Abstract
Remote C─H functionalization of heterocyclic biaryls will be of great importance in synthesis and medicinal chemistry. Through adjusting the geometric relationship of the directing atom and target C─H bonds, two new catalytic templates have been developed to enable the functionalization of the more hindered ortho-C─H bonds of heterobiaryls bearing directing heteroatom at the meta- or para-positions, affording unprecedented site-selectivity. The use of template chaperone also overcomes product inhibition and renders the directing templates catalytic. The utility of this protocol was demonstrated by olefination of heterocyclic biaryls with various substituents, overriding conventional steric and electronic effects. These ortho-C─H olefinated heterobiaryls are sterically hindered and can often be challenging to prepare through aryl-aryl coupling reactions.
Keywords: heterobiaryl, C─H activation, olefination, site-selectivity, palladium
Graphical Abstract

The site-selective C─H functionalizations of 3- and 4-phenylpyridine biaryls at sterically disfavored ortho positions were realized by developing two new catalytic template.
Introduction
Achieving site selectivity in remote C─H functionalization is challenging owing to the presence of multiple C─H bonds that bear insufficient difference in electronic and steric environments.[1] While non-directed C─H functionalizations of electronically or sterically biased arenes can afford site-selectivity with an appropriate ligand-supported catalyst,[2] directing effect through substrate-catalyst interactions is an indispensable tool for controlling site selectivity as elucidated in enzyme catalysis.[3] By engineering the distance and geometric relationship between C─H bonds and the directing atom, we have developed a U-shaped template to achieve meta- and para-selectivity of various arene substrates.[4] The origin of the selectivity is derived from the size and geometry of the privileged macrocyclophane transition states. However, the use of covalently attached directing templates is not practical. Most importantly, synthetic substrates such as heterocycles do not always have a chemical handle to install the template covalently.[5]
While our efforts to extend the covalently attached templates to catalytic templates for Pd(II) catalysts are in progress, several innovative attempts have been made in the fields of iridium and rhodium catalysis by employing hydrogen bonds, electrostatic interactions and ion-pair interactions to achieve remote directed selectivity.[6] Notably, Kanai disclosed the first example of remote Ir-catalyzed meta-C─H borylation via a macrocyclophane transition state assembled by hydrogen bonding.[6c] Recently, Nakao and our group reported two examples of Ir and Ni-catalyzed meta-C─H borylation and alkenylation of pyridine by utilizing Lewis acid-base interaction to anchor the heterocyclic substrates.[7] In 2017, our group disclosed a bifunctional template strategy to achieve palladium-catalyzed remote C─H olefination of heterocyclic substrates bearing a pyridine or quinoline motif (Scheme 1a).[5a] The two metal centers played different roles: one was used as anchor that reversibly coordinated to heterocyclic substrates, and the other was directed by the nitrile or pyridine directing group of the template to cleave target C─H bonds through the assembled macrocyclophane transition state. This template architecture could be explored to enable various C─H functionalizations of quinoline and isoquinoline motifs.[8] Although extensive studies have been reported to address ortho- and meta-C─H functionalizations of 2-phenylpyridines, site-selective C─H activation of other heterobiaryls is still elusive (Scheme 1b).[9] Encouraged by the strong coordinating pyridine-type directing groups for remote C─H functionalization of aza-arenes,[10] we envisioned that such heterocycles could also be employed by engineering the distance and geometry of the template to accommodate the macrocyclophane-like transition state. In particular, we are interested in achieving unconventional site-selectivity of heterocyclic biaryls. 3-Phenyl and 4-phenylpyridine motifs are found in numerous biologically active compounds, including drug molecules and natural products.[11] Herein, we report the development of two new templates that could selectively direct the functionalization of sterically hindered ortho-C─H bonds of 3-phenyl and 4-phenylpyridine motifs (Scheme 1c). Although meta-C─H olefination of 3-phenylpyridine was reported by our group using a template with bis-amide backbone (Scheme 1a),[5a] selective ortho C─H functionalization of heterocylic biaryls bearing nitrogen at meta- or para-positions has not been successful to date.
