Abstract
PURPOSE
Few cancer centers systematically engage patients with evidence-based tobacco treatment despite its positive effect on quality of life and survival. Implementation strategies directed at patients, clinicians, or both may increase tobacco use treatment (TUT) within oncology.
METHODS
We conducted a four-arm cluster-randomized pragmatic trial across 11 clinical sites comparing the effect of strategies informed by behavioral economics on TUT engagement during oncology encounters with cancer patients. We delivered electronic health record (EHR)–based nudges promoting TUT across four nudge conditions: patient only, clinician only, patient and clinician, or usual care. Nudges were designed to counteract cognitive biases that reduce TUT engagement. The primary outcome was TUT penetration, defined as the proportion of patients with documented TUT referral or a medication prescription in the EHR. Generalized estimating equations were used to estimate the parameters of a linear model.
RESULTS
From June 2021 to July 2022, we randomly assigned 246 clinicians in 95 clusters, and collected TUT penetration data from their encounters with 2,146 eligible patients who smoke receiving oncologic care. Intent-to-treat (ITT) analysis showed that the clinician nudge led to a significant increase in TUT penetration versus usual care (35.6% v 13.5%; OR = 3.64; 95% CI, 2.52 to 5.24; P < .0001). Completer-only analysis (N = 1,795) showed similar impact (37.7% clinician nudge v 13.5% usual care; OR = 3.77; 95% CI, 2.73 to 5.19; P < .0001). Clinician type affected TUT penetration, with physicians less likely to provide TUT than advanced practice providers (ITT OR = 0.67; 95% CI, 0.51 to 0.88; P = .004).
CONCLUSION
EHR nudges, informed by behavioral economics and aimed at oncology clinicians, appear to substantially increase TUT penetration. Adding patient nudges to the implementation strategy did not affect TUT penetration rates.
INTRODUCTION
Continued tobacco smoking by patients with cancer worsens quality of life (QoL) and reduces survival.1-3 Smoking accelerates tumor growth, disease progression, tumor resistance to treatment, and treatment-related toxicities.4-7 Yet, >50% of patients with cancer who smoked before their diagnosis continue to smoke after diagnosis and during treatment.8 Routine evidence-based tobacco use treatment (TUT) reduces cancer-specific and all-cause mortality, reduces treatment-related toxicity, and improves QoL among patients receiving cancer care.1 Therefore, the 2021 US Preventive Services Task Force, Healthy People 2030, the Department of Health and Human Services, and the Surgeon General have recommended that clinicians ask all adults about tobacco use, advise them to quit, and provide evidence-based treatment.9-12 Likewise, the National Comprehensive Cancer Network,3 American Society of Clinical Oncology,13 and the American Association for Cancer Research14 encourage oncologists to provide evidence-based TUT.
CONTEXT
Key Objective
Are nudges informed by behavioral economics during oncology encounters with patients with cancer effective for increasing engagement in tobacco use treatment (TUT)?
Knowledge Generated
Clinician nudges aimed at counteracting omission bias and delivered via the electronic health record resulted in a significant (>3-fold) increase in TUT engagement. The addition of a patient nudge did not affect TUTS engagement rates. Among clinicians, advanced practice providers were more likely than physicians to engage in TUT. White and non-White patients were equally engaged in TUT by the clinician nudge.
Relevance (S.B. Wheeler)
-
Clinician-focused nudges can provide important and timely cues to action that result in more patients engaging in TUT. Such nudges may be a low-cost, low-risk way to optimize use of tobacco cessation services during clinical encounters.*
*Relevance section written by JCO Associate Editor Stephanie B. Wheeler, PhD, MPH.
Despite the importance of TUT, only half of the cancer centers consistently identify patient tobacco use,15 and fewer systematically engage patients in evidence-based TUT.16 In response to this practice gap, the National Cancer Institute launched the Cancer Center Cessation Initiative (C3i) to help centers develop ways to identify and engage patients who smoke.17 One major C3i objective is overcoming clinician, patient, and health system barriers by integrating TUT into cancer care. An earlier C3i implementation strategy studied at Penn Medicine focused on maximizing tobacco use screening and referral, on the basis of the Ask-Advise-Connect model.18 Because lack of clinician experience is a frequently cited barrier to TUT,19-21, our initial strategy used an automated default electronic health record (EHR) referral to our TUT Service (TUTS). This effort significantly improved TUT engagement, with TUTS referrals rising from 0% at baseline to 34% during the 6-month postimplementation period. Although this suggested that clinician behavior was modifiable, the study was not a randomized trial and >60% of default referral orders were declined, implicating additional implementation barriers.22
Implementation efforts to promote TUT engagement within oncology may be enhanced using behavioral economics, which has helped improve patient outcomes and transform health care delivery across a wide range of activities.23-26 Specific clinician-related barriers likely decrease TUT engagement, including clinician pessimism regarding the ability to help patients stop using tobacco, misconceptions about patient resistance, and implicit biases regarding patients’ capacities to volitionally alter the course of illness.27 These motivators are related to clinician willingness to invest effort in help-giving28-30 and may prevent acquisition of new skills.31 Individuals with cancer face unique challenges when engaging in tobacco cessation efforts, including low self-efficacy, low perceived benefits of quitting, and perceived treatment risk.32-34 Furthermore, racial and ethnic minorities and individuals with low socioeconomic status (SES) suffer disproportionately from targeted tobacco marketing, have diminished access to evidence-based TUT, and report poorer response to TUT.35-39 Thus, social inequities may also affect implementation and TUT engagement.
