Abstract
Purpose of review
This review provides an overview of the management and treatment landscape of inclusion body myositis (IBM), while highlighting the current challenges and future directions.
Recent findings
IBM is a slowly progressive myopathy that predominantly affects patients over the age of 40, leading to increased morbidity and mortality. Unfortunately, a definitive cure for IBM remains elusive. Various clinical trials targeting inflammatory and some of the noninflammatory pathways have failed. The search for effective disease-modifying treatments faces numerous hurdles including variability in presentation, diagnostic challenges, poor understanding of pathogenesis, scarcity of disease models, a lack of validated outcome measures, and challenges related to clinical trial design. Close monitoring of swallowing and respiratory function, adapting an exercise routine, and addressing mobility issues are the mainstay of management at this time.
Summary
Addressing the obstacles encountered by patients with IBM and the medical community presents a multitude of challenges. Effectively surmounting these hurdles requires embracing cutting-edge research strategies aimed at enhancing the management and treatment of IBM, while elevating the quality of life for those affected.
Keywords: clinical trial design, inclusion body myositis, individualized medicine
INTRODUCTION
Inclusion body myositis (IBM) is the most prevalent muscle disease primarily affecting individuals above the age of 40. The disease has a male predominance occurring nearly twice as frequently in males compared to females [1]. Although variability in presentation exists, IBM most commonly presents with muscle weakness predominantly affecting deep finger flexors and/or knee extensors [2]. IBM is associated with increased morbidity and mortality [3▪]. Pharmacologic treatments tried to date have been relatively unsuccessful in modifying disease course without a currently recognized cure [4]. The lack of treatment can be attributed to various challenges spanning from diagnostic challenges to the development of targeted therapies based on a comprehensive understanding of the underlying disease mechanisms. This article examines the diagnostic and management challenges faced by patients with IBM and provides an overview of the current treatment landscape.
Box 1.
no caption available
CLINICAL PRESENTATION AND ASSOCIATED CHALLENGES
IBM is clinically characterized by chronic, slowly progressive weakness, often asymmetric, presenting with predominant finger flexor and/or knee extensor involvement [2,5–8]. However, less common presentations of this disease can occur in approximately 14% of patients including isolated dysphagia, foot drop, proximal upper limb weakness, axial weakness with head drop, and facial diplegia [9▪,10,11]. Furthermore, IBM may be diagnosed even at a preclinical stage manifesting with elevated creatine kinase levels [9▪]. The lingering course, the variable presentations, and the distal asymmetric weakness, mimicking a mononeuropathy (e.g. ulnar) or a motor neuron disorder, can be challenging for providers to recognize and result in diagnostic delay with time from symptom onset to diagnosis ranging from 4 to 9 years on average [9▪,12].
DIAGNOSIS AND DIAGNOSTIC CHALLENGES
Diagnosis is established based on a combination of clinical features and muscle biopsy findings. Several diagnostic criteria have been published, all requiring a combination of clinical and histopathological features [13–23]. All included diagnostic categories share very high specificity of more than 97% with variable sensitivity ranging from 11% to 84% [23]. The high specificity is in part due to the distinctive histopathological and clinical findings when present. The European Neuromuscular Centre (ENMC) 2013 IBM diagnostic criteria remain one of the most widely used criteria [20]. The mandatory clinical criteria include age of onset later than 45 years, duration of symptoms more than 12 months, and serum creatine kinase levels not more than 15 times the upper limit of normal [20]. Clinical features include weakness of the finger flexors more than shoulder abductors and/or weakness of the quadriceps more than or equal to hip flexors [20]. Although the prominent involvement of deep finger flexors and/or quadriceps are typical in IBM, they are not pathognomonic as other myopathies can have predilection to these muscles (Table 1). Additional challenges are posed in patients with onset of weakness beyond finger flexors and quadriceps as the differential for such presentations is wider.
Table 1.
Myopathies that can mimic IBM based on reported patterns of weakness
| Prominent finger flexion weakness | Prominent knee extension weakness | |
| Myopathies on the spectrum of IBM | ||
| Granulomatous myopathy [107,108] | + | + |
| Polymyositis with mitochondrial pathology [109] | + | + |
| Acquired myopathies | ||
| AL amyloidosis [110] | + | − |
| Inherited myopathy with overlapping phenotype with IBM | ||
| Myotonic dystrophy type 1 [111–113] | + | + |
| Myotonic dystrophy type 2 [114] | + | +/− |
| Dysferlinopathies [115,116] | + | + |
| Dystrophinopathies [117] | + | + |
| Filaminopathy [118] | + | + |
| Limb girdle muscular dystrophy D3 [119] | + | + |
| VCP myopathy [120] | + | + |
| GNE myopathy [121] | + | – |
| Myofibrillar myopathies [122,123] | + | +/– |
| Inherited myopathies with a distinct phenotype but reported rare cases overlapping with IBM | ||
| MYH7 myopathy [124] | + | + |
| Pompe disease [125] | + | + |
| LAMA2 muscular dystrophy [126] | + | – |
| ACTA1 myopathy [127] | + | + |
Pathologic criteria for IBM include endomysial lymphocytic infiltration invading nonnecrotic muscle fibers referred to as autoaggressive inflammation, the presence of rimmed vacuoles, and evidence of protein deposits [20]. A challenge in histopathologic diagnosis is that rimmed vacuoles and congophilic deposits may be absent on muscle biopsy in patients clinically diagnosed with IBM in up to 25% of patients [24]. Selecting the appropriate muscle to biopsy can also be challenging. Choosing a muscle that is only moderately weak is necessary to avoid a false negative result if the muscle is only mildly affected or end-stage (nondiagnostic) muscle changes if the muscle is severely affected [24,25]. Patients may require a repeat muscle biopsy to confirm the diagnosis [9▪]. Although relatively benign, a muscle biopsy may result in complications such as bleeding or hematoma formation, muscle herniation with activity if the wound is not carefully closed, and/or wound dehiscence [26]. Additionally, other myopathies such as multisystem proteinopathies and myofibrillary myopathies may contain rimmed vacuoles or protein accumulations and can be confused for IBM on pathologic grounds when inflammation is absent or sparce [27,28].
Electrodiagnostic testing through nerve conduction studies and needle electromyography (EMG) is typically performed during diagnostic work up and could help define the nature of the underlying process (neuropathic versus myopathic), rule out disease mimickers, and select a target for biopsy. However, in IBM, long duration high amplitude motor unit potentials (neuropathic) are often present mixed with short duration, low amplitude, complex units (myopathic) [29,30]. This may result in test misinterpretation further delaying the diagnosis.
