Abstract
Objectives:
To detect the expression and significance of GSDMD-N (gasdermin D N-terminal) in breast cancer, along with pyroptosis effector protein NLRP3 (nucleotide-binding oligomerization domain-like receptor protein 3), and determine their relationship with the clinicopathological characteristics of breast cancer.
Methods:
From January 2014 to December 2014, NLRP3 and GSDMD-N expression in 90 breast carcinoma organism samples and 30 paracancer tissues in the Department of Pathology. The First Affiliated Hospital of Bengbu Medical College was assessed using immunohistochemistry. The method of Kaplan–Meier was employed for the sake of comparing the survival between NLRP3 and GSDMD-N protein low and high expression groups. Among the breast cancerous organisms, the relationship between the expression of NLRP3 and GSDMD-N, corresponding adjacent tissues, and various clinicopathological features was analyzed using the χ2 and Spearman rank correlation tests.
Results:
In the 90 breast cancer tissue samples, the pyrolysis pathway effector proteins GSDMD-N and NLRP3 were actively associated; and, expression intensities of NLRP3 and GSDMD-N were shown to be correlated with breast cancer. In addition, the clinicopathological features of patients were shown to be correlated with breast cancer. Notably, the higher the expressions of NLRP3 and GSDMD-N, the lower the risk of death of patients with breast cancer and the better the prognosis.
Conclusion:
The expression of the pyrolysis effector proteins NLRP3 and GSDMD-N in breast cancer tissues may take the lead in tumor prognosis in patients with breast cancer.
Keywords: breast cancer, effector proteins, GSDMD-N, NLRP3, pyroptosis
1. Introduction
Pyrolysis is a recently discovered, novel category of programmed cell death that is different from apoptosis.[1] In 2000, Brennan and Cookson first discovered the pyrolysis of macrophages infected with Salmonella typhimurium.[2] Pyroptosis is a programmed cell death pathway characterized by the formation of pores in the cell membrane, followed by cell swelling, release of cellular contents, secretion of inflammatory factors, and ultimately cell lysis, in response to various external stimuli. Since the discovery of the key execution protein GSDMD,[3] scientists have gained a good understanding of the mechanism of pyroptosis. The pathways of pyroptosis mainly include the classical pathway and the nonclassical pathway. The classical pathway involves the activation of the inflammasome by caspase-1. Activated caspase-1 cleaves gasdermin D protein (GSDMD) to generate gasdermin D N-terminal (GSDMD-N), which forms oligomers on the cell membrane. GSDMD-N oligomers serve as pore-forming channels that facilitate the release of activated IL-18, IL-1β, and other cytokines from the cytoplasm to the extracellular space, triggering an inflammatory response and leading to cell disintegration. The inflammasome, which consists of components such as pro-caspase-1, NLRs, AIM2, pyrin, and ASC, plays a crucial role in inducing pyroptosis.
Pyrocytosis has been a popular research topic recently.[4–10] It generally occurs at the cellular level; however, it is unclear whether pyrolysis can take the lead in the treatment along with the prevision of sick people suffering from breast carcinoma. Based on the classical pathway of cell pyroptosis, we have chosen nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), a member of the NLRs family of molecules, and the N-terminal domain of the cell pyroptosis execution protein GSDMD as our research subjects. This research aimed to explore the correlation between expression levels of pyroptosis pathway proteins and the clinicopathological features of breast carcinoma, as well as their relationship with the prognosis of patients suffering from breast carcinoma.
2. Materials and methods
2.1. Study design and samples
Ninety cases of archived breast carcinoma specimens, as well as 30 cases with neighboring tissues confirmed by the Department of Pathology, The First Affiliated Hospital of Bengbu Medical College throughout 2014, were collected. The pathological grade and clinical stage of all patients with breast cancer were based on the diagnostic criteria of the World Health Organization’s “Breast and Female Reproductive Organ Tumor Pathology and Genetics” from 2003. The collected cases were followed up until death or until January 2020. This study was approved by the institutional ethical review board of First Affiliated Hospital of Bengbu Medical College.
