Abstract
The activities of the oxazolidinone antibacterial agents eperezolid (PNU-100592) and linezolid (PNU-100766) were compared with that of vancomycin against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureus (n = 200), coagulase-negative staphylococci (n = 100), and vancomycin-susceptible and -resistant Enterococcus faecalis and Enterococcus faecium (n = 50). Eperezolid and linezolid demonstrated good in vitro inhibitory activity, regardless of methicillin susceptibility for staphylococci (MIC at which 90% of the isolates are inhibited [MIC90] range, 1 to 4 μg/ml) or vancomycin susceptibility for enterococci (MIC90 range, 1 to 4 μg/ml). In time-kill studies, eperezolid and linezolid were bacteriostatic in action. A postantibiotic effect of 0.8 ± 0.5 h was demonstrated for both eperezolid and linezolid against S. aureus, S. epidermidis, E. faecalis, and E. faecium.
Eperezolid (PNU-100592 [formally U-100592]) and linezolid (PNU-100766 [formally U-100766]) are members of the new synthetic class of antibacterial compounds known as the oxazolidinones. Initial screening of these compounds indicated that they are active against a variety of gram-positive organisms, including methicillin-resistant strains of Staphylococcus aureus and Staphylococcus epidermidis; Enterococcus spp., including vancomycin-resistant strains; and Streptococcus spp., including viridans streptococci and penicillin-resistant pneumococci. These compounds also demonstrate activity against Corynebacterium spp., Bacteroides fragilis, Moraxella catarrhalis, Listeria monocytogenes, and strains of Mycobacterium tuberculosis (1, 2–4, 9, 10, 12, 14, 16). Although the exact mechanism of action is unknown, structure-activity investigations have determined that these compounds are bacteriostatic and exert their mechanism of action by protein synthesis inhibition (4–6). In addition, spontaneous mutations resulting in resistance among staphylococci occur rarely, and there appears to be no cross-resistance between these compounds and other antibacterial agents (10, 17). Newer agents with unique activity against multi-drug-resistant gram-positive organisms are clearly needed. We investigated the in vitro activities of the oxazolidinones eperezolid and linezolid versus that of vancomycin against various clinical strains of methicillin-susceptible and -resistant staphylococci and vancomycin-susceptible and -resistant enterococci.
Susceptibility-grade powders for eperezolid and linezolid were supplied by Pharmacia and Upjohn, Inc., Kalamazoo, Mich. Vancomycin susceptibility powder was purchased commercially (Sigma Chemical Co., St. Louis, Mo.).
Clinical isolates of Staphylococcus aureus, S. epidermidis, various other coagulase-negative staphylococci, E. faecalis, and E. faecium were collected over a 6-month period from hospitalized patients at Detroit Receiving Hospital and University Health Center, Detroit, Mich. Methicillin susceptibility was determined by the oxacillin disk method (13).
MICs were determined by a microdilution method with Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented (SMHB) with calcium (25 mg/liter) and magnesium (12.5 mg/liter). Susceptibility testing for each drug was performed according to the guidelines of the National Committee for Clinical Laboratory Standards (13).