The utilization of imaging techniques in the diagnosis of myopathies has been on the rise. In IBM, muscle MRI has been proven valuable in supporting the diagnosis due to its ability to detect a characteristic pattern of muscle involvement (Fig. 1) [31,32]. Additionally, muscle MRI can aid in selecting a muscle for biopsy. However, it is important to consider certain limitations, including the time-consuming and expensive nature of MRI, challenges in discerning the pattern at early and advanced stages of the disease, and the need for independent interpretation by neurologists or rheumatologists, as most radiologists may not be well versed in the various patterns associated with myopathies. Moreover, muscle ultrasound is emerging as a promising tool, especially helpful in detecting subclinical muscle involvement of the deep finger flexors, quadriceps, and medial gastrocnemius [33].
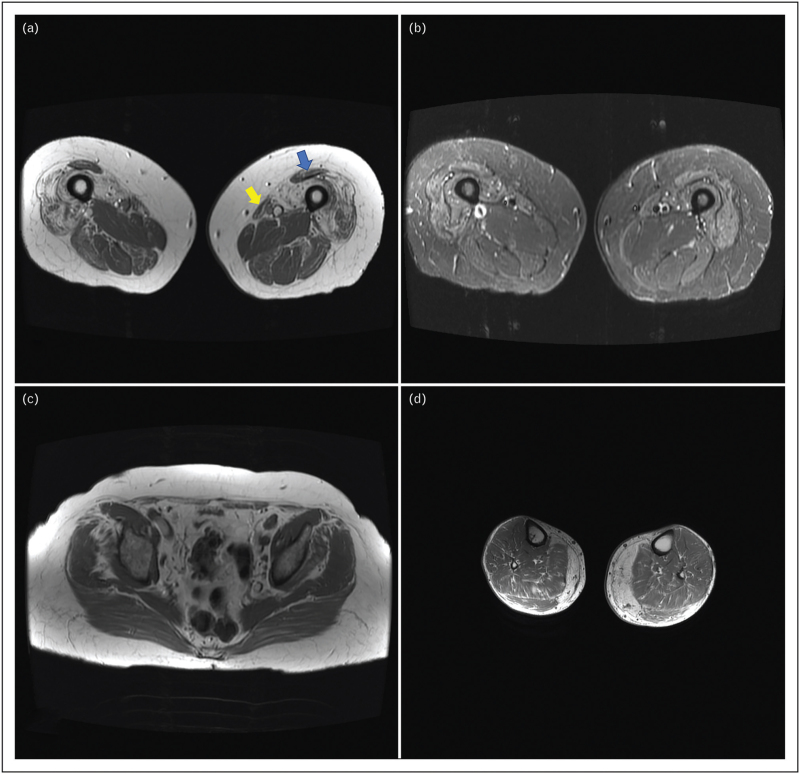
FIGURE 1.
MRI findings in inclusion body myositis. Typical muscle MRI findings in IBM: T1 axial images (a, c, d) and FSE-IR sequence (b). (a) Atrophy and fatty replacement of the distal quadriceps, predominantly involving the vastus medialis and lateralis, worse on the right, with sparing of the rectus femoris (blue arrow) and involvement of the sartorius (yellow arrow). In contrast, there is relative sparing of pelvic muscles (c) and more proximal parts of the quadriceps creating a proximal to distal gradient in the quadriceps (not shown). Increased water content in distal quadriceps (b) indicating inflammation (equivalent to a T2 sequence). In the leg compartment, there is prominent involvement of the medial gastrocnemius (d).
Last, the only currently available serum diagnostic biomarker for IBM is cytosolic 5’-nucleotidase 1A (cN-1A) antibody. It has a limited sensitivity ranging from 30% to 50% [34,35]. Although overall specificity was originally reported to be high (>90%), cN-1A antibodies can also been found in others diseases such as dermatomyositis, Sjogren's syndrome, antisynthetase syndrome, immune mediated necrotizing myopathy, and systemic lupus erythematous [36,37]. As a result, the specificity for the antibody currently ranges between 30–80% [38]. Nevertheless, while cN-1A antibody should not be used as a standalone test to diagnose IBM, a positive result helps raise suspicion for the diagnosis, especially in patients with atypical presentations or at a preclinical stage [9▪].
LIMITED THERAPEUTIC TARGETS
Another challenge facing the development of a disease-modifying treatment of IBM is the lack of detailed understanding of its pathogenesis [39]. Regardless of which is the cause versus the consequence, once the disease manifests clinically, the vast majority of patients display complex histopathological findings featuring inflammation, mitochondrial dysfunction, disrupted autophagy, and protein aggregation. Review of disease mechanisms is beyond the scope of this article and is nicely summarized in several articles [39–42]. Most clinical trials targeted various aspects of the inflammatory or immune response as detailed below in the treatment landscape section. However, the highly differentiated cytotoxic CD8+ T cells, displaying markers of senescence such as KLRG1, are thought to evade conventional immunosuppressive or immunomodulatory therapies [43,44]. Current phase 1 and phase 2/3 trials (clinical trial NCT05721573) targeting these KLRG1 positive T cells are ongoing [45]. However, detailed knowledge of the noninflammatory pathways is lagging behind posing a major challenge to identifying therapeutic targets. Drugs with broad rather than targeted mechanisms of action have been tried as detailed below [39,41].
LIMITED DISEASE MODELS
The availability of validated disease models to understand pathogenesis and develop treatments is an important aspect in translational research. Modeling monogenic inherited diseases via gene editing is more straightforward compared to diseases such as IBM. Models for hereditary multisystem proteinopathies (MSP), mainly VCP mouse models, have been used in IBM [24,46,47]. Although also known as hereditary inclusion body myopathies, VCP and other MSP disorders are distinct entities with different clinical features and populations at risk compared to IBM. A MCK-βAPP model has been reported [48]. Furthermore, cholesterol-fed rabbits and nematode C. elegans have also been proposed [49,50]. More recently, a xenograft animal model was developed where human muscle from IBM patients was transplanted into immunodeficient mice [51▪▪]. The xenograft recapitulated the canonical histopathological features of IBM. However, given the nature of these models, functional and behavioral evaluations cannot be performed.
Modeling the inflammatory component is also challenging. There are reported animal models for idiopathic inflammatory myopathies but not IBM. Existing models encompass infection-related models of myositis [virus models such as Ross River virus (RRV) infection and Coxsackie virus B], antigen-induced, and myosin and C protein induced myositis models [52–56]. Although these models result in muscle inflammation mimicking human inflammatory myopathies, they are unlikely to recapitulate IBM features which involve CD8+- T-cell driven endomysial inflammation and noninflammatory findings.
