Abstract
Objective:
Adverse childhood experiences (ACEs) contribute to elevations in neuropsychiatric and neurocognitive symptoms in HIV+ adults. Emerging data suggest that exposures to threat-related and deprivation-related ACEs may have differential impacts on function, with threat exposure contributing to neuropsychiatric symptoms, and deprivation contributing to executive dysfunction. Yet, it remains unclear how specific types of ACEs impact neuropsychiatric and neurocognitive symptoms in HIV+ adults. Hence, the current study examined whether these two dimensions of adversity contribute differentially to neuropsychiatric symptoms and executive dysfunction in HIV+ adults.
Methods:
We included a sample of demographically-matched HIV+ (N=72) and HIV-negative (N=85) adults. Standardized self-report measures assessed threat-related (interpersonal violence) and deprivation-related (poverty/neglect) ACEs, as well as neuropsychiatric symptoms (depression, anxiety, apathy). A brief battery of neuropsychological tests assessed executive functions.
Results:
Compared to HIV-negative participants, HIV+ participants reported significantly higher rates of threat exposure (51% vs. 67%, p=.04), while rates of deprivation did not differ significantly (8% vs. 13%, p=.38). In the HIV+ sample, threat exposure was associated with neuropsychiatric symptoms (p<.01) but not executive dysfunction (p=.75). By contrast, deprivation was associated with executive dysfunction, at a trend level (p=.09), but not with neuropsychiatric symptoms (p=.70).
Conclusions:
Our data suggest that, relative to HIV-negative samples, HIV+ samples experience higher rates of threat-related ACEs, which contribute to neuropsychiatric symptom elevations. Moreover, our preliminary findings suggest that different types of ACEs could be associated with different profiles of neuropsychiatric and neurocognitive difficulty in HIV+ adults, highlighting the importance of considering dimensions of adversity in future studies.
Keywords: Adverse Childhood Experiences, Early-Life Stress, Childhood Abuse, Childhood Trauma, Child Neglect, Affect
Introduction
Relative to HIV-negative adults, adult persons living with HIV (PLWH) experience higher rates of neuropsychiatric symptoms, including depression, anxiety, and apathy (Benton, 2008; Bing et al., 2001; Bogdanova, Diaz-Santos, & Cronin-Golomb, 2010; Clark, Cohen, Westbrook, Devlin, & Tashima, 2010; Clark et al., 2015; Do et al., 2014; Morrison et al., 2002), as well as neurocognitive difficulties (Clark & Cohen, 2010; Heaton et al., 2010), particularly in the area of executive function (EF) (Heaton et al., 2011). Notably, a burgeoning line of research has begun to reveal that, in adult PLWH, high levels of early-life stress (ELS) exposure contribute to increases in neuropsychiatric and neurocognitive symptoms (Clark, Arce Rentería, Hegde, & Morgello, 2018; Clark et al., 2012; Clark, Sweet, Morgello, Philip, & Cohen, 2017; Spies, Fennema-Notestine, Archibald, Cherner, & Seedat, 2012; Womersley, Seedat, & Hemmings, 2017). These recent findings align with a larger body of research indicating that high ELS exposure increases vulnerability to neuropsychiatric and neurocognitive symptoms in non-HIV samples (Hedges & Woon, 2011; Pechtel & Pizzagalli, 2011; Philip et al., 2013; Teicher & Samson, 2016; Teicher, Samson, Anderson, & Ohashi, 2016).
There is substantial evidence to suggest that levels of ELS exposure are elevated in HIV+ cohorts relative to non-HIV samples (Machtinger, Wilson, Haberer, & Weiss, 2012; Paxton, Myers, Hall, & Javanbakht, 2004; Spies, Afifi, et al., 2012). Although few studies have conducted direct comparisons of ELS exposure rates in demographically-matched HIV+ and HIV-negative samples (Reisner, Falb, & Mimiaga, 2011), rates of abuse and other forms of ELS in HIV+ cohorts range from 32% to 76% (Simoni & Ng, 2000; Spies, Afifi, et al., 2012; Villar-Loubet et al., 2014; Walton et al., 2011; Whetten et al., 2006), compared to 14% to 32% reported in non-HIV samples (Spies, Afifi, et al., 2012). Collectively, these studies suggest that HIV+ samples experience an increased burden of ELS exposure relative to non-HIV samples. Such data underscore the critical need to understand the effects of high ELS in HIV+ samples.
Yet, in order to develop appropriate prevention and intervention strategies for PLWH who have experienced high ELS we may need to develop a more nuanced understanding of the effects of ELS exposure on functional outcomes in this population. Studies examining the effects of high ELS on neuropsychiatric and neurocognitive outcomes in PLWH have primarily focused on the total number of adverse childhood experiences (ACEs), due in part to the evidence that ACEs commonly co-occur (Dong et al., 2004). Such an approach assumes that different types of adversity impact development in a similar manner, but converging evidence from animal and human research suggests that different types of ACEs may have differential effects on neuropsychiatric and neurocognitive outcomes (McLaughlin, Sheridan, & Lambert, 2014). Furthermore, because ACEs commonly co-occur, investigations that examine only cumulative effects fail to tease apart, and can thus obscure, the independent impacts that specific types of adversities have on different outcomes (Lambert, King, Monahan, & McLaughlin, 2017).
An emerging line of research examining two dimensions of ACEs, threat exposure (experiences that represent a threat to one’s physical integrity) and deprivation (an absence of expected cognitive and social inputs) (Sheridan & McLaughlin, 2014), hypothesizes that threat-related stressors may contribute uniquely to affective dysfunction, whereas deprivation-related stressors may have greater effects on cognition (e.g., associative learning) (McLaughlin, Sheridan, & Nelson, 2017), including higher-order EFs (Lambert et al., 2017; Letkiewicz, Funkhouser, & Shankman, 2021). While it remains unclear whether a true double dissociation exists, this hypothesis is supported by data indicating that experiences of abuse in childhood significantly increase the risk of developing depression and anxiety in adulthood (Li, D’Arcy, & Meng, 2016), whereas studies examining the effects of early-life deprivation (e.g., low socioeconomic status, physical neglect, institutionalization) commonly reveal significant effects on EF, including reductions in working memory, cognitive flexibility, inhibition, and planning (Bos, Fox, Zeanah, & Nelson III, 2009; Farah et al., 2006; Haft & Hoeft, 2017; McLaughlin, 2016; Raver, Blair, & Willoughby, 2013). This differential pattern of effects is also reflected in data from animal research and human neuroimaging studies, which indicate that threat-related experiences are associated with abnormalities in brain regions involved in affective processing (e.g., amygdala), whereas early-life deprivation has greater impacts on neural regions implicated in EF (e.g., dorsolateral prefrontal cortex, anterior cingulate) (McCrory et al., 2013; McCrory et al., 2011; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015; McLaughlin et al., 2014; Sheridan, Sarsour, Jutte, D’Esposito, & Boyce, 2012).
