Abstract
The low overall survival rates of breast cancer (BC) patients in sub-Saharan Africa (SSA) are driven by regionally differing tumor biology, advanced tumor stages at diagnosis and limited access to therapy. However, it is not known whether regional differences in the composition of the tumor microenvironment (TME) exist and affect patients’ prognosis. In this international, multicentre cohort study, 1,237 formalin-fixed, paraffin-embedded BC samples, including samples of the ‘African Breast Cancer-Disparities in Outcomes (ABC-DO) Study’, were analyzed. The immune cell phenotypes, their spatial distribution in the TME and immune escape mechanisms of BC samples from SSA and Germany (n=117) were investigated using histomorphology, conventional and multiplex immunohistochemistry (IHC), and RNA expression analysis. The data revealed no regional differences in the number of tumor-infiltrating lymphocytes (TILs) in the 1,237 SSA BC samples, while the distribution of TILs in different BC IHC subtypes showed regional diversity, particularly when compared to German samples. Higher TIL densities were associated with better survival in the SSA cohort (n=400), but regional differences concerning the predictive value of TILs existed. High numbers of CD163+ macrophages and CD3+CD8+ T cells accompanied by reduced cytotoxicity, altered IL10 and IFNγ levels and downregulation of MHC class I components were predominantly detected in BC samples from Western SSA. Features of non-immunogenic BC phenotypes were associated with reduced patient survival (n=131). We therefore conclude that regional diversity in the distribution of BC subtypes, TME composition and immune escape mechanisms should be considered for therapy decisions in SSA and the design of personalized therapies.
Keywords: breast cancer, tumor microenvironment, survival, sub-Saharan Africa, immune escape
Introduction
Cancer is an increasing public health concern in sub-Saharan Africa (SSA), with an increase of 70% predicted by 2030, including an increase in female breast cancer (BC) (1–3). Due to diagnosis at an advanced stage, a weak health infrastructure and limited treatment options, the survival rates of BC patients in SSA are particularly low (4–6). Furthermore, in the US, BC patients of African-American ancestry have a poorer prognosis than their Caucasian counterparts, even after accounting for differences in access to healthcare (7). Despite limited information regarding BC patients from SSA, geographical diversity in BC biology in this area has been suggested (8,9). Since the SSA population is highly heterogeneous in terms of genetic variation, differences in immunological features have been proposed as predictors of the overall survival (OS) of SSA patients (10,11).
BC has been characterized as a heterogeneous disease comprising hormone receptor (HR)+Her2–, HR+Her2+, HR–Her2+ and triple-negative tumors that differ regarding their prognosis (12). Due to the heterogeneous distribution of BC subtypes in SSA, regional differences have been suggested (8,13–17). In addition, the quality of tumor processing is highly variable between countries, which may also negatively influence data interpretation (13).
The composition of the TME driven by intrinsic and extrinsic factors may also influence BC patients’ prognosis in SSA (18–20). Intrinsic immune escape strategies for BC include downregulation of major histocompatibility complex (MHC) class I and upregulation of co-inhibitory immune checkpoint molecules, such as programmed death ligand 1 (PD-L1), accompanied by reduced antitumoral immune responses and worse patient outcomes (20). In addition, the TME influences tumor progression, the response to therapy and outcomes (21). While an increased frequency of immune suppressive cells, a low frequency of CD8+ T lymphocytes and impaired cytotoxic immune responses are associated with reduced survival of BC patients, higher frequencies of tumor-infiltrating lymphocytes (TILs) are correlated with a favourable prognosis of BC (22).
Currently, whether TIL counts in BC tumors differ between ethnicities is the subject of controversial discussion. One study demonstrated only subtle differences within triple-negative tumors (23), whereas another study reported substantially higher frequencies of pro-tumorigenic, suppressive immune cells in BC patients with African-American ancestry (24). So far, to the best of our knowledge, there are no published data focusing on the biological diversity of the TME of BC from SSA. Therefore, the aim of this international, multicentre study was to decipher TME variations and immune escape strategies in 1,237 BC patient samples from 10 countries in SSA and to compare them with European BC samples and to analyse their impact on mortality.
Materials and Methods
Patients’ characteristics and study design
Formalin-fixed and paraffin-embedded (FFPE) tumor tissue blocks of 1,497 female BC patients from SSA were prospectively collected between 2006 and 2020 across 10 countries (Table 1). Samples were collected by local pathologists, the African Cancer Registry Network (AFCRN) and the International Agency for Research on Cancer (IARC). When possible, patient’s clinical data and follow-up data (n = 400) were obtained. The patients from Southern Africa (SA) and Western Africa (WA) with available survival data were part of the ABC-DO study (6), and these survival data were truncated at 3 years. Due to the lack of detailed information on staging, all tumors >5 cm or with known metastasis were defined as advanced-stage tumors. Based on non-representative tumor areas, 250 BC samples were excluded, resulting in the inclusion of 1,237 BC samples in the present study (Supplementary Figure S1). Histopathological diagnoses of BC were made according to the WHO classification of Breast Tumors 5th edition (25), including histopathological grading according Elston and Ellis. All samples were analysed by conventional immunohistochemistry (IHC) using antibodies (Ab) directed against the ER, PR, Her2 and Ki-67 (Supplementary Table S1). As a control, 117 German BC samples with similar proportions of IHC subtypes were collected at the Institute of Pathology of the Martin-Luther-University Halle-Wittenberg, Germany, 67/117 BC samples are part of the PiA-study (NCT01592825) (26). This research weas performed in accordance with the Declaration of Helsinki. All data related to clinical samples were approved by local ethical institutions, as listed in Supplementary Table S2. If possible, informed written consent for the use of samples for research purposes was obtained from patients.