LONG-TERM OUTCOMES AND SURVIVAL
IBM is associated with both increased morbidity and mortality. The median time to using a cane is about 5 years and to using a wheelchair is about 10.5 years [57]. Over time, all patients eventually require the use of a wheelchair [58]. An annual decline of 3–5% in grip strength, 3.7% in overall strength, and 6.3% in IBM functional rating scale (IBMFRS) have been reported [58,59]. The median time to onset of dysphagia has been cited as approximately 6 years and can be seen in approximately two thirds of patients [57]. Patients with IBM are also shown to have an increased mortality [57–59]. IBM patients had a 10 year survival rate of 36% of index compared to 50% in control patients [57]. The most common cause of death in IBM arises from respiratory compromise and dysphagia (aspiration pneumonia) [60]. Furthermore, patients with IBM are more likely to have a superimposed peripheral neuropathy, Sjogren's syndrome, or T-cell large granular lymphocytic leukemia than the general population [3▪]. The slow progressive course and survival would be challenging to capture in a clinical trial.
TREATMENT LANDSCAPE
Although, there is currently no pharmacologic cure for IBM, current treatment strategies primarily revolve around implementing supportive measures to address symptoms such as dysphagia, respiratory compromise, muscle weakness, and limited mobility [24].
Dysphagia is a common symptom in IBM patients and can be debilitating [61]. Dysphagia-targeted interventions may provide temporary relief, such as myotomy or dilation of a cricopharyngeal bar when patients have a significant obstructive components [62,63]. Botox injections remain controversial and may pose some safety concerns [61,64]. There is anecdotal evidence for IVIG improving dysphagia in IBM [65,66]. Most importantly, regular evaluation by a speech and swallow therapist would ensure proper dietary modification to avoid aspiration.
Neuromuscular respiratory insufficiency usually occurs at advanced disease stages. Screening for and managing respiratory involvement is crucial. Patients often need referral to pulmonary and/or sleep medicine specialists who can assist with initiation and management of noninvasive ventilation in the appropriate setting [24].
The biggest challenge facing treatment of IBM to date is the lack of disease-modifying therapy. Over 20 unique drug therapies or combinations have been studied in IBM as summarized in Table 2 and further detailed below.
Table 2.
Pharmacologic clinical trials in inclusion body myositis (IBM)
| First author, year | Drug | Mechanism | Primary outcome measure(s) | Study design |
| Witting, 2022 [64] | Botox | Neuromuscular junction blockade | Dysphagia questionnaire, time cold-water swallow test, and subjective dysphagia status | Pilot study |
| Machado, 2022 [85] | Arimoclomol | Amplification of heat shock protein expression | IBMFRS | Phase 2/3 |
| Coudert, 2022 [80] | Testosterone + exercise | Anti-inflammatory and increases muscle mass | Blood biomarkers | Pilot study |
| Benveniste, 2021 [81] | Sirolimus | mTOR inhibitor | Maximal voluntary isometric knee extension strength | Phase 2b |
| Amato, 2021 [84] | Bimagrumab | Activin type 2 receptor-targeted monoclonal antibody | 6MWT and safety | Phase 2b |
| Kosmidis, 2019 [76] | Canakinumab | IL-1beta-targeted monoclonal antibody | QMT | Pilot study |
| Mendell, 2017 [83] | Follistatin gene therapy | Increases muscle mass | 6MWT | Pilot study |
| Schmidt, 2016 [75] | Alemtuzumab | CD52-targeted monoclonal antibody | Muscle tissue biomarkers | Pilot study |
| Saperstein, 2016 [74] | Natalizumab | Monoclonal antibody that targets the α4 chain (CD49) of the adhesion molecules α4β1 and α4β7-integrin | MMT, QMT, and quality of life measures | Pilot study |
| Corbett, 2015 [82] | Triheptanoin | Anaplerotic therapy | 6MWT, IBMFRS, and MMT | Pilot study |
| Kosmidis, 2013 [73] | Anakinra | Interleukin-1 receptor-targeted monoclonal antibody | MMT and grip strength | Pilot study |
| Sancricca, 2011 [79)] | Simvastatin | Potential immunomodulatory and anti-inflammatory | IBMFRS and assessment tools proposed by International Myositis Assessment Collaborative Study group | Pilot study |
| Sultan, 2008 [72] | Rituximab | CD20-targeted monoclonal antibody | QMT and CK level | Pilot study |
| Dastmalchi, 2008 [71] | Infliximab | TNF alpha-targeted monoclonal antibody | Myositis disease activity score and MRI | Pilot study |
| Barohn, 2006 [77] | Etanercept | Soluble TNF alpha receptor antagonist | QMT | Pilot study |
| Muscle study group, 2004 [70] | Avenox | INFbeta-1a-targeted monoclonal antibody | Safety and tolerability | Pilot study |
| Lindberg, 2003 [128] | Methotrexate and ATG anti-T-lymphocyte globulin | Broad immunosuppression | QMT | Pilot study |
| Rutkove, 2002 [78] | Oxandrolone | Anti-inflammatory and potentially immunomodulatory | MVICT, MMT, and functional performance testing | Pilot study |
| Badrising, 2002 [69] | Methotrexate | Broad immunosuppressant | QMT | Pilot study |
| Dalakas, 2001 [68] | IVIG + prednisone | Immunomodulation (IVIG) and broad immunosuppression (prednisone) | QMT and MRC | Pilot study |
| Walter, 2000 [66] | IVIG | Immunomodulation | MMT, NSS, patient assessment, outstretched arm time, and EMG | Pilot study |
| Leff, 1993 [67] | Azathioprine + methotrexate | Broad immunosuppression | MMT, activities of daily living questionnaire, and serum muscle associated enzymes | Pilot study |
6MWT, 6 min walk test; CK, creatine kinase; EMG, electromyography; IBMFRS, inclusion body myositis functional rating scale; MMT, manual muscle testing; MRC, medical research council; MRI, magnetic resonance imaging; MVICT, maximal voluntary isometric contraction testing; NSS, neuromuscular symptom score; QMT, quantitative muscle testing.
Immunosuppressive/immunomodulatory therapies included azathioprine, methotrexate, IVIG, IVIG + prednisone, interferon beta 1a, etanercept, infliximab, anakinra, natalizumab, alemtuzumab, and canakimumab [66–77]. Drugs with pleiomorphic mechanism of action, including an anti-inflammatory effects, such as sirolimus, oxandrolone, simvastatin, and more recently testosterone in tandem with exercise training have also been tried [78–80]. Sirolimus inhibits mTOR and has pleiotropic effects on cell metabolism, autophagy, and mitochondrial function [81]. Additional investigational treatments included drugs to increase muscle mass such as follistatin gene therapy and bimagrumab, and arimoclomol addressed protein homeostasis by prolonging the activation of Heat Shock Factor-1 augmenting heat shock protein levels [82–85]. Currently, randomized controlled trials for sirolimus (clinical trial NCT04789070) and ABC008 a monoclonal antibody that selectively depletes cytotoxic T cells (clinical trial NCT05721573) are underway with highly anticipated results.
