn W. Telomeric associations and loss of telomeric DNA repeats in renal tumors. Genes Chromosomes Cancer. 1993;6:178–181. doi: 10.1002/gcc.2870060308. [DOI] [PubMed] [Google Scholar]
- 74.Mehle C, Ljungberg B, Roos G. Telomere shortening in renal cell carcinoma. Cancer Res. 1994;54:236–241. [PubMed] [Google Scholar]
- 75.Dahse R, Fiedler W, Junker K, Schlichter A, Schubert J, Claussen U. Telomerase activity and telomere lengths: Alterations in renal cell carcinomas. Kidney Int. 1999;56:1289–1290. doi: 10.1046/j.1523-1755.1999.00688.x. [DOI] [PubMed] [Google Scholar]
- 76.Pal D, Sharma U, Khajuria R, Singh SK, Kakkar N, Prasad R. Augmented telomerase activity, reduced telomere length and the presence of alternative lengthening of telomere in renal cell carcinoma: Plausible predictive and diagnostic markers. Gene. 2015;562:145–151. doi: 10.1016/j.gene.2015.02.079. [DOI] [PubMed] [Google Scholar]
- 77.Callahan CL, Schwartz K, Ruterbusch JJ, Shuch B, Graubard BI, Lan Q, Cawthon R, Baccarelli AA, Chow WH, Rothman N, et al. Leukocyte telomere length and renal cell carcinoma survival in two studies. Br J Cancer. 2017;117:752–755. doi: 10.1038/bjc.2017.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hofmann JN, Lan Q, Cawthon R, Hosgood HD, III, Shuch B, Moore LE, Rothman N, Chow WH, Purdue MP. A prospective study of leukocyte telomere length and risk of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:997–1000. doi: 10.1158/1055-9965.EPI-13-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hofmann JN, Baccarelli A, Schwartz K, Davis FG, Ruterbusch JJ, Hoxha M, McCarthy BJ, Savage SA, Wacholder S, Rothman N, et al. Risk of renal cell carcinoma in relation to blood telomere length in a population-based case-control study. Br J Cancer. 2011;105:1772–1775. doi: 10.1038/bjc.2011.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Svenson U, Ljungberg B, Roos G. Telomere length in peripheral blood predicts survival in clear cell renal cell carcinoma. Cancer Res. 2009;69:2896–2901. doi: 10.1158/0008-5472.CAN-08-3513. [DOI] [PubMed] [Google Scholar]
- 81.Morais M, Dias F, Teixeira AL, Medeiros R. Telomere length in renal cell carcinoma: The Jekyll and Hyde biomarker of ageing of the kidney. Cancer Manag Res. 2020;12:1669–1679. doi: 10.2147/CMAR.S211225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Machiela MJ, Hofmann JN, Carreras-Torres R, Brown KM, Johansson M, Wang Z, Foll M, Li P, Rothman N, Savage SA, et al. Genetic variants related to longer telomere length are associated with increased risk of renal cell carcinoma. Eur Urol. 2017;72:747–754. doi: 10.1016/j.eururo.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Martino M, Taus C, Lucca I, Hofbauer SL, Haitel A, Shariat SF, Klatte T. Association of human telomerase reverse transcriptase gene polymorphisms, serum levels, and telomere length with renal cell carcinoma risk and pathology. Mol Carcinog. 2016;55:1458–1466. doi: 10.1002/mc.22388. [DOI] [PubMed] [Google Scholar]
- 84.Svenson U, Grönlund E, Söderström I, Sitaram RT, Ljungberg B, Roos G. Telomere length in relation to immunological parameters in patients with renal cell carcinoma. PLoS One. 2013;8:e55543. doi: 10.1371/journal.pone.0055543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Endén K, Tainio J, Hou M, Suominen A, Pakarinen M, Huang T, Söder O, Jalanko H, Jahnukainen K, Jahnukainen T. Telomere length regulators are activated in young men after pediatric kidney transplantation compared to healthy controls and survivors of childhood cancer-A cross-sectional study. Pediatr Transplant. 2019;23:e13550. doi: 10.1111/petr.13550. [DOI] [PubMed] [Google Scholar]
- 86.HaydeéCottliar AS, Noriega MF, Narbaitz M, Rodríguez A, Slavutsky IR. Association between telomere length and BCL2 gene rearrangements in low- and high-grade non-Hodgkin lymphomas. Cancer Genet Cytogenet. 2006;171:1–8. doi: 10.1016/j.cancergencyto.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 87.Widmann TA, Herrmann M, Taha N, König J, Pfreundschuh M. Short telomeres in aggressive non-Hodgkin's lymphoma as a risk factor in lymphomagenesis. Exp Hematol. 2007;35:939–946. doi: 10.1016/j.exphem.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Lan Q, Cawthon R, Shen M, Weinstein SJ, Virtamo J, Lim U, Hosgood HD, III, Albanes D, Rothman N. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:7429–7433. doi: 10.1158/1078-0432.CCR-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Machiela MJ, Lan Q, Slager SL, Vermeulen RC, Teras LR, Camp NJ, Cerhan JR, Spinelli JJ, Wang SS, Nieters A, et al. Genetically predicted longer telomere length is associated with increased risk of B-cell lymphoma subtypes. Hum Mol Genet. 2016;25:1663–1676. doi: 10.1093/hmg/ddw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee JJ, Nam CE, Cho SH, Park KS, Chung IJ, Kim HJ. Telomere length shortening in non-Hodgkin's lymphoma patients undergoing chemotherapy. Ann Hematol. 2003;82:492–495. doi: 10.1007/s00277-003-0691-4. [DOI] [PubMed] [Google Scholar]
- 91.Remes K, Norrback KF, Rosenquist R, Mehle C, Lindh J, Roos G. Telomere length and telomerase activity in malignant lymphomas at diagnosis and relapse. Br J Cancer. 2000;82:601–607. doi: 10.1054/bjoc.1999.0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adamson DJ, King DJ, Haites NE. Significant telomere shortening in childhood leukemia. Cancer Genet Cytogenet. 1992;61:204–206. doi: 10.1016/0165-4608(92)90088-P. [DOI] [PubMed] [Google Scholar]
- 93.Engelhardt M, Ozkaynak MF, Drullinsky P, Sandoval C, Tugal O, Jayabose S, Moore MA. Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia. 1998;12:13–24. doi: 10.1038/sj.leu.2400889. [DOI] [PubMed] [Google Scholar]
- 94.Borssén M, Cullman I, Norén-Nyström U, Sundström C, Porwit A, Forestier E, Roos G. hTERT promoter methylation and telomere length in childhood acute lymphoblastic leukemia: Associations with immunophenotype and cytogenetic subgroup. Exp Hematol. 2011;39:1144–1151. doi: 10.1016/j.exphem.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y, Fang M, Sun X, Sun J. Telomerase activity and telomere length in acute leukemia: Correlations with disease progression, subtypes and overall survival. Int J Lab Hematol. 2010;32:230–238. doi: 10.1111/j.1751-553X.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- 96.Eskandari E, Hashemi M, Naderi M, Bahari G, Safdari V, Taheri M. Leukocyte telomere length shortening, hTERT genetic polymorphisms and risk of childhood acute lymphoblastic leukemia. Asian Pac J Cancer Prev. 2018;19:1515–1521. doi: 10.22034/APJCP.2018.19.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sheng X, Zhang L, Luo D, Tong N, Wang M, Fang Y, Li J, Zhang Z. A common variant near TERC and telomere length are associated with susceptibility to childhood acute lymphoblastic leukemia in Chinese. Leuk Lymphoma. 2012;53:1688–1692. doi: 10.3109/10428194.2012.671482. [DOI] [PubMed] [Google Scholar]