Similarly, recent data from an fMRI study conducted with an adolescent/young adult sample (13-20 years old) showed that deprivation was independently associated with prefrontal and parietal activation during an EF task (working memory), whereas levels of threat were unrelated to these measures (Sheridan, Peverill, Finn, & McLaughlin, 2017). In another study conducted in a sample of young children (4-7 years old), researchers found that deprivation, but not threat, was associated with reductions in EF (cognitive control), whereas threat, but not deprivation, was associated with affective functions (fear learning) (Machlin, Miller, Snyder, McLaughlin, & Sheridan, 2019). While some studies report that both threat-related and deprivation-related childhood adversities impact affective symptoms in non-clinical adult samples (Chu, Williams, Harris, Bryant, & Gatt, 2013; Li et al., 2016), and deprivation may impact affective brain regions in children (Noble, Houston, Kan, & Sowell, 2012), there is evidence that some of the observed overlap in the effects of threat and deprivation on mental health outcomes may arise due to a lack of controlling for comorbid exposures. For example, data from a study conducted with adolescents (16-17 years old) suggest that threat exposure is associated with emotion regulation abilities, but not cognitive control, whereas deprivation exposure (poverty) is associated with cognitive control, but not emotion regulation (Lambert et al., 2017). Notably, the specificity of these associations was not apparent when researchers assessed each adversity type in isolation. That is, the specificity of these associations was only revealed when using a multivariate approach that controlled for co-occurring exposures. Taken as a whole, these data suggest that an examination of the unique contributions of specific types of ACEs, grouped according to their underlying dimensions, may provide a more nuanced understanding of the relation between ELS and functional outcomes in PLWH.
We currently lack a comprehensive understanding of ELS-related effects in PLWH. Relatively few studies have examined ELS effects on mental health outcomes in PLWH, despite known elevations in neuropsychiatric and neurocognitive symptoms relative to HIV-negative samples and overlaps in HIV-related and ELS-related neuropathology (Clark et al., 2015; Cohen, Grieve, et al., 2006). Because PLWH exhibit greater neuropsychiatric and neurocognitive symptoms in the context of stress relative to HIV-negative samples (e.g., Clark et al., 2017; Watson et al., 2019), we sought to gain a better understanding of whether and how ELS effects, specifically early-life threat and deprivation exposures, impact mental health outcomes in PLWH. Our first aim in this preliminary study was to compare the rates of threat-related and deprivation-related ACEs reported in a cohort of adult PLWH and HIV-negative adults matched on key demographic factors associated with ELS risk (e.g., race) (Slopen et al., 2016). Our second goal was to utilize a multivariate approach to simultaneously examine whether two dimensions of adversity, threat and deprivation, contribute differentially to neuropsychiatric symptoms and executive dysfunction in PLWH. We predicted that threat exposure would be associated with greater neuropsychiatric symptoms and deprivation with greater EF impairments. Altogether, these analyses aim to better characterize the nature of, and specific risks associated with, ELS in PLWH.
Methods
Participants
Seventy-two adult PLWH and 85 HIV-negative control (HC) adults were recruited from the Mount Sinai Hospital in New York, NY and The Miriam Hospital in Providence, RI. One investigator (USC) oversaw all procedures. The Institutional Review Boards at the Icahn School of Medicine at Mount Sinai and The Miriam Hospital approved this research. All participants gave their informed written consent and were financially compensated for their time.
Inclusion criteria required that participants were between 21 and 70 years of age, right-handed, completed 8 or more years of education, and were native English speakers. All participants obtained a score of ≥ 25 points on the Mini-Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975). HIV serostatus was documented by ELISA and confirmed by Western blot test. All participants were enrolled in studies of ELS exposure; hence, the sample was selected such that degree of high ELS exposure was roughly balanced within and across our groups (Table 1). Here, as in prior studies (Clark et al., 2018; Clark et al., 2012; Clark et al., 2017; Seckfort et al., 2008), high ELS exposure was defined as an endorsement of 3 or more ACEs on the Early Life Stress Questionnaire (ELSQ) (Cohen, Paul, et al., 2006) and low ELS as an endorsement of fewer than 3 ACEs.
Table 1.
Demographic, neuropsychiatric, and cognitive characteristics of the participant groups
| HIV+ (N=72) |
HC (N=85) |
||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Mean | SD | Mean | SD | F/t/χ2 | df | p | |
| Demographic Characteristics | |||||||
| Recruitment Site (% New York, NY) | 76 | 81 | 0.54 | 1 | 0.46 | ||
| % Providence, RI | 24 | 19 | |||||
| Age (years) | 44.38 | 9.74 | 47.87 | 11.64 | 2.02 | 155 | 0.05 |
| % Male | 64 | 58 | 0.64 | 1 | 0.43 | ||
| WTAR (SS) | 100.42 | 16.96 | 104.35 | 14.43 | 1.54 | 150 | 0.13 |
| Racial Composition (% Caucasian) | 18 | 20 | 0.10 | 1 | 0.76 | ||
| % African American | 69 | 61 | |||||
| % Asian American | 1 | ||||||
| % Native American | 1 | 1 | |||||
| % Bi/Multiracial | 7 | 9 | |||||
| % Other | 3 | 8 | |||||
| Ethnic Composition (% Hispanic) | 14 | 24 | 2.34 | 1 | 0.13 | ||
| % Hepatitis C Positive | 13 | 3 | 5.58 | 1 | 0.02 | ||
| KMSK – Alcohol (/13) | 7.64 | 3.49 | 5.82 | 3.60 | 3.19 | 155 | <0.01 |
| KMSK – Cocaine (/16) | 6.43 | 6.38 | 2.00 | 4.45 | 4.96 | 123.8 | <0.01 |
| KMSK – Opiate (/13) | 0.82 | 2.50 | 0.58 | 2.06 | 0.67 | 155 | 0.51 |
| Current Stress – PSS (/56) | 17.01 | 7.81 | 17.61 | 8.88 | 0.44 | 155 | 0.66 |
| % with High ELS Status | 56 | 46 | 1.46 | 1 | 0.23 | ||
| Number of ACEs | 3.39 | 2.62 | 2.80 | 2.70 | 1.38 | 155 | 0.17 |
| % with Threat Exposure | 67 | 51 | 4.14 | 1 | 0.04 | ||
| % with Deprivation Exposure | 13 | 8 | 0.78 | 1 | 0.38 | ||
| HIV-Disease Variables | |||||||
| Nadir CD4 (cells/μL) | 235.90 | 209.23 | |||||
| Current CD4 (cells/μL) | 664.58 | 328.77 | |||||
| Current log10 HIVL | 1.97 | 1.03 | |||||
| % with HIVL Below 50 Copies/mL | 57 | ||||||
| Length of HIV Infection (years) | 15.15 | 7.05 | |||||
| % on ARV Medications | 100 | ||||||
| Neuropsychiatric & Executive Function Indices | |||||||
| Neuropsychiatric Composite (z-score)1 | 0.23 | 0.87 | 0.00 | 0.85 | 3.45 | 1,142 | 0.07 |
| Executive Function Composite (z-score)1 | −0.54 | 1.28 | −0.35 | 1.14 | 1.45 | 1,141 | 0.23 |
Note: ACEs = adverse childhood experiences; ARV = antiretroviral; ELS = early life stress; HC = HIV-negative Control; HIVL = HIV viral load; KMSK = Kreek-McHugh-Schluger-Kellogg scale; PSS = Perceived Stress Scale; SS = Standard Score; WTAR = Wechsler Test of Adult Reading.
Analysis controls for demographic variables on which the groups differed significantly (KMSK-A, KMSK-C, hepatitis C status).
