Abstract
Objective
The aim of the study is to examine the impact of maternal interpregnancy body mass index (BMI) change on subsequent offspring mortality risk.
Study Design
This is a retrospective cohort study of women who had two consecutive live singleton deliveries of at least 20 weeks’ gestation from the Utah Population Database. Our exposure was defined as interpregnancy BMI change from the date of first delivery to the conception date of subsequent pregnancy. We categorized BMI change as: < – 1, − 1 to 0, 0 to <1 (reference), 1 to 2, 2 to 4, ≥4 kg/m2. Our primary outcome was all-cause age-specific mortality during four time periods: neonatal (≤28 days), infant (29 days to <1 year old), childhood ((≥1 to <5 years old), and late childhood (5 to <18 years old). We also examined mortality specifically attributed to congenital anomalies. Analyses used Cox proportional hazard models stratified by full term (≥37 weeks) and preterm (<37 weeks) deliveries. All models were adjusted for relevant confounders.
Results
Of 266,752 women, among full-term deliveries, women with a BMI increase of 4 kg/m2 or more had an increased risk of neonatal mortality in their subsequent pregnancy (hazard ratio or HR = 1.72, 95% confidence interval or CI: 1.23–2.41) Women who lost 1 kg/m2 or more between deliveries also had increased neonatal mortality (HR = 1.46, 95% CI: 1.04–2.05). There were no differences in infant, early, or late childhood mortality by interpregnancy BMI change. Maternal interpregnancy interval weight loss of 1 kg/m2 or more and weight gain of 4 kg/m2 also had increased risk of mortality associated with congenital anomalies or conditions arising during the neonatal period following their subsequent delivery.
Conclusion
Women with significant interpregnancy weight gain and modest weight loss have a significant increased risk of neonatal mortality following their subsequent pregnancy.
Keywords: neonatal mortality, weight gain, weight loss, obesity, congenital anomalies
Approximately two-thirds of adults in the United States are overweight (body mass index [BMI]: 25–29.9 kg/m2) or obese (BMI ≥30 kg/m2).1 Postpartum weight retention is a risk factor for obesity: one-third of women who were of normal weight prepregnancy become overweight or obese after delivery, and almost half of women retain 10 pounds or more following delivery.2 Women who were overweight or obese prior to pregnancy retain a greater proportion of weight gain during gestation compared with normal weight women.3 These results suggest that retained weight following a delivery is an important contributor to the obesity epidemic.
Weight gain between deliveries is associated with increased risk of cesarean delivery,4 hypertensive disorders of pregnancy,5 and gestational diabetes.6 The risk of these adverse maternal outcomes is increased among obese and overweight women compared with those who are normal weight.7 Less is known about the role of interpregnancy weight gain and neonatal outcomes. Among obese women, interpregnancy weight gain is associated with increased birth weight.8 A Belgian population-based study demonstrated that weight loss of 1 kg/m2 or more between pregnancies decreased the risk of macrosomia in some women,9 but another study showed weight loss also increases the risk of a low-birthweight infant,10 a known risk factor for neonatal mortality.11 Among normal weight women, interpregnancy weight gain is associated with increased risk of stillbirth12 and infant mortality.13
In this study, our aim was to determine whether interpregnancy weight change was associated with increased risk of neonatal, infant, childhood, and late childhood mortality.
Materials and Methods
This is a retrospective cohort study of women with two successive singleton deliveries in Utah from 1989 to 2014, with data obtained from the Utah Population Database (UPDB). The UPDB is a unique health data resource that receives data about residents in Utah, including biodemographic, health, economic, and birth outcome data; birth, marriage, and death certificates; and medical records from the two largest health care systems in the state. Approval for this study was obtained on August 11, 2016, from the University of Utah Institutional Review Board, protocol no.: 00091642.
