Abstract
Background and Aims:
Despite NAFLD being the most prevalent liver disease globally, currently there are no FDA-approved treatments, and weight loss through caloric restriction and enhanced physical activity is the recommended treatment strategy. Intermittent fasting (IF) has been proposed as an alternative strategy with additional cardiometabolic benefits. In this systematic review and meta-analysis, we evaluated the anthropometric, biochemical, and hepatic impacts of IF in patients with NAFLD.
Methods:
MEDLINE, EMBASE, Cochrane Central, and conference abstracts were searched for IF interventions in adults with NAFLD until April 2, 2023. Meta-analysis with a random effects model was used to compare pre-intervention and post-intervention changes in anthropometric, biochemical, and hepatic end points in the IF intervention group with the control group. Publication bias was assessed using Egger’s test.
Results:
Fourteen studies were included in the systematic review and ten in the meta-analysis (n = 840 participants, 44.64% male). Studies varied in modalities for NAFLD diagnosis, duration of IF (4–52 weeks), and type of IF (5:2 diet, modern alternate-day fasting, time-restricted eating, or religious fasting). Body weight, body mass index, and waist to hip ratio all significantly improved following fasting intervention (p< 0.05). Adults with NAFLD showed an improvement in serum alanine transaminase, aspartate aminotransferase, hepatic steatosis (controlled attenuation parameter measured by vibration-controlled transient elastography), and hepatic stiffness (measured by vibration-controlled transient elastography) after fasting intervention (p < 0.05).
Conclusions:
There is limited, but moderate- to high-quality evidence to suggest that IF can improve hepatic end points and promote weight loss in adults with NAFLD. Larger randomized controlled studies with extended duration are needed to further validate our findings.
INTRODUCTION
As a consequence of the increasing prevalence of obesity, type 2 diabetes, and related metabolic conditions, NAFLD has become one of the most important causes of chronic liver disease worldwide, affecting 24% of the global population1 and increasing the risk of all-cause mortality.1,2 While there are several therapeutics in Phase III clinical trials, there are no currently approved medical therapies for NAFLD. Weight loss through caloric restriction and increased physical activity remains the backbone of therapy.3–5 However, caloric restriction may be challenging to maintain in the long term,6 an important consideration given the significant impact of an individual’s dietary choices and adherence on the degree of weight loss.7,8 To optimize NAFLD management and increase the likelihood of favorable hepatic outcomes, a variety of options for dietary interventions are likely needed to satisfy individual patient needs.
Emerging animal and human data suggest that some of the health benefits of caloric restriction may be attributable to the metabolic switch from fed to fasting states as opposed to an overall reduction in caloric intake.9–11 Thus, it is plausible that certain dietary interventions, such as intermittent fasting (IF) where one alternates between fasting and fed states, may confer significant weight loss, hepatic, and overall metabolic health benefits in patients with NAFLD.
IF has emerged as one of the most popular diets for weight loss in recent years, encompassing any dietary pattern with a short-term period of little to no caloric intake followed by a period of ad libitum consumption.12 While both caloric restriction and IF may result in a decreased caloric intake over a 24-hour period, a substantial reduction in caloric intake is not integral to IF. The most commonly described methods include 5:2 diet (limited calories consumed for 2 days each week with no restrictions on the remaining 5), modern alternate-day fasting (MADF) (a fasting day with anywhere from zero calories to ~25% of energy needs alternated with an ad libitum feeding day), time-restricted feeding (TRF) (reducing the daily feeding window to a specific number of hours per day, usually 4–10 h, and fasting for the remaining hours of the day), and religious fasting (eg, Ramadan).
IF is effective for weight loss, similar to caloric restriction,13–18 and improves the overall metabolic health.12,19 However, there remains a lack of evidence-based guidelines for its use for the treatment of NAFLD. In this systematic review with meta-analysis and meta-regression, we evaluate the impact of IF on anthropometric, biochemical, and hepatic end points in adults with NAFLD.
METHODS
This study was conducted according to the guidelines of the preferred reporting items for systematic reviews and meta-analysis (PRISMA, http://links.lww.com/HC9/A422).20
Search strategy
MEDLINE, EMBASE, and Cochrane CENTRAL were searched up until April 2, 2023. In addition, the following journals were searched for published conference abstracts: Journal of Hepatology, Hepatology, and Gastroenterology. Free-text and index terms for “NAFLD” and “fasting” were combined. The search terms were a combination of free-text and medical subject headings including NAFLD, NASH, or NAFL. Studies identified were then combined with the set operator “AND” to include studies identified by the following free-text and medical subject headings terms: fasting, IF, time-restricted eating, time-restricted feeding, and 5:2 diet. The full search strategy is available in Appendix A, http://links.lww.com/HC9/A423. No date, language, or article type restrictions were included in the search strategy. In addition, reference lists of existing systematic reviews and relevant papers were checked. Search results were imported into Covidence, an online platform for conducting systematic reviews and metanalyses, and duplicate entries were automatically removed.
Study selection and eligibility criteria
All steps of study selection were performed by 2 investigators (Marcia Lange and Devika Nadkarni) who independently applied pre-defined inclusion and exclusion criteria (Supplemental Table 1, http://links.lww.com/HC9/A423). The inclusion criteria included any study with an IF type intervention in adults with NAFLD and at least 1 end point of interest. End points of interest included body weight (kg), body mass index (BMI), waist circumference (WC) (cm), waist to hip ratio (WHR), serum alanine transaminase (ALT) (IU/L), serum aspartate aminotransferase (AST) (IU/L), lipid profile (HDL, LDL, cholesterol, or triglycerides) (mg/dL), homeostatic model assessment for insulin resistance (HOMA-IR), fasting glucose, liver steatosis (as measured by controlled attenuation parameter (CAP), magnetic resonance intrahepatic lipid content, or histology), and liver stiffness (as measured by vibration-controlled transient elastography or magnetic resonance elastography). Titles and abstracts were first screened, then eligible articles were subject to full-text review. Case reports and case series were removed during the full-text review. Disagreements at any step were resolved by discussion and involvement of a third investigator (Carolyn Newberry, Sonal Kumar, or Tatyana Kushner). Concordance was quantified using Cohen’s Kappa at both steps of study selection.