Exclusion criteria included self-reported history of uncorrected abnormal vision; developmental disability; learning disability; major psychiatric illness (e.g., bipolar disorder, posttraumatic stress disorder, major depressive disorder, generalized anxiety disorder, attention deficit hyperactivity disorder); neurological illness affecting the central nervous system (e.g., stroke, progressive multifocal leukoencephalopathy); and traumatic head injury with loss of consciousness >10 minutes. Substance use exclusion criteria were reported current alcohol dependence as per the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998); use of heroin/opiates or any intravenous drug within the past 6 months; use of cocaine within the past month; and positive urine toxicology at the time of assessment (cocaine, opiates, methamphetamine, amphetamine, benzodiazepine, barbiturates, methadone, oxycodone).
Demographic Measures
Antiretroviral (ARV) use and nadir CD4 levels (i.e., the lowest ever CD4 T-cell count) and were obtained via self-report (Buisker, Dufour, & Myers, 2015; Cunningham, Rana, Shapiro, & Hays, 1997) and verified against the medical record. All PLWH were prescribed ARV medications. Current CD4 levels and plasma HIV viral loads (HIVL) were measured at the study visit. HIVL was log 10 transformed to normalize the distribution. Participants were assessed for hepatitis C virus (HCV) infection, defined as positive HCV antibody. HCV data were not available for 2 HIV+ and 8 HC participants. Alcohol and drug use histories for all participants were quantified using the Kreek-McHugh-Schluger-Kellogg scale (KMSK) (Kellogg et al., 2003), which provided 3 subscales characterizing lifetime consumption of alcohol (KMSK-A), cocaine (KMSK-C), and opiates (KMSK-O). The Wechsler Test of Adult Reading (WTAR) estimated intellectual function (Wechsler, 2001); scaled scores were derived using published normative data. WTAR scores were not available for 3 HIV+ and 2 HC participants. The Perceived Stress Scale (PSS) (S. Cohen, Kamarck, & Mermelstein, 1983) measured levels of current stress. See Table 1 for group characteristics.
Threat and Deprivation
ACEs were assessed using the Early Life Stress Questionnaire (ELSQ) (Cohen, Paul, et al., 2006), a widely-used measure (Baker et al., 2013; Clark et al., 2018; Clark et al., 2012; Clark et al., 2017; Cohen, Grieve, et al., 2006; Paul et al., 2008; Seckfort et al., 2008) that assesses the occurrence of 17 ACEs (e.g., parental divorce, sexual abuse) prior to age 18 years. To capture experiences related to the dimension of threat, consistent with prior research (Lambert et al., 2017), we examined items associated with experiences of interpersonal violence in the school, home, or neighborhood setting. Seven ACEs were included in this domain (sexual, physical, or emotional abuse; domestic abuse; bullying; family conflict; warfare). As in prior studies (Lambert et al., 2017), individuals who endorsed any ACE within this domain were assigned a threat score of 1; all others were assigned a threat score of 0. To capture experiences related to the overarching dimension of deprivation, consistent with prior research (Lambert et al., 2017; McLaughlin et al., 2014; Sheridan & McLaughlin, 2014), we examined items related to poverty and physical neglect. Three items from the ELSQ were included in this domain (not having enough to eat; lack of care provided by parent/caretaker; having to wear dirty clothes). As in prior studies (Lambert et al., 2017), individuals who endorsed any ACE within this domain were assigned a deprivation score of 1; all others were assigned a deprivation score of 0.
Neuropsychiatric Measures
Neuropsychiatric symptoms known to be elevated in PLWH were examined (Benton, 2008; Bing et al., 2001; Bogdanova et al., 2010; Ciesla & Roberts, 2001; Clark et al., 2012; Clark et al., 2010; Clark et al., 2017; Clark et al., 2015; Do et al., 2014; Morrison et al., 2002), including levels of current depression, anxiety, and apathy, quantified using the Center for Epidemiological Studies-Depression Scale (CESD) (Radloff, 1977), the Beck Anxiety Inventory (BAI) (Beck, Epstein, Brown, & Steer, 1988), and the Apathy Evaluation Scale (AES) (Marin, Biedrzycki, & Firinciogullari, 1991), respectively. As in prior studies (Clark et al., 2018; Clark et al., 2017), for each participant, z-scores were calculated for each measure based on the mean of the HC group for that measure; the three z-scores were then averaged to create a composite index score for each participant. Accordingly, this index z-score represents the overall degree of neuropsychiatric difficulty reported across the three domains assessed. To verify this, we conducted a principal components analysis (PCA) on the three measures (Bartlett’s test: χ2= 174.7, p < 0.001; KMO = 0.564). Only one component had an eigenvalue over 1; this component explained 70% of the total variance (all communalities >0.572). Across the entire sample, correlations between the three measures were significant (all rs >.339, ps < .001). In both the HIV+ and HC groups, component scores correlated strongly with composite index scores (HIV: r[72] = 0.99, p < 0.001; HC: r[85] = 0.99, p < 0.001), supporting the use of the composite (z-score) index in subsequent analyses. Higher scores indicate greater neuropsychiatric symptoms.
Executive Function Measures
Three neurocognitive tests sensitive in detecting HIV-related EF impairments (Heaton et al., 1995) were administered, including the Trail Making Test (TMT), Part B (Reitan & Davison, 1974), a test of verbal fluency (FAS) (Aita et al., 2019; Borkowski, Benton, & Spreen, 1967), and the WAIS-III Letter-Number Sequencing (LNS) (Wechsler, 1997), consistent with prior studies (Castelo, Courtney, Melrose, & Stern, 2007; Clark et al., 2012). Raw scores were computed to z-scores based on published normative data (Spreen & Strauss, 1998; Tombaugh, 2004; Wechsler, 1997), which permit comparisons with prior studies and which include age (TMT, FAS, WAIS-III) and education (TMT) corrections. Z-scores were averaged to compute a domain-specific composite score for each participant (Castelo et al., 2007; Clark et al., 2012). Hence, this score represents an overall average EF ability score. To verify this, we conducted a PCA on the three measures (Bartlett’s test: χ2= 67.5, p < 0.001; KMO = 0.663). Only one component had an eigenvalue over 1; this component explained 60% of the total variance (all communalities >0.597). Across the entire sample, correlations between the three measures were significant (all rs > .390, ps < .001). In both the HIV+ and HC groups, component scores correlated strongly with composite index scores (HIV: r[72] = 0.95, p < 0.001; HC: r[84] = 0.94, p < 0.001), supporting the use of the composite (z-score) index in subsequent analyses. Composite scores were not available for one HC participant. Lower scores indicate greater executive dysfunction.
Statistical Analyses
Differences in demographic and clinical factors between groups were assessed using independent-samples t-tests and chi-square tests. HIV+ and HC group differences in neuropsychiatric symptoms and EF were assessed using ANCOVAs; in these models, demographic variables on which the groups differed significantly were included as covariates. Partial eta-squared (ηp2) was used as an indicator of effect size, where values of .01, .06, and .14 indicate small, medium, and large effects, respectively (J. Cohen, 1988). Rates of threat and deprivation exposure in HIV+ and HC samples were compared using chi-square tests.