Scheme 1.
Remote C─H functionalization of heterocyclic systems.
3-Phenyl and 4-phenylpyridine motifs are found in numerous biologically active compounds, including drug molecules and natural products.[11] Herein, we report the development of two new templates that could selectively direct the functionalization of sterically hindered ortho-C─H bonds of 3-phenyl and 4-phenylpyridine motifs (Scheme 1c). Although meta-C─H olefination of 3-phenylpyridine was reported by our group using a template with bis-amide backbone (Scheme 1a),[5a] selective ortho C─H functionalization of heterocylic biaryls bearing nitrogen at meta- or para-positions has not been successful to date.
Notably, ortho-C─H bonds of these heterobiaryl compounds are more challenging to functionalize than the less hindered meta and para-C─H bonds.[12] Complementary to the previously reported methods, this protocol provides an alternative method for the synthesis of ortho-substituted heterobiaryl compounds.
Results and Discussion
We began our investigation by tuning the geometric structure of directing templates (Table 1). Only a trace amount of olefination product was observed using previously reported templates with benzonitrile directing groups (T1 and T2). In addition to a nitrile directing group, pyridine and quinoline were selected as directing groups attached to the template backbone because of their stronger directing ability and a more rigid geometry. Because these heterobiaryl compounds are more geometrically flexible than quinoline substrates, various pyridine, quinoline and isoquinoline-based templates were prepared and carefully adjusted to achieve the precise recognition of distance and geometry. T3-T5 were found to be effective with 3-phenylpyridine motifs, showing various degrees of efficiency. Notably, using T6 as the template, meta olefination product was obtained as the major product, with a ratio of 80/20 (meta/others). The optimized template T4 afforded the desired ortho-olefinated product in 73% yield as the major product. Although T7 and T8 are unreactive to 3-phenylpyridine, they were found to be the optimal templates for ortho-C─H olefination of 4-phenylpyridine, affording the corresponding olefination product in moderate yields and moderate ortho-selectivity. Reactivity and ortho-selectivity could be improved by further optimization.
Table 1.

|
Conditions: 1). 1a (0.1 mmol, 1.0 equiv.), Pd(OAc)2 (1.0 equiv.), T4 (1.0 equiv.), acetone (0.1 mL), 90 °C for 1 h then evaporate acetone. 2). Ethyl acrylate (3.0 equiv.), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), Ag2CO3 (2.5 equiv.), HFIP (1.0 mL), 90 °C, under air, 48 h. See SI for work-up procedures.
The yield and ratio of regioisomers were determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard.
Yields for ortho-olefinated product of 4-phenylpyridine, ortho/others = 75/25.
With the optimal directing templates for 3-phenyl and 4-phenylpyridine determined, we next tried to minimize the amount of these directing templates by adding a template chaperone. The concept of template chaperone (TC) was firstly proposed in our recent research.[10] While at least 1.0 equiv. of template is required to avoid catalyst inhibition from the strong binding of heterocyclic products, we hypothesized that they could coordinate to the two different templates reversibly. For example, upon functionalization of substrates by coordinating to the directing templates, they could then be transferred to the template chaperone as products, thus avoiding product inhibition. Using 3-phenylpyridine as the model substrate and T4 as the directing template, we could decrease the amount of the directing templates to 0.15 equiv. by adding 0.85 equiv. of a template chaperone (TC-Pd-CN) (See SI for results of other transition metal template chaperones, such as Cu, Ni, Fe and Zn complexes). Importantly, unlike the directing templates, the template chaperone is easily synthesized, purified and recovered.
