Abstract
A stroke due to underlying intracranial large artery occlusion, which is atherosclerotic in nature, is known as intracranial atherosclerotic disease (ICAD). It is important to recognize that ischaemic stroke due to ICAD differs from extracranial disease and other stroke aetiologies and requires a nuanced approach. It is a significant cause of stroke worldwide, and severe symptomatic ICAD can present challenges from a therapeutic standpoint, including recurrent ischaemic stroke despite optimal management. Furthermore, exploring the underlying pathophysiological mechanisms responsible for the disease may be necessary while considering treatment options. This narrative review aims to provide an all-encompassing overview of this disease. Epidemiology and clinical pathophysiology will be explored in detail. The findings of large clinical trials will serve as a guide to finding the most optimized management strategies. Another critical question that arises is the treatment of acute ischaemic stroke due to large vessel occlusion with underlying intracranial atherosclerosis, is the treatment and clinical diagnosis the same as for other aetiologies of stroke (i.e. extracranial disease and nonvalvular atrial fibrillation)? Consequently, secondary prevention of patients with ischaemic stroke or transient ischaemic attack will be divided into medical therapy, risk factor control, and endovascular and surgical treatment options.
Keywords: alteplase, angioplasty, atherosclerotic disease, intracranial, ischaemic stroke
Introduction
Highlights
Atherosclerotic disease of the major intracranial vessels is a particularly important cause of stroke, as a symptomatic intracranial atherosclerotic disease has a high recurrence rate despite medical treatment.
The goal of therapy in intracranial atherosclerotic disease induced acute ischaemic stroke is to restore blood flow to brain regions that are not yet infracted.
In the treatment of acute ischaemic stroke with large vessel occlusion, accurate and early diagnostic strategies need to be elucidated, which would then lead to development of better prospective studies for rescue therapy.
One of the leading causes of death and disability in the United States is due to stroke1. Atherosclerotic disease of the major intracranial vessels is a particularly important cause of stroke, as a symptomatic intracranial atherosclerotic disease (ICAD) has a high recurrence rate despite medical treatment2. Risk factors for ICAD include hypertension, smoking, diabetes mellitus, and hyperlipidemia3. Interestingly ICAD prognosis has also been linked with metabolic syndrome, reduced adiponectin, and increased C-reactive protein levels4. The purpose of this review is to highlight management strategies for ICAD based on currently available evidence. Databases of Medline, PubMed, Web of Science, and Embase were searched to include observational studies, reviews, and clinical trials.
Epidemiology
ICAD appears to be a part of 8–10% of strokes in the United States and ~30–50% in Asian countries5,6. The Northern Manhattan Stroke Study (NMSS) demonstrated that the annual incidence of ICAD was much higher in Black and Hispanic patients than in white patients. Yet lifestyle and risk factor management differences should also be considered when evaluating these racial differences5. When considering the racial distribution of the global population, ICAD is likely the most common stroke subtype7.
Pathophysiology
Atherosclerosis is a complex disease process that leads to the development of both intracranial and extracranial disease within large arteries8. The process starts with fatty particles deposited into the endothelium, which progresses to combine inflammatory, smooth muscle, and connective tissue cells, forming a plaque9. Several distinct mechanisms lead to ischaemic stroke/transient ischaemic attack (TIA) when this process occurs in intracranial vessels.
The first is thrombosis of the vessel itself, which occludes the parent artery. Secondly, artery-to-artery embolus in which debris can break off from a plaque and occlude another artery. The third mechanism is progressive luminal stenosis leading to reduced blood flow from the stenosed vessel. Last is branch occlusion, which involves the plaque obstructing the orifice of smaller neighbouring vessels, often referred to as branch occlusive disease or branch atheromatous disease10 (Fig. 1).
Figure 1.