- 98.Takauchi K, Tashiro S, Ohtaki M, Kamada N. Telomere reduction of specific chromosome translocation in acute myelocytic leukemia. Jpn J Cancer Res. 1994;85:127–130. doi: 10.1111/j.1349-7006.1994.tb02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sieglová Z, Zilovcová S, Cermák J, Ríhová H, Brezinová D, Dvoráková R, Marková M, Maaloufová J, Sajdová J, Brezinová J, et al. Dynamics of telomere erosion and its association with genome instability in myelodysplastic syndromes (MDS) and acute myelogenous leukemia arising from MDS: A marker of disease prognosis? Leuk Res. 2004;28:1013–1021. doi: 10.1016/j.leukres.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 100.Lansdorp PM. Maintenance of telomere length in AML. Blood Adv. 2017;1:2467–2472. doi: 10.1182/bloodadvances.2017012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y, Wang T, Dagnall C, Haagenson M, Spellman SR, Hicks B, Jones K, Lee SJ, Savage SA, Gadalla SM. Relative telomere length before hematopoietic cell transplantation and outcome after unrelated donor hematopoietic cell transplantation for acute leukemia. Biol Blood Marrow Transplant. 2017;23:1054–1058. doi: 10.1016/j.bbmt.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swiggers SJ, Kuijpers MA, de Cort MJ, Beverloo HB, Zijlmans JM. Critically short telomeres in acute myeloid leukemia with loss or gain of parts of chromosomes. Genes Chromosomes Cancer. 2006;45:247–256. doi: 10.1002/gcc.20286. [DOI] [PubMed] [Google Scholar]
- 103.Behrens YL, Thomay K, Hagedorn M, Ebersold J, Schmidt G, Lentes J, Davenport C, Schlegelberger B, Göhring G. Jumping translocations: Short telomeres or pathogenic TP53 variants as underlying mechanism in acute myeloid leukemia and myelodysplastic syndrome? Genes Chromosomes Cancer. 2019;58:139–148. doi: 10.1002/gcc.22665. [DOI] [PubMed] [Google Scholar]
- 104.Watts JM, Dumitriu B, Hilden P, Kishtagari A, Rapaport F, Chen C, Ahn J, Devlin SM, Stein EM, Rampal R, et al. Telomere length and associations with somatic mutations and clinical outcomes in acute myeloid leukemia. Leuk Res. 2016;49:62–65. doi: 10.1016/j.leukres.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li AM, Hyagu S, Maze D, Schreiber R, Sirrs S, Stockler-Ipsiroglu S, Sutherland H, Vercauteren S, Schultz KR. Prolonged granulocyte colony stimulating factor use in glycogen storage disease type 1b associated with acute myeloid leukemia and with shortened telomere length. Pediatr Hematol Oncol. 2018;35:45–51. doi: 10.1080/08880018.2018.1440675. [DOI] [PubMed] [Google Scholar]
- 106.Aalbers AM, Calado RT, Young NS, Zwaan CM, Wu C, Kajigaya S, Coenen EA, Baruchel A, Geleijns K, de Haas V, et al. Telomere length and telomerase complex mutations in pediatric acute myeloid leukemia. Leukemia. 2013;27:1786–1789. doi: 10.1038/leu.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song DY, Kim JA, Jeong D, Yun J, Kim SM, Lim K, Park SN, Im K, Choi S, Yoon SS, Lee DS. Telomere length and its correlation with gene mutations in chronic lymphocytic leukemia in a Korean population. PLoS One. 2019;14:e0220177. doi: 10.1371/journal.pone.0220177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jebaraj BMC, Tausch E, Landau DA, Bahlo J, Robrecht S, Taylor-Weiner AN, Bloehdorn J, Scheffold A, Mertens D, Böttcher S, et al. Short telomeres are associated with inferior outcome, genomic complexity, and clonal evolution in chronic lymphocytic leukemia. Leukemia. 2019;33:2183–2194. doi: 10.1038/s41375-019-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomay K, Fedder C, Hofmann W, Kreipe H, Stadler M, Titgemeyer J, Zander I, Schlegelberger B, Göhring G. Telomere shortening, TP53 mutations and deletions in chronic lymphocytic leukemia result in increased chromosomal instability and breakpoint clustering in heterochromatic regions. Ann Hematol. 2017;96:1493–1500. doi: 10.1007/s00277-017-3055-1. [DOI] [PubMed] [Google Scholar]
- 110.Steinbrecher D, Jebaraj BMC, Schneider C, Edelmann J, Cymbalista F, Leblond V, Delmer A, Ibach S, Tausch E, Scheffold A, et al. Telomere length in poor-risk chronic lymphocytic leukemia: Associations with disease characteristics and outcome. Leuk Lymphoma. 2018;59:1614–1623. doi: 10.1080/10428194.2017.1390236. [DOI] [PubMed] [Google Scholar]
- 111.Strefford JC, Kadalayil L, Forster J, Rose-Zerilli MJ, Parker A, Lin TT, Heppel N, Norris K, Gardiner A, Davies Z, et al. Telomere length predicts progression and overall survival in chronic lymphocytic leukemia: Data from the UK LRF CLL4 trial. Leukemia. 2015;29:2411–2414. doi: 10.1038/leu.2015.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dos Santos P, Panero J, Palau Nagore V, Stanganelli C, Bezares RF, Slavutsky I. Telomere shortening associated with increased genomic complexity in chronic lymphocytic leukemia. Tumour Biol. 2015;36:8317–8324. doi: 10.1007/s13277-015-3556-2. [DOI] [PubMed] [Google Scholar]
- 113.Palma M, Parker A, Hojjat-Farsangi M, Forster J, Kokhaei P, Hansson L, Osterborg A, Mellstedt H. Telomere length and expression of human telomerase reverse transcriptase splice variants in chronic lymphocytic leukemia. Exp Hematol. 2013;41:615–626. doi: 10.1016/j.exphem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 114.Sellmann L, de Beer D, Bartels M, Opalka B, Nückel H, Dührsen U, Dürig J, Seifert M, Siemer D, Küppers R, et al. Telomeres and prognosis in patients with chronic lymphocytic leukaemia. Int J Hematol. 2011;93:74–82. doi: 10.1007/s12185-010-0750-2. [DOI] [PubMed] [Google Scholar]
- 115.Roos G, Kröber A, Grabowski P, Kienle D, Bühler A, Döhner H, Rosenquist R, Stilgenbauer S. Short telomeres are associated with genetic complexity, high-risk genomic aberrations, and short survival in chronic lymphocytic leukemia. Blood. 2008;111:2246–2252. doi: 10.1182/blood-2007-05-092759. [DOI] [PubMed] [Google Scholar]
- 116.Lin TT, Letsolo BT, Jones RE, Rowson J, Pratt G, Hewamana S, Fegan C, Pepper C, Baird DM. Telomere dysfunction and fusion during the progression of chronic lymphocytic leukemia: Evidence for a telomere crisis. Blood. 2010;116:1899–1907. doi: 10.1182/blood-2010-02-272104. [DOI] [PubMed] [Google Scholar]
- 117.Ojha J, Codd V, Nelson CP, Samani NJ, Smirnov IV, Madsen NR, Hansen HM, de Smith AJ, Bracci PM, Wiencke JK, et al. ENGAGE Consortium Telomere Group. Genetic variation associated with longer telomere length increases risk of chronic lymphocytic leukemia. Cancer Epidemiol Biomarkers Prev. 2016;25:1043–1049. doi: 10.1158/1055-9965.EPI-15-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wysoczanska B, Dratwa M, Gebura K, Mizgala J, Mazur G, Wrobel T, Bogunia-Kubik K. Variability within the human TERT gene, telomere length and predisposition to chronic lymphocytic leukemia. Onco Targets Ther. 2019;12:4309–4320. doi: 10.2147/OTT.S198313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Furtado FM, Scheucher PS, Santana BA, Scatena NF, Calado RT, Rego EM, Matos DM, Falcão RP. Telomere length analysis in monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia Binet A. Braz J Med Biol Res. 2017;50:e6019. doi: 10.1590/1414-431x20176019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang L, Kost SEF, Beiggi S, Zhang Y, Schmidt R, Nugent Z, Marshall A, Banerji V, Gibson SB, Johnston JB. Buccal cell telomere length is not a useful marker for comorbidities in chronic lymphocytic leukemia. Leuk Res. 2019;86:106220. doi: 10.1016/j.leukres.2019.106220. [DOI] [PubMed] [Google Scholar]