Data extraction
If multiple reports were available for a study, for the purposes of data extraction, full-length manuscripts were prioritized over conference abstracts or presentations. Data were independently extracted in duplicate by 2 reviewers (Marcia Lange and Devika Nadkarni) using a standardized data collection form. Any differences in extracted data were discussed with a third reviewer (Carolyn Newberry, Sonal Kumar, or Tatyana Kushner) and resolved. Study characteristics including location, duration, study design, type of fasting intervention, diagnostic modality for NAFLD diagnosis, and number of study participants were recorded. Participant characteristics and information on end points of interest was also collected. If necessary, further information was obtained by directly contacting the original investigators.
Quality assessment and risk of bias
Quality assessments were conducted by 2 independent reviewers (Marcia Lange and Devika Nadkarni) using ROBINS-I for nonrandomized studies and RoB 2 for randomized controlled trials (RCTs).
Synthesis methods
Data were synthesized by tabulating the study intervention characteristics and comparing them against the planned groups for each synthesis. As necessary, differing units of measurement for end points of interest were uniformized. For example, triglyceride measurements were converted from standard international U (mmol/L) to mg/dL by multiplying by 88.57.21,22 Fasting glucose was expressed in mg/dL and converted from standard international U (mmol/L) by multiplying by 18.02.23 Total cholesterol, HDL, and LDL measurements were converted from standard international units (mmol/L) to mg/dL by multiplying by 38.67.
Data analysis
Data were analyzed using the meta package in R and the PRISMA flow diagram was generated using the PRISMA2020, http://links.lww.com/HC9/A422 package.24 Pre-intervention and post-intervention changes in end points and standard deviations (SD) for change were calculated based on equations outlined in the Cochrane Handbook.25 Mean difference was calculated for each end point of interest using a random effects model to pool the data. Two-sided p values < 0.05 were considered statistically significant. Study weights were assigned using the inverse variance method. Heterogeneity was reported quantitatively with the I2 statistic, and p values for heterogeneity were assigned using Q-tests with a significance cutoff of p < 0.1. Heterogeneity was defined as low (0%–40%), moderate (41%–60%), or substantial (>60%). Meta-regression models were generated for all end points with statistically significant heterogeneity. Recognizing that the type of IF intervention, study duration, or study country may influence the results, subgroup analyses were conducted. Publication bias was assessed for end points with at least 5 studies using Egger’s regression test, with significant publication bias defined as a p < 0.05.
RESULTS
Study characteristics
We screened 6284 records for title and abstract, with moderate agreement (Cohen’s k: 0.52) (Figure 1). Of these, 6135 were deemed irrelevant and 149 reports were retrieved for detailed full-text review. One hundred thirty-five studies were excluded (predominantly due to an alternative dietary intervention not within the definition of IF and its subtypes) with fair concordance (Cohen’s k: 0.30). Bibliographies of reviews and key articles were also searched, yielding 2 additional studies.26,27 After collating reports from the same studies, 14 studies were included in the review: 10 randomized control trials, (Cai 2019 included two different IF interventions), 2 double-arm nonrandomized studies, 2 single-arm studies, and 1 retrospective case-control study.26–39 Included studies were published between the years 2014 and 2023.
FIGURE 1.
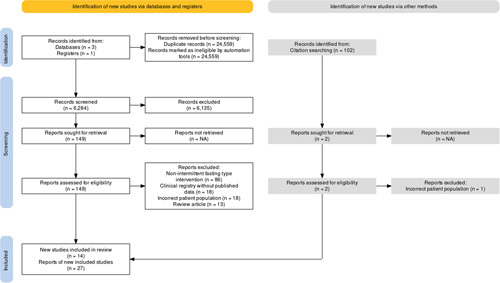
PRISMA flowchart of search for study inclusion in the systematic review and meta-analysis. Abbreviations: PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Systematic review and descriptive analysis
The majority of studies took place in Middle Eastern (40.00%) or Asian (26.67%) countries. The diagnostic modality for NAFLD varied: 42.86% of studies used imaging (mainly ultrasound), 14.29% used transient elastography with CAP, 14.29% relied on chart review and diagnosis codes, and 7.14% relied on elevated transaminases. Two studies did not clarify how NAFLD was diagnosed.34,38 None of the studies included patients diagnosed with NAFLD or NASH by liver biopsy. The types of IF intervention studied included the following: 4 evaluating religious fasting (Ramadan), 5 TRF, 3 MADF, and 3 evaluating the 5:2 diet. One study evaluated both MADF and TRF.29 Within those that conducted the 5:2 diet, 2 studies implemented a 5:2 diet where participants spent 2 nonconsecutive days per week of fasting, consuming roughly 500 kcal/day.30,38 The third 5:2 study had participants fasting on 2 consecutive days per week, where 25% of the recommended calorie intake was consumed over a 2-hour period from 12:00 to 2:00 p.m.30,40 Fasting interventions lasted a median 12 weeks (IQR 4, 12, range 4–52). Seven studies documented participant adherence to the fasting diet, primarily through use of 72-hour food recall diaries and regular follow-up with a dietician.27,30–32,34,35,39,40 The reported dropout rate in the fasting intervention group was < 10% for all studies. All end points of interest were found in at least a subset of the studies, except for magnetic resonance intrahepatic lipid content, histology assessing steatosis and/or fibrosis, and magnetic resonance elastography. Six studies assessed liver steatosis and fatty liver improvement following fasting intervention: 2 studies measured steatosis by vibration-controlled transient elastography/CAP,30,40 2 graded liver steatosis based on imaging pre-intervention and post-intervention (by steatosis grading),27,31 and the final 2 used magnetic resonance intrahepatic lipid content to assess fatty liver improvement.35,39 Table 1 provides a detailed overview of each study in the systematic review.
TABLE 1.