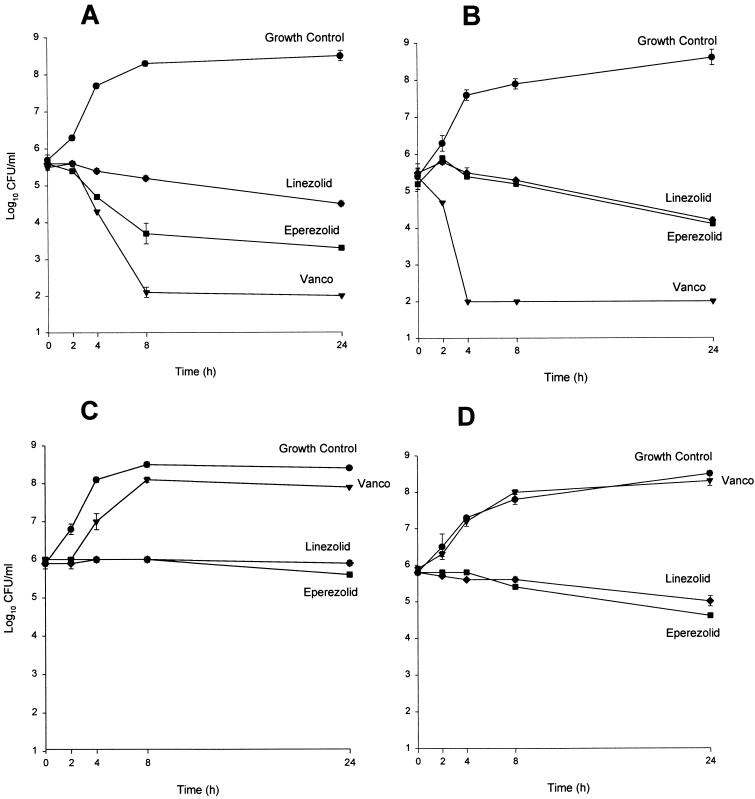
The bactericidal activities of eperezolid and linezolid were compared to that of vancomycin by use of time-kill analyses. Four representative clinical isolates (methicillin-resistant S. aureus R323, methicillin-susceptible S. epidermidis R264, vancomycin-resistant E. faecalis R581, and vancomycin-resistant E. faecium R20) were evaluated. The test strains were grown overnight at 35°C in SMHB and diluted to yield a starting inoculum of 106 CFU/ml. Sufficient stock antibiotic solution was added to achieve a desired concentration of four times the respective MICs. Growth controls were prepared in a similar fashion with substitution of the appropriate medium in place of the stock antibiotic solution. All tubes were incubated at 35°C with constant rotation for 24 h. Samples (0.1 ml) were removed at 0, 4, 8, and 24 h; diluted at least 250-fold with 0.9% sodium chloride to reduce antibiotic carryover; and plated on tryptic soy agar (Difco). The limit of detection for this method is 30 CFU/plate, corresponding to 300 CFU/ml (11). At time points at which bacterial counts were expected to be below limits of detection, 0.1-ml samples were placed in 10 ml of cold 0.9% sodium chloride and filtered by a 0.45-μm-pore-diameter filter (Millipore, Bedford, Mass.). Filters were placed aseptically on tryptic soy agar and incubated for 24 h. The limit of detection for this method is 10 CFU/plate, corresponding to 100 CFU/ml (11). All time-kill-curve experiments were performed in duplicate.
The presence of a postantibiotic effect (PAE) was determined for eperezolid, linezolid, and vancomycin for representatives of each group of organisms by the method described by Craig and Gudmundsson (3). An overnight growth of S. aureus, S. epidermidis, E. faecalis, or E. faecium was diluted into fresh SMHB to 106 CFU/ml and then incubated on a rotor at 37°C for 3 to 4 h until the logarithmic growth phase was achieved. At the end of this period, the inoculum size was determined, and each tube containing the test organisms was then exposed to eperezolid, linezolid, or vancomycin at the MIC and at four times the MIC for 1 h at 37°C on a rotor. One test tube of each organism was also used as a growth control and was subjected to the same procedures as described above but was not exposed to the antibiotic. Following incubation with the antibiotic, the cultures, including the growth controls, were diluted 1:1,000 into 10 ml of fresh prewarmed SMHB and reincubated at 37°C. Samples were removed in duplicate every 1.0 h and plated onto tryptic soy agar to determine the PAE. Each PAE experiment was performed in duplicate. The duration of the PAE was calculated by the equation PAE = T − C, where T is the time required for the CFU count in the culture exposed to antibiotic to increase 1 log10 unit above the count observed immediately after antibiotic removal and C is the time required for the CFU count in the control to increase 1 log10 unit above the count observed immediately after the same procedure used on the test culture for the antibiotic removal.