In contrast, while pharmacologic therapies have had little to no benefit in IBM, several nonpharmacologic strategies have had successes [24]. Blood flow restricted resistance training for 12 weeks in IBM patients increased muscle strength on testing indicating specific strength training exercises may be beneficial for this group of patients [86]. Other exercises programs including community exercise and home exercise programs have also increased patient exercise capacity and preserved muscle function in IBM [87,88]. Medical devices such as cyborg hybrid assistive limb therapy and selective ankle foot orthoses have also improved ambulatory function in IBM [89,90]. An expiratory muscle strength training device to improve swallowing has also been considered [91]. Additionally, self-management training and shared medical appointments have improved social endurance and healthcare quality of life [92,93]. However, current standard-of-care guidelines in IBM are lacking.
TRIAL OUTCOME MEASURES
Six outcome measures have been used as primary and several others as secondary in IBM clinical trials [94▪]. The primary outcome measures included the 6-min walk distance (6MWD), IBMFRS, quantitative muscle testing (QMT) and maximal voluntary isometric contraction testing (MVICT), manual muscle testing (MMT), and thigh muscle volume measured by MRI. Each of these measures has its own challenges, and there is no currently available outcome measure validated in IBM with content able to capture all the aspects of the disease and reflect bulbar, upper limb, and lower limb function.
For instance, the 6-min walk distance test was designed to assess exercise capacity in pulmonary diseases and may be influenced by other nonneuromuscular conditions [95]. It also has a major flooring effect and cannot be used in IBM patients who have already lost ambulation [94▪]. Muscle strength testing, manually or via dynamometry, lacks standardization with marked intra- and inter-rater variability. In contrast, the IBMFRS has better content than others capturing multiple domains of disease severity [96]. This scale has been validated in IBM [97,98]. A recent study has confirmed that the intrarater and interrater reliability for scoring using IBMFRS is high with intraclass correlation coefficients for video ratings >0.9 [99▪]. However, the IBMFRS is a clinician-administered questionnaire, not designed according to patient-reported outcome measures standards, and a relatively significant change in function is needed in order to achieve a one point change on the scale. There is a critical need to develop standardized, validated outcome measure in IBM and novel patient reported outcomes (PROs) according to FDA PRO guidelines [100]. Additionally, surrogate outcome measures may play a crucial role in monitoring progression and evaluating treatment efficacy. Quantitative muscle MRI has proven valuable in quantifying muscle loss over time, while correlating with decline in physical performance [101▪▪]. However, establishing the minimal clinically important difference is yet to be determined.
From a clinical trial design perspective, a balance between cost and having a long enough trial is needed to avoid type II errors. While the main reason for failed trials could solely be the lack of effectiveness of the investigated drug, the duration of several trials was 6 month or less which is suboptimal in such a chronic, slowly progressive disease [66,102,103]. Furthermore, given the rarity of IBM, stratifying groups by variables of interest (sex, race, distribution of weakness, disease severity etc.) would make such trials not feasible as stratification should be accounted for during sample size calculation as it requires penalization of the p-value. Platform trials offer the advantage of sharing a placebo arm and testing several investigational medicine products simultaneously, which improves recruitment and saves costs. On the downside, any design flaw would impact several trials.
Additional limitations of clinical trials exist. Evaluation of the outcome measures used in clinical trials is often based on averaged estimates, such as mean difference and standard deviations, which may not fully capture the individual-level effect. Furthermore, the clinical trial setting is different from real life scenarios, and the placebo group's rate of decline may not align with natural history studies. Innovative designs to address these limitations have been proposed, including pragmatic trials and n-of-1 trials, both of which have their challenges not addressed in this article. The intervention in pragmatic trials is embedded in healthcare system workflow, and data are collected from electronic health records in routine clinical visits [104]. The n-of-1 clinical trial design is the closest to the concept of individualized medicine. It uses the same methodology of parallel group trials to ensure scientific rigor while generating clinically relevant treatment outcomes tailored to the patient involved [105,106].
CONCLUSION
In conclusion, inclusion body myositis (IBM) is a chronic progressive muscle disease with an unclear underlying cause. Despite its significant impact on patient morbidity and mortality, there is currently no recognized cure or disease-modifying therapy available for IBM. Clinicians, patients, and scientists face numerous challenges in the treatment of this disease including the variable clinical phenotypes at presentation and difficulties in early-stage diagnosis. A major obstacle in addressing IBM lies in the limited understanding of its pathogenesis, which in turn limits the development of targeted treatment therapies. Additionally, innovative trial outcome measures and designs are likely needed to facilitate the development of more effective treatments.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Callan A, Capkun G, Vasanthaprasad V, et al. A Systematic review and meta-analysis of prevalence studies of sporadic inclusion body myositis. J Neuromuscul Dis 2017; 4:127–137. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter S, Karpati G, Heller I, Eisen A. Inclusion body myositis: a distinct variety of idiopathic inflammatory myopathy. Neurology 1978; 28:8–17. [DOI] [PubMed] [Google Scholar]
- 3▪.Naddaf E, Shelly S, Mandrekar J, et al. Survival and associated comorbidities in inclusion body myositis. Rheumatology (Oxford) 2022; 61:2016–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]; A population-based study demonstrating decreased survival in patients with inclusion body myositis compared to population controls. Peripheral neuropathy, Sjogren syndrome and T-cell large granulocytic leukemia were more common in IBM group than population controls.
- 4.Leclair V, Lundberg IE. Recent clinical trials in idiopathic inflammatory myopathies. Curr Opin Rheumatol 2017; 29:652–659. [DOI] [PubMed] [Google Scholar]
- 5.Lotz BP, Engel AG, Nishino H, et al. Inclusion body myositis. Observations in 40 patients. Brain 1989; 112 (Pt 3):727–747. [DOI] [PubMed] [Google Scholar]
- 6.Sayers ME, Chou SM, Calabrese LH. Inclusion body myositis: analysis of 32 cases. J Rheumatol 1992; 19:1385–1389. [PubMed] [Google Scholar]
- 7.Badrising UA, Maat-Schieman ML, van Houwelingen JC, et al. Inclusion body myositis. Clinical features and clinical course of the disease in 64 patients. J Neurol 2005; 252:1448–1454. [DOI] [PubMed] [Google Scholar]
- 8.Naddaf E, Barohn RJ, Dimachkie MM. Inclusion body myositis: update on pathogenesis and treatment. Neurother 2018; 15:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9▪.Alamr M, Pinto MV, Naddaf E. Atypical presentations of inclusion body myositis: clinical characteristics and long-term outcomes. Muscle Nerve 2022; 66:686–693. [DOI] [PubMed] [Google Scholar]; This study systematically evaluated the atypical presentations of IBM beyond finger flexion and knee extension weakness. Atypical presentations occurred in 14% of IBM patients with isolated dysphagia being the most common.