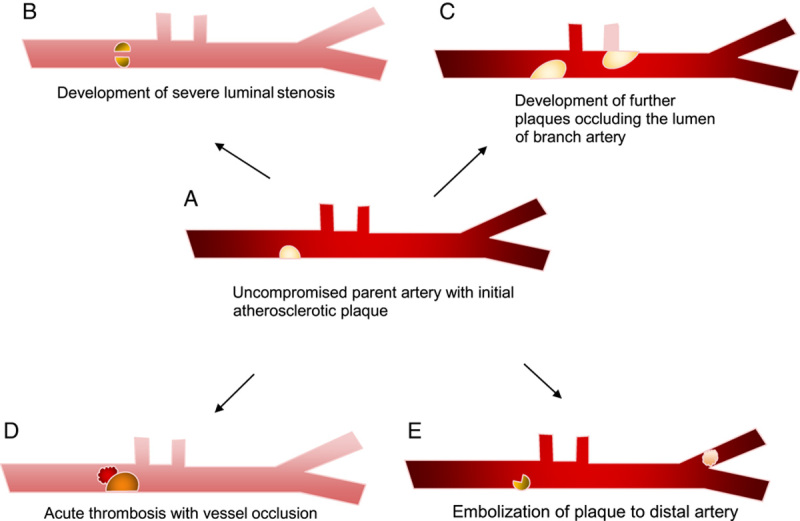
Modelling the progression of atherosclerosis on varying stroke mechanisms in intracranial atherosclerotic disease.
Treatment of acute ischaemic stroke (AIS) due to ICAD
The goal of therapy in AIS is to restore blood flow to brain regions that are not yet infracted. Intravenous alteplase is the primary treatment for AIS if initiated within 4.5 h11. In the underdeveloped world, the number of stroke patients receiving r-tPA is exceedingly low. In developing nations, the main challenges to thrombolysis therapy include prehospital delay, budgetary constraints, and a lack of infrastructure. Although the use of intravenous alteplase is getting common, developing countries should focus on primary and secondary stroke prevention strategies12. Mechanical thrombectomy (MT) is indicated for AIS with large vessel occlusion (LVO) in the anterior circulation. Five randomized controlled trials from 2010 to late 2014 demonstrated the benefit of MT to patients with AIS due to LVO in the anterior circulation13. Further trials have demonstrated select patients can benefit from MT for up to 24 h14. Generally, MT for posterior circulation LVO has shown to be equal to standard medical care; however, this may have been due to low sample sizes, and MT can be considered for LVO in the posterior circulation15,16.
Although there can be many reasons for the failure of MT and the long-term prognosis of patients undergoing an endovascular procedure depends on several variables, in treating AIS with LVO, underlying ICAD is considered the primary reason for the failure of MT17–20. This could be because the primary mechanism of ICAD-LVO is in situ thrombosis, which an unstable atherosclerotic plaque environment may characterize. One can hypothesize the differences between extracting an embolus (potentially from another source, i.e. cardiac) using thrombectomy in a healthy vascular environment compared to an acutely inflamed and atherosclerotic artery21,22.
Before addressing potential treatment options for ICAD-LVO, it is essential to understand the approach to diagnosis. There can be potentially four sources of LVO: ICAD, extracranial artery atherosclerotic embolism/plaque rupture, cardioembolic and cryptogenic embolism23. In the acute phase, one of the most important features of patient outcomes is the concept of time to treatment/reperfusion; as such, a limited number of diagnostic investigations can be considered optimal to perform before thrombectomy24,25. Non-contrast computed tomography scan is performed, followed by perfusion studies, yet these methods only diagnose AIS with LVO but not specifically ICAD-LVO. Currently, using digital subtraction angiography with the presence of a truncal type of occlusion is the criteria used to diagnose ICAD-LVO26. Therefore, frequently the diagnosis of ICAD-LVO is treatment dependant. This provides a challenge in performing prospective studies in AIS due to ICAD-LVO.