The activities of eperezolid and linezolid compared to that of vancomycin are shown in Table 1. For S. aureus, vancomycin was one- to twofold more active than eperezolid and linezolid. The oxazolidinones were equipotent to vancomycin against all coagulase-negative staphylococci tested. Compound eperezolid was at least one- to twofold more active than linezolid against coagulase-negative staphylococci. In time-kill studies, eperezolid and linezolid displayed bacteriostatic action against all isolates tested. As expected, vancomycin displayed bactericidal activity against S. aureus and S. epidermidis but not E. faecalis or E. faecium (Fig. 1).
TABLE 1.
Eperezolid (PNU-100592), linezolid (PNU-100766), and vancomycin activities against selected pathogens
| Organism (no. of isolates) | Agent | MIC (μg/ml)a
|
||
|---|---|---|---|---|
| MIC range | 50% | 90% | ||
| S. aureus | ||||
| Methicillin susceptible (100) | Eperezolid | 1.0–8.0 | 2.0 | 2.0 |
| Linezolid | 1.0–8.0 | 2.0 | 4.0 | |
| Vancomycin | 0.25–1.0 | 0.5 | 1.0 | |
| Methicillin resistant (100) | Eperezolid | 1.0–8.0 | 2.0 | 4.0 |
| Linezolid | 1.0–8.0 | 2.0 | 4.0 | |
| Vancomycin | 0.25–2.0 | 1.0 | 1.0 | |
| S. epidermidis | ||||
| Methicillin-susceptible (24) | Eperezolid | 0.5–2.0 | 1.0 | 1.0 |
| Linezolid | 1.0–2.0 | 1.0 | 2.0 | |
| Vancomycin | 0.25–2.0 | 1.0 | 2.0 | |
| Methicillin-resistant (35) | Eperezolid | 0.5–2.0 | 1.0 | 1.0 |
| Linezolid | 0.5–4.0 | 1.0 | 2.0 | |
| Vancomycin | 0.5–2.0 | 1.0 | 2.0 | |
| Coagulase-negative staphylococcus (non-S. epidermidis) | ||||
| Methicillin-susceptible (25) | Eperezolid | 0.5–2.0 | 1.0 | 1.0 |
| Linezolid | 0.5–2.0 | 1.0 | 2.0 | |
| Vancomycin | 0.13–2.0 | 1.0 | 1.0 | |
| Methicillin-resistant (16) | Eperezolid | 1.0–2.0 | 1.0 | 1.0 |
| Linezolid | 1.0–2.0 | 1.0 | 2.0 | |
| Vancomycin | 0.5–2.0 | 1.0 | 2.0 | |
| E. faecalis (25)b | Eperezolid | 1.0–4.0 | 1.0 | 4.0 |
| Linezolid | 1.0–4.0 | 2.0 | 4.0 | |
| Vancomycin | 0.5–>64.0 | 1.0 | >64.0 | |
| E. faecium (25)b | Eperezolid | 0.5–4.0 | 2.0 | 2.0 |
| Linezolid | 0.5–4.0 | 2.0 | 2.0 | |
| Vancomycin | 0.5–>64.0 | >64.0 | >64.0 | |
50% and 90%, MICs for 50 and 90% of the isolates, respectively.
Includes 20 vancomycin-resistant isolates.
FIG. 1.
Time-kill experiments performed in duplicate. Results are means ± standard deviations. (A and B) Methicillin-resistant S. aureus (R323) and methicillin-susceptible S. epidermidis (R264), respectively. (C and D) Vancomycin (Vanco)-resistant E. faecalis (R581) and E. faecium (R20), respectively.