Summary of studies included in systematic review
| References | Country | Study design | Modality for NAFLD diagnosis | N | Intervention duration (wk) | Intervention group/comparison group | Duration of fasting for intervention group | Mean age of intervention group/comparison group | % Male of intervention group/comparison group | Mean baseline BMI of intervention group/comparison group | Measured end points |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arabi 201532 | Iran | Single arm | Ultrasound | 50 | 4 | Religious/none | 16 h daily | 40.52 (10.9)/− | 66.0/− | 29.5 (2.40)/− | BMI, WC, FBS, ALT, AST, TC, TG, HDL, LDL |
| Badran 202233 | Egypt | Single arm | Ultrasound | 98 | 4 | TRF/none | 16 h daily | Unknown | 23.5/− | 37.03 (6.56)/− | BMI, BW, WC, FBS, HOMA-IR, ALT, AST, TC, TG, HDL, LDL |
| Cai 2019 (ADF)a 29 | China | RCT | Ultrasound | 169 | 12 | MADF/SoC | 24 h every other day | 35.5 (4.42)/34.54 (6.96) | 36.8/29.11 | 26.12 (2.21)/26.34 (2.73) | BMI, WC, FBS, CAP, TC, TG, HDL, LDL, LSM |
| Cai 2019 (TRF)a 29 | China | RCT | Ultrasound | 174 | 12 | TRF/SoC | 16 h daily | 33.56 (6.23)/34.54 (6.96) | 30.53/29.11 | 26.76 (1.59)/26.34 (2.73) | BMI, WC, FBS, CAP, TC, TG, HDL, LDL, LSM |
| DiBattista 202034 | United Kingdom | Nonrandomized double arm | Unknown | 16 | 4 | TRF/ Noncompleters b | 16 h daily | - | - | 38.16/43.16 | BMI, BW |
| Ebrahimi 2020a 27 | Iran | RCT | Chart review | 83 | 4 | Religious/NF | 16 h daily | 37.59 (7.06)/35.8 (7.33) | 59.5/78 | 30.09 (4.49)/28.2 (2.5) | BMI, BW, WC,WHR, FBS, HOMA-IR, ALT, AST, TC, TG, HDL, LDL |
| Ezpeleta 2023a 35 | United States | RCT | MRI | 80 | 12 | MADF/NF or MADF + exercise | 21 h every other day | 44(16)/44(12) | 20/20 | 36(8)/36(6) | BW, FBS, HOMA-IR, ALT, AST, TC, TG, HDL, LDL |
| Holmer 2021a 30 | Sweden | RCT | Imaging or transient elastography | 44 | 12 | 5:2 Diet/LCHF or SoC | 24 h on 2 nonconsecutive days per week | 57 (10)/56 (9) | 52.0/29.0 | 32.3 (2.7)/32.9 (5.2) | BMI, BW, WHR, HOMA-IR, ALT, AST, TC, TG, HDL, LDL, LSM |
| Johari 2019a 31 | Malaysia | RCT | Elevated transaminases | 39 | 8 | MADF/NF | 24 h every other dayc | 45.33 (10.77)/52.6 (12.03) | 72.73/90 | 31.6 (5.19)/28.21 (3.32) | BMI, BW, FBS, ALT, AST, TC, TG, HDL, LDL, LSM |
| Kord Varkaneh 2022a 40 | Iran | RCT | Transient elastography | 44 | 12 | 5:2 Diet/NF | 24 h on 2 nonconsecutive days per week | 46.42 (13.35)/44.17 (4.9) | 57.1/65.2 | 30.42 (2.27)/30.6 (3.09) | BMI, BW, WC, FBS, HOMA-IR, CAP, LSM |
| Mack 2014a 36 | Australia | RCT | Ultrasound | 32 | 12 | TRF/SoC | 16 h daily | − | − | 29/30 | BMI, BW, WC, HOMA-IR, ALT, AST, TC, TG, HDL, LDL, CAP, LSM |
| Mari 2021a 26 | Israel | Retrospective case control | Ultrasound | 155 | 4 | Religious/NF | 16 h daily | 51.8 (20.9)/52.6 (19.3) | 52.7/51.8 | 36.7 (7.1)/34.3 (6.3) | BMI, HOMA-IR, ALT, AST |
| Rahimi 2017a 37 | Iran | Nonrandomized double arm | Chart review | 60 | 4 | Religious/NF | 16 h daily | 46.03 (11.72)/49.58 (10.96) | 73.5/53.8 | 29.46 (4.52)/30.08 (4.12) | BMI, BW, ALT |
| Wei 202339 | China | RCT | MRI | 88 | 52 | TRF/Caloric Restriction | 16 h daily | 32.3(10.5)/31.7(8.3) | 53/58 | 32.2(3.4)/32.2(3.2) | BMI, BW, WC, FBS, HOMA-IR, ALT, AST, TC, TG, HDL, LDL, LSM |
| Xiao 202238 | China | RCT | Unknown | 31 | 24 | 5:2 Diet/liraglutide | 24 h on 2 nonconsecutive days per week | 42.87 (6.84)/− | 51.0/− | 30.44 (1.42)/− | BMI, BW, WC, FBS, HOMA-IR, ALT, AST, HDL, LDL |
Indicates which studies were included in the meta-analysis (on the basis of being double-arm studies with a nonfasting, nonpharmaceutical intervention comparison group).
Noncompleters were participants who did not complete the study.
Fasting was defined as consumption of 70% of calories.
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; BW, body weight; CAP, controlled attenuation parameter steatosis measurement; FBS, fasting blood sugar; HOMA-IR, homeostatic model assessment for insulin resistance; LSM, liver stiffness measurement; MADF, modern alternate-day fasting; NF, nonfasting; RCT, randomized controlled trial; SoC, standard of care; TC, total cholesterol; TG, total triglycerides; TRF, time-restricted feeding; WC, waist circumference; WHR, waist to hip ratio.
Quality assessment and risk of bias
Risk-of-bias assessments of all 10 studies included in the meta-analysis are shown in Figure 2. Among the RCTs, the overall risk of bias was low, with nearly all studies reporting adequate randomization and allocation. However, due to the nature of dietary interventions, some concern for bias was conferred due to being unable to blind participants and at times researchers to the intervention. Of the double-arm observational studies included in the meta-analysis, overall risk of bias was moderate. The most serious concern for bias came from potential confounding bias; neither of the studies adjusted nor controlled for confounding variables in the study design or during analysis.26,37
FIGURE 2.