Typically, management involves implementing rescue therapy after failure to recanalize the target vessel after thrombectomy; this involves intra-arterial antiplatelets, anti-thrombotic, angioplasty, stenting, or a combination of these treatments27–29. However, there is yet to be a consensus on how best to utilize each rescue modality in various clinical scenarios. One approach describes visualizing the lesion post-thrombectomy and basing treatment on re-occlusion or residual stenosis. In cases of persistent stenosis, stenting is performed, and in cases of re-occlusion, angioplasty is done first, followed by stenting as necessary, with a specific strategy to avoid angioplasty in perforator-associated segments30. Deep perforator-type infarcts or small scattered infarcts should not be treated with angioplasty or stenting. In contrast, border zone-type or large territorial infarcts warrant intervention with either angioplasty or stenting31.
As demonstrated above, ICAS-LVO presents unique hurdles, and current promising evidence comes mainly from retrospective studies. Furthermore, identifying a subgroup of patients to perform prospective studies is difficult because of the challenges in establishing an early (pre-treatment) diagnosis of ICAD-LVO (Fig. 2).
Figure 2.

Treatment algorithm for intracranial atherosclerotic disease. MT, mechanical thrombectomy; TIA, transient ischaemic attack; TPA, Tissue plasminogen activator. *Rescue treatments include intra-arterial antiplatelet, antithrombotics or angioplasty and/or stenting.
Secondary prevention
Medical therapy
Anti-thrombotic therapy
The use of anti-thrombotic agents is intuitive when dealing with atherosclerotic disease. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial compared the safety and efficacy of warfarin and aspirin in patients with ICAD. The trial recruited patients with 50–99% stenosis of a major intracranial artery who suffered TIA or stroke within 90 days. The trial concluded that warfarin was associated with higher rates of adverse events and provided no benefit over aspirin32. The Stenting and Aggressive Medical Therapy for Intracranial Stenosis (SAMMPRIS) trial enlisted patients with 70–99% stenosis of a major intracranial artery who suffered TIA or stroke 30 days prior to entry. In the aggressive medical treatment arm (AMT) of the group, the use of dual antiplatelet therapy (DAPT) with aspirin and clopidogrel for the first 90 days demonstrated a stroke and death at 1 year rate of 12.2%33,34. Compared to the subgroup of patients in the WASID trial (70–99% intracranial artery stenosis), the recurrent rate of stroke of patients on DAPT appears to be significantly lower (12% vs. 23% at 1 year). It is important to note that this effect can also be attributed to superior risk factor control in WASID vs. SAMMPRIS patients35.
In the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial, patients were assigned clopidogrel and aspirin or aspirin monotherapy within 24 h of the onset of TIA or stroke, subgroup analysis of patients with ICAS in CHANCE shows that patients treated with DAPT had a lower rate of recurrent stroke at 90 days compared to monotherapy, but this result was not statistically significant36.
The clopidogrel plus aspirin for infarction reduction in acute stroke or transient ischaemic attack patients with significant artery stenosis and micro embolic signals (CLAIR) trial aimed to measure micro embolic signalling in patients of recent stroke who were either on DAPT or aspirin alone, many of the patients had symptomatic intracranial stenosis in the internal carotid artery or the middle cerebral artery. The trial demonstrated that combination therapy was more effective than aspirin alone in reducing micro embolic signals37.
The studies mentioned above suggest that DAPT is likely beneficial for patients with ICAD though further study to define optimal duration may be necessary. Therefore, based on AHA recommendations, DAPT with aspirin and clopidogrel for up to 90 days, followed by aspirin monotherapy, is recommended for patients with significant artery stenosis of 70–90%38.
An interesting point to note from clopidogrel use in acute coronary syndromes is clopidogrel resistance. Often a strategy of switching therapies to Ticagrelor has been shown to be effective39. Clopidogrel resistance has also been suggested in a sub-study of the CHANCE trial40. An important question is whether recurrent stroke in ICAD patients, despite optimal therapy with clopidogrel and aspirin, can be attributed to clopidogrel resistance. Furthermore, should these patients be treated with Ticagrelor or Prasugrel before trying other therapies?