- 10.Salam S, Morrow JM, Howard R, et al. Two emerging phenotypes of atypical inclusion body myositis: illustrative cases. Clin Exp Rheumatol 2023; 41:340–347. [DOI] [PubMed] [Google Scholar]
- 11.Needham M, Mastaglia FL. Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet neurol 2007; 6:620–631. [DOI] [PubMed] [Google Scholar]
- 12.Phillips BA, Zilko PJ, Mastaglia FL. Prevalence of sporadic inclusion body myositis in Western Australia. Muscle Nerve 2000; 23:970–972. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese LH, Mitsumoto H, Chou SM. Inclusion body myositis presenting as treatment-resistant polymyositis. Arthritis Rheum 1987; 30:397–403. [DOI] [PubMed] [Google Scholar]
- 14.Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med 1991; 325:1487–1498. [DOI] [PubMed] [Google Scholar]
- 15.Mastaglia FL, Phillips BA. Idiopathic inflammatory myopathies: epidemiology, classification, and diagnostic criteria. Rheum Dis Clin North Am 2002; 28:723–741. [DOI] [PubMed] [Google Scholar]
- 16.Tawil R, Griggs RC. Inclusion body myositis. Curr Opin Rheumatol 2002; 14:653–657. [DOI] [PubMed] [Google Scholar]
- 17.Griggs RC, Askanas V, DiMauro S, et al. Inclusion body myositis and myopathies. Ann Neurol 1995; 38:705–713. [DOI] [PubMed] [Google Scholar]
- 18.Hilton-Jones D, Miller A, Parton M, et al. Inclusion body myositis. MRC centre for neuromuscular diseases, IBM workshop, London, 13 June 2008. Neuromuscul Disord 2010; 20:142–147. [DOI] [PubMed] [Google Scholar]
- 19.Benveniste O, Hilton-Jones D. International workshop on inclusion body myositis held at the institute of myology, Paris, on 29 May 2009. Neuromuscul Disord 2010; 20:414–421. [DOI] [PubMed] [Google Scholar]
- 20.Rose MR, Group EIW. 188th ENMC international workshop: inclusion body myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord 2013; 23:1044–1055. [DOI] [PubMed] [Google Scholar]
- 21.Verschuuren JJ, Badrising UA, Wintzen AR. Emery AEH, et al. Inclusion body myositis. Diagnostic Criteria for Neuromuscular Disorders. London: Royal Society of Medicine Press, European Neuromuscular Centre; 1997. 81–84. [Google Scholar]
- 22.Badrising UA, Maat-Schieman M, van Duinen SG, et al. Epidemiology of inclusion body myositis in the Netherlands: a nationwide study. Neurology 2000; 55:1385–1387. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd TE, Mammen AL, Amato AA, et al. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology 2014; 83:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naddaf E. Inclusion body myositis: update on the diagnostic and therapeutic landscape. Front Neurol 2022; 13:1020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alhammad RM, Naddaf E. Myopathies presenting with head drop: clinical spectrum and treatment outcomes. Neuromuscul Disord 2020; 30:128–136. [DOI] [PubMed] [Google Scholar]
- 26.Hart MG, Santarius T, Trivedi RA. Muscle and nerve biopsy for the neurosurgical trainee. Br J Neurosurg 2013; 27:727–734. [DOI] [PubMed] [Google Scholar]
- 27.Broccolini A, Mirabella M. Hereditary inclusion-body myopathies. Biochim Biophys Acta 2015; 1852:644–650. [DOI] [PubMed] [Google Scholar]
- 28.Selcen D. Myofibrillar myopathies. Neuromuscul Disord 2011; 21:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto MV, Laughlin RS, Klein CJ, et al. Inclusion body myositis: correlation of clinical outcomes with histopathology, electromyography and laboratory findings. Rheumatology (Oxford) 2022; 61:2504–2511. [DOI] [PubMed] [Google Scholar]
- 30.Dimachkie MM, Barohn RJ. Inclusion body myositis. Curr Neurol Neurosci Rep 2013; 13:321. [DOI] [PubMed] [Google Scholar]
- 31.Tasca G, Monforte M, De Fino C, et al. Magnetic resonance imaging pattern recognition in sporadic inclusion-body myositis. Muscle Nerve 2015; 52:956–962. [DOI] [PubMed] [Google Scholar]
- 32.Zubair AS, Salam S, Dimachkie MM, et al. Imaging biomarkers in the idiopathic inflammatory myopathies. Front Neurol 2023; 14:1146015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albayda J, van Alfen N. Diagnostic value of muscle ultrasound for myopathies and myositis. Curr Rheumatol Rep 2020; 22:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pluk H, van Hoeve BJ, van Dooren SH, et al. Autoantibodies to cytosolic 5’-nucleotidase 1A in inclusion body myositis. Ann Neurol 2013; 73:397–407. [DOI] [PubMed] [Google Scholar]
- 35.Larman HB, Salajegheh M, Nazareno R, et al. Cytosolic 5’-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol 2013; 73:408–418. [DOI] [PubMed] [Google Scholar]
- 36.Ikenaga C, Findlay AR, Goyal NA, et al. Clinical utility of anticytosolic 5’-nucleotidase 1A antibody in idiopathic inflammatory myopathies. Ann Clin Transl Neurol 2021; 8:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbert MK, Stammen-Vogelzangs J, Verbeek MM, et al. Disease specificity of autoantibodies to cytosolic 5’-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Ann Rheum Dis 2016; 75:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amlani A, Choi MY, Tarnopolsky M, et al. Anti-NT5c1A autoantibodies as biomarkers in inclusion body myositis. Front Immunol 2019; 10:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mammen AL. Inclusion body myositis: autoimmune or myodegenerative disease? Neurology 2022; 98:521–522. [DOI] [PubMed] [Google Scholar]
- 40.Snedden AM, Kellett KAB, Lilleker JB, et al. The role of protein aggregation in the pathogenesis of inclusion body myositis. Clin Exp Rheumatol 2022; 40:414–424. [DOI] [PubMed] [Google Scholar]
- 41.Askanas V, Engel WK. Inclusion-body myositis, a multifactorial muscle disease associated with aging: current concepts of pathogenesis. Curr Opin Rheumatol 2007; 19:550–559. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol 2019; 15:257–272. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt J, Rakocevic G, Raju R, Dalakas MC. Upregulated inducible co-stimulator (ICOS) and ICOS-ligand in inclusion body myositis muscle: significance for CD8+ T cell cytotoxicity. Brain 2004; 127 (Pt 5):1182–1190. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg SA, Pinkus JL, Kong SW, et al. Highly differentiated cytotoxic T cells in inclusion body myositis. Brain 2019; 142:2590–2604. [DOI] [PubMed] [Google Scholar]