- 121.Kaifie A, Schikowsky C, Vasko T, Kraus T, Brümmendorf TH, Ziegler P. Additional benefits of telomere length (TL) measurements in chronic lymphocytic leukemia. Leuk Lymphoma. 2019;60:541–543. doi: 10.1080/10428194.2018.1482544. [DOI] [PubMed] [Google Scholar]
- 122.Iwama H, Ohyashiki K, Ohyashiki JH, Hayashi S, Kawakubo K, Shay JW, Toyama K. The relationship between telomere length and therapy-associated cytogenetic responses in patients with chronic myeloid leukemia. Cancer. 1997;79:1552–1560. doi: 10.1002/(SICI)1097-0142(19970415)79:8<1552::AID-CNCR17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 123.Brümmendorf TH, Holyoake TL, Rufer N, Barnett MJ, Schulzer M, Eaves CJ, Eaves AC, Lansdorp PM. Prognostic implications of differences in telomere length between normal and malignant cells from patients with chronic myeloid leukemia measured by flow cytometry. Blood. 2000;95:1883–1890. doi: 10.1182/blood.V95.6.1883. [DOI] [PubMed] [Google Scholar]
- 124.Bouillon AS, Ventura Ferreira MS, Awad SA, Richter J, Hochhaus A, Kunzmann V, Dengler J, Janssen J, Ossenkoppele G, Westerweel PE, et al. Telomere shortening correlates with leukemic stem cell burden at diagnosis of chronic myeloid leukemia. Blood Adv. 2018;2:1572–1579. doi: 10.1182/bloodadvances.2018017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boultwood J, Fidler C, Shepherd P, Watkins F, Snowball J, Haynes S, Kusec R, Gaiger A, Littlewood TJ, Peniket AJ, Wainscoat JS. Telomere length shortening is associated with disease evolution in chronic myelogenous leukemia. Am J Hematol. 1999;61:5–9. doi: 10.1002/(SICI)1096-8652(199905)61:1<5::AID-AJH2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 126.Drummond M, Lennard A, Brûmmendorf T, Holyoake T. Telomere shortening correlates with prognostic score at diagnosis and proceeds rapidly during progression of chronic myeloid leukemia. Leuk Lymphoma. 2004;45:1775–1781. doi: 10.1080/10428190410001693542. [DOI] [PubMed] [Google Scholar]
- 127.Keller G, Brassat U, Braig M, Heim D, Wege H, Brümmendorf TH. Telomeres and telomerase in chronic myeloid leukaemia: Impact for pathogenesis, disease progression and targeted therapy. Hematol Oncol. 2009;27:123–129. doi: 10.1002/hon.901. [DOI] [PubMed] [Google Scholar]
- 128.Boultwood J, Peniket A, Watkins F, Shepherd P, McGale P, Richards S, Fidler C, Littlewood TJ, Wainscoat JS. Telomere length shortening in chronic myelogenous leukemia is associated with reduced time to accelerated phase. Blood. 2000;96:358–361. doi: 10.1182/blood.V96.1.358. [DOI] [PubMed] [Google Scholar]
- 129.Caocci G, Greco M, Delogu G, Secchi C, Martino B, Labate C, Abruzzese E, Trawinska MM, Galimberti S, Orru F, et al. Telomere length shortening is associated with treatment-free remission in chronic myeloid leukemia patients. J Hematol Oncol. 2016;9:63. doi: 10.1186/s13045-016-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Samassekou O, Ntwari A, Hébert J, Yan J. Individual telomere lengths in chronic myeloid leukemia. Neoplasia. 2009;11:1146–1154. doi: 10.1593/neo.09836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nikolouzakis TK, Falzone L, Lasithiotakis K, Krüger-Krasagakis S, Kalogeraki A, Sifaki M, Spandidos DA, Chrysos E, Tsatsakis A, Tsiaoussis J. Current and future trends in molecular biomarkers for diagnostic, prognostic, and predictive purposes in non-melanoma skin cancer. J Clin Med. 2020;9:2868. doi: 10.3390/jcm9092868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Llorca-Cardeñosa MJ, Peña-Chilet M, Mayor M, Gomez-Fernandez C, Casado B, Martin-Gonzalez M, Carretero G, Lluch A, Martinez-Cadenas C, Ibarrola-Villava M, Ribas G. Long telomere length and a TERT-CLPTM1 locus polymorphism association with melanoma risk. Eur J Cancer. 2014;50:3168–3177. doi: 10.1016/j.ejca.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 133.Anic GM, Sondak VK, Messina JL, Fenske NA, Zager JS, Cherpelis BS, Lee JH, Fulp WJ, Epling-Burnette PK, Park JY, Rollison DE. Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer Epidemiol. 2013;37:434–439. doi: 10.1016/j.canep.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bodelon C, Pfeiffer RM, Bollati V, Debbache J, Calista D, Ghiorzo P, Fargnoli MC, Bianchi-Scarra G, Peris K, Hoxha M, et al. On the interplay of telomeres, nevi and the risk of melanoma. PLoS One. 2012;7:e52466. doi: 10.1371/journal.pone.0052466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han J, Qureshi AA, Prescott J, Guo Q, Ye L, Hunter DJ, De Vivo I. A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. 2009;129:415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nan H, Du M, De Vivo I, Manson JE, Liu S, McTiernan A, Curb JD, Lessin LS, Bonner MR, Guo Q, et al. Shorter telomeres associate with a reduced risk of melanoma development. Cancer Res. 2011;71:6758–6763. doi: 10.1158/0008-5472.CAN-11-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burke LS, Hyland PL, Pfeiffer RM, Prescott J, Wheeler W, Mirabello L, Savage SA, Burdette L, Yeager M, Chanock S, et al. Telomere length and the risk of cutaneous malignant melanoma in melanoma-prone families with and without CDKN2A mutations. PLoS One. 2013;8:71121. doi: 10.1371/journal.pone.0071121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Caini S, Raimondi S, Johansson H, De Giorgi V, Zanna I, Palli D, Gandini S. Telomere length and the risk of cutaneous melanoma and non-melanoma skin cancer: A review of the literature and meta-analysis. J Dermatol Sci. 2015;80:168–174. doi: 10.1016/j.jdermsci.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 139.Rachakonda S, Srinivas N, Mahmoudpour SH, Garcia-Casado Z, Requena C, Traves V, Soriano V, Cardelli M, Pjanova D, Molven A, et al. Telomere length and survival in primary cutaneous melanoma patients. Sci Rep. 2018;8:10947. doi: 10.1038/s41598-018-29322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Viceconte N, Dheur MS, Majerova E, Pierreux CE, Baurain JF, van Baren N, Decottignies A. Highly aggressive metastatic melanoma cells unable to maintain telomere length. Cell Rep. 2017;19:2529–2543. doi: 10.1016/j.celrep.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 141.Menin C, Bojnik E, Del Bianco P, Elefanti L, Gianesin K, Keppel S, Stagni C, Mocellin S, Vecchiato A, De Rossi A. Differences in telomere length between sporadic and familial cutaneous melanoma. Br J Dermatol. 2016;175:937–943. doi: 10.1111/bjd.14652. [DOI] [PubMed] [Google Scholar]