Weight change was defined as the difference in BMI, from the start of first pregnancy to the estimated date of conception of subsequent pregnancy, using the best clinical gestation date available. To assist with clinical translation of the results, BMI change was categorized as loss of 1 kg/m2 or greater, loss of 1 kg/m2 to no change, gain of 0 to <1 kg/m2, gain of 1 to <2 kg/m2, gain of 2 to <4 kg/m2, and gain of 4 kg/m2 or more.
The primary outcomes examined were all-cause age-specific mortality evaluated across four time periods: neonatal (≤28 days of life), infant (29 days to <1 year old), childhood (≥1 to <5 years old), and late childhood (5 to <18 years old). We stratified deliveries by term or preterm delivery (defined as ≥37 weeks or <37 weeks at delivery, respectively) a priori. Offspring were only included in the age-specific models if they survived until the beginning of the time period, and if data were available. Secondary outcomes included cause-specific mortality from any congenital anomalies in each of the four time periods.
For bivariate models, one-way analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables were used. Cox’s proportional hazard models were used to assess mortality risk, adjusting for potential confounders including maternal age (years), race, ethnicity, maternal education (less than high school, high school grad, any college), marital status, smoking during pregnancy, diabetes, hypertension, assisted reproduction at second delivery, interpregnancy interval (months), sex (male or female), gestational age of second birth and whether the second child was small and large for gestational age birthweight (defined as weight less than and greater than 10th percentile for gestational age in weeks). A test of trend for weight change ordered categories was also conducted for each Cox model. Using collinearity diagnostic tests according to Belsey et al14 where we examine condition indices and the proportion of the variance of regression estimate accounted for by each principal component, we find no harmful levels of collinearity among the covariates. All analyses were performed in R version 3.5 (https://www.r-project.org). All confidence intervals (CIs) were based on Type I error of 0.05.