- 45.Goel N, Needham M, Soler-Ferran D, et al. Depletion of klrg1+ T cells in a first-in-human clinical trial of ABC008 in inclusion body myositis (IBM). Ann Rheum Dis 2022; 81: (Suppl 1): 1008–1009. [Google Scholar]
- 46.Weihl CC, Miller SE, Hanson PI, Pestronk A. Transgenic expression of inclusion body myopathy associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum Mol Genet 2007; 16:919–928. [DOI] [PubMed] [Google Scholar]
- 47.Nalbandian A, Llewellyn KJ, Badadani M, et al. A progressive translational mouse model of human valosin-containing protein disease: the VCP(R155H/+) mouse. Muscle Nerve 2013; 47:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugarman MC, Yamasaki TR, Oddo S, et al. Inclusion body myositis-like phenotype induced by transgenic overexpression of beta APP in skeletal muscle. Proc Natl Acad Sci USA 2002; 99:6334–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rebolledo DL, Minniti AN, Grez PM, et al. Inclusion body myositis: a view from the caenorhabditis elegans muscle. Mol Neurobiol 2008; 38:178–198. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Ghribi O, Geiger JD. Rabbits fed cholesterol-enriched diets exhibit pathological features of inclusion body myositis. Am J Physiol Regul Integr Comp Physiol 2008; 294:R829–R835. [DOI] [PubMed] [Google Scholar]
- 51▪▪.Britson KA, Ling JP, Braunstein KE, et al. Loss of TDP-43 function and rimmed vacuoles persist after T cell depletion in a xenograft model of sporadic inclusion body myositis. Sci Transl Med 2022; 14:eabi9196. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors reported nuclear clearance and cytoplasmic aggregation of TDP-43 in muscle biopsies from patients with IBM. Additionally, the study described a xenograft mouse model for IBM that recapitulated all the histopathological features of the disease. Furthermore, rimmed vacuoles and loss of TDP-43 function persisted after T cell reduction within IBM xenogragts.
- 52.Allenbach Y, Solly S, Gregoire S, et al. Role of regulatory T cells in a new mouse model of experimental autoimmune myositis. Am J Pathol 2009; 174:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ascherman DP. Animal models of inflammatory myopathy. Curr Rheumatol Rep 2012; 14:257–263. [DOI] [PubMed] [Google Scholar]
- 54.Morrison TE, Fraser RJ, Smith PN, et al. Complement contributes to inflammatory tissue destruction in a mouse model of Ross River virus-induced disease. J Virol 2007; 81:5132–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandager MM, Nugent JL, Schulz WL, et al. Interactions between multiple genetic determinants in the 5’ UTR and VP1 capsid control pathogenesis of chronic postviral myopathy caused by coxsackievirus B1. Virology 2008; 372:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katsumata Y, Harigai M, Sugiura T, et al. Attenuation of experimental autoimmune myositis by blocking ICOS-ICOS ligand interaction. J Immunol 2007; 179:3772–3779. [DOI] [PubMed] [Google Scholar]
- 57.Shelly S, Mielke MM, Mandrekar J, et al. Epidemiology and natural history of inclusion body myositis: a 40-year population-based study. Neurology 2021; 96:e2653–e2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox FM, Titulaer MJ, Sont JK, et al. A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 2011; 134 (Pt 11):3167–3175. [DOI] [PubMed] [Google Scholar]
- 59.Sangha G, Yao B, Lunn D, et al. Longitudinal observational study investigating outcome measures for clinical trials in inclusion body myositis. J Neurol Neurosurg Psychiatry 2021; 13:13. [DOI] [PubMed] [Google Scholar]
- 60.Molberg O, Dobloug C. Epidemiology of sporadic inclusion body myositis. Curr Opin Rheumatol 2016; 28:657–660. [DOI] [PubMed] [Google Scholar]
- 61.Ambrocio KR, Garand KLF, Roy B, et al. Diagnosing and managing dysphagia in inclusion body myositis: a systematic review. Rheumatology (Oxford) 2023; 00:1–18. [DOI] [PubMed] [Google Scholar]
- 62.Hoesseini A, Honings J, Taus-Mohamedradja R, et al. Outcomes of endoscopic cricopharyngeal myotomy with CO2 laser surgery: a retrospective study of 47 patients. Head Neck 2016; 38:1022–1027. [DOI] [PubMed] [Google Scholar]
- 63.Murata KY, Kouda K, Tajima F, Kondo T. Balloon dilation in sporadic inclusion body myositis patients with Dysphagia. Clin Med Insights Case Rep 2013; 6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witting N, Daugaard D, Prytz S, et al. Botulinum toxin treatment improves dysphagia in patients with oculopharyngeal muscular dystrophy and sporadic inclusion body myositis. J Neurol 2022; 269:4154–4160. [DOI] [PubMed] [Google Scholar]
- 65.Dalakas MC, Sonies B, Dambrosia J, et al. Treatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled study. Neurology 1997; 48:712–716. [DOI] [PubMed] [Google Scholar]
- 66.Walter MC, Lochmuller H, Toepfer M, et al. High-dose immunoglobulin therapy in sporadic inclusion body myositis: a double-blind, placebo-controlled study. J Neurol 2000; 247:22–28. [DOI] [PubMed] [Google Scholar]
- 67.Leff RL, Miller FW, Hicks J, et al. The treatment of inclusion body myositis: a retrospective review and a randomized, prospective trial of immunosuppressive therapy. Medicine (Baltimore) 1993; 72:225–235. [DOI] [PubMed] [Google Scholar]
- 68.Dalakas MC, Koffman B, Fujii M, et al. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM. Neurology 2001; 56:323–327. [DOI] [PubMed] [Google Scholar]
- 69.Badrising UA, Maat-Schieman MLC, Ferrari MD, et al. Comparison of weakness progression in inclusion body myositis during treatment with methotrexate or placebo. Ann Neurol 2002; 51:369–372. [DOI] [PubMed] [Google Scholar]
- 70.Muscle Study G. Randomized pilot trial of high-dose betaINF-1a in patients with inclusion body myositis. Neurology 2004; 63:718–720. [DOI] [PubMed] [Google Scholar]
- 71.Dastmalchi M, Grundtman C, Alexanderson H, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis 2008; 67:1670–1677. [DOI] [PubMed] [Google Scholar]
- 72.Sultan SM, Ng KP, Edwards JCW, et al. Clinical outcome following B cell depletion therapy in eight patients with refractory idiopathic inflammatory myopathy. Clin Exp Rheumatol 2008; 26:887–893. [PubMed] [Google Scholar]