A primary aim was to assess whether early-life threat and deprivation exposures impact neuropsychiatric and neurocognitive outcomes in PLWH. Accordingly, we examined the association of threat and deprivation exposure to our outcome measures (neuropsychiatric symptom z-scores; EF z-scores) in the HIV+ sample using two linear regression analyses, one for each outcome measure. In each model, the outcome measure was entered as the dependent variable, and threat and deprivation scores were entered as predictor variables. Hence, each model examined the unique associations of threat and deprivation exposure to the outcome variable of interest. Covariates included demographic and clinical factors that differed significantly between HIV+ groups with and without threat or deprivation exposure (current CD4, prior alcohol use) to control for the potential effects that these factors might have on the dependent variable, and thus better assess unique associations between ACE exposures and the dependent variable. Multicollinearity checks were run for all regression models to ensure that correlations between predictor variables did not exceed acceptable limits (tolerance <0.2) (Menard, 1995). All statistical analyses were conducted using SPSS (version 24).
Results
Demographic Measures
Demographic data for the HIV+ and HC groups are reported in Table 1, including group means and statistics. HIV+ and HC groups did not differ significantly with respect to estimated intelligence (WTAR, p=.13), current stress levels (PSS, p=.66), or racial composition (p=.76); however, relative to the HC group, the HIV+ group reported higher levels of prior alcohol (p<.01) and cocaine (p<.01) use, and had higher rates of HCV infection (p=.02).
Among the HIV+ sample, we examined differences in demographic and clinical factors in those with and without threat exposure. Compared to HIV+ participants without threat exposure (N=24), those with threat exposure (N=48) exhibited higher current CD4 levels (499.7 vs. 747.0 cells/μL, respectively; t[70]=3.20, p<.01) and prior alcohol use (KMSK-A, 6.21 vs. 8.35, respectively; t[70]=2.55, p=.01); however, groups did not differ significantly in age (p=.84), sex (p=.86), estimated intelligence (p=.32), racial composition (p=.13), ethnicity (p=.81), current stress (p=.06), prior history of cocaine (p=.08) or opioid (p=.87) use, HCV status (p=.95), nadir CD4 (p=.31), or HIVL (p=.07). Comparisons of HIV+ participants with and without deprivation exposure (N=9 and N=63, respectively) were non-significant (ps >.05).
Threat and Deprivation
The HIV+ group reported higher rates of threat exposure than the HC group (67% vs. 51%, respectively; p=.04), while rates of deprivation did not differ significantly across the groups (13% vs. 8%, respectively; p=.38) (Table 1). Figure 1 shows the rates of threat, deprivation, and comorbid exposures in the HIV+ and HC groups. In addition, Table 2 shows exposure rates for individual ACEs in the HIV+ and HC groups.
Figure 1.

Percent of participants reporting early-life threat, deprivation, and comorbid exposures in the HIV+ and HC groups.
Table 2.
Percent of participants reporting threat-related and deprivation-related adverse childhood experiences (ACE) in the HIV+ and HC groups.
| HIV+ (N=72) |
HC (N=85) |
|
|---|---|---|
|
| ||
| % | % | |
| Threat | 67 | 51 |
| family conflict | 29 | 28 |
| bullied | 35 | 28 |
| warfare | 7 | 6 |
| domestic abuse | 14 | 7 |
| emotional abuse | 24 | 19 |
| physical abuse | 15 | 13 |
| sexual abuse | 26 | 9 |
|
| ||
| Deprivation | 13 | 8 |
| not enough to eat | 6 | 6 |
| neglect/lack of care | 7 | 4 |
| wore dirty clothes | 1 | 1 |
Neuropsychiatric Measures
The HIV+ group exhibited trend-level elevations in neuropsychiatric symptoms relative to the HC group when covarying for demographic variables on which the groups differed significantly (KMSK-A, KMSK-C, HCV status) (F[1,142]=3.45, p=.07, ηp2=.02) (Table 1).
Executive Function Measures
Rates of executive dysfunction did not differ significantly between HIV+ and HC participants (F[1,154]=.95, p=.33, ηp2=.01), even when covarying for demographic variables on which the groups differed significantly (KMSK-A, KMSK-C, HCV status) (p=.23, ηp2=.01) (Table 1).
Relation of Threat and Deprivation to Neuropsychiatric & Executive Functions in PLWH
A multivariate approach (Lambert et al., 2017) was used to examine whether threat and deprivation contributed uniquely to neuropsychiatric symptom elevations in PLWH. The model predicting neuropsychiatric symptoms revealed that threat exposure was significantly associated with neuropsychiatric symptom levels when controlling for deprivation (B=.59, 95% Confidence Interval[CI]=.17 – 1.01, β=.32 [t=2.79, p<.01]) (Figure 2). By contrast, deprivation was unrelated to neuropsychiatric symptoms when controlling for threat (B=.12, CI= −.49 – .72, β=.04 [t=0.39, p=.70]). In this model, threat exposure accounted for 11% of the variance in neuropsychiatric symptoms, whereas deprivation accounted for less than 1%. The association between threat and neuropsychiatric symptoms remained significant (B=.68, CI=.20 – 1.15, β=.37 [t=2.83, p<.01]) even after controlling for variables on which the HIV+ exposure groups differed significantly (current CD4, KMSK-A). In exploratory post-hoc analyses, this model was rerun using each neuropsychiatric measure as the dependent variable to examine the specificity of the observed association; results from all three models revealed significant associations with threat (CESD: p<.05; BAI: p<.01; AES: p<.05) but not deprivation (ps >.05).
Figure 2.

Threat (violence) exposure is associated with greater affective symptoms in HIV+ adults. Note: rpb = point-biserial correlation coefficient; effects unadjusted for deprivation exposure.
The model predicting EF in PLWH revealed a trend-level association between deprivation and executive dysfunction when controlling for threat (B= −.80, CI= −1.71 – .12, β= −.21 [t=1.74, p=.09]) (Figure 3). By contrast, threat was unrelated to EF when controlling for deprivation (B= −.10, CI= −.74 – .54, β= −.04 [t=0.32, p=.75]). In this model, deprivation accounted for 4% of the variance in executive abilities, whereas threat accounted for less than 1%. The association between deprivation and EF remained at a trend level (B= −.77, CI= −1.70 – .16, β= −.20 [t=1.66, p=.10]) even after controlling for variables on which the HIV+ exposure groups differed significantly (current CD4, KMSK-A). In exploratory post-hoc analyses, this model was rerun using each EF measure as the dependent variable. Results revealed trend-level associations between deprivation and Trails B (TMT: p=.10; FAS: p>.05; LNS: p>.05); associations with threat were non-significant in all three models (ps >.05).
Figure 3.