Results
After excluding women with missing or out-of-range data, we identified 266,752 women who delivered two successive singletons between 1989 and 2014 (Fig. 1). In our cohort, 14.3% had BMI loss of 1 kg/m2 or greater, 14.3% loss of 1 kg/m2 to no change, 28.1% gain of 0 to <1 kg/m2, 14.0% gain of 1 to <2 kg/m2, 15.6% gain of 2 to <4 kg/m2, and 13.8% gain of 4 kg/m2 or more. Table 1 shows cohort characteristics and their association with weight change between pregnancies. The majority (92.9%) of the subsequent deliveries were at term. Preterm delivery was significantly associated with weight change (p < 0.001). Maternal BMI, age, race/ethnicity, education, hypertension, diabetes, and smoking were all significantly associated with weight change, as was the interpregnancy interval.
Fig. 1.
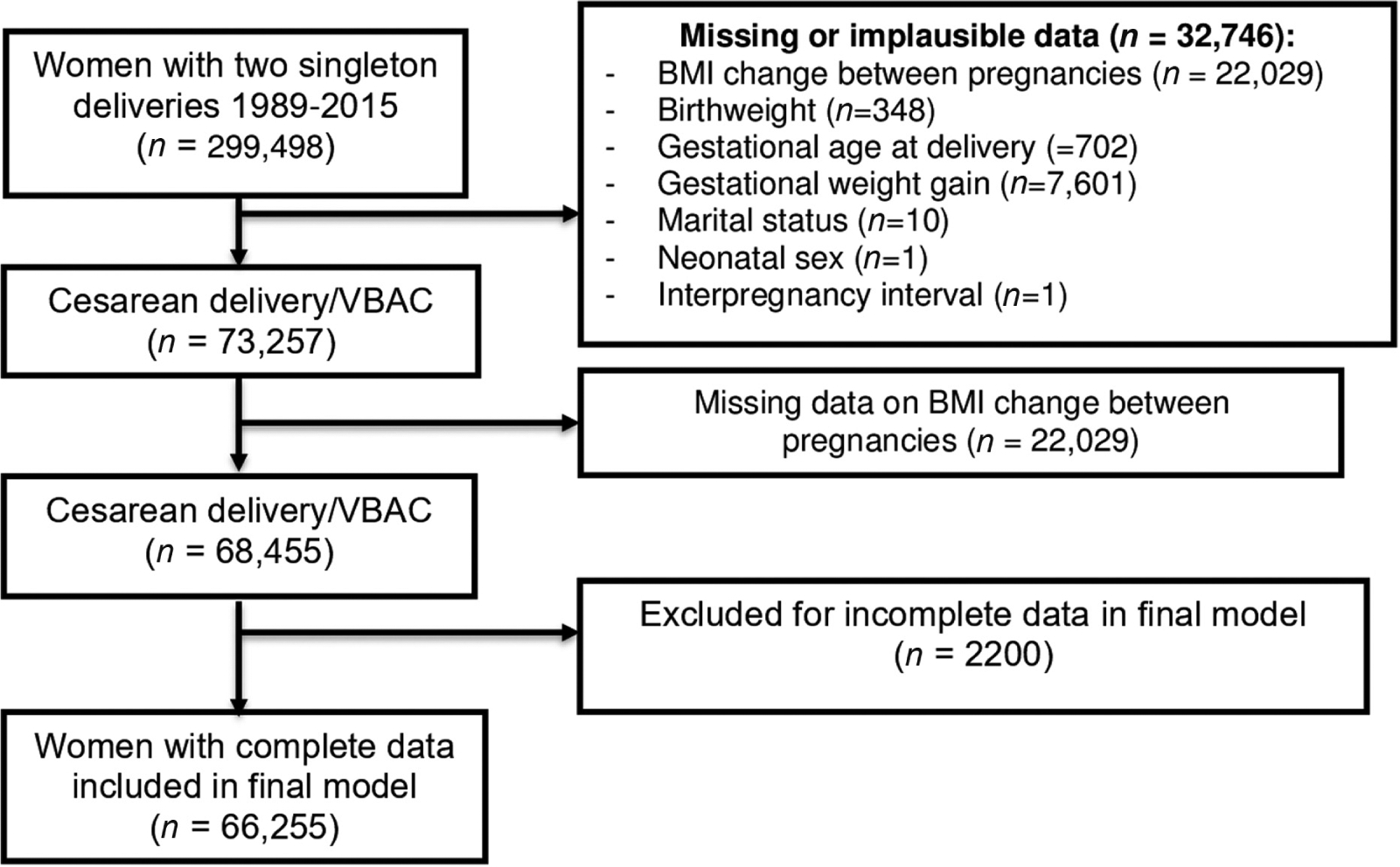
Study cohort selection from Utah Population Database (1989–2015).
Table 1.
Correlates of weight change between pregnancies
| Variable | Lost 1 kg/m2 or more (N = 38,193) | Lost 1 kg/m2 to no change (N = 38,018) | Gained 0 kg/m2 to <1 kg/m2 (N = 74,916) | Gained 1 to <2 kg/m2 (N = 37,438) | Gained 2 to <4 kg/m2 (N = 41,654) | Gained 4 kg/m2 or more (N = 36,745) | p-Valuea |
|---|---|---|---|---|---|---|---|
| Maternal BMI (kg/m2)a | 22.9 ± 4.8 | 22.0 ± 4.0 | 22.5 ±4.0 | 24.2 ± 4.4 | 26.4 ± 4.8 | 31.3 ± 5.9 | <0.001 |
| Race/ethnicity: | <0.001 | ||||||
| White non-Hispanic | 29,598 (77.7%) | 31,684 (83.5%) | 61,940 (82.9%) | 29,380 (78.7%) | 31,211 (75.1%) | 25,767 (70.2%) | |
| Black non-Hispanic | 127 (0.3%) | 87 (0.2%) | 173 (0.2%) | 126 (0.3%) | 166 (0.4%) | 178 (0.5%) | |
| Hispanic | 6,314 (16.6%) | 4,326 (11.4%) | 8,648 (11.6%) | 5,712 (15.3%) | 7,759 (18.7%) | 8,520 (23.2%) | |
| Asian | 356 (0.9%) | 415 (1.1%) | 945 (1.3%) | 509 (1.4%) | 441 (1.1%) | 203 (0.6%) | |