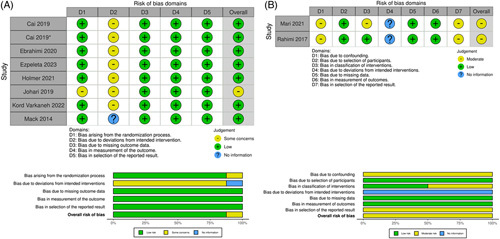
Risk of bias assessment for studies included in meta-analysis. (A) Risk of bias assessment for nonrandomized double-arm studies using ROBINS-I; (B) Risk of bias assessment for randomized controlled studies using RoB2.
Overview of meta-analysis
Only double-arm studies with a nonfasting, nonpharmaceutical intervention comparison group were included in the meta-analysis portion of the review due to high risk of bias from single-arm studies.26,27,29–31,35–37,40 Ten studies were included in the meta-analysis for a pooled total of 840 participants (44.64% male). The mean age of the IF group was 41.72 years (SD 8.13) and for the comparison group was 42.27 years (SD 8.83).
Effect of intermittent fasting on anthropometric end points
A summary of the impact of IF on anthropometric outcomes in adults with NAFLD is shown in Figure 3. A pooled analysis of 9 studies that assessed pre-intervention and post-intervention changes in body weight showed a significant decrease of −2.66 kg (95% CI: −3.81 to −1.52, p < 0.001) with considerable heterogeneity (I2 = 80.8%, p < 0.001).27,29,31,35–37 Similarly, IF also reduced BMI by −0.77 kg/m2 (95% CI: −1.15 to −0.38, p < 0.001) in a pooled analysis of 10 studies showing moderate but significant heterogeneity (I2 = 49%, p = 0.03).26,27,29–31,35–37,40 Pooled analyses also demonstrated a statistically significant decrease in waist to hip ratio (WHR: −0.0036, 95% CI: −0.01 to −0.0011, p < 0.01),27,30 and a nonsignificant reduction in WC (WC: −0.61, 95% CI: −1.53 to 0.31, p = 0.19).29,35,40 There was no significant heterogeneity for either WC or WHR (WC: I2 = 0%, p = 0.64; WHR: I2 = 0%, p = 0.52).
FIGURE 3.
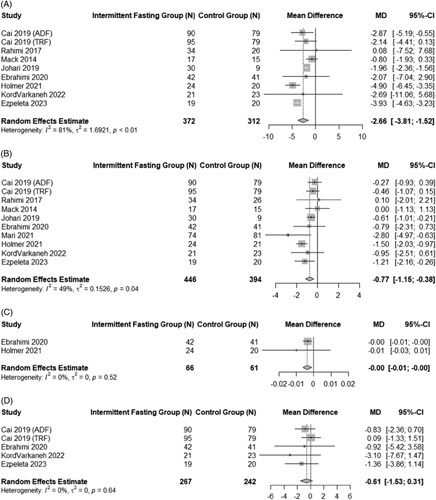
Pooled analysis of the impact of intermittent fasting on anthropometric end points. Forest plots correspond to pooled analysis of body weight (A), BMI (B), WHR (C), and WC (D). Abbreviations: BMI, body mass index; WC, waist circumference; WHR, waist to hip ratio.
Effect of intermittent fasting on biochemical end points
Figure 4 summarizes the impact of IF on lipid profiles of adults with NAFLD. Data showed that IF led to a nonsignificant change in total serum cholesterol of −6.38 mg/dL (95% CI: −14.71 to 1.94, p = 0.13) with moderate heterogeneity (I2 = 47%, p = 0.08).27,29,30,35,40 Total serum triglycerides were significantly reduced by a mean difference of −20.91 mg/dL (95% CI: −35.10 to −6.72, p < 0.01) with low heterogeneity (I2 = 10%, p = 0.36).27,29–31,35,40 IF interventions also had a slight but statistically significant impact on serum LDL (mean difference: −0.35 mg/dL, 95% CI: −0.61 to −0.08, p < 0.01; I2 = 0%, p = 0.98).27,29–31,35,40 HDL was not impacted by IF (−0.00 mg/dL 95% CI: −0.08 to 0.08, p = 1.00) with low heterogeneity (I2 = 40.00%, p = 0.13).27,29–31,35,40
FIGURE 4.
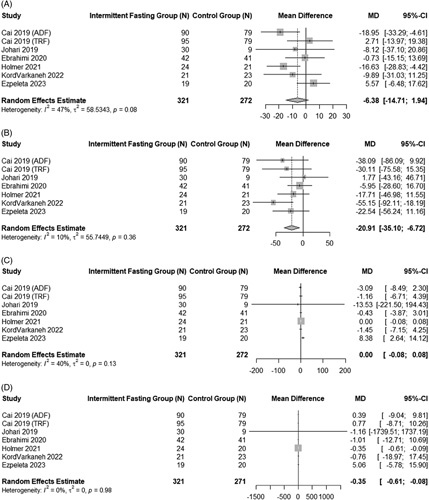
Pooled analysis of the impact of intermittent fasting on biochemical end points. Forest plots correspond to pooled analysis of total cholesterol (A), triglycerides (B), HDL (C), and LDL (D).
In contrast to the control arm, IF arms had a nonsignificant reduction in fasting blood glucose levels (−0.71 mg/dL, 95% CI: −3.06 to 1.64, p = 0.55) in a pooled analysis of 6 studies, with no heterogeneity (I2 = 0%, p = 0.48).27,29,31,35,40 HOMA-IR showed a statistically significant decrease by −0.69 (95% CI: −1.02 to −0.36, p < 0.001).26,27,30,35,40 There was no significant heterogeneity (I2 = 0%, p = 0.92). Forest plots for these 2 end points are found in Figure 5.
FIGURE 5.