We designed this pragmatic trial to test the effectiveness of patient- and clinician-directed implementation strategies, or nudges, informed by behavioral economics and delivered through the EHR, to counteract heuristics that reduce the likelihood of engaging in TUT. We compared the effects of nudges directed at patients, clinicians, or both to usual care on rates of TUT. We also performed a preliminary examination of patient and clinician characteristics that may moderate the impact of nudges on rates of TUT, including characteristics that may have important implications for health and social inequities, such as patient age, sex, race/ethnicity, and neighborhood-level SES.
METHODS
Design and Setting
We conducted a cluster-randomized pragmatic trial across five hospitals and six clinics within Penn Medicine's Abramson Cancer Center.40 Clinician clusters were randomized into four arms: clinician nudge, patient nudge, both clinician and patient nudge, or usual care. Patients were nested within clinician clusters. The primary outcome was penetration of TUT, defined as the provision of a patient referral for the TUTS program or the provision of tobacco use medication during cancer care. This study was approved by the University of Pennsylvania Institutional Review Board. Since this was a pragmatic trial to improve use of evidence-based tobacco treatments, the study represented minimal participant risk, and a waiver of informed consent was granted.
Participant Eligibility
The clinician sample included physicians and advanced practice providers (APPs) within medical, radiation, and gynecologic oncology clinics. Eligibility criteria for clinicians included (1) currently practicing at an included site; (2) prescribing authority in Pennsylvania or New Jersey; (3) cared for ≥1 patient who used tobacco within the 30-day period preceding recruitment; and (4) English-speaking. Eligibility criteria for patients included any International Classification of Diseases-10 cancer diagnosis, self-reported current tobacco use assessed by staff initiating the visit, a scheduled appointment with a participating clinician, and English-speaking. Patients were accrued as they were seen by an eligible clinician.
Study Procedures
Clinician enrollment proceeded in two steps: (1) announcement of study initiation at staff meetings, with opportunity to ask questions about design and impact on workflow, and (2) a personalized email delivered to all eligible clinicians reiterating study methods and providing instructions for opting out. All eligible clinicians (N = 246) were enrolled, with none opting out when offered. Patient enrollment began with a positive tobacco use assessment at the first patient visit within the study period, termed the index visit. Assessment of patient tobacco exposure included the 30-day period preceding the index visit, and was accomplished using a standardized Best Practice Alert (BPA) activated within the EHR during the check-in and vital sign workflow.22 The next scheduled visit with a clinician in a cluster randomly assigned to that same arm was termed the subsequent visit, during which clinicians received a care guidance BPA at the point of care if randomly assigned to the clinician nudge or the clinician and patient nudge (Data Supplement [Figs 1 and 2], online only). Clinicians randomly assigned to the patient nudge arm received no care guidance BPA. Instead, their patients received an electronic message 24-72 hours before the subsequent visit encouraging them to speak with the clinician about TUT. Patient nudges were delivered through myPennMedicine, the patient portal used by >75% of patients with cancer. If randomly assigned to the both-nudges arm, both patients and clinicians received nudges as above. Usual care subsequent visits proceeded without either nudge.
Intervention Content
Clinician Nudge
The findings from our preliminary work examining physician preferences toward TUT revealed a strong preference for interventions perceived as effective.41 We previously showed that strategies minimizing well-established cognitive biases such as omission bias—the tendency to focus on the potential harm of action more than that of inaction—change physician behavior more than strategies solely aiming to increase knowledge of TUTS availability.42 We developed a BPA that targeted this bias by directly reminding clinicians that “[t]reating tobacco dependence without delay can improve cancer care outcomes” and facilitating an instant referral to TUTS (Data Supplement [Figs 1 and 2]). Clinicians were required to either accept the defaulted send option, or to deselect the command and actively choose the do not send option. Clinicians opting out were required to provide a justification using an available checklist or free text. The BPA did not restrict clinicians' ability to directly provide TUT by either prescribing medication or referring for TUT through alternative mechanisms.
Patient Nudge
Status quo bias, or sticking with a current choice even if better alternatives exist, can reduce patient willingness to engage in TUT.43 The patient nudge included information specific to an upcoming appointment with the oncology clinician and encouraged patients to discuss TUT with their clinician by emphasizing the importance of TUT to their care, delivered via the online portal (Data Supplement [Figs 1 and 2]).
Usual Care
Clinicians can refer to TUTS or offer TUT on their own without prompting. Our previous evidence suggests this rarely, if ever, happens.22
Measures
During preparation for this trial, we identified alternate workflows that clinicians used to provide tobacco use medications without referring patients to the TUT program.40 We chose an inclusive, pragmatic outcome definition of penetration to capture all observable TUT behaviors. Thus, the primary implementation outcome was penetration of TUT, defined as the proportion of patients who received either a treatment referral (via the BPA or elsewhere in the EHR workflow) or a prescription for tobacco treatment medication (ie, nicotine replacement, varenicline, or bupropion). We collected patient-level data, including age, sex, and race/ethnicity, from the EHR. We also calculated neighborhood-level SES at the census tract level using the Yost score, a validated composite index of SES incorporating variables such as education, income, and occupation.44 Clinician-level data included sex, race, clinician type (physician v APP), and specialty. Practice-level data included geographic location.
Random Assignment
We randomized by clinician clusters identified on the basis of paired connections between physicians and APPs within networks of practice colleagues. Clusters were formed between clinicians with overlapping patient pools to reduce cross-cluster contamination. The clusters were not site-specific as many clinicians worked at multiple sites. We formed 95 clusters from 246 clinicians. Patients were nested under clinician clusters and assigned to an arm based upon the clinician they saw at their index visit, preventing crossover.