- 73.Kosmidis ML, Alexopoulos H, Tzioufas AG, Dalakas MC. The effect of anakinra, an IL1 receptor antagonist, in patients with sporadic inclusion body myositis (sIBM): a small pilot study. J Neurol Sci 2013; 334 (1–2):123–125. [DOI] [PubMed] [Google Scholar]
- 74.Saperstein D, Levine T. Interim analysis of a pilot trial of natalizumab in inclusion body myositis. Neurology Conference: 68th American Academy of Neurology Annual Meeting, AAN 2016; 86: (Suppl. 1): [Google Scholar]
- 75.Schmidt K, Kleinschnitz K, Rakocevic G, et al. Molecular treatment effects of alemtuzumab in skeletal muscles of patients with IBM. BMC Neurol 2016; 16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kosmidis ML, Pikazis D, Vlachoyiannopoulos P, et al. Trial of canakinumab, an IL-1beta receptor antagonist, in patients with inclusion body myositis. Neurol Neuroimmunol Neuroinflamm 2019; 6:e581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barohn RJ, Herbelin L, Kissel JT, et al. Pilot trial of etanercept in the treatment of inclusion-body myositis. Neurology 2006; 66: (Suppl 1): S123–S124. [DOI] [PubMed] [Google Scholar]
- 78.Rutkove SB, Parker RA, Nardin RA, et al. A pilot randomized trial of oxandrolone in inclusion body myositis. Neurology 2002; 58:1081–1087. [DOI] [PubMed] [Google Scholar]
- 79.Sancricca C, Mora M, Ricci E, et al. Pilot trial of simvastatin in the treatment of sporadic inclusion-body myositis. Neurol Sci 2011; 32:841–847. [DOI] [PubMed] [Google Scholar]
- 80.Coudert JD, Slater N, Sooda A, et al. Immunoregulatory effects of testosterone supplementation combined with exercise training in men with inclusion body myositis: a double-blind, placebo-controlled, cross-over trial. Clin 2022; 11:e1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benveniste O, Hogrel JY, Belin L, et al. Sirolimus for treatment of patients with inclusion body myositis: a randomised, double-blind, placebo-controlled, proof-of-concept, phase 2b trial. Lancet Rheumatol 2021; 3:e40–e48. [DOI] [PubMed] [Google Scholar]
- 82.Corbett AJ, Garg N, Crosbie A, Borges K. A pilot study of triheptanoin treatment in sporadic inclusion body myositis (SIBM). Ann Neurol 2015; (19):S102. [Google Scholar]
- 83.Mendell JR, Sahenk Z, Al-Zaidy S, et al. Follistatin gene therapy for sporadic inclusion body myositis improves functional outcomes. Mol Ther 2017; 25:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amato AA, Hanna MG, Machado PM, et al. Efficacy and safety of bimagrumab in sporadic inclusion body myositis: long-term extension of RESILIENT. Neurology 2021; 96:e1595–e1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Machado P, Dimachkie M, Barohn R, et al. Phase 2/3 study of arimoclomol in IBM. J 2022; 23: (Suppl): S8. [Google Scholar]
- 86.Jorgensen AN, Jensen KY, Nielsen JL, et al. Effects of blood-flow restricted resistance training on mechanical muscle function and thigh lean mass in sIBM patients. Scand J Med Sci Sports 2022; 32:359–371. [DOI] [PubMed] [Google Scholar]
- 87.Wallace A, Pietrusz A, Dewar E, et al. Evaluating the benefits of community based aerobic training on the physical health and well being of people with neuromuscular disease. Muscle and Nerve 2016; 54: (Suppl 1): S5.27588585 [Google Scholar]
- 88.Arnardottir S, Alexanderson H, Lundberg IE, Borg K. Sporadic inclusion body myositis: pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reaction. J Rehabil Med 2003; 35:31–35. [DOI] [PubMed] [Google Scholar]
- 89.Nakajima T, Sankai Y, Takata S, et al. Cybernic treatment with wearable cyborg Hybrid Assistive Limb (HAL) improves ambulatory function in patients with slowly progressive rare neuromuscular diseases: a multicentre, randomised, controlled crossover trial for efficacy and safety (NCY-3001). Orphanet J Rare Dis 2021; 16:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernhardt K, Oh T, Kaufman K. Stance control orthosis trial in patients with inclusion body myositis. Prosthet Orthot Int 2011; 35:39–44. [DOI] [PubMed] [Google Scholar]
- 91.Mohannak N, Pattison G, Radich B, et al. Exploring the efficacy of the expiratory muscle strength trainer to improve swallowing in inclusion body myositis: a pilot study. Neuromuscul Disord 2020; 30:294–300. [DOI] [PubMed] [Google Scholar]
- 92.Veenhuizen Y, Cup EHC, Groothuis JT, et al. Effectiveness and cost-effectiveness of a self-management group program to improve social participation in patients with neuromuscular disease and chronic fatigue: protocol of the Energetic study. BMC Neurol 2015; 15: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seesing FM, Drost G, Groenewoud J, et al. Shared medical appointments improve QOL in neuromuscular patients: a randomized controlled trial. Neurology 2014; 83:240–246. [DOI] [PubMed] [Google Scholar]
- 94▪.Roy B, Lucchini M, Lilleker JB, et al. Current status of clinical outcome measures in inclusion body myositis: a systematised review. Clin Exp Rheumatol 2023; 41:370–378. [DOI] [PubMed] [Google Scholar]; This systematized review summarized the outcome measures used in IBM and discusses their limitations.
- 95.Agarwala P, Salzman SH. Six-minute walk test: clinical role, technique, coding, and reimbursement. Chest 2020; 157:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dimachkie MM, Barohn RJ. Inclusion body myositis. Neurol Clin 2014; 32:629–646. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goyal NA, Greenberg SA, Cauchi J, et al. Correlations of disease severity outcome measures in inclusion body myositis. Neuromuscul Disord 2022; 32:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jackson CE, Barohn RJ, Gronseth G, et al. Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve 2008; 37:473–476. [DOI] [PubMed] [Google Scholar]
- 99▪.Symonds T, Randall J, Lloyd-Price L, et al. Study to assess content validity and interrater and intrarater reliability of the inclusion body myositis functional rating scale. Neurol Clin Pract 2023; 13:e200168. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study evaluated the validity and reliability of the Inclusion Body Myositis Functional Rating Scale and reported sound content validity and interrater and intrarater reliability.