The oxazolidinones represent a unique class of synthetic antimicrobials that have activity against a wide variety of problematic pathogens, including methicillin-resistant staphylococci and vancomycin-resistant enterococci (7, 9, 10, 12, 14, 16, 17). Other unique features of these compounds include their novel mechanism of action, for which they do not display cross-resistant activity with other classes of antimicrobials, and the fact that the spontaneous rate of mutation to resistance for these compounds is very low. This may translate to a low incidence of resistance developing during therapy (4–6, 10, 17). Our susceptibility data are similar to those reported by other investigators in that the MICs of eperezolid, in general, were 1 to 2 dilutions lower than those of linezolid against coagulase-negative staphylococci (10). However, this difference in susceptibility was minimal and was not noted for S. aureus or for the enterococci tested. Previous investigators have demonstrated that eperezolid and linezolid display bacteriostatic activity and do not display concentration-dependent killing (9). Our time-kill-curve experiments also demonstrated bacteriostatic activity, as opposed to the bactericidal activity displayed by vancomycin for vancomycin-susceptible organisms. We demonstrated that both oxazolidinones possess a PAE and that the PAEs of eperezolid and linezolid were similar (Table 2). The PAE was greater at four times the MIC (range, 0.2 to 1.4 h) than at the MIC (0.1 to 0.8 h) for both compounds against all organisms tested. This was also true for vancomycin against staphylococci (four times the MIC, 1.1 to 2.9; MIC, 0 to 1.9 h). The PAE for eperezolid and linezolid was considerably lower against the E. faecalis isolate than against the E. faecium and staphylococci isolates. This was an interesting finding, since the MICs for E. faecalis tended to be slightly higher than those for the E. faecium isolates tested. This trend was also apparent upon visual inspection of the killing curves. A similar phenomenon has been reported with the investigational antibiotic RP 59500 (15). Currently, the mechanism behind the apparent differences in activity of RP 59500 against E. faecium versus E. faecalis is not known. The combination of an antibiotic’s pharmacokinetic profile and its PAE is important in determining the most appropriate dosing interval. Our data indicate that the PAEs for these two oxazolidinones are relatively short. However, based upon preliminary pharmacokinetic studies which have reported a serum half-life of 6 h for the oxazolidinones, the PAE, which has a tendency to be longer in vivo, may allow these agents to be given at intervals of 8 to 12 h or longer (8). Human studies to determine the appropriate dosage range based upon pharmacokinetic-pharmacodynamic relationships such as area under the concentration-time curve–MIC and time above the MIC are needed to assess the true potential of this novel class of antimicrobials.
TABLE 2.
PAEs for eperezolid (PNU-100592), linezolid (PNU-100766), and vancomycin activities against selected pathogens
| Organism | Agent | PAE (h)a
|
|
|---|---|---|---|
| 1× MIC | 4× MIC | ||
| S. aureus R323 (methicillin resistant) | Eperezolid | 0.5 ± 0.1 | 0.9 ± 0.2 |
| Linezolid | 0.5 ± 0.2 | 0.6 ± 0.0 | |
| Vancomycin | 1.9 ± 0.0 | 2.9 ± 0.0 | |
| S. epidermidis R264 (methicillin susceptible) | Eperezolid | 0.2 ± 0.3 | 0.9 ± 0.2 |
| Linezolid | 0.3 ± 0.5 | 1.1 ± 0.9 | |
| Vancomycin | 0.0 ± 0.0 | 2.3 ± 0.2 | |
| E. faecalis R581 (vancomycin resistant) | Eperezolid | 0.1 ± 0.0 | 0.2 ± 0.0 |
| Linezolid | <0.1 ± 0.4 | 0.1 ± 0.0 | |
| Vancomycin | NDb | ND | |
| E. faecium R20 (vancomycin resistant) | Eperezolid | 0.7 ± 0.4 | 1.2 ± 0.6 |
| Linezolid | 0.8 ± 0.5 | 1.4 ± 0.3 | |
| Vancomycin | ND | ND | |
Results are means ± standard deviations for two PAE experiments.
ND, not done.
Acknowledgments
This study was supported in part by a grant from Pharmacia and Upjohn, Kalamazoo, Mich.