To elucidate the key components of this reaction, we carried out several control experiments. Using 1a as the model substrate, less than 5% of olefination products with several regioisomers were observed in the absence of any templates (Table 2, entry 2). Replacing T4 with TPG also only gave a trace amount of products, indicating the essential directing effect of T4 (entry 3). Using 1.0 equiv. of T4 instead of a combination of 0.85 equiv. TPG-Pd-CN and 0.15 equiv. T4 gave the similar reactivity. However, adopting less reactive 1i as model substrate, yields of the corresponding olefination products dropped to 35%, probably due to the strong coordination of excess isoquinoline directing groups (entry 4). Decreasing the amount of directing templates T4 from 1.0 equiv. to 20 mol% led to less than 5% yield (entry 5). No product was observed in the absence of palladium acetate (entry 6). Moreover, increasing the loading of palladium acetate to 110 mol% without using any templates only gave trace amount of olefination products (entry 7).
Table 2.

|

|
|||
|---|---|---|---|---|
| Entry | Variations from standard conditions | Yield of 3a (ortho) |
Yield of 31 (ortho) |
|
| 1 | none | 76% | 60% |

|
| 2 | no any templates | 5% (mixture) | 5% (mixture) | |
| 3 | 1.0 equiv. TPG, no T4 | 5% (mixture) | 5% (mixture) | |
| 4 | 1.0 equiv. T4, no TPG | 73% | 35% | |
| 5 | 0.2 equiv. T4, no TPG | <5% | <5% | |
| 6 | no Pd(OAc)2 | 0% | 0% | |
| 7 | no any templates, 110 mol% Pd(OAc)2 | 5% (mixture) | 5% (mixture) | |
Conditions: 1). 1a, 1i (0.1 mmol, 1.0 equiv.), Pd(OAc)2 (15 mol%), TPG-Pd-CN (0.85 equiv.), T4 (0.15 equiv.), acetone (0.1 mL), 90 °C for 1 h then evaporate acetone. 2). Ethyl acrylate (3.0 equiv.), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), Ag2CO3 (2.5 equiv.), HFIP (1.0 mL), 90 °C, under air, 48 h. See SI for work-up procedures.
The yield and ratio of regioisomers were determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard (ortho/others > 90/10).
With the best conditions established, we set out to investigate the substrate scope of 3-phenyl and 4-phenylpyridines. Using ethyl acrylate as the coupling partner and T4 as the directing template, a variety of 3-phenylpyridines bearing different substituents were examined to afford the ortho-olefinated products in moderate to high yields and high regioselectivity (Table 3). Mono ortho-olefinated 3-phenylpyridine (3a) was isolated in 71% yield, with an regioselectivity of 88/6/6 (o/m/p regioisomers). Methyl substituent on meta position of the phenyl ring was also tolerated to provide the desired ortho products in 65% yield, however, two ortho-olefinated regioisomers with a ratio of 75/25 were observed for this meta-substituted substrate (3b). Other substituents including chloro, methoxy and ester at the meta-position afforded the same seletivity, albeit in lower yields (3c-3e). This protocol could tolerate a wide range of substituents, including electron-withdrawing groups that usually deactivate C─H bonds on the phenyl ring.[5,11] Chloro, amido, trifluoromethyl, and ester groups were well tolerated to afford the corresponding mono ortho-olefination products in good yields, exhibiting excellent ortho site-selectivity and mono selectivity (3f, 3g, 3i and 3j). Different substituents on the pyridine ring were also tested. Fluoro and methyl group at the ortho (C6) position of the pyridine ring were well tolerated, providing the corresponding products in high yields and mono ortho-selectivity (3h, 3i and 3j). Substrates bearing methoxy, methyl, fluoro and trifluoromethyl groups on the meta position of pyridine were studied and the corresponding ortho-olefinated products were obtained in 58% to 83% yields, with high ortho-selectivity (3k-3p). Olefination of substrates with substituents on the ortho position of the phenyl ring, as well as ortho (C2) and para positions of pyridine generated sterically demanding ortho-olefinated products in moderate to high yields (3q, 3r and 3s). To further explore the scope of this protocol, we also tested heterocycles other than pyridine. 