Table 1:
Characteristics of BC samples analysed
| Parameters | Eastern SSA n = 444 (35.9%) | Central SSA n = 99 (8.0%) | Southern SSA n = 242 (19.6%) | Western SSA n = 452 (36.5%) | Caucasians n = 117 |
|---|---|---|---|---|---|
|
| |||||
| Countries (N) | Ethiopia (289)* | Congo (99) | Namibia (137)* | Ivory Coast (94) | Germany (117) |
| Malawi (16) | South Africa (105)* | Mali (83) | |||
| Mauritius (72) | Nigeria (275)* | ||||
| Uganda (67)* | |||||
|
| |||||
| Woman of Black Ethnicity (%) | 100% | >95% | >95% | >95% | 0% |
|
| |||||
| Mean age (range) | 46.6 (16–97) | 49.1 (27–86) | 55.5 (25–97) | 47.6 (22–91) | 64.3 (35–85) |
| Tumor size (cm) | |||||
| <5 (% of known) | 173 (64.6%) | 7 (13.2%) | 100 (42.7%) | 72 (37.9%) | 71 (60.7%) |
| >5 (% of known) | 95 (35.4%) | 46 (96.8%) | 134 (57.3%) | 118 (62.1%) | 46 (39.3%) |
| Unknown | 176 | 46 | 8 | 190 | NA |
|
| |||||
| Simplified tumor stage | |||||
| Early | 192 (43.2%) | 7 (7.0%) | 100 (41.2%) | 87 (19.2%) | 71 (60.7%) |
| Advanced | 139 (31.1%) | 46 (46.5%) | 134 (55.4%) | 168 (37.2%) | 46 (39.3%) |
| Unknown | 113 (25.5%) | 46 (46.5%) | 8 (3.3%) | 197 (43.6%) | 0 (0.0%) |
|
| |||||
| Available survival data | 139 (31.3%) | 0 (0,0%) | 192 (79.3%) | 69 (16.2%) | 0 (0.0%) |
|
| |||||
| Number of patients alive after 3 years follow-up | 80 (57.5%) | NA | 129 (67.2%) | 26 (35.6%) | NA |
|
| |||||
| Histology | |||||
| NST | 365 (82.2%) | 89 (89.8%) | 194 (80.2%) | 390 (86.2%) | 87 (74.4%) |
| non NST | 79 (17.8%) | 10 (10.2%) | 48 (19.8%) | 62 (13.8%) | 30 (25.6%) |
|
| |||||
| Grading | |||||
| G1 | 23 (5.2%) | 3 (3.0%) | 40 (16.5%) | 28 (6.2%) | 18 (15.4%) |
| G2 | 182 (41.0%) | 38 (38.4%) | 101 (41.7%) | 188 (41.6%) | 64 (54.7%) |
| G3 | 237 (53.4%) | 58 (58.6%) | 96 (41.8%) | 236 (52.2%) | 35 (29.9%) |
|
| |||||
| IHC subtype | |||||
| Luminal A-like | 132 (29.7%) | 28 (28.3%) | 89 (36.8%) | 89 (19.7%) | 72 (61,5.0%) |
| Luminal B-like | 163 (36.7%) | 37 (37.4%) | 94 (38.8%) | 113 (25.0%) | 21 (18,0%) |
| Her2+ | 48 (10.8%) | 13 (13.1%) | 17 (7.0%) | 72 (15.9%) | 7 (6.0%) |
| TNBC | 101 (22.7%) | 21 (21.2%) | 42 (17.4%) | 178 (39.4%) | 17 (14.5%) |
|
| |||||
| TILs | |||||
| Low | 210 (47.2%) | 49 (49.5%) | 140 (57.9%) | 219 (48.5%) | 60 (51.3%) |
| Intermediate | 161 (36.6%) | 41 (41.4%) | 73 (30.2%) | 182 (40.3%) | 47 (40.2%) |
| High | 73 (16.3%) | 9 (9.1%) | 29 (12.0%) | 51 (11.3%) | 10 (8.5%) |
|
| |||||
| PD-L1 expression by TILs | |||||
| Negative | 186 (41.9%) | 34 (34.3%) | 98 (40.5%) | 260 (57.5%) | 89 (76.2%) |
| Positive | 176 (39.6%) | 63 (63.6%) | 73 (30.2%) | 169 (37.4%) | 28 (23.8%) |
| Missing | 82 (18.5%) | 2 (2.1%) | 71 (29.3%) | 23 (5.1%) | 0 (0.0%) |
|
| |||||
| MHC class I expression | |||||
| HC (range) | 137.3 (0–300) | 201.1 (30–300) | 148.9 (10–300) | 113.3 (0–300) | 175.5 (10–300) |
| β2-m (range) | 166.5 (0–300) | 182.8 (0–300) | 193.0 (10–300) | 123.1 (0300) | 186.6 (40–300) |
Follow-up data were available from countries marked with an asterisk ().
Abbreviations Table 1: ß2-microglobuline, ß2-m, heavy chain, HC; human epidermal growth factor receptor 2 positive, Her2+; major histocompatibility complex, MHC; not applicable, N, number; NA; programmed death ligand-1, PD-L1; tumor-infiltrating lymphocytes, TILs; triple-negative BC, TNBC
Quality score
In order to quantify tissue quality, a quality score was developed using the following parameters: (i) general visual impression (from +1 for ‘bad’ to +5 points for ‘very good’); (ii) staining of non-neoplastic breast glands for ER (−1 or +1 point); (iii) staining of proliferating cells (internal control via stained mitosis) using an anti-Ki-67 ab (−1 or +1 point) and (iv) whether the tissue detached during the staining procedure (−1 or +1 point). Samples with scores <2 points were excluded.
Histopathology and IHC
BC subtypes were classified according to the surrogate IHC subtype classification published in 2013 (12). TILs were analysed on haematoxylin and eosin (H&E) slides as described elsewhere (27). IHC staining was performed on a Bond III automated immunostainer (Leica Biosystems Nussloch GmbH, Wetzlar, Germany) using the Bond Polymer Refine Detection Kit (DS9800-CN). Antibodies against Beta-2-microgloblin (β2-m), MHC class I heavy chain (HC), programmed death receptor (PD)-1, PD-L1, perforin, granzyme B, phosphorylated signal transducer and activator of transcription (pSTAT)1 and tapasin (tpn) (Supplementary Table S1) were applied as recommended by the manufacturer. PD-L1 testing was performed with the CAL10 antibody clone (Zytomed), which showed a good correlation with the widely used SP142 antibody clone (Ventana) (28). Immunostaining was assessed by using semi-quantitative H-scoring as described elsewhere (29).
RNA isolation and expression analysis
Prior to RNA isolation, the BC tissues were microdissected from 505 samples. Microdissection was performed by a pathologist using a Zeiss axioscope 5 microscope (Zeiss, Germany) and tumor tissue was marked with a pencil to exclude pre-existing breast parenchyma. For RNA isolation, two to four 10-μm-thick tissue slides were used. Deparaffinization of FFPE tissues was performed with 2 × xylene for 5 min, followed by incubation in 96% and 70% ethanol for 2 min each. Proteinase K digestion (Qiagen, cat#19131) was performed for up to 2 h at room temperature followed by 15 min at 80°C. RNA was isolated with a Qiagen miRNeasy FFPE Kit (Qiagen, cat#217504) according to the manufacturer’s instructions. RNA expression analysis was performed using the NanoString nCounter XT Assay according to the Hybridization protocol for the nCounter XT CodeSet Gene Expression Assay (NanoString nCounter, Seattle, WA, USA) and data analysed with the nCounter Expression Data Analysis Guide (MAN- C0011–04 from 2017). From each sample, 400 ng of FFPE-derived RNA were used. All steps were performed as described in nCounter XT Assay User Manual (MAN-10023–11 from 2016). For the analysis a custom codeset design was used and all respective genes are summerized in Supplementary Table S3. The expression levels were evaluated with the NanoStringNorm (https://github.com/sgrote/NanoStringNormalizeR/) R package.
Construction of tissue microarrays (TMAs)
FFPE tissues from 374 patients with intermediate to very high tissue quality and with sufficient material were included in TMAs containing two 0.6-mm tissue cylinders of each donor block using a manual tissue arrayer (Beecher Instruments Inc., Sun Prairie, WI, USA). Two tissue cores from the tumor centre and the invasive margin were selected and arrayed on a recipient paraffin block.
Multispectral imaging (MSI), spatial distribution analysis and immunoscoring
The frequency and localization of immune cell subpopulations and cancer cells from 374 samples was determined by MSI and is representatively shown in Figure 1. The staining procedure was performed as recently described (30). The Ab panel included CD3, CD8, FOXP3, CD20, CD163 and panCK (Supplementary Table S1). For imaging the PerkinElmer Vectra Polaris platform was employed. Cell segmentation and phenotyping were performed using the inForm software (PerkinElmer Inc., USA, Version 2.4). The frequency and spatial distribution of cell populations were analysed using an R script (https://github.com/akoyabio).