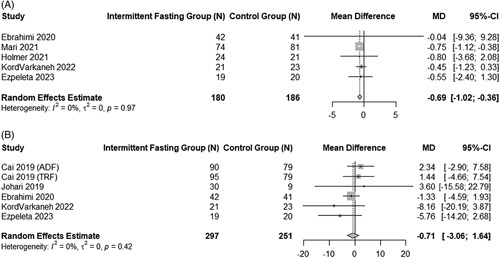
Pooled analysis of the impact of intermittent fasting on HOMA-IR and fasting glucose. Forest plots correspond to pooled analysis of HOMA-IR (A) and fasting blood glucose level (B). Abbreviations: HOMA-IR, homeostatic model assessment for insulin resistance.
Effect of intermittent fasting on hepatic end points
Forest plots for the effect of IF on hepatic end points can be found in Figure 6. ALT significantly improved in adults with NAFLD following fasting intervention (mean difference: −6.02 IU/L, 95% CI: −11.52 to −0.52, p= 0.03, I2 = 75%, p < 0.01).26,27,30,35,37,40 Serum AST levels also showed a significant improvement but with high between-study heterogeneity (mean difference: −5.27 IU/L, 95% CI: −7.88 to −2.67, p < 0.001; I2 = 60%, p = 0.03).26,30,31,35,40 In addition to biochemical markers of liver health, 2 studies, both of 12 weeks duration, reported on liver steatosis (CAP, dB/m) and 6 studies on liver stiffness (kPa). The pooled estimate comparing fasting intervention groups to control groups for liver steatosis showed a −24.71 dB/m (95% CI: −47.40 to −2.03, p = 0.03) reduction in CAP score.30,40 Between-study heterogeneity was not significant (I2 = 30.1%, p = 0.23). Liver stiffness was also positively impacted by IF interventions (mean difference: −0.59 kPa, 95% CI: −0.99 to −0.20, p < 0.01).29–31,36,40 Heterogeneity was not significant (I2 = 0%, p = 0.52).
FIGURE 6.
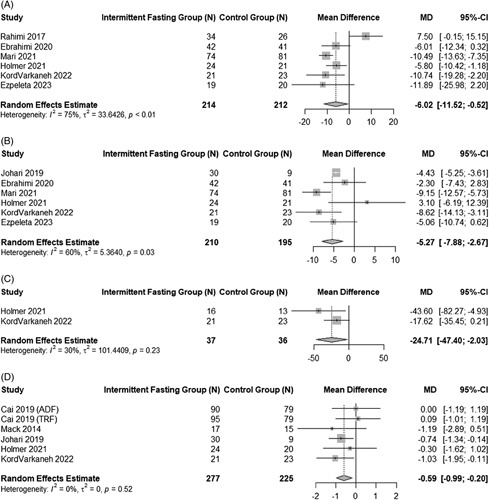
Pooled analysis of the impact of intermittent fasting on liver-specific end points. Forest plots for pooled analysis of ALT (A), AST (B), liver steatosis (C), and liver stiffness (D). Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase.
Sensitivity analyses
Sensitivity analyses were conducted for each end point whenever 2 or more studies were available (Supplemental Table 2, http://links.lww.com/HC9/A423). We conducted sensitivity analyses by (1) excluding studies where NAFLD was not diagnosed by imaging, (2) excluding studies of < 12 weeks duration, (3) excluding studies ≥ 12 weeks, (4) excluding non-RCT studies, and (5) excluding based on intervention type.
All sensitivity analyses for BMI demonstrated stability in the meta-analysis outcome, except for sensitivity analysis wherein only religious fasting interventions were included. Religious fasting intervention-only analysis showed a nonsignificant reduction in BMI, as opposed to a significant reduction as was found in primary analysis. These findings were similarly demonstrated for body weight. Excluding studies < 12 weeks duration or only 5:2 diet interventions resulted in the significant reduction in HOMA-IR becoming nonsignificant though similar in magnitude.
For total cholesterol, including 5:2 diet-only studies resulted in an increased magnitude in the mean difference as well as a significant reduction (5:2 diet-only: −14.94 mg/dL, p < 0.01; all studies: −6.38 mg/dL, p = 0.13). Excluding studies ≥ 12 weeks greatly reduced the magnitude of the mean difference for total triglycerides (excluding ≥12 wk: −4.39 mg/dL, p = 0.67; all studies: −20.91 mg/dL, p < 0.01). Comparably, excluding studies ≥ 12 weeks led to an increase rather than decrease in LDL following fasting intervention (excluding ≥12 wk: 1.81, p = 0.53; all studies: −0.35, p < 0.01).
On sensitivity analyses for both ALT and AST, excluding studies ≥12 weeks or choosing religious fasting-only studies resulted in an attenuation of the magnitude of the mean difference as well as a shift toward nonsignificance. In addition, for ALT, removal of nonhigh-quality RCTs demonstrated a smaller and nonsignificant mean difference (excluding non-RCTs: −4.80 IU/L, p = 0.15; all studies: −6.02 IU/L, p= 0.03). Analyses of the stability of liver stiffness outcomes demonstrated that excluding studies < 12 weeks duration, excluding studies that did not diagnose NAFLD by imaging, excluding non-high-quality RCTs, or including MADF-only intervention studies all resulted in the mean difference no longer being significant and a reduction in its magnitude.
Across all sensitivity analyses, waist circumference, liver steatosis as measured by CAP, HDL, and fasting blood glucose had outcomes consistent with findings from the primary analysis. Sensitivity analyses could not be conducted for WHR due to the low number of studies with WHR data.
Meta-regression
We conducted meta-regression analyses for all end points with statistically significant heterogeneity (prior to sensitivity analyses) (Supplemental Table 3, http://links.lww.com/HC9/A423). Age, baseline BMI, and proportion of male participants were the moderators inputted into the regression models. Age was significantly associated with the effect size for BMI, accounting for 96.80% of the heterogeneity seen on pooled analysis (Age β = −0.06, p < 0.001). In a multivariate meta-regression model with age and proportion of male participants as moderators, this model accounted for 100% of the heterogeneity on pooled analysis for body weight (age β = −0.14, p < 0.05; sex β = +0.04, p < 0.0001). On univariate analysis, proportion of male participants accounted for 68.84% of the heterogeneity in variations in the effect size for ALT (% Male β = + 0.38, p = 0.02). However, on multivariate analysis, this association was not preserved, and none of the moderators were found to have statistically significant associations with the effect size for ALT. For AST, baseline BMI was significantly associated with its effect size on univariate analysis (baseline BMI β = −0.68, p = 0.02). Similar to ALT, on multivariate meta-regression, the association was not preserved, and overall the model did not account for the heterogeneity found on meta-analysis.