5-Phenyl pyrimidine and 3-phenylquinoline motifs were also well tolerated by this method, providing the corresponding products in 52% to 90% yields, with excellent ortho-selectivity (3t to 3x). A biologically active compound, bearing the same core structure as oxazolidinone-class antibiotics Tedizolid and Linezolid,[13] was examined to showcase the applicability of this protocol, affording the desired ortho-olefinated product as the major product in 77% yield, with an 85/15 ortho-selectivity (3y). Using 3-(o-methylphenyl) pyridine, 3-(naphthalen-1-yl) pyridine and quinoline as substrates, multiple olefination products and low selectivity were observed (3zg, 3zh and 3zi). This protocol provides an efficient way to access ortho-functionalized heterobiaryls and paves the way for molecular editing of such heterocyclic compounds.[5a] Besides, 4-phenylpyridine substrates were also tested under the optimal conditions using T8 as the directing template (Table 4). Simple 4-phenylpyridine was olefinated in 39% yield at desired ortho position, with a ratio of 74/13/13 o/m/p products and less than 5% di products were observed (4a). Fluoro substituent on meta position of the phenyl ring was tolerated to provide the desired ortho products in 48% yield, however, two ortho-olefinated regioisomers with a ratio of 66/34 were observed (4b). 2-Methyl-4-phenylpyridine without any substituents on the phenyl ring gave 51% yield of ortho-olefinated product with a 77/23 ortho-selectivity (4c). A wide range of para substituents on the phenyl ring, from electron-donating to electron-withdrawing, were studied to give 43% to 80% yields with > 90/10 ortho-selectivity, (4d-4f, 4h, 4j). However, chloro substituent on para position of the phenyl ring gave a decreased ortho-selectivity (4g). 3-Fluoro-4-phenylpyridine afforded a 1:1 mixture of ortho and meta-olefination products (4i).
Table 3.

|
Conditions: Step 1). 1a-1v (0.1 mmol, 1.0 equiv.), Pd(OAc)2 (15 mol%), TPG-Pd-CN (0.85 equiv.), T4 (0.15 equiv.), acetone (0.1 mL), 90 °C for 1 h then evaporate acetone. Step 2). Ethyl acrylate (3.0 equiv.), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), Ag2CO3 (2.5 equiv.), HFIP (1.0 mL), 90 °C, under air, 48 h. See SI for work-up procedures.
Isolated yields for ortho-olefinated product, di-products were observed in less than 5% yield. The percentages under each structure indicate the yields and selectivity of the mono-ortho-olefinated product. The ortho selectivity was determined by 1H NMR analysis of the reaction mixture (assisted with GC-MS analysis); the variance is estimated to be within 5%.
Using 15 mol% Pd(OAc)2 and 30 mol% Ac-Gly-OH in Step 2.
olefination on quinoline ring was also observed as one of the byproducts.
di-olefination product was observed in 10% yield.
Table 4.

|
Conditions: Step 1). 2a-2j (0.1 mmol, 1.0 equiv.), Pd(OAc)2 (20 mol%), TPG-Pd-CN (0.8 equiv.), T8 (0.2 equiv.), acetone (0.1 mL), 90 °C for 1 h then evaporate acetone. Step 2). Ethyl acrylate (3.0 equiv.), Pd(OAc)2 (10 or 15 mol%), Ac-Gly-OH (20 or 30 mol%), Ag2CO3 (2.5 equiv.), HFIP (1.0 mL), 90 °C, under air, 48 h. See SI for work-up procedures.