Statistical Analysis
A sample of 900 patients provided ≥80% power to detect an 11% improvement in our primary outcome (eg, from 34% referral rate for current estimates to a clinically relevant 45%), using a two-sided type 1 error rate of 5% and an interclass correlation of 0.07, for planned comparisons between usual care and each nudge arm. We analyzed our binary outcome using logistic regression with generalized estimating equations (GEE). The study design is factorial, and models contained binary predictor terms for clinician and/or patient nudges. We included covariates from patients (eg, race) and clinicians (eg, clinician type) and controlled for time between visits in days. We controlled for type 1 error inflation by hierarchical testing, starting with the overall model significance, followed by effects of each nudge. Once we fitted the main effects model, we tested for interaction between nudges and retained the interaction term if significant. We followed the primary modeling with post hoc assessments of predictors, including our a priori interest in race, to assess interactions with study arm. Our primary analysis was intent-to-treat (ITT) so that all patients who completed the subsequent visit were included regardless of whether all interventions were received (N = 2,146). In a secondary analysis, we examined a GEE model that included only encounters wherein all nudges were received as intended (ie, a completer-only analysis; N = 1,795).
RESULTS
Sample Characteristics and Covariates
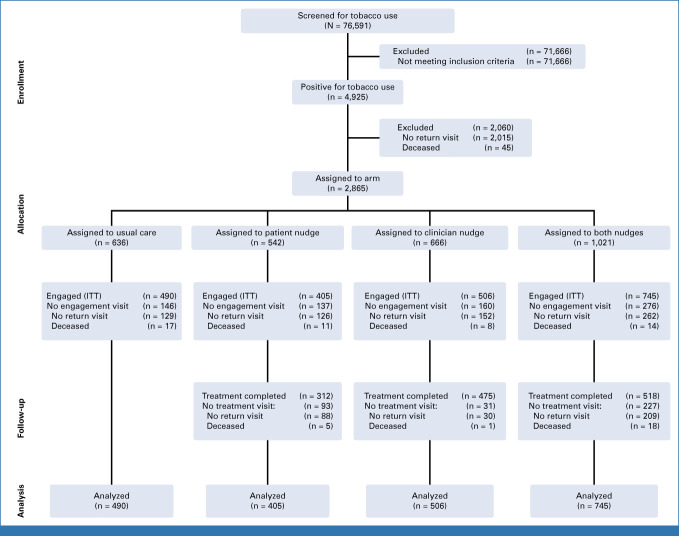
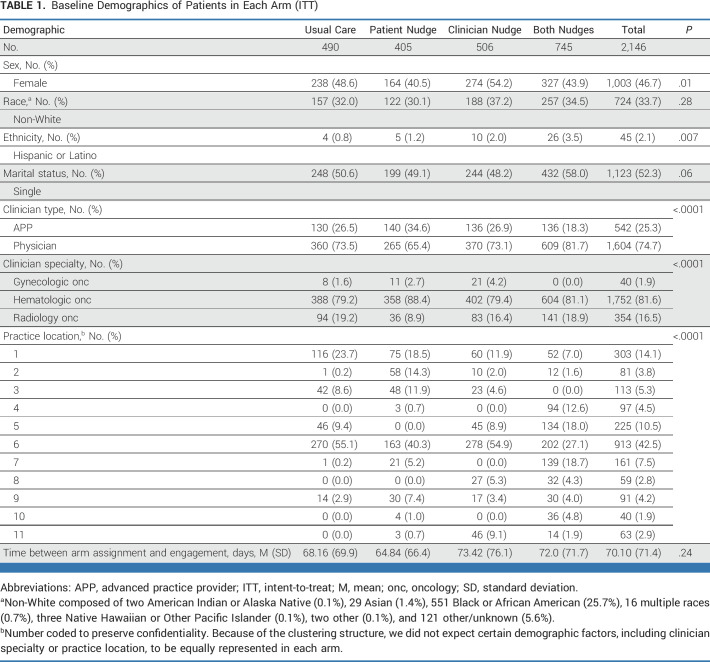
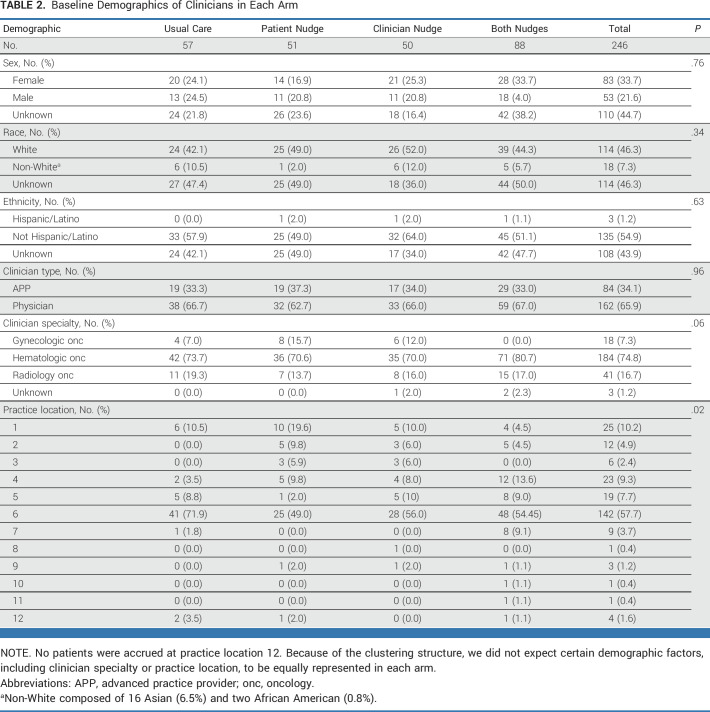
Figure 1 shows the accrual and randomization data from this trial. Seventy-six thousand five hundred ninety-one patients were screened for current tobacco use between June 2021 and July 2022. Of the 4,925 (6.4%) patients who screened positive for tobacco use, 2,865 (58%) had a scheduled visit with a clinician enrolled in this study; 2,146 patients (75%) completed a subsequent clinic visit and formed the ITT sample. Average time between index and subsequent visits was 70.1 days (standard deviation = 71.4). However, accelerated visit schedules prevented some in the ITT sample from receiving nudges before their subsequent visit (n = 351), yielding a completer-only sample of 1,795. Tables 1 and 2 show the sample characteristics.