| Other race | 1,700 (4.5%) | 1,420 (3.7%) | 3,049 (4.1%) | 1,614 (4.3%) | 1,983 (4.8%) | 2,013 (5.5%) | |
| Maternal age (years)a | 27.1 ± 4.6 | 27.3 ± 4.5 | 27.4 ± 4.6 | 27.2 ±4.7 | 27.0 ± 4.8 | 26.8 ± 4.9 | <0.001 |
| Maternal educationa | <0.001 | ||||||
| < High school | 5,075 (13.3%) | 3,197 (8.4%) | 6,026 (8.0%) | 4,157 (11.1%) | 5,745 (13.8%) | 6,849 (18.6%) | |
| High school graduate | 10,715 (28.1%) | 9,108 (24.0%) | 18,823 (25.1%) | 10,949 (29.2%) | 13,917 (33.4%) | 14,147 (38.5%) | |
| College | 22,403 (58.7%) | 25,713 (67.6%) | 50,067 (66.8%) | 22,332 (59.7%) | 21,992 (52.8%) | 15,749 (42.9%) | |
| Married at the time of deliverya | 32,336 (84.7%) | 34,067 (89.6%) | 67,166 (89.7%) | 32,449 (86.7%) | 35,102 (84.3%) | 29,081 (79.1%) | <0.001 |
| Maternal hypertensiona | 1,481 (3.9%) | 1,123 (3.0%) | 2,311 (3.1%) | 1,452 (3.9%) | 2,178 (5.2%) | 2,965 (8.1%) | <0.001 |
| Maternal diabetesa | 1,144 (3.0%) | 755 (2.0%) | 1,610 (2.1%) | 1,107 (3.0%) | 1,473 (3.5%) | 2,112 (5.7%) | <0.001 |
| Smokinga | 3,268 (8.6%) | 2,172 (5.7%) | 4,050 (5.4%) | 2,333 (6.2%) | 3,061 (7.3%) | 3,694 (10.1%) | <0.001 |
| Interpregnancy interval (months) | 34.7 ± 21.3 | 33.1 ± 18.9 | 33.5 ± 19.2 | 37.3 ± 23.2 | 40.5 ± 26.0 | 49.9 ± 34.2 | <0.001 |
| Assisted reproductive technologyb | 436 (1.1%) | 462 (1.2%) | 850 (1.1%) | 469 (1.3%) | 497 (1.2%) | 463 (1.3%) | 0.335 |
| Male sexb | 19,492 (51.0%) | 19,368 (50.9%) | 38,675 (51.6%) | 19,275 (51.5%) | 21,422 (51.4%) | 18,964 (51.6%) | 0.188 |
| Preterm delivery (<37 wk gestation)b | 2,889 (7.6%) | 2,642 (6.9%) | 5,011 (6.7%) | 2,637 (7.0%) | 2,898 (7.0%) | 2,741 (7.5%) | <0.001 |
| Small for gestational age birthweightb | 3,779 (9.9%) | 3,780 (9.9%) | 7,338 (9.8%) | 3,762 (10.0%) | 4,203 (10.1%) | 3,689 (10.0%) | 0.580 |
Abbreviations: BMI, body mass index; kg/m2, kilogram/meter squared.
p-Values are from a chi-square test for categorical variables, one-way analysis of variance for continuous variables.
At subsequent delivery/during subsequent pregnancy.