- 100.Health USDo, Human Services FDACfDE, Research, et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006; 4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101▪▪.Laurent D, Riek J, Sinclair CDJ, et al. Longitudinal changes in MRI muscle morphometry and composition in people with inclusion body myositis. Neurology 2022; 99:e865–e876. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study quantified the longitudinal changes in muscle MRI in patients with IBM and demonstrated the correlation of MRI changes with physical performance, supporting he use of muscle MRI as a surrogate outcome measure in clinical trials of IBM.
- 102.Dalakas MC, Sonies B, Koffman B, et al. High-dose intravenous immunoglobulin (IVIg) combined with Prednisone in the treatment of patients with inclusion-body myositis (IBM): a double-blind, randomised controlled trial. Neurology 1997; 48:A332. [Google Scholar]
- 103.Amato AA, Sivakumar K, Goyal N, et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology 2014; 83:2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016; 375:454–463. [DOI] [PubMed] [Google Scholar]
- 105.Lillie EO, Patay B, Diamant J, et al. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med 2011; 8:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fountzilas E, Tsimberidou AM, Vo HH, Kurzrock R. Clinical trial design in the era of precision medicine. Genome Med 2022; 14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Larue S, Maisonobe T, Benveniste O, et al. Distal muscle involvement in granulomatous myositis can mimic inclusion body myositis. J Neurol Neurosurg Psychiatry 2011; 82:674–677. [DOI] [PubMed] [Google Scholar]
- 108.Miyazaki M, Mori-Yoshimura M, Yamamoto T, et al. Chronic sarcoid myopathy mimicking sporadic inclusion body myositis. Clin Neurol Neurosurg 2019; 182:84–86. [DOI] [PubMed] [Google Scholar]
- 109.Kleefeld F, Uruha A, Schanzer A, et al. Morphologic and molecular patterns of polymyositis with mitochondrial pathology and inclusion body myositis. Neurology 2022; 99:e2212–e2222. [DOI] [PubMed] [Google Scholar]
- 110.Smestad C, Monstad P, Lindboe CF, Mygland A. Amyloid myopathy presenting with distal atrophic weakness. Muscle Nerve 2004; 29:605–609. [DOI] [PubMed] [Google Scholar]
- 111.Bouchard JP, Cossette L, Bassez G, Puymirat J. Natural history of skeletal muscle involvement in myotonic dystrophy type 1: a retrospective study in 204 cases. J Neurol 2015; 262:285–293. [DOI] [PubMed] [Google Scholar]
- 112.Garibaldi M, Nicoletti T, Bucci E, et al. Muscle magnetic resonance imaging in myotonic dystrophy type 1 (DM1): Refining muscle involvement and implications for clinical trials. Eur J Neurol 2022; 29:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heskamp L, van Nimwegen M, Ploegmakers MJ, et al. Lower extremity muscle pathology in myotonic dystrophy type 1 assessed by quantitative MRI. Neurology 2019; 92:e2803–e2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Day JW, Ricker K, Jacobsen JF, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology 2003; 60:657–664. [DOI] [PubMed] [Google Scholar]
- 115.Fatehi F, Nafissi S, Urtizberea JA, et al. Dysferlinopathy in Iran: clinical and genetic report. J Neurol Sci 2015; 359:256–259. [DOI] [PubMed] [Google Scholar]
- 116.Diaz-Manera J, Fernandez-Torron R, J. LL, et al. Muscle MRI in patients with dysferlinopathy: pattern recognition and implications for clinical trials. J Neurol Neurosurg Psychiatry 2018; 89:1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beltran Papsdorf T, Howard JF, Jr, Chahin N. Late-onset Becker muscular dystrophy: refining the clinical features and electrophysiological findings. Muscle Nerve 2015; 52:885–887. [DOI] [PubMed] [Google Scholar]
- 118.Duff RM, Tay V, Hackman P, et al. Mutations in the N-terminal actin-binding domain of filamin C cause a distal myopathy. Am J Hum Genet 2011; 88:729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berardo A, Lornage X, Johari M, et al. HNRNPDL-related muscular dystrophy: expanding the clinical, morphological and MRI phenotypes. J Neurol 2019; 266:2524–2534. [DOI] [PubMed] [Google Scholar]
- 120.Peyer AK, Kinter J, Hench J, et al. Novel valosin containing protein mutation in a Swiss family with hereditary inclusion body myopathy and dementia. Neuromuscul Disord 2013; 23:149–154. [DOI] [PubMed] [Google Scholar]
- 121.Haghighi A, Nafissi S, Qurashi A, et al. Genetics of GNE myopathy in the non-Jewish Persian population. Eur J Hum Genet 2016; 24:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reilich P, Schoser B, Schramm N, et al. The p.G154S mutation of the alpha-B crystallin gene (CRYAB) causes late-onset distal myopathy. Neuromuscul Disord 2010; 20:255–259. [DOI] [PubMed] [Google Scholar]
- 123.Penisson-Besnier I, Talvinen K, Dumez C, et al. Myotilinopathy in a family with late onset myopathy. Neuromuscul Disord 2006; 16:427–431. [DOI] [PubMed] [Google Scholar]
- 124.Roda RH, Schindler AB, Blackstone C, et al. Laing distal myopathy pathologically resembling inclusion body myositis. Ann Clin Transl Neurol 2014; 1:1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bandyopadhyay S, Wicklund M, Specht CS. Novel presentation of Pompe disease: inclusion-body myositis-like clinical phenotype. Muscle Nerve 2015; 52:466–467. [DOI] [PubMed] [Google Scholar]
- 126.Nicolau S, Liewluck T, Milone M. Myopathies with finger flexor weakness: not only inclusion-body myositis. Muscle Nerve 2020; 62:445–454. [DOI] [PubMed] [Google Scholar]
- 127.Liewluck T, Niu Z, Moore SA, et al. ACTA1-myopathy with prominent finger flexor weakness and rimmed vacuoles. Neuromuscul Disord 2019; 29:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lindberg C, Trysberg E, Tarkowski A, Oldfors A. Anti-T-lymphocyte globulin treatment in inclusion body myositis: a randomized pilot study. Neurology 2003; 61:260–262. [DOI] [PubMed] [Google Scholar]