FIG 1.
CONSORT diagram. For the assigned to usual care arm, treatment visit = 0 because of the nature of the intervention design. There are no nudges to be delivered. In other words, treatment is completed with the engaged visit, and there are no additional treatment visits. ITT, intent-to-treat.
TABLE 1.
Baseline Demographics of Patients in Each Arm (ITT)
TABLE 2.
Baseline Demographics of Clinicians in Each Arm
For patients who received the patient nudge, 55% opened the message, and, of those, 85% opened it on the day it was sent (average time to open = 1.39 days). Patients who did not receive the nudge were older (62.9 v 62.2; F[1,2144] = 5.03; P = .02), and more likely to be male (17.8% v 14.7%; χ2[1] = 3.997; P = .047), non-White (20.2% v 14.4%; χ2[1] = 11.3; P = .001), Hispanic (33.3% v 16%; χ2[1] = 7.95; P = .004), single (18.7% v 13.8%; χ2[1] = 9.53; P = .002), or seen by a gynecologic oncologist (30% v 15.5% v 18.9%; χ2[2] = 7.13; P = .03).
Models of TUT
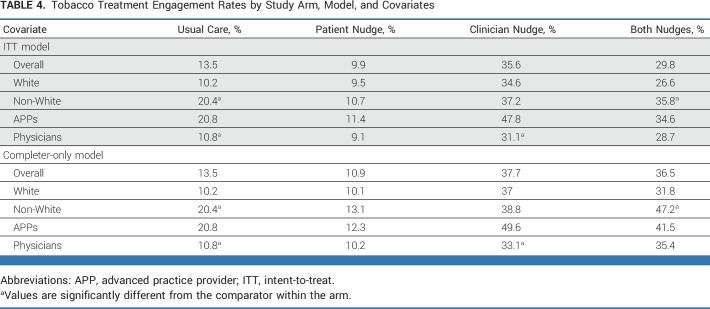
All models controlled for variables in Tables 1 and 2 that were different across arms except for location in the ITT model since that variable was missing for participants for whom the nudge was not delivered. Controlling for covariates, both the ITT and completer-only models predicting TUT penetration were significant (ITT: χ2[12] = 115.94; P < .0001; completers: χ2[11] = 130.46; P < .0001). Although neither model showed significant interaction effects for the clinician and patient nudges, the clinician nudge arm main effect was a significant predictor of TUT penetration (Table 3). Compared with usual care, the clinician nudge yielded about a 3-fold increase in rates of penetration of TUT (Fig 2 and completer—Table 4).
TABLE 3.
Generalized Estimating Equations Predicting TUT Engagement
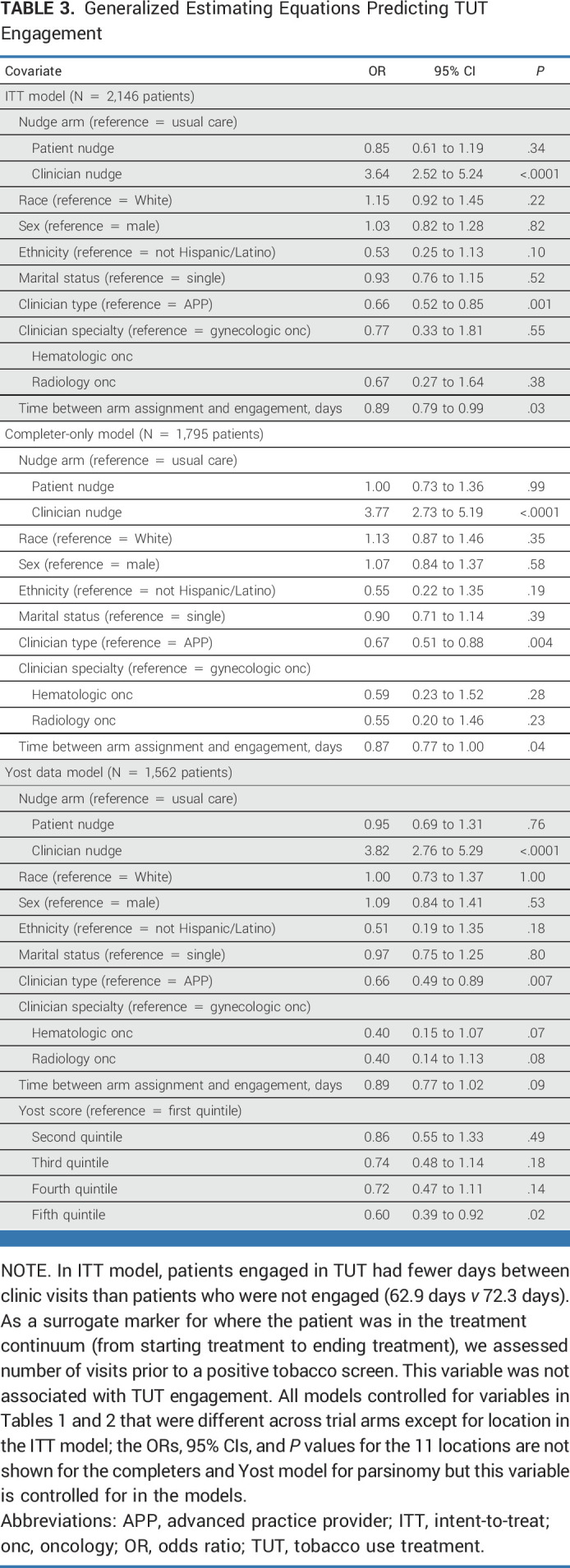
FIG 2.