2.2. Reagents
Antibodies used in this study included rabbit antihuman NLRP3 polyclonal antibody (Proteintech), and rabbit antihuman GSDMD-N polyclonal antibody (Affinity). Additional reagents included the DAB chromogenic kit and ElivisionTM Plus Kit (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou).
2.3. Immunohistochemical staining method and results
All breast cancer tissue samples and control paracancer tissue samples were fixed with a 4% neutral formalin solution, paraffin-embedded, and consecutively sectioned at 4 µm intervals. Thereafter, they were dewaxed, washed with a xylene solution, and dehydrated with a gradient of ethanol concentrations. Immunohistochemical staining was performed using the Elivision TM Plus Kit in the light of the producer`s guidelines. There existed a sample known positive for the positive control. For the negative control, samples were incubated in phosphate-buffered saline instead of antibody.
The proportion of the positive cells, coupled with staining intensity, was scored comprehensively. Stained cells were recorded as a percentage of all counted cells, and scored as blow: 0 points, ≤5% the positive cells; 1 point, 5% to 25% the positive cells; 2 points, 26% to 50% the positive cells; 3 points, 51% to 75% the positive cells; and 4 points, >70% the positive cells. According to the appearance of the cells after staining, there were: no yellow, light-yellow particles, brown particles, or dark brown staining with scores of 0, 1, 2, 3, and 4 points. According to the standard, each slice was randomly selected from 5 fields 400 times; every field was scored for the proportion of the stained cells and that of the intensity of staining with the following categorization: ≥6 points, high expression; <6 points, low expression. Immunohistochemical staining results were performed by 2 senior diagnosticians by means of 1 independent double-blind approach.
2.4. Statistical analysis
The approach of Kaplan–Meier was employed for the purpose of making a comparison with the survival between low and high expression groups of NLRP3 and GSDMD-N. The patients’ ages from whom breast cancer tissues were derived (≥50 years old vs <50 years old), pathological TNM grade (grade I, grade II, or grade III), clinical stage (stage I, stage II, or stage III), tumor dimensions (<2.0-cm vs ≥2.0-cm), metastasis of lymph node (metastasis vs non-metastasis), NLRP3 expression (high vs low expression), GSDMD-N expression (high vs low expression), and other parameters of the clinicopathology were included into the multi-factor model for analysis. Distinction between the 2 groups were dissected by means of log-rank testing. In breast cancer tumor tissues, the correlation between the expressions of NLRP3 and GSDMD-N, corresponding adjacent tissues, and various clinicopathological parameters was analyzed using the χ2 and Spearman rank correlation tests. The statistical dissections were implemented by means of the SPSS 25.0 (IBM Corp., Armonk, NY). A P-value < 0.05 illustrated a statistically significant difference.
3. Results
3.1. Sample characteristics
All the patients from whom the breast cancer samples were derived were female, had not received preoperative chemotherapy or radiotherapy, and were 33 to 75 years of age, with a middle age of 51 years old. The longest time of follow-up was 72 months with the shortest follow-up being 60 months. The clinicopathological data of all breast cancer cases are shown in Table 1.
Table 1.
NLRP3 and GSDMD-N protein expression in breast cancer and their relationship with clinically relevant parameters.