In The Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and ASA for Prevention of Stroke and Death (THALES) trial, a subgroup of patients who were found to have ipsilateral atherosclerotic stenosis was found to have a higher absolute risk and more significant absolute risk reduction of stroke or death at 30 days, compared to those without41. However, it is challenging to attribute stroke or TIA due to ICAD in this group. Therefore, more definitive studies on Ticagrelor with symptomatic ICAD patients are necessary.
Direct oral anticoagulants (apixaban, rivaroxaban) are well-documented in venous thromboembolism and atrial fibrillation42. Their use in preventing stroke in ICAD patients compared to DAPT has not yet been studied.
Risk factor control
Adequate management of hypertension, hyperlipidemia, obesity, diabetes, and smoking is important for cerebrovascular disease, including ICAD. Analysis of the WASID trial has highlighted the importance of elevated blood pressure and cholesterol levels in patients with ICAD with an increased risk of stroke43. Likewise, in the analysis of SAMMPRIS, raised blood pressure, cholesterol, and physical inactivity should be treated in patients with ICAD. Of particular note is that physical inactivity was the strongest predictor of favourable outcomes35. A systolic blood pressure (SBP) target of less than 140 mmHg is recommended for patients with hypertension, which should be treated with pharmacologic and non-pharmacologic strategies44.
How intense the SBP should be controlled or targets lower than less than 140 mmHg has not been established. Some data suggest lower blood pressure targets may be harmful in the setting of ICAS. In one study, patients with symptomatic internal carotid or middle cerebral artery occlusion were divided into intensive (SBP 124.6 ± 10 mmHg) and moderate (SBP 132.3 ±10 mmHg) blood pressure control groups and changes in white matter lesions at the whole forebrain between baseline and 24 weeks was measured using fluid attenuation inversion recovery. The intensive BP control group failed to prove noninferiority compared to the modest BP group and may increase ischaemic lesion volume45.
Patients with a history of stroke or TIA should be prescribed high-intensity statin therapy. (20) A low density lipoprotein-cholesterol goal of less than 70 mg/dl has shown benefit in a trial of 2860 patients, in comparison to a range of 90–110 mg/dl46.
Endovascular treatments
Angioplasty and stenting
The SAMMPRIS trial was a large multicenter randomized control trial with the primary purpose of comparing aggressive medical management (AMT) using DAPT with clopidogrel and aspirin alone to angioplasty and stenting (using the Wingspan system) and AMT in ICAD. The trial was stopped prematurely due to the high rate of stroke/death in the stenting group33,34. Based on the study results above, stenting and angioplasty are not recommended for patients with a first stroke or TIA, which is attributable to ICAD.
Following SAMMPRIS, the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) trial, which used a balloon-mounted stent rather than the Wingspan system, with similar criteria and 250 planned patients enroled in two arms of the study with either medical therapy alone or balloon-expandable stent and medical therapy, with the primary endpoint being Stroke or TIA in the qualifying artery within 12 months of enrolment. This trial was stopped prematurely, too, due to a higher rate of stroke in the intervention arm47.
The Wingspan Stent System Post Market Surveillance (WEAVE) study was a post-market surveillance study of the Wingspan stent that included 152 patients. The trial aimed to assess complications within 72 h of the procedure. The trial was stopped early due to a lower-than-expected periprocedural event rate of 2.6%, compared to the SAMMPRIS 14.7%48. Of note, all the patients in the WEAVE trial had symptomatic ICAD with 70–99% stenosis and had failed medical therapy. The question arises: with lower periprocedural events and strict patient selection (Stroke/TIA while on optimal medical management), could there be a role for stenting in ICAD?