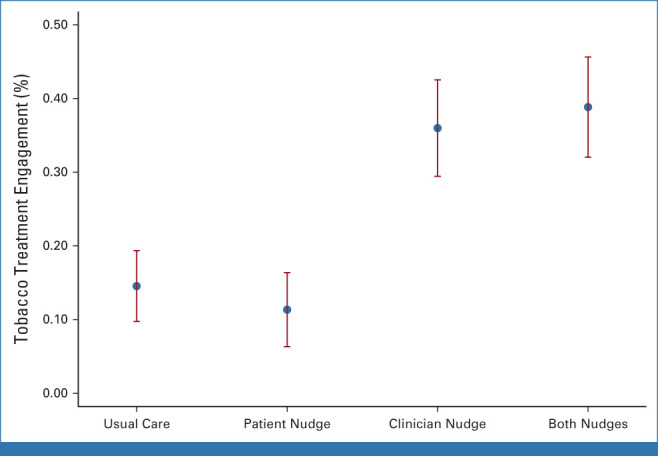
Rates of tobacco treatment engagement across treatment arms (ITT model). ITT, intent-to-treat.
TABLE 4.
Tobacco Treatment Engagement Rates by Study Arm, Model, and Covariates
In both models, clinician type was associated with TUT penetration rates (Table 3). There was no difference between APPs and physicians within the patient-only and combined nudge arms (Table 4), but higher penetration rates were found for APPs in the usual care (χ2[1] = 7.51; P = .007) and the clinician-only (χ2[1] = 11.85; P = .001) arms. Likewise, in the completer-only model, patients seen by APPs experienced higher TUT rates in the usual care (χ2[1] = 7.51; P = .007) and the clinician nudge (χ2[1] = 10.78; P = .001) arms.
Race was not associated with penetration of TUT in the models (Table 3). However, on the basis of our a priori interest in assessing equity, our post hoc analyses suggested an uneven impact of race across arms in the completers model (Table 4): non-White patients had significantly higher TUT rates in the usual care (χ2[1] = 8.97; P = .003) and both-nudges (χ2[1] = 11.12; P = .01) arms. This pattern of TUT penetration across arms and race was similar in the ITT model: higher TUT rates were observed in the usual care (χ2[1] = 8.97; P = .003) and both-nudges (χ2[1] = 6.65; P = .011) arms, but not in the patient or clinician nudge arms, suggesting that the influence of race on the completer-only model may have been a proxy for the influence of race across all arms. Restricting the completer-only model to include only the subset of patients for whom geocoding data were available through the EHR (n = 1,562) and adding Yost score as a covariate did not affect the main effects and showed that TUT penetration was lower in the highest SES group (Table 4).
DISCUSSION
Nudges delivered through the EHR are a well-established way to affect clinical behaviors and can have a substantial effect on patient and implementation outcomes.45 Changes to the way information is offered at the point of decision making are considered ethically acceptable if opting out of the default option remains easy and the range of clinical choices is preserved.46 In previous examples, clinician nudges using behavioral economic principles helped increase adherence to targeted guidelines, including for statin initiation,47 vaccination,48 and lung cancer screening.49 To our knowledge, this is the first study in oncology to compare implementation strategies to improve TUT using behavioral economic nudges aimed at clinicians, patients, or both. It builds upon our previous work and targets theoretically informed biases among both clinicians and patients, addressing known barriers to tobacco cessation treatment in this high-risk population. Overall, our results indicate that clinician nudges, aimed at counteracting omission bias and using default options and accountable justification of deviation, significantly increase the odds of TUT during cancer care—an effect that can benefit patients in both the short and long term.50
The impact of patient-directed nudges is less well established, and depends on the style of the nudge and the nature of the target problem.51,52 When considering nudging tobacco dependence treatment in cancer care, a shift in pre-existing assumptions is clearly warranted.53 Because smoking is stigmatized, it remained unclear what impact a patient-directed nudge might have on the clinical interaction. A nudge perceived as overly directive or judgmental might discourage engagement during the clinic visit or, in the worst case, undermine therapeutic relationships.54 Our patient nudge attempted to frame TUT positively and counteract the influence of status quo bias. Although our clinician nudge used techniques higher on the ladder of nudge interventions, our patient nudge used a lower-potency approach.55 Our inability to identify a discernable effect of the patient nudge may be a function of potency, characteristics of the electronic message transmission vehicle, or the specific cognitive bias addressed. Race was not a significant predictor of TUT penetration in the models but our post hoc assessment of this relationship indicated race could be a factor in TUT penetration. This observation was difficult to explain but may have been due to clustering across a geographically large and diverse health system. Our observations on race may have been confounded by SES as well, with a significant inverse relationship between SES quintile and TUT penetration. In either case, nudges did not exacerbate traditional inequities that typically affect health care delivery.
When nudges are introduced to clinician workflows, they are particularly effective for increasing TUT penetration when APPs are handling clinical encounters. This may be due to training or perspective, with APPs engaging in this element of clinical care more readily than their physician counterparts. It is also likely that the details of cancer care workflow confound this association; physicians may be more likely to perform initial evaluations while APPs are more likely to perform follow-up care. If so, the over-representation of TUT among APPs may be a function of pragmatic care planning concerns.
