Helicobacter pylori is the causative agent of chronic superficial gastritis and contributes to the pathogenesis of peptic ulcer disease, mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma (4). The antimycotic miconazole has been shown to be bacteriostatically active against H. pylori (6, 8); however, details of the action of the substance are not known. The aim of the present study was therefore to investigate the morphological changes induced by miconazole.
H. pylori IMMi 95-451 was cultured microaerophilically in 3.0 ml of brain heart infusion broth (CM 225; Oxoid, Basingstoke, United Kingdom) supplemented with 10% newborn calf serum (no. 14-416 A; Serva, Heidelberg, Germany) and 0.25% yeast extract (no. 30-000-49; Flow, Meckenheim, Germany) (BNY broth) in tissue culture flasks with a volume of 50 ml (no. 690160; Greiner, Frickenhausen, Germany). The BNY broth cultures reached a cell density of at least 3 × 108 cells per ml. To obtain cultures with >90% intact and viable cells, two transfers of 1.5 ml of H. pylori culture in 1.5 ml of fresh BNY broth were made after 24 and 48 h. To the 48-h cultures, miconazole (Biotrend, Cologne, Germany) was added to reach a final concentration of 16 μg/ml. After another 24 h of incubation, 3.0-ml suspensions of treated cultures and controls without antibiotic were fixed for 1 h in 2% glutaraldehyde in cacodylate buffer (100 mmol) and then in 1% osmium tetroxide and 2% uranyl acetate. The organisms were deposited by centrifugations for 2 min at 8,000 × g. The final cell pellet was dehydrated in acetone and embedded in glycidether (Epon 812). Sections were mounted on copper grids and stained for 20 s with 4% lead citrate. Microphotographs were obtained with a Zeiss EM 10 transmission electron microscope at an accelerating voltage of 60 kV.
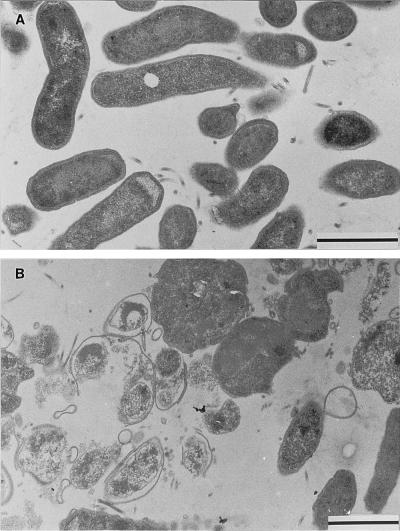
Most of the untreated control cells appeared as slightly curved or straight bacilli. The cells with intact membranes and a dense cytoplasm had a diameter of about 0.5 μm (Fig. 1A). Cultures exposed to miconazole for 24 h revealed few bacteria with intact shape and structure. Most cells were swollen, with diameters up to 1.5 μm, or heavily destroyed (Fig. 1B). However, intact flagellar and submembranous dense structures were still found in the treated cells. Round cell forms with intact cytoplasms and membranes were rarely found in treated cultures.
FIG. 1.
Effect of miconazole on the structure of H. pylori cells (strain IMMi 95-451). (A) Typically curved bacteria with polar flagellae in untreated controls are seen. (B) Swollen and destroyed bacteria predominate after treatment for 24 h with 16 μg of miconazole per ml. Bar represents 1 μm.
The inhibitory activity of miconazole (MIC range of 2 to 32 μg/ml) and of other nitroimidazole antimycotics against H. pylori was recently noted (6–8). Until now, bactericidal activity of miconazole against H. pylori has not been documented. The electron microscopic investigations prove the destructive action of miconazole on H. pylori. The replacement of the normal bacilliform morphology by cell wall blebbing, lytic cells, and vesiculation was similar to the changes seen with beta-lactam antibiotics, shown in an earlier study (2). However, in contrast to the observations of Berry et al. (3) on the effects of amoxicillin against H. pylori, round or coccoid cell forms were not induced by the miconazole treatment.
Imidazole antimycotics impair ergosterol synthesis of the cytoplasmic membrane in yeasts and molds. It can be speculated that these substances may interfere with cholesterol metabolism in H. pylori. This is unique and characteristic of this organism (1, 5).
REFERENCES
- 1.Ansorg R, Müller K-D, von Recklinghausen G, Nalik H P. Cholesterol binding of Helicobacter pylori. Zentralbl Bakteriol. 1992;276:323–329. doi: 10.1016/s0934-8840(11)80538-4. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong J A, Wee S H, Goodwin C S, Wilson D H. Response of Campylobacter pyloridis to antibiotics, bismuth and an acid-reducing agent in vitro—an ultrastructural study. J Med Microbiol. 1987;24:343–350. doi: 10.1099/00222615-24-4-343. [DOI] [PubMed] [Google Scholar]
- 3.Berry V, Jennings K, Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob Agents Chemother. 1995;39:1859–1861. doi: 10.1128/aac.39.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J. Helicobacter pylori and related organisms. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone; 1995. pp. 1956–1964. [Google Scholar]
- 5.Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K. Unique cholesteryl glycosides in Helicobacter pylori: composition and structural analysis. J Bacteriol. 1995;177:5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rautelin H, Renkonen O-V, Kosunen T U. In vitro activity of antifungal azoles against Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1992;11:273–274. doi: 10.1007/BF02098101. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 7.von Recklinghausen G, di Maio C, Ansorg R. Activity of the antimycotic ketoconazole against Helicobacter pylori. J Antimicrob Chemother. 1992;30:238–240. doi: 10.1093/jac/30.2.238. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 8.von Recklinghausen G, di Maio C, Ansorg R. Activity of antibiotics and azole antimycotics against Helicobacter pylori. Zentralbl Bakteriol. 1993;280:279–285. doi: 10.1016/s0934-8840(11)80966-7. [DOI] [PubMed] [Google Scholar]