| NLRP3 | GSDMD-N | |||||
|---|---|---|---|---|---|---|
| Low expression | High expression | P-value | Low expression | High expression | P-value | |
| Age (years) | ||||||
| <50 | 22 (48.89%) | 23 (51.11%) | .673 | 22 (48.89%) | 23 (51.11%) | .398 |
| ≥50 | 20 (44.44%) | 25 (55.56%) | 26 (57.78%) | 19 (42.22%) | ||
| Pathological grade | ||||||
| I | 2 (16.67%) | 10 (83.33%) | .001 | 4 (33.33%) | 8 (66.67%) | .002 |
| II | 23 (40.35%) | 34 (59.65%) | 26 (45.61%) | 31 (54.39%) | ||
| III | 17 (80.95%) | 4 (19.05%) | 18 (85.71%) | 3 (14.29%) | ||
| Tumor size | ||||||
| <2 cm | 5 (26.32%) | 14 (73.68%) | .045 | 6 (31.58%) | 13 (68.42%) | .032 |
| ≥2 cm | 37 (52.11%) | 34 (47.89%) | 42 (59.15%) | 29 (40.85%) | ||
| Lymph node metastasis | ||||||
| No | 13 (31.71%) | 28 (68.29%) | .009 | 16 (39.02%) | 25 (60.98%) | .013 |
| Yes | 29 (59.18%) | 20 (40.82%) | 32 (65.31%) | 17 (34.69%) | ||
| Pathological TNM grade | ||||||
| I | 11 (31.43%) | 24 (68.57%) | .011 | 11 (31.43%) | 24 (68.57%) | .001 |
| II | 29 (54.72%) | 24 (45.28%) | 35 (66.04%) | 18 (33.96%) | ||
| III | 2 (100.00%) | 0 (0.00%) | 2 (100.00%) | 0 (0.00%) | ||
GSDMD-N = gasdermin D N-terminal, NLRP3 = nucleotide-binding oligomerization domain-like receptor protein 3.
3.2. Expression of NLRP3 and its relationship with the clinicopathological features of breast cancer
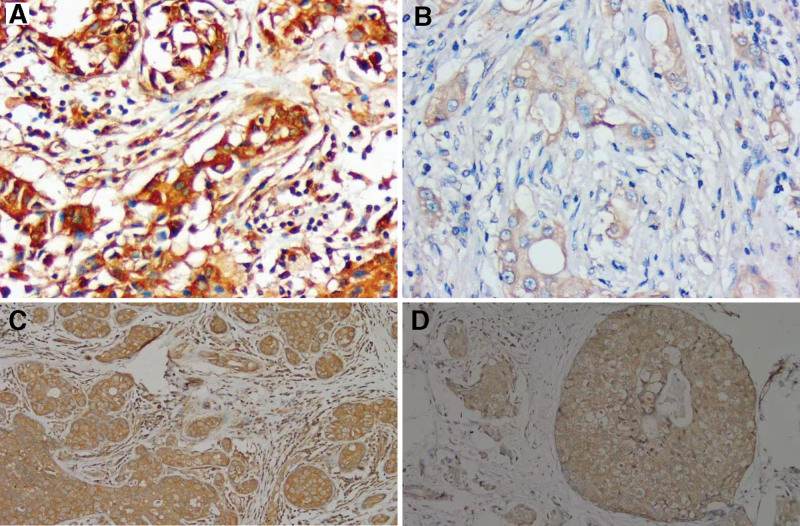
The high expression ratio of NLRP3 in 90 breast carcinoma samples was 53.33% (48/90, Fig. 1A), and the low expression ratio was 46.67% (42/90, Fig. 1B). In contrast, the highest and lowest expression rates of NLRP3 in adjacent tissues were 83.33% (25/30) and 16.67% (5/30), respectively. As for the high and low expression groups, the distinction were statistically obvious (P < .05). In breast cancer tissues, when the expression intensity of NLRP3 was higher, the tumor size was smaller. In addition, high NLRP3 expression intensity was correlated with lower clinical stage, lower pathological grade of the tumor tissue, and lower incidence of lymph node transfer (all P < .05). No statistically significant correlation was noticed between NLRP3 protein expression intensity and age sick people suffering from the breast carcinoma (P > .05, Table 1).
Figure 1.
Expression of nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) and gasdermin D N-terminal (GSDMD-N) protein in breast cancers tissues (Elivision TM Plus Kit; original magnification, ×200). (A) NLRP3 is highly expressed in breast cancer tissues. (B) There is a low expression of NLRP3 in breast cancer. (C) GSDMD-N is highly expressed in breast cancer tissues. (D) There is a low expression of GSDMD-N in breast cancer.