Among all neonates, among term neonates, both weight loss of 1 kg/m2 and weight gain ≥4 kg/m2 were associated with higher risk of all-cause mortality in the neonatal period (hazard ratio [HR] = 1.46, 95% CI: 1.04–2.05 for weight loss; HR = 1.72, 95% CI: 1.23–2.41 for weight gain ≥4 kg/m2; Table 2). Results were similar in significance and magnitude for mortality specifically due to congenital anomalies (HR = 2.55, 95% CI: 1.39–4.66 for weight loss; HR = 2.30, 95% CI: 1.19–4.45 for weight gain ≥4 kg/m2). Mortality for pre-term neonates born was not associated with weight change between pregnancies.
Table 2.
Maternal interpregnancy BMI change and neonatal (birth to 28 ≤d) mortality of a subsequent child
| Full term (≥37 wk gestation) N = 247,940 | Preterm (<37 wk gestation) N = 18,812 | |||
|---|---|---|---|---|
| All-cause mortality | Hazard rate ratioa | 95% CI | Hazard rate ratioa | 95% CI |
| Weight change: | ||||
| Lost 1 kg/m2 or more | 1.46 | 1.04–2.05 | 1.05 | 0.83–1.32 |
| Lost 1 kg/m2 to no change | 1.14 | 0.79–1.65 | 1.17 | 0.93–1.47 |
| Gained 0 kg/m2 to <1 kg/m2 | 1.00 | 1.00 | ||
| Gained 1 to <2 kg/m2 | 1.11 | 0.77–1.62 | 0.83 | 0.64–1.07 |
| Gained 2 to <4 kg/m2 | 1.29 | 0.91–1.82 | 1.04 | 0.82–1.33 |
| Gained 4 kg/m2 or more | 1.72 | 1.23–2.41 | 0.97 | 0.75–1.25 |
| Trend | 1.04 | 0.97–1.11 | 0.97 | 0.93–1.02 |
| Mortality from congenital malformations | ||||
| Weight change: | ||||
| Lost 1 kg/m2 or more | 2.55 | 1.39–4.66 | 1.06 | 0.65–1.73 |
| Lost 1 kg/m2 to no change | 1.65 | 0.85–3.20 | 1.00 | 0.60–1.66 |
| Gained 0 kg/m2 to <1 kg/m2 | 1.00 | 1.00 | ||
| Gained 1 to <2 kg/m2 | 1.20 | 0.57–2.53 | 0.98 | 0.58–1.63 |
| Gained 2 to <4 kg/m2 | 1.70 | 0.88–3.29 | 0.69 | 0.39–1.23 |
| Gained 4 kg/m2 or more | 2.30 | 1.19–4.45 | 0.67 | 0.36–1.23 |
| Trend | 0.98 | 0.86–1.11 | 0.91 | 0.82–1.02 |
Abbreviation: kg/m2, kilogram/meter squared.
These models control for whether the neonate was small-for-gestational age, large-for-gestational age, gestational age at delivery (weeks), neonatal sex, interpregnancy interval, assisted reproduction, and maternal race/ethnicity, age, education, marital status, smoking, diabetes, and hypertension during the subsequent pregnancy.
Weight change was not associated with all-cause mortality and mortality from congenital anomalies in the infant period (29 days to 1 year) among term infants, as well as preterm infants, with the exception of weight gain of 2 to <4 kg/m2 (HR = 0.42, 95% CI: 0.20–0.89) for all-cause mortality (Table 3). During the early childhood period (ages 1–5 years), no individual categories of weight change were associated with either all-cause or congenital anomaly mortality in either full term or preterm children, although the tests of trend were significant (HR = 1.37, 95% CI: 1.03–1.82 for preterm all-cause mortality; HR = 1.52, 95% CI: 1.11–2.07 for mortality due to congenital anomalies for full-term infants; data not shown). Interpregnancy weight change is largely unassociated with late childhood mortality for either preterm or full-term infants (data not shown).
Table 3.