- 142.Kammori M, Takubo K, Nakamura K, Furugouri E, Endo H, Kanauchi H, Mimura Y, Kaminishi M. Telomerase activity and telomere length in benign and malignant human thyroid tissues. Cancer Lett. 2000;159:175–181. doi: 10.1016/S0304-3835(00)00547-4. [DOI] [PubMed] [Google Scholar]
- 143.Matthews P, Jones CJ, Skinner J, Haughton M, de Micco C, Wynford-Thomas D. Telomerase activity and telomere length in thyroid neoplasia: Biological and clinical implications. J Pathol. 2001;194:183–193. doi: 10.1002/path.848. [DOI] [PubMed] [Google Scholar]
- 144.Dong C, Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer. 2001;92:144–150. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1147>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 145.Jendrzejewski J, Tomsic J, Lozanski G, Labanowska J, He H, Liyanarachchi S, Nagy R, Ringel MD, Kloos RT, Heerema NA, de la Chapelle A. Telomere length and telomerase reverse transcriptase gene copy number in patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:1876–1880. doi: 10.1210/jc.2011-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Capezzone M, Cantara S, Marchisotta S, Filetti S, De Santi MM, Rossi B, Ronga G, Durante C, Pacini F. Short telomeres, telomerase reverse transcriptase gene amplification, and increased telomerase activity in the blood of familial papillary thyroid cancer patients. J Clin Endocrinol Metab. 2008;93:3950–3957. doi: 10.1210/jc.2008-0372. [DOI] [PubMed] [Google Scholar]
- 147.Capezzone M, Cantara S, Marchisotta S, Busonero G, Formichi C, Benigni M, Capuano S, Toti P, Pazaitou-Panayiotou K, Caruso G, et al. Telomere length in neoplastic and nonneoplastic tissues of patients with familial and sporadic papillary thyroid cancer. J Clin Endocrinol Metab. 2011;96:1852–1856. doi: 10.1210/jc.2011-1003. [DOI] [PubMed] [Google Scholar]
- 148.He M, Bian B, Gesuwan K, Gulati N, Zhang L, Nilubol N, Kebebew E. Telomere length is shorter in affected members of families with familial nonmedullary thyroid cancer. Thyroid. 2013;23:301–307. doi: 10.1089/thy.2012.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gramatges MM, Morton LM, Yasui Y, Arnold MA, Neglia JP, Leisenring WM, Machiela MJ, Dagnall CL, Chanock SJ, Armstrong GT, et al. Telomere length-associated genetic variants and the risk of thyroid cancer in survivors of childhood cancer: A report from the childhood cancer survivor study (CCSS) Cancer Epidemiol Biomarkers Prev. 2019;28:417–419. doi: 10.1158/1055-9965.EPI-18-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li J, An C, Zheng H, Lei T, Zhang N, Zheng Y, Yang M. Leukocyte telomere length and risk of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2019;104:2712–2718. doi: 10.1210/jc.2018-02471. [DOI] [PubMed] [Google Scholar]
- 151.Graham MK, Meeker A. Telomeres and telomerase in prostate cancer development and therapy. Nat Rev Urol. 2017;14:607–619. doi: 10.1038/nrurol.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu J, Chang WS, Tsai CW, Bau DT, Xu Y, Davis JW, Thompson TC, Logothetis CJ, Gu J. Leukocyte telomere length is associated with aggressive prostate cancer in localized prostate cancer patients. EBioMedicine. 2020;52:102616. doi: 10.1016/j.ebiom.2019.102616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsai CW, Chang WS, Xu J, Xu Y, Huang M, Pettaway C, Bau DT, Gu J. Leukocyte telomere length is associated with aggressive prostate cancer in localized African American prostate cancer patients. Carcinogenesis. 2020;41:1213–1218. doi: 10.1093/carcin/bgaa070. [DOI] [PubMed] [Google Scholar]
- 154.Hu R, Hua XG, Jiang QC. Associations of telomere length in risk and recurrence of prostate cancer: A meta-analysis. Andrologia. 2019;51:e13304. doi: 10.1111/and.13304. [DOI] [PubMed] [Google Scholar]
- 155.Luxton JJ, McKenna MJ, Lewis AM, Taylor LE, Jhavar SG, Swanson GP, Bailey SM. Telomere length dynamics and chromosomal instability for predicting individual radiosensitivity and risk via machine learning. J Pers Med. 2021;11:188. doi: 10.3390/jpm11030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gu CY, Jin SM, Qin XJ, Zhu Y, Bo D, Lin GW, Shi GH, Ye DW. Genetic variants in RTEL1 influencing telomere length are associated with prostate cancer risk. J Cancer. 2019;10:6170–6174. doi: 10.7150/jca.35917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hurwitz LM, Heaphy CM, Joshu CE, Isaacs WB, Konishi Y, De Marzo AM, Isaacs SD, Wiley KE, Platz EA, Meeker AK. Telomere length as a risk factor for hereditary prostate cancer. Prostate. 2014;74:359–364. doi: 10.1002/pros.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Julin B, Shui I, Heaphy CM, Joshu CE, Meeker AK, Giovannucci E, De Vivo I, Platz EA. Circulating leukocyte telomere length and risk of overall and aggressive prostate cancer. Br J Cancer. 2015;112:769–776. doi: 10.1038/bjc.2014.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Renner W, Krenn-Pilko S, Gruber HJ, Herrmann M, Langsenlehner T. Relative telomere length and prostate cancer mortality. Prostate Cancer Prostatic Dis. 2018;21:579–583. doi: 10.1038/s41391-018-0068-3. [DOI] [PubMed] [Google Scholar]
- 160.Svenson U, Roos G, Wikström P. Long leukocyte telomere length in prostate cancer patients at diagnosis is associated with poor metastasis-free and cancer-specific survival. Tumour Biol. 2017;39:1010428317692236. doi: 10.1177/1010428317692236. [DOI] [PubMed] [Google Scholar]
- 161.Wulaningsih W, Astuti Y, Matsuguchi T, Anggrandariyanny P, Watkins J, PILAR Research Network Circulating Prostate-specific antigen and telomere length in a nationally representative sample of men without history of prostate cancer. Prostate. 2017;77:22–32. doi: 10.1002/pros.23245. [DOI] [PubMed] [Google Scholar]
- 162.Heaphy CM, Haffner MC, Graham MK, Lim D, Davis C, Corey E, Epstein JI, Eisenberger MA, Wang H, De Marzo AM, et al. Telomere lengths differ significantly between small-cell neuroendocrine prostate carcinoma and adenocarcinoma of the prostate. Hum Pathol. 2020;101:70–79. doi: 10.1016/j.humpath.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mirabello L, Huang WY, Wong JY, Chatterjee N, Reding D, Crawford ED, De Vivo I, Hayes RB, Savage SA. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8:405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Joshu CE, Peskoe SB, Heaphy CM, Kenfield SA, Mucci LA, Giovannucci EL, Stampfer MJ, Yoon G, Lee TK, Hicks JL, et al. Current or recent smoking is associated with more variable telomere length in prostate stromal cells and prostate cancer cells. Prostate. 2018;78:233–238. doi: 10.1002/pros.23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Heaphy CM, Joshu CE, Barber JR, Davis C, Zarinshenas R, De Marzo AM, Lotan TL, Sfanos KS, Meeker AK, Platz EA, et al. Racial difference in prostate cancer cell telomere lengths in men with higher grade prostate cancer: A clue to the racial disparity in prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev. 2020;29:676–680. doi: 10.1158/1055-9965.EPI-19-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Luu HN, Long J, Wen W, Zheng Y, Cai Q, Gao YT, Zheng W, Shu XO. Association between genetic risk score for telomere length and risk of breast cancer. Cancer Causes Control. 2016;27:1219–1228. doi: 10.1007/s10552-016-0800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kroupa M, Rachakonda S, Vymetalkova V, Tomasova K, Liska V, Vodenkova S, Cumova A, Rossnerova A, Vodickova L, Hemminki K, et al. Telomere length in peripheral blood lymphocytes related to genetic variation in telomerase, prognosis and clinicopathological features in breast cancer patients. Mutagenesis. 2020;35:491–497. doi: 10.1093/mutage/geaa030. [DOI] [PubMed] [Google Scholar]