3.3. Expression of GSDMD-N and its relationship with the clinicopathological features of breast cancer
The high expression GSDMD-N ratio in breast carcinoma group was 46.67% (42/90, Fig. 1C), and low GSDMD-N expression ratio was 53.33% (48/90, Fig. 1D). In contrast, the high and low expression rates of GSDMD-N in the adjacent controls were 13.33% (4/30) and 86.67% (26/30), separately. It was the distinctions between 2 groups that were significant statistically (P < .05). GSDMD-N expression intensity didn`t correlate with the age of patients with breast carcinoma (P > .05). In the breast cancer samples, increased expression intensity of GSDMD-N, combined with smaller tumor size, correlated with lower pathological grade, lower clinical stage, and lower incidence of lymph node metastasis (P < .05, Table 1).
3.4. Expression intensities of NLRP3 and GSDMD-N in breast cancer tissue
The Spearman correlation between expression intensities of GSDMD-N and NLRP3 in 90 breast cancer tissues illuminated an evident positive relationship (R = 0.339, P = .001 [<.05], Table 2).
Table 2.
Correlation between NLRP3 and GSDMD-N proteins in 90 breast cancer tissues.
| NLRP3 | ||
|---|---|---|
| Low expression | High expression | |
| GSDMD-N | ||
| Low expression | 30 | 18 |
| High expression | 12 | 30 |
| r | 0.339* | |
| P-value | .001 | |
GSDMD-N = gasdermin D N-terminal, NLRP3 = nucleotide-binding oligomerization domain-like receptor protein 3.
Positive correlation.
3.5. Cox multi-factor analysis
The consequences of regression analysis indicated that the age of sick people suffering from the breast cancer did not have a significant impact on prognosis. However, the pathological grade; pathological TNM grade; tumor stage, tumor size; and lymphatic metastasis all had a significant positive correlation with bad prevision (all P < .05). NLRP3 and GSDMD-N expression also displayed a significant correlation with poor prognosis (P < .05); the lower the expression levels, the higher the risk of death (both P < .05) (Table 3).
Table 3.
Results of multivariate analysis of 90 patients with breast cancer.
| B | SE | Wald | P-value | RR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Age | −0.218 | 0.496 | 0.194 | .660 | 0.804 | 0.304 | 2.124 |
| Pathological grade | 1.458 | 0.564 | 6.689 | .010 | 4.299 | 1.424 | 12.980 |
| Tumor size | 2.199 | 0.487 | 20.365 | .000 | 9.012 | 3.468 | 23.417 |
| Lymph node metastasis | 1.531 | 0.633 | 5.851 | .016 | 4.621 | 1.337 | 15.969 |
| Pathological TNM staging | 1.217 | 0.505 | 5.805 | .016 | 3.376 | 1.255 | 9.082 |
| GSDMD-N | −2.116 | 0.750 | 7.949 | .005 | 0.121 | 0.028 | 0.525 |
| NLRP3 | −3.180 | 1.030 | 9.537 | .002 | 0.042 | 0.006 | 0.313 |
CI = confidence interval, GSDMD-N = gasdermin D N-terminal, NLRP3 = nucleotide-binding oligomerization domain-like receptor protein 3, RR = relative risk, SE = standard error.