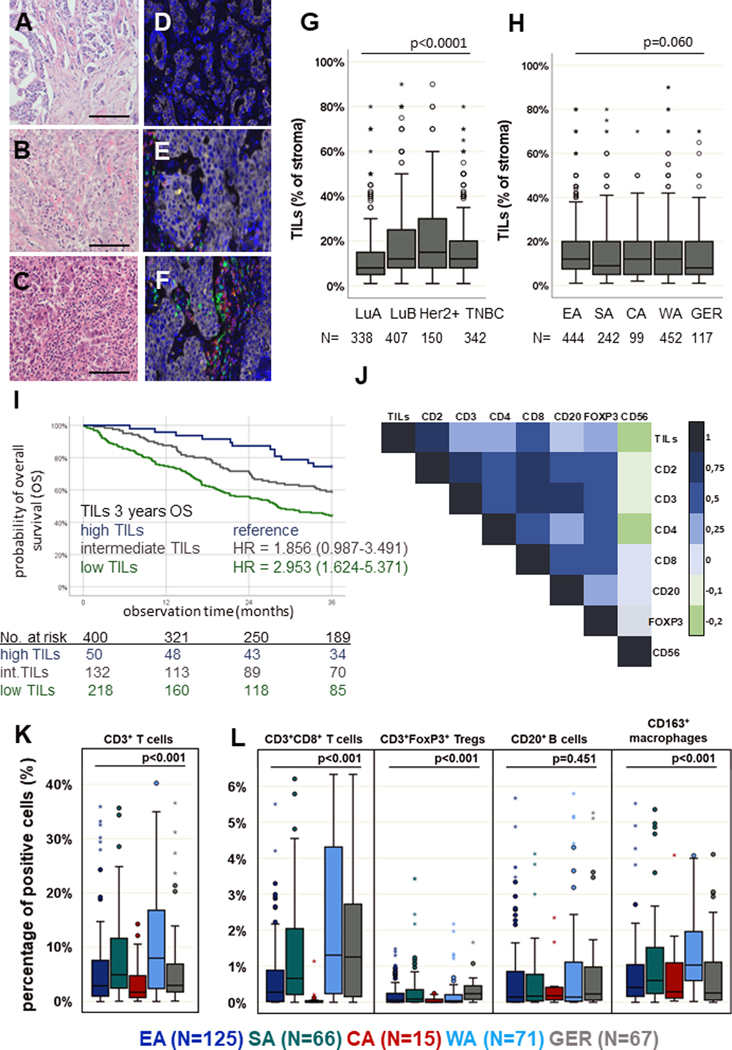
Figure 1: Tumor-infiltrating lymphocytes (TILs) and their quantitative distribution within the tumor microenvironment (TME) of BC samples.
Representative H&E staining of FFPE BC samples with low (A), intermediate (B) and high (C) infiltration of TILs. Scale bars depict 20 μm. In total, TILs were analyzed in 1,237 samples. MSI was performed on 374 samples with representatively shown pictures with low (D), intermediate (E) and high (F) infiltration of TILs. MSI allows visualization of immune cell subpopulations including CD3+CD8– T cells (yellow), CD3+CD8+ T cells (red), CD3+FOXP3+ T cells (turquoise), CD163+ macrophages (orange) and CD20+ B cells (green) as well as pan-cytokeratin+ cancer cells (grey). (G) Boxplots (line represents the mean) indicating that TILs were found to be higher in luminal-B-like (LuB), human epidermal growth factor receptor 2 positive (Her2+) and triple-negative BC (TNBC) subtypes, compared to luminal-A-like (LuA) (p < 0.0001, multivariate analysis). (H) No differences in the quantity of TILs in different regions in sub-Saharan Africa (SSA) were detected (Eastern Africa, EA; Southern Africa, SA; Central Africa, CA; Western Africa, WA), while the TIL frequency was tendentially lower in German (GER) BC samples. (I) Kaplan-Meier curve: the amounts of TILs indicates a prognostic impact with poorer survival in BC with lower numbers of TILs (p = 0.001, log-rank Test). (J) Pearson correlation map with association levels: a higher numbers of TILs are linked with increased frequencies of all immune cell subpopulations analysed. Pearson correlation coefficients are represented by different colours defined in the scale bar on the right side of the correlation map. The immune cell subpopulations analysed by MSI revealed higher numbers of CD3+ T cells (K), CD3+CD8+ T cells and CD163+ macrophages (M2) (L) in WA. No relevant difference in the frequency of CD20+ B cells was shown. However, in GER samples, the frequency of Tregs was slightly higher when compared to those in all regions of SSA. Except for the log-rank tests, all p-values were estimated in multivariate analyses and are adjusted for differences in age, grading, quality score, stage, IHC subtype and region of origin as confounding factors. All p-values are shown in the graphs.
Statistics
The Mann–Whitney U test was employed to compare clinical data. Variables originating from gene expression analyses were assessed by multivariate regression using generalized linear models (Binomial, Poisson or Gamma families, depending on the response variable) in order to estimate odds ratios (OR) and confidence intervals (CI) while adjusting for the following confounding factors: age (continuous variable, by year), grading (binary variable, grade 3 vs. 1 or 2), quality score (discrete quantitative variable, 1 to 10), stage (binary variable, advanced vs. early), IHC subtype (categorical variable) and region of origin (categorical variable). Higher OR denoted concordance between higher gene expression values and increasing variable values (age, grading, quality and stage) or as compared to a reference category (‘luminal A-like’ in the case of IHC subtype and ‘East’ in the case of Region). Missing age entries were imputed using the median age of patients of the country of origin. Otherwise, patients with missing information in any other variable were excluded from regression analyses. Multivariate regressions were performed using the statsmodels library for Python (www.statsmodels.org). Linear correlations between variables were estimated as Pearson’s correlations. Survival analyses were performed on 400 patients from SSA (recruited between 2005 and 2017, maximum follow-up time of 36 months) using Kaplan–Meier estimators and with differences calculated with log-rank tests (unadjusted) or Cox proportional hazard models (adjusted for confounding factors). Cox proportional hazard models were implemented and plotted alongside the corresponding Kaplan–Meier curves using the Lifelines library for Python (https://lifelines.readthedocs.io/). Except when stated otherwise, all statistical analyses were performed using IBM SPSS Statistics or GraphPad Prism v9.
Data Availability Statement
The data generated in this study are available in the manuscript and its supplementary files or upon request from the corresponding author.
Results
General epidemiological characteristics
Regional variations in clinical, pathologic and immunologic parameters of BC patients are shown in Table 1. Among women with tumors of known stage, early-stage tumors were the least common in samples from Central Africa (CA) (13%) but higher in those from WA (38%), SA (43%) and Eastern Africa (EA) (58%). Regional differences in BC subtypes exceeded 10% absolute differences for luminal A–like tumors (ranging from 19% of tumors in WA to 37% in SA), luminal B–like (25% of WA tumors compared to 37%–38% in all other regions) and triple negative breast cancer (TNBC) (almost 40% in WA vs 17%–23% in other regions).
BC subtype–specific regional differences in the frequency of TILs
The prevalence of TILs was assessed in 1,237 BC samples. Univariate analysis showed low (1%–9.9%) or intermediate (10%–39.9%) infiltration of TILs in most BC samples, while only a few cases demonstrated high TIL infiltration (>40%), as shown in Figure 1A–C. An increased frequency of TILs was found in luminal B–like (adjusted OR: 1.42, 95% CI: 1.23–1.64), Her2+ (OR: 1.48, 95% CI: 1.23–1.79) and TNBC (OR: 1.25, 95% CI: 1.07–1.46) when compared to the luminal A–like subtype (Figure 1G and Supplementary Figure S2A). In multivariable analysis, neither regional differences in the TIL frequency within SSA (Figure 1H) nor an association with the tissue quality, tumor stage, menopausal status or age was detected (Supplementary Figure S2A), but higher grading and the luminal B–like, Her2+ and TNBC subtypes were associated with higher TIL densities. However, after adjustment for age and clinical features, no relevant differences in TIL frequencies could be demonstrated in comparison to GER samples (n = 117). Of note, the distribution of TILs within the respective IHC subtypes showed regional diversity, with only GER samples showing the highest TIL densities in TNBC (Supplementary Figure S2B). Low TILs correlated with a worse survival probability independently of other clinico-pathological parameters (n = 400, Figure 1I, HR = 2.953 [95% CI: 1.624–5.371], p = 0.005). As shown in Supplementary Figure S2C, TILs are a prognostic marker particularly in ER– tumors. Moreover, the prognostic impact of TILs was the highest in EA, while in SA and WA the significance was lower (Supplementary Figure S2D).