- 168.Samavat H, Xun X, Jin A, Wang R, Koh WP, Yuan JM. Association between prediagnostic leukocyte telomere length and breast cancer risk: The Singapore Chinese Health Study. Breast Cancer Res. 2019;21:50. doi: 10.1186/s13058-019-1133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pellatt AJ, Wolff RK, Torres-Mejia G, John EM, Herrick JS, Lundgreen A, Baumgartner KB, Giuliano AR, Hines LM, Fejerman L, et al. Telomere length, telomere-related genes, and breast cancer risk: The breast cancer health disparities study. Genes Chromosomes Cancer. 2013;52:595–609. doi: 10.1002/gcc.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang Z, Zhang Z, Guo Y, Shui H, Liu G, Jin T, Wang H. Shorter telomere length is associated with increased breast cancer risk in a Chinese Han population: A Case-Control analysis. J Breast Cancer. 2018;21:391–398. doi: 10.4048/jbc.2018.21.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shen J, Terry MB, Gurvich I, Liao Y, Senie RT, Santella RM. Short telomere length and breast cancer risk: A study in sister sets. Cancer Res. 2007;67:5538–5544. doi: 10.1158/0008-5472.CAN-06-3490. [DOI] [PubMed] [Google Scholar]
- 172.Pavanello S, Varesco L, Gismondi V, Bruzzi P, Bolognesi C. Leucocytes telomere length and breast cancer risk/susceptibility: A case-control study. PLoS One. 2018;13:e0197522. doi: 10.1371/journal.pone.0197522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duggan C, Risques R, Alfano C, Prunkard D, Imayama I, Holte S, Baumgartner K, Baumgartner R, Bernstein L, Ballard-Barbash R, et al. Change in peripheral blood leukocyte telomere length and mortality in breast cancer survivors. J Natl Cancer Inst. 2014;106:dju035. doi: 10.1093/jnci/dju035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ceja-Rangel HA, Sánchez-Suárez P, Castellanos-Juárez E, Peñaroja-Flores R, Arenas-Aranda DJ, Gariglio P, Benítez-Bribiesca L. Shorter telomeres and high telomerase activity correlate with a highly aggressive phenotype in breast cancer cell lines. Tumour Biol. 2016;37:11917–11926. doi: 10.1007/s13277-016-5045-7. [DOI] [PubMed] [Google Scholar]
- 175.Kammori M, Sugishita Y, Okamoto T, Kobayashi M, Yamazaki K, Yamada E, Yamada T. Telomere shortening in breast cancer correlates with the pathological features of tumor progression. Oncol Rep. 2015;34:627–632. doi: 10.3892/or.2015.4063. [DOI] [PubMed] [Google Scholar]
- 176.Barczak W, Rozwadowska N, Romaniuk A, Lipińska N, Lisiak N, Grodecka-Gazdecka S, Książek K, Rubiś B. Telomere length assessment in leukocytes presents potential diagnostic value in patients with breast cancer. Oncol Lett. 2016;11:2305–2309. doi: 10.3892/ol.2016.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eyüboğlu İP, Yenmiş G, Bingöl EN, Yüksel Ş, Tokat F, Özbek P, Güllü Amuran G, Yakıcıer C, Akkiprik M. Next-generation sequencing identifies BRCA1 and/or BRCA2 mutations in Women at high hereditary risk for breast cancer with shorter telomere length. OMICS. 2020;24:5–15. doi: 10.1089/omi.2019.0103. [DOI] [PubMed] [Google Scholar]
- 178.Badie S, Escandell JM, Bouwman P, Carlos AR, Thanasoula M, Gallardo MM, Suram A, Jaco I, Benitez J, Herbig U, et al. BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat Struct Mol Biol. 2010;17:1461–1469. doi: 10.1038/nsmb.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thorvaldsdottir B, Aradottir M, Stefansson OA, Bodvarsdottir SK, Eyfjörd JE. Telomere length is predictive of breast cancer risk in BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2017;26:1248–1254. doi: 10.1158/1055-9965.EPI-16-0946. [DOI] [PubMed] [Google Scholar]
- 180.Ennour-Idrissi K, Maunsell E, Diorio C. Telomere length and breast cancer prognosis: A systematic review. Cancer Epidemiol Biomarkers Prev. 2017;26:3–10. doi: 10.1158/1055-9965.EPI-16-0343. [DOI] [PubMed] [Google Scholar]
- 181.Garland SN, Johnson B, Palmer C, Speck RM, Donelson M, Xie SX, DeMichele A, Mao JJ. Physical activity and telomere length in early stage breast cancer survivors. Breast Cancer Res. 2014;16:413. doi: 10.1186/s13058-014-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alhareeri AA, Archer KJ, Fu H, Lyon DE, Elswick RK, Jr, Kelly DL, Starkweather AR, Elmore LW, Bokhari YA, Jackson-Cook CK. Telomere lengths in women treated for breast cancer show associations with chemotherapy, pain symptoms, and cognitive domain measures: A longitudinal study. Breast Cancer Res. 2020;22:137. doi: 10.1186/s13058-020-01368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mirabello L, Garcia-Closas M, Cawthon R, Lissowska J, Brinton LA, Pepłońska B, Sherman ME, Savage SA. Leukocyte telomere length in a population-based case-control study of ovarian cancer: A pilot study. Cancer Causes Control. 2010;21:77–82. doi: 10.1007/s10552-009-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kuhn E, Meeker AK, Visvanathan K, Gross AL, Wang TL, Kurman RJ, Shih IeM. Telomere length in different histologic types of ovarian carcinoma with emphasis on clear cell carcinoma. Mod Pathol. 2011;24:1139–1145. doi: 10.1038/modpathol.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vajpeyi R. WHO Classification of Tumours: Pathology and genetics of tumours of the breast and female genital organs. J Clin Pathol. 2005;58:671–672. [Google Scholar]
- 186.Martinez-Delgado B, Anowsky K, Inglada-Perez L, de la Hoya M, Caldes T, Vega A, Blanco A, Martin T, Gonzalez-Sarmiento R, Blasco M, et al. Shorter telomere length is associated with increased ovarian cancer risk in both familial and sporadic cases. J Med Genet. 2012;49:341–344. doi: 10.1136/jmedgenet-2012-100807. [DOI] [PubMed] [Google Scholar]