REFERENCES
- 1.Barry A L. In vitro evaluation of DuP 105 and DuP 721, two new oxazolidinone antimicrobial agents. Antimicrob Agents Chemother. 1988;32:150–152. doi: 10.1128/aac.32.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brumfitt W, Hamilton-Miller J M T. Antibacterial oxazolidinones. In vitro activity of a new analogue, E3709. Diagn Microbiol Infect Dis. 1992;15:621–624. doi: 10.1016/0732-8893(90)90040-3. [DOI] [PubMed] [Google Scholar]
- 3.Craig W A, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 403–431. [Google Scholar]
- 4.Daly J S, Eliopolous G M, Reiszner E, Moellering R C., Jr Mechanism of action and activity of DuP 105 and DuP 721, new oxazolidinone compounds. J Antimicrob Chemother. 1988;21:721–730. doi: 10.1093/jac/21.6.721. [DOI] [PubMed] [Google Scholar]
- 5.Daly J S, Eliopolous G M, Willey S, Moellering R C., Jr Mechanism of action and in vitro and in vivo activities of S-6123, a new oxazolidinone compound. Antimicrob Agents Chemother. 1988;32:1341–1346. doi: 10.1128/aac.32.9.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eustice D C, Felman P A, Zajac I, Slee A M. Mechanism of action of DuP 721: inhibition of an early event during initiation of protein synthesis. Antimicrob Agents Chemother. 1988;32:1218–1222. doi: 10.1128/aac.32.8.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford C W, Hamel J C, Wilson D M, Moerman J K, Stapert D, Yancey R J, Jr, Hutchinson D K, Barbachyn M R, Brickner S J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antimicrobial agents against experimental bacterial infections. Antimicrob Agents Chemother. 1996;40:1508–1513. doi: 10.1128/aac.40.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford, C. W. Personal communication.
- 9.Jones R N, Johnson D M, Erwin M E. In vitro antimicrobial activities and spectra of U-100592 and U-100766, two novel fluorinated oxazolidinones. Antimicrob Agents Chemother. 1996;40:720–726. doi: 10.1128/aac.40.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaatz G W, Seo S M. In vitro activities of oxazolidinone compounds U100592 and U100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1996;40:799–801. doi: 10.1128/aac.40.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGrath B J, Kang S L, Kaatz G W, Rybak M J. Bactericidal activities of teicoplanin, vancomycin, and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob Agents Chemother. 1994;38:2034–2040. doi: 10.1128/aac.38.9.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulazimoglu L, Drenning S D, Yu V L. In vitro activities of two novel oxazolidinones (U100592 and U100766), a new fluoroquinolone (trovafloxacin), and dalfopristin-quinupristin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1996;40:2428–2430. doi: 10.1128/aac.40.10.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Approved standard M7-A2. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 2nd ed. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 14.Neu H C, Novelli A, Saha G, Chin N-X. In vitro activities of two oxazolidinone antimicrobial agents, DuP 721 and DuP 105. Antimicrob Agents Chemother. 1988;32:580–583. doi: 10.1128/aac.32.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pechere, J. C. 1992. In vitro-activity of RP 59500, a semisynthetic streptogramin, against staphylococci and streptococci. J. Antimicrob. Chemother. 30(Suppl. A):15–18. [DOI] [PubMed]
- 16.Slee A M, Wuonola M A, McRipley R J, Zajac I, Zawada M J, Bartholomew P T, Gregory W A, Forbes M. Oxazolidinones, a new class of synthetic antibacterial agents: in vitro and in vivo activities of DuP 105 and DuP 721. Antimicrob Agents Chemother. 1987;31:1791–1797. doi: 10.1128/aac.31.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zurenko G E, Yagi B H, Schaadt R D, Allison J W, Kilburn J O, Glickman S E, Hutchinson D K, Barbachyn M R, Brickner S J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother. 1996;40:839–845. doi: 10.1128/aac.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]