Correlation of the TIL frequency with the immune cell composition of BC
The density of TILs was positively correlated with CD2, CD3, CD4, CD8, CD20 and FOXP3 expression (Figure 1J, p < 0.0001), while CD56 expression was relatively lower. Using multiplex IHC, the frequency and localization of CD3+, CD3+CD8–, CD3+CD8+, CD3+FOXP3+ T cells, CD20+ B cells and CD163+ M2 macrophages in relation to each other as well as to panCK+ BC cells were analysed (Figure 1D–F). A positive correlation was found between the number of TILs and the frequency of CD3+, CD3+CD8–, CD3+CD8+, CD3+FOXP3+ T cells and CD20+ B cells (p < 0.0001), while no association between TILs and CD163+ M2 macrophages was detected (p = 0.242, Figure 1D–F).
Regional differences in the immune cell repertoire of BC
Regarding immune cell composition, WA BC samples had a higher mean frequency of CD3+ T cells (particularly CD3+CD8+) than EA and GER BC samples (OR: 1.93, 65% CI: 1.26–2.94, Figure 1K and Supplementary Figure S3. In all intrinsic BC subtypes, the proportion of CD3+CD8+ T cells was the highest in WA within SSA, but comparable with GER samples. The percentage of CD20+ B cells and CD3+FOXP3+ T cells in BC samples did not differ between SSA regions, whereas the frequency of CD163+ M2 macrophages was higher in samples from WA (OR: 1.92, 95% CI: 1.42–2.61, Figure 1M and Supplementary Figure S3C). The link between TME and tumor-specific features upon adjustment for confounders is given in Supplementary Figure S2A and S3A–C.
Altered spatial distribution of immune cell subpopulations in BC of distinct regions
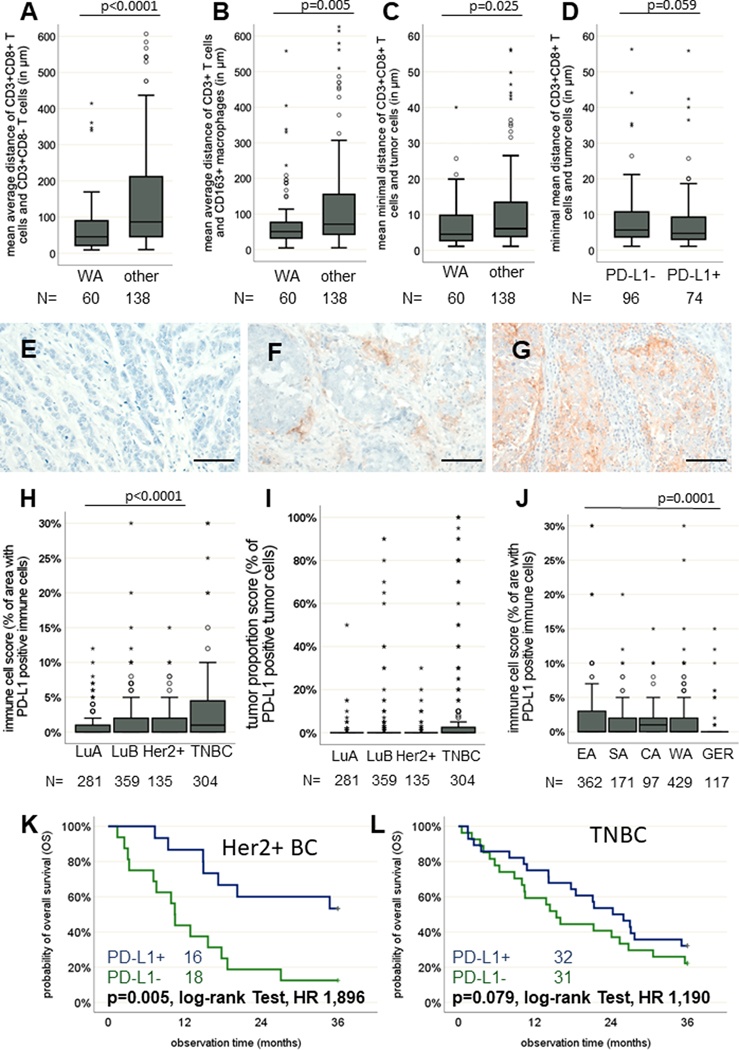
The frequency of the different immune cell subpopulations was on average higher at the invasive margin than in the tumor centre. Increased numbers of CD163+ macrophages were found in the tumor centre of WA samples (p = 0.001). Spatial distribution analysis revealed a shorter mean distance of 74.8 μm between CD3+CD8– and CD3+CD8+ T cells in WA samples compared to a mean distance of 155.1 μm in BC samples from all other regions (Figure 2 A, p < 0.0001). Additionally, a closer proximity of CD163+ macrophages to CD3+ T-cell subsets was found in BC samples from WA, with an average of 86.5 μm compared to 128.6 μm in samples from other regions (Figure 2B, p = 0.005). Regarding the minimal distance between tumor cells and CD3+CD8+ T cells, closer contact was found in WA samples, with an average of 9.4 μm compared to 14.2 μm in samples from other regions (Figure 2C, p = 0.025). The minimal distance between tumor cells and CD3+CD8+ T cells was closer in tumors positive for PD-L1 (Figure 2D, p = 0.059).
Figure 2: Spatial immune cell distribution in synopsis with PD-L1 expression in BC of SSA.
Boxplots (line represents the mean) showing that regardless of PD-L1 expression, a closer proximity of CD3+CD8− and CD3+CD8+ T cells is evident for Western SSA (WA) samples (A). A closer spatial proximity of CD3+ T cells and CD163+ macrophages exist in WA (B). Finally, a closer spatial proximity of CD3+CD8+ T cells and tumor cells was present in WA samples as well (C). Depending on the PD-L1 status, minor differences in the spatial proximity of CD3+CD8+ T cells and tumor cells could be shown, with a slightly higher proximity in PD-L1-positive samples (D). Representative IHC staining of BC samples without (E) and with low (F) and high (G) PD-L1 expression. Scale bars depict 50 μm. The prevalence of both BC samples positive for PD-L1, analysed with the immune cell score (H) and the tumor proportion score (I), was higher in triple-negative BC (TNBC). However, only minor differences were seen in the respective regions within SSA (J), while PD-L1 expression was significantly lower in GER samples. Kaplan-Meier curves show better OS of patients with PD-L1 BC in both (K) Her2+ and (L) TNBC subtypes. Except for the log-rank tests, all p-values were estimated in multivariate analyses and are adjusted for differences in age, grading, quality score, stage, IHC subtype and region of origin as confounding factors.
BC subtype–specific PD-L1 expression and its clinical relevance
The immune inhibitory molecule PD-L1, known to be frequently overexpressed in cancer (31) was assessed in 1,059 BC samples (Figure 2E–G). PD-L1 staining of tumor cells was found in 28.3% of the BC samples. In all, 45.4% of BC samples expressed PD-L1 on TILs, and less frequently on tumor cells (Figure 2H–I), which both positively correlated with the number of TILs (p < 0.0001) and the frequency of CD3+ T cells (p = 0.004). In general, a higher proportion of PD-L1+ samples was demonstrated in TNBC (OR: 2.96, 95% CI: 1.86–4.71) than in luminal A–like IHC subtypes (Figure 2H), which was only slightly increased in Her2+ (OR: 1.53, 95% CI: 0.87–4.71) and luminal B–like (OR: 1.25, 95% CI: 0.79–1.97). Furthermore, slightly lower expression of PD-L1 was observed in WA samples (Supplementary Figure S4A) as well as in GER samples. PD-L1 expression had no impact on BC patients’ outcomes in the total cohort but was correlated with improved survival in Her2+ and TNBC subtypes (Figure 2K–L, HR: 1.90 and 1.19, respectively).