Maternal interpregnancy BMI change and infant mortality (29 d to <1 y) of a subsequent child
| Full term (≥37 wk gestation) N = 235,011 | Preterm (<37 wk gestation) N = 17,157 | |||
|---|---|---|---|---|
| All-cause mortality | Hazard rate ratioa,b | 95% CI | Hazard rate ratioa,b | 95% CI |
| Weight change | ||||
| Lost 1 kg/m2 or more | 1.17 | 0.82–1.68 | 0.76 | 0.43–1.35 |
| Lost 1 kg/m2 to no change | 0.90 | 0.60–1.35 | 0.74 | 0.40–1.36 |
| Gained 0 kg/m2 to <1 kg/m2 | 1.00 | 1.00 | ||
| Gained 1 to <2 kg/m2 | 1.41 | 0.99–1.99 | 0.79 | 0.44–1.43 |
| Gained 2 to <4 kg/m2 | 1.20 | 0.85–1.72 | 0.42 | 0.20–0.89 |
| Gained 4 kg/m2 or more | 1.35 | 0.94–1.94 | 1.18 | 0.69–2.02 |
| Trend | 1.06 | 0.98–1.13 | 1.03 | 0.91–1.15 |
| Mortality from congenital malformations | ||||
| Weight change | ||||
| Lost 1 kg/m2 or more | 1.10 | 0.46–2.59 | 0.94 | 0.31–2.80 |
| Lost 1 kg/m2 to no change | 1.29 | 0.58–2.87 | 0.84 | 0.26–2.73 |
| Gained 0 kg/m2 to <1 kg/m2 | 1.00 | 1.00 | ||
| Gained 1 to <2 kg/m2 | 1.27 | 0.56–2.91 | 0.39 | 0.08–1.81 |
| Gained 2 to <4 kg/m2 | 1.33 | 0.59–2.99 | 0.57 | 0.15–2.14 |
| Gained 4 kg/m2 or more | 2.16 | 0.99–4.68 | 1.03 | 0.33–3.20 |
| Trend | 1.12 | 0.96–1.32 | 0.96 | 0.76–1.21 |
These models control for whether the neonate was small-for-gestational age, large-for-gestational age, gestational age at delivery (weeks), neonatal sex, interpregnancy interval, assisted reproduction, and maternal race/ethnicity, age, education, marital status, smoking, diabetes, and hypertension during the subsequent pregnancy.
Models include only those infants who were alive at 29 d following delivery.
Discussion
In this study, we show that interpregnancy weight loss of ≥1 kg/m2 and interpregnancy weight gain of ≥4 kg/m2 are associated with neonatal all-cause mortality as well as mortality from congenital anomalies among full term infants. Once we examined offspring mortality beyond the neonatal period, the association between interpregnancy weight change was less consistent.
Our study confirms an increased risk of neonatal mortality with significant interpregnancy weight gain.13,15 Our study establishes that this association does not persist beyond the neonatal period. Our study advances the literature by establishing that congenital anomalies may be one mechanism linking significant interpregnancy weight gain and neonatal mortality. Our study also establishes that modest weight loss is a risk factor for neonatal mortality, a finding which is less common in the literature.
We postulate that maternal interpregnancy weight change may affect the intrauterine environment, which may subsequently lead to adverse fetal development and neonatal mortality. Underdiagnosed diabetes and impaired glucose tolerance, both more common among obese women, may lead to a higher likelihood of fetal anomalies in a subsequent pregnancy.16 Another possible explanation for these findings is that it is more difficult to detect fetal anomalies via ultrasound in obese women,17 perhaps making it more likely women will carry anomalous fetuses to term, or clinicians will be less prepared to care for these neonates following delivery. The lack of association between maternal interpregnancy weight change and infant, early childhood, and late childhood mortality is expected as these deaths are likely attributable primarily to causes such as sudden infant death syndrome, infections, and accidents.18,19
With regard to weight loss as a risk factor for neonatal mortality, while there is some evidence that maternal weight loss is associated with decreased birthweight8 and increased risk of a growth restricted infant,9 we controlled for these factors with these data. Previous research shows that in addition to affecting birthweight, maternal weight loss also leads to changes in the size and structure of the placenta,20 which may have an as-yet unknown effect on perinatal and neonatal mortality, and may be an alternate explanation for these findings. Finally, the association between maternal weight and congenital anomalies may be U-shaped, with both underweight and overweight women exhibiting higher rates of congenital anomalies as compared with normal weight woman, which may explain our results.21
Interpregnancy weight gain may result from postpartum weight retention from the initial pregnancy as well as weight gained once the initial pregnancy is over. Excess gestational weight gain in the initial pregnancy is associated with increased postpartum weight retention.22 Women who are overweight prior to an index pregnancy also gain more weight between pregnancies.23 This excess weight from all sources can lead to increased risk of hypertensive disorders,24 gestational diabetes,6,25 and, again, changes in the size of the placenta,20,26 which may lead to increased risk of neonatal death. While we were able to control for some of these factors (such as diabetes), other factors such as changes in the placenta may lie on the causal pathway, and warrant further investigation.