Subgroup analyses
Given the variety of IF interventions within the meta-analysis, a subgroup analysis evaluating the influence of each type of IF intervention (5:2 diet, religious, MADF, and TRF) on liver-specific end points was conducted (Supplemental Table 4, http://links.lww.com/HC9/A423). There were no significant differences between the various IF intervention types for ALT (p = 0.64), AST (p = 0.89), or liver stiffness (p = 0.55). Liver steatosis was excluded from this analysis as this outcome was only present in 2 studies, both of which used the 5:2 diet for the intervention arm. To assess the potential impact of intervention duration, we compared hepatic end points for studies greater than or less than 8 weeks duration (Supplemental Table 5, http://links.lww.com/HC9/A423). Analysis did not reveal any statistically significant differences in the 2 subgroups for any of the hepatic end points (ALT p = 0.49; AST p = 0.79; liver stiffness p = 0.52). Subgroup analysis for liver steatosis was limited by the only 2 studies reporting on steatosis being of similar duration. Finally, study location (East Asia vs. Middle East vs. Other) did not impact ALT (p = 0.79), AST (p = 0.43), liver stiffness (p = 0.51), or liver steatosis (p = 0.23) (Supplemental Table 6, http://links.lww.com/HC9/A423). East Asia included China and Malaysia; Middle East included Iran and Israel; Other included studies in any other regions of the world.
Publication bias
For end points with at least 5 studies, funnel plots were generated (Supplemental Figures 1-3, http://links.lww.com/HC9/A424; http://links.lww.com/HC9/A423; http://links.lww.com/HC9/A425; http://links.lww.com/HC9/A423; http://links.lww.com/HC9/A426; http://links.lww.com/HC9/A423) and Egger’s regression test was calculated. There was no evidence of publication bias for any of the anthropometric, biochemical, or hepatic end points with at least 5 studies.
DISCUSSION
In this systematic review with meta-analysis, we evaluated the impact of IF on various anthropometric, biochemical, and hepatic end points in adults with NAFLD. We demonstrated that existing literature provides moderate to high-quality evidence to suggest that IF of at least 4 weeks may have a beneficial effect on anthropometric, biochemical, and hepatic end points in NAFLD. The majority of studies were conducted in Eastern patient populations, comprising 67% of the pooled patient cohort. Pooled analyses from RCTs and double-arm studies showed that IF promotes weight loss and abdominal fat loss, as supported by the significant reductions in body weight, BMI, and WHR. Cardiometabolic measures of health, such as total triglycerides and LDL levels, were also positively impacted following fasting intervention. Importantly, in the context of NAFLD, IF reduced all hepatic end points. Various sensitivity analyses were conducted that demonstrated robustness in the outcomes for the anthropometric end points, though findings from sensitivity analyses for biochemical and hepatic end points should be interpreted with caution due to low statistical power from the few studies included. Although studies on interventions of longer duration are needed, current literature supports the potential use of IF as a treatment modality in NAFLD, for both its hepatic and extrahepatic benefits.
Pooled data, with no evidence of publication bias, demonstrated that IF in adults with NAFLD effectively reduces body weight, BMI, and WHR. IF’s ability to promote weight loss in adults with NAFLD supports its use as a potential alternative therapeutic option to calorically restricted diets. It is also important to consider that NAFLD exists in a spectrum of conditions all linked to metabolic syndrome, including obesity, type 2 diabetes, and coronary artery disease, and prior studies have shown that IF promotes weight loss as well as cardiometabolic health.14,17,41 Thus, for patients with NAFLD and concomitant metabolic syndrome, IF may be a more advantageous treatment option than caloric restriction. In addition, our finding that IF may lead to a reduction in WHR is significant in the context of both NAFLD and overall metabolic health since abdominal (visceral) obesity, irrespective of body weight or BMI, has been recognized as one of the predominant risk factors for metabolic syndrome.42,43
Our meta-analysis demonstrated that even short-term IF in adults with NAFLD can improve liver steatosis and stiffness, as well as ALT and AST, although this may have been influenced by IF type. For instance, the objectives for fasting are strikingly contrasted between an individual who is fasting for religious reasons such as Ramadan (and who is eating calorically rich meals during the feeding periods) and an individual who is fasting with the goal of weight loss. Dietary choices and adherence may vary greatly, which potentially masked the true magnitude of IF’s impact on liver end points. This is further supported by our finding that although studies of religious fasting interventions were shown to not significantly affect hepatic end points, the 5:2 diet and MADF did (Supplemental Table 4, http://links.lww.com/HC9/A423). Future studies of IF and NAFLD should focus on 5:2 diet, MADF, and TRF interventions of longer duration (>12 wk) with the goal of evaluating whether the magnitude of improvement in hepatic end points increases with length of fasting intervention and whether this reduction becomes more clinically significant over time.
Interestingly, the hepatic benefits of IF may not necessarily be due to weight loss, but rather through the metabolic switch that occurs between fasting and fed states.9–11 This metabolic switch promotes the release and use of fatty acids and ketone bodies as an energy source rather than glucose. Ketone bodies are known to be potent signaling molecules, implicated in the regulation of numerous major cellular pathways that confer resistance to stress and disease as well as improve organ function.19,44 While the exact mechanism behind how IF promotes liver health remains unclear, in animals, data have shown that the beneficial effects of IF on liver metabolism and inflammation are preserved regardless of changes in dietary intake or weight loss.45 Taken together, while IF and caloric restriction type diets may be considered equivalent for weight loss,46 IF should be viewed distinctly due to its potential to confer specific improvements in liver health and steatosis independently of weight regulation.