Low regional differences in the function of immune cells
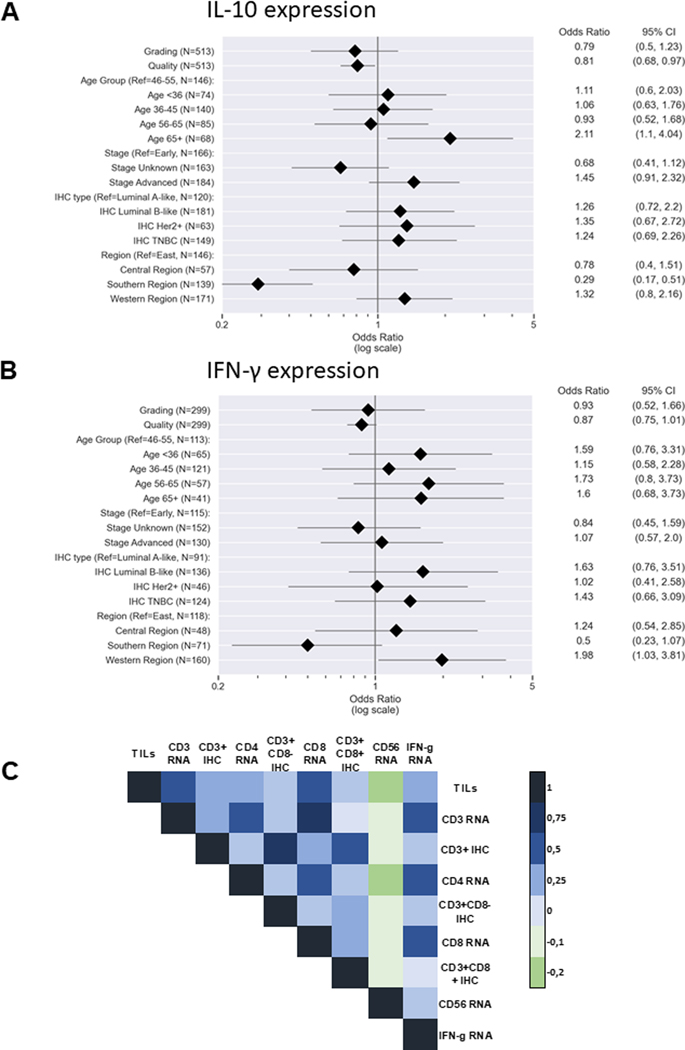
Since RNA expression analyses revealed low or undetectable expression of IFN-γ, IL-2, IL-7, IL-10 and IL-15 in samples of low tissue quality, only BC samples of intermediate to very high quality were evaluated for cytokine expression (n = 505). The expression of IL-2, IL-7 and IL15 was very low in all BC samples analysed, whereas higher IL-10 and IFN-γ mRNA expression levels were detected in advanced-stage tumors (Figure 3A–B), with the lowest expression of IL-10 in samples from SA and slightly higher expression in samples from WA (Figure 3A). The IFN-γ expression levels were highly variable in WA samples, with a three-fold higher mean value compared to BC samples from EA (OR: 1.98, 95% CI: 1.03–3.81, Figure 3B). Higher IFN-γ expression was positively correlated with the TIL density (p < 0.001, Figure 3C). The expression of the cytotoxic markers granzyme B and perforin was low, ranging between 0 and 10%, with the lowest mean frequencies of perforin in samples from WA (Supplementary Figure S4B–C).
Figure 3: Cytokine expression and its influence on immune cell infiltration.
The expression of (A) interleukin (IL)-10 and (B) interferon-gamma (IFN-γ) is visualized with forest plots. Only samples with detectable RNA of the respective genes were considered. The different sample sizes correspond to the number of specimens for which data were available for all variables examined in each test. Pearson correlation map (C) showing the connection of IFN-γ expression and tumor-infiltrating lymphocytes (TILs) and immune cell subpopulations. Pearson correlation coefficient is displayed by different colours defined in the scale bar on the right-hand side of the figure.
Regional differences in the frequency of MHC class I–mediated immune escape in BC
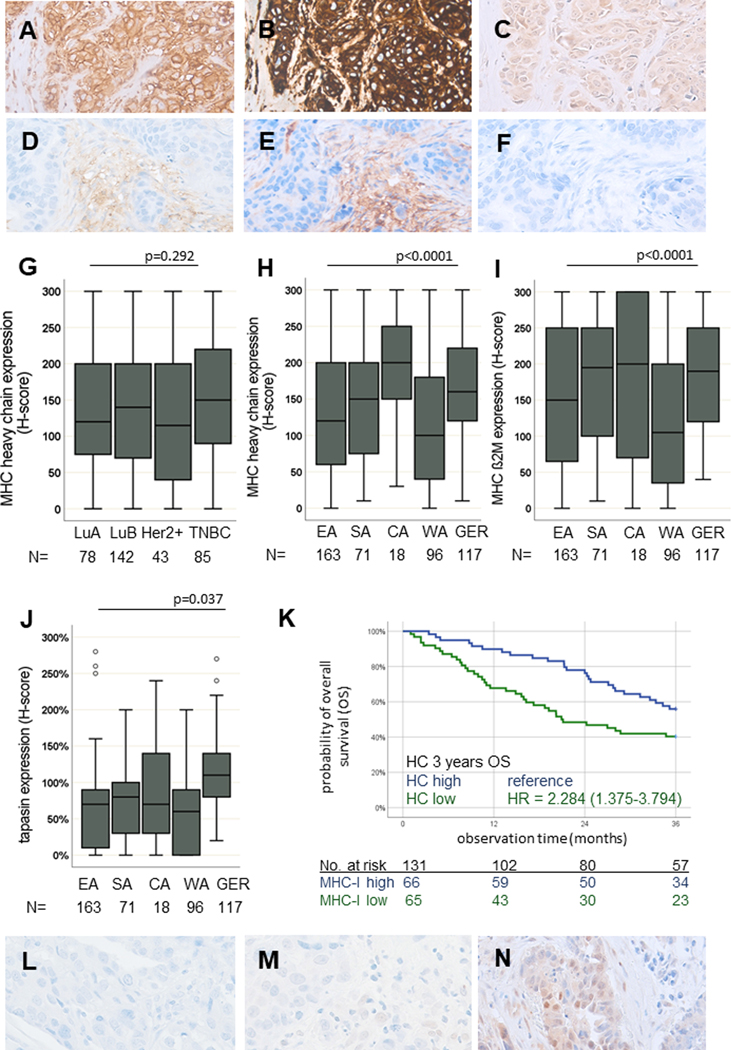
Since IFN-γ is a regulator of MHC class I components (32), the expression of the MHC class I HC, β2-m and the APM component tpn was determined (Figure 4A–F). Lower expression of these proteins was detected in BC samples from WA as compared to those from SA (OR: 2.06, 95% CI: 1.02–4.16), CA (OR: 7.72, 95% CI: 1.98–30.18) and Germany (OR: 1.00, 95% CI: 1.0–1.0, Figure 4H–J, Supplementary Figure S5A). No major differences in MHC class I HC and β2-m expression were detected regarding the BC subtypes (HC: p = 0.292, β2-m: p = 0.190, Figure 4G), age, grading or clinical stage (Supplementary Figure S5A–B). Furthermore, BC samples with lower expression of the MHC class I HC (p = 0.006) showed poorer patient survival rates independently of other clinico-pathological parameters (n = 131, Figure 4K, Supplementary Figure S6). Since basal MHC class I expression depends on functional IFN-Ɣ signalling (33), BC samples were also analysed for pSTAT1 (Figure 4L–N), which was positively correlated with IFNG RNA expression (p = 0.006), with the highest expression in BC samples from CA and SA.
Figure 4: JAK/STAT signalling and MHC class I pathway component expression in SSA.