Our study has many strengths, including a large cohort from a diverse population and detailed clinical records linked to vital statistics data. Given the retrospective nature of this cohort, we were able to examine the association between weight change and offspring mortality across several age intervals, not just in the neonatal period. We were also able to control for multiple potential confounders of the relationship between interpregnancy weight change and mortality, including preterm delivery, hypertension, diabetes, baseline BMI, and smoking. By controlling for confounders such as baseline BMI and maternal co-morbidities, we are able to establish that it is likely interpregnancy weight change that drives an increased risk in neonatal mortality.
Our study also has several limitations. As is always the case with vital statistics data, attribution error may occur in mortality cause, although this is unlikely to affect our estimates of all-cause mortality. Selection bias may be present in terms of women who choose to remain in Utah for both deliveries and are subsequently captured in the database. We also cannot rule out the possibility of residual confounding, particularly as the interval between delivery and death increases. These data come from Utah only; these findings may not be applicable in other settings. Finally, all associations are correlational; causality is not assured.
In conclusion, independent of BMI, interpregnancy weight change may be a modifiable risk factor for neonatal mortality, particularly with regard to decreasing the risk of congenital anomalies.
Key Points.
Significant weight gain between deliveries increases the risk of neonatal death.
Modest weight loss between deliveries increases the risk of neonatal death.
This risk may be partially explained by increased risk of congenital malformations.
Funding
The authors thank the Pedigree and Population Resource of the Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance, and support of the UPDB. They also acknowledge partial support for the UPDB through grant P30 CA2014 from the National Cancer Institute, University of Utah and from the University of Utah’s Program in Personalized Health and Center for Clinical and Translational Science. Additional funding was received intramural seed grant from the Diabetes and Metabolism Research Center at the University of Utah. MCS is supported by Women’s Reproductive Health Research (WRHR K12, 1K12 HD085816) Career Development Program.
Footnotes
Conflict of Interest
None declared.