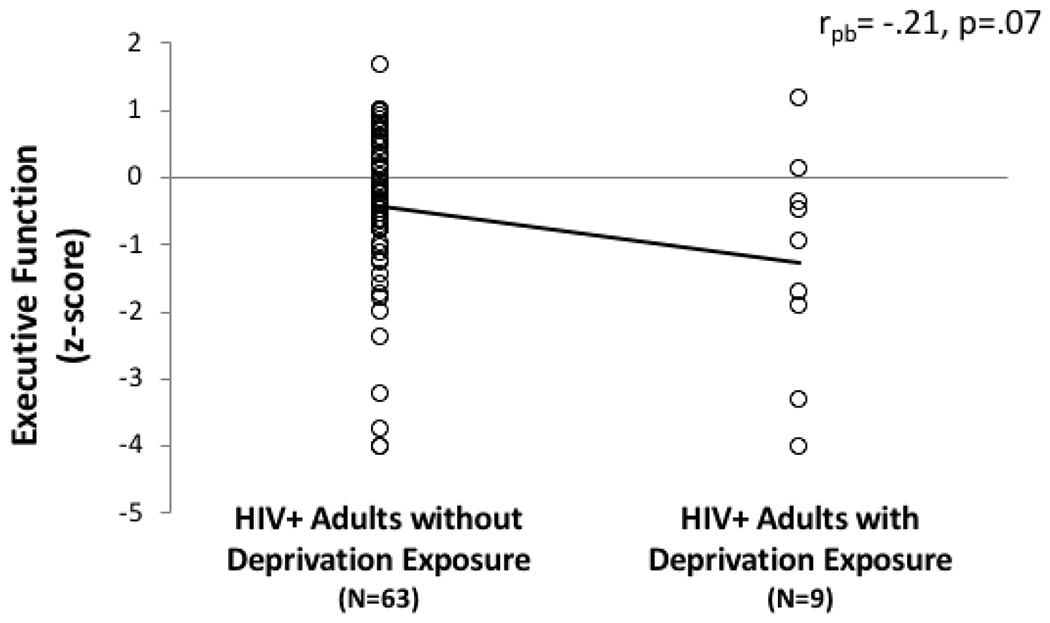
Deprivation (poverty/neglect) is associated with greater executive dysfunction in HIV+ adults. Note: rpb = point-biserial correlation coefficient; effects unadjusted for threat exposure.
Discussion
To our knowledge, this is the first study conducted to compare rates of exposure to different dimensions of adversity in a demographically-matched sample of HIV+ and HC adults. Our data revealed elevated rates of threat-related ACEs (e.g., sexual abuse; domestic abuse; bullying) in adult PLWH relative to HC adults. This elevation was observed despite the fact that rates of high ELS exposure did not differ between these two groups. That is, although the HIV+ and HC groups were matched with respect to the overall degree of ELS burden, differences in the rate of exposure to specific types of ACEs were still observed, where a higher rate of threat-related adversities was present in the HIV+ group than in the HC group (67% vs. 51%, respectively). This finding is consistent with prior reports indicating elevated rates of abuse and trauma in HIV+ cohorts (Machtinger et al., 2012; Spies, Afifi, et al., 2012). Accordingly, our results suggest that, in HIV+ samples, the nature of ELS exposure may differ from that of HC samples, with threat-related adversities being more prevalent in HIV+ than in HC samples. Importantly, these differences could impact the ways in which ELS-related effects are expressed in HIV+ and HC samples, as prior data indicate that threat-related exposures are independently associated with affective dysregulation in non-HIV samples (Lambert et al., 2017).
Consistent with this notion, in the HIV+ sample we observed that threat-related ACEs exhibited unique associations with neuropsychiatric symptom elevations, accounting for 11% of the variance in neuropsychiatric symptoms, whereas deprivation was unrelated to neuropsychiatric symptoms. Our findings thus implicate threat-related ACEs as unique contributing factors in ELS-related neuropsychiatric symptom elevations previously reported in PLWH (Clark et al., 2017). Additionally, we also observed unique trend-level associations between early-life deprivation and executive dysfunction in adult PLWH, whereas threat was unrelated to EF. These results are consistent with an emerging literature describing links between deprivation and EF difficulties in non-HIV samples (Farah, 2017; Haft & Hoeft, 2017; Letkiewicz et al., 2021). Notably, this differential pattern of associations aligns with findings from neuroimaging studies, which indicate that early-life threat exposure (violence, trauma) is associated with aberrant patterns of activation in regions implicated in affective response (e.g., amygdala), whereas early-life deprivation is more commonly associated with abnormalities in the structure and function of brain regions implicated in EF (e.g., dorsolateral prefrontal cortex, anterior cingulate) (McCrory et al., 2013; McCrory et al., 2011; McLaughlin et al., 2015; McLaughlin et al., 2014; Sheridan et al., 2012). Accordingly, future studies that investigate whether threat-related and deprivation-related ACEs are associated with unique neural effects in HIV+ samples may be warranted to enhance our understanding of the pathophysiological sequelae of ELS in the context of HIV.
Data from prior studies in non-HIV samples suggest that the developmental processes underlying these associations may be partly dissociable. Exposures to threat-related ACEs result in aberrant processing of affective stimuli, including elevated reactivity to and attention towards potential threats (van Marle, Hermans, Qin, & Fernandez, 2009). It is hypothesized that such reactions may be adaptive in unsafe environments, but become problematic over time, as they are associated with physiological processes (e.g., HPA axis dysregulation) that increase risk for psychopathology (e.g., depression, anxiety) (Danese & Lewis, 2017; Heim, Newport, Mletzko, Miller, & Nemeroff, 2008; Milojevich, Norwalk, & Sheridan, 2019; Shea, Walsh, Macmillan, & Steiner, 2005; Teicher et al., 2016). Early-life deprivation, on the other hand, is thought to be associated with a decrease in exposures to environmental stimuli (e.g., cognitive inputs, language with complicated syntax, learning opportunities, other cognitively enriching activities) that promote the elaboration of cognitive abilities over time in the typically developing brain (Sheridan & McLaughlin, 2014; Spratt et al., 2012). Yet, a complete separation between these two dimensions is unlikely, as responses to many ACEs involve cross-dimensional aspects. For example, threat exposures (e.g., abuse) are sometimes associated with a reduction in environmental engagement (e.g., social isolation, withdrawal behaviors) (Elliott, Cunningham, Linder, Colangelo, & Gross, 2005), while deprivation-related ACEs (e.g., neglect) can involve insecure attachments with caretakers/parental figures (Cyr, Euser, Bakermans-Kranenburg, & Van Ijzendoorn, 2010) that have the potential to impact the development of affective functions. Nevertheless, there is burgeoning evidence that early-life exposures to threat and deprivation may be associated with unique, developmentally relevant trajectories that involve specific effects on brain and behavior (Lambert et al., 2017; McLaughlin, 2016). In this context, our findings provide initial evidence to suggest that future investigations aimed at teasing apart and elucidating these effects in PLWH are warranted.
Our findings have potential clinical implications, as we observed unique associations between ACE exposures and mental health outcomes in HIV+ adults. Hence, our data suggest that assessing for early-life adversity, particularly threat and deprivation exposures, may assist in the identification of PLWH who may require more frequent screening and/or in-depth assessment of psychological and cognitive functions, and who may benefit from preventative and supportive services (e.g., psychotherapy). Interventions tailored to addressing specific threat-related ACEs (e.g., sexual abuse) have been shown to reduce neuropsychiatric symptoms in PLWH (Seedat, 2012; Sikkema et al., 2007). Such findings align with data from non-HIV samples suggesting psychotherapeutic interventions (e.g., cognitive-behavioral therapy, mindfulness) could be particularly effective for adults who have experienced ACEs (Blalock et al., 2013; Korotana, Dobson, Pusch, & Josephson, 2016; Nemeroff et al., 2003; Zobel et al., 2011). Additional research is needed to further investigate this possibility in PLWH.