A recent multicenter randomized clinical trial enroled 263 patients with ICAD who suffered stroke or TIA while on DAPT and were divided into two arms, intervention with a drug-eluting stent (DES) or bare metal stent. The results showed the DES group had a significantly low rate of ischaemic stroke recurrence from day 31 to 1 year compared to the bare metal stent group, 0.8% vs. 6.9%49. Recently, the China Angioplasty & Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) trial compared stenting plus medical therapy versus medical therapy alone in patients with symptomatic severe intracranial atherosclerotic stenosis. The study concluded that among patients with a transient ischaemic attack or ischaemic stroke due to symptomatic severe intracranial atherosclerotic stenosis, the addition of percutaneous transluminal angioplasty and stenting to medical therapy, compared with medical therapy alone, resulted in no significant difference in the risk of stroke or death within one month or stroke in the suiting artery territory beyond 30 days through 1 year50.
Submaximal angioplasty
Submaximal angioplasty is an angioplasty technique which uses slow expansion of a smaller balloon by at least 50–80% of the normal vessel diameter. Recent meta-analysis showed 9 studies with 408 interventions in 395 patients with a periprocedural complication rate of 5 percent, and rate of stroke or death beyond 30 days to be ~4% in patients with symptomatic non-emergent ICAD. Submaximal angioplasty may be a safe and effective strategy to manage ICAD however further larger studies are needed to validate this51.
Surgical treatments
Encephaloduroarteriosynangiosis (EDAS)
Bypass surgery for ICAD had been considered previously, with the joining of the superficial temporal artery to the middle temporal artery compared to the best medical care. The study failed to demonstrate extracranial to intracranial anastomosis effectively prevents cerebral ischaemia for ICAD52. EDAS is a novel indirect revascularization method in which the superficial temporal artery is dissected while still connected to the external carotid artery, it is then placed near the middle cerebral artery, and over time the process of neovascularisation takes place and provides new flow to the intracranial compartment. The EDAS revascularization for symptomatic intracranial atherosclerotic steno-occlusive (ERSIAS) trial is a prospective performance criterion trial of EDAS plus intensive medical management and is currently advancing into phase-IIb/III53. With further advancement, EDAS could develop as a new therapeutic option for ICAD.
Conclusion
Intracranial atherosclerosis is a complex and significant factor in the development of stroke and presents unique challenges which require further inquiry. In the treatment of AIS with large vessel occlusion, accurate and early diagnostic strategies need to be elucidated, which would then lead to development of better prospective studies for rescue therapy. In terms of secondary prevention, there has been much progress throughout the field and the landmark study of SAMMPRIS demonstrated the importance of risk factor control and superiority of medical management, yet despite great insight into medical treatment, patients remain at high risk for recurrent stroke. Further analysis raised questions as to what the role does endovascular treatments play in secondary prevention of ICAD, and if certain class of patients can benefit from them. Therefore, further progress can be made in both medical and interventional strategies.
Ethical approval
Ethical approval is not required at our institution to publish a narrative review.
Consent
N/A—Narrative Review.
Source of funding
This study received no external funding.
Author contribution
R.A. and H.M. conceived and designed the study. R.A. and H.M. were responsible for data collection and acquisition of data. R.S.B., A.G., and M.K. performed the literature review. R.A., H.M., R.S.B., and A.G. wrote the initial manuscript. H.M. and M.K. critically revised the manuscript. All authors have approved the final manuscript.
Conflicts of interest disclosure
The authors declare that there is no conflict of interest regarding the publication of this narrative review.
Research registration unique identifying number (UIN)
N/A—Narrative Review.
Guarantor
Dr. Meraj Kamal.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article
Published online 14 August 2023
Contributor Information
Rehan Ahmed, Email: rehanahmed7348@gmail.com.
Hamza Maqsood, Email: hamzamaqsood381@gmail.com.
Rochaknaveen Singh Bains, Email: rochakbains05@gmail.com.