There are key strengths and limitations of this work. We used a pragmatic design and engaged both patients and clinicians—a key strength.56 There is potential for these implementation strategies to be both highly impactful and generalizable to other clinical settings and systems. Because our outcomes focused on clinical behaviors that are generally the result of a negotiated plan between clinician and patient, one key limitation is that clinician decision making may be moderated by unmeasured patient refusals. Also, given the multidisciplinary nature of cancer care, the potential for confounding because of contamination was present despite our a priori efforts to minimize its effect (eg, penetration influenced by frequency and/or variety of clinical visits). Finally, about 17% of patient nudges were not effectively delivered and more remained unread. Patients who did not receive the nudge may have had more advanced stages of disease necessitating accelerated visit schedules, no online portal account created, or another health care access–related disparity. This represents a significant opportunity for enhancement. Nevertheless, the results were remarkably consistent across the ITT and completer-only models.
Overall, this well-powered, rigorous pragmatic study demonstrates that implementation strategies, informed by behavioral economics within the EHR to counter biases that reduce health behavior engagement, can significantly increase the penetration of TUT in oncology care and do so without exacerbating health inequities in care delivery. Future work is needed to test the ability to generalize these findings to other health systems and to optimize the patient-directed nudge. Such work can continue to demonstrate the impact of implementation science and behavioral economics for enhancing the quality of cancer care to promote the best possible clinical outcomes for patients.
Rinad S. Beiads
Honoraria: Optum/UnitedHealth Group
Consulting or Advisory Role: OptumLabs, Optum/UnitedHealth Group
Justin Bekelman
Stock and Other Ownership Interests: Reimagine Care
Honoraria: National Comprehensive Cancer Network
Consulting or Advisory Role: UnitedHealthcare, Reimagine Care, AstraZeneca, Healthcare Foundry
Research Funding: Loxo@Lilly (Inst), Gilead Sciences (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending: machine learning systems using electronic health record data and patient-reported outcomes; 17/972,105
Callie Scott
Employment: Cohere Health, Humana
David A. Asch
Employment: VAL Health
Leadership: VAL Health
Stock and Other Ownership Interests: VAL Health
Honoraria: Deloitte, Boehringer Ingelheim, Mars Veterinary Health
Research Funding: United Health Group (Inst)
Jessica Chen
Employment: University of Pennsylvania
Lawrence N. Shulman
Consulting or Advisory Role: Genentech
Research Funding: Celgene (Inst), Independence Blue Cross (Inst)
Katharine A. Rendle
Honoraria: MJH Healthcare Holdings, LLC
Consulting or Advisory Role: Merck
Research Funding: Pfizer (Inst), AstraZeneca
Travel, Accommodations, Expenses: MJH Healthcare Holdings, LLC
Krisda H. Chaiyachati
Employment: Penn Medicine, Verily, The Children's Hospital of Philadelphia
Leadership: Intend Health Strategies
Stock and Other Ownership Interests: Verily
Travel, Accommodations, Expenses: The American Telemedicine Association
Oluwadamilola Fayanju
Research Funding: Gilead Sciences
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the AcademyHealth and NIH's 15th Annual Conference on the Science of Dissemination and Implementation in Health, Washington, DC, December 11-14, 2022.
SUPPORT
Supported by a grant from the National Cancer Institute (P50 CA244690).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Participant data reported in the article, after deidentification, will be available at the time of publication, along with a data dictionary. A methodologically rigorous proposal to use the data should be provided to the corresponding author, Brian Jenssen (JenssenB@chop.edu). Data will be made available with limited investigator support after investigator approval of the proposal and approval by the University of Pennsylvania Perelman School of Medicine.
AUTHOR CONTRIBUTIONS
Conception and design: Brian P. Jenssen, Robert Schnoll, Rinad S. Beidas, Justin Bekelman, Anna-Marika Bauer, Tierney Fisher, Callie Scott, Peter Gabriel, David A. Asch, Alison M. Buttenheim, Jessica Chen, Julissa Melo, Lawrence N. Shulman, Alicia B.W. Clifton, Katharine A. Rendle, Rachel C. Shelton, E. Paul Wileyto, Frank T. Leone
Financial support: Robert Schnoll, Rinad S. Beidas, Justin Bekelman
Administrative support: Robert Schnoll, Rinad S. Beidas, Anna-Marika Bauer, Julissa Melo, Alicia B.W. Clifton, Adina Lieberman, Tasnim Salam, Daniel Blumenthal, Daniel Ragusano
Provision of study materials or patients: Robert Schnoll, Dwayne Grant, Randall Oyer
Collection and assembly of data: Brian P. Jenssen, Robert Schnoll, Anna-Marika Bauer, Sarah Evers-Casey, Tierney Fisher, Jody Nicoloso, Peter Gabriel, Jessica Chen, Michael Horst, Adina Lieberman, Tasnim Salam, E. Paul Wileyto, Sue Ware, Daniel Ragusano, Frank T. Leone
Data analysis and interpretation: Brian P. Jenssen, Robert Schnoll, Justin Bekelman, Tierney Fisher, Peter Gabriel, Alison M. Buttenheim, Dwayne Grant, Michael Horst, Randall Oyer, Lawrence N. Shulman, Katharine A. Rendle, Krisda H. Chaiyachati, Rachel C. Shelton, Oluwadamilola Fayanju, E. Paul Wileyto, Daniel Blumenthal, Daniel Ragusano, Frank T. Leone
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cluster Randomized Pragmatic Clinical Trial Testing Behavioral Economic Implementation Strategies to Improve Tobacco Treatment for Patients With Cancer Who Smoke
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rinad S. Beiads
Honoraria: Optum/UnitedHealth Group
Consulting or Advisory Role: OptumLabs, Optum/UnitedHealth Group
Justin Bekelman
Stock and Other Ownership Interests: Reimagine Care
Honoraria: National Comprehensive Cancer Network
Consulting or Advisory Role: UnitedHealthcare, Reimagine Care, AstraZeneca, Healthcare Foundry
Research Funding: Loxo@Lilly (Inst), Gilead Sciences (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending: machine learning systems using electronic health record data and patient-reported outcomes; 17/972,105
Callie Scott
Employment: Cohere Health, Humana
David A. Asch
Employment: VAL Health
Leadership: VAL Health
Stock and Other Ownership Interests: VAL Health