Several issues merit further consideration. First, although significant group-related differences in the rates of threat-related ACEs were detected, significant group differences in deprivation were not observed. Our sample was recruited with the intention of balancing rates of high ELS exposure (≥3 ACEs) within and across our groups, which likely affected the rates at which individual ACEs were observed in our sample. While the average number of ACEs in the current HIV+ group was generally in line with prior randomly selected HIV+ samples (Clark et al., 2018; Clark et al., 2012; Clark et al., 2017) (3.4 vs. 3.3, respectively), the average number of ACEs in the HC group was slightly higher than that reported in prior large-scale studies (Cohen, Paul, et al., 2006) (2.8 vs. 2.2, respectively), which suggests an overestimation of exposure rates in our HC sample. Accordingly, additional investigations conducted in community-based samples are needed to provide greater clarity regarding the degree to which rates of early-life threat and deprivation exposures differ in HIV+ and HC samples. Nevertheless, as noted above, we suggest that the observation of significantly higher rates of threat-related ACEs in PLWH relative to HC adults, even in the context of high ELS exposure rates that do not differ significantly between these groups, only further underscores the relevance of threat-related ACEs to HIV+ samples. Second, it is possible that the relatively low rate of deprivation in our HIV+ sample, and associated reductions in power, may have impacted our ability to detect associations with outcome variables beyond the level of a trend, as the low frequency of those experiencing deprivation only (vs. deprivation and threat combined) reduces our ability to ascertain individual effects. Future studies conducted with larger samples, as well as those using different methods to assess deprivation (e.g., estimations of socioeconomic status during childhood), are needed to clarify this issue. Similarly, studies that utilize a continuous rather than a binary approach to quantifying threat and deprivation exposures could better assess potential dose-response effects (LaNoue, George, Helitzer, & Keith, 2020). Lastly, levels of neuropsychiatric symptoms and executive dysfunction in the HIV+ group, while elevated, did not differ significantly from the HC group – findings that diverge from prior reports (Clark et al., 2017; Heaton et al., 2011); however, as discussed above, our sample was somewhat unique in that rates of high ELS exposure were roughly balanced in the HIV+ and HC groups, which may have elevated levels of executive dysfunction and neuropsychiatric symptomology in our HC sample. Also, the degree of variability in the HIV+ and HC groups’ scores suggests the potential presence of latent subgroups for whom ACE-related effects may be more prominent. Future studies with larger sample sizes are needed to further examine these possibilities.
In summary, findings from this study suggest that examining different dimensions of adversity may enhance our understanding of the specific consequences of ELS exposure in PLWH. Using this approach, we found that PLWH experienced higher rates of threat-related ACEs, which contributed uniquely to neuropsychiatric symptom elevations. By contrast, we found that deprivation demonstrated unique trend-level associations with executive dysfunction in PLWH. Our results thus illuminate the ways in which different types of early-life adversity elevate the risk of neuropsychiatric and neurocognitive symptoms in HIV+ samples. To our knowledge, this is the first report of independent associations between different dimensions of ACEs and mental health outcomes in PLWH. Our results support the need for further clinical research in this population; if confirmed in larger community-based samples, such findings may be applied to more precisely identify PLWH at risk for developing neuropsychiatric and neurocognitive symptoms. Importantly, future studies that investigate whether threat and deprivation are associated with unique neural and/or pathophysiological mechanisms in HIV+ samples (Womersley et al., 2017) appear warranted as such studies may help to refine interventions aimed at addressing ELS-related maladies in adult PLWH. Lastly, future studies using longitudinal approaches will be needed to clarify the direction of the observed effects, as well as the potential role of contributory mechanisms (e.g., sleep dysfunction) (Agorastos, Pervanidou, Chrousos, & Baker, 2019; Syed & Nemeroff, 2017; Taylor, 2010).
Funding Sources.
This work was supported by grants from the National Institutes of Health (Grants K23 MH096628 [USC], R25 MH080663 [USC], UL1 TR000067 [USC], P30 DA027827 [USC]). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the NIH.
Footnotes
Conflicts of Interest. All authors reported no biomedical financial interests or potential conflicts of interest.
References
- Agorastos A, Pervanidou P, Chrousos GP, & Baker DG (2019). Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Front Psychiatry, 10, 118. doi: 10.3389/fpsyt.2019.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aita SL, Beach JD, Taylor SE, Borgogna NC, Harrell MN, & Hill BD (2019). Executive, language, or both? An examination of the construct validity of verbal fluency measures. Appl Neuropsychol Adult, 26(5), 441–451. doi: 10.1080/23279095.2018.1439830 [DOI] [PubMed] [Google Scholar]
- Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, & Paul RH (2013). Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging Behav, 7(2), 196–203. doi: 10.1007/s11682-012-9215-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown GK, & Steer RA (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. [DOI] [PubMed] [Google Scholar]
- Benton TD (2008). Depression and HIV/AIDS. Curr Psychiatry Rep, 10(3), 280–285. [DOI] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, … Shapiro M (2001). Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry, 58(8), 721–728. doi:yoa9450 [pii] [DOI] [PubMed] [Google Scholar]
- Blalock JA, Minnix JA, Mathew AR, Wetter DW, McCullough JP, & Cinciripini PM (2013). Relationship of childhood trauma to depression and smoking outcomes in pregnant smokers. J Consult Clin Psychol, 81(5), 821–830. doi: 10.1037/a0033381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova Y, Diaz-Santos M, & Cronin-Golomb A (2010). Neurocognitive correlates of alexithymia in asymptomatic individuals with HIV. Neuropsychologia, 48(5), 1295–1304. doi: 10.1016/j.neuropsychologia.2009.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski JG, Benton AL, & Spreen O (1967). Word fluency and brain damage. Neuropsychologia, 5(2), 135–140. [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, & Nelson CA III (2009). Effects of early psychosocial deprivation on the development of memory and executive function. Front Behav Neurosci, 3, 16. doi: 10.3389/neuro.08.016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisker TR, Dufour MS, & Myers JJ (2015). Recall of Nadir CD4 Cell Count and Most Recent HIV Viral Load Among HIV-Infected, Socially Marginalized Adults. AIDS Behav, 19(11), 2108–2116. doi: 10.1007/s10461-015-1018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo JM, Courtney MG, Melrose RJ, & Stern CE (2007). Putamen hypertrophy in nondemented patients with human immunodeficiency virus infection and cognitive compromise. Arch Neurol, 64(9), 1275–1280. doi: 10.1001/archneur.64.9.1275 [DOI] [PubMed] [Google Scholar]
- Chu DA, Williams LM, Harris AW, Bryant RA, & Gatt JM (2013). Early life trauma predicts self-reported levels of depressive and anxiety symptoms in nonclinical community adults: relative contributions of early life stressor types and adult trauma exposure. J Psychiatr Res, 47(1), 23–32. doi: 10.1016/j.jpsychires.2012.08.006 [DOI] [PubMed] [Google Scholar]
- Ciesla JA, & Roberts JE (2001). Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry, 158(5), 725–730. [DOI] [PubMed] [Google Scholar]