Isolated yields for ortho-olefinated product, di-products were observed in less than 5% yield. The percentages under each structure indicate the yields and selectivity of the mono-ortho-olefinated product. The ortho selectivity was determined by 1H NMR analysis of the reaction mixture (assisted with GC-MS analysis); the variance is estimated to be within 5%. For 4a, 52% yield of desired ortho product was obtained when using 15 mol% Pd(OAc)2 and 30 mol% Ac-Gly-OH in Step 2 (o/others = 3/1). For 4c, the combined yield of meta and para isomers is 12% (one is 7%, the other is 5%). For 4k, the combined yield of meta and para isomers is 10%. For 4l, the combined yield of meta and para isomers is 10% (5% meta and 5% para products).
Combined yield of ortho and meta-olefinated products.
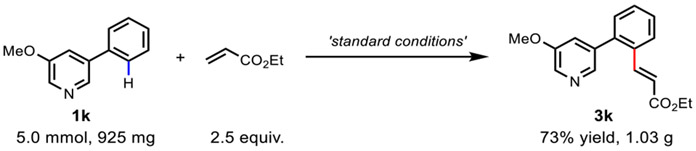
In addition, the scope of olefination reagents was evaluated. Using 3-methoxy-5-phenylpyridine (1h) as the model substrate, we tested various α, β-unsaturated olefins. Reactions with acrylate derivatives proceeded in > 80% yields as well as superior ortho-selectivity (3z and 3za). Olefins bearing other electron-withdrawing groups such as amide, ketone, phosphonate, and sulfone were compatible with our reaction conditions, affording the corresponding mono ortho-olefinated products in 45% to 86% yields (3zb-3ze). Electron-deficient styrene can also be used as a coupling partner, yielding the desired product in 90% (3zf). Two additional reactive olefins were examined using 2-methyl-4-phenylpyridine (2c) as the model substrate, giving the desired products in 58% and 54% yields, respectively (4k, 4l). To further showcase the utility of this method, gram scale experiment was carried out using 5.0 mmol of 1h (925 mg) as the substrate. The desired ortho-olefination product was isolated in 73% yield with superior ortho-selectivity (1.03g, 3k) (Scheme 2).
Scheme 2.
Gram-scale reaction.
Based on our previous template-directed C-H activation,[10] the directed pathway could lead to minor regioisomers, although non-directed pathway could also erode regioselectivity. A plausible mechanism for this remote C─H olefination is outlined in Scheme 3. Template exchange of heterocyclic substrate and olefinated product between the directing template (T4 or T8) and the template chaperone enables high reactivity in the presence of a catalytic amount of the directing template.[10] Treating the crude complex mixture with 4-dimethylaminopyridine and then with methanesulfonic acid in acetonitrile,[5a] 97% of the template chaperone (TC-Pd-CN) was recovered and used as a recyclable reagent for this remote C─H olefination reaction (See SI for template recovery procedure).
Scheme 3.
Proposed catalytic cycle.
Conclusion
In conclusion, we have achieved site-selective remote C─H functionalization of 3- and 4-phenylpyridine biaryls, with prefered reactivity at sterically disfavored ortho positions, another step towards broad molecular editing of various sites in synthetic substrates. Although ortho-selectivity is lower than 90% in some cases, this protocol offers an alternative and direct approach to achieve these sterically demanding biaryl compounds, especially in the context of late-stage functionalization. This reaction features broad substrate scope and functional group compatibility.
Supplementary Material
Acknowledgements
We gratefully acknowledge The Scripps Research Institute and the NIH (National Institute of General Medical Sciences grant R01 GM102265) for financial support. We gratefully acknowledge Dr. Jason Chen (TSRI), Brittany Sanchez (TSRI) and Emily Sturgell (TSRI) for HRMS analysis and compound purification.
Footnotes
Supporting information for this article is given via a link at the end of the document.
Conflict of interest
The authors declare no conflict of interest.