3.6. Survival analysis
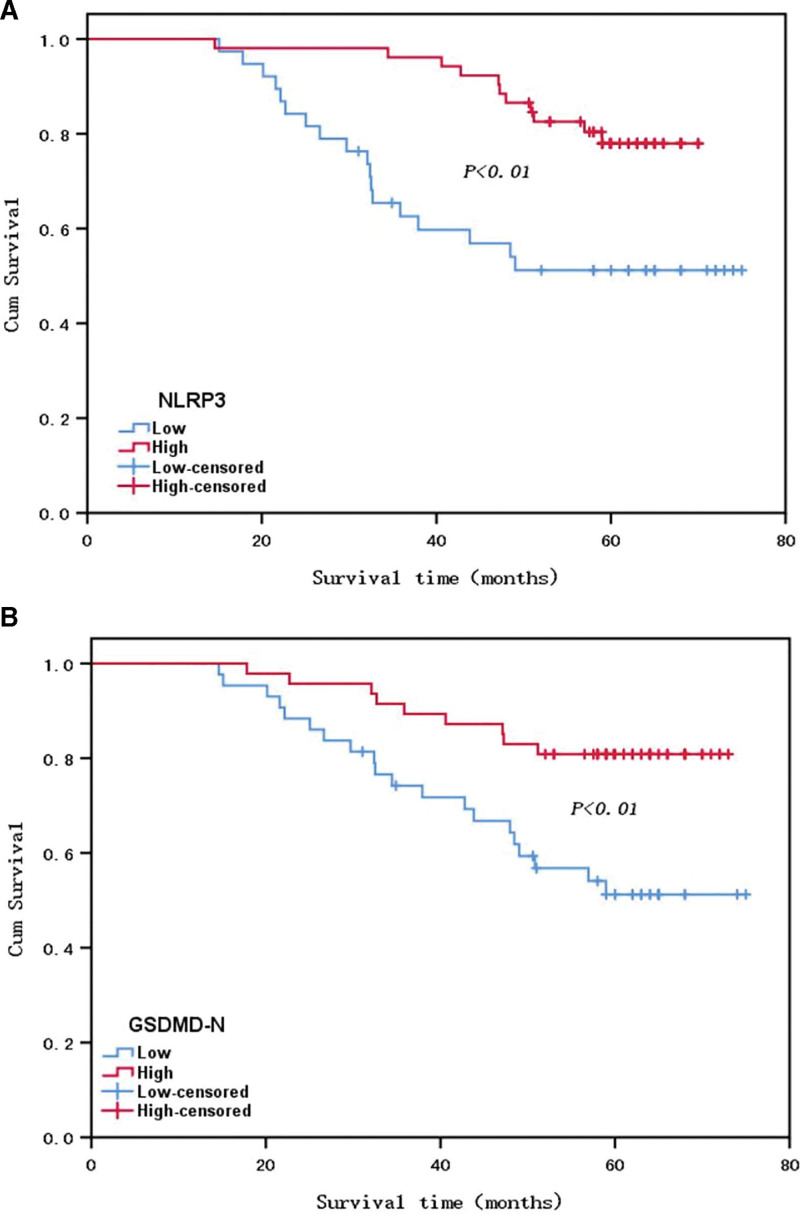
As to the 90 individuals with breast carcinoma in this study, their 5-year survival ratio was 70% (63/90). The Kaplan–Meier analysis of survival illustrated that the overall survival of individuals with breast cancer of the high NLRP3 expression team evidently surpassed that of the low expression team (P < .05, Fig. 2A). Similarly, the overall survival of individuals with breast cancer of the GSDMD-N high expression team evidently surpassed that of the low expression team (P < .05, Fig. 2B).
Figure 2.
Survival curves of patients with breast cancer in the high and low expression groups of NLRP3 (A) and gasdermin D N-terminal (GSDMD-N) (B) proteins.