- 187.Falandry C, Horard B, Bruyas A, Legouffe E, Cretin J, Meunier J, Alexandre J, Delecroix V, Fabbro M, Certain MN, et al. Telomere length is a prognostic biomarker in elderly advanced ovarian cancer patients: A multicenter GINECO study. Aging (Albany NY) 2015;7:1066–1076. doi: 10.18632/aging.100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang M, Prescott J, Poole EM, Rice MS, Kubzansky LD, Idahl A, Lundin E, De Vivo I, Tworoger SS. Prediagnosis leukocyte telomere length and risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:339–345. doi: 10.1158/1055-9965.EPI-16-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, Edwards SL, Pickett HA, Shen HC, Smart CE, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45:371–384. 384e1–2. doi: 10.1038/ng.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Terry KL, Tworoger SS, Vitonis AF, Wong J, Titus-Ernstoff L, De Vivo I, Cramer DW. Telomere length and genetic variation in telomere maintenance genes in relation to ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:504–512. doi: 10.1158/1055-9965.EPI-11-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kotsopoulos J, Prescott J, De Vivo I, Fan I, Mclaughlin J, Rosen B, Risch H, Sun P, Narod SA. Telomere length and mortality following a diagnosis of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2603–2606. doi: 10.1158/1055-9965.EPI-14-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Antoun S, Atallah D, Tahtouh R, Assaf MD, Moubarak M, Ayoub EN, Chahine G, Hilal G. Glucose restriction combined with chemotherapy decreases telomere length and cancer antigen-125 secretion in ovarian carcinoma. Oncol Lett. 2020;19:1338–1350. doi: 10.3892/ol.2019.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Victorelli S, Passos JF. Telomeres and cell senescence-size matters not. EBioMedicine. 2017;21:14–20. doi: 10.1016/j.ebiom.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim B, Ryu KJ, Lee S, Kim T. Changes in telomere length and senescence markers during human ovarian tissue cryopreservation. Sci Rep. 2021;11:2238. doi: 10.1038/s41598-021-81973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang DK, Ngan HY, Cheng RY, Cheung AN, Liu SS, Tsao SW. Clinical significance of telomerase activation and telomeric restriction fragment (TRF) in cervical cancer. Eur J Cancer. 1999;35:154–160. doi: 10.1016/S0959-8049(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 196.Maida Y, Kyo S, Forsyth NR, Takakura M, Sakaguchi J, Mizumoto Y, Hashimoto M, Nakamura M, Nakao S, Inoue M. Distinct telomere length regulation in premalignant cervical and endometrial lesions: Implications for the roles of telomeres in uterine carcinogenesis. J Pathol. 2006;210:214–223. doi: 10.1002/path.2038. [DOI] [PubMed] [Google Scholar]
- 197.Chen X, Wei S, Ma H, Jin G, Hu Z, Suping H, Li D, Hang D, Wu X, Li N. Telomere length in cervical exfoliated cells, interaction with HPV genotype, and cervical cancer occurrence among high-risk HPV-positive women. Cancer Me. 2019;8:4845–4851. doi: 10.1002/cam4.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 199.Deng Z, Wang Z, Xiang C, Molczan A, Baubet V, Conejo-Garcia J, Xu X, Lieberman PM, Dahmane N. Formation of telomeric repeat-containing RNA (TERRA) foci in highly proliferating mouse cerebellar neuronal progenitors and medulloblastoma. J Cell Sci. 2012;125:4383–4394. doi: 10.1242/jcs.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Oh BK, Keo P, Bae J, Ko JH, Choi JS. Variable TERRA abundance and stability in cervical cancer cells. Int J Mol Med. 2017;39:1597–1604. doi: 10.3892/ijmm.2017.2956. [DOI] [PubMed] [Google Scholar]
- 201.Déjardin J, Kingston RE. Purification of proteins associated with specific genomic Loci. Cell. 2009;136:175–186. doi: 10.1016/j.cell.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kappei D, Butter F, Benda C, Scheibe M, Draškovič I, Stevense M, Novo CL, Basquin C, Araki M, Krastev DB, et al. HOT1 is a mammalian direct telomere repeat-binding protein contributing to telomerase recruitment. EMBO J. 2013;32:1681–1701. doi: 10.1038/emboj.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou S, Xiao Y, Zhuang Y, Liu Y, Zhao H, Yang H, Xie C, Zhou F, Zhou Y. Knockdown of homeobox containing 1 increases the radiosensitivity of cervical cancer cells through telomere shortening. Oncol Rep. 2017;38:515–521. doi: 10.3892/or.2017.5707. [DOI] [PubMed] [Google Scholar]
- 204.Du J, Xue W, Ji Y, Zhu X, Gu Y, Zhu M, Wang C, Gao Y, Dai J, Ma H, et al. U-shaped association between telomere length and esophageal squamous cell carcinoma risk: A case-control study in Chinese population. Front Med. 2015;9:478–486. doi: 10.1007/s11684-015-0420-0. [DOI] [PubMed] [Google Scholar]
- 205.Xing J, Ajani JA, Chen M, Izzo J, Lin J, Chen Z, Gu J, Wu X. Constitutive short telomere length of chromosome 17p and 12q but not 11q and 2p is associated with an increased risk for esophageal cancer. Cancer Prev Res (Phila) 2009;2:459–465. doi: 10.1158/1940-6207.CAPR-08-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zheng YL, Hu N, Sun Q, Wang C, Taylor PR. Telomere attrition in cancer cells and telomere length in tumor stroma cells predict chromosome instability in esophagealsquamous cell carcinoma: A genome-wide analysis. Cancer Res. 2009;69:1604–1614. doi: 10.1158/0008-5472.CAN-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lin SW, Abnet CC, Freedman ND, Murphy G, Risques R, Prunkard D, Rabinovitch P, Pan QJ, Roth MJ, Wang GQ, et al. Measuring telomere length for the early detection of precursor lesions of esophageal squamous cell carcinoma. BMC Cancer. 2013;13:578. doi: 10.1186/1471-2407-13-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li Z, Song Y, Xu Y, Shen Y, Zhang N, Yang M, Yu D. Identification of Leukocyte telomere length-related genetic variants contributing to predisposition of Esophageal Squamous Cell Carcinoma. J Cancer. 2020;11:5025–5031. doi: 10.7150/jca.45165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lu Y, Yan C, Du J, Ji Y, Gao Y, Zhu X, Yu F, Huang T, Dai J, Ma H, et al. Genetic variants affecting telomere length are associated with the prognosis of esophageal squamous cell carcinoma in a Chinese population. Mol Carcinog. 2017;56:1021–1029. doi: 10.1002/mc.22567. [DOI] [PubMed] [Google Scholar]
- 210.Shi J, Sun F, Peng L, Li B, Liu L, Zhou C, Han J, Zhang L, Zhou L, Zhang X, et al. Leukocyte telomere length-related genetic variants in 1p34.2 and 14q21 loci contribute to the risk of esophageal squamous cell carcinoma. Int J Cancer. 2013;132:2799–2807. doi: 10.1002/ijc.27959. [DOI] [PubMed] [Google Scholar]
- 211.Hao XD, Yang Y, Song X, Zhao XK, Wang LD, He JD, Kong QP, Tang NL, Zhang YP. Correlation of telomere length shortening with TP53 somatic mutations, polymorphisms and allelic loss in breast tumors and esophageal cancer. Oncol Rep. 2013;29:226–236. doi: 10.3892/or.2012.2098. [DOI] [PubMed] [Google Scholar]
- 212.Yu Q, Yang J, Liu B, Li W, Hu G, Qiu H, Huang L, Xiong H, Yuan X. Combined effects of leukocyte telomere length, p53 polymorphism and human papillomavirus infection on esophageal squamous cell carcinoma in a Han Chinese population. Cancer Epidemiol. 2014;38:569–575. doi: 10.1016/j.canep.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 213.Wennerström EC, Risques RA, Prunkard D, Giffen C, Corley DA, Murray LJ, Whiteman DC, Wu AH, Bernstein L, Ye W, et al. Leukocyte telomere length in relation to the risk of Barrett's esophagus and esophageal adenocarcinoma. Cancer Med. 2016;5:2657–2665. doi: 10.1002/cam4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pan W, Du J, Shi M, Jin G, Yang M. Short leukocyte telomere length, alone and in combination with smoking, contributes to increased risk of gastric cancer or esophageal squamous cell carcinoma. Carcinogenesis. 2017;38:12–18. doi: 10.1093/carcin/bgw111. [DOI] [PubMed] [Google Scholar]