To our knowledge, our study represents one of the first systematic reviews and meta-analyses evaluating the effect of IF in adults with NAFLD. One of the main strengths is the inclusion criteria, which resulted in a well-characterized population focusing on NAFLD, as opposed to NAFLD and NASH for instance. Our review was also limited to IF without any kind of exercise or physical activity intervention, allowing us to characterize the specific impact of this dietary intervention. Moreover, 1 prior systematic review and meta-analysis was conducted by Yin et al. (2021), demonstrating similar statistically significant decreases in body weight, BMI, and liver biochemical tests, though no significant differences in liver stiffness nor triglyceride and cholesterol levels when comparing IF and nonfasting groups, and the study did not report on liver steatosis.47 With the publication of newer RCTs since this prior review, our study provides more comprehensive and robust conclusions on liver-specific and anthropometric end points.
Despite its strengths, our review has some limitations. Firstly, the small number of studies in the review and predominantly Asian study population require that our findings be interpreted with some caution. Secondly, the absence of a single, consistent diagnostic modality for NAFLD across studies is another limitation. The use of radiographic modalities and serum markers to diagnose NAFLD in the studies, in place of liver biopsy, may have impacted which participants under study “truly” had NAFLD. Further heterogeneity was likely introduced by the differing types and length of IF interventions. In an attempt to mitigate and further explore this heterogeneity, we conducted subgroup analyses that did not show evidence of study duration, fasting intervention type, or study location (Middle East vs. East Asia vs. Other) influencing our findings. However, the limited number of studies makes it difficult to draw robust conclusions from the subgroup analysis.
Given the growing worldwide burden of NAFLD, the severity of outcomes with progression to fibrosis, and the difficulty patients have in adhering to long-term calorically restricted diets, new therapeutic options are needed. Our systematic review with meta-analysis supports the use of IF as a promising alternative for the treatment of NAFLD due to its promotion of weight loss and improvement of liver steatosis. Future studies of IF and NAFLD should focus on longer follow-up periods to better elucidate the associated impacts of this dietary intervention and validate our findings. The sustainability of this intervention and its impact on fibrosis would be of interest. In addition, RCTs comparing IF to the recommended hypocaloric diet in adults with NAFLD are needed to determine the optimal diet for NAFLD.
Supplementary Material
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the Digestive Disease Research Foundation.
Preliminary data previously presented at EASL International Liver Congress in June 2022 and at the NAFLD Summit in September 2022.
FUNDING INFORMATION
Marcia Lange received research funding from the Digestive Disease Research Foundation
CONFLICTS OF INTEREST
Tatyana Kushner advises and received grants from Gilead. She advises Abbvie and Bausch. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; BW, body weight; CAP, controlled attenuation parameter; FBS, fasting blood sugar; HOMA-IR, homeostatic model assessment for insulin resistance; IF, intermittent fasting; LSM, liver stiffness measurement; MADF, modern alternate-day fasting; NF, nonfasting; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCT, randomized clinical trial; SoC, standard of care; TC, total cholesterol; TG, total triglycerides; TRF, time-restricted feeding; WC, waist circumference; WHR, waist to hip ratio.
Marcia Lange and Devika Nadkarni are Co-first authors.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Marcia Lange, Email: marcia.lange@icahn.mssm.edu.
Devika Nadkarni, Email: devika.nadkarni@icahn.mssm.edu.
Lily Martin, Email: lily.martin@mssm.edu.
Carolyn Newberry, Email: can9054@med.cornell.edu.
Sonal Kumar, Email: sok9028@med.cornell.edu.
Tatyana Kushner, Email: Tatyana.kushner@mssm.edu.
REFERENCES
- 1.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Samala N, Chalasani N. CAP and LSM as determined by VCTE are independent predictors of all-cause mortality in the US adult population. Hepatology. 2023;77:1241–1252. [DOI] [PubMed] [Google Scholar]
- 3.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378.e5; quiz e14-e15. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson N, Keating S, George J. Exercise and the liver: implications for therapy in fatty liver disorders. Semin Liver Dis. 2012;32:65–79. [DOI] [PubMed] [Google Scholar]
- 6.Scheen AJ. The future of obesity: new drugs versus lifestyle interventions. Expert Opin Investig Drugs. 2008;17:263–267. [DOI] [PubMed] [Google Scholar]
- 7.Alhassan S, Kim S, Bersamin A, King AC, Gardner CD. Dietary adherence and weight loss success among overweight women: results from the A-TO-Z weight loss study. Int J Obes. 2008;32:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. [DOI] [PubMed] [Google Scholar]
- 9.Duregon E, Pomatto-Watson LCDD, Bernier M, Price NL, de Cabo R. Intermittent fasting: from calories to time restriction. GeroScience. 2021;43:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longo VD, Panda S. Fasting, Circadian Rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson MP, de Cabo R. Effects of intermittent fasting on health, aging, and disease. Reply N Engl J Med. 2020;382:1773–1774. [DOI] [PubMed] [Google Scholar]
- 12.Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Cardiometabolic Benefits of Intermittent Fasting. Annu Rev Nutr. 2021;41:333–361. [DOI] [PubMed] [Google Scholar]
- 13.Sogawa H, Kubo C. Influence of short-term repeated fasting on the longevity of female (NZB x NZW)F1 mice. Mech Ageing Dev. 2000;115:61–71. [DOI] [PubMed] [Google Scholar]
- 14.Welton S, Minty R, O'driscoll T, Willms H, Poirier D, Madden S, et al. Intermittent fasting and weight loss: Systematic review. Can Fam Physician. 2020;66:117–125. https://www.ncbi.nlm.nih.gov/pubmed/32060194 [PMC free article] [PubMed] [Google Scholar]
- 15.Patikorn C, Roubal K, Veettil SK, Chandran V, Pham T, Lee YY, et al. Intermittent fasting and obesity-related health outcomes: An umbrella review of meta-analyses of randomized clinical trials. JAMA Netw Open. 2021;4:e2139558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu S, Surampudi P, Rosharavan B, Chondronikola M. Intermittent fasting as a nutrition approach against obesity and metabolic disease. Curr Opin Clin Nutr Metab Care. 2020;23:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgundvaag E, Mak J, Kramer CK. Metabolic Impact of Intermittent Fasting in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Interventional Studies. J Clin Endocrinol Metab. 2021;106:902–911. [DOI] [PubMed] [Google Scholar]
- 18.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med. 2017;177:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Cabo R, Mattson MP. Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med. 2019;381:2541–2551. [DOI] [PubMed] [Google Scholar]
- 20.PRISMA. Accessed January 1, 2022. https://www.prisma-statement.org/
- 21.Conventional Units - International Units. Global RPH: The Clinician’s Ultimate Reference. Published October 10, 2017. Accessed September 19, 2022. https://globalrph.com/medical/conventional-units-international-units/
- 22.Cholesterol Units Converter. Omni Calculator. Published July 14, 2022. Accessed September 19, 2022. mnicalculator.com/health/choles.