References:
- [1].Meng G, Lam NYS, Lucas EL, Saint-Denis TG, Verma P, Chekshin N, Yu J-Q, J. Am. Chem. Soc 2020, 142, 10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wedi P, van Gemmeren M, Angew. Chem., Int. Ed 2018, 57, 13016; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2018, 130, 13198. [Google Scholar]
- [3].a) For selected examples on directed C(sp2)─H activation: Ackermann L, Vicente R, Kapdi AR, Angew. Chem., Int. Ed 2009, 48, 9792; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2009, 121, 9976; [Google Scholar]; b) Sambiagio C, Schonbauer D, Blieck R, Dao-Huy T, Pototschnig G, Schaaf P, Wiesinger T, Zia MF, Wencel-Delord J, Besset T, Maes BUW, Schnurch MA, Chem. Soc. Rev 2018, 47, 6603; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Rej S, Chatani N, Angew. Chem., Int. Ed 2019, 58, 8304; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2019, 131, 8390. [Google Scholar]
- [4].(a) Selected examples: Leow D, Li G, Mei T-S, Yu J-Q, Nature 2012, 486, 518; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wan L, Dastbaravardeh N, Li G, Yu J-Q, J. Am. Chem. Soc 2013, 135, 18056; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yang G, Lindovska P, Zhu D, Kim J, Wang P, Tang R-Y, Movassaghi M, Yu J-Q, J. Am. Chem. Soc 2014, 136, 10807; [DOI] [PubMed] [Google Scholar]; d) Chu L, Shang M, Tanaka K, Chen Q, Pissarnitski N, Streckfuss E, Yu J-Q, ACS Cent. Sci 2015, 1, 394; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Dey A, Sinha SK, Achar TK, Maiti D, Angew. Chem., Int. Ed 2019, 58, 10820; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2019, 131,10934; [Google Scholar]; f) Xu H-J, Kang Y-S, Shi H, Zhang P, Chen Y-K, Zhang B, Liu Z-Q, Zhao J, Sun W-Y, Yu J-Q, Lu Y, J. Am. Chem. Soc 2019, 141, 76; [DOI] [PubMed] [Google Scholar]; g) Dutta U, Maiti S, Pimparkar S, Maiti S, Gahan LR, Krenske EH, Lupton DW, Maiti D, Chem. Sci 2019, 10, 7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].(a) Zhang Z, Tanaka K, Yu J-Q, J.-Q, Nature 2017, 543, 538; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu L-Y, Qiao JX, Yeung K-S, Ewing WR, Yu J-Q, J. Am. Chem. Soc 2019, 141, 14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].(a) Davis HJ, Phipps RJ, Chem. Sci 2017, 8, 864; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Roosen PC, Kallepalli VA, Chattopadhyay B, Singleton DA, Maleczka RE, Smith MR, J. Am. Chem. Soc 2012, 134, 11350; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kuninobu Y, Ida H, Nishi M, Kanai M, Nat. Chem 2015, 7, 712; [DOI] [PubMed] [Google Scholar]; d) Davis HJ, Genov GR, Phipps RJ, Angew. Chem., Int. Ed 2017, 56, 13351; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem 2017, 129, 13536; [Google Scholar]; e) Mihai MT, Davis HJ, Genov GR, Phipps RJ, ACS Catal. 2018, 8, 3764; [Google Scholar]; f) Hoque ME, Bisht R, Halder C, Chattopadhyay B, J. Am. Chem. Soc 2017, 139, 7745; [DOI] [PubMed] [Google Scholar]; g) Li HL, Kuninobu Y, Kanai M, Angew. Chem., Int. Ed 2017, 56, 1495; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2017, 129, 1517; [Google Scholar]; h) Lu X, Yoshigoe Y, Ida H, Nishi M, Kanai M, Kuninobu Y, ACS Catal. 2019, 9, 1705; [Google Scholar]; i) Bisht R, Hoque ME, Chattopadhyay B, Angew. Chem., Int. Ed 2018, 57, 15762; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2018, 130, 15988; [Google Scholar]; j) Goswami N, Sinda SK, Mondal P, Adhya S, Datta A, Maiti D, DOI: 10.26434/chemrxiv-2022-4v1l4. [DOI] [Google Scholar]