A version of this paper was presented at the 38th Annual Meeting of the Society for Maternal-Fetal Medicine, Dallas, TX, January 29, 2018 to February 3rd, 2018: abstract no.: 724.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endres LK, Straub H, McKinney C, et al. ; Community Child Health Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol 2015;125(01):144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketterl TG, Dundas NJ, Roncaioli SA, Littman AJ, Phipps AI. Association of pre-pregnancy BMI and postpartum weight retention before second pregnancy, Washington State, 2003–2013. Matern Child Health J 2018;22(09):1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dude AM, Lane-Cordova AD, Grobman WA. Interdelivery weight gain and risk of cesarean delivery following a prior vaginal delivery. Am J Obstet Gynecol 2017;217(03):373.e1–373.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynes C, McLain AC, Yeung EH, Albert P, Liu J, Boghossian NS. Interpregnancy weight change and adverse maternal outcomes: a retrospective cohort study. Ann Epidemiol 2017;27(10):632–637. e5, e635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol 2011;117(06):1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane JM, White J, Murphy P, Burrage L, Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J Obstet Gynaecol Can 2009;31(01):28–35 [DOI] [PubMed] [Google Scholar]
- 8.Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal R, Hopkins SA. The impact of interpregnancy weight change on birthweight in obese women. Am J Obstet Gynecol 2013;208(03):205.e1–205.e7 [DOI] [PubMed] [Google Scholar]
- 9.Bogaerts A, Ameye L, Martens E, Devlieger R. Weight loss in obese pregnant women and risk for adverse perinatal outcomes. Obstet Gynecol 2015;125(03):566–575 [DOI] [PubMed] [Google Scholar]
- 10.Bogaerts A, Van den Bergh BRH, Ameye L, et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol 2013;122(05):999–1009 [DOI] [PubMed] [Google Scholar]
- 11.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med 1985;312(02): 82–90 [DOI] [PubMed] [Google Scholar]
- 12.Whiteman VE, Crisan L, McIntosh C, et al. Interpregnancy body mass index changes and risk of stillbirth. Gynecol Obstet Invest 2011;72(03):192–195 [DOI] [PubMed] [Google Scholar]
- 13.Cnattingius S, Villamor E. Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet 2016;387(10018):558–565 [DOI] [PubMed] [Google Scholar]
- 14.Belsey DA, Kuh E, Welsch RE. Regression Diagnostics. New York, NY: John Wiley & Sons; 1980 [Google Scholar]
- 15.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 2006;368(9542):1164–1170 [DOI] [PubMed] [Google Scholar]
- 16.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009;301(06):636–650 [DOI] [PubMed] [Google Scholar]
- 17.Best KE, Tennant PW, Bell R, Rankin J. Impact of maternal body mass index on the antenatal detection of congenital anomalies. BJOG 2012;119(12):1503–1511 [DOI] [PubMed] [Google Scholar]
- 18.Ely DM, Hoyert DL. Differences between rural and urban areas in mortality rates for the leading causes of infant death: United States, 2013–2015. NCHS Data Brief 2018;(300):1–8 [PubMed] [Google Scholar]
- 19.National Vital Statistics System NCfHS. CDC. 10 leading causes of death by age group, United States—2016. 2018. Accessed August 9, 2018 at: https://www.cdc.gov/injury/wisqars/LeadingCauses.html [Google Scholar]
- 20.Wallace JM, Bhattacharya S, Campbell DM, Horgan GW. Interpregnancy weight change impacts placental weight and is associated with the risk of adverse pregnancy outcomes in the second pregnancy. BMC Pregnancy Childbirth 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rankin J, Tennant PW, Stothard KJ, Bythell M, Summerbell CD, Bell R. Maternal body mass index and congenital anomaly risk: a cohort study. Int J Obes 2010;34(09):1371–1380 [DOI] [PubMed] [Google Scholar]
- 22.Hutcheon JA, Chapinal N, Bodnar LM, Lee L. The INTERGROWTH- 21st gestational weight gain standard and interpregnancy weight increase: a population-based study of successive pregnancies. Obesity (Silver Spring) 2017;25(06):1122–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace JM, Bhattacharya S, Horgan GW. Weight change across the start of three consecutive pregnancies and the risk of maternal morbidity and SGA birth at the second and third pregnancy. PLoS One 2017;12(06):e0179589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Getahun D, Ananth CV, Oyelese Y, Chavez MR, Kirby RS, Smulian JC. Primary preeclampsia in the second pregnancy: effects of changes in prepregnancy body mass index between pregnancies. Obstet Gynecol 2007;110(06): 1319–1325 [DOI] [PubMed] [Google Scholar]
- 25.Sorbye LM, Skjaerven R, Klungsoyr K, Morken NH. Gestational diabetes mellitus and interpregnancy weight change: a population-based cohort study. PLoS Med 2017;14(08):e1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace JM, Horgan GW, Bhattacharya S. Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies. Placenta 2012;33(08):611–618 [DOI] [PubMed] [Google Scholar]