- 23.Blood Sugar Conversion Calculator. MD App. Published May 27, 2017. Accessed September 19, 2022. https://www.mdapp.co/blood-sugar-conversion-calculator-71/
- 24.Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Systematic Reviews. 2022;18:e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. www.training.cochrane.org/handbook (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from. [Google Scholar]
- 26.Mari A, Khoury T, Baker M, Said Ahmad H, Abu Baker F, Mahamid M. The Impact of Ramadan Fasting on Fatty Liver Disease Severity: A Retrospective Case Control Study from Israel. Isr Med Assoc J. 2021;23:94–98. https://www.ncbi.nlm.nih.gov/pubmed/33595214 [PubMed] [Google Scholar]
- 27.Ebrahimi S, Gargari BP, Aliasghari F, Asjodi F, Izadi A. Ramadan fasting improves liver function and total cholesterol in patients with nonalcoholic fatty liver disease. Int J Vitam Nutr Res. 2020;90:95–102. [DOI] [PubMed] [Google Scholar]
- 28.Aliasghari F, Izadi A, Gargari BP, Ebrahimi S. The effects of ramadan fasting on body composition, blood pressure, glucose metabolism, and markers of inflammation in NAFLD patients: An Observational Trial. J Am Coll Nutr. 2017;36:640–645. [DOI] [PubMed] [Google Scholar]
- 29.Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019;19:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmer M, Lindqvist C, Petersson S, Moshtaghi-Svensson J, Tillander V, Brismar TB, et al. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - A randomised controlled trial. JHEP Rep. 2021;3:100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johari MI, Yusoff K, Haron J, Nadarajan C, Ibrahim KN, Wong MS, et al. A Randomised controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Sci Rep. 2019;9:11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arabi SM, Zarifi SH, Nematy M, Safarian M. The effect of ramadan fasting on non-alcoholic fatty liver disease (NAFLD) patients. J Fast Health. 2015;3:74–80. [Google Scholar]
- 33.Badran H, Elsabaawy M, Sakr A, Eltahawy M, Elsayed M, Elsabaawy D, et al. Impact of intermittent fasting on laboratory, radiological, and anthropometric parameters in NAFLD patients. Clin Exp Hepatol. 2022;8:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Battista E, Yeoman A, Everitt J, Williams S, Kenkre J. The treating liver study: Preliminary findings of a “time re-focused eating” feasibility study for obesity and non-alcoholic fatty liver disease. Obes Rev. 2020;21:S211. [Google Scholar]
- 35.Ezpeleta M, Gabel K, Cienfuegos S, Kalam F, Lin S, Pavlou V, et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: A randomized controlled trial. Cell Metab. 2023;35:56–70.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mack A, Hodge A, Tuck C, Tchongue J, Holt D, Sievert W, Moore GT. Non-alcoholic fatty liver disease intermittent fasting time intervention (NIFTI): fasting without calorie restriction improves hepatic transient elastography, visceral adiposity and insulin resistance compared to standard care. J Gastroenterol Hepatol. 2014;29(S2):68–101. [Google Scholar]
- 37.Rahimi H, Emad Habibi M, Gharavinia A, Hasan Emami M, Baghaei A, Tavakol N. Effect of Ramadan Fastin on Alanine Aminotransferase (ALT) in Non-Alcoholic Fatty Liver Disease (NAFLD). J Fast Health. 2017;5:107–112. [Google Scholar]
- 38.Xiao Y, Liu Y, Zhao L, Zhou Y. Effect of 5:2 Fasting Diet on Liver Fat Content in Patients with Type 2 Diabetic with Nonalcoholic Fatty Liver Disease. Metab Syndr Relat Disord. 2022;20:459–465. [DOI] [PubMed] [Google Scholar]
- 39.Wei X, Lin B, Huang Y, Yang S, Huang C, Shi L, et al. Effects of time-restricted eating on nonalcoholic fatty liver disease: The TREATY-FLD Randomized Clinical Trial. JAMA Netw Open. 2023;6:e233513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kord Varkaneh H, Salehi Sahlabadi A, Găman MA, Rajabnia M, Sedanur Macit-Çelebi M, Santos HO, et al. Effects of the 5:2 intermittent fasting diet on non-alcoholic fatty liver disease: a randomized controlled trial. Front Nutr. 2022;9:948655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales-Suarez-Varela M, Collado Sánchez E, Peraita-Costa I, Llopis-Morales A, Soriano JM. Intermittent fasting and the possible benefits in obesity, diabetes, and multiple sclerosis: a Systematic Review of Randomized Clinical Trials. Nutrients. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Björntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. [DOI] [PubMed] [Google Scholar]
- 43.Ruderman NB, Schneider SH, Berchtold P. The “metabolically-obese,” normal-weight individual. Am J Clin Nutr. 1981;34:1617–1621. [DOI] [PubMed] [Google Scholar]
- 44.Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. [DOI] [PubMed] [Google Scholar]
- 45.Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients. 2019;11:2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients. 2019;11:2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin C, Li Z, Xiang Y, Peng H, Yang P, Yuan S, et al. Effect of Intermittent Fasting on Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Front Nutr. 2021;8:709683. [DOI] [PMC free article] [PubMed] [Google Scholar]