- Clark US, Arce Rentería M, Hegde RR, & Morgello S (2018). Early life stress-related elevations in reaction time variability are associated with brain volume reductions in HIV+ adults. Frontiers in Behavioral Neuroscience, 12(6). doi: 10.3389/fnbeh.2018.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, & Cohen RA (2010). Brain dysfunction in the era of combination antiretroviral therapy: implications for the treatment of the aging population of HIV-infected individuals. Curr Opin Investig Drugs, 11(8), 884–900. [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Sweet LH, Gongvatana A, Hana GN, Westbrook ML, … Tashima KT (2012). Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. J Int Neuropsychol Soc, 18(4), 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Westbrook ML, Devlin KN, & Tashima KT (2010). Facial emotion recognition impairments in individuals with HIV. J Int Neuropsychol Soc, 16(6), 1127–1137. doi: 10.1017/S1355617710001037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Sweet LH, Morgello S, Philip NS, & Cohen RA (2017). High early life stress and aberrant amygdala activity: risk factors for elevated neuropsychiatric symptoms in HIV+ adults. Brain Imaging Behav, 11(3), 649–665. doi: 10.1007/s11682-016-9542-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Walker KA, Cohen RA, Devlin KN, Folkers AM, Pina MJ, & Tashima KT (2015). Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia, 70, 263–271. doi: 10.1016/j.neuropsychologia.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, … Williams LM (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry, 59(10), 975–982. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Paul RH, Stroud L, Gunstad J, Hitsman BL, McCaffery J, … Gordon E (2006). Early life stress and adult emotional experience: an international perspective. Int J Psychiatry Med, 36(1), 35–52. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. J Health Soc Behav, 24(4), 385–396. [PubMed] [Google Scholar]
- Cunningham WE, Rana HM, Shapiro MF, & Hays RD (1997). Reliability and validity of self-report CD4 counts-in persons hospitalized with HIV disease. J Clin Epidemiol, 50(7), 829–835. doi: 10.1016/s0895-4356(97)00061-9 [DOI] [PubMed] [Google Scholar]
- Cyr C, Euser EM, Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2010). Attachment security and disorganization in maltreating and high-risk families: a series of meta-analyses. Dev Psychopathol, 22(1), 87–108. doi: 10.1017/S0954579409990289 [DOI] [PubMed] [Google Scholar]
- Danese A, & Lewis SJ (2017). Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology, 42(1), 99–114. doi: 10.1038/npp.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, … Skarbinski J (2014). Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One, 9(3), e92842. doi: 10.1371/journal.pone.0092842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, … Giles WH (2004). The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl, 28(7), 771–784. doi: 10.1016/j.chiabu.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Elliott GC, Cunningham SM, Linder M, Colangelo M, & Gross M (2005). Child physical abuse and self-perceived social isolation among adolescents. J Interpers Violence, 20(12), 1663–1684. doi: 10.1177/0886260505281439 [DOI] [PubMed] [Google Scholar]
- Farah MJ (2017). The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron, 96(1), 56–71. doi: 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, … Hurt H (2006). Childhood poverty: specific associations with neurocognitive development. Brain Res, 1110(1), 166–174. doi: 10.1016/j.brainres.2006.06.072 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Haft SL, & Hoeft F (2017). Poverty’s Impact on Children’s Executive Functions: Global Considerations. New Dir Child Adolesc Dev, 2017(158), 69–79. doi: 10.1002/cad.20220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, … Grant I (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, … Grant I (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17(1), 3–16. doi: 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, … Group TH (1995). The HNRC 500-Neuropsychology of HIV infection at different disease stages. . Journal of the International Neuropsychological Society, 1(3), 231–251. [DOI] [PubMed] [Google Scholar]
- Hedges DW, & Woon FL (2011). Early-life stress and cognitive outcome. Psychopharmacology (Berl), 214(1), 121–130. doi: 10.1007/s00213-010-2090-6 [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, & Nemeroff CB (2008). The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology, 33(6), 693–710. doi: 10.1016/j.psyneuen.2008.03.008 [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, … Kreek MJ (2003). The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend, 69(2), 137–150. doi:S0376871602003083 [pii] [DOI] [PubMed] [Google Scholar]
- Korotana LM, Dobson KS, Pusch D, & Josephson T (2016). A review of primary care interventions to improve health outcomes in adult survivors of adverse childhood experiences. Clin Psychol Rev, 46, 59–90. doi: 10.1016/j.cpr.2016.04.007 [DOI] [PubMed] [Google Scholar]
- Lambert HK, King KM, Monahan KC, & McLaughlin KA (2017). Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Dev Psychopathol, 29(3), 929–940. doi: 10.1017/S0954579416000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaNoue MD, George BJ, Helitzer DL, & Keith SW (2020). Contrasting cumulative risk and multiple individual risk models of the relationship between Adverse Childhood Experiences (ACEs) and adult health outcomes. BMC Med Res Methodol, 20(1), 239. doi: 10.1186/s12874-020-01120-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letkiewicz AM, Funkhouser CJ, & Shankman SA (2021). Childhood maltreatment predicts poorer executive functioning in adulthood beyond symptoms of internalizing psychopathology. Child Abuse Negl, 118, 105140. doi: 10.1016/j.chiabu.2021.105140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, D’Arcy C, & Meng X (2016). Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: systematic review, meta-analysis, and proportional attributable fractions. Psychol Med, 46(4), 717–730. doi: 10.1017/S0033291715002743 [DOI] [PubMed] [Google Scholar]
- Machlin L, Miller AB, Snyder J, McLaughlin KA, & Sheridan MA (2019). Differential Associations of Deprivation and Threat With Cognitive Control and Fear Conditioning in Early Childhood. Front Behav Neurosci, 13, 80. doi: 10.3389/fnbeh.2019.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger EL, Wilson TC, Haberer JE, & Weiss DS (2012). Psychological trauma and PTSD in HIV-positive women: a meta-analysis. AIDS Behav, 16(8), 2091–2100. doi: 10.1007/s10461-011-0127-4 [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, & Firinciogullari S (1991). Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res, 38(2), 143–162. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, … Viding E (2013). Amygdala activation in maltreated children during pre-attentive emotional processing. British Journal of Psychiatry, 202(4), 269–276. doi: 10.1192/bjp.bp.112.116624 [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, & Viding E (2011). Heightened neural reactivity to threat in child victims of family violence. Curr Biol, 21(23), R947–948. doi: 10.1016/j.cub.2011.10.015 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA (2016). Future Directions in Childhood Adversity and Youth Psychopathology. J Clin Child Adolesc Psychol, 45(3), 361–382. doi: 10.1080/15374416.2015.1110823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child Maltreatment and Neural Systems Underlying Emotion Regulation. J Am Acad Child Adolesc Psychiatry, 54(9), 753–762. doi: 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev, 47, 578–591. doi: 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Nelson CA (2017). Neglect as a Violation of Species-Expectant Experience: Neurodevelopmental Consequences. Biol Psychiatry, 82(7), 462–471. doi: 10.1016/j.biopsych.2017.02.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard S (1995). Applied Logistic Regression Analysis. Thousand Oaks, CA: Sage. [Google Scholar]