Honoraria: Deloitte, Boehringer Ingelheim, Mars Veterinary Health
Research Funding: United Health Group (Inst)
Jessica Chen
Employment: University of Pennsylvania
Lawrence N. Shulman
Consulting or Advisory Role: Genentech
Research Funding: Celgene (Inst), Independence Blue Cross (Inst)
Katharine A. Rendle
Honoraria: MJH Healthcare Holdings, LLC
Consulting or Advisory Role: Merck
Research Funding: Pfizer (Inst), AstraZeneca
Travel, Accommodations, Expenses: MJH Healthcare Holdings, LLC
Krisda H. Chaiyachati
Employment: Penn Medicine, Verily, The Children's Hospital of Philadelphia
Leadership: Intend Health Strategies
Stock and Other Ownership Interests: Verily
Travel, Accommodations, Expenses: The American Telemedicine Association
Oluwadamilola Fayanju
Research Funding: Gilead Sciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.US Department of Health and Human Services : The health consequences of smoking—50 years of progress: A report of the surgeon general, 2014. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/ [DOI] [PubMed]
- 2.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2015. CA Cancer J Clin 65:5-29, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Shields PG, Herbst RS, Arenberg D, et al. : Smoking cessation, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 14:1430-1468, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Condoluci A, Mazzara C, Zoccoli A, et al. : Impact of smoking on lung cancer treatment effectiveness: A review. Future Oncol 12:2149-2161, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Vawda N, Banerjee RN, Debenham BJ: Impact of smoking on outcomes of HPV-related oropharyngeal cancer treated with primary radiation or surgery. Int J Radiat Oncol Biol Phys 103:1125-1131, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Kelemen LE, Warren GW, Koziak JM, et al. : Smoking may modify the association between neoadjuvant chemotherapy and survival from ovarian cancer. Gynecol Oncol 140:124-130, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Chang EHE, Braith A, Hitsman B, et al. : Treating nicotine dependence and preventing smoking relapse in cancer patients. Expert Rev Qual Life Cancer Care 2:23-39, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Land SR, Toll BA, Moinpour CM, et al. : Research priorities, measures, and recommendations for assessment of tobacco use in clinical cancer Research. Clin Cancer Res 22:1907-1913, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Preventive Services Task Force; Krist AH Davidson KW et al. : Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA 325:265-279, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Tobacco use—Healthy people 2030. health.gov. https://health.gov/healthypeople/objectives-and-data/browse-objectives/tobacco-use
- 11. Fiore MC, Jaén CR, Baker TB, et al. : Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD, US Department of Health and Human Services, Public Health Service, 2008. https://www.ncbi.nlm.nih.gov/books/NBK63952/
- 12. US Department of Health and Human Services : Smoking cessation: A report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf [DOI] [PubMed]
- 13.Nekhlyudov L, Lacchetti C, Davis NB, et al. : Head and neck cancer survivorship care guideline: American Society of Clinical Oncology clinical practice guideline endorsement of the American Cancer Society guideline. J Clin Oncol 35:1606-1621, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Toll BA, Brandon TH, Gritz ER, et al. : Assessing tobacco use by cancer patients and facilitating cessation: An American Association for Cancer Research policy statement. Clin Cancer Res 19:1941-1948, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein AO, Ripley-Moffitt CE, Pathman DE, et al. : Tobacco use treatment at the U.S. National Cancer Institute’s designated Cancer Centers. Nicotine Tob Res 15:52-58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siu AL: Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement for interventions for tobacco smoking cessation. Ann Intern Med 163:622-634, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Croyle RT, Morgan GD, Fiore MC: Addressing a core gap in cancer care—The NCI moonshot program to help oncology patients stop smoking. N Engl J Med 380:512-515, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidrine JI, Shete S, Cao Y, et al. : Ask-advise-connect: A new approach to smoking treatment delivery in health care settings. JAMA Intern Med 173:458-464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren GW, Marshall JR, Cummings KM, et al. : Addressing tobacco use in patients with cancer: A survey of American Society of Clinical Oncology members. J Oncol Pract 9:258-262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren GW, Marshall JR, Cummings KM, et al. : Practice patterns and perceptions of thoracic oncology providers on tobacco use and cessation in cancer patients. J Thorac Oncol 8:543-548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhargava M: Perspective. What happens when the doctor blames you for your own cancer? Washington Post. https://www.washingtonpost.com/outlook/what-happens-when-the-doctor-blames-you-for-your-own-cancer/2019/01/11/2791611e-14ff-11e9-90a8-136fa44b80ba_story.html
- 22.Jenssen BP, Leone F, Evers-Casey S, et al. : Building systems to address tobacco use in oncology: Early benefits and opportunities from the cancer center cessation initiative. J Natl Compr Cancer Netw 17:638-643, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loewenstein G, Brennan T, Volpp KG: Asymmetric paternalism to improve health behaviors. JAMA 298:2415-2417, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Halpern SD, Ubel PA, Asch DA: Harnessing the power of default options to improve health care. N Engl J Med 357:1340-1344, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Patel MS, Volpp KG: Leveraging insights from behavioral economics to increase the value of health-care service provision. J Gen Intern Med 27:1544-1547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoong SL, Hall A, Stacey F, et al. : Nudge strategies to improve healthcare providers’ implementation of evidence-based guidelines, policies and practices: A systematic review of trials included within Cochrane systematic reviews. Implement Sci 15:50, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder SA: What to do with a patient who smokes. JAMA 294:482-487, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Weiner B: On sin versus sickness. A theory of perceived responsibility and social motivation. Am Psychol 48:957-965, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Weiner B: An attributional theory of achievement motivation and emotion. Psychol Rev 92:548-573, 1985 [PubMed] [Google Scholar]