- [7].a) Yang L, Uemura N, Nakao Y, J. Am. Chem. Soc 2019, 141, 7972; [DOI] [PubMed] [Google Scholar]; b) Zhang T, Luan Y-X, Lam N, Li J-F, Li Y, Ye M, Yu J-Q, Nat. Chem 2021, 13, 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shi H, Lu Y, Weng J, Bay KL, Chen X, Tanaka K, Verma P, Houk KN, Yu J-Q, Nat. Chem 2020, 12, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) For selected examples of transition metal-catalyzed ortho- and meta-C─H functionalizations of 2-phenylpyridines: Sambiagio C, Schönbauer D, Blieck R, Dao-Huy T, Pototschnig G, Schaaf P, Wiesinger T, Zia MF, Wencel-Delord J, Besset T, Maes BUW, Schnürch M, Chem. Soc. Rev 2018, 47, 6603; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Kohn G, Whittlesey MK, Frost CG, J. Am. Chem. Soc 2011, 133, 19298; [DOI] [PubMed] [Google Scholar]; c) Hofmann N, Ackermann L, J. Am. Chem. Soc 2013, 135, 5877; [DOI] [PubMed] [Google Scholar]; d) Yu Q, Hu L, Wang Y, Zheng S, Huang J, Angew. Chem. Int. Ed 2015, 54, 15284; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2015, 127, 15499; [Google Scholar]; e) Fan Z, Ni J, Zhang A, J. Am. Chem. Soc 2016, 138, 8470; [DOI] [PubMed] [Google Scholar]; f) Fan Z, Lu H, Cheng Z, Zhang A, Chem. Commun 2018, 54, 6008; [DOI] [PubMed] [Google Scholar]; g) Gandeepan P, Koeller J, Korvorapun K, Mohr J, Ackermann L, Angew. Chem. Int. Ed 2019, 58, 9820; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2019, 131, 9925. [Google Scholar]
- [10].Fan Z, Chen X, Tanaka K, Park HS, Lam NYS, Wong JJ, Houk KN, Yu J-Q, Nature 2022, 610, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].a) Wang P, Verma P, Xia G-Q, Shi J, Qiao JX, Tao S, Cheng PTW, Poss MA, Farmer ME, Yeung K-S, Yu J-Q, Nature 2017, 551, 489; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen H, Wedi P, Meyer T, Tavakoli G, van Gemmeren M, Angew. Chem., Int. Ed 2018, 57, 2497; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2018, 130, 2523; [Google Scholar]; c) Zhao D, Xu P, Ritter T, Chem. 2019, 5, 97; [Google Scholar]; d) Liu L-Y, Yeung K-S, Yu J-Q, Chem. Eur. J 2019, 25, 2199; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Mondal A, Chen H, Flӓmig L, Wedi P. van Gemmeren M, J. Am. Chem. Soc 2019, 141, 18662. [DOI] [PubMed] [Google Scholar]
- [12].a) Zhang Y-H, Yu J-Q, J. Am. Chem. Soc 2009, 131, 14654; [DOI] [PubMed] [Google Scholar]; b) Fang L, Saint-Denis TG, Taylor BLH, Ahlquist S, Hong K, Liu S, Han L, Houk KN, Yu J-Q, J. Am. Chem. Soc 2017, 139, 10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Roger C, Roberts JA, Muller L, Clin. Pharmacokinet 2018, 57, 559; [DOI] [PubMed] [Google Scholar]; b) Burdette SD, Trotman R, Clin. Infect. Dis 2015, 61, 1315; [DOI] [PubMed] [Google Scholar]; c) Limaye RP, Patil AN, J. Clin. Diagn. Res 2016, 10, FC17; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hoskin JL, Al-Hasan Y, Sabbagh MN, Nicotine Tob. Res 2019, 21, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.