- Milojevich HM, Norwalk KE, & Sheridan MA (2019). Deprivation and threat, emotion dysregulation, and psychopathology: Concurrent and longitudinal associations. Dev Psychopathol, 31(3), 847–857. doi: 10.1017/S0954579419000294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison MF, Petitto JM, Ten Have T, Gettes DR, Chiappini MS, Weber AL, … Evans DL (2002). Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry, 159(5), 789–796. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, … Keller MB (2003). Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A, 100(24), 14293–14296. doi: 10.1073/pnas.2336126100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012). Neural correlates of socioeconomic status in the developing human brain. Dev Sci, 15(4), 516–527. doi: 10.1111/j.1467-7687.2012.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Henry L, Grieve SM, Guilmette TJ, Niaura R, Bryant R, … Gordon E (2008). The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr Dis Treat, 4(1), 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton KC, Myers HF, Hall NM, & Javanbakht M (2004). Ethnicity, serostatus, and psychosocial differences in sexual risk behavior among HIV-seropositive and HIV-seronegative women. AIDS Behav, 8(4), 405–415. doi: 10.1007/s10461-004-7325-2 [DOI] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl), 214(1), 55–70. doi: 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Carpenter LL, Kuras YI, … Niaura RS (2013). Early life stress is associated with greater default network deactivation during working memory in healthy controls: a preliminary report. Brain Imaging Behav, 7(2), 204–212. doi: 10.1007/s11682-012-9216-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement, 1(3), 385 – 401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Raver CC, Blair C, & Willoughby M (2013). Poverty as a predictor of 4-year-olds’ executive function: new perspectives on models of differential susceptibility. Dev Psychol, 49(2), 292–304. doi: 10.1037/a0028343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner SL, Falb KL, & Mimiaga MJ (2011). Early Life Traumatic Stressors and the Mediating Role of PTSD in Incident HIV Infection Among US Men, Comparisons by Sexual Orientation and Race/Ethnicity: Results From the NESARC, 2004–2005. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0b013e31821d36b4 [DOI] [PubMed] [Google Scholar]
- Reitan RM, & Davison LA (1974). Clinical neuropsychology: Current status and applications. Oxford England: V. H. Winston & Sons. [Google Scholar]
- Seckfort DL, Paul R, Grieve SM, Vandenberg B, Bryant RA, Williams LM, … Gordon E (2008). Early life stress on brain structure and function across the lifespan: A preliminary study. Brain Imaging and Behavior, 2(1), 49–58. [Google Scholar]
- Seedat S (2012). Interventions to improve psychological functioning and health outcomes of HIV-infected individuals with a history of trauma or PTSD. Curr HIV/AIDS Rep, 9(4), 344–350. doi: 10.1007/s11904-012-0139-3 [DOI] [PubMed] [Google Scholar]
- Shea A, Walsh C, Macmillan H, & Steiner M (2005). Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology, 30(2), 162–178. doi: 10.1016/j.psyneuen.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 59 Suppl 20, 22–33;quiz 34-57. [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2014). Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci, 18(11), 580–585. doi: 10.1016/j.tics.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Peverill M, Finn AS, & McLaughlin KA (2017). Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Dev Psychopathol, 29(5), 1777–1794. doi: 10.1017/S0954579417001390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Sarsour K, Jutte D, D’Esposito M, & Boyce WT (2012). The impact of social disparity on prefrontal function in childhood. PLoS One, 7(4), e35744. doi: 10.1371/journal.pone.0035744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema KJ, Hansen NB, Kochman A, Tarakeshwar N, Neufeld S, Meade CS, & Fox AM (2007). Outcomes from a group intervention for coping with HIV/AIDS and childhood sexual abuse: reductions in traumatic stress. AIDS Behav, 11(1), 49–60. doi: 10.1007/s10461-006-9149-8 [DOI] [PubMed] [Google Scholar]
- Simoni JM, & Ng MT (2000). Trauma, coping, and depression among women with HIV/AIDS in New York City. AIDS Care, 12(5), 567–580. doi: 10.1080/095401200750003752 [DOI] [PubMed] [Google Scholar]
- Slopen N, Shonkoff JP, Albert MA, Yoshikawa H, Jacobs A, Stoltz R, & Williams DR (2016). Racial Disparities in Child Adversity in the U.S.: Interactions With Family Immigration History and Income. Am J Prev Med, 50(1), 47–56. doi: 10.1016/j.amepre.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Spies G, Afifi TO, Archibald SL, Fennema-Notestine C, Sareen J, & Seedat S (2012). Mental health outcomes in HIV and childhood maltreatment: a systematic review. Syst Rev, 1, 30. doi: 10.1186/2046-4053-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies G, Fennema-Notestine C, Archibald SL, Cherner M, & Seedat S (2012). Neurocognitive deficits in HIV-infected women and victims of childhood trauma. AIDS Care, 24(9), 1126–1135. doi: 10.1080/09540121.2012.687813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt EG, Friedenberg SL, Swenson CC, Larosa A, De Bellis MD, Macias MM, … Brady KT (2012). The Effects of Early Neglect on Cognitive, Language, and Behavioral Functioning in Childhood. Psychology (Irvine), 3(2), 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, & Strauss E (1998). A compendium of neuropsychological tests: Administration, norms, and commentary (2nd ed.). New York, NY, US: Oxford University Press. [Google Scholar]
- Syed SA, & Nemeroff CB (2017). Early Life Stress, Mood, and Anxiety Disorders. Chronic Stress (Thousand Oaks), 1. doi: 10.1177/2470547017694461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE (2010). Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A, 107(19), 8507–8512. doi:1003890107 [pii] 10.1073/pnas.1003890107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, & Samson JA (2016). Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry, 57(3), 241–266. doi: 10.1111/jcpp.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci, 17(10), 652–666. doi: 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Tombaugh TN (2004). Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol, 19(2), 203–214. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, & Fernandez G (2009). From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry, 66(7), 649–655. doi: 10.1016/j.biopsych.2009.05.014 [DOI] [PubMed] [Google Scholar]
- Villar-Loubet OM, Illa L, Echenique M, Cook R, Messick B, Duthely LM, … Potter J (2014). Prenatal and mental health care among trauma-exposed, HIV-infected, pregnant women in the United States. J Assoc Nurses AIDS Care, 25(1 Suppl), S50–61. doi: 10.1016/j.jana.2013.06.006 [DOI] [PubMed] [Google Scholar]
- Walton G, Co SJ, Milloy MJ, Qi J, Kerr T, & Wood E (2011). High prevalence of childhood emotional, physical and sexual trauma among a Canadian cohort of HIV-seropositive illicit drug users. AIDS Care, 23(6), 714–721. doi: 10.1080/09540121.2010.525618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CW, Sundermann EE, Hussain MA, Umlauf A, Thames AD, Moore RC, … Moore DJ (2019). Effects of trauma, economic hardship, and stress on neurocognition and everyday function in HIV. Health Psychol, 38(1), 33–42. doi: 10.1037/hea0000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997). WAIS-III administration and scoring manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (2001). Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Whetten K, Leserman J, Lowe K, Stangl D, Thielman N, Swartz M, … Van Scoyoc L (2006). Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. Am J Public Health, 96(6), 1028–1030. doi: 10.2105/AJPH.2005.063263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womersley JS, Seedat S, & Hemmings SMJ (2017). Childhood maltreatment and HIV-associated neurocognitive disorders share similar pathophysiology: a potential sensitisation mechanism? Metab Brain Dis. doi: 10.1007/s11011-017-0062-9 [DOI] [PubMed] [Google Scholar]
- Zobel I, Kech S, van Calker D, Dykierek P, Berger M, Schneibel R, & Schramm E (2011). Long-term effect of combined interpersonal psychotherapy and pharmacotherapy in a randomized trial of depressed patients. Acta Psychiatr Scand, 123(4), 276–282. doi: 10.1111/j.1600-0447.2010.01671.x [DOI] [PubMed] [Google Scholar]