4. Discussion
Cell pyrolysis is involved in the emergence and advancement of many diseases, including neurological diseases, infectious diseases, inflammatory immune diseases, cardiovascular diseases, metabolic diseases.[4–10] The classic pyrolysis pathway is regulated caspase-1. In contrast, the nonclassical pathway of pyrolysis is regulated by caspase-4/5/11. The classic pyrolysis pathway is currently the most widely studied and is initiated by the pattern recognition receptor to form inflammasomes to mediate pyrolysis. Pattern recognition receptors include transmembrane TLRs (Toll-like receptors), cytosolic proteins, along with the C-type lectin receptors,[11,12] which motivates the body’s intrinsic immune reaction through identifying pathogen-related molecular models and interacting with them. Apoptosis-related speck-like protein containing CARD (ASC) is 1 particular adaptor protein which comprises an N-terminal PYD (pyrin domain) as well as a CARD (C-terminal caspase recruitment domain). In the classic pyrolysis pathway, the pattern recognition receptor first indirectly connects to the precursor of caspase-1 through ASC to produce the inflammasome. This kind of inflammasome can activate the precursor of caspase-1 to make it active caspase-1, and caspase-1 is able to cleave the interleukin (IL)-18 precursor as well as IL-1β precursor into mature IL-18 and IL-1β, respectively. Caspase-1 simultaneously cleaves the gasdermin D (GSDMD) protein, cutting the N-terminal and C-terminal domains of the GSDMD protein. After being divided, the GSDMD-N-terminal (GSDMD-N) protein migrates from the cytoplasm to the cell membrane, and remains on the cell membrane where it accumulates form a membrane hole, thereby destroying the integrity of the cell membrane, and causing the cell to undergo pyroptosis. Mature IL-18 and IL-1β are secreted from the membrane pores to the cellular exterior and gather vast quantities of the inflammatory cells outside the cell to expand the inflammatory response.[12–17] The nonclassic pyrolysis pathway is mainly directly recognized by caspase-4/5/11 and combined with the endotoxin lipopolysaccharide of gram-negative bacteria. After being activated by oligomerization, it shows the corresponding protease activity.[18] The activated caspase-4/5/11 separates the GSDMD-N protein by cleaving the GSDMD protein which forms a membrane hole in the cell membrane to induce cell pyrolysis.[19–21]
NLRP3 is a crucial family member for the nucleotide-binding oligomerization domain-like receptor. It is an important pattern recognition receptor that can recognize pathogens, microorganisms, endogenous danger signals, and certain metabolites. NLRP3 is assembled into the NLRP3 inflammasome through ASC and the caspase-1 precursor. The inflammasome of NLRP3 is presently the attractive for study. The typical NLRP3 activating course is split into 2 phases: the first stage of TLR4 signaling pathway adjusted by pre-activation stage of increased transcription levels of NLRP3 and IL-1β precursors, and the second stage of NLRP3 posttranscriptional modification activation, in which nonclassic NLRP3 activation directly recognizes lipopolysaccharide through caspase-11. During the pyroptosis, NLRP3 plays an essential part in the host immune defense process; however, excessive inflammation and pyroptosis can cause irreversible damage to the body.[22–24]
Breast cancer has become one of the most common contributors to carcinoma deaths in female patients, and is a malignant carcinoma with one of the highest incidence rates among females.[25] There are no typical clinical manifestations in the early stages of breast cancer; therefore, most individuals with breast cancer are entering the middle or late phases when they see a doctor. Recently, the incidence of breast cancer in China has shown an upward trend,[26–28] which seriously threatens women’s lives. With the development of medicine, research on breast cancer has gradually improved. At present, there are many effective methods for treating breast cancer. Molecular targeted therapy for breast cancer has also been widely used in clinical treatments and experiments. The study of genes has also been a dominant center of study. Within current studies, the immunohistochemical ElivisionTM Plus method was employed with the aim of exploring the expression of the pyrolysis pathway proteins NLRP3 and GSDMD-N in 90 breast cancer tissue samples. We found the following: When the pathological TNM grade of breast cancer tumor tissue was higher, the TNM stage was higher. In addition, the larger the mass was, the lower expression intensities of GSDMD-N and NLRP3 were. Expression intensities of GSDMD-N and NLRP3 in the non-metastasis group obviously exceeded that in metastasis team. Analysis of Kaplan–Meier survival indicated that the overall survival time was significantly lower in low expression team of GSDMD-N and NLRP3 than in high expression group. The dissection of the Spearman relationship illustrated a positive relationship between expressions of GSDMD-N and NLRP3 in breast cancer tissue. This relationship is consistent with the pyrolysis pathway. The aforementioned results indicate that pyrolysis plays an essential part in the occurrence, promotion, invasion, as well as breast cancer metastasis. Tumor cell pyrolysis is beneficial for prognosis and therapy of individuals with breast cancer. Currently, the incidence of tumors is increasing every year, but there is still no effective way to cure tumors. Therefore, people are eager to develop new and more effective treatments. The discovery of pyrolysis represents a new breakthrough, and the role of pyrolysis in tumors is gradually attracting attention. As a form of programmed cell death, pyrolysis means that the growth of tumor cells can be inhibited, resulting in death, thus reducing tumor cells in patients with breast cancer. Pyrolysis may be a novel mechanism of antitumor immunity.