Representative IHC stainings of BC samples with MHC class I HC (A, D), β2-m (B, E) and the APM component tpn (C, F) are shown with no or weak and strong staining patterns, respectively. A lower expression of HC (H), β2-m (I) and tpn (J) was found in WA, while no differences can be demonstrated in the respective intrinsic subtypes (G). (K) The prognostic influence of MHC class I expression is representatively shown for HC. Kaplan-Meier curve illustrates poor survival in patients with low HC expression in SSA (p = 0.006, log-rank test). Activation of IFN-γ signalling via phosphorylated pSTAT1 is shown with representative low, intermediate and high nuclear expression analysed by IHC (L-M). All p-values were estimated in multivariate analyses and are adjusted for differences in age, grading, quality score, stage, IHC subtype and region of origin as confounding factors.
Discussion
Genetic, immunologic, environmental and socioeconomic factors are known to influence BC pathophysiology and disease progression (12,21,34). So far, regional differences in BC biology have been proposed to be due to the ethnicity-dependent distribution of intrinsic BC subtypes (9,15,16,34–36). Studies on BC in women of African ancestry, mainly performed in the US, suggest more aggressive BCs with a higher proportion of TNBC linked with a poor outcome when compared to BC patients of European ancestry (10,11,23,24,37,38). It is noteworthy that African Americans do not represent the regional diversity in SSA, since the vast majority of this population has its ancestry in Western, Central-Western and South-Western SSA (39,40). However, a study comparing Tanzanian, African American and European American BC patients also demonstrated higher proportions of more aggressive intrinsic BC subtypes in EA (41). Although the TCGA database has provided information about the genomic landscape of BC, including TNBC, these databases mainly comprise BC specimens from women of European ancestry and only a low number of BC cases of African ancestry (42). Recent results based on the Nigerian Breast Cancer Study of TNBC using whole-exome sequencing demonstrated twice as many TP53 mutations, a higher frequency of GATA3 mutations and a lower frequency of PIK3CA mutations when compared to Caucasians (9,43–45). In addition, aberrant regulation of DNA damage repair (DDR) genes was shown in BC of women of African ancestry (38), which can lead to the activation of the G2–M checkpoint (46). Next to regional differences in the mutational landscape in SSA, an altered TME composition was previously reported in US BC patients depending on their ethicity, with only subtle differences in the amounts of TILs, but increased frequencies in immune suppressive cell subpopulations, such as Tregs and M2 macrophages, have been suggested (24,47). However, to the best of our knowledge, no data exist regarding regional TME diversity and immune escape mechanisms in SSA.
In this study, regional TME differences and their impact on the survival of BC patients from 10 SSA countries were analysed by determination of the number of TILs as well as the composition and function of the immune cell repertoire. Since tissue quality and its variation across centres is a potential biasing factor, a scoring system was established to quantify tissue quality in order to include it in the modelling and thus prevent false-negative results.
While the TIL counts in BC were comparable within the SSA regions, their mean values were higher in all SSA regions when compared with BC samples from GER. This is in line with studies from the US showing a higher TIL frequency in African Americans than in European Americans (48). By contrast, analysis of the immune cell repertoire demonstrated regional diversity, with higher numbers of CD3+ T cells and CD163+ M2 macrophages in WA. These results are in line with data on BC in African American women, reporting higher levels of these immune cell subsets (23,24), in which the majority of patients probably have WA ancestry (39,40). Moreover, an ancestry-associated gene expression profile of TNBC in women of African descent with significantly higher levels of CD3+ T cells in African Americans and patients from Ghana when compared to European Americans and Ethiopian patients was demonstrated (49). This study confirmed previous reports that increased numbers of TILs are a favourable prognostic factor in BC (27,50). However, TILs showed a more pronounced prognostic value in ER– BC in the present study like in previous studies as well (51), with differences in their prognostic significance between SSA regions. A higher predictive value of TIL frequencies was shown in EA, whereas analysis of TIL frequencies in WA showed no significant influence on survival. Findings from two prospective clinical trials in the United States also revealed that African-American patients had higher proportions of high TILs and no influence of increased TILs on disease-free-survival (52,53).
MSI revealed a higher proximity of CD3+CD8+ T cells and cancer cells in WA samples, with a mean distance of 9.4 μm compared to a distance of 14.2 μm in the other regions, suggesting a stronger interaction between tumor and immune cells in WA samples. The prognostic impact of the spatial distribution of immune to tumor cells is confirmed with a radius of 10 μm, while others implicate 30 μm as biologically relevant (54–56). Although a positive prognostic value is generally attributed to a high proportion of CD3+CD8+ T cells (57), the highest mean frequency of this immune cell subpopulation in SSA was detectable in WA BC cases despite their unfavourable outcome. Interestingly, the frequency of CD3+CD8+ T cells in WA was comparable with the mean frequency of samples from GER. The unfavourable outcome in WA BC might be explained by T-cell exhaustion or dysfunction, known to be more pronounced in African Americans (58). Functional status of CD8+ T cells in this study demonstrated lower expression of cytotoxic markers, but the T-cell exhaustion marker PD-1 was not altered when compared to that of German BC samples. Additionally, high levels of IL-10 and reduced IL-2, IL-7 and IL-15 are known to be involved in impaired T-cell proliferation (59), which might be in line with the highest frequency of immunosuppressive M2-type CD163+ macrophages, which secrete IL-10 (60), found among WA BC samples. In addition, IFN-γ produced by T and NK cells (43) exhibited higher expression levels in WA BC samples. This was positively correlated with the expression of PD-L1 by TILs and tumor cells and MHC class I antigens by tumor cells known to be upregulated by IFN-γ (61). A higher PD-L1 surface expression in TILs, which routinely has to be analysed prior to immunotherapy in BC (62), was further associated with improved survival in the Her2+ BC subtype and TNBC. It is also noteworthy that minor regional differences, including slightly lower PD-L1 expression in TILs, were detected in BC samples from WA, whereas a significant downregulation of MHC class I APM components was demonstrated in WA compared to that of other SSA regions. In particular, the MHC class I HC and tpn showed lower expression levels in BC samples from WA when compared to all other regions in SSA and to German BC samples, which reflect a more pronounced MHC class I–mediated immune escape of BC in the WA region (18,19,63–67).
Limitations
It is noteworthy that SSA regions are intrinsically variable in terms of genetic, environmental, lifestyle and socioeconomic parameters and other factors, which were not available for our cohort. Furthermore, several indicators suggest that the samples included for each region were not unbiased, e.g. among patients with a known tumor stage, the higher proportion of early-stage tumors in EA than in SA would not be expected at the population level. Therefore, adjustment for stage was included in all multivariable analysis. Moreover, quality issues with the available tissues are a significant problem in SSA countries. Additionally, due to lack of adequate material in our study, it was not possible to perform genetic analysis to assess regional differences. Therefore, prospective studies are urgently needed to overcome these limitations and confirm the results obtained.
Conclusion
In addition to a higher proportion of ER− and biologically more aggressive tumors in WA (13) when compared with other SSA regions as well as European patients, the TME composition and expression of immune modulator molecules in WA show an unfavourable pattern. This possibly contributes to the particularly low survival rates of BC patients in WA. Thus, immune profiling of BC in SSA underlines the clinical relevance of the TME and immune escape strategies linked to BC patient outcomes. Considerable diversity is seen regarding these features between SSA regions. However, beyond the biological relevance shown in this study, further investigations are urgently required to identify the underlying immune regulatory mechanisms in BC patients of WA origin. This study highlights the relevance and importance of multicentre studies to analyse regional diversities in more detail, which might shape the design of clinical trials and therapeutic options.
Supplementary Material
Synopsis:
The authors report regional diversity in the distribution of breast cancer subtypes and immune cell composition in sub-Saharan Africa (SSA) that was associated with a higher prevalence of non-immunogenic breast–cancer phenotypes in Western SSA.