- 215.Tahara T, Tahara S, Horiguchi N, Kawamura T, Okubo M, Ishizuka T, Yamada H, Yoshida D, Ohmori T, Maeda K, et al. Telomere length in leukocyte DNA in gastric cancer patients and its association with Clinicopathological features and prognosis. Anticancer Res. 2017;37:1997–2001. doi: 10.21873/anticanres.11543. [DOI] [PubMed] [Google Scholar]
- 216.Hou L, Savage SA, Blaser MJ, Perez-Perez G, Hoxha M, Dioni L, Pegoraro V, Dong LM, Zatonski W, Lissowska J, et al. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:3103–3109. doi: 10.1158/1055-9965.EPI-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Qu F, Li R, He X, Li Q, Xie S, Gong L, Ji G, Lu J, Bao G. Short telomere length in peripheral blood leukocytes predicts poor prognosis and indicates an immunosuppressive phenotypegastric cancer patients. Mol Oncol. 2015;9:727–739. doi: 10.1016/j.molonc.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang Z, Koh WP, Jin A, Wang R, Yuan JM. Telomere length and risk of developing gastric adenocarcinoma: The Singapore Chinese Health Study. Gastric Cancer. 2018;2:598–605. doi: 10.1007/s10120-017-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjærg-Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105:459–468. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- 220.Liu X, Bao G, Huo T, Wang Z, He X, Dong G. Constitutive telomere length and gastric cancer risk: Case-control analysis in Chinese Han population. Cancer Sci. 2009;100:1300–1305. doi: 10.1111/j.1349-7006.2009.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu Y, Lei T, Zhang N, Zheng Y, Kou P, Shang S, Yang M. Leukocyte telomere length and risk of gastric cardia adenocarcinoma. Sci Rep. 2018;8:14584. doi: 10.1038/s41598-018-32954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tahara T, Shibata T, Kawamura T, Horiguchi N, Okubo M, Nakano N, Ishizuka T, Nagasaka M, Nakagawa Y, Ohmiya N. Telomere length shortening in gastric mucosa is a field effect associated with increased risk of gastric cancer. Virchows Arch. 2016;469:19–24. doi: 10.1007/s00428-016-1948-3. [DOI] [PubMed] [Google Scholar]
- 223.Heo YR, Lee JH. Association between telomere length and PIK3CA amplification in gastric cancer. Clin Exp Med. 2018;18:133–134. doi: 10.1007/s10238-017-0465-2. [DOI] [PubMed] [Google Scholar]
- 224.Lili M, Yuxiang F, Zhongcheng H, Ying S, Ru C, Rong X, Jiang L. Genetic variations associated with telomere length affect the risk of gastric carcinoma. Medicine (Baltimore) 2020;99:e20551. doi: 10.1097/MD.0000000000020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Du J, Zhu X, Xie C, Dai N, Gu Y, Zhu M, Wang C, Gao Y, Pan F, Ren C, et al. Telomere length, genetic variants and gastric cancer risk in a Chinese population. Carcinogenesis. 2015;36:963–970. doi: 10.1093/carcin/bgv075. [DOI] [PubMed] [Google Scholar]
- 226.Choi BJ, Yoon JH, Kim O, Choi WS, Nam SW, Lee JY, Park WS. Influence of the hTERT rs2736100 polymorphism on telomere length in gastric cancer. World J Gastroenterol. 2015;21:9328–9336. doi: 10.3748/wjg.v21.i31.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Campa D, Matarazzi M, Greenhalf W, Bijlsma M, Saum KU, Pasquali C, van Laarhoven H, Szentesi A, Federici F, Vodicka P, et al. Genetic determinants of telomere length and risk of pancreatic cancer: A PANDoRA study. Int J Cancer. 2019;144:1275–1283. doi: 10.1002/ijc.31928. [DOI] [PubMed] [Google Scholar]
- 228.Duell EJ. Telomere length and pancreatic cancer risk: Breaking down the evidence. Gut. 2017;66:1. doi: 10.1136/gutjnl-2016-313156. [DOI] [PubMed] [Google Scholar]
- 229.Luu HN, Huang JY, Wang R, Adams-Haduch J, Jin A, Koh WP, Yuan JM. Association between leukocyte telomere length and the risk of pancreatic cancer: Findings from a prospective study. PLoS One. 2019;14:e0221697. doi: 10.1371/journal.pone.0221697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Campa D, Mergarten B, De Vivo I, Boutron-Ruault MC, Racine A, Severi G, Nieters A, Katzke VA, Trichopoulou A, Yiannakouris N, et al. Leukocyte telomere length in relation to pancreatic cancer risk: A prospective study. Cancer Epidemiol Biomarkers Prev. 2014;23:2447–2454. doi: 10.1158/1055-9965.EPI-14-0247. [DOI] [PubMed] [Google Scholar]
- 231.Mormile R. Telomere length and pancreatic cancer risk-letter. Cancer Epidemiol Biomarkers Prev. 2017;26:1157. doi: 10.1158/1055-9965.EPI-17-0225. [DOI] [PubMed] [Google Scholar]
- 232.Skinner HG, Gangnon RE, Litzelman K, Johnson RA, Chari ST, Petersen GM, Boardman LA. Telomere length and pancreatic cancer: A case-control study. Cancer Epidemiol Biomarkers Prev. 2012;21:2095–2100. doi: 10.1158/1055-9965.EPI-12-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang R, Zhao J, Xu J, Liu F. Association of peripheral leukocyte telomere length and its variation with pancreatic cancer and colorectal cancer risk in Chinese population. Oncotarget. 2016;7:38579–38585. doi: 10.18632/oncotarget.9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Antwi SO, Bamlet WR, Broderick BT, Chaffee KG, Oberg A, Jatoi A, Boardman LA, Petersen GM. Genetically predicted telomere length is not associated with pancreatic cancer risk. Cancer Epidemiol Biomarkers Prev. 2017;26:971–974. doi: 10.1158/1055-9965.EPI-17-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Antwi SO, Bamlet WR, Rabe KG, Cawthon RM, Umudi I, Druliner BR, Sicotte H, Oberg AL, Jatoi A, Boardman LA, Petersen GM. Leukocyte telomere length and its interaction with germline variation in Telomere-Related genes in relation to pancreatic adenocarcinoma risk. Cancer Epidemiol Biomarkers Prev. 2020;29:1492–1500. doi: 10.1158/1055-9965.EPI-19-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hamada T, Yuan C, Bao Y, Zhang M, Khalaf N, Babic A, Morales-Oyarvide V, Cochrane BB, Gaziano JM, Giovannucci EL, et al. Prediagnostic leukocyte telomere length and pancreatic cancer survival. Cancer Epidemiol Biomarkers Prev. 2019;28:1868–1875. doi: 10.1158/1055-9965.EPI-19-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bao Y, Prescott J, Yuan C, Zhang M, Kraft P, Babic A, Morales-Oyarvide V, Qian ZR, Buring JE, Cochrane BB, et al. Leucocyte telomere length genetic variants at the TERT gene region and risk of pancreatic cancer. Gut. 2017;66:1116–1122. doi: 10.1136/gutjnl-2016-312510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Posch A, Hofer-Zeni S, Klieser E, Primavesi F, Naderlinger E, Brandstetter A, Filipits M, Urbas R, Swiercynski S, Jäger T, et al. Hot Spot TERT promoter mutations are rare in sporadic pancreatic neuroendocrine Neoplasms and associated with telomere length and epigenetic expression patterns. Cancers (Basel) 2020;12:1625. doi: 10.3390/cancers12061625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mormile R. Leukocyte telomere length and pancreatic cancer survival: A consequence of activation of IL-6 signaling pathway in the carcinogenic process? J Gastrointest Cancer. 2020;51:720–721. doi: 10.1007/s12029-020-00364-5. [DOI] [PubMed] [Google Scholar]