We are proud that there has been progress in treating tumors through the induction of cell pyroptosis. The research team at Wuhan University has developed a novel chemotherapy-photodynamic therapy combination treatment to enhance the efficacy of tumor immunotherapy. Their new regimen, which combines chemotherapy and photodynamic therapy, significantly improves the tumor targeting of traditional chemotherapy drugs and photosensitizers by forming a new type of engineered nanocarrier system. These nanocarriers can accumulate in large quantities at the tumor site while distributing less in normal tissues, thus avoiding systemic toxicity. Furthermore, after the nanocarriers accumulate in the tumor microenvironment, they can induce tumor cell pyroptosis, release inflammatory factors and tumor-associated antigens, activate antigen-presenting cells, and initiate adaptive immune responses. After combined immunotherapy, this regimen can significantly enhance the effectiveness of PD-1 blockade therapy, suppress tumor growth, and prevent tumor recurrence. This research indicates that enhancing tumor immunotherapy efficacy through the induction of tumor cell pyroptosis provides new insights for improving future immunotherapy regimens.[29]
5. Conclusions
Expression of the classic pyrolysis pathway proteins NLRP3 and GSDMD-N may be linked to the prognosis of individuals with breast carcinoma, and further, more in-depth studies thereof are still needed. The specific mechanism of pyrolysis has also not yet been fully elucidated and requires profound research. The pyrolysis pathway presents an attractive novel approach to breast cancer therapy that is expected to further improve the survival and cure rates of patients with breast cancer.
Acknowledgements
This study was supported by the Bengbu Medical College Graduate Research Innovation Program (Byycx1975).
Author contributions
Conceptualization: Ligao Wu.
Data curation: Ligao Wu.
Formal analysis: Ligao Wu.
Funding acquisition: Ligao Wu.
Investigation: Ligao Wu, Jinjie Liu.
Methodology: Ligao Wu, Jinjie Liu, Qing Zhu.
Project administration: Jinjie Liu, Qing Zhu.
Resources: Xia Wu, Jinjie Liu, Yuanli Huang, Qing Zhu.
Software: Xia Wu, Yuanli Huang, Qing Zhu.
Supervision: Ligao Wu, Yuanli Huang, Qing Zhu.
Validation: Yuanli Huang.
Writing – original draft: Ligao Wu.
Writing – review & editing: Ligao Wu.
Abbreviations:
- GSDMD-N
- gasdermin D N-terminal
- NLRP3
- nucleotide-binding oligomerization domain-like receptor protein 3
LW and XW contributed equally to this work.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Wu L, Wu X, Liu J, Huang Y, Zhu Q. Expression and significance of effector proteins NLRP3 and gasdermin D N-terminal protein in the pyrolysis pathway in breast cancer. Medicine 2023;102:40(e35440).
Contributor Information
Xia Wu, Email: 2199902701@QQ.COM.
Jinjie Liu, Email: 8945263@163.COM.
Yuanli Huang, Email: 545628629@163.COM.
Qing Zhu, Email: 49143313@QQ.COM.