Acknowledgements:
We want to thank all patients who provided tumor samples and pathology staff at the sites in SSA for providing the tissue material. We thank Maria Heise for excellent secretarial help. We thank Christine Fathke (MD) and Linda Dießel (MD) for support in rare histomorphological diagnoses and Sarah Voigtländer and Andreas Wilfer for excellent laboratory organization. Where authors are identified as personnel of the International Agency for Research on Cancer or WHO, the authors alone are responsible for the views expressed in this article, which do not necessarily represent the decisions, policies or views of the International Agency for Research on Cancer or WHO.
Funding:
This work was supported by grants from the Else-Kröner-Foundation (EJK, 2018_HA31SP), Susan G. Komen-Foundation (EJK, GTDR16378013, VM, IIR13264158), German Cancer Aid (BS, Integrate-TN, 70113450), the German Research Council (BS, Se581/33-1), German Academic Exchange Service (EJK, ID57216764), Ministry for Economic Cooperation and Development and the Else-Kröner-Fresenius Foundation (EJK, ID81256434), Hoffmann-La Roche Ltd (EJK, 27.5.2014) and the National Cancer Institute (VM, R01CA244559).
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019. Apr 15;144(8):1941–53. [DOI] [PubMed] [Google Scholar]
- 2.Hamdi Y, Abdeljaoued-Tej I, Zatchi AA, Abdelhak S, Boubaker S, Brown JS, et al. Cancer in Africa: The Untold Story. Front Oncol. 2021;11:650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021. May;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 4.Weiner CM, Mathewos A, Addissie A, Ayele W, Aynalem A, Wondemagegnehu T, et al. Characteristics and follow-up of metastatic breast cancer in Ethiopia: A cohort study of 573 women. Breast Edinb Scotl. 2018. Dec;42:23–30. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie F, Zietsman A, Galukande M, Anele A, Adisa C, Parham G, et al. Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer - disparities in outcomes (ABC-DO) study. Int J Cancer. 2018. Apr 15;142(8):1568–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack V, McKenzie F, Foerster M, Zietsman A, Galukande M, Adisa C, et al. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. Lancet Glob Health. 2020. Sep;8(9):e1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol Off J Am Soc Clin Oncol. 2006. Mar 20;24(9):1342–9. [DOI] [PubMed] [Google Scholar]
- 8.Anyigba CA, Awandare GA, Paemka L. Breast cancer in sub-Saharan Africa: The current state and uncertain future. Exp Biol Med Maywood NJ. 2021. Jun;246(12):1377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansari-Pour N, Zheng Y, Yoshimatsu TF, Sanni A, Ajani M, Reynier JB, et al. Whole-genome analysis of Nigerian patients with breast cancer reveals ethnic-driven somatic evolution and distinct genomic subtypes. Nat Commun. 2021. Nov 26;12(1):6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002. Apr 24;287(16):2106–13. [DOI] [PubMed] [Google Scholar]
- 11.Albain KS, Unger JM, Crowley JJ, Coltman CA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009. Jul 15;101(14):984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011. Aug;22(8):1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng A, McCormack V, dos-Santos-Silva I. Receptor-Defined Subtypes of Breast Cancer in Indigenous Populations in Africa: A Systematic Review and Meta-Analysis. Adami HO, editor. PLoS Med. 2014. Sep 9;11(9):e1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Effi AB, Aman NA, Koui BS, Koffi KD, Traoré ZC, Kouyate M. Immunohistochemical determination of estrogen and progesterone receptors in breast cancer: relationship with clinicopathologic factors in 302 patients in Ivory Coast. BMC Cancer. 2017. Feb 7;17(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantelhardt EJ, Mathewos A, Aynalem A, Wondemagegnehu T, Jemal A, Vetter M, et al. The prevalence of estrogen receptor-negative breast cancer in Ethiopia. BMC Cancer. 2014. Dec;14(1):895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jemal A, Fedewa SA. Is the prevalence of ER-negative breast cancer in the US higher among Africa-born than US-born black women? Breast Cancer Res Treat. 2012. Oct;135(3):867–73. [DOI] [PubMed] [Google Scholar]
- 17.Desalegn Z, Yohannes M, Porsch M, Stückrath K, Anberber E, Santos P, et al. Intrinsic subtypes in Ethiopian breast cancer patient. Breast Cancer Res Treat. 2022. Dec;196(3):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Y, Ma D, Zhao S, Suo C, Shi J, Xue MZ, et al. Multi-Omics Profiling Reveals Distinct Microenvironment Characterization and Suggests Immune Escape Mechanisms of Triple-Negative Breast Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2019. Aug 15;25(16):5002–14. [DOI] [PubMed] [Google Scholar]
- 19.Bates JP, Derakhshandeh R, Jones L, Webb TJ. Mechanisms of immune evasion in breast cancer. BMC Cancer. 2018. May 11;18(1):556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil Del Alcazar CR, Alečković M, Polyak K. Immune Escape during Breast Tumor Progression. Cancer Immunol Res. 2020. Apr;8(4):422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018. Jan;19(1):40–50. [DOI] [PubMed] [Google Scholar]
- 22.Tomioka N, Azuma M, Ikarashi M, Yamamoto M, Sato M, Watanabe KI, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer Tokyo Jpn. 2018. Jan;25(1):34–42. [DOI] [PubMed] [Google Scholar]
- 23.O’Meara T, Safonov A, Casadevall D, Qing T, Silber A, Killelea B, et al. Immune microenvironment of triple-negative breast cancer in African-American and Caucasian women. Breast Cancer Res Treat. 2019. May;175(1):247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim G, Pastoriza JM, Condeelis JS, Sparano JA, Filippou PS, Karagiannis GS, et al. The Contribution of Race to Breast Tumor Microenvironment Composition and Disease Progression. Front Oncol. 2020;10:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organisation mondiale de la santé, editor. Breast tumours. 5th ed. Geneva: OMS; (World health organization classification of tumours). [Google Scholar]
- 26.Schüler K, Bethmann D, Kaufhold S, Hartung C, Stückrath K, Lantzsch T, et al. Prognostic Value of Tumour-Infiltrating Lymphocytes in an Unselected Cohort of Breast Cancer Patients. Diagn Basel Switz. 2022. Oct 18;12(10):2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015. Feb;26(2):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldelli E, Hodge KA, Bellezza G, Shah NJ, Gambara G, Sidoni A, et al. PD-L1 quantification across tumor types using the reverse phase protein microarray: implications for precision medicine. J Immunother Cancer. 2021. Oct;9(10):e002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995. Sep;48(9):876–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer M, Vaxevanis C, Bethmann D, Massa C, Pazaitis N, Wickenhauser C, et al. Multiplex immunohistochemistry as a novel tool for the topographic assessment of the bone marrow stem cell niche. Methods Enzymol. 2020;635:67–79. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 2016;9:5023–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28(3–4):239–60. [DOI] [PubMed] [Google Scholar]
- 33.Respa A, Bukur J, Ferrone S, Pawelec G, Zhao Y, Wang E, et al. Association of IFN-gamma signal transduction defects with impaired HLA class I antigen processing in melanoma cell lines. Clin Cancer Res Off J Am Assoc Cancer Res. 2011. May 1;17(9):2668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiagge EM, Ulintz PJ, Wong S, McDermott SP, Fossi SI, Suhan TK, et al. Multiethnic PDX models predict a possible immune signature associated with TNBC of African ancestry. Breast Cancer Res Treat. 2021. Apr;186(2):391–401. [DOI] [PubMed] [Google Scholar]
- 35.Roy I, Othieno E. Breast carcinoma in Uganda: microscopic study and receptor profile of 45 cases. Arch Pathol Lab Med. 2011. Feb;135(2):194–9. [DOI] [PubMed] [Google Scholar]