Azouba Gulraiz, Email: azouba.gulriaz@gmail.com.
Meraj Kamal, Email: merajkamaln66@gmail.com.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528; [published correction appears in Circulation. 2020 Jan 14;141(2):e33]. [DOI] [PubMed] [Google Scholar]
- 2.Yaghi S, Rostanski SK, Boehme AK, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol 2016;73:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013;12:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res 2017;120:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. Northern Manhattan Stroke Study Stroke 1995;26:14–20. [DOI] [PubMed] [Google Scholar]
- 6.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006;1:158–159. [DOI] [PubMed] [Google Scholar]
- 7.Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396–2399. [DOI] [PubMed] [Google Scholar]
- 8.Cipolla MJ. The Cerebral Circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2009. Chapter 2, Anatomy and Ultrastructure. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53086/ [PubMed]
- 9.Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers 2019;5:56. [DOI] [PubMed] [Google Scholar]
- 10.Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke 2014;16:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–1703. [DOI] [PubMed] [Google Scholar]
- 12.Ghandehari K. Barriers of thrombolysis therapy in developing countries. Stroke Res Treat 2011;2011:686797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 14.Baron JC. Selection of patients for thrombectomy in the extended time window. JAMA Neurol 2021;78:1051–1053. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 2020;19:115–122. [DOI] [PubMed] [Google Scholar]
- 16.Langezaal LCM, van der Hoeven EJRJ, Mont’Alverne FJA, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med 2021;384:1910–1920. [DOI] [PubMed] [Google Scholar]
- 17.Baek JH, Kim BM, Heo JH, et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke 2018;49:2088–2095. [DOI] [PubMed] [Google Scholar]
- 18.Tsang ACO, Orru E, Klostranec JM, et al. Thrombectomy outcomes of intracranial atherosclerosis-related occlusions. Stroke 2019;50:1460–1466. [DOI] [PubMed] [Google Scholar]
- 19.Al Kasab S, Almallouhi E, Alawieh A, et al. Outcomes of rescue endovascular treatment of emergent large vessel occlusion in patients with underlying intracranial atherosclerosis: insights from STAR. J Am Heart Assoc 2021;10:e020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobrocky T, Kaesmacher J, Bellwald S, et al. Stent-retriever thrombectomy and rescue treatment of M1 occlusions due to underlying intracranial atherosclerotic stenosis: cohort analysis and review of the literature. Cardiovasc Intervent Radiol 2019;42:863–872. [DOI] [PubMed] [Google Scholar]
- 21.Yang WJ, Wong KS, Chen XY. Intracranial atherosclerosis: from microscopy to high-resolution magnetic resonance imaging. J Stroke 2017;19:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin NS, Benavides S, Starkman S, et al. Autopsy findings after intracranial thrombectomy for acute ischemic stroke: a clinicopathologic study of 5 patients. Stroke 2010;41:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Kasab S, Holmstedt CA, Jauch EC, et al. Acute ischemic stroke due to large vessel occlusion. Emerg Med Rep 2018;39:13–22. [Google Scholar]
- 24.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–1288. [DOI] [PubMed] [Google Scholar]
- 25.Menon BK, Sajobi TT, Zhang Y, et al. Analysis of workflow and time to treatment on thrombectomy outcome in the endovascular treatment for small core and proximal occlusion ischemic stroke (ESCAPE) randomized, controlled trial. Circulation 2016;133:2279–2286. [DOI] [PubMed] [Google Scholar]
- 26.Baek JH, Kim BM. Angiographical identification of intracranial, atherosclerosis-related, large vessel occlusion in endovascular treatment. Front Neurol 2019;10:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JG, Suh DC, Song Y, et al. Direct stenting of intracranial atherosclerosis-related acute large vessel occlusion. Clin Neuroradiol 2021;31:833–841. [DOI] [PubMed] [Google Scholar]