- 240.Kroupa M, Rachakonda SK, Liska V, Srinivas N, Urbanova M, Jiraskova K, Schneiderova M, Vycital O, Vymetalkova V, Vodickova L, et al. Relationship of telomere length in colorectal cancer patients with cancer phenotype and patient prognosis Relationship of telomere length in colorectal cancer patients with cancer phenotype and patient prognosis. Br J Cance. 2019;121:344–350. doi: 10.1038/s41416-019-0525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lopez-Doriga A, Valle L, Alonso MH, Aussó S, Closa A, Sanjuan X, Barquero D, Rodríguez-Moranta F, Sanz-Pamplona R, Moreno V. Telomere length alterations in microsatellite stable colorectal cancer and association with the immune response. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2992–3000. doi: 10.1016/j.bbadis.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 242.Piñol-Felis C, Fernández-Marcelo T, Viñas-Salas J, Valls-Bautista C. Telomeres and telomerase in the clinical management of colorectal cancer. Clin Transl Oncol. 2017;19:399–408. doi: 10.1007/s12094-016-1559-0. [DOI] [PubMed] [Google Scholar]
- 243.Balc'h EL, Grandin N, Demattei MV, Guyétant S, Tallet A, Pagès JC, Ouaissi M, Lecomte, Charbonneau M. Measurement of telomere length in colorectal cancers for improved molecular diagnosis. Int J Mol Sci. 2017;18:1871. doi: 10.3390/ijms18091871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Luu HN, Qi M, Wang R, Adams-Haduch J, Miljkovic I, Opresko PL, Jin A, Koh WP, Yuan JM. Association between leukocyte telomere length and colorectal cancer risk in the Singapore Chinese Health Study. Clin Transl Gastroenterol. 2019;10:1–9. doi: 10.14309/ctg.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Peacock SD, Massey TE, Vanner SJ, King WD. Telomere length in the colon is related to colorectal adenoma prevalence. PLoS One. 2018;13:e0205697. doi: 10.1371/journal.pone.0205697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Naing C, Aung K, Lai PK, Mak JW. Association between telomere length and the risk of colorectal cancer: A meta-analysis of observational studies. BMC Cancer. 2017;17:24. doi: 10.1186/s12885-016-2997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Aljarbou F, Almobarak A, Binrayes A, Alamri HM. The expression of telomere-related proteins and DNA damage response and their association with telomere length in colorectal cancer in Saudi patients. PLoS One. 2018;13:e0197154. doi: 10.1371/journal.pone.0197154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Park WJ, Bae SU, Heo YR, Jung SJ, Lee JH. Telomere shortening in non-tumorous and tumor mucosa is independently related to colorectal carcinogenesis in precancerous lesions. Int J Mol Epidemiol Genet. 2017;8:53–58. [PMC free article] [PubMed] [Google Scholar]
- 249.Li J, Chang J, Tian J, Ke J, Zhu Y, Yang Y, Gong Y, Zou D, Peng X, Yang N, et al. A Rare Variant P507L in TPP1 interrupts TPP1-TIN2 interaction, influences telomere length, and confers colorectal cancer risk in Chinese population. Cancer Epidemiol Biomarkers Prev. 2018;27:1029–1035. doi: 10.1158/1055-9965.EPI-18-0099. [DOI] [PubMed] [Google Scholar]
- 250.Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. Depression and telomere length: A meta-analysis. J Affect Disord. 2016;191:237–247. doi: 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.AlAhwal MS, Zaben FA, Sehlo MG, Khalifa DA, Al-Aama JY, Edris S, Ashy JA, Koenig HG. Depression and telomere length in colorectal cancer patients in Saudi Arabia. Asian J Psychiatr. 2019;40:130–131. doi: 10.1016/j.ajp.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 252.Ko E, Seo HW, Jung G. Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology. 2018;67:1378–1391. doi: 10.1002/hep.29604. [DOI] [PubMed] [Google Scholar]
- 253.Ma LJ, Wang XY, Duan M, Liu LZ, Shi JY, Dong LQ, Yang LX, Wang ZC, Ding ZB, Ke AW. Telomere length variation in tumor cells and cancer-associated fibroblasts: Potential biomarker for hepatocellular carcinoma. J Pathol. 2017;243:407–417. doi: 10.1002/path.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lee HW, Park TI, Jang SY, Park SY, Park WJ, Jung SJ, Lee JH. Clinicopathological characteristics of TERT promoter mutation and telomere length in hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e5766. doi: 10.1097/MD.0000000000005766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ningarhari M, Caruso S, Hirsch TZ, Bayard Q, Franconi A, Védie AL, Noblet B, Blanc JF, Amaddeo G, Ganne N, et al. Telomere length is key to hepatocellular carcinoma diversity and telomerase addiction is an actionable therapeutic target. J Hepatol. 2021;74:1155–1166. doi: 10.1016/j.jhep.2020.11.052. [DOI] [PubMed] [Google Scholar]
- 256.Zeng H, Wu HC, Wang Q, Yang HI, Chen CJ, Santella RM, Shen J. Telomere length and risk of hepatocellular carcinoma: A nested Case-control study in Taiwan cancer screening program cohort. Anticancer Res. 2017;37:637–644. doi: 10.21873/anticanres.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cheng Y, Yu C, Huang M, Du F, Song C, Ma Z, Zhai X, Yang Y, Liu J, Bei JX, et al. Genetic association of telomere length with hepatocellular carcinoma risk: A Mendelian randomization analysis. Cancer Epidemiol. 2017;50:39–45. doi: 10.1016/j.canep.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 258.Feng W, Yu D, Li B, Luo OY, Xu T, Cao Y, Ding Y. Paired assessment of liver telomere lengths in hepatocellular cancer is a reliable predictor of disease persistence. Biosci Rep. 2017;37:BSR20160621. doi: 10.1042/BSR20160621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nikolouzakis TK, Stivaktakis PD, Apalaki P, Kalliantasi K, Sapsakos TM, Spandidos DA, Tsatsakis A, Souglakos J, Tsiaoussis J. Effect of systemic treatment on the micronuclei frequency in the peripheral blood of patients with metastatic colorectal cancer. Oncol Lett. 2019;17:2703–2712. doi: 10.3892/ol.2019.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nikolouzakis TK, Vakonaki E, Stivaktakis PD, Alegakis A, Berdiaki A, Razos N, Souglakos J, Tsatsakis A, Tsiaoussis J. Novel prognostic biomarkers in metastatic and locally advanced colorectal cancer: Micronuclei frequency and telomerase activity in peripheral blood lymphocytes. Front Oncol. 2021;11:683605. doi: 10.3389/fonc.2021.683605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Guterres AN, Villanueva J. Targeting telomerase for cancer therapy. Oncogene. 2020;39:5811–5824. doi: 10.1038/s41388-020-01405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.