References
- [1].Broz P. Immunology: caspase target drives pyroptosis. Nature. 2015;526:642–3. [DOI] [PubMed] [Google Scholar]
- [2].Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. [DOI] [PubMed] [Google Scholar]
- [3].Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. [DOI] [PubMed] [Google Scholar]
- [4].McKenzie BA, Dixit VM, Power C. Fiery cell death: pyroptosis in the central nervous system. Trends Neurosci. 2020;43:55–73. [DOI] [PubMed] [Google Scholar]
- [5].Al Mamun A, Ara Mimi A, Wu Y, et al. Pyroptosis in diabetic nephropathy. Clin Chim Acta. 2021;523:131–43. [DOI] [PubMed] [Google Scholar]
- [6].He B, Nie Q, Wang F, et al. Role of pyroptosis in atherosclerosis and its therapeutic implications. J Cell Physiol. 2021;236:7159–75. [DOI] [PubMed] [Google Scholar]
- [7].Brokatzky D, Mostowy S. Pyroptosis in host defence against bacterial infection. Dis Model Mech. 2022;15:dmm049414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wei Y, Yang L, Pandeya A, et al. Pyroptosis-induced inflammation and tissue damage. J Mol Biol. 2022;434:167301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tian K, Yang Y, Zhou K, et al. The role of ROS-induced pyroptosis in CVD. Front Cardiovasc Med. 2023;10:1116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mulla J, Katti R, Scott MJ. The role of Gasdermin-D-mediated pyroptosis in organ injury and its therapeutic implications. Organogenesis. 2023;19:2177484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sarhan J, Liu BC, Muendlein HI, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115:E10888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nozaki K, Li L, Miao EA. Innate sensors trigger regulated cell death to combat intracellular infection. Annu Rev Immunol. 2022;40:469–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bernard EM, Broz P. Activation and manipulation of inflammasomes and pyroptosis during bacterial infections. Biochem J. 2022;479:867–82. [DOI] [PubMed] [Google Scholar]
- [14].Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27:673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Elias EE, Lyons B, Muruve DA. Gasdermins and pyroptosis in the kidney. Nat Rev Nephrol. 2023;19:337–50. [DOI] [PubMed] [Google Scholar]
- [16].Hachim MY, Khalil BA, Elemam NM, et al. Pyroptosis: the missing puzzle among innate and adaptive immunity crosstalk. J Leukoc Biol. 2020;108:323–38. [DOI] [PubMed] [Google Scholar]
- [17].Ryder CB, Kondolf HC, O’Keefe ME, et al. Chemical modulation of gasdermin-mediated pyroptosis and therapeutic potential. J Mol Biol. 2022;434:167183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aki T, Funakoshi T, Unuma K, et al. Inverse regulation of GSDMD and GSDME gene expression during LPS-induced pyroptosis in RAW264.7 macrophage cells. Apoptosis. 2022;27:14–21. [DOI] [PubMed] [Google Scholar]
- [19].Mascarenhas DPA, Cerqueira DM, Pereira MSF, et al. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog. 2017;13:e1006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Al Mamun A, Suchi SA, Aziz MA, et al. Pyroptosis in acute pancreatitis and its therapeutic regulation. Apoptosis. 2022;27:465–81. [DOI] [PubMed] [Google Scholar]
- [21].Place DE, Kanneganti TD. Recent advances in inflammasome biology. Curr Opin Immunol. 2018;50:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Coll RC, Schroder K, Pelegrín P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43:653–68. [DOI] [PubMed] [Google Scholar]
- [23].Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18:2114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lucas-Ruiz F, Peñín-Franch A, Pons JA, et al. Emerging role of NLRP3 inflammasome and pyroptosis in liver transplantation. Int J Mol Sci. 2022;23:14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferlay J, Ervik M, Lam F, et al. Global cancer observatory:cancer today [EB/OL]. (2020/01/01) [2022/11/07]. Available at: https://gco.iarc.fr/today.
- [26].Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lei S, Zheng R, Zhang S, et al. Breast cancer incidence and mortality in women in China: temporal trends and projections to 2030. Cancer Biol Med. 2021;18:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shi Z, Lin J, Wu Y, et al. Burden of cancer and changing cancer spectrum among older adults in China: trends and projections to 2030. Cancer Epidemiol. 2022;76:102068. [DOI] [PubMed] [Google Scholar]
- [29].Xiao Y, Zhang T, Ma X, et al. Microenvironment Responsive Prodrug Induced Pyroptosis Boosts Cancer Immunotherapy. Adv Sci (Weinh). 2021;8:e2101840. [DOI] [PMC free article] [PubMed] [Google Scholar]