- 36.Brandão M, Guisseve A, Bata G, Alberto M, Ferro J, Garcia C, et al. Breast cancer subtypes: implications for the treatment and survival of patients in Africa-a prospective cohort study from Mozambique. ESMO Open. 2020. Oct;5(5):e000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015. Apr;15(4):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazumder A, Jimenez A, Ellsworth RE, Freedland SJ, George S, Bainbridge MN, et al. The DNA damage repair landscape in Black women with breast cancer. Ther Adv Med Oncol. 2022;14:17588359221075458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salas A, Carracedo A, Richards M, Macaulay V. Charting the ancestry of African Americans. Am J Hum Genet. 2005. Oct;77(4):676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zakharia F, Basu A, Absher D, Assimes TL, Go AS, Hlatky MA, et al. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2009;10(12):R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mremi A, Broadwater G, Jackson K, Amsi P, Mbulwa C, Hyslop T, et al. Breast cancer in Tanzanian, black American, and white American women: An assessment of prognostic and predictive features, including tumor infiltrating lymphocytes. PloS One. 2019;14(11):e0224760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan J, Hu Z, Mahal BA, Zhao SD, Kensler KH, Pi J, et al. Integrated Analysis of Genetic Ancestry and Genomic Alterations across Cancers. Cancer Cell. 2018. Oct 8;34(4):549–560.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ademuyiwa FO, Tao Y, Luo J, Weilbaecher K, Ma CX. Differences in the mutational landscape of triple-negative breast cancer in African Americans and Caucasians. Breast Cancer Res Treat. 2017. Feb;161(3):491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hercules SM, Liu X, Bassey-Archibong BBI, Skeete DHA, Smith Connell S, Daramola A, et al. Analysis of the genomic landscapes of Barbadian and Nigerian women with triple negative breast cancer. Cancer Causes Control CCC. 2022. Jun;33(6):831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen JW, Murugesan K, Newberg JY, Sokol ES, Savage HM, Stout TJ, et al. Comparison of PIK3CA Mutation Prevalence in Breast Cancer Across Predicted Ancestry Populations. JCO Precis Oncol. 2022. Nov;6:e2200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vakili-Samiani S, Khanghah OJ, Gholipour E, Najafi F, Zeinalzadeh E, Samadi P, et al. Cell cycle involvement in cancer therapy; WEE1 kinase, a potential target as therapeutic strategy. Mutat Res. 2022;824:111776. [DOI] [PubMed] [Google Scholar]
- 47.Sawe RT, Kerper M, Badve S, Li J, Sandoval-Cooper M, Xie J, et al. Aggressive breast cancer in western Kenya has early onset, high proliferation, and immune cell infiltration. BMC Cancer. 2016. Mar 10;16:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marczyk M, Qing T, O’Meara T, Yagahoobi V, Pelekanou V, Bai Y, et al. Tumor immune microenvironment of self-identified African American and non-African American triple negative breast cancer. NPJ Breast Cancer. 2022. Jul 22;8(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martini R, Delpe P, Chu TR, Arora K, Lord B, Verma A, et al. African Ancestry-Associated Gene Expression Profiles in Triple-Negative Breast Cancer Underlie Altered Tumor Biology and Clinical Outcome in Women of African Descent. Cancer Discov. 2022. Nov 2;12(11):2530–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J Clin Oncol. 2019. Mar 1;37(7):559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oshi M, Patel A, Wu R, Le L, Tokumaru Y, Yamada A, et al. Enhanced immune response outperform aggressive cancer biology and is associated with better survival in triple-negative breast cancer. NPJ Breast Cancer. 2022. Aug 9;8(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klar N, Gray RJ, Adams S, Sparano JA, Goldstein LJ, DeMichele AM, et al. Abstract P1–08-35: Stromal tumor infiltrating lymphocytes analysis by race and ethnicity in triple negative breast cancers from 2 phase III randomized adjuvant breast cancer trials: ECOG-ACRIN E2197 and E1199. Cancer Res. 2022. Feb 15;82(4_Supplement):P1–08-35-P1-08–35. [Google Scholar]
- 53.Sayed S, Yang XR, Govender D, Breast Health Study group. Abstract P1–02-13: Tumour infiltrating lymphocytes (TILs) and immune composition in breast cancer patients from Kenya: Spatial distributions and associations with risk factors and tumour characteristics. Cancer Res. 2022. Feb 15;82(4_Supplement):P1–02-13-P1-02–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsakiroglou AM, Fergie M, Oguejiofor K, Linton K, Thomson D, Stern PL, et al. Spatial proximity between T and PD-L1 expressing cells as a prognostic biomarker for oropharyngeal squamous cell carcinoma. Br J Cancer. 2020. Feb;122(4):539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng Z, Bethmann D, Kappler M, Ballesteros-Merino C, Eckert A, Bell RB, et al. Multiparametric immune profiling in HPV- oral squamous cell cancer. JCI Insight. 2017. Jul 20;2(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauer M, Vaxevanis C, Al-Ali HK, Jaekel N, Naumann CLH, Schaffrath J, et al. Altered Spatial Composition of the Immune Cell Repertoire in Association to CD34+ Blasts in Myelodysplastic Syndromes and Secondary Acute Myeloid Leukemia. Cancers. 2021. Jan 7;13(2):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rapoport BL, Galon J, Nayler S, Fugon A, Martel M, Mlecnik B, et al. 1984P Tumour infiltrating lymphocytes in early breast cancer: High levels of CD3, CD8 cells and Immunoscore® are associated with pathological CR and time to progression in patients undergoing neo-adjuvant chemotherapy. Ann Oncol. 2020. Sep;31:S1112. [Google Scholar]
- 58.Yao S, Cheng TYD, Elkhanany A, Yan L, Omilian A, Abrams SI, et al. Breast Tumor Microenvironment in Black Women: A Distinct Signature of CD8+ T-Cell Exhaustion. J Natl Cancer Inst. 2021. Aug 2;113(8):1036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raeber ME, Zurbuchen Y, Impellizzieri D, Boyman O. The role of cytokines in T-cell memory in health and disease. Immunol Rev. 2018. May;283(1):176–93. [DOI] [PubMed] [Google Scholar]
- 60.Rőszer T Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seliger B Basis of PD1/PD-L1 Therapies. J Clin Med. 2019. Dec 8;8(12):E2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erber R, Hartmann A. Understanding PD-L1 Testing in Breast Cancer: A Practical Approach. Breast Care Basel Switz. 2020. Oct;15(5):481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torigoe T, Asanuma H, Nakazawa E, Tamura Y, Hirohashi Y, Yamamoto E, et al. Establishment of a monoclonal anti-pan HLA class I antibody suitable for immunostaining of formalin-fixed tissue: unusually high frequency of down-regulation in breast cancer tissues. Pathol Int. 2012. May;62(5):303–8. [DOI] [PubMed] [Google Scholar]
- 64.Palmisano GL, Pistillo MP, Capanni P, Pera C, Nicolò G, Salvi S, et al. Investigation of HLA class I downregulation in breast cancer by RT-PCR. Hum Immunol. 2001. Feb;62(2):133–9. [DOI] [PubMed] [Google Scholar]
- 65.Steven A, Seliger B. The Role of Immune Escape and Immune Cell Infiltration in Breast Cancer. Breast Care Basel Switz. 2018. Mar;13(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gil Del Alcazar CR, Huh SJ, Ekram MB, Trinh A, Liu LL, Beca F, et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017. Oct;7(10):1098–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Zhang Q, Yu C, Cao Y, Zuo Y, Yang L. Immune cell infiltration-based signature for prognosis and immunogenomic analysis in breast cancer. Brief Bioinform. 2021. Mar 22;22(2):2020–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available in the manuscript and its supplementary files or upon request from the corresponding author.