- 30.Weiner B, Perry RP, Magnusson J: An attributional analysis of reactions to stigmas. J Pers Soc Psychol 55:738-748, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Bazerman M: Judgment in Managerial Decision Making (ed 6). Hoboken, NJ, John Wiley and Sons, 2006 [Google Scholar]
- 32.Schnoll RA, Rothman RL, Newman H, et al. : Characteristics of cancer patients entering a smoking cessation program and correlates of quit motivation: Implications for the development of tobacco control programs for cancer patients. Psychooncology 13:346-358, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Schnoll RA, Rothman RL, Lerman C, et al. : Comparing cancer patients who enroll in a smoking cessation program at a comprehensive cancer center with those who decline enrollment. Head Neck 26:278-286, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Martinez E, Tatum KL, Weber DM, et al. : Issues related to implementing a smoking cessation clinical trial for cancer patients. Cancer Causes Control 20:97-104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulak JA, Cornelius ME, Fong GT, et al. : Differences in quit attempts and cigarette smoking abstinence between Whites and African Americans in the United States: Literature review and results from the international tobacco control US survey. Nicotine Tob Res 18:S79-S87, 2016. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landrine H, Corral I, Campbell KM: Racial disparities in healthcare provider advice to quit smoking. Prev Med Rep 10:172-175, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez D, Carlos HA, Adachi-Mejia AM, et al. : Retail tobacco exposure: Using geographic analysis to identify areas with excessively high retail density. Nicotine Tob Res 16:155-165, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drope J, Liber AC, Cahn Z, et al. : Who’s still smoking? Disparities in adult cigarette smoking prevalence in the United States. CA Cancer J Clin 68:106-115, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Levinson AH: Where the U.S. tobacco epidemic still rages: Most remaining smokers have lower socioeconomic status. J Health Care Poor Underserved 28:100-107, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Jenssen BP, Schnoll R, Beidas R, et al. : Rationale and protocol for a cluster randomized pragmatic clinical trial testing behavioral economic implementation strategies to improve tobacco treatment rates for cancer patients who smoke. Implement Sci 16:72, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evers-Casey S, Schnoll R, Jenssen BP, et al. : Implicit attribution of culpability and impact on experience of treating tobacco dependence. Health Psychol 38:1069-1074, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leone FT, Evers-Casey S, Graden S, et al. : Academic detailing interventions improve tobacco use treatment among physicians working in underserved communities. Ann Am Thorac Soc 12:854-858, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tversky A, Kahneman D: Advances in prospect theory: Cumulative representation of uncertainty. J Risk Uncertain 5:297-323, 1992 [Google Scholar]
- 44.Yost K, Perkins C, Cohen R, et al. : Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12:703-711, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Patel MS, Volpp KG, Asch DA: Nudge units to improve the delivery of health care. N Engl J Med 378:214-216, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blumenthal-Barby JS, Burroughs H: Seeking better health care outcomes: The ethics of using the “nudge”. Am J Bioeth 12:1-10, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Patel MS, Kurtzman GW, Kannan S, et al. : Effect of an automated patient dashboard using active choice and peer comparison performance feedback to physicians on statin prescribing: The PRESCRIBE cluster randomized clinical trial. JAMA Netw Open 1:e180818, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gatwood J, Brookhart A, Kinney O, et al. : Impact of patient and provider nudges on addressing herpes zoster vaccine series completion. Vaccine 41:778-786, 2023 [DOI] [PubMed] [Google Scholar]
- 49.Barnes AJ, Groskaufmanis L, Thomson NB: Promising approaches from behavioral economics to improve patient lung cancer screening decisions. J Am Coll Radiol 13:1566-1570, 2016 [DOI] [PubMed] [Google Scholar]
- 50.National Cancer Institute : Treating smoking in cancer patients: An essential component of cancer care. Division of Cancer Control and Population Sciences (DCCPS), 2022. https://cancercontrol.cancer.gov/brp/tcrb/monographs/monograph-23
- 51.Adusumalli S, Kanter GP, Small DS, et al. : Effect of nudges to clinicians, patients, or both to increase statin prescribing: A cluster randomized clinical trial. JAMA Cardiol 8:23-30, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Möllenkamp M, Zeppernick M, Schreyögg J: The effectiveness of nudges in improving the self-management of patients with chronic diseases: A systematic literature review. Health Policy 123:1199-1209, 2019 [DOI] [PubMed] [Google Scholar]
- 53.Richter KP, Ellerbeck EF: It’s time to change the default for tobacco treatment. Addiction 110:381-386, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Reach G: Patient education, nudge, and manipulation: Defining the ethical conditions of the person-centered model of care. Patient Prefer Adherence 10:459-468, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Last BS, Buttenheim AM, Timon CE, et al. : Systematic review of clinician-directed nudges in healthcare contexts. BMJ Open 11:e048801, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shortreed SM, Rutter CM, Cook AJ, et al. : Improving pragmatic clinical trial design using real-world data. Clin Trials 16:273-282, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Participant data reported in the article, after deidentification, will be available at the time of publication, along with a data dictionary. A methodologically rigorous proposal to use the data should be provided to the corresponding author, Brian Jenssen (JenssenB@chop.edu). Data will be made available with limited investigator support after investigator approval of the proposal and approval by the University of Pennsylvania Perelman School of Medicine.