- 28.Gross BA, Desai SM, Walker G, et al. Balloon-mounted stents for acute intracranial large vessel occlusion secondary to presumed atherosclerotic disease: evolution in an era of supple intermediate catheters. J Neurointerv Surg 2019;11:975–978. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Lin M, Wang S, et al. Primary angioplasty and stenting may be superior to thrombectomy for acute atherosclerotic large-artery occlusion. Interv Neuroradiol 2018;24:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Kasab S, Almallouhi E, Spiotta AM. Rescue endovascular treatment for emergent large vessel occlusion with underlying intracranial atherosclerosis: current state and future directions. Front Neurol 2021;12:734971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JS, Lee SJ, Hong JM, et al. Endovascular treatment of large vessel occlusion strokes due to intracranial atherosclerotic disease. J Stroke 2022;24:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352:1305–1316. [DOI] [PubMed] [Google Scholar]
- 33.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993–1003; [published correction appears in N Engl J Med. 2012 Jul 5;367(1):93]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014;383:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turan TN, Nizam A, Lynn MJ, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology 2017;88:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Wong KS, Leng X, et al. Dual antiplatelet therapy in stroke and ICAS: Subgroup analysis of CHANCE. Neurology 2015;85:1154–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489–497. [DOI] [PubMed] [Google Scholar]
- 38.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–2236; [published correction appears in Stroke. 2015 Feb;46(2):e54]. [DOI] [PubMed] [Google Scholar]
- 39.Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation 2010;121:1188–1199. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA 2016;316:70–78. [DOI] [PubMed] [Google Scholar]
- 41.Amarenco P, Denison H, Evans SR, et al. Ticagrelor added to aspirin in acute nonsevere ischemic stroke or transient ischemic attack of atherosclerotic origin. Stroke 2020;51:3504–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almarshad F, Alaklabi A, Bakhsh E, et al. Use of direct oral anticoagulants in daily practice. Am J Blood Res 2018;8:57–72. [PMC free article] [PubMed] [Google Scholar]
- 43.Chaturvedi S, Turan TN, Lynn MJ, et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology 2007;69:2063–2068. [DOI] [PubMed] [Google Scholar]
- 44.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021;52:e364–e467; [published correction appears in Stroke. 2021 Jul;52(7):e483-e484]. [DOI] [PubMed] [Google Scholar]
- 45.Park JM, Kim BJ, Kwon SU, et al. Intensive blood pressure control may not be safe in subacute ischemic stroke by intracranial atherosclerosis: a result of randomized trial. J Hypertens 2018;36:1936–1941. [DOI] [PubMed] [Google Scholar]
- 46.Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med 2020;382:9. [DOI] [PubMed] [Google Scholar]
- 47.Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA 2015;313:1240–1248. [DOI] [PubMed] [Google Scholar]
- 48.Wabnitz AM, Derdeyn CP, Fiorella D, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. 2019;50:143–147. 10.1161/strokeaha.118.020840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jia B, Zhang X, Ma N, et al. Comparison of drug-eluting stent with bare-metal stent in patients with symptomatic high-grade intracranial atherosclerotic stenosis: a randomized clinical trial. JAMA Neurol 2022;79:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao P, Wang T, Wang D, et al. Effect of stenting plus medical therapy vs medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis: The CASSISS Randomized Clinical Trial. JAMA 2022;328:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stapleton CJ, Chen YF, Shallwani H, et al. Submaximal angioplasty for symptomatic intracranial atherosclerotic disease: a meta-analysis of peri-procedural and long-term risk. Neurosurgery 2020;86:755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med 1985;313:1191–1200. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez NR, Jiang H, Lyden P, et al. Encephaloduroarteriosynangiosis (EDAS) revascularization for symptomatic intracranial atherosclerotic steno-occlusive (ERSIAS) Phase-II objective performance criterion trial. Int J Stroke 2